Abstract
Aims
Vitamin D deficiency has been associated with some disorders including cardiovascular diseases. Dyslipidemia is a major risk factor for cardiovascular diseases. However, data about the relationships between vitamin D and lipids are inconsistent. The relationship of vitamin D and Atherogenic Index of Plasma (AIP), as an excellent predictor of level of small and dense LDL, has not been reported. The objective of this study was to investigate the effects of vitamin D status on serum lipids in Chinese adults.
Methods
The study was carried out using 1475 participants from the Center for Physical Examination, 306 Hospital of PLA in Beijing, China. Fasting blood samples were collected and serum concentrations of 25(OH)D, total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) were measured. AIP was calculated based on the formula: log [TG/HDL-C]. Multiple linear regression analysis was used to estimate the associations between serum 25(OH)D and lipids. The association between the occurrences of dyslipidemias and vitamin D levels was assessed by multiple logistic regression analysis. Confounding factors, age and BMI, were used for the adjustment.
Results
The median of serum 25(OH)D concentration was 47 (27–92.25) nmol/L in all subjects. The overall percentage of 25(OH)D ≦ 50 nmol/L was 58.5% (males 54.4%, females 63.7%). The serum 25(OH)D levels were inversely associated with TG (β coefficient = -0.24, p < 0.001) and LDL-C (β coefficient = -0.34, p < 0.001) and positively associated with TC (β coefficient = 0.35, p < 0.002) in men. The associations between serum 25(OH)D and LDL-C (β coefficient = -0.25, p = 0.01) and TC (β coefficient = 0.39, p = 0.001) also existed in women. The serum 25(OH)D concentrations were negatively associated with AIP in men (r = -0.111, p < 0.01) but not in women. In addition, vitamin D deficient men had higher AIP values than vitamin D sufficient men. Furthermore, the occurrences of dyslipidemias (reduced HDL-C, elevated TG and elevated AIP) correlated with lower 25(OH)D levels in men, whereas the higher TC and LDL-C associated with higher 25(OH)D levels in women.
Conclusion
It seems that the serum 25(OH)D levels are closely associated with the serum lipids and AIP. Vitamin D deficiency may be associated with the increased risk of dyslipidemias, especially in men. The association between vitamin D status and serum lipids may differ by genders.
Introduction
Vitamin D is an essential fat-soluble vitamin with multiple functions. The main source of vitamin D in humans is exposure of the skin to sunlight. In skin 7-dehydrocholesterol can be converted to previtamin D3 after absorbing solar ultraviolet B radiation and then sequentially hydroxylated into 25(OH)D and 1,25-dihydroxyvitamin D3 (an active form) by hydroxylases in the liver and kidney. Vitamin D can be also ingested in the diet or by oral supplements [1]. Besides its classical physiological function of regulation of calcium and bone metabolism, vitamin D is suggested to have many other functions such as modulating immune function, anti-inflammatory activity, suppressing the rennin-angiotensin system and reducing insulin resistance [2–5]. Currently, vitamin D deficiency has been suggested to be associated with some cardiovascular health problems. Low levels of 25(OH)D are independently associated with increased mortality in subjects with cardiovascular disease (CVD) [6]. A meta-analysis of observational studies has shown that decreases in 25(OH)D by 16 ng/dL confer a 16% greater risk for hypertension [7]. A multitude of observations suggest that raising blood levels of 25(OH)D reduces the risk of hypertension, stroke and myocardial infarction [5, 8, 9]. Lipid/lipoprotein abnormalities which refers to raised levels of TC, TG and LDL-C and decreased levels of HDL-C have been identified to be important risk factors of atherosclerosis and CVD [10]. It has been confirmed that lowering of serum cholesterol results in a reduction in cardiovascular morbidity [11]. Whether vitamin D could influence CVD by affecting lipid profile has not been thoroughly studied. Previous studies have suggested that there is a relationship between 25(OH)D levels and serum lipids. However, the results are inconsistent. A cross-sectional study by Jorde et al showed that there were positive associations between serum 25(OH)D levels and TC, HDL-C and LDL-C and a negative association between serum 25(OH)D and TG among 8018 subjects in Norway [12]. Data from the study of Gaddipati VC suggests that serum 25(OH)D levels are negatively associated with TC, TG and LDL-C, and positively associated with HDL-C in Americans [13]. In addition, small and dense LDL (sdLDL) is found to deposit more readily on the arterial wall compared to LDL-C [14]. AIP (log[TG/HDL-C]), as an excellent predictor of levels of sdLDL-C, has been reported to correlate to atherosclerosis and coronary artery disease (CAD) [15]. The potential of AIP to predict cardiovascular risk has been shown in some studies [15, 16]. However, to date, there is no data reported about the relationship between vitamin D and AIP. Therefore the present study was to investigate the relationship of serum 25(OH)D with serum lipids and AIP.
Subjects and Methods
Subjects
This study was conducted in participants recruited from the Center for Physical Examination, 306 Hospital of PLA in Beijing, China from August 2013 to December 2013, and with no history of malignancies, myocardial infarction, stroke, diabetes, severe liver diseases, kidney disease, and other diseases that affect serum vitamin D levels such as Cushing syndrome, hyperparathyroidism and hyperthyroidism. We planned to evaluate 1708 participants, but only 1475 were analyzed (Fig 1). We also excluded individuals who had supplementation of vitamin D, vitamin D analogues, and any drugs that could affect calcium and phosphorus metabolism and lipid lowering drugs. Authors did not have access to the identifying information of participants during or after data collection.
