ABSTRACT
Effective translation of breakthrough discoveries into innovative products in the clinic requires proactive mitigation or elimination of several drug development challenges. These challenges can vary depending upon the type of drug molecule. In the case of therapeutic antibody candidates, a commonly encountered challenge is high viscosity of the concentrated antibody solutions. Concentration-dependent viscosity behaviors of mAbs and other biologic entities may depend on pairwise and higher-order intermolecular interactions, non-native aggregation, and concentration-dependent fluctuations of various antibody regions. This article reviews our current understanding of molecular origins of viscosity behaviors of antibody solutions. We discuss general strategies and guidelines to select low viscosity candidates or optimize lead candidates for lower viscosity at early drug discovery stages. Moreover, strategies for formulation optimization and excipient design are also presented for candidates already in advanced product development stages. Potential future directions for research in this field are also explored.
KEYWORDS: Electrostatics, hydrophobicity, high concentration, intermolecular interactions, monoclonal antibody solution, networks, viscosity
Abbreviations
- ADC
antibody-drug conjugate
- B22
osmotic second virial coefficient
- c
concentration of a solute
- Cp
centipoise
- CDR
complementarity determining region
- η
viscosity of solution
- ηsp
specific viscosity of solution
- [η]
intrinsic viscosity of the solute
- η0
viscosity of solution at zero solute concentration
- kH
Huggins constant in Huggins equation of viscosity
- kB
Boltzmann constant
- kD
diffusion interaction parameter
- kS
change in sedimentation coefficient for a unit change in concentration
- M
molar mass of the solute
- mAb
monoclonal antibody
- v'
partial specific volume of the solute
- ϕ
volume fraction of a solute in solution
- T
temperature
- rH
hydrodynamic radius of a solute
- υ
a parameter to account for non-spherical nature of solute in the Ross and Minton equation for viscosity
- k1
contribution of monomers in viscosity of a solution
- k2
contribution of pairwise interactions in viscosity of a solution
- Fab
fragment antigen binding
- Fc
fragment crystalizable
- D
diffusion constant
- D0
Diffusivity of a solute at its infinite dilution
- mL
milliliter
- mg
milligram
- f
a factor that depends on the dimensions of ellipsoids
- V
volume fraction of the ellipsoids in a solution
- pI
Isoelectric point
- Fv
variable region of an antibody
- ZFv
charge on variable region
- ξFv
zeta-potential of Fv
- pIFv
isoelectric point of variable region
- pHformulation
Formulation pH
- DLVO
Derjaguin-Landau-Vervey-Overbeek
- PMF
Potential of mean force
Introduction
In last three decades, the United States Food and Drug Administration (US FDA) has approved approximately 125 biologic drugs,1 and market share of biologic drugs is estimated to rise up to 20% by 2017.2 Among these products, four approved in 2014 are expected to accrue more than $1 billion each in sales revenue before 2020.3 Biologic drugs typically refer to recombinant human or plasma-derived proteins, hormones, enzymes, vaccines, as well as antibody based therapeutics,4 which include monoclonal antibodies (mAbs), antibody-drug conjugates, and Fc-fusion proteins. Therapeutic mAbs have been developed to treat numerous human maladies such as arthritis, ankylosing spondylitis, Crohn's disease, chronic plaque psoriasis, cryopyrin-associated periodic syndromes, cancers, and postmenopausal osteoporosis.5-7 High specificity, low non-mechanism toxicity and manageable immunogenicity8 make mAbs suitable for commercial drug product development. In fact, mAbs have been compared to the ideal of a magic bullet proposed by Paul Ehrlich because of their high target specificity.9
Presently, intravenous (IV) infusion is the most commonly used route of administration for mAb drug products. However, an alternative route, subcutaneous administration, is being increasingly used for patients with chronic diseases because frequent dosing may be needed over their lifetimes. Subcutaneous injections can be self-administered by the patients using ready-to-use pre-filled syringes or pens /auto-injectors and delivery devices. Such devices are advantageous for better patient comfort. Moreover, the concentrated mAb drug solutions contained in these devices may provide the additional benefit of longer intervals between the injections (and more effective pharmacokinetic profile for the antibody). Such innovations can help reduce healthcare costs by minimizing hospital (or clinic) visits, increasing patient compliance and adherence.10 A critical consideration in the development of concentrated antibody formulations for subcutaneous administration is the limited volume (1—1.5 mL) available in the subcutaneous space.11 This necessitates development of stable, highly concentrated antibody formulations. For example, an antibody that requires subcutaneous dosing at 2 mg per kilogram of patient weight will need to be formulated at a concentration of 130—200 mg per mL to accommodate a 100 kg patient. However, such highly concentrated antibody solutions can demonstrate high viscosities in some cases,10 which can lead to serious difficulties in their development as drug products suitable for subcutaneous administration 10 because high viscosity may increase the injection time and pain at the site of injection, adversely affecting patient compliance.12,13 High viscosity of a concentrated antibody solution also poses difficulties during bioprocessing of the drug substance. During downstream ultrafiltration and diafiltration steps, high viscosity of the antibody solution may increase the back pressure of the pump and decrease the transmembrane flux, which may increase the processing time, destabilize the drug substance and increase manufacturing costs.14,15 A detailed discussion on the role of viscosity in the manufacturability of concentrated antibody solutions can be found in the review by Shire.15
A number of research and development activities must be undertaken to support development of highly concentrated antibody drug products suitable for subcutaneous delivery. First, high-throughput computational and experimental tools that can rapidly estimate concentration-dependent viscosity behavior of therapeutic mAb candidates need to be developed. These tools should be applied at early stages of drug discovery to identify mAb candidates vulnerable to demonstrating high viscosities at high concentrations (e.g., above 100 mg per mL). Depending upon the stage of a project and availability of alternatives with comparable biologic activity, such candidates may be either de-prioritized or suitably optimized. Second, understanding the roles played by a lead candidate's sequence and structure in determining its high concentration solution behavior is essential to optimize the lead candidate for reduced viscosity while maintaining its biologic activity and stability. Third, the role of formulation components and their interaction with protein molecule in controlling the solution viscosity must be clearly understood to support discovery or rational design of viscosity lowering excipients suitable for subcutaneous delivery.
In recent years, both industrial and academic researchers have devoted considerable efforts to understand the molecular origins of concentration-dependent viscosity behaviors of mAbs. Here, we review these efforts in the context of an emerging concept called developability of biologic drug products. We begin with a description of solution viscosity and then discuss how intermolecular interactions dictate concentration-dependent viscosity behaviors of mAbs. Viscosity behaviors of molecular solutions have been studied for several decades, and it is impossible to summarize the whole body of scientific literature within this review. Instead, we provide a focused perspective on approaches to understand viscosity behaviors of antibody solutions at the molecular level and propose general strategies to mitigate viscosity issues during development of high concentration therapeutic antibody drug products. High viscosity of concentrated antibody formulations is the primary focus here, although we recognize that many other challenges associated with the development of high concentration antibody formulations, such as opalescence, solubility and aggregation, can also potentially delay the time to market. Additionally, this review focuses on Newtonian viscosity behavior of antibody solutions even though recent reports in literature have identified non-Newtonian behavior in certain antibody solutions13,16,17 and the increase in viscosity due to accumulation of antibody molecules on the air/water interface.18
Macroscopic and microscopic descriptions of solution viscosity
At the macroscopic level, viscosity is the rate of transfer of momentum in a liquid.19 At the microscopic level, solution viscosity is the resistance to solute mobility as illustrated in Fig. 1.
Figure 1.
Stokes' law for viscous drag on a spherical solute. This law can be extended to non-spherical solutes.92
Briefly, the Stokes-Einstein equation connects solution viscosity (η) with microscopic quantities such as diffusion coefficient (D) of spherical solute molecules.20
| (1) |
Where, kB is the Boltzmann constant, T is temperature and rH is the hydrodynamic radius.
Einstein 20 derived the viscosity of a dilute solution of a spherical solute,
| (2) |
where, is the viscosity of solution at zero solute concentration, and ϕ is the volume fraction of the solute. Later, Jeffery extended this to a solution of ellipsoids,21
| (3) |
where f is a factor dependent on dimensions of the ellipsoids, and V is volume fraction of the ellipsoids. The equations above describe viscosity behaviors of dilute solutions with the assumption that the solute molecules do not experience the presence of one another. That is, the effects due to molecular crowding and solute-solute interactions are ignored.
Molecular crowding is an important consideration in understanding the physical behavior of solutions because each solute molecule diminishes the available volume to other solute molecules.22 Mooney's equation incorporates effects of molecular crowding,23
| (4) |
where, k is the crowding factor. While Mooney's equation accounts for molecular crowding, it assumes that solute molecules remain inert to one another except for crowding the available space, and therefore it ignores intermolecular interactions among the solute molecules. Ross and Minton modified Mooney's equation to include short-ranged intermolecular interactions,24
| (5) |
where, υ is the shape factor for the solute and [η] is the intrinsic viscosity. The Ross and Minton equation24 does not take into account long-range intermolecular interactions. In the case of macromolecules of biological interest such as mAbs, both the short-ranged and long-ranged intermolecular interactions play a key role in determining their concentration-dependent viscosity behaviors.25 Therefore, the following relationship is commonly used for describing concentration-dependent viscosity behaviors of macromolecular solutions,26
| (6) |
Here, coefficients , and so on represent the contributions of monomers, dimeric and higher-order clusters of macromolecules.26 Briefly, for a dilute solution of spheres, the coefficient is the intrinsic viscosity, the effect of solute molecule on the flow of solvent around it, and is the effect of one spherical solute on the flow around a second spherical solute.27,28 Therefore, is influenced by the pairwise distribution of the solutes in the solution. Computer algorithms can now predict k1 with reasonable accuracy from crystal structure of a protein because k1, i.e., intrinsic viscosity, heavily depends on the overall shape of the molecule.29
If the cubic and higher order powers of concentration in the above equation are ignored, then the equation becomes similar to that derived by Huggins for viscosity of a dilute solution of long-chain polymers,28
| (7) |
where, is specific viscosity, c is concentration of the solute, kH is a polymer, and solvent and temperature-dependent constant.
