Hydrogen production catalyzed by the extremely anaerobic enzyme [FeFe]-hydrogenase in air-grown microalgae reports on microoxic niches within the thylakoid stroma.
Abstract
Photosynthetic hydrogen production in the microalga Chlamydomonas reinhardtii is catalyzed by two [FeFe]-hydrogenase isoforms, HydA1 and HydA2, both irreversibly inactivated upon a few seconds exposure to atmospheric oxygen. Until recently, it was thought that hydrogenase is not active in air-grown microalgal cells. In contrast, we show that the entire pool of cellular [FeFe]-hydrogenase remains active in air-grown cells due to efficient scavenging of oxygen. Using membrane inlet mass spectrometry, 18O2 isotope, and various inhibitors, we were able to dissect the various oxygen uptake mechanisms. We found that both chlororespiration, catalyzed by plastid terminal oxidase, and Mehler reactions, catalyzed by photosystem I and Flavodiiron proteins, significantly contribute to oxygen uptake rate. This rate is considerably enhanced with increasing light, thus forming local anaerobic niches at the proximity of the stromal face of the thylakoid membrane. Furthermore, we found that in transition to high light, the hydrogen production rate is significantly enhanced for a short duration (100 s), thus indicating that [FeFe]-hydrogenase functions as an immediate sink for surplus electrons in aerobic as well as in anaerobic environments. In summary, we show that an anaerobic locality in the chloroplast preserves [FeFe]-hydrogenase activity and supports continuous hydrogen production in air-grown microalgal cells.
Photosynthetic hydrogen production is limited to microbial photosynthetic organisms, such as Chlamydomonas reinhardtii, and is absent from terrestrial plants (Prince and Kheshgi, 2005). The reasons for this absence are vague, but they could be related to differences in the growth environments. Microalgae naturally reside in ponds, wet soils, and lakes (Clowez et al., 2015), and have a tendency to swim into deeper water layers as a defense mechanism against intense light. Hence, they occasionally face fluctuating levels of oxygen in their proximate liquid or soil surroundings. In contrast, terrestrial plants have a constant atmospheric oxygen concentration in their surroundings. Thus, the difference in oxygen concentration might be the selective pressure for preservation or loss of the hydrogen-producing enzyme [FeFe]-hydrogenase in microalgae and terrestrial plants, respectively.
Microalgal hydrogen production is catalyzed anaerobically by the oxygen-sensitive [FeFe]-hydrogenase (Ghirardi et al., 1997; Stiebritz and Reiher, 2012), which is reduced by ferredoxin, a multipotent electron carrier, and functions in three pathways. The first takes place in the dark, via a fermentative route, and involves pyruvate ferredoxin reductase (Dubini et al., 2009). The second is the photosynthetic route, which takes place in light at the stromal side of the photosystem I complex (PSI; Winkler et al., 2010). PSI is reduced by linear electron flow (LEF) that originates at the photosystem II complex (PSII). PSII absorbs and uses light energy to split water molecules into electrons, protons, and oxygen. Electron transport through the cytochrome b6f complex (cytb6f) facilitates proton pumping from the stroma into the lumen, thus maintaining a proton gradient necessary for ATP production through ATPsyntase. In a third pathway, LEF originating at NDAPH dehydrogenase complex type II (NDA2) receives electrons from starch breakdown and reduces the plastoquinone pool bypassing water splitting at PSII (Jans et al., 2008). Here, as in the formers, ferredoxin mediates electron flow to [FeFe]-hydrogenase.
Chloroplast oxygen uptake through chlororespiration, illustrated in Figure 1, takes place upstream to PSI and involves plastid terminal oxidases (PTOX1 and PTOX2; Houille-Vernes et al., 2011). In addition, chloroplast oxygen uptake through the Mehler reaction takes place directly at PSI (Mehler, 1951a; Asada, 2000) or via ferredoxin resulting in water release. Recently, an additional Mehler-like reaction was found. This reaction is carried out by FLVA and FLVB, two newly isolated diiron flavoproteins, which are reduced by NADPH and convert oxygen directly into water (not shown in the scheme; Jokel et al., 2015).