Fig 1. Flow diagram of participant enrollment.
Anthropometric and laboratory measurements
The routine measurement of weights (at least to 0.1 kg) and heights (at least to 0.1 cm) was determined by a height and weight measurement instrument. Body mass index (BMI) was calculated based on the formula: BMI = weight (kg)/height (m)2.
Fasting blood samples were collected and, centrifuged, and serum was stored at -80°C until use. TC, TG, HDL-C and LDL-C were determined by an enzymatic colorimetric method using an automatic biochemical analyzer [17] (OlympsAu640; Shimotogari, Nagizumi-Chu, Sunto-Gun, Shizuoka, Japan). The intra- and inter-assay coefficients of variation (CVs) for TC were <4% and <5%, respectively. The CVs for TG, LDL-C and HDL-C were <5% (intra-assay) and <6% (inter-assay). The cut-off values of dyslipidemia for TC, TG, LDL-C and HDL-C were set at > 5.7 mmol/L, > 1.7 mmol/L, > 3.36 mmol/L, and < 0.9 mmol/L respectively. These values are normally used as references for our hospital to diagnose dyslipidemia. AIP value was calculated as the logarithm to the base 10 of the ratio of TG to HDL-C (log[TG/HDL-C]). DobiásováoviDL-C]). DobiDobise values are normally used as references for our hospital to diag [18]. Therefore, AIP > 0.15 was regarded as abnormal value in the present study. 25(OH)D, as the metabolizing and the primary circulating form of vitamin D, can be regarded as the best indicator of serum vitamin D status, due to its unique structure, which possesses long half-life and is stable [19]. Serum concentration of 25(OH)D was determined using an enzyme-linked immunosorbent assay kit following the manufacturer's instructions [20] (Immunodiagnostic Systems Ltd, Boldon, Tyne & Wear, United Kingdom). The absorbance was read at 450 nm using a microplate reader (BIO-RAD 550, BioTek, Vermont, USA). The intra-assay CV and inter-assay CV were 4.6% and 6.7% respectively. Although there has been debate over which level of serum 25(OH)D reflects optimum vitamin D status, the Institute of Medicine concluded that the serum 25(OH)D levels > 50 nmol/L could cover the requirements of at least 97.5% of the population which was based on assuring bone health [21]. In our study 25(OH)D levels ≦ 50nmol/L and 25(OH)D levels > 50nmol/L were considered as vitamin D deficiency and vitamin D sufficiency respectively.
Ethics statement
The protocol of this study was approved by the Ethics Committee of the 306 Hospital of Chinese People's Liberation Army. Written consent was obtained from all participants.
Statistical analysis
Statistical analysis was performed using Statistical Product and Service Solutions (SPSS) software version 16.0 (SPSS Inc, Chicago, IL, USA). Descriptive characteristics for participants were presented as medians (5th-95th) for non-normally distributed continuous variables and expressed as means (SD) for normally distributed continuous variables which were determined by a Kolmogorov-Smirnov test. The Mann-Whitney U test (non-normally distributed variables), t test (normally distributed variables) and the chi square test (categorical variables) were used to compare the differences between groups. Multiple linear regression analysis was carried out to estimate the associations between serum 25(OH)D concentration (independent variable) and serum lipids (dependent variables: TC, TG, LDL-C, HDL-C). Effect modification by sex was evaluated by stratified analyses of covariance. The test for statistical interaction was added with sex × TC, sex × TG, sex × LDL-C and sex × HDL-C into the multiple regression. TG and HDL-C were transformed from non-normally distributed variables to normally distributed variables after a logarithmic transformation. We analyzed the association between 25(OH)D and AIP using Spearman's rank correlation coefficient. Logistic regression analysis was further used to evaluate the associations between the occurrences of dyslipidemias and 25(OH)D levels and data was expressed as odds ratios (OR) with 95% confidence intervals (CI). All p values were two tailed and < 0.05 were considered to be statistically significant.
Result
General characteristics
Clinical and anthropometric characteristics of participants were summarized in Table 1. The overall percentage of vitamin D deficiency was 58.5% (males 54.4%, females 63.7%). While, males had significantly higher values in age, BMI, TG, LDL-C, 25(OH)D and lower HDL-C compared to females (Table 1).