It is impossible to list all the equations that have been used in literature to describe solution viscosity. In 1962, Rutgers 30 listed 96 equations and classified them into various categories such as theoretical, semi-empirical, empirical, Einsteinian, logarithmic, and polynomial. Sudduth31 then showed that many of these equations differ only in the degree to which they account for intermolecular interactions.
From the above discussion, it is clear that intermolecular interactions among solute molecules play an important role in determining solution viscosity. The intermolecular interactions in turn depend on characteristics of the solute molecules (see below). In the next sections, we compare concentration-dependent viscosity behaviors of two antibodies and interpret their differences in terms of the intermolecular interactions formed by the mAbs.
Concentration-dependent viscosity behaviors of mAb solutions
Fig. 2 presents concentration-dependent viscosity curves of two antibody solutions under identical formulation conditions (i.e., same formulation buffer, pH, temperature, and excipients). At 150 mg per mL, mAb2 has significantly higher viscosity than mAb1. From the drug product development perspective, mAb1 is suitable for development as a high concentration drug product because its solution shows low viscosities at high concentrations. Because experimental conditions for both the mAbs are identical, the differences in their solution viscosity behaviors must arise from differences in the intermolecular interactions, antibody networks and higher-order structures formed by the mAbs in their respective solutions. The intermolecular interactions among antibody molecules include both pairwise and higher-order interactions involving multiple molecules. The pairwise intermolecular interactions, expected to prevail at dilute concentrations, are related to experimentally measurable quantities such as osmotic second virial coefficients (B22) and diffusion interaction parameter (kD). However, as the concentrations rise, the higher-order interactions are also expected to contribute significantly toward solution viscosity. The following sections describe both pairwise and higher-order intermolecular interactions in antibody solutions, and Table S1 in Supplementary Material describes various physical quantities that can help with understanding viscosity behaviors of these solutions.
Figure 2.
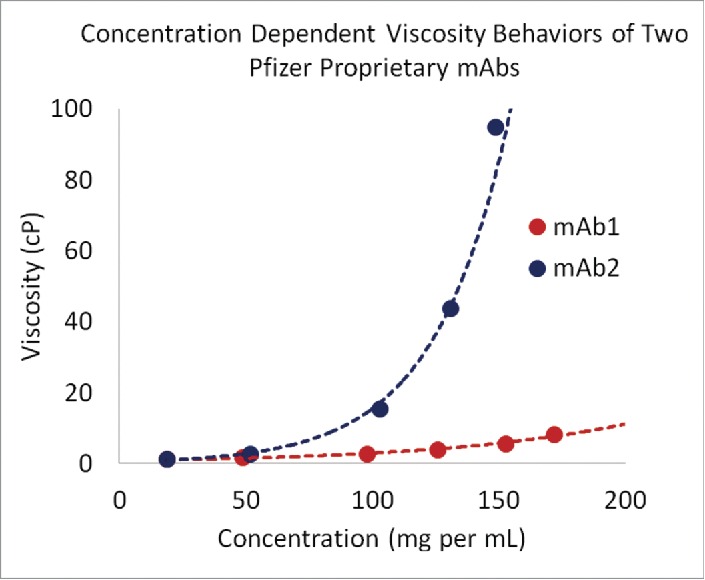
Concentration dependence of viscosity of antibody solutions under identical buffer conditions. Sequence—structure differences may be able to explain viscosity behavior.
Theoretical foundations and experimentally measurable quantities to describe pairwise intermolecular interactions
In literature, intermolecular interactions arising due to the polarizabilities of molecules have been often referred to as either dispersion interactions or van der Waals interactions. The two terms are used interchangeably in the following discussion.
Derjaguin-Landau-Vervey-Overbeek (DLVO) theory uses pairwise intermolecular interactions to rationalize fundamental features of colloidal systems.32 This theory can be extended to explain proteins solution behaviors as well.33 In the simplest form of this theory, Lifshitz-Hamaker approach describes van der Waals interactions and Debye–Hückel approximation describes electrostatic interactions.19 In both the Lifshitz-Hamaker approach and Debye–Hückel approximation, solvent is a continuum that screens the intermolecular interactions among the solute molecules. The intermolecular interactions arising from van der Waals interactions are always attractive.19 However, pairwise electrostatic interactions may be repulsive, neutral or attractive depending on the overall charge and charge distribution on solute molecules. This theory assumes that distance dependence of pairwise potential of mean force (PMF) is sufficient to explain solution behavior.19 PMF between two molecules, located at a separation r, is the reversible work required to bring these molecules from infinite separation to the distance r. A plot of PMF versus r may have multiple minima of differing potential energies separated by kinetic barriers that must be overcome to transition from one minimum to another. The depths of these energy minima and kinetic barriers determine the reversibility of intermolecular associations between the solute molecules. The effects of salts, charge distribution, and van der Waals interactions on this plot can then be studied to understand protein solution behavior.19,32,33
Another simplified theoretical framework specially designed for rapid assessment of intermolecular interactions in protein solutions is proximity energies.34 The proximity energy framework focuses on interactions between macromolecules such as proteins and antibodies. It is assumed that the pairwise interactions between two macromolecules become significant when they are proximal to each other. Two macromolecules are considered to be proximal when the separation between them is of the same order of magnitude as their molecular dimensions.
Both proximity energies framework and DLVO theory rely predominantly on pairwise intermolecular interactions among solute molecules to explain the solution characteristics. These theories can be experimentally tested by measuring osmotic second virial coefficient (B22). B22 is a measure of the strength of pairwise intermolecular interactions among the solute molecules in a solution. A positive value for B22 indicates repulsive pairwise intermolecular interactions between two solute monomers, while a negative value suggests attractive pairwise interactions.
In addition to its relationship with pairwise interactions and self-association of solute molecules, B22 is also related to diffusion. The diffusion coefficient is often written in terms of a polynomial of solute concentration.35 In the dilute concentration limit, the polynomial becomes:
| (8) |
where D is diffusion coefficient at concentration c, D0 is diffusion coefficient at infinite dilution and kD is diffusion interaction parameter.
Assuming a polynomial dependence for the friction coefficient, Harding et al.35 have established the following relationship between kD and B22,
| (9) |
where, M is the molecular weight of the solute, kS is the change in sedimentation coefficient per unit change in concentration, and is the partial specific volume of the solute.
Equation 9 is valid under the following three assumptions: 1) the friction coefficient is linearly correlated with solute concentration and the slope of the linear fit is kS; 2) the diffusion coefficient is also linearly correlated with solute concentration and the slope of the linear fit is kD; and 3) contributions of higher-order virial coefficients are nearly zero at dilute concentrations. This relationship provides an indirect estimate of pairwise intermolecular interactions using diffusion interaction parameter, kD.
Colloidal description of protein solutions can generate hypotheses for protein solution behavior relevant to drug development. This description is commonly applied to develop quantitative screens for protein-protein interactions. Yadav et al.36 suggested −5.1 mL per gm as cutoff for kD between repulsive and attractive interactions in antibody solutions by measuring hydrodynamic volume of an IgG1 via static light scattering. The intermolecular interactions are considered to be attractive if kD < −5.1 mL per gm, otherwise they are considered repulsive. However, Tessier and coworkers 37 suggested a cutoff at approximately −8 mL per gm. Note that these cutoff values depend upon peculiarities of antibody molecules, and are expected to change from molecule to molecule. The thermodynamic behaviors of concentrated antibody solutions can be modified by several factors, including formulation buffer components, molecular frameworks, isotypes, and drug discovery as well as manufacturing platforms.