Figure 1.
Schematic illustration of the oxygen uptake mechanism in the chloroplast. Water splitting at PSII generates molecular oxygen, protons, and LEF. Electrons are transferred from PSII to the plastoquinone pool (QA; QB) from which the electron flow is transferred either to oxygen uptake in PTOX or to cytb6f. From cytb6f, electrons are transferred to plastocyanin (PC), which in turn reduces PSI that consumes oxygen directly via the Mehler reaction. The inhibitor DCMU specifically blocks electron output from PSII to the plastoquinone pool. Hence, upon addition of DCMU, the chloroplast oxygen uptake is omitted, without affecting mitochondrial respiration (not shown). The inhibitor DBMIB specifically blocks electron transfer output from cytb6f, inhibiting oxygen consumption by the Mehler reaction, catalyzed by PSI, without affecting PTOX chlororespiration. n-PG is a specific inhibitor of PTOX.
Until recently it was accepted that active [FeFe]-hydrogenase in C. reinhardtii is absent in air-grown cultures, and its accumulation is induced only upon anoxia, with a maximal accumulation observed after 3 h of nitrogen sparging (Happe et al., 1994) or 72 h of sulfur deprivation (Winkler et al., 2002). Accordingly, atmospheric levels of oxygen completely inactivate the catalytic site of [FeFe]-hydrogenase within a few seconds (Ghirardi et al., 2007). In contrast, Kosourov and Seibert (Kosourov and Seibert, 2009) observed continuous hydrogen production from C. reinhardtii cultures embedded in films under ambient atmosphere, although the oxygen levels within the films were not determined. In further support of this, recent reports have suggested that [FeFe]-hydrogenase is capable of aerobic activity in strains of Chlorella. It was shown that Chlorella vulgaris could maintain hydrogen production under atmospheres of 21% (Hwang et al., 2014) or 15% (Chader et al., 2009) oxygen. The active pool of hydrogenase was estimated at ∼30 units per mg of dry weight (Hwang et al., 2014). Last, in a recent paper by Godaux et al. (Godaux et al., 2015), the authors claim that in a transition from dark anoxia to light, high rate of hydrogen production decreased to lower rate, before the onset of oxygen accumulation. Hence, they conclude that a competition for electrons with downstream processes such as FNR and CEF govern the hydrogen production rate rather than oxygen concentration. All of these findings contradict the common view since the oxygen sensitivity of [FeFe]-hydrogenase is well documented (Ghirardi et al., 1997). Furthermore, it is unclear why the transcripts of C. reinhardtii hydrogenase and its maturases, Hyd EF and G, are present under aerobiosis and increase under anaerobiosis induced by sulfur deprivation by roughly 20%, as we analyzed from published RNAseq data (González-Ballester et al., 2010).
The mechanism underlying [FeFe]-hydrogenase activity in air-grown cultures is yet to be resolved. We have therefore used the model organism C. reinhardtii, cultivated in air, to examine whether an active pool of [FeFe]-hydrogenase indeed exists, as well as to explore the mechanism(s) protecting [FeFe]-hydrogenase under aerobic conditions. We present evidence showing hydrogen production for durations longer than expected in strictly aerobic cultures of C. reinhardtii. We further demonstrate the coupling of aerobic hydrogen production with the photosynthetic electron transport chain and dissect the mechanisms protecting [FeFe]-hydrogenase activity under aerobic conditions.
RESULTS
Aerobic Hydrogen Production
To study steady-state hydrogen production rate, 50-mL suspensions of wild-type C. reinhardtii (strain CC-124) at 2.5 µg (chl)/mL were cultivated in aerated 100-mL flasks (Fig. 2A, inset) with constant stirring and under three light intensities (77, 155, and 600 µE m−2 s−1; hereafter µE). To ensure full aerobiosis in these conditions, we quantified the concentration of dissolved oxygen using oxygen electrode and Winkler reaction (Winkler, 1888). Furthermore, we analyzed the headspace gas using a gas chromatograph. Growth at all irradiances showed constant atmospheric levels of oxygen in the headspace of the growth vessels as well as ∼250 µm dissolved oxygen in the growth media (Supplemental Fig. S1); the same oxygen concentration was measured for the positive control—a cell-free aerobic growth media.
