Abstract
Vitamin D has been previously recognized to play important roles in human immune system and function. In the pulmonary system, vitamin D regulates the function of antimicrobial peptides, especially cathelicidin/LL-37. Human cathelicidin/LL-37 is a bactericidal, bacteriostatic, and antiviral endogenous peptide with protective immune functions. Chronic exposure to excessive alcohol has the potential to reduce levels of vitamin D (inactive vitamin D [25(OH)D3] and active vitamin D [1, 25(OH)2D3]) and leads to downregulation of cathelicidin/LL-37. Alcohol-mediated reduction of LL-37 may be partly responsible for increased incidence of more frequent and severe respiratory infections among subjects with alcohol use disorder (AUD). The objective of this study was to investigate the mechanisms by which alcohol exerts its influence on vitamin D metabolism. In addition, the aim was to establish associations between chronic alcohol exposures, levels of pulmonary vitamin D, and cathelicidin/LL-37 using broncho-alveolar lavage fluid samples of subjects with AUD and healthy controls. Findings from the experiment showed that levels of inactive vitamin D (25(OH)D3), active vitamin D (1, 25(OH)2D3), cathelicidin/LL-37, and CYP27B1 proteins were significantly reduced (P < 0.05) when compared with the matched healthy control group. However, CYP2E1 was elevated in all the samples examined. Chronic exposure to alcohol has the potential to reduce the levels of pulmonary vitamin D and results in subsequent downregulation of the antimicrobial peptide, LL-37, in the human pulmonary system.
Keywords: Excessive ethanol, vitamin D, cathelicidin, pneumonia, CYP2E1, CYP27B1
Introduction
In the United States, from 2006 to 2010, excessive use of alcohol has led to approximately 88,000 deaths and 2.5 million years of potential life lost each year.1 Furthermore, excessive drinking has been responsible for 1 in 10 deaths among working-age adults between the ages of 20 and 64 years. The economic costs of excessive alcohol consumption in 2010 were estimated at $249 billion.2,3 According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA) fact sheet, drinking habit that becomes severe is given the medical diagnosis of “alcohol use disorder” (AUD). Approximately 17 million adults (7.2%) in the United States aged 18 years and older had an AUD in 2012, representing 11.2 million men and 5.7 million women. In addition, in 2012, an estimated 855,000 adolescents aged 12–17 years had an AUD diagnosis. AUD is characterized by 11 criteria according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, published by the American Psychiatry Association in 2013.4,5 These symptoms/criteria include the following: evidence of social impairment, being physically hazardous to self and others, having psychological issues, showing signs and symptoms of withdrawal, engaging in copious amount of drinks in order to be high and in disregard to injury, having interpersonal issues, manifesting evidences of obligation impairment, devoting enormous amount of time in getting drunk, having uncontrolled desire to drink alcohol even when it is highly injurious and inconvenient, having histories of attempts and unsuccessful efforts in quitting drinking, and finally, requiring large amount of alcohol and longer period to get drunk. A manifestation of two or more of these symptoms qualifies an individual to be diagnosed with AUD.4,5 The lifetime AUD prevalence is 29.1% (68.5 million people) and the 12-month prevalence is 13.9% (32.6 million adults).5–9
Published studies have shown that chronic ethanol overconsumption interferes with activities and functions of essential vitamins and nutrients, including folic acid and vitamins D, C, and E.10,11 Chronic ethanol consumption also has a deleterious effect on lung function. People who consumed excessive alcohol have been shown to develop more frequent and severe upper respiratory tract infections, community-acquired pneumonia, and acute respiratory distress syndrome.9,12,13
According to studies conducted in murine animal models, compared with the control group, 1,25(OH)2D3 levels in 8-week ethanol-fed mice showed statistically significant reductions (42%) in broncho-alveolar lavage fluid (BALF) samples of mice. In the United States, the number of individuals with AUDs who die from bacterial pneumonia annually approximates those who die due to more widely acknowledged alcohol-related conditions, including pancreatitis and trauma. People who chronically overconsume alcohol are prone to lung infection, particularly severe bacterial lung infection.6,7,14–20
Our experiment is significant by investigating these associations using human BALF samples. The objective of this study was to analyze and compare the cellular contents of BALF obtained from patients who chronically consume alcohol excessively and have been diagnosed as having an AUD, as compared with healthy controls who do not consume alcohol excessively.
Methods
BALF experiments
Biological samples of BALF were obtained from Colorado Pulmonary-Alcohol Research Consortium (CoPARC) Biobank. In addition, demographic and respiratory health survey results collected from ethanol overconsumers surveyed at the consortium sites (hospitals in Atlanta and Denver) were obtained from CoPARC. CoPARC was established to develop new interventions for individuals with AUD with the aim of decreasing their predisposition to pneumonia infection. With the support of the NIAAA, CoPARC has been funded to conduct translational investigations to complement and extend basic science observations pertaining to subjects with AUD.
Upper airway bronchial washings and lower airway BALF samples from subjects with AUD and healthy controls were analyzed for vitamin D metabolites, cathelicidin/LL-37, and selected Phase I metabolizing enzymes (CYP2E1, CY27B1) involved in vitamin D metabolic pathways by utilizing techniques described in assay protocols. Samples from subjects and controls were added to the appropriate labeled tubes. The BALF samples were treated and analyzed according to the enzyme-linked immunosorbent assay protocol for different protein concentrations. The ELISA protocol involves transferring duplicate samples to the appropriate wells for culture and incubation. Addition of diluted streptavidin–peroxidase and tetramethyl-benzidine (TMB) substrate to the cultured samples. Next was the addition of 1 mL of 25-D biotin solution, diluted control (CTRL), 200 µL of enzyme conjugate, and 200 µL of TMB substrate to enhance protein response as demonstrated by color development. The color development was stopped by the addition of 100 µL of stop solution (HCl). The absorbance was measured at 450 nm using the microplate reader within 10 minutes of adding stop solution.