Table 1. Subject characteristics.
| Variables | All (n = 1475) | Men (n = 829) | Women (n = 646) | P -value |
|---|---|---|---|---|
| Age | 39 (24–64) | 40 (25–64.5) | 38 (24–64) | <0.001b |
| BMI (kg/m2) | 24.57 (3.67) | 25.69 (20.24–31.51) | 22.38 (18.36–28.87) | <0.001b |
| TC (mmol/L) | 4.73 (3.49–6.31) | 4.78 (0.85) | 4.76 (0.86) | 0.58a |
| TG (mmol/L) | 1.17 (0.51–3.61) | 1.39 (0.59–4.35) | 0.93 (0.47–2.49) | <0.001b |
| LDL-C (mmol/L) | 3.01 (0.79) | 3.12 (0.80) | 2.86 (0.76) | <0.001a |
| HDL-C (mmol/L) | 1.29 (0.89–2.01) | 1.18 (0.85–1.74) | 1.47 (1.01–2.18) | <0.001b |
| 25(OH)D (nmol/L) | 47 (27–92.25) | 48 (28–97) | 43 (25–85) | <0.001b |
| AIP | -0.02 (0.32) | 0.10 (0.29) | -0.18 (0.28) | <0.001a |
BMI, body mass index; TC, Total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein; HDL-C, high-density lipoprotein; 25(OH)D, 25-hydroxyvitamin D; AIP, atherogenic index of plasma.
aStudent’s t–test was used to compare the mean values of normally distributed variables.
bThe Mann-Whitney U test was used to examine non-normally distributed variables.
P values were used to assess the differences between males and females.
Age, TG, HDL-C and 25(OH)D in men, women and all participants, BMI in men and women and TC in all participants were presented as medians (5th-95th). BMI in all participants, TC in men and women as well as LDL-C and AIP in men, women and all participants were expressed as means (SD).
The association between serum 25(OH)D concentrations and lipids in men and women
Multiple regression analysis was used to assess the associations between serum 25(OH)D concentrations and lipids (Table 2). In males 25(OH)D concentrations were negatively associated with TG and LDL-C and positively associated with TC after adjusting for age and BMI. Per 10 nmol/L increase in serum 25(OH)D was associated with decreases of 1.74 mmol/L in TG and 0.34mmol/L in LDL-C and an increase of 0.35 mmol/L in TC. We also found an apparently negative association between 25(OH)D and LDL-C and a positive association between 25(OH)D and TC in women after adjustment for age and BMI. Each 10 nmol/L increment in 25(OH)D concentration was associated with a decrease of 0.25 mmol/L in LDL-C and an increase of 0.39 mmol/L in TC in women. Whereas there was no significant association between 25(OH)D and HDL-C either in males or in females. Substantial sex differences existed, the effect modification by sex was not significant with respect to TC, TG, LDL-C and HDL-C (p > 0.05).
Table 2. The association between serum 25(OH)D concentrations and serum lipids in males and females after adjusting for age and BMI.
| Men (n = 829) | Women (n = 646) | |||
|---|---|---|---|---|
| Variables | β ariablesati | p-value | β coefficient | p-value |
| TC | 0.35 | 0.002 | 0.39 | 0.001 |
| TG | -0.24 | <0.001 | -0.09 | 0.118 |
| LDL-C | -0.34 | <0.001 | -0.25 | 0.01 |
| HDL-C | -0.07 | 0.196 | -0.09 | 0.176 |
TC, Total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein; HDL-C, high-density lipoprotein.
25(OH)D, TG and HDL-C were log transformed for the analysis.
βcoefficient is a standardized coefficient in multiple linear regression analysis.
The correlation between vitamin D status and AIP
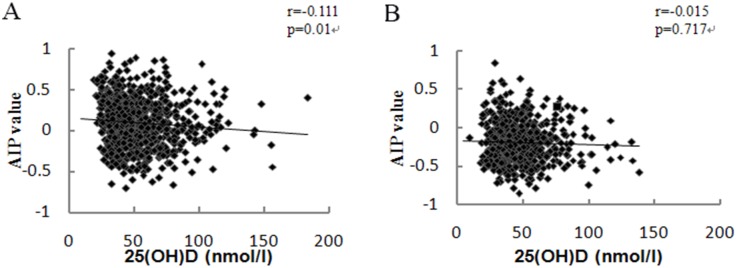
The association between vitamin D and AIP was analyzed using Spearman's Rank Correlation Coefficient. The serum 25(OH)D concentrations were negatively associated with AIP in men. However there were no significant associations between 25(OH)D concentrations and AIP in women (Fig 2).
Fig 2. The correlation between 25(OH)D and AIP.
Scatter plot showing the relations between serum 25(OH)D concentrations and AIP in men (A) and women (B).
The comparison of lipid profiles at different vitamin D status
To investigate whether the relationship between serum 25(OH)D and serum lipids differs by vitamin D status, we categorized the participants into two subgroups of vitamin D deficiency and vitamin D sufficiency. The results showed that TG and AIP were significantly higher in men with vitamin D deficiency compared to those in men with vitamin D sufficiency (Table 3). Furthermore, The percentages of dyslipidemias and abnormal AIP values by vitamin D status were also presented in Table 3. The incidences of elevated TG, reduced HDL and elevated AIP were higher in vitamin D deficient men than those in vitamin D sufficient men. However, there were no significant differences with these parameters between the groups in females.