Figure 2.
Photosynthetic activity and quantification of [FeFe]-hydrogenase in air-cultivated C. reinhardtii cells. A, Steady-state hydrogen production rates measured by MIMS in wild-type cultures cultivated under three light intensities: 77, 155, and 600 µE. Inset, Cells were grown in flasks stoppered with a sponge completely permeable to air, with constant light and stirring. B, LEF from PSII to hydrogen recorded upon transition from 77 µE to 1200 µE of either wild-type cells in the presence (broken green line) or absence (solid green line) of the PSII donor side inhibitor DCMU. The mutant hydEF-1 was used as negative control (black). C, Inhibition of hydrogenase by the inhibitor CO shuts down hydrogen production (green bar) in air-grown cells upon transition from 77 µE to 1200 µE at the onset of the recording. Photosynthetic oxygen production was not affected by the addition of CO (blue bars). D, Quantification of [FeFe]-hydrogenase in cells cultivated aerobically under 77, 155, and 600 µE. Top, An immunoblot performed using anti-HydA antibody. Purified [FeFe]-hydrogenase (5 ng of HydA) was used as marker and reference for band intensity quantification. Equal amounts of total protein were loaded in each lane. [FeFe]-hydrogenase quantities were normalized either to ng HydA per µg total protein (brown), shown in the left y axis, or to ng HydA per 1 million cells (orange), shown in the right y axis. The cultivation light intensities are shown at the bottom of each lane. All experiments were carried out in triplicates.
Hydrogen production and oxygen production/uptake rates were investigated using a membrane inlet mass spectrometer, which monitors multiple gas traces in real time and thus can discriminate between isotopes of the same gas. In this study we simultaneously analyzed the traces of H2 (Mus et al., 2005), stable oxygen isotopes 16O2 and 18O2, and Ar (Radmer and Kok, 1976; Kana et al., 1994; Tchernov et al., 1997). We found that the wild-type strain (Fig. 2, A and B), but not the negative control, a hydEF-1 mutant lacking [FeFe]-hydrogenase activity (Fig. 2B), exhibited hydrogen production rates that were inversely dependent on light intensity (Fig. 2A).
To ascertain the source of electrons responsible for hydrogen production, we inhibited the LEF from water, the electron donor of PSII, to [FeFe]-hydrogenase, using dichlorophenyl dimethyl urea (DCMU), which blocks electron output from PSII (schematically illustrated in Fig. 1). We observed no hydrogen production upon addition of DCMU at growth light intensity (77 µE) that was increased to 1200 µE at the onset of measurement, indicating that LEF was the principal electron source for hydrogen production (Fig. 2B).
To validate that the observed hydrogen production is indeed catalyzed by the [FeFe]-hydrogenase enzyme and that the enzyme pool is at the active, oxygen-sensitive “ox state” (Adamska-Venkatesh et al., 2014), we added the [FeFe]-hydrogenase inhibitor CO at growth light intensity (77 µE) that was increased to 1200 µE at the onset of measurement. CO blocks the active site of the hydrogenase, forming an Hox-CO state (Lubitz et al., 2014). A complete inhibition of hydrogen production was observed, while photosynthetic oxygen evolution was uninterrupted (Fig. 2C).