The original proteins present in the samples were measured using Protein A280 in NANODROP 2000/2000c (Thermo Fisher Scientific Inc.).
Bronchoscopy
Study participants were given consent forms that explained the procedure and the risks to them before being enrolled for this study. Participants’ vital signs, including heart rate, blood pressure, breathing rate, and the level of oxygen in the blood, were measured as baseline data. Participants were instructed to neither eat at least six hours prior to the procedure nor have any alcohol to drink 24 hours before the procedure.
A small plastic catheter (intravenous, IV) was placed in a vein in their arm, and about 2.5 mL of blood was collected. In addition, participant’s saliva was collected by having them gargle for a total of 60 seconds with 10 mL of salt water. After gargling, the participants were asked to spit the salt water into a cup.
A local anesthetic agent (lidocaine) was sprayed in their throats and noses. This was performed to prevent coughing and discomfort. Fentanyl or midazolam was given through the IV catheter to help to relax the participants.
The bronchoscope is made of a flexible fiber-optic material and has a light source and a viewing device or camera at the end. This tube was passed through the nose and down the throat to reach the lung. It was held steady in a part of the lung. First, up to three soft, flexible brushes were inserted one at a time through the bronchoscope to gently scrape off cells from inside the lung. The brushes were removed after brushing the inside of the lungs. Next, 1 1/2 ounces of sterile salt water (saline) was injected through the bronchoscope into the lung. This was performed up to six times. The salt water was immediately taken out through the bronchoscope and collected in a container. The total amount of time that each procedure took from start to finish for the bronchoscopy procedure was about four hours. The actual procedure took about 20 minutes. Participants were watched for about one more hour after the procedure and then discharged when they were fully awake and stable.
The bronchoscopy test was conducted at the University of Colorado Hospital, Colorado Clinical and Translational Sciences Institute, for the study participants at the Denver center and Veterans Hospital for the study participants at the Atlanta center.
Oral wash and BALF processing (alcoholics and controls)
Oral wash and saline control samples from the study participants were transferred to sterile 15-mL conical tubes and centrifuged at 4 °C, 14,000 × g for 20 minutes. The supernatant was discarded, and the pellet was resuspended in 0.5-mL sterile phosphate-buffered saline. This was then transferred to a labeled 2-mL clear micro-centrifuge tube (Fisher #05-408-138). The samples were vortexed on high setting for 1 minute and stored at −80 °C.
Subjects and controls
Denver center
Subjects with AUD have been identified using a validated survey. Subjects and controls were recruited voluntarily in Denver and Atlanta. Prior to enrollment, they were provided with a consent form that was read and explained to them. The subjects were also allowed to study the consent forms and research protocols extensively, before they were allowed to sign and enroll in the research. They underwent research sampling in the morning following the admission to ensure that issues with alcohol withdrawal have been appropriately addressed. Subjects with AUD were monitored closely for alcohol withdrawal using a standardized Clinical Institute Withdrawal Assessment for Alcohol protocol and provided symptom-driven benzodiazepines as needed. Moreover, daily multivitamin, thiamine, and folate were provided to participants. Daily rounds by the site principal investigator on admitted subjects were conducted. After protocol completion, subjects were discharged with a responsible adult, or after 24 hours have elapsed since bronchoscopy. Subjects with AUD were reimbursed with supermarket gift cards that cannot be used for the purchase of alcohol. In order to reduce confounders in our research, we controlled for cigarette and marijuana smoking by excluding participants who reported that they have these behaviors. After controlling for confounders, participants were reduced to 40: 20 subjects with AUD and 20 control subjects.
AUD subjects
Inclusion criteria
The inclusion criteria were based on the Alcohol Use Disorders Identification Test (AUDIT) score of ≥8 for men, ≥5 for women; whose last alcohol-containing beverage was consumed within the seven days prior to enrollment. AUDIT is a 10-question test developed by the World-Health-Organization-sponsored collaborative project to determine whether a person may be at risk for alcohol abuse problems.21 In order to score the AUDIT, point values of each answer choice are summed together and then interpreted based on the following criteria:21,22 (a) a score of eight or more in men (seven in women) indicates a strong likelihood of hazardous or harmful alcohol consumption. (b) A score of 20 or more is suggestive of alcohol dependence (although some authors quote scores of more than 13 in women and 15 in men as indicating likely dependence).23 Participants who smoke cigarettes and marijuana were excluded from the study’s sample.
Exclusion criteria
The exclusion criteria (in addition to cigarette and marijuana users) included history of comorbidity requiring daily medication (except hypertension), concurrent illicit drug use, abnormal chest radiograph, spirometry remarkable for a forced expiratory volume in one second or forced vital capacity of <80% predicted, and age <18 years or >55 years.
Controls subjects
Control subjects were also recruited and pair matched to subjects with AUD based on age and gender.
Inclusion criteria
The inclusion criteria for control subjects were based on an AUDIT score of <2 for men, <1 for women.
Exclusion criteria
Control subjects were ineligible to participate if they meet any of the criteria set forth for subjects with AUDs as listed earlier.
Atlanta center
We also obtained some BALF samples from the center in Atlanta, Georgia, in order to compare the levels of enzymes and BALF response experiments based on geography, race, and gender. Most of the samples obtained from Atlanta were not controlled for cigarette smoking.