Both, kD and B22 are good indicators of viscosity of antibody solutions at concentrations near 150 mg per mL. Saito et al. 38suggested the following general guideline: Antibodies with B22 greater than mL mol per gm2 have solution viscosities lower than 20 cP at 150 mg per mL, provided B22 and viscosity measurements are performed in the same formulation.38 In a separate study, Connolly et al.39 measured viscosities of 29 antibodies in the concentration range 0 to 200 mg per mL. The formulation conditions were the same for all these antibodies. The kD values were found to correlate well with solution viscosities at concentrations below 200 mg per mL. Additional data from Binabaji et al. confirmed the validity of this general guideline.40 In the data from Binabaji et al., mAbs that met the B22 criterion of Saito et al. had low solution viscosities at 150 mg per mL. However, Binabaji et al. also reported that B22 is insufficient to predict solution viscosities when mAb concentrations rise above 200 mg per mL. Furthermore, the authors demonstrated that osmotic third virial coefficient (B33), interactions involving three mAbs, is a good predictor of solution viscosity for antibody concentrations up to 300 mg per mL. In qualitative agreement with the findings of Binabaji and coworkers, Lilyestrom et al. also noted that clusters consisting of as many as nine antibody molecules are present in an antibody solution at 175 mg per mL that has viscosity of approximately 450 cP.41 Additionally, recent computer simulations also suggested that higher-order antibody networks can form in certain antibody solutions at concentrations above 100 mg per mL.42
The higher order networks and structures within liquids can be probed by oscillatory rheometry experiments using a time variant stress. If the period of oscillation of this stress (inverse of the oscillation frequency) is sufficiently greater than the liquid's relaxation time, then the liquid dissipates all the energy provided to it by the external stress, a gas-like property. But, if this period is smaller than or equal to the liquid's relaxation time, then the liquid stores a fraction of the energy provided to it by the external stress, a solid-like property. Kalonia and coworkers have pioneered studies on the uses of ultrasonic rheometry for protein-protein interactions that are relevant to viscosity behaviors of highly concentrated antibody solutions.43,44 These studies experimentally measure ultrasonic storage modulus, which is related to storage of energy in the solution. Kalonia and coworkers have suggested ultrasonic storage modulus as an easy measure of protein-protein interactions in highly concentrated solutions. This is useful because the measurements of B22 and kD can only be performed in dilute solutions, and are therefore not a direct measure of protein-protein interactions at high concentrations.
The above discussion has been focused on the colloidal descriptions of intermolecular interactions and their role in determining viscosity behaviors of concentrated antibody solutions. However, Sarangapani et al. recently presented evidence that a colloidal description of protein solutions based solely on intermolecular interactions may be incomplete.45-47 The colloidal descriptions of macromolecular viscosity make the following assumptions: 1) a macromolecular solution is monodisperse, i.e., solute molecules have uniform size and shape; 2) the charges on macromolecular surface are uniformly distributed; and 3) the macromolecule is a rigid globule with little conformational flexibility. The authors show that these assumptions become particularly invalid at high concentrations, and therefore caution against using purely colloidal explanations of molecular origins of viscosity of protein solutions. Specifically, using bovine serum albumin as a model protein, these authors report that the protein's three-dimensional (3D) structure changes significantly at high concentrations compared to the one in dilute solution. Similar structural deviations may also occur in highly concentrated antibody solutions. The consequences of these structural changes should be included as additional factors while studying antibody rheology because intrinsic viscosity and friction coefficients of solutes are dependent on molecular size and shape.
The changes in 3D structures may be related to non-native aggregation, and recent reports show that non-native aggregation can increase viscosity of protein solutions. Nicoud et al.48 show that the aggregate fractal dimension of a mAb increases as concentration rises. Barnett et al.49 used the protein α-chymotrypsinogen as a model system to illustrate that the 3D structure of the protein changes with increasing concentration. In this case, monomer loss is followed by increase in viscosity. The authors then suggest that the mechanism of protein aggregation can be used to predict the viscosity of a protein solution in a concentration-dependent manner. Amin et al.50 have reviewed the mechanism and experimental characterization of native and non-native aggregates as well as particulate formation. The authors highlight that concentration-dependent formation of macromolecular aggregates may also contribute to the viscosity behaviors of protein solutions.
Taken together two complementary descriptions emerge from the above discussion. The colloidal description seeks to explain concentration-dependent viscosity behavior in terms of intermolecular interactions, assuming that the molecular conformations, and therefore the charge distributions on the molecular surface, remain the same at all concentrations. This suggests that highly concentrated antibody solutions contain an equilibrated mixture of pairwise and higher-order antibody interactions, with the equilibrium shifting in favor of higher-order interactions as antibody concentrations rise. Therefore, characterizing configurations of the antibody networks and higher-order oligomeric structures, enabled by the underpinning intermolecular interactions, in molecular details is critical to improve our understanding of concentration-dependent viscosity behaviors of the antibody solutions. In contrast, the molecular description emphasizes the need to consider changes in molecular structure at high concentration and their consequences. The emphasis is on the conformational dynamics of the solute molecules and non-native aggregation. The molecular and colloidal descriptions are not mutually exclusive of each other. Instead, they are complementary. Both molecular and colloidal descriptions are needed to fully understand solution behaviors of antibody molecules at different concentrations. Computational biophysical tools are the only methods currently available that can be used to study both of them. Atomistic molecular dynamics simulations can provide insights into conformational flexibilities of the antibody molecules and coarse-grained simulations of antibody solutions can prove useful in study of higher-order network formation and aggregation, while currently available light scattering and imaging experiments continue to evolve.
Multi-scale molecular simulations of antibody solutions to understand higher-order intermolecular interactions
In principle, concentration-dependent intermolecular interactions in antibody solutions can be studied in full atomic details via computer simulations. However, practical hurdles exist. An antibody molecule consists of approximately 1,400 amino acid residues or 20,000 atoms (including the hydrogen atoms). Adding explicit solvent molecules can increase the total number of atoms in a simulation box to anywhere between 400,000 and 1,000,000 depending upon type of simulation box and size of solvation shell used. Such simulations require large computing resources. Hence, only a few atomistic molecular dynamics (MD) simulations on a single full-length antibody molecule in explicit solvent have been performed,51-55 and only one study describing atomistic simulations on two full-length antibody molecules in the simulation box, to study pairwise intermolecular interactions, has been reported so far.56 While atomistic MD simulations of solutions containing several full length antibody molecules are currently impractical, coarse-grained MD simulations can provide a faster, but less accurate, understanding of intermolecular interactions in antibody solutions. In such simulations, groups of atoms, residues or even whole structural domains are represented by a single “bead”, depending upon the level of coarse-graining required. Besides reducing the number of particles in a simulation, coarse-graining macromolecules smoothens their energy landscapes.57 In general, coarse-graining of a biological macromolecule should be performed in a manner that retains reasonably accurate descriptions of the properties of interest while ignoring the others.57-59
Chaudhari et al.60,61 studied concentration-dependent solution behavior of two antibodies with different levels of coarse-graining and Buck et al.42 extended Chaudhari's approach to four different mAbs. Because of the small number of beads per antibody molecule and absence of explicit solvent molecules, these authors were able to put several hundred antibody molecules in a simulation box and perform molecular dynamics simulations over micro-second time scales. These studies found formation of antibody clusters, networks and higher-order oligomers to be consistent with increased viscosity of antibody solutions. Moreover, the simulations were able to pin-point the underlying cause. The presence of domain level electrostatic complementarities among the antibody molecules leads to formation of the antibody networks and higher-order structures.42 Such networks impart “solid-like” properties to the highly concentrated antibody solutions.42 The density to saturation ratios of these networks are conceptually related to modulus of elasticity. Therefore, antibody molecules capable of forming these networks are able to resist solution deformation under shear stress and demonstrate increased viscosities at high concentrations.42 Fig. 3 shows a cartoon representing network formation. Additionally, coarse-gained representations of antibody molecules that include greater details are also feasible. For example, in the MARTINI coarse-grained model, every amino acid is represented as one bead.62-64
Figure 3.

A pictorial representation of ideas expressed by Kumar and coworkers.42 An antibody is depicted as an electrostatic multipole. These electrostatic multipoles lead to higher-order network formation and higher viscosity.
Brownian dynamics simulations, in addition to coarse-grained molecular dynamics simulations, can also provide a faster route to understanding intermolecular interactions in protein solutions. These simulations are performed in absence of the solvent. The effect of solvent molecules is represented by an effective friction force, and a stochastic differential equation instead of Newtonian equation of motion is integrated 65-68 for the dynamics of the solute molecules. Additionally, a rigid body representation of the solute molecules and simplified forms of force-field parameters can also decrease the demands on computer resources and time.65 Brownian dynamics simulations have been employed to predict diffusion of antibodies in solution. Balbo et al.69 studied a murine antibody (PDB entry: 1IGT) solution at high concentrations (greater than 200 mg per mL) with fluorescence correlation spectroscopy and Brownian dynamics simulations. Translation diffusion coefficients measured from experiments and estimated by computer simulations were found to be in reasonable agreement. The authors concluded that molecular shape and size are major determinants of the protein diffusion. These simulations can also estimate higher-order intermolecular interactions and pairwise potential of mean force.65,70 For example, Wade and coworkers 70 have used Brownian dynamics simulations to detect concentration-dependent oligomer formation in the solutions of myoglobin, hemoglobin A, and sickle-cell hemoglobin S.
Differences in intermolecular interactions formed by antibody molecules arise from their hydrophobic and electrostatic attributes
Why, under identical formulation conditions, do solutions of some antibodies contain clusters, networks and higher-order structures while those of other antibodies do not? The answer to this question lies in differences in the molecular conformational dynamics of different antibodies, as well as in the intermolecular interactions formed by different antibodies. These differences, in turn, arise from differences in the amino acid sequences and structural properties of the antibody molecules. Electrostatic attributes such as net charge, distribution of partial atomic charges, pI and dipole moment of a full length antibody molecule and its component domains make important contributions toward determining nature, frequency and duration of intermolecular interactions formed in solution at a given antibody concentration. Hydrophobic interactions, on the other hand, are due to the tendency of biological macromolecules to exclude water and self-associate.71 The hydrophobic interactions drive self-association when the molecules are close by and there is shape complementarity at the interface between the interacting partners.71 In contrast, the electrostatic interactions can form at all intermolecular distances.