Cellular Content of Hydrogenase
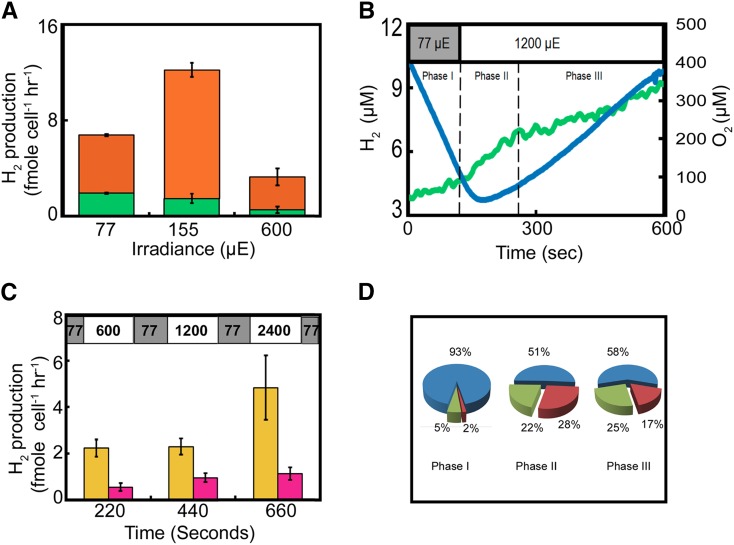
Previous studies using Chlamydomonas detected both [FeFe]-hydrogenase transcripts (Forestier et al., 2003; González-Ballester et al., 2010) and protein (Forestier et al., 2003; Hemschemeier et al., 2008) in air-grown microalgal cells, but did not detect [FeFe]-hydrogenase activity under these conditions. As shown above, our measurements revealed a steady rate of hydrogen production in air-grown Chlamydomonas cells. To further support this observation, we determined the cellular content and activity of [FeFe]-hydrogenase pools in microalgal samples directly withdrawn from the aerated cultivation flasks. [FeFe]-hydrogenase contents were analyzed by immunoblot using a commercial antibody against HydA1 and revealed ∼6 fmol of enzyme per 1 × 106 cells in the irradiance range tested (Fig. 2D; Supplemental Fig. S2). Since the content of chlorophyll and protein per cell is modulated under different irradiance, [FeFe]-hydrogenase content was normalized per cell. The [FeFe]-hydrogenase pool per cell slightly decreased above irradiance of 77 µE. To further study the observed [FeFe]-hydrogenase activity independently of photosynthetic electron transfer, which could be limiting, we lysed the cells and used a chemical electron donor. Cell extracts were exposed to an excess of reduced methyl viologen (MV), which serves as an artificial electron donor for [FeFe]-hydrogenase (Meuser et al., 2012). [FeFe]-hydrogenase activity was observed in all cultures within the irradiance range tested (Fig. 3A, orange bars). Interestingly, net oxygen production under steady growth light intensity was observed only when light irradiance reached 600 µE (Supplemental Fig. S3). This increase had a direct impact on [FeFe]-hydrogenase, as indicated by the drastic decrease in its activity (Fig. 3A, orange bars). Still, [FeFe]-hydrogenase activity was not totally eliminated under 600 µE, suggesting that mitochondrial respiration and chloroplast O2 uptake can effectively form a microoxic environment at the stromal side of the thylakoid membrane, even under continuous growth under high light.
Figure 3.
[FeFe]-hydrogenase activity under fluctuating light in air-cultivated C. reinhardtii cells. A, The measured photosynthetic (green) versus chemical (orange) hydrogen production rates in cells cultivated aerobically (as shown in the inset of Fig. 2A) under varying irradiance. B, Recorded traces of net hydrogen (green) and oxygen (blue) kinetics as a function of irradiance. The top x axis shows the light intensity at each time point. Dissolved gases in the samples were measured simultaneously to illumination for 100 s under 77 µE (phase I), followed by a continuous illumination under 1200 µE. The onset of the transition to 1200 µE, lasting ∼100 S, where hydrogen production is maximal, is defined as phase II. The remaining period at 1200 µE is defined as phase III. C, Hydrogen production under fluctuating light. Photosynthetic hydrogen production rates during light irradiance of 77 µE punctuated each 2 min with 3 min of high irradiance (600, 1200, or 2400 µE). Phase II (yellow) and phase III (pink) are shown for each high light intensity. All experiments were carried out in triplicates. D, Pie graphs showing the divergence of oxygen consumption between the various mechanisms under the three phases shown in B. The percentages were calculated using the rates shown in Table I. Hence, while mitochondrial respiration (blue) was constant, 160 [µmol (O2) mg−1 h−1], PTOX (green) and Mehler reactions (red) were significantly increased in a transition to high light.