Table 3. The comparison of characteristics at different vitamin D status in males and females.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| 25(OH)Dent vitam (451) | 25(OH)D>50nmol/L (378) | p-value | 25(OH)DLnt vitam (413) | 25(OH)D>50nmol/L (233) | p-value | |
| Age | 39 (24–62) | 40 (25–67) | 0.01b | 37 (24–64.35) | 39 (24–63) | 0.59 b |
| BMI (kg/m2) | 25.93 (3.37) | 25.73 (3.30) | 0.40a | 22.2 (18.27–29.06) | 22.72 (18.41–28.32) | 0.23 b |
| TC (mmol/L) | 4.80 (0.85) | 4.76 (0.85) | 0.59 a | 4.71 (0.88) | 4.83 (0.83) | 0.87 a |
| TG (mmol/L) | 1.51 (0.596–4.4) | 1.31 (0.59–4.25) | 0.01 b | 0.93 (0.48–2.16) | 0.93 (0.46–2.64) | 0.36 b |
| LD-L-C (mmol/L) | 3.15 (0.76) | 3.08 (0.85) | 0.18 a | 2.79 (1.65–4.18) | 2.84 (1.72–4.23) | 0.38 b |
| HDL-C (mmol/L) | 1.17 (0.81–1.73) | 1.19 (0.88–1.75) | 0.13 b | 1.46 (0.99–2.21) | 1.48 (1.06–2.11) | 0.48 b |
| AIP | 0.12 (0.30) | 0.07 (0.28) | 0.01 a | -0.18 (0.27) | -0.17 (0.29) | 0.59 a |
| Elevated TC %(n) | 13.08% (59) | 16.74% (39) | 0.08 | 12.34% (51) | 16.73% (39) | 0.13c |
| Elevated TG %(n) | 43.46% (196) | 33.86% (128) | 0.01 | 11.86% (49) | 15.88% (37) | 0.15c |
| Elevated LDL-C %(n) | 9.97% (45) | 5.82% (22) | 0.24 | 22.76% (94) | 26.61% (62) | 0.29c |
| Reduced HDL-C %(n) | 36.14% (163) | 32.01% (121) | 0.03 | 2.18% (9) | 0.89% (2) | 0.34c |
| Elevated AIP %(n) | 46.12% (208) | 37.83% (143) | 0.02 | 13.38 (47) | 11.59% (27) | 0.52c |
25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; TC, Total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein; HDL-C, high-density lipoprotein; AIP, atherogenic index of plasma.
Differences in data between the different vitamin D groups was analyzed by astudent’s t–test for normally distributed continuous variables, bMann-Whitney U test for non-normally distributed continuous variables or cchi square test for the categorical variables.
Age, TG and HDL-C in men, women and all participants, and BMI and LDL-C in women were presented as medians (5th-95th). BMI and LDL-C in men and TC and AIP in men, women and all participants were expressed as means (SD).
The association between the occurrences of dyslipidemias and 25(OH)D levels
Unadjusted OR and age and BMI adjusted OR for dyslipidemias and elevated AIP by serum 25(OH)D levels in men and women were presented in Table 4. After adjusting for age and BMI, the OR for the elevated TG, reduced HDL-C and elevated AIP decreased significantly in vitamin D sufficient men compared with vitamin D deficient men. In contrast, in women, the OR for the incidence of dyslipidemias or elevated AIP showed no significant difference between vitamin D sufficient group and vitamin D deficient group.
Table 4. Odds ratio of dyslipidemias and elevated AIP by serum 25(OH)D levels in men and women.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| 25(OH)D in men and wom | 25(OH)D>50nmol/L (378) | p-value | 25(OH)DL (378) and wom | 25(OH)D>50nmol/L (233) | p-value | |
| Elevated TC | ||||||
| Unadjusted | 1.000 | 0.943 (0.626–1.422) | 0.781 | 1.000 | 1.427 (0.908–2.242) | 0.123 |
| Adjusted | 1.000 | 0.909 (0.600–1.376) | 0.652 | 1.000 | 1.423 (0.901–2.278) | 0.129 |
| Elevated TG | ||||||
| Unadjusted | 1.000 | 0.666 (0.502–0.884) | 0.005 | 1.000 | 1.402 (0.885–2.223) | 0.150 |
| Adjusted | 1.000 | 0.612 (0.450–0.831) | 0.002 | 1.000 | 1.453 (0.887–2.382) | 0.138 |
| Elevated LD-L-C | ||||||
| Unadjusted | 1.000 | 0.832 (0.623–1.111) | 0.212 | 1.000 | 1.230 (0.849–1.782) | 0.273 |
| Adjusted | 1.000 | 0.838 (0.625–1.123) | 0.237 | 1.000 | 1.223 (0.825–1.814) | 0.315 |
| Reduced HDL-C | ||||||
| Unadjusted | 1.000 | 0.558 (0.328–0.947) | 0.031 | 1.000 | 0.389 (0.083–1.814) | 0.229 |
| Adjusted | 1.000 | 0.570 (0.333–0.975) | 0.04 | 1.000 | 0.426(0.089–2.035) | 0.285 |
| Elevated AIP | ||||||
| Unadjusted | 1.000 | 0.711 (0.538–0.939) | 0.016 | 1.000 | 1.021 (0.617–1.688) | 0.937 |
| Adjusted | 1.000 | 0.667 (0.492–0.905) | 0.009 | 1.000 | 1.023 (0.600–1.746) | 0.932 |
25(OH)D, 25-hydroxyvitamin D; TC, Total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein; HDL-C, high-density lipoprotein; AIP, atherogenic index of plasma.
The OR and p-value for the prevalence of dyslipidemias and elevated AIP between the two levels of serum 25(OH)D were analyzed using logistic regression after adjustment for age and BMI. Data are presented as OR (95% CI).