High concentration antibody drug products are typically formulated in the pH range of 5.2 to 6.3.72 Within this pH range, all the constant domains (except CH3 domain in IgG4 mAbs) have positive net charge, as shown by calculations of Li et al.71 that were performed using multiple crystal structures of antibodies and antibody components. This observation suggests that intermolecular electrostatic interactions involving only the constant domains of the mAbs are dominantly repulsive, and therefore variations in viscosity behaviors of different antibodies of a given isotype can be reasonably explained based on the differences in the molecular attributes of their variable regions. Consistently, the net charge, pI, zeta-potential and aggregation propensity of the Fv portions were used by Li et al.71 to explain concentration-dependent viscosity behaviors of antibody solutions, within the above stated formulation pH range. In an independent study, Dill and coworkers also arrived at similar conclusion.73
Kumar and coworkers have developed a profile-based method to screen antibody molecules based on attributes of their Fv portions.71 A pictorial representation of this method is provided in Fig. 4, and a brief description of antibody structure is provided in the Supplementary Material. In profile 1, antibodies have positive charge on Fv (ZFv), positive zeta-potential on Fv (ξFv), and pI of Fv (pIFv) > formulation pH (pHformulation). Therefore, overall electrostatic interactions among the antibody molecules are predominantly repulsive. Solutions of such antibodies are likely to show low to moderate viscosities at 150 mg per mL.71 In profile 2, ZFv and ξFv are close to zero and pIFv ≈ pHformulation, the intermolecular electrostatic interactions among such antibody molecules are still predominantly repulsive because of the positive net charge on constant regions, like in profile 1. However, electrostatic repulsions are weaker for the profile 2 antibodies, and the roles of hydrophobic interactions and shape complementarities become more significant toward viscosity outcomes of their high concentration solutions.71 In such cases, the antibody solutions are likely to show moderate viscosities at high concentrations.71 In the profile 3, the antibody molecules have negative ZFv, negative ξFv, and pIFv < pHformulation. Such antibodies are likely to show moderate to high viscosities at 150 mg per mL 71 because of electrostatic complementarities between the variable and constant regions. In the case of profile 3 antibodies, electrostatic interactions and hydrophobicity work in tandem. These profiles were deduced using concentration-dependent viscosity curves of 11 mAbs obtained under identical formulation and experimental conditions.71 Further tests using different sets consisting of 19 and 43 antibodies showed the validity of these qualitative profiles (data not shown).
Figure 4.

Profile-based method to screen antibody molecules for viscosity. This method can be used to rapidly screen antibody molecules with homology modeling of the Fv portion. The antibody molecules can be divided into three profiles based on the properties of the Fv region: positive net charge on Fv, positive zeta-potential of Fv, and pI of Fv greater than pH of the formulation buffer (profile 1, left-most molecule); nearly zero net charge on Fv, nearly zero zeta-potential of Fv, and pI of Fv near formulation pH (profile 2, middle molecule); negative net charge, negative zeta-potential, and pI of Fv greater than formulation pH (profile 3, right-most molecule). In case of profile 1, repulsions can overwhelm hydrophobic effect and a low to moderate viscosity can be expected. In profile 2, weaker repulsions are dominated by stronger hydrophobicity, and moderate viscosity can be expected. In profile 3, hydrophobicity and electrostatic attractions act in tandem. Higher viscosity should be anticipated and such molecules can be red-flagged.
Strategies to lower viscosity in highly concentrated antibody solutions: applying the knowledge of molecular underpinnings of viscosity to drug product development
The previous sections of this review described our current understanding of molecular origins of high viscosity in concentrated antibody solutions. In this section, we discuss drug discovery and development stage specific strategies to consistently produce low viscosity high concentration therapeutic antibody drug products. These strategies, summarized in Table 1, have emerged naturally from the previously discussed molecular underpinnings of viscosity of macromolecular solutions. Strategies I and II can be applied at early stages of drug discovery to select and optimize antibody candidates for their compatibility with platform formulations. If no platform formulations have been predefined, then formulation optimization (Strategy III) can also be performed at early stages as part of Strategy I and/or II.
Table 1.
Drug discovery and development stage specific strategies to reduce viscosities of highly concentrated antibody solutions.*
| Strategy | Stage of drug discovery and development | Potential approach to reduce viscosity | Additional considerations |
|---|---|---|---|
| I | Early discovery stage. Several candidates (10—100) are available and platform formulations have been defined | Rank antibody candiates for viscosity at high concentration using computational and experimental techniques | Prioritize the candidates that show low viscosities at high concentrations, if all other attributes are comparable |
| II | Lead candidate is identified but sequence optimization for easier developability, in platform formulations, is allowed | Generate variants of the lead candidate for reduced viscosity via rational design | Test variants for biological activity and other developability attributes |
| III | Lead candidate is already in advanced drug development stages and/or no sequence changes are allowed | Optimize the formulation and/or use viscosity reducing excipient(s) | Formulation change at advanced stages of drug development may be time consuming and costly because the reformulated drug product may need to be tested again for stability and safety. Use of novel excipient(s) may require additional safety considerations |
Refer to text for applications of these strategies.
Strategy I (Selection of low viscosity antibody candidates)
This is the most proactive and cost-effective strategy, and it should be applied at early stages of drug discovery when typically 10—100 potential lead candidates are available.74 At such stages, the antibody drug candidates that are likely to show low viscosities at high concentrations should be prioritized if all other attributes, such as potency, safety, physicochemical stability, and pharmacodynamics/pharmacokinetics, are also similar. It is pertinent to mention here that these requirements vary on a case-by-case basis because target product profiles differ across drug discovery and development projects. Computational high throughput tools capable of ranking antibody molecules according to their high concentration viscosity profiles, in platform formulation(s), can be appropriately used at such early stages of drug discovery. The computational tools have the advantage that they require no materials, and can therefore rank order large antibody sequence libraries for viscosity and other developability considerations even before any experimental work is undertaken.
Strategy II (Optimization of antibody lead candidate(s) for lower viscosity)
This option can be exercised at a stage of the drug discovery where the number of lead candidates is reduced to 1—5 or no more than 10 molecules. At this stage, the amino acid sequence of an antibody can be optimized to reduce viscosity while maintaining its biological activity and physicochemical stability. This option requires an advanced understanding of sequence—structure properties of the drug candidates and access to protein engineering and molecular biology facilities.75
Strategy III (Optimization of antibody formulations)
This option is most suitable for candidates at advanced drug development stages where amino acid sequence of the candidate molecule cannot be changed, but there is still scope for formulation optimization. Formulation components such as buffer, pH, excipients and salts can be optimized to yield lower viscosity for the drug product. However, a change in formulation can be very costly and resource intensive at such late stages because a formulation change shall trigger additional long term stability, safety and efficacy studies on the reformulated drug product. In essence, this is the least pro-active, most risky strategy as its tries to ‘fix' the viscosity issue, only if it arises.
Below, we examine literature reports pertinent to these viscosity reducing strategies.
Strategies I and II: Effects of sequence and structure attributes of an antibody on its viscosity behavior
Both electroviscous effects and protein-protein interactions can influence the concentration-dependent viscosity behavior of a macromolecule.19,25 For example, Raut et al.76 have identified the importance of electroviscous effects on the viscosity behavior of dual-variable domain immunoglobulin, a molecule larger than a conventional monoclonal antibody molecule. In a conventional antibody, electroviscous effects originate due to charge and charge distribution on the antibody. Total charge and charge distribution on the surface of an antibody govern its interactions with the solvent as well as inter-domain interactions within the antibody. These interactions are important determinants of the size of the solvation shell around the antibody molecule, and therefore its hydrodynamic radius and intrinsic viscosity. Charge and charge distribution are antibody sequence and structure dependent. Therefore, to understand the role of sequence and electroviscous effects in antibody solutions, Yadav et al.36 studied four positively charged antibodies at pH 6.0 at room temperature in histidine-hydrochloride buffer. They measured viscosity as a function of antibody concentration in the range 0 to 225 mg per mL. They also performed experimental measurements of zeta-potential and diffusion interaction parameter kD by measuring mutual diffusion in the concentration range 0–12 mg per mL. Based on their results, the authors concluded that protein-protein interactions, not electroviscous effects, lead to increased viscosities at high concentrations in antibody solutions. Additionally, the authors were also able to rationalize attractive intermolecular interactions in terms of total charge on the antibody molecules and suggested that greater positive charge on the antibody molecules leads to greater repulsions, and therefore to reduced viscosity. In another study involving mutation in antibody sequences, Shire and coworkers77 studied two antibodies that are different only in their complementarity-determining regions (CDRs), but one antibody had significantly higher viscosity than the other at 100 mg per mL. The authors then produced several mutants by exchanging residues in CDRs of the two antibodies and found that the greatest reduction in viscosity occurs when CDR residues from both the light and heavy chains were exchanged simultaneously from low to high viscosity antibody. This report suggested that CDRs could play a greater role in antibody intermolecular interactions than any other region of the antibody. Note that CDRs are found within the Fv region, and several viscosity prediction methods are being developed using this region. For example, Kumar and coworkers71 have developed a method that profiles antibody molecules based on the properties of the Fv region. Sharma and coworkers,73 used charge on the Fv region, charge difference between VL and VH domains, and total hydrophobicity of the amino acids in the Fv region to predict viscosity at 180 mg per mL concentration. Agrawal and coworkers78 have developed another method for viscosity prediction at high concentrations using the distribution of the negatively charged residues in the Fv region. All these methods use structural models of the Fv portions, obtained from homology modeling, for viscosity prediction at high concentration. Such methods can be further improved by using equilibrated molecular dynamics simulations to generate conformational ensembles and compute average molecular properties of the Fv regions. Use of full-length antibody models may further improve accuracy of the viscosity prediction methods due to improved descriptions of electrostatic multipole effects that arise from the differences in charges on individual antibody domains.