To estimate the fraction of [FeFe]-hydrogenase driven by photosynthetic electron transfer from the total active fraction, chemically measured using cell extract and the artificial electron donor MV, we plotted the in vivo photosynthetic hydrogen production rate in the light (Fig. 3A, green bars) versus its chemically measured rate (Fig. 3A, orange bars; Meuser et al., 2012). Notably, a large fraction of the active [FeFe]-hydrogenase pool did not function as an electron sink in air-cultivated cells (Fig. 3A, green bars versus orange bars), raising the question as to its function.
Hydrogenase Functions as an Electron Sink in a Transition to High Light
A sudden increase in irradiance may create an increased electron pressure on the electron transfer chain, especially when light-dependent oxygen consumption does not function properly, e.g. under low oxygen. In such cases, active [FeFe]-hydrogenase might take part in a first-line defense to immediately dissipate excess electrons (White and Melis, 2006; Hemschemeier et al., 2008). We challenged this assumption aerobically by exposing cells grown under 77 µE (“phase I” in Fig. 3B) to a continuous intense light irradiance of 1200 µE while simultaneously determining the hydrogen and oxygen kinetics. A short-lived burst of hydrogen production (∼100 s; “phase II” in Fig. 3B) preceded the onset of oxygen evolution. Interestingly, following the 100-s burst, the basal hydrogen production rate was maintained throughout the exposure to high light irradiance (“phase III” in Fig. 3B). To study whether this phenomenon can continue for a longer extant, we recorded the kinetics for additional 30 min. We observed that both hydrogen and oxygen were accumulating simultaneously even when oxygen concentration was as high as 350 µm (Supplemental Fig. S4).
To further investigate the role of [FeFe]-hydrogenase under fluctuating light intensities, we measured the hydrogen production rates in cells cultivated under 77 µE and then exposed to fluctuating light, consisting of 3 min at high irradiance of 600, 1200, and 2400 µE, punctured with relaxation periods of 2 min at 77 µE. As shown in Figure 3C, the hydrogen production rate at phase II (yellow bars) upon each exposure to high irradiance reached up to 6 fmol H2 cell−1 h−1, which clearly indicates that the [FeFe]-hydrogenase pool in microalgae is in a “standby” state, ready to dissipate excess electrons upon sudden exposure to high light. Thus, it seems that [FeFe]-hydrogenase provides an immediate electron sink that chronologically precedes oxygen accumulation and gains maximal thrust only at the onset of transition into high light. In addition, net oxygen production that was simultaneously measured showed that PSII was active at all light intensities (Supplemental Table S1). Hence, the increased net oxygen production rates alongside higher irradiance imply that a mechanism involving strong photoinhibition is unlikely.
Determination of Oxygen Consumption Pathways
The unanticipated hydrogen production in air-grown microalgae observed under both stable and fluctuating light intensities suggests efficient oxygen consumption at the stromal side of PSI, where [FeFe]-hydrogenase naturally resides. Oxygen produced upon water splitting at PSII may be consumed by mitochondrial respiration and by chloroplast oxygen uptake (illustrated in Fig. 1). Chloroplast oxygen uptake takes place before PSI by chlororespiration driven by PTOX, and at PSI by Mehler and Mehler-like reactions (Mehler, 1951a, 1951b; Badger et al., 1980; Houille-Vernes et al., 2011; Jokel et al., 2015). To differentiate between these possibilities, we analyzed the oxygen