Discussion
Vitamin D is a multifunction vitamin. Vitamin D deficiency has been suggested to be associated with a number of conditions including cardiovascular disease [22, 23]. The dyslipidemia which is characterized by elevations of LDL, raised TG, and decreases in HDL-C has been well-established as a CVD risk factor [24, 25]. Vitamin D may impact cardiovascular health through influencing serum lipids. Our results showed that in male participants, serum 25(OH)D concentrations had a strong negative association with TG and LDL-C after adjusting relevant confounders such as age and BMI. These findings add to the evidence that serum lipids correlate with levels of 25(OH)D, a finding that has been reported in previous studies [26–28].
We also found that vitamin D deficient men had higher TG values compared to vitamin D sufficient men. Moreover, the higher incidences of elevated TG and reduced HDL-C were associated with the lower 25(OH)D levels in males. These results further suggest that vitamin D deficiency may be associated with the increased risk of dyslipidemias. The associations between vitamin D and serum lipids have been extensively studied in different populations. Jungert A et al found that 25(OH)D levels were positively associated with HDL-C and inversely associated with TC, HDL-C, LDL-C:HDL-C and TC:HDL-C among the elderly women in Germany [28]. Data from the study of Karhapää et al suggests that serum 25(OH)D levels are negatively associated with TC, TG and LDL-C in middle-aged Finnish males [27]. The findings from Sun’s study of 136 Japanese males aged 20–79 years indicate that vitamin D is inversely correlated with TG and LDL-C:HDL-C [26]. Results from the study of Skaaby et al showed that a 10 nmol/l higher level of 25(OH)D was associated with a decrease in TG (0.52%) and VLDL-C (0.66%) in Danish adults [29]. They also found that there was a causal effect of higher vitamin D status on a more favorable lipid profile [30]. These results suggest that associations between the levels of vitamin D and serum lipid profiles exist among different populations and maintaining vitamin D sufficiency seems to have a beneficial effect on serum lipids. This potential association still needs further study and clarification.
sdLDL-C is more easily to deposit on the arterial wall by binding to glycoprotein compared to LDL-C [31]. Elevated sdLDL-C is predominantly responsible for cholesterol depositing and decreasing in clearance of LDL-C [14]. What’s more, sdLDL-C prefers to transform into oxidized low density lipoprotein which would be taken up by macrophages to form foam cells. Therefore, sdLDL-C becomes one of the major causative factors of arteriosclerosis and cardiovascular disease [32]. AIP, as a transformation of TG/HDL-C, was first introduced by DobiásováoviDobieda transformation of TG/HDL-C, was first <Year>sdLDL-C [33]. In addition, elevating TG and/or decreasing HDL-C could cause AIP to rise. Hypertriglyceridemia and/or hypo-HDL cholesterolemia as special types of dyslipidemia are thought to be high risk factors for atherosclerosis and CAD [34, 35]. AIP has been shown to be a more useful marker of atherogenicity and cardiovascular risk than single LDL-C or TC [36, 37]. AIP, as an index of dyslipidaemia to predict the risk of developing atherosclerosis and CVD, has been applied in some studies [15, 16]. In our study we observed that levels of 25(OH)D had a negative association with AIP and elevated AIP values were associated with lower 25(OH)D levels in males. The data further suggests that the improvement of vitamin D status may have favorable potential in reducing the risk of dyslipidemias.
However, how vitamin D influences lipid profile is not clear yet. Previous data has suggested that increasing intestinal calcium absorption could reduce synthesis and secretion of hepatic TG [38]. Vitamin D could inhibit synthesis and secretion of TG through stimulating intestinal calcium absorption. It has also been suggested that increased level of intestinal calcium could reduce intestinal absorption of fatty acid due to the formation of insoluble calcium-fatty complexes. Serum levels of LDL-C would be reduced by the decreased absorption of fat, particularly saturated fatty acids [39]. In addition, calcium could promote the conversion of cholesterol into bile acids and thereby reduce the level of cholesterol [40]. Other studies have proved that high level of parathyroid hormone (PTH) could result in TG elevating and higher concentrations of 25(OH)D suppress serum PTH levels [41, 42]. Therefore, vitamin D could influence TG concentrations by regulating PTH levels. In addition, previous studies have provided a strong evidence that vitamin D deficiency may be associated with impaired b-cell function and insulin resistance which could affect lipoprotein metabolism and lead to an increase in TG level and a decrease in HDL-C level [43–45]. In addition, vitamin D has been suggested to be involved in lipid metabolism such as the synthesis of bile acid in the liver [46], suggesting that vitamin D may affect the regulation of lipids directly.
In the present study, we observed that the associations between serum 25(OH)D levels and serum lipids were more pronounced in males than in females, which was also found in Yin’s study [47]. Previous studies have shown that serum 25(OH)D levels have no significant relationship with lipid profile among postmenopausal women [48]. The difference of hormone and hormonal sensitivity of the target tissue between genders could impact lipid metabolism differently. Life style difference such as smoking, alcohol consumption, sun exposure and physical activity may also contribute to the dissimilar results between males and females.
In addition, the results in Table 2 showed that 25(OH)D was positively associated with TC and negatively associated with LDL-C, which was also showed in Bolland’s study [49]. Total cholesterol consists of several components including LDL-C, HDL-C and VLDL-C [50]. The interactions between 25(OH)D and each of these components might be different. The association between TC and 25(OH)D is a composite outcome of the associations between 25(OH)D and each of these components in TC. Therefore, the association between 25(OH)D and TC could be different from the association between 25(OH)D and any one of the cholesterol components.