Human subject protection
The sample collection of BALF in Denver and Atlanta was approved by the University of Colorado Institutional Review Board (IRB) and Veterans Affairs (VA) hospital, Atlanta, respectively. The overall study was approved by a memorandum of understanding between the University of Colorado and Tulane University IRB.
Statistical analysis
All quantitative experiments were performed independently and in triplicates (minimum of three separate times). Experimental data were analyzed using GraphPad Prism Version 5.00 for Windows (GraphPad Software Inc., San Diego, CA, USA). For each experiment, the data obtained were used to approximate a statistical model using nonlinear regression. The mean and standard deviations were reported to compare subjects and controls. When comparing three or more groups, a repeated measure analysis of variance (P < 0.05) followed by Bonferroni’s post hoc test was used to control for multiple comparisons. We used two-tailed Student’s t-test to identify any difference in the means between the two groups (subjects versus control). All results were graphed to visually represent gradual measurement changes within each group and between each group. A P (probability) value of < 0.05 was considered statistically significant.
Responses from the CoPARC questionnaire of subjects with AUD and healthy control were analyzed using a chi-square analysis on SPSS Statistics 19 program (IBM Corporation).
Results
BALF levels of 25(OH)D3 and 1,25 (OH)2D3 in AUD and control subjects (Denver center)
When compared with the control group, the level of 25(OH)D3 was reduced by 40% among subjects with AUD. In a similar trend, approximately 35% reduction was observed in the concentration of 1, 25 (OH)2D3 recorded among subjects with AUD when compared with healthy controls (Fig. 1).
Figure 1.
BALF levels of 25(OH)D3 and 1,25 (OH)2D3 in AUD and control subjects.
Notes: Left: Level of 25(OH)D3 in BALF samples of AUD subjects was statistically reduced by 40% when compared to BALF samples from healthy control subjects. Right: BALF samples of AUD subjects showed 35% reduction in the level of 1,25 (OH)2D3 when compared to healthy control subjects. NB: Ethanol group represents the AUD groups.
Cathelicidin/LL-37 levels in upper and lower BALF samples of AUD and control subjects
When compared with the control group, the concentration of LL-37 proteins in the upper BALF of subjects with AUD was reduced (not statistically significant). A similar nonstatistically significant reduction was observed when LL-37 protein level was quantified in lower BALF samples of subjects with AUD when compared with the healthy control group (Fig. 2).
Figure 2.
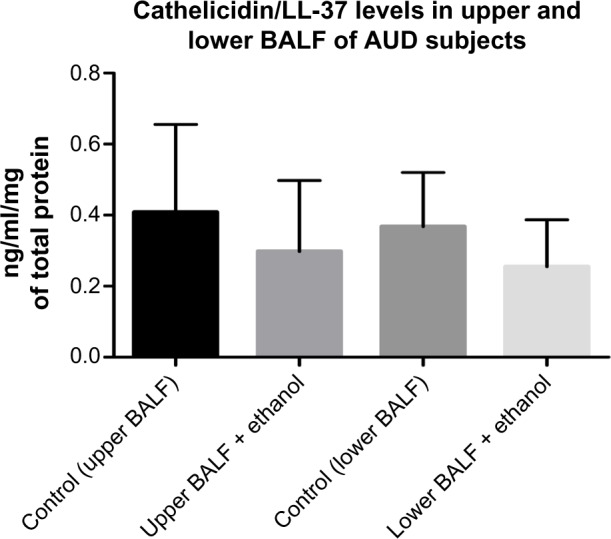
Cathelicidin/LL-37 levels in Upper and Lower BALF samples of AUD and control.
Notes: Levels of LL-37 were reduced (not statistically) in both upper and lower BALF samples of AUD subjects when compared to their respective healthy control subjects. In both scenarios, lower levels of LL-37 were recorded in the lower BALF samples of the study participants when compared to the upper BALF samples from the same study population, (n = 40)
CYP2E1 levels in BALF samples of AUD and control subjects
When we analyzed upper BALF samples of AUD and control subjects for the concentration of CYP2E1 proteins, we observed an increased level (40%) of this enzyme (not significantly) in BALF samples from subjects with AUD when compared with control subjects. We did not observe any significant difference in the levels of CYP2E1 in the lower BALF samples of subjects with AUD (slight 5% increase) when compared with the control group (Fig. 3).
Figure 3.
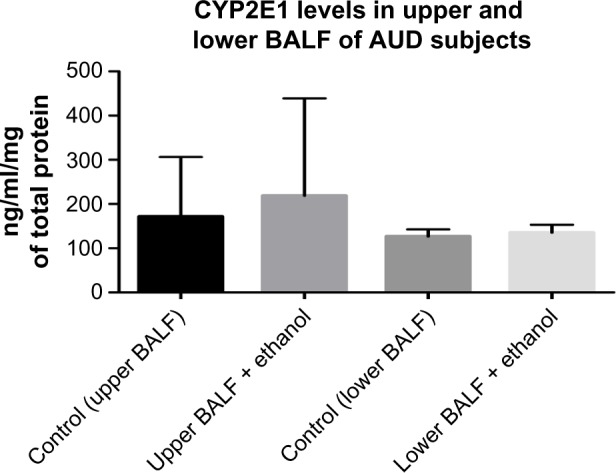
CYP2E1 protein levels in BALF samples of AUD and Control subjects.
Notes: The level of CYP2E1 was increased (non-statistically) by 40% in the upper BALF samples of AUD subjects when compared to healthy control subjects. Similar trend was observed in the lower BALF samples of AUD subjects with an increase (non-statistically) of about 5% when compared to the control group (n = 40).