In three closely related studies, Kumar and coworkers42,71,75 identified the molecular attributes that correlate with viscosity in highly concentrated antibody solutions, rationalized their observations using a putative mechanism involving antibody network formation gleaned from coarse-grained simulations, and then used this knowledge to rationally design viscosity reducing mutants of an antibody. They obtained concentration-dependent viscosity curves of eleven different antibodies under standardized formulation conditions,71 and interpreted this experimental data in terms of sequence and structural attributes of the mAbs and their components. An empirical equation, derived from the data, connected pI and aggregation propensity of the Fv regions with viscosity of the mAbs at 150 mg per mL. The findings from this data analysis71 and coarse grained simulations42 were combined to rationally design viscosity lowering variants of an antibody that demonstrated high viscosities at concentrations above 100 mg per mL.75 A negatively charged patch and an aggregation-prone region (APR) on the Fv portion of the viscosity-challenged antibody were disrupted via single point mutations. The APR was disrupted via two different single-point mutations, V44K and L45K. In the case of the negatively charged patch, different mutations were designed to reverse the charge on the patch (E59K, E59R) and to neutralize the negative charge (E59Y). The antibody variants were tested for viscosity, solubility, diffusion, and biological activity. Surprisingly, charge neutralization rather than charge reversal yielded a mutant that showed the greatest reduction in viscosity. Disruption of the APR also resulted in reduced viscosity for the variant V44K, but the extent of reduction was insignificant. Both the mutants retain biological activity as demonstrated by in vitro enzyme-linked immunosorbent assay experiments. In summary, this study dissected the contributions of hydrophobic and electrostatic intermolecular interactions, and suggested that rational design strategies for optimizing antibodies for reduced viscosity should focus on the surface charge distribution of their Fv regions.
Strategy III: Optimizing formulation for reduced viscosity
To optimize the formulation of an antibody drug candidate for lower viscosity, it is necessary to understand the effect of formulation components, such as buffer, pH, salt, surfactant and other excipients, on intermolecular interactions. The effect of formulation components on aggregation, stability, and solubility has been studied in greater detail than the effect on viscosity of antibody solutions. However, recent studies have provided some useful insights into the role of formulation components in viscosity of antibody solutions. Shire and coworkers79 studied the role of formulation pH and salt concentration on intermolecular interactions of an antibody. They estimated intermolecular interactions by measuring diffusion interaction parameter (kD). The authors also performed electrophoretic experiments to measure zeta-potential and pI of the antibody. They found that kD tends to be the most negative (attractive interactions) near the pI, and becomes more positive away from the pI (repulsive interactions). When the solution pH is away from the pI of the antibody, addition of salts makes intermolecular interactions less repulsive due to increased electrostatic screening. In a complementary study, Scherer80 studied the role of salts (NaCl, NaSCN, and ArgCl) on B22 of an antibody in a solution. The B22 of this antibody in a co-solute free solution was mL mol per gm2. However, by adding these co-solutes in the solution Scherer was able to achieve a B22 more positive than mL mol per gm2. Moreover, Esfandiary et al.81 studied the role of hydrophobic and electrostatic interactions in the mechanism of antibody self-association. The authors found that self-association is likely driven by interactions of anions with positively charged residues of an antibody with some support from hydrophobic interactions. The authors also studied solutions of Fab and Fc fragments of the antibody and concluded that Fab-Fab interactions contribute more than Fab-Fc or Fc-Fc interactions toward the antibody self-association. Interestingly, the authors find that antibody self-association is anion specific, thus suggesting a possibility of changing the degree of self-association in antibody solutions by using different salts.
In addition to changing formulation pH and salt concentration, excipients can also be added to the formulation to reduce the viscosity of an antibody drug candidate at high concentrations. Klibanov and coworkers82 have studied the effect of hydrophobic salts on the viscosity of antibody solutions at 100 mg per mL concentration. The authors tested 43 different salts and achieved up to 10-fold reduction in viscosity using salts of camphor sulfonic acid. Kamerzell et al.83 have also studied polar salts, dimethyl sulfoxide and dimethylacetamide. The authors found that these salts decrease the viscosity of the antibody solutions by preferentially solvating hydrophobic residues and disrupting intermolecular interactions. He and coworkers 84 have studied the role of sugars (trehalose, sorbitol, sucrose, xylose, galactose, fructose, and glucose) on the viscosity behaviors of antibody solutions. The authors found that sugars increase the viscosity of antibody solutions at high concentrations. Further, the disaccharides (trehalose and sucrose) increase the viscosity more than the monosaccharides (sorbitol, xylose, galactose, fructose, and glucose). The authors also found that increases in temperature or the concentration of sodium chloride decrease the viscosity of antibody solutions. The decrease in viscosity due to salt is often explained by shielding of electrostatic interactions by the salts.
In addition to salts and sugars, amino acids and small peptides can be used to decrease viscosity of concentrated antibody solutions. Wang and coworkers 85 studied the role of amino acids (alanine, proline, valine, glycine, serine, histidine hydrochloride, lysine hydrochloride, arginine hydrochloride, sodium glutamate), salts (sodium chloride, sodium acetate, sodium sulfate, ammonium chloride), urea and guanidine hydrochloride on the concentration-dependent viscosity behaviors of two antibody solutions. They found that urea and nonpolar amino acids have insignificant effects on viscosity behaviors of the antibody solutions. However, the salts and charged amino acids in their salt forms reduce the viscosity substantially. The authors further studied the physicochemical environment of amino acids by fluorescence spectroscopy to determine the role of aromatic amino acids in the concentration dependent viscosity behavior of antibody solutions. The physicochemical environment of the aromatic residues remains unchanged in the presence of excipients, which rules out interactions between the aromatic groups and the excipients as a likely cause of decrease in viscosity.
In two related reports, Inoue and coworkers86,87 studied the effect of viscosity-reducing excipients (lysine hydrochloride, arginine hydrochloride, methionine, threonine, valine, glycine, proline, serine, alanine, histidine, sodium chloride, and guanidine hydrochloride) on the viscosity behaviors of human and bovine albumins as well as IgGs. The authors found that charged amino acids and salts decrease the viscosity more than the nonpolar amino acids. The molecular mechanisms of viscosity reduction by arginine hydrochloride and lysine hydrochloride are similar to salts; they also reduced viscosity by shielding the electrostatic interactions between macromolecules. However, the authors also find that the same excipient can increase or decrease the viscosity of an antibody solution depending on the concentration of the excipient in the solution. Using salts of arginine, Trout and coworkers88 have highlighted the effect of anions on protein aggregation, and found that ArgH(H2PO4) reduced protein aggregation to the greatest extent, while ArgHSCN shows the least reduction. Similarly, some anions may be able to reduce viscosity of antibody solutions more than the others.
In summary, a variety of excipients and formulation components can be used to reduce viscosity. A recent patent file by Bowen and coworkers89 lists more than 25 excipients and small peptides to reduce viscosity of antibody solutions.
Future Directions
The proportion of biologic drug candidates in the pipelines of leading pharmaceutical companies has increased substantially in the past decade. Proactive elimination or mitigation of product development challenges such as high viscosity can help reduce the cost of drug development by facilitating efficient translation of drug candidates into marketed products. Therefore, it is desirable for researchers interested in improving developability of therapeutic mAb candidates to devise a repertoire of innovative solutions that are capable of: 1) rapidly identifying drug candidates with potential developability issues; 2) suggesting rational sequence optimizations to eliminate or mitigate potential developability issues in the lead candidates without negatively affecting their biological activity; and 3) discovering or rationally designing safe excipients to mitigate developability challenges, which can help meet target product profiles for candidates already in late-stage drug development, as well as facilitate new opportunities for product life-cycle management. In this regard, reducing the viscosity of highly concentrated antibody solutions is an active area of both experimental and computational research. Diverse solutions to the problem are being explored and validated. However, the number of antibody molecules studied in literature reports is relatively small. In the coming years, these tools shall be further perfected as larger datasets of the antibody solution behaviors become available.
From the perspective of therapeutic drug product development, quantitatively predicting the concentration-dependent viscosity curve of a therapeutic mAb candidate in a platform formulation, solely from its amino acid sequence or structure is currently a challenge. Meeting this challenge shall enable rapid screening (at the early stages of drug discovery) of antibody molecules with potential viscosity issues. Molecular assessment activities involving the experiments performed under standardized conditions can help identify antibody candidates with potential developability issues. Feeding the results from such activities into molecular attribute databases can enable derivation of statistical correlations between viscosity of highly concentrated antibody solutions and molecular level descriptors of antibody sequence—structure. Initial attempts in this direction are already yielding promising results.71,73 Along with predicting the viscosity curves, the ability to reliably predict the concentration threshold above which the viscosity of an antibody solution shall become unacceptably high (e.g., >20 cP) can provide valuable inputs to early-stage drug candidate selection and design.