In the current study the percentage of serum 25(OH)D ≦ 50 nmol/L in participants was 58.5%. A previous study has shown that the prevalence of vitamin D deficiency accounted for 66.2% among middle-aged Chinese population [47]. A study from the United States of America demonstrated that half of the subjects had vitamin D deficiency [49]. More recently, A Vitezova et al showed 57% of the participants in a Netherlands study had vitamin D deficiency [51]. A study from Sun X et al showed that 78.7% of Japanese male participants were 25(OH)D-deficiency [26].These results suggest that vitamin D deficiency is a common problem in many populations.
Limits and Strength
Several limitations of this study should be considered when interpret these results. First of all, as with the nature of all the cross-sectional studies, the results could not be reflection of causality. Further investigations are needed to establish the causal relationship between vitamin D and serum lipid levels. For example, interventional studies should be taken into consideration. Secondly, all participants in our study were from Beijing, which limits the generalized application of the results to the whole country. Thirdly, although our study took into account the potential confounding factors (age and BMI), there were still some other confounding variables that may have an influence on the relationship between vitamin D and serum lipids such as waist circumference, sun exposure, physical activity, smoking, etc. In addition, the associations between vitamin D and lipids were possibly influenced by levels of calcium and parathyroid hormone which we did not measure. Despite the above limitations, to the best of our knowledge, the present study is the first data evidence for investigating the association of serum 25(OH)D levels with AIP.
Conclusions
In summary, Vitamin D deficiency is common among adults living in Beijing. Serum 25(OH)D levels seem correlated with the serum lipids. Vitamin D deficiency may be associated with the increased risk of dyslipidemias. The relationship of vitamin D status and serum lipids may differ by genders. Large randomized, placebo-controlled trials will be required to better-understand the relationship between vitamin D and serum lipid profiles.
Supporting Information
(XLSX)
Acknowledgments
The authors would like to thank the participants of the staff of the Center for Special Medicine and Experimental Research, 306th Hospital of PLA and Center for Physical Examination, 306th Hospital of PLA for their assistance and support in data collection.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Schnatz PF, Nudy M, Jiang X, Demko JE, Appt SE. Vitamin D deficiency and cardiovascular disease in postmenopausal women: contributions from human and nonhuman primate studies. Menopause. 2015. January 5 10.1097/GME.0000000000000399 [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011. January;12(1):4–18. . [DOI] [PubMed] [Google Scholar]
- 3.Delvin E, Souberbielle JC, Viard JP, Salle B. Role of vitamin D in acquired immune and autoimmune diseases. Critical reviews in clinical laboratory sciences. 2014. August;51(4):232–47. 10.3109/10408363.2014.901291 [DOI] [PubMed] [Google Scholar]
- 4.Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei AR, Larijani B. Correlation between vitamin D3 deficiency and insulin resistance in pregnancy. Diabetes/metabolism research and reviews. 2008. Jan-Feb;24(1):27–32. 10.1002/dmrr.737 [DOI] [PubMed] [Google Scholar]
- 5.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. The Journal of clinical investigation. 2002. July;110(2):229–38. Pubmed Central PMCID: 151055. 10.1172/JCI15219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Archives of internal medicine. 2008. June 23;168(12):1340–9. 10.1001/archinte.168.12.1340 [DOI] [PubMed] [Google Scholar]
- 7.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. Journal of hypertension. 2011. April;29(4):636–45. 10.1097/HJH.0b013e32834320f9 [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? Journal of the American College of Cardiology. 2008. December 9;52(24):1949–56. 10.1016/j.jacc.2008.08.050 [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008. January 29;117(4):503–11. Pubmed Central PMCID: 2726624. 10.1161/CIRCULATIONAHA.107.706127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polkowska A, Glowinska-Olszewska B, Tobiaszewska M, Bossowski A. [Risk factors for cardiovascular disease in children with type 1 diabetes in 2000–2010 in Podlasie Province]. Pediatric endocrinology, diabetes, and metabolism. 2015;20(2):47–54. 10.18544/PEDM-20.02.0002 [DOI] [PubMed] [Google Scholar]
- 11.The Lipid Research Clinics Coronary Primary Prevention Trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. Jama. 1984. January 20;251(3):365–74. . [PubMed] [Google Scholar]
- 12.Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. European journal of clinical nutrition. 2010. December;64(12):1457–64. 10.1038/ejcn.2010.176 [DOI] [PubMed] [Google Scholar]