CYP27B1 levels in BALF of subjects with AUD and controls
Levels of CYP27B1, the primary vitamin D-activating enzyme, was significantly reduced by approximately 60% in the upper BALF samples of subjects with AUD when compared with the control group. However, in the lower BALF samples, the reduction in CYP27B1 enzymes was not statistically significant (reduced by 20%) between subjects with AUD and the matched control groups (Fig. 4).
Figure 4.
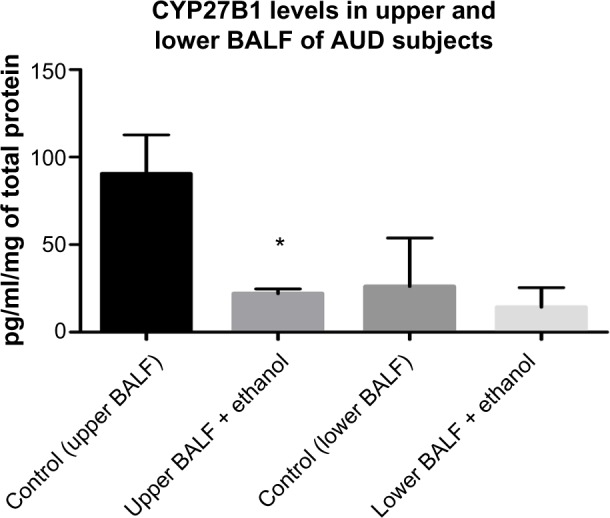
CYP27B1 Levels in BALF of AUD Subjects and Control.
Notes: The level of CYP27B1 was significantly reduced statistically by 60% in the upper BALF samples of AUD subjects when compared to healthy control subjects. In the lower BALF samples of AUD subject, a 20% reduction (non-statistically) was observed when compared to the control group (n = 40).
Levels of 1, 25 (OH)2D3 in BALF samples of AUD and control subjects (Atlanta center)
When compared with the control group, the concentration of 1, 25 (OH)2D3 in the BALF samples of subjects with AUD was statistically reduced by approximately 50%, following a similar trend previously observed among the study participants in Denver (Fig. 5).
Figure 5.
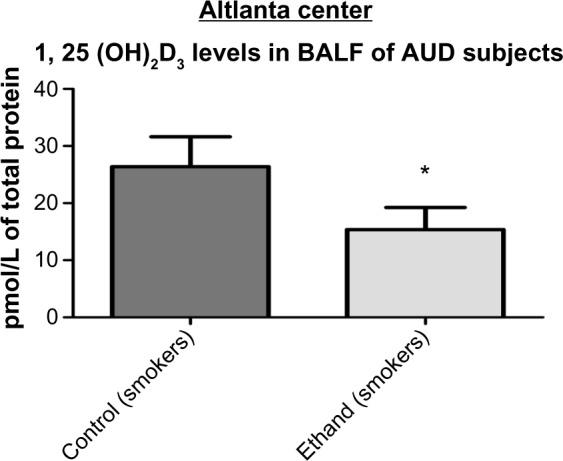
Levels of 1, 25 (OH)2D3 in BALF samples of AUD and control subjects Levels of 1, 25 (OH)2D3 in the BALF samples of AUD subjects were statistically reduced by 50% (from 28 to 14 pmol/L of total protein) when compared to the control group P < 0.0028 < 0.05 (n = 33).
Comparing levels of 1, 25 (OH)2D3 between BALF samples of AUD and control subjects from Denver and Atlanta
When we compared the levels of 1, 25 (OH)2D3 among the study participants from two different study centers, Denver and Atlanta, we observed a significant reduction among the study participants in Atlanta, in both subjects with AUD and control subjects, when compared with the study participants in Denver (Fig. 6).
Figure 6.
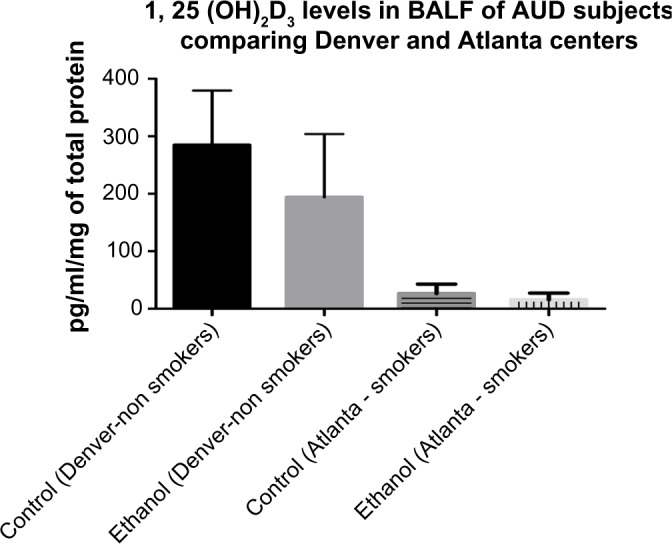
Comparing levels of 1, 25 (OH)2D3 between BALF samples of AUD and control subjects from Denver and Atlanta.
Notes: When compared to control subjects in Denver, the levels of 1, 25 (OH)2D3 in BALF samples of control subjects in Atlanta were reduced statistically by more than 80%. Similarly, when comparing levels of 1, 25 (OH)2D3 in BALF samples of AUD study participants, the 1, 25 (OH)2D3 levels among the Atlanta group was statistically reduced by 65% when compared to the Denver group, P* 0.0001 (as indicated by the *and ∼ sign).