Two complementary strategies could guide future research in this area. Firstly, a detailed knowledge of molecular sequence—structure motifs present in the antibody molecules that drive intermolecular interactions, antibody networks and cluster formations at high concentrations is required. Coarse-grained multi-copy molecular dynamics simulations suggest that electrostatically driven intermolecular interactions and formation of antibody networks and clusters, promoted by the negatively charged Fv regions, leads to high viscosities in antibody solutions.42 However, atomistic details of antibody network configurations that enable such intermolecular interactions are yet to be gleaned. Particularly, the roles played by positively and negatively charged surface patches and shape complementarities in promoting intermolecular interactions among the antibody molecules and their concentration dependence have not been clearly understood so far. Secondly, computational tools of atomistic and coarse grained modeling, simulations and protein design can be utilized to suggest minor sequence modifications to an antibody candidate such that its potency and physicochemical stability are retained. Initial attempts to rationally design such antibody variants have been successful.73,75,77 Further delineating such rational strategies will be very useful toward suitably improving solution behaviors of highly valuable lead candidates. As discussed earlier, a large and positive osmotic second virial coefficient is a good indicator of lower viscosity of antibody solutions at 150 mg per mL concentration. Rapid computational methods that can estimate osmotic second virial coefficient of an antibody in a solution need to be developed and used to screen antibody candidates with developability issues. Such estimates can also be used to identify viscosity reducing mutations in an antibody candidate.
Although out of the scope of this review, we note that alternative approaches to the problems associated with highly viscous drugs are also being developed. For example, the subcutaneous space available for drug administration can be expanded. Technologies such as Enhanze™, a recombinant human hyaluronidase from Halozyme, Inc.., can increase the volume available in the subcutaneous space and ameliorate the need to develop highly concentrated drug products. This technology is being investigated for biologic drug products.90,91
Supplementary Material
Disclosure of potential conflicts of interest
The authors are employees of Pfizer Inc.
Acknowledgments
DST thanks Pfizer postdoctoral program for financial support. The authors acknowledge discussions with several Pfizer colleagues on topics of viscosity in antibody solutions.
References
- 1.Miller KL, Lanthier M. Regulatory watch: Innovation in biologic new molecular entities: 1986-2014. Nat Rev Drug Discov 2015; 14:83; PMID:25633785; http://dx.doi.org/ 10.1038/nrd4535 [DOI] [PubMed] [Google Scholar]
- 2.IMS Health Global Spending on Medicines. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/Corporate/IMS%20Health%20Institute/Reports/Global_Use_of_Meds_Outlook_2017/Biologics_Market.pdf. July132015 [Google Scholar]
- 3.Mullard A. 2014 FDA drug approvals. Nat Rev Drug Discov 2015; 14:77-81; PMID:25633781; http://dx.doi.org/ 10.1038/nrd4545 [DOI] [PubMed] [Google Scholar]
- 4.USFDA What Are "Biologics" Questions and Answers. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CBER/ucm133077.htm. July 8, 2015 [Google Scholar]
- 5.Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010; 9:767-74; PMID:20811384; http://dx.doi.org/ 10.1038/nrd3229 [DOI] [PubMed] [Google Scholar]
- 6.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10:317-27; PMID:20414205; http://dx.doi.org/ 10.1038/nri2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-87; PMID:22437872; http://dx.doi.org/ 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 8.Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol 2005; 23:1073-8; PMID:16151394; http://dx.doi.org/ 10.1038/nbt0905-1073 [DOI] [PubMed] [Google Scholar]
- 9.Buttel IC, Chamberlain P, Chowers Y, Ehmann F, Greinacher A, Jefferis R, Kramer D, Kropshofer H, Lloyd P, Lubiniecki A, et al.. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals 2011; 39:100-9; PMID:21353596; http://dx.doi.org/ 10.1016/j.biologicals.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 10.Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci 2004; 93:1390-402; PMID:15124199; http://dx.doi.org/ 10.1002/jps.20079 [DOI] [PubMed] [Google Scholar]
- 11.Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Controlled Release 2006; 114:230-41; PMID:16876899; http://dx.doi.org/ 10.1016/j.jconrel.2006.05.027 [DOI] [PubMed] [Google Scholar]
- 12.Friess H, Langrehr J, Oettle H, Raedle J, Niedergethmann M, Dittrich C, Hossfeld D, Stoger H, Neyns B, Herzog P, et al.. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer 2006; 6:285; PMID:17156477; http://dx.doi.org/ 10.1186/1471-2407-6-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rathore N, Pranay P, Bernacki J, Eu B, Ji W, Walls E. Characterization of protein rheology and delivery forces for combination products. J Pharmaceutical Sci 2012; 101:4472-80; PMID:22941931; http://dx.doi.org/ 10.1002/jps.23297 [DOI] [PubMed] [Google Scholar]
- 14.Goswami S, Wang W, Arakawa T, Ohtake S. Developments and challenges for mAb-based therapeutics. Antibodies 2013; 2:452-500; http://dx.doi.org/ 10.3390/antib2030452 [DOI] [Google Scholar]
- 15.Shire SJ. Formulation and manufacturability of biologics. Curr Opin Biotechnol 2009; 20:708-14; PMID:19880308; http://dx.doi.org/ 10.1016/j.copbio.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 16.Fischer I, Schmidt A, Bryant A, Besheer A. Calculation of injection forces for highly concentrated protein solutions. International journal of pharmaceutics 2015; 493:70-4; PMID:26211901; http://dx.doi.org/ 10.1016/j.ijpharm.2015.07.054 [DOI] [PubMed] [Google Scholar]
- 17.Allmendinger A, Fischer S, Huwyler J, Mahler HC, Schwarb E, Zarraga IE, Mueller R. Rheological characterization and injection forces of concentrated protein formulations: an alternative predictive model for non-Newtonian solutions. Eur J Pharm Biopharm 2014; 87:318-28; PMID:24560966; http://dx.doi.org/ 10.1016/j.ejpb.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 18.Pathak JA, Sologuren RR, Narwal R. Do clustering monoclonal antibody solutions really have a concentration dependence of viscosity? Biophys J 2013; 104:913-23; PMID:23442970; http://dx.doi.org/ 10.1016/j.bpj.2013.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg JC. An Introduction to Interfaces and Colloids, the bridge to nanoscience. World Scientific Publishing Company Private Limited; 2010 [Google Scholar]
- 20.Lauffer MA. Motion in viscous liquids: Simplified derivations of the Stokes and Einstein equations. J Chem Edu 1981; 58:250; http://dx.doi.org/ 10.1021/ed058p250 [DOI] [Google Scholar]
- 21.Junk M, Illner R. A new derivation of Jeffery's equation. J Math Fluid Mech 2007; 9:455-88; http://dx.doi.org/ 10.1007/s00021-005-0208-0 [DOI] [Google Scholar]
- 22.Zhou HX, Rivas G, Minton AP. Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Ann Rev Biophys 2008; 37:375-97; PMID:18573087; http://dx.doi.org/ 10.1146/annurev.biophys.37.032807.125817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mooney M. The viscosity of a concentrated suspension of spherical particles. J Colloid Sci 1951; 6:162-70; http://dx.doi.org/ 10.1016/0095-8522(51)90036-0 [DOI] [Google Scholar]
- 24.Ross PD, Minton AP. Hard quasispherical model for the viscosity of hemoglobin solutions. Biochem Biophys Res Commun 1977; 76:971-6; PMID:20088; http://dx.doi.org/ 10.1016/0006-291X(77)90950-0 [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Nguyen MD, Andya JD, Shire SJ. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J Pharm Sci 2005; 94:1928-40; PMID:16052543; http://dx.doi.org/ 10.1002/jps.20347 [DOI] [PubMed] [Google Scholar]
- 26.Creighton TE. The physical and chemical basis of molecular biology. Helvetian Press, 2010 [Google Scholar]
- 27.Simha R. Effect of concentration on the viscosity of dilute solutions. Transactions N York Acad Sci 1949; 11:96; http://dx.doi.org/ 10.1111/j.2164-0947.1949.tb00137.x [DOI] [PubMed] [Google Scholar]
- 28.Huggins ML. The viscosity of dilute solutions of long-chain molecules. IV. dependence on concentration. J Am Chem Society 1942; 64:2716-8; http://dx.doi.org/ 10.1021/ja01263a056 [DOI] [Google Scholar]