- 13.Gaddipati VC, Bailey BA, Kuriacose R, Copeland RJ, Manning T, Peiris AN. The relationship of vitamin D status to cardiovascular risk factors and amputation risk in veterans with peripheral arterial disease. Journal of the American Medical Directors Association. 2011. January;12(1):58–61. 10.1016/j.jamda.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 14.Zhu XW, Deng FY, Lei SF. Meta-analysis of Atherogenic Index of Plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Primary care diabetes. 2015. February;9(1):60–7. 10.1016/j.pcd.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 15.Frohlich J, Dobiasova M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clinical chemistry. 2003. November;49(11):1873–80. . [DOI] [PubMed] [Google Scholar]
- 16.Onat A, Can G, Kaya H, Hergenc G. "Atherogenic index of plasma" (log10 triglyceride/high-density lipoprotein-cholesterol) predicts high blood pressure, diabetes, and vascular events. Journal of clinical lipidology. 2010. Mar-Apr;4(2):89–98. 10.1016/j.jacl.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 17.Shojaei Nik MH, Darabi M, Ziaee A, Hajmanoochehri F. Serum Phospholipase A2-IIA, hs-CRP, and Lipids in Women With Subclinical Hypothyroidism. International journal of endocrinology and metabolism. 2014. July;12(3):e16967 Pubmed Central PMCID: 4166036. 10.5812/ijem.16967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobiasova M. [AIP—atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice]. Vnitrni lekarstvi. 2006. January;52(1):64–71. . [PubMed] [Google Scholar]
- 19.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005. July;16(7):713–6. 10.1007/s00198-005-1867-7 [DOI] [PubMed] [Google Scholar]
- 20.Enko D, Kriegshauser G, Stolba R, Worf E, Halwachs-Baumann G. Method evaluation study of a new generation of vitamin D assays. Biochemia medica. 2015;25(2):203–12. Pubmed Central PMCID: 4470105. 10.11613/BM.2015.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, et al. IOM committee members respond to Endocrine Society vitamin D guideline. The Journal of clinical endocrinology and metabolism. 2012. April;97(4):1146–52. 10.1210/jc.2011-2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nsengiyumva V, Fernando ME, Moxon JV, Krishna SM, Pinchbeck J, Omer SM, et al. The association of circulating 25-hydroxyvitamin D concentration with peripheral arterial disease: A meta-analysis of observational studies. Atherosclerosis. 2015. December;243(2):645–51. 10.1016/j.atherosclerosis.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 23.Heidari B, Nargesi AA, Hafezi-Nejad N, Sheikhbahaei S, Pajouhi A, Nakhjavani M, et al. Assessment of serum 25-hydroxy vitamin D improves coronary heart disease risk stratification in patients with type 2 diabetes. American heart journal. 2015. September;170(3):573–9 e5. 10.1016/j.ahj.2015.06.017 [DOI] [PubMed] [Google Scholar]
- 24.Dokic B, Donovic N, Tadic B, Nikolic D. Factors and Estimation of Risk for Cardiovascular Diseases among Patients in Primary Health Care in Central Serbia. Central European journal of public health. 2015. September;23(3):195–9. 10.21101/cejph.a4009 [DOI] [PubMed] [Google Scholar]
- 25.Mazidi M, Rezaie P, Chaudhri O, Karimi E, Nematy M. The effect of Ramadan fasting on cardiometabolic risk factors and anthropometrics parameters: A systematic review. Pakistan journal of medical sciences. 2015. Sep-Oct;31(5):1250–5. 10.12669/pjms.315.7649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun X, Cao ZB, Tanisawa K, Ito T, Oshima S, Ishimi Y, et al. Associations between the Serum 25(OH)D Concentration and Lipid Profiles in Japanese Men. Journal of atherosclerosis and thrombosis. 2015;22(4):355–62. 10.5551/jat.26070 [DOI] [PubMed] [Google Scholar]
- 27.Karhapaa P, Pihlajamaki J, Porsti I, Kastarinen M, Mustonen J, Niemela O, et al. Diverse associations of 25-hydroxyvitamin D and 1,25-dihydroxy-vitamin D with dyslipidaemias. Journal of internal medicine. 2010. December;268(6):604–10. 10.1111/j.1365-2796.2010.02279.x [DOI] [PubMed] [Google Scholar]
- 28.Jungert A, Roth HJ, Neuhauser-Berthold M. Associations of serum 25-hydroxycholecalciferol and parathyroid hormone with serum lipids differ by sex and vitamin D status. Public health nutrition. 2015. June;18(9):1684–91. 10.1017/S1368980014002286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skaaby T, Husemoen LL, Pisinger C, Jorgensen T, Thuesen BH, Fenger M, et al. Vitamin D status and changes in cardiovascular risk factors: a prospective study of a general population. Cardiology. 2012;123(1):62–70. 10.1159/000341277 [DOI] [PubMed] [Google Scholar]