Demographic distribution of study participants from Denver center
With regard to race among subjects with AUD from the study population in Denver center, 45% were Hispanic or Latino, while 55% were non-Hispanic or Latino. Among the controls, 10% were Hispanic or Latino, while 90% were non-Hispanic or Latino (Table 1A). The ethnic distribution among the study participants followed a similar trend as was observed in Table 1A. Among subjects with AUD, 10% were American Indian or Alaska native, 35% were native Hawaiian or other Pacific Islander, 45% were White, while 10% of the study population did not indicate their ethnicity. Among the control group, 15% were Black or African American, while the remaining 85% were White (Table 1B). Among subjects with AUD, 25% were females, while we had 75% of males participating in the study. In the control group, 75% were males and 25% were females (Table 1C).
Table 1.
Racial, ethnic, and gender distribution of study participants (Denver center).
| NO | FACTORS | AUD SUBJECTS | CONTROL SUBJECTS |
|---|---|---|---|
| A | Race | ||
| Hispanic or Latino | 9 | 2 | |
| Not Hispanic or Latino | 11 | 18 | |
| Don’t know | – | – | |
| Refused | – | – | |
| Total | 20 | 20 | |
| B | Ethnicity | ||
| American Indian/Alaska native | 2 | – | |
| Asian | – | – | |
| Black or African American | – | 3 | |
| Native Hawaiian or other Pacific Islander | 7 | – | |
| White | 9 | 17 | |
| Don’t know | – | – | |
| Refused | – | – | |
| Unchecked | 2 | – | |
| Total | 20 | 20 | |
| C | Gender | ||
| Male | 15 | 15 | |
| Female | 5 | 5 | |
| Total | 20 | 20 |
Notes: (A) Among AUD subjects, 45% were Hispanic or Latino, while 55% were Not-Hispanic or Latino. Among the control subjects, 10% were Hispanic or Latino, while 90% were Not-Hispanic or Latino. (B) Among AUD subjects, 10% were American Indian or Alaska Native, 35% were Native Hawaiian or other Pacific Islander, 45% were white and 10% did not indicate their ethnicity. Among the control group, 15% were Black or African American, while 85% were White. (C) Among AUD subjects, 25% were female, while the remaining 75% were male. The same distribution was observed among the control subjects. Overall, among the study participants, 75% were male and 25% were female.
Demographic distribution of study participants from Atlanta center
In terms of racial distribution in the Atlanta center, approximately 91% of the study participants were African American, while 9% were Caucasian. Among the control population, 87.5% were African American while the remaining 12.5% of the study population were White (Table 2A). Among the study participants (AUD) from Atlanta center, 86% were males and the remaining 14% were females (Table 2B). Among the matched healthy controls, there were no females at all, where it was a 100% male participation.
Table 2.
Racial, ethnic, and gender distribution of study participants (Atlanta center).
| NO | FACTOR | AUD SUBJECTS | CONTROL SUBJECTS |
|---|---|---|---|
| A | Race | ||
| African American | 20 | 14 | |
| White | 2 | 2 | |
| Total | 22 | 16 | |
| B | Gender | ||
| Male | 19 | 16 | |
| Female | 3 | 0 | |
| Total | 22 | 16 |
Notes: (A) Among AUD subjects, 9.1% were White and the remaining 90.9% were African Americans. Among the control subjects, 11% were White and the rest, 89% were African Americans. (B) Among the AUD subjects, approximately 14% were female, while 86% were male. Among the control subjects, there were no female, all the participants were 100% male. In total, 92% of the study participants were male, while 8% were female.
Discussion
Chronic exposure to excessive ethanol has various potential adverse and deleterious outcomes in human systems. According to the earlier studies, antibacterial peptides and proteins remain core network of epithelial defense barrier that helps protect against bacterial invasion.14,24–27 As shown by our experiment, the levels of both 25(OH)D3 and 1, 25 (OH)2D3 were reduced among subjects with AUD when compared with healthy controls (Fig. 1). This is probably due to adverse effects of excessive ethanol exposure on the metabolism of vitamin D by depleting enzymes involved in converting 25 (OH)D3 to 1, 25 (OH)2D3 (Fig. 4). Other published works have also shown that there were no statistical changes by chronic ethanol overconsumption in the serum levels of 25(OH)D3; however, statistically significant reduction was observed in the lung tissue levels of 25(OH)D3 when compared with the control group.13 As shown by our results (Fig. 2), these earlier studies alluded to increase in the concentration of antimicrobial peptide activity in BALF samples from healthy control subjects and patients with sarcoidosis. Following chronic ingestion of excessive alcohol, the levels of LL-37 proteins were reduced in upper and lower BALF samples of subjects with AUD, when compared with their respective controls. This further corroborates our hypotheses that alcohol has an inhibitory effect on vitamin D metabolism and subsequently the upregulation of LL-37, antimicrobial peptides. These antimicrobial peptides, LL-37, are aggregated in alveolar macrophages, bronchial epithelial cells, and bronchial glands, and they are activated to recruit other antimicrobial peptides in fighting off invading pathogens in the pulmonary system, further confirming their defensive functions in airway mucosa.14,24,28–32 Studies have shown that lower levels of 25 (OH)D3 and consequently, 1, 25 (OH)2D3, can result in reduced immune function and response, leading to an increase in the prevalence of community-acquired and bacterial pneumonia among vulnerable populations, such as subjects with AUD.29,33 Ethanol disruption of enzymes involved in vitamin D metabolism is mediated by CYP2E1, an enzyme that is involved in ethanol metabolism directly or by generating reactive oxidative metabolites such as malondialdehyde, 4-hydroxynonenal, and hydroxyethyl radical.13,34 This is probably responsible for the high levels of CP2E1 observed in BALF samples of subjects with AUD when compared with the control group (Fig. 3).
The BALF samples obtained from Denver center were controlled for smoking. This is because numerous studies have shown that smoking is a potent cofounder that contributes to vitamin D deficiency among vulnerable populations.27,35–45 However, because of the resulting small sample size after controlling for smoking, we were also interested in documenting the effects of smoking on the analysis of BALF samples when smoking was not controlled for as a potential confounder. Hence, samples obtained from Atlanta, Georgia, were not controlled for smoking.