- 29.Aragon S. A precise boundary element method for macromolecular transport properties. J Computational Chem 2004; 25:1191-205; PMID:15116362; http://dx.doi.org/ 10.1002/jcc.20045 [DOI] [PubMed] [Google Scholar]
- 30.Rutgers IR. Relative viscosity and concentration. Rheol Acta 1962; 2:305-48; http://dx.doi.org/ 10.1007/BF01976051 [DOI] [Google Scholar]
- 31.Sudduth RD. A generalized model to predict the viscosity of solutions with suspended particles. I. J Applied Polymer Sci 1993; 48:25-36; http://dx.doi.org/ 10.1002/app.1993.070480104 [DOI] [Google Scholar]
- 32.Israelachvili JN. Intermolecular and surface forces. Elsevier; 2011 [Google Scholar]
- 33.Nicoud L, Owczarz M, Arosio P, Morbidelli M. A multiscale view of therapeutic protein aggregation: A colloid science perspective. Biotechnol J 2015; 10:367-78; PMID:25772395; http://dx.doi.org/ 10.1002/biot.201400858 [DOI] [PubMed] [Google Scholar]
- 34.Laue T. Proximity energies: a framework for understanding concentrated solutions. J Mol Recognition 2012; 25:165-73; PMID:22407980; http://dx.doi.org/ 10.1002/jmr.2179 [DOI] [PubMed] [Google Scholar]
- 35.Harding SE, Johnson P. The concentration-dependence of macromolecular parameters. Biochem J 1985; 231:543-7; PMID:4074322; http://dx.doi.org/ 10.1042/bj2310543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav S, Shire SJ, Kalonia DS. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J Pharm Sci 2010; 99:4812-29; PMID:20821382; http://dx.doi.org/ 10.1002/jps.22190 [DOI] [PubMed] [Google Scholar]
- 37.Jayaraman J, Wu J, Brunelle MC, Cruz AM, Goldberg DS, Lobo B, Shah A, Tessier PM. Plasmonic measurements of monoclonal antibody self-association using self-interaction nanoparticle spectroscopy. Biotechnol Bioeng 2014; 111:1513-1520 [DOI] [PubMed] [Google Scholar]
- 38.Saito S, Hasegawa J, Kobayashi N, Kishi N, Uchiyama S, Fukui K. Behavior of monoclonal antibodies: relation between the second virial coefficient (B (2)) at low concentrations and aggregation propensity and viscosity at high concentrations. Pharm Res 2012; 29:397-410; PMID:21853361; http://dx.doi.org/ 10.1007/s11095-011-0563-x [DOI] [PubMed] [Google Scholar]
- 39.Connolly BD, Petry C, Yadav S, Demeule B, Ciaccio N, Moore JM, Shire SJ, Gokarn YR. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J 2012; 103:69-78; PMID:22828333; http://dx.doi.org/ 10.1016/j.bpj.2012.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binabaji E, Ma J, Zydney AL. Intermolecular interactions and the viscosity of highly concentrated monoclonal antibody solutions. Pharm Res 2015; 32(9):3102; PMID:25832501 [DOI] [PubMed] [Google Scholar]
- 41.Lilyestrom WG, Yadav S, Shire SJ, Scherer TM. Monoclonal antibody self-association, cluster formation, and rheology at high concentrations. J Phys Chem B 2013; 117:6373-84; PMID:23560896; http://dx.doi.org/ 10.1021/jp4008152 [DOI] [PubMed] [Google Scholar]
- 42.Buck PM, Chaudhri A, Kumar S, Singh SK. Highly viscous antibody solutions are a consequence of network formation caused by domain-domain electrostatic complementarities: insights from coarse-grained simulations. Mol Pharm 2015; 12:127-39; PMID:25383990; http://dx.doi.org/ 10.1021/mp500485w [DOI] [PubMed] [Google Scholar]
- 43.Saluja A, Badkar AV, Zeng DL, Kalonia DS. Ultrasonic rheology of a monoclonal antibody (IgG2) solution: implications for physical stability of proteins in high concentration formulations. J Pharm Sci 2007; 96:3181-95; PMID:17588261; http://dx.doi.org/ 10.1002/jps.20970 [DOI] [PubMed] [Google Scholar]
- 44.Saluja A, Badkar AV, Zeng DL, Nema S, Kalonia DS. Ultrasonic storage modulus as a novel parameter for analyzing protein-protein interactions in high protein concentration solutions: correlation with static and dynamic light scattering measurements. Biophys J 2007; 92:234-44; PMID:17028129; http://dx.doi.org/ 10.1529/biophysj.106.095174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prausnitz J. The fallacy of misplaced concreteness. Biophys J 2015; 108:453-4; PMID:25650910; http://dx.doi.org/ 10.1016/j.bpj.2014.11.3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarangapani P, Jones RL, Hudson S, Pathak JA. The pH and concentration dependence of protein-protein interactions, conformation, and viscosity in crowded protein solutions. Biophys J 2013; 106:665a-6a; http://dx.doi.org/ 10.1016/j.bpj.2013.11.3685 [DOI] [Google Scholar]
- 47.Sarangapani Prasad S, Hudson Steven D, Jones Ronald L, Douglas Jack F, Pathak Jai A. Critical examination of the colloidal particle model of globular proteins. Biophys J 2015; 108:724-37; PMID:25650939; http://dx.doi.org/ 10.1016/j.bpj.2014.11.3483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicoud L, Lattuada M, Yates A, Morbidelli M. Impact of aggregate formation on the viscosity of protein solutions. Soft Matter 2015; 11:5513-22; PMID:26061258; http://dx.doi.org/ 10.1039/C5SM00513B [DOI] [PubMed] [Google Scholar]
- 49.Barnett GV, Qi W, Amin S, Neil Lewis E, Roberts CJ. Aggregate structure, morphology and the effect of aggregation mechanisms on viscosity at elevated protein concentrations. Biophy Chem 2015; 207:21-9; PMID:26284891; http://dx.doi.org/ 10.1016/j.bpc.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 50.Amin S, Barnett GV, Pathak JA, Roberts CJ, Sarangapani PS. Protein aggregation, particle formation, characterization & rheology. Curr Opin Colloid Interface Sci 2014; 19:438-49; http://dx.doi.org/ 10.1016/j.cocis.2014.10.002 [DOI] [Google Scholar]
- 51.Voynov V, Chennamsetty N, Kayser V, Helk B, Trout BL. Predictive tools for stabilization of therapeutic proteins. MAbs 2009; 1:580-2; PMID:20068399; http://dx.doi.org/ 10.4161/mabs.1.6.9773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chennamsetty N, Voynov V, Kayser V, Helk B, Trout BL. Design of therapeutic proteins with enhanced stability. Proc Natl Acad Sci U S A 2009; 106:11937-42; PMID:19571001; http://dx.doi.org/ 10.1073/pnas.0904191106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandt JP, Patapoff TW, Aragon SR. Construction, MD simulation, and hydrodynamic validation of an all-atom model of a monoclonal IgG antibody. Biophys J 2010; 99:905-13; PMID:20682269; http://dx.doi.org/ 10.1016/j.bpj.2010.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Kumar S, Buck PM, Singh SK. Impact of de-glycosylation and thermal stress on conformational stability of a full length murine IgG2a monoclonal antibody: Observations from molecular dynamics simulations. Proteins 2012; 81:443-460 [DOI] [PubMed] [Google Scholar]
- 55.Kortkhonjia E, Brandman R, Zhou JZ, Voelz VA, Chorny I, Kabakoff B, Patapoff TW, Dill KA, Swartz TE. Probing antibody internal dynamics with fluorescence anisotropy and molecular dynamics simulations. MAbs 2013; 5:306-22; PMID:23396076; http://dx.doi.org/ 10.4161/mabs.23651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lapelosa M, Patapoff TW, Zarraga IE. Molecular simulations of the pairwise interaction of monoclonal antibodies. J Phys Chem B 2014; 118:13132-41; PMID:25350229; http://dx.doi.org/ 10.1021/jp508729z [DOI] [PubMed] [Google Scholar]
- 57.Saunders MG, Voth GA. Coarse-graining methods for computational biology. Ann Rev Biophy 2013; 42:73-93; PMID:23451897; http://dx.doi.org/ 10.1146/annurev-biophys-083012-130348 [DOI] [PubMed] [Google Scholar]
- 58.Takada S. Coarse-grained molecular simulations of large biomolecules. Curr Opin Struct Biol 2012; 22:130-7; PMID:22365574; http://dx.doi.org/ 10.1016/j.sbi.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 59.Kamerlin SC, Vicatos S, Dryga A, Warshel A. Coarse-grained (multiscale) simulations in studies of biophysical and chemical systems. Ann Rev Phys Chem 2011; 62:41-64; PMID:21034218; http://dx.doi.org/ 10.1146/annurev-physchem-032210-103335 [DOI] [PubMed] [Google Scholar]
- 60.Chaudhri A, Zarraga IE, Kamerzell TJ, Brandt JP, Patapoff TW, Shire SJ, Voth GA. Coarse-grained modeling of the self-association of therapeutic monoclonal antibodies. J Phys Chem B 2012; 116:8045-57; PMID:22694284; http://dx.doi.org/ 10.1021/jp301140u [DOI] [PubMed] [Google Scholar]
- 61.Chaudhri A, Zarraga IE, Yadav S, Patapoff TW, Shire SJ, Voth GA. The role of amino acid sequence in the self-association of therapeutic monoclonal antibodies: insights from coarse-grained modeling. J Phys Chem B 2013; 117:1269-79; PMID:23316912; http://dx.doi.org/ 10.1021/jp3108396 [DOI] [PubMed] [Google Scholar]