- 30.Skaaby T, Husemoen LL, Martinussen T, Thyssen JP, Melgaard M, Thuesen BH, et al. Vitamin D status, filaggrin genotype, and cardiovascular risk factors: a Mendelian randomization approach. PloS one. 2013;8(2):e57647 Pubmed Central PMCID: 3584055. 10.1371/journal.pone.0057647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen H, Xu L, Lu J, Hao T, Ma C, Yang H, et al. Correlation between small dense low-density lipoprotein cholesterol and carotid artery intima-media thickness in a healthy Chinese population. Lipids in health and disease. 2015;14(1):137 Pubmed Central PMCID: 4625741. 10.1186/s12944-015-0143-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho Y, Lee SG, Jee SH, Kim JH. Hypertriglyceridemia is a major factor associated with elevated levels of small dense LDL cholesterol in patients with metabolic syndrome. Annals of laboratory medicine. 2015. November;35(6):586–94. Pubmed Central PMCID: 4579102. 10.3343/alm.2015.35.6.586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobiasova M, Frohlich J. [The new atherogenic plasma index reflects the triglyceride and HDL-cholesterol ratio, the lipoprotein particle size and the cholesterol esterification rate: changes during lipanor therapy]. Vnitrni lekarstvi. 2000. March;46(3):152–6. . [PubMed] [Google Scholar]
- 34.Tuteja S, Rader DJ. High-density lipoproteins in the prevention of cardiovascular disease: changing the paradigm. Clinical pharmacology and therapeutics. 2014. July;96(1):48–56. 10.1038/clpt.2014.79 [DOI] [PubMed] [Google Scholar]
- 35.Rosenson RS, Brewer HB Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nature reviews Cardiology. 2015. September 1 10.1038/nrcardio.2015.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, et al. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Archives of internal medicine. 2001. December 10–24;161(22):2685–92. . [DOI] [PubMed] [Google Scholar]
- 37.Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clinical biochemistry. 2001. October;34(7):583–8. . [DOI] [PubMed] [Google Scholar]
- 38.Cho HJ, Kang HC, Choi SA, Ju YC, Lee HS, Park HJ. The possible role of Ca2+ on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biological & pharmaceutical bulletin. 2005. August;28(8):1418–23. . [DOI] [PubMed] [Google Scholar]
- 39.Christensen R, Lorenzen JK, Svith CR, Bartels EM, Melanson EL, Saris WH, et al. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2009. July;10(4):475–86. 10.1111/j.1467-789X.2009.00599.x [DOI] [PubMed] [Google Scholar]
- 40.Vaskonen T, Mervaala E, Sumuvuori V, Seppanen-Laakso T, Karppanen H. Effects of calcium and plant sterols on serum lipids in obese Zucker rats on a low-fat diet. The British journal of nutrition. 2002. March;87(3):239–45. 10.1079/BJNBJN2001508 [DOI] [PubMed] [Google Scholar]
- 41.Song SJ, Si S, Liu J, Chen X, Zhou L, Jia G, et al. Vitamin D status in Chinese pregnant women and their newborns in Beijing and their relationships to birth size. Public health nutrition. 2013. April;16(4):687–92. 10.1017/S1368980012003084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. The American journal of clinical nutrition. 2009. May;89(5):1321–7. 10.3945/ajcn.2008.27004 [DOI] [PubMed] [Google Scholar]
- 43.Karnchanasorn R, Ou HY, Chiu KC. Plasma 25-hydroxyvitamin D levels are favorably associated with beta-cell function. Pancreas. 2012. August;41(6):863–8. 10.1097/MPA.0b013e31823c947c [DOI] [PubMed] [Google Scholar]
- 44.Howard BV. Insulin resistance and lipid metabolism. The American journal of cardiology. 1999. July 8;84(1A):28J–32J. . [DOI] [PubMed] [Google Scholar]
- 45.Tai ES, Emmanuel SC, Chew SK, Tan BY, Tan CE. Isolated low HDL cholesterol: an insulin-resistant state only in the presence of fasting hypertriglyceridemia. Diabetes. 1999. May;48(5):1088–92. . [DOI] [PubMed] [Google Scholar]
- 46.Jiang W, Miyamoto T, Kakizawa T, Nishio SI, Oiwa A, Takeda T, et al. Inhibition of LXRalpha signaling by vitamin D receptor: possible role of VDR in bile acid synthesis. Biochemical and biophysical research communications. 2006. December 8;351(1):176–84. 10.1016/j.bbrc.2006.10.027 [DOI] [PubMed] [Google Scholar]
- 47.Yin X, Sun Q, Zhang X, Lu Y, Sun C, Cui Y, et al. Serum 25(OH)D is inversely associated with metabolic syndrome risk profile among urban middle-aged Chinese population. Nutrition journal. 2012;11:68 Pubmed Central PMCID: 3508942. 10.1186/1475-2891-11-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pirro M, Manfredelli MR, Helou RS, Scarponi AM, Schillaci G, Bagaglia F, et al. Association of parathyroid hormone and 25-OH-vitamin D levels with arterial stiffness in postmenopausal women with vitamin D insufficiency. Journal of atherosclerosis and thrombosis. 2012;19(10):924–31. . [DOI] [PubMed] [Google Scholar]
- 49.Bolland MJ, Bacon CJ, Horne AM, Mason BH, Ames RW, Wang TK, et al. Vitamin D insufficiency and health outcomes over 5 y in older women. The American journal of clinical nutrition. 2010. January;91(1):82–9. 10.3945/ajcn.2009.28424 [DOI] [PubMed] [Google Scholar]
- 50.Birtcher KK, Ballantyne CM. Cardiology patient page. Measurement of cholesterol: a patient perspective. Circulation. 2004. September 14;110(11):e296–7. 10.1161/01.CIR.0000141564.89465.4E [DOI] [PubMed] [Google Scholar]
- 51.Vitezova A, Zillikens MC, van Herpt TT, Sijbrands EJ, Hofman A, Uitterlinden AG, et al. Vitamin D status and metabolic syndrome in the elderly: the Rotterdam Study. European journal of endocrinology / European Federation of Endocrine Societies. 2015. March;172(3):327–35. 10.1530/EJE-14-0580 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.