As a result of the analysis, we discovered that subjects with AUD who smoke cigarettes have statistically significant lower levels of 1, 25 (OH)2D3 when compared with controls who do not drink alcohol, but smoke cigarettes (Fig. 5). Our results established that excessive consumption of ethanol and smoking has far worse adverse effects on vitamin D metabolism and consequently less efficient immune response and function in the pulmonary system.
When compared, BALF samples from Denver, Colorado, had higher levels of 1, 25 (OH)2D3 when compared with samples from Atlanta, Georgia. This further confirmed that smoking, a potential confounding factor, contributes (mechanism not known) to disruption of vitamin D metabolism (Fig. 6). We plan to investigate this mechanism in a future research project.
Public health significance
One of the objectives of this study was to evaluate the public health significance of the association between social determinants, inactive vitamin D (25(OH)D3), active vitamin D (1,25(OH)2 D3), and antimicrobial peptide cathelicidin/LL-37 among subjects with AUD and matched healthy controls, highlighting its causal implications on increased susceptibility to respiratory infections.
Our results showed that the levels of 1, 25 (OH)2D3 were lower among the study participants from Atlanta center, who were predominantly African Americans (Table 2), when compared with the study participants from Denver center, who were predominantly White (Table 1). All the study participants from Denver center analyzed in our experiment were also nonsmokers. Earlier studies have documented similar results among US adults. Vitamin D deficiency was common in the US population, especially among minorities.4,5,10,46–53 A study by Forrest and Stuhldreher showed that among elder adults, 41% had less than 25 ng/mL of 25 (OH)D3 and among women with osteoporosis, 64% had less than 30 ng/mL of 25 (OH)D3. Among African American adults, 61% had less than 15 ng/mL of 25 (OH)D3. Vitamin D deficiency is defined by levels of 25(OH)D3 less than 20 ng/mL (50 nmol/L). The study concluded that African American adults had the highest prevalence rate of vitamin D deficiency (82.1%) followed by Hispanics adults (62.9%). In terms of race, men were significantly more likely to have vitamin D deficiency than women. This trend was observed among our study participants from Atlanta Center with 92% males and 8% females (Table 2).
Overall, the published studies have shown that minority populations had 9.6 times increased risk of developing vitamin D deficiency when compared with the rest of population in the United States.10,27,39,42–46,52,54–58
Vitamin D deficiency has also been associated with high prevalence of chronic obstructive pulmonary disease (COPD) especially among smokers when compared with non-COPD healthy smokers.38,59 While 31% of healthy smokers showed vitamin D deficiency (inactive vitamin D <20 ng/mL), 39%, 47%, and 69% of patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1, 2, and 3, respectively, exhibited vitamin D deficiency. Furthermore, as many as 77% of patients with GOLD stage 4 of COPD exhibited vitamin D deficiency. When the levels of 25(OH)D3 were lowered to <10 ng/mL, only 2% of healthy smokers showed vitamin D deficiency at this level, compared with 4.3%, 8.1%, 8%, and 13.3% of patients with GOLD stage ranging from 1 to 4, respectively, who were severely deficient in vitamin D.31,52,59–61 This was similar to the result observed in our experiment, which showed that the levels of 1, 25 (OH)2D3 were significantly low among the study participants from Atlanta center, all of whom were smokers (Figs. 5 and 6).
In conclusion, our study confirmed that excessive exposure to ethanol, race, and cigarette smoking play significant causal roles in the onset of vitamin D deficiency among vulnerable populations.
One of the challenges of this study and analysis was the small sample size. While this may not negatively impact on the mechanism of action, we hope to investigate these observations in a larger population size and make more elaborate translational conclusions toward understanding associations between excessive ethanol exposure, vitamin D, and cathelicidin/LL-37.
Conclusion
Our results have clearly shown that minority populations have increased risk of vitamin D deficiency as seen from the results obtained from the analysis of the BALF samples. Smoking also lowers the levels of vitamin D among minority populations.25,43,58,59,62 More public health targeted interventions will be helpful among minority populations in terms of increasing awareness and sensitization on the need to consume vitamin D dietary supplements.
Overall, we have shown from our results that excessive consumption of alcohol can significantly lower the levels of inactive vitamin D (25(OH)D3), active vitamin D (1, 25(OH)2 D3), and antimicrobial peptide cathelicidin/LL-37 among subjects with AUD, especially in minority populations. This can lead to increased pulmonary morbidity and reduced ability of the pulmonary system to fight infections.
Acknowledgments
The authors are grateful to Drs. Ellen Burnham and Ashish Mehta for providing Broncho Alveolar Lavage Fluid (BALF) samples for this study.
Footnotes
ACADEMIC EDITOR: Timothy Kelley, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1180 words, excluding any confidential comments to the academic editor.