kinetics during the three phases of hydrogen production shown in Figure 3B. Since 16O2 is continuously produced under light by the water-splitting activity of PSII, accurate oxygen consumption was measured using injections of known amounts of an 18O2 isotope that is not produced in vivo (Radmer et al., 1978). To calculate the magnitude of oxygen uptake by chlororespiration, we subtracted the 18O2 consumption rate measured in the presence of 2,5-dibromo-6-isopropyl-3-methyl-1,4-benzoquinone (DBMIB) + DCMU, when only respiration is active, from the 18O2 uptake rate measured in the presence of DBMIB alone, when both respiration and chlororespiration are active (dark controls are shown in Supplemental Fig. S5). To further validate if the chlororespiration can be attributed to PTOX activity, DCMU was replaced with n-propyl gallate (n-PG). Hence, we subtracted the 18O2 uptake rate measured in the presence of DBMIB + n-PG from the rate measured in the presence of DBMIB. As shown in Table I, chlororespiration and 18O2 uptake rate by PTOX are highly similar, supporting the notion that PTOX is the principle oxygen consumer upstream to PSI, notably, the measured PTOX rates under steady state are in agreement with Cournac et al. (2002). However, the rate of chlororespiration significantly increased upon transition from medium (77 µE) to high light (1200µE; Table I), compensating for the increased oxygen production by PSII. Finally, we analyzed the oxygen uptake by Mehler and Mehler-like reactions. The Mehler contributions were calculated by subtracting the chlororespiration rate from the total chloroplast 18O2 uptake rate that was determined by subtracting mitochondrial respiration in light (18O2 uptake in the presence of DCMU) from the gross oxygen consumption in light. Here, as for PTOX, a significant enhancement in the Mehler reaction rate was observed upon transition from medium (77 µE) (Table I, phase I) to high light (1200 µE; Table I, phase II), compensating for the increased oxygen production by PSII. At later time points (phase III), the Mehler rate was decreased, while oxygen consumption by PTOX remained constant (Table I, phase III), resulting in a net oxygen release (Table I, phase III). It is notable that in transition from medium (77 µE) to high light (1200 µE), as much as 50% of the oxygen produced by PSII was consumed by the chloroplast oxygen uptake pathways (Fig. 3D).
Table I. Oxygen kinetics during the three phases observed following a sharp transition from medium to high light.
Dissection of the independent elements of oxygen kinetics in a transition from irradiance of 77 µE to 1200 µE was conducted in three phases using 18O2 isotope in the presence or absence of the inhibitors DCMU, DBMIB, and n-PG.
| Phase | I | II | III |
|---|---|---|---|
| Rates [µmol (O2) mg−1 h−1] ± se | |||
| Calculated gross | 93 | 302 | 285 |
| Net O2 release | −72.64 ± 15.91 | 0.42 ± 2 | 20.85 ± 5.5 |
| Thylakoid total | −11.35 ± 9.84 | −148.43 ± 33.2 | −110.62 ± 42.19 |
| Chlororespiration (DBMIB + DCMU) | −8.7 | −64.73 ± 12.58 | |
| PTOX (DBMIB + n-PG) | −8.8 ± 3.4 | −72.83 ± 16.84 | |
| Calculated Mehler reactions | −2.55 | −84 | −46 |
| Mitochondria dark | −162 ± 10 | ||
| Mitochondria light (+DCMU) | −154 ± 34 | ||
DISCUSSION
In this work we show that [FeFe]-hydrogenase activity in air-grown Chlamydomonas cultures is evident due to anaerobic niches within the thylakoid membranes. Still, [FeFe]-hydrogenase could be aerobically active due to (1) intrinsic tolerance or (2) fast [FeFe]-hydrogenase protein synthesis that could not take place under strictly aerobic conditions (Happe et al., 1994; Winkler et al., 2002).