- 62.Marrink SJ, Risselada HJ, Yefimov S, Tieleman DP, de Vries AH. The MARTINI force field: coarse grained model for biomolecular simulations. J Phys Chem B 2007; 111:7812-24; PMID:17569554; http://dx.doi.org/ 10.1021/jp071097f [DOI] [PubMed] [Google Scholar]
- 63.Marrink SJ, Tieleman DP. Perspective on the Martini model. Chem Soc Rev 2013; 42:6801-22; PMID:23708257; http://dx.doi.org/ 10.1039/c3cs60093a [DOI] [PubMed] [Google Scholar]
- 64.Periole X, Marrink SJ. The Martini coarse-grained force field. Methods Mol Biol 2013; 924:533-65; PMID:23034762; http://dx.doi.org/ 10.1007/978-1-62703-017-5_20 [DOI] [PubMed] [Google Scholar]
- 65.Martinez M, Bruce NJ, Romanowska J, Kokh DB, Ozboyaci M, Yu X, Öztürk MA, Richter S, Wade RC. SDA 7: A modular and parallel implementation of the simulation of diffusional association software. J Computational Chem 2015; 36:1631-1645; PMID:26123630; http://dx.doi.org/ 10.1002/jcc.23971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erban R. From molecular dynamics to Brownian dynamics. Proc Math Phys Eng Sci 2014; 470:20140036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen MP, Tildesley DJ. Computer simulation of liquids. Oxford: Science Publications; 1986. [Google Scholar]
- 68.Doyle P, Underhill P. Brownian Dynamics Simulations of Polymers and Soft Matter In: Yip S, ed. Handbook of Materials Modeling: Springer; Netherlands, 2005:2619-30 [Google Scholar]
- 69.Balbo J, Mereghetti P, Herten DP, Wade RC. The shape of protein crowders is a major determinant of protein diffusion. Biophys J 2013; 104:1576-84; PMID:23561534; http://dx.doi.org/ 10.1016/j.bpj.2013.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mereghetti P, Wade RC. Atomic detail Brownian Dynamics simulations of concentrated protein solutions with a mean field treatment of hydrodynamic interactions. J Phys Chem B 2012; 116:8523-33; PMID:22594708; http://dx.doi.org/ 10.1021/jp212532h [DOI] [PubMed] [Google Scholar]
- 71.Li L, Kumar S, Buck PM, Burns C, Lavoie J, Singh SK, Warne NW, Nichols P, Luksha N, Boardman D. Concentration dependent viscosity of monoclonal antibody solutions: explaining experimental behavior in terms of molecular properties. Pharm Res 2014; 31:3161-78; PMID:24906598; http://dx.doi.org/ 10.1007/s11095-014-1409-0 [DOI] [PubMed] [Google Scholar]
- 72.Warne NW. Development of high concentration protein biopharmaceuticals: the use of platform approaches in formulation development. Eur J Pharm Biopharm 2011; 78:208-12; PMID:21406226; http://dx.doi.org/ 10.1016/j.ejpb.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 73.Sharma VK, Patapoff TW, Kabakoff B, Pai S, Hilario E, Zhang B, Li C, Borisov O, Kelley RF, Chorny I, et al.. In silico selection of therapeutic antibodies for development: viscosity, clearance, and chemical stability. Proc Natl Acad Sci U S A 2014; 111:18601-6; PMID:25512516; http://dx.doi.org/ 10.1073/pnas.1421779112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zurdo J. Developability assessment as an early de-risking tool for biopharmaceutical development. Pharmaceutical Bioprocessing 2013; 1:29-50; http://dx.doi.org/ 10.4155/pbp.13.3 [DOI] [Google Scholar]
- 75.Nichols P, Li L, Kumar S, Buck PM, Singh SK, Goswami S, Balthazor B, Conley TR, Sek D, Allen MJ. Rational design of viscosity reducing mutants of a monoclonal antibody: hydrophobic vs. electrostatic inter-molecular interactions. MAbs 2015; 7:212-30; PMID:25559441; http://dx.doi.org/ 10.4161/19420862.2014.985504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raut A, Kalonia D. Viscosity Analysis of Dual Variable Domain Immunoglobulin Protein Solutions: Role of Size, Electroviscous Effect and Protein-Protein Interactions. Pharmaceutical Res 2015; 33:1-12; PMID:25168518 [DOI] [PubMed] [Google Scholar]
- 77.Yadav S, Sreedhara A, Kanai S, Liu J, Lien S, Lowman H, Kalonia DS, Shire SJ. Establishing a link between amino acid sequences and self-associating and viscoelastic behavior of two closely related monoclonal antibodies. Pharm Res 2011; 28:1750-64; PMID:21626060; http://dx.doi.org/ 10.1007/s11095-011-0410-0 [DOI] [PubMed] [Google Scholar]
- 78.Agrawal NJ, Helk B, Kumar S, Mody N, Sathish HA, Samra HS, Buck PM, Li L, Trout BL. Computational tool for the early screening of monoclonal antibodies for their viscosities. MABS 2015; 8:1-6; PMID:26399600; http://dx.doi.org/ 10.1080/19420862.2015.1099773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yadav S, Liu J, Shire SJ, Kalonia DS. Specific interactions in high concentration antibody solutions resulting in high viscosity. J Pharm Sci 2010; 99:1152-68; PMID:19705420; http://dx.doi.org/ 10.1002/jps.21898 [DOI] [PubMed] [Google Scholar]
- 80.Scherer TM. Cosolute effects on the chemical potential and interactions of an IgG1 monoclonal antibody at high concentrations. J Phys Chem B 2013; 117:2254-66; PMID:23330570; http://dx.doi.org/ 10.1021/jp3091717 [DOI] [PubMed] [Google Scholar]
- 81.Esfandiary R, Parupudi A, Casas-Finet J, Gadre D, Sathish H. Mechanism of reversible self-association of a monoclonal antibody: role of electrostatic and hydrophobic interactions. J Pharm Sci 2015; 104:577-86; PMID:25407315; http://dx.doi.org/ 10.1002/jps.24237 [DOI] [PubMed] [Google Scholar]
- 82.Guo Z, Chen A, Nassar R, Helk B, Mueller C, Tang Y, Gupta K, Klibanov A. Structure-activity relationship for hydrophobic salts as viscosity-lowering excipients for concentrated solutions of monoclonal antibodies. Pharm Res 2012; 29:3102-9; PMID:22692671; http://dx.doi.org/ 10.1007/s11095-012-0802-9 [DOI] [PubMed] [Google Scholar]
- 83.Kamerzell TJ, Pace AL, Li M, Danilenko DM, McDowell M, Gokarn YR, Wang YJ. Polar solvents decrease the viscosity of high concentration IgG1 solutions through hydrophobic solvation and interaction: formulation and biocompatibility considerations. J Pharm Sci 2013; 102:1182-93; PMID:23359242; http://dx.doi.org/ 10.1002/jps.23453 [DOI] [PubMed] [Google Scholar]
- 84.He F, Woods C, Litowski J, Roschen L, Gadgil H, Razinkov V, Kerwin B. Effect of Sugar Molecules on the Viscosity of High Concentration Monoclonal Antibody Solutions. Pharmaceutical Res 2011; 28:1552-60; PMID:21573867; http://dx.doi.org/ 10.1007/s11095-011-0388-7 [DOI] [PubMed] [Google Scholar]
- 85.Wang S, Zhang N, Hu T, Dai WG, Feng X, Zhang X, Qian F. Viscosity-Lowering Effect of Amino Acids and Salts on Highly Concentrated Solutions of two IgG1 Monoclonal Antibodies. Mol Pharmaceutics 2015; 12(12):4478-87; PMID:26528726; http://dx.doi.org/24268865 10.1021/acs.molpharmaceut.5b00643 [DOI] [PubMed] [Google Scholar]
- 86.Inoue N, Takai E, Arakawa T, Shiraki K. Arginine and lysine reduce the high viscosity of serum albumin solutions for pharmaceutical injection. J Biosci Bioengineering 2014; 117:539-43; PMID:24268865; http://dx.doi.org/ 10.1016/j.jbiosc.2013.10.016 [DOI] [PubMed] [Google Scholar]
- 87.Inoue N, Takai E, Arakawa T, Shiraki K. Specific Decrease in Solution Viscosity of Antibodies by Arginine for Therapeutic Formulations. Mol Pharmaceutics 2014; 11:1889-96; PMID:24689736; http://dx.doi.org/ 10.1021/mp5000218 [DOI] [PubMed] [Google Scholar]
- 88.Schneider CP, Shukla D, Trout BL. Arginine and the Hofmeister Series: The Role of Ion–Ion Interactions in Protein Aggregation Suppression. The J Phy Chem B 2011; 115:7447-58; PMID:21568311; http://dx.doi.org/ 10.1021/jp111920y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bowen MN, Liu J, Patel AR. Compositions and methods useful for reducing the viscosity of protein-containing formulations. United States, 2013; The patent publication number is WO 2011/139718 A1. [Google Scholar]
- 90.Kang D, Jadin L, Nekoroski T, Drake F, Zepeda M. Recombinant human hyaluronidase PH20 (rHuPH20) facilitates subcutaneous infusions of large volumes of immunoglobulin in a swine model. Drug Deliv and Transl Res 2012; 2:254-64; http://dx.doi.org/ 10.1007/s13346-012-0065-3 [DOI] [PubMed] [Google Scholar]
- 91.Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of Subcutaneous and Intravenous Administration of Trastuzumab: A Phase I/Ib Trial in Healthy Male Volunteers and Patients With HER2-Positive Breast Cancer. J Clin Pharmacol 2013; 53:192-201; PMID:23436264; http://dx.doi.org/ 10.1177/0091270012436560 [DOI] [PubMed] [Google Scholar]
- 92.Berg HC. Random walks in biology Princeton: University Press, 1983 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