FUNDING: Dr. McCaskill is supported by the National Institute of Health (NI H) Research grant (1KO1HL121041) and Louisiana Board of Regents, LEQSF. Dr. Ashish Mehta is supported by a Career Development Award (1IK2CX000643) from the Department of Veterans Affairs (Clinical Science Research and Development). The contents of this report do not represent the view of the Department of Veterans Affairs or the United States Government. Dr. Ellen Burnham’s COPARC is supported by National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health, award number R24AA019661; with additional support provided by the Colorado Clinical and Translational Sciences Institute on the University of Colorado Anschutz Medical Campus, funded by NIH/NCATS award, number UL1TR001082. The authors confirm that the funders had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: OO, TH, AM, MM. Analysed the data: OO, TH, MM. Wrote the first draft of the manuscript: OO. Contributed to the writing of the manuscript: OO, MM. Agree with manuscript results and conclusions: OO, TH, AM, ML, MM. Jointly developed the structure and arguments for the paper: OO, ML, MM. Made critical revisions and approved final version: OO, TH, AM, ML, MM. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Centers for Disease Control and Prevention . Alcohol Related Disease Impact (ARDI) Application. Atlanta, GA: CDC; 2013. Available at: http://apps.nccd.cdc.gov/DACH_ARDI/Default.aspx. [Google Scholar]
- 2.Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the united states. Prev Chronic Dis. 2014;11:E109. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 national and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49(5):e73–9. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72(8):757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant BF, Goldstein RB, Smith SM, et al. The alcohol use disorder and associated disabilities interview schedule-5 (AUDADIS-5): reliability of substance use and psychiatric disorder modules in a general population sample. Drug Alcohol Depend. 2015;148:27–33. doi: 10.1016/j.drugalcdep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the US, 2006. Am J Prev Med. 2011;41(5):516–24. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 7.Caregaro L, Alberino F, Amodio P, et al. Malnutrition in alcoholic and virus-related cirrhosis. Am J Clin Nutr. 1996;63(4):602–9. doi: 10.1093/ajcn/63.4.602. [DOI] [PubMed] [Google Scholar]
- 8.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system – a review. Alcohol Clin Exp Res. 1998;22(9):1927–42. [PubMed] [Google Scholar]
- 9.Engs RC, Aldo-Benson M. The association of alcohol consumption with self-reported illness in university students. Psychol Rep. 1995;76(3):727–36. doi: 10.2466/pr0.1995.76.3.727. [DOI] [PubMed] [Google Scholar]
- 10.Kent JC, Devlin RD, Gutteridge DH, Retallack RW. Effect of alcohol on renal vitamin D metabolism in chickens. Biochem Biophys Res Commun. 1979;89(1):155–61. doi: 10.1016/0006-291x(79)90957-4. [DOI] [PubMed] [Google Scholar]
- 11.Leevy CM, Moroianu ŞA. Nutritional aspects of alcoholic liver disease. Clin Liver Dis. 2005;9(1):67–81. doi: 10.1016/j.cld.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155(15):1649–54. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 13.McCaskill ML, Hottor HT, Sapkota M, Wyatt TA. Dietary diallyl disulfide supplementation attenuates ethanol-mediated pulmonary vitamin D speciate depletion in C57Bl/6 mice. BMC Nutr. 2015;1:18. doi: 10.1186/s40795-015-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agerberth B, Grunewald J, Castaños-Velez E, et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med. 1999;160(1):283–90. doi: 10.1164/ajrccm.160.1.9807041. [DOI] [PubMed] [Google Scholar]
- 15.Bailey KL, Wyatt TA, Romberger DJ, Sisson JH. Alcohol functionally upregulates toll-like receptor 2 in airway epithelial cells. Alcohol Clin Exp Res. 2009;33(3):499–504. doi: 10.1111/j.1530-0277.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baines M. Vitamin C and exposure to alcohol. Int J Vitam Nutr Res Suppl. 1982;23:287–93. [PubMed] [Google Scholar]
- 17.Bals R, Goldman MJ, Wilson JM. Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect Immun. 1998;66(3):1225–32. doi: 10.1128/iai.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger U, Wilson P, McClelland R, et al. Immunocytochemical detection of 1, 25-dihydroxyvitamin D receptors in normal human tissues*. J Clin Endocrinol Metab. 1988;67(3):607–13. doi: 10.1210/jcem-67-3-607. [DOI] [PubMed] [Google Scholar]
- 19.Bucki R, Leszczyńska K, Namiot A, Sokołowski W. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch Immunol Ther Exp (Warsz) 2010;58(1):15–25. doi: 10.1007/s00005-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 20.Capps JA, Coleman GH. Influence of alcohol on prognosis of pneumonia in cook county hospital. J Am Med Assoc. 1923;80(11):750–2. [Google Scholar]
- 21.Bohn MJ, Babor TF, Kranzler HR. The alcohol use disorders identification test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423–32. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- 22.Kitchens JM. Does this patient have an alcohol problem? JAMA. 1994;272(22):1782–7. [PubMed] [Google Scholar]
- 23.Nadjarian AH. An evaluation of screening, brief intervention, and referral to treatment in emergency departments. 2013. [Google Scholar]
- 24.Agerberth B, Guðmundsson G. Antimicrobial Peptides and Human Disease. Springer; 2006. Host antimicrobial defence peptides in human disease; pp. 67–90. [DOI] [PubMed] [Google Scholar]
- 25.Rehm J, Gmel G, Sempos CT, Trevisan M. Alcohol-related morbidity and mortality. Mouth. 2002;140(208):C00–97. [PMC free article] [PubMed] [Google Scholar]
- 26.Raiten DJ, Picciano MF. Vitamin D and health in the 21st century: bone and beyond. Executive summary. Am J Clin Nutr. 2004;80(6 Suppl):1673S–7S. doi: 10.1093/ajcn/80.6.1673S. [DOI] [PubMed] [Google Scholar]
- 27.Zasloff M. Fighting infections with vitamin D. Nat Med. 2006;12(4):388–90. doi: 10.1038/nm0406-388. [DOI] [PubMed] [Google Scholar]
- 28.Frohm M, Agerberth B, Hangar G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272(24):15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 29.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–65. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 31.Kocarnik J. Increased vitamin D intake and decreased lung cancer risk for never smokers. Sci Spotlight. 2013;3(10) [Google Scholar]
- 32.Larrick JW, Hirata M, Zhong J, Wright SC. Anti-microbial activity of human CAP18 peptides. Immunotechnology. 1995;1(1):65–72. doi: 10.1016/1380-2933(95)00006-2. [DOI] [PubMed] [Google Scholar]
- 33.White M, Mankan A, Lawless MW, O’Dwyer MJ, McManus R, Ryan T. Mortality in humans with pneumonia and sepsis is related to an uncompensated anti-inflammatory response to infection. Arch Intern Med. 2008;168(13):1468–9. doi: 10.1001/archinte.168.13.1468-b. [DOI] [PubMed] [Google Scholar]
- 34.Niemelä O, Parkkila S, Pasanen M, Viitala K, Villanueva JA, Halsted CH. Induction of cytochrome P450 enzymes and generation of protein-aldehyde adducts are associated with sex-dependent sensitivity to alcohol-induced liver disease in micropigs. Hepatology. 1999;30(4):1011–7. doi: 10.1002/hep.510300413. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Y, Niyonsaba F, Ushio H, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human α-defensins from neutrophils. Br J Dermatol. 2007;157(6):1124–31. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 36.Zasloff M. Antimicrobial peptides in health and disease. N Engl J Med. 2002;347(15):1199–9. doi: 10.1056/NEJMe020106. [DOI] [PubMed] [Google Scholar]
- 37.Yamini S, West KP, Jr, Wu L, Dreyfuss ML, Yang DX, Khatry SK. Circulating levels of retinol, tocopherol and carotenoid in nepali pregnant and postpartum women following long-term beta-carotene and vitamin A supplementation. Eur J Clin Nutr. 2001;55(4):252–9. doi: 10.1038/sj.ejcn.1601152. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355(9204):618–21. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 39.Weber G, Heilborn JD, Jimenez CIC, Hammarsjö A, Törmä H, Ståhle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124(5):1080–2. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Walter G, Herting E, Agerberth B, Johansson J. Antibacterial activities of the cathelicidins prophenin (residues 62 to 79) and LL-37 in the presence of a lung surfactant preparation. Antimicrob Agents Chemother. 2004;48(6):2097–100. doi: 10.1128/AAC.48.6.2097-2100.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T, Shankar K, Bucci TJ, Warbritton A, Mehendale HM. Diallyl sulfide inhibition of CYP2E1 does not rescue diabetic rats from thioacetamide-induced mortality. Toxicol Appl Pharmacology. 2001;173(1):27–37. doi: 10.1006/taap.2001.9165. [DOI] [PubMed] [Google Scholar]
- 42.Sugden J, Davies J, Witham M, Morris A, Struthers A. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25(3):320–5. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 43.Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41(5):293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patek AJ., Jr Alcohol, malnutrition, and alcoholic cirrhosis. Am J Clin Nutr. 1979;32(6):1304–12. doi: 10.1093/ajcn/32.6.1304. [DOI] [PubMed] [Google Scholar]
- 45.Manari A, Preedy V, Peters T. Nutritional intake of hazardous drinkers and dependent alcoholics in the UK. Addict Biol. 2003;8(2):201–10. doi: 10.1080/1355621031000117437. [DOI] [PubMed] [Google Scholar]
- 46.Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31(1):48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Ginde AA, Mansbach JM, Camargo CA. Vitamin D, respiratory infections, and asthma. Curr Allergy Asthma Rep. 2009;9(1):81–7. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
- 48.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natal Cancer Inst. 2006;98(7):451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 49.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 50.Heulens N, Korf H, Cielen N, et al. Vitamin D deficiency exacerbates COPD-like characteristics in the lungs of cigarette smoke-exposed mice. Respir Res. 2015;16:110. doi: 10.1186/s12931-015-0271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes D, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158(1):20–5. doi: 10.1111/j.1365-2249.2009.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kakarala RR, Chandana SR, Harris SS, Kocharla LP, Dvorin E. Prevalence of vitamin D deficiency in uninsured women. J Gen Intern Med. 2007;22(8):1180–3. doi: 10.1007/s11606-007-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lebowitz MD. Respiratory symptoms and disease related to alcohol consumption. Am Rev Respir Dis. 1981;123(1):16–9. doi: 10.1164/arrd.1981.123.1.16. [DOI] [PubMed] [Google Scholar]
- 54.Lieber CS. Alcohol, liver, and nutrition. J Am Coll Nutr. 1991;10(6):602–32. doi: 10.1080/07315724.1991.10718182. [DOI] [PubMed] [Google Scholar]
- 55.Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2(3):205–9. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- 56.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502–21. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner RT, Aloia RC, Segel LD, Hannon KS, Bell NH. Chronic alcohol treatment results in disturbed vitamin D metabolism and skeletal abnormalities in rats. Alcohol Clin Exp Res. 1988;12(1):159–62. doi: 10.1111/j.1530-0277.1988.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 58.Wyatt TA, Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L575–81. doi: 10.1152/ajplung.2001.281.3.L575. [DOI] [PubMed] [Google Scholar]
- 59.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179(8):630–6. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 60.Kim M, Fujiki R, Kitagawa H, Kato S. 1α, 25 (OH) 2 D 3-induced DNA methylation suppresses the human CYP27B1 gene. Mol Cell Endocrinol. 2007;265:168–73. doi: 10.1016/j.mce.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Kurylowicz A. The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources. 16th ed. Croatia: InTech; 2011. Impact of air pollution on vitamin D status and related health consequences; pp. 17–40. [Google Scholar]
- 62.Vuolo L, Di Somma C, Faggiano A, Colao A. Vitamin D and cancer. Front Endocrinol. 2012;3:58. doi: 10.3389/fendo.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]