Concerning intrinsic tolerance, the known mechanisms for partial “oxygen-tolerance” of [FeFe]-hydrogenase were shown only in vitro and involve reversible inactivation (Vincent et al., 2005; Stripp et al., 2009; Stiebritz and Reiher, 2012). Yet, our data clearly show that the cellular [FeFe]-hydrogenase pool is active, as evident from the increased hydrogen evolution rates during the transition from low to high light. Furthermore, we show that the entire [FeFe]-hydrogenase pool is at the CO/O2-sensitive ox state since the [FeFe]-hydrogenase activity was completely blocked by the addition of external CO, ceasing all hydrogen production at the transition from 77 µE to 1200 µE (Fig. 2C). Thus, we did not observe an inactive population of [FeFe]-hydrogenase, rendering the possibility of intrinsic oxygen tolerance unlikely. To understand how the activity of such an oxygen-sensitive enzyme is possible in air-grown microalgae, we dissected the oxygen uptake mechanisms, namely, mitochondrial respiration and gross chloroplast oxygen uptake, which consists of chlororespiration catalyzed by PTOX, and Mehler reactions. We found that the combined oxygen uptake activity of these pathways keeps the oxygen level within the stromal face of PSI to a minimum, which enables [FeFe]-hydrogenase activity resulting in hydrogen production in air-grown microalgae. In support of our findings of microoxic niches in the chloroplast, one can observe some similarity to other photosynthetic organisms; for example, the protection of another strictly anaerobic enzyme, nitrogenase, in the cyanobacteria Anabaena is carried out by an efficient removal of oxygen by the Mehler-like reaction. Recently, Ermakova et al. (2014) showed that Flv3B protects nitrogenase by performing light-induced O2 uptake, which maintains microoxic conditions inside of the heterocysts.
Interestingly, we found that a major fraction of the [FeFe]-hydrogenase pool is idle but active. Hence, its full capacity is revealed only upon transition from low to high light. The period of full [FeFe]-hydrogenase activity is short, around 100 s, suggesting that [FeFe]-hydrogenase participates in a first line of defense for the immediate dissipation of the increased electron flux in fluctuating light environments. Still, hydrogen production accounted for less than 1% of this initial electron flux. Thus, it appears that under aerobic conditions, the conversion of oxygen into water, rather than hydrogen production, is the prime sink for excess electrons generated under sharp repetitive transitions into high light. Taken together, these findings suggest that higher plants have probably not preserved this enzyme due to a constant atmospheric oxygen level in their surroundings, which enables, under high irradiance, efficient dissipation of excess electrons via PTOX and Mehler reactions. Thus, the fluctuating oxygen concentrations in the proximity of microalgae could have formed an evolutionary pressure to preserve an additional mechanism, namely, [FeFe]-hydrogenase, to confront instances such as under low oxygen, where the chlororespiration catalyzed by PTOX and Mehler reactions cannot function properly.
CONCLUSION
Due to [FeFe]-hydrogenase oxygen sensitivity, we exploited [FeFe]-hydrogenase as an oxygen reporter. The presence of [FeFe]-hydrogenase activity in air-grown microalgae indicates a microoxic locality in the thylakoid stroma, where the enzyme resides.
We show that in air-cultivated Chlamydomonas cells, [FeFe]-hydrogenase (1) is active due to efficient oxygen consumption fed by LEF at the stromal side of PSI, and (2) functions as a minor alternative sink for excess electrons, especially upon transition to high light.
In relation to futuristic algal hydrogen production, it is widely accepted that the main barrier of the engineering efforts toward photosynthetic hydrogen production is thought to be the oxygen sensitivity of [FeFe]-hydrogenase. Our findings, of the microoxic locality at the thylakoid stroma, might be used as a platform for reengineering of the natural oxygen-scavenging pathways. Such modifications, if successful, could facilitate a continuous production of hydrogen in air-grown microalgae.
MATERIALS AND METHODS
Organisms and Growth Conditions
Chlamydomonas reinhardtii wild-type strain CC-124 (mt- [137C]) and the hydEF-1 mutant or the hydA1-hydA2 double mutant were cultivated on 1.5% (w/v) agar plates with Tris-Acetate-Phosphate (TAP) medium. The cultures were maintained at 24.5°C under 77 µE cool-white light. For all experiments, 50 mL of microalgal suspension were cultivated aerobically in aerated 100-mL flasks under constant stirring using a magnetic bar. Cell density was kept below 2.5 µg (chl)/mL.
Determination of Cell Density and Chlorophyll Concentration
Cell density was determined using a Neubauer hemocytometer. Chlorophyll was extracted and measured as previously described (Jeffrey and Humphrey, 1975).
Determination of [FeFe]-Hydrogenase Content by Western Blot
Soluble proteins were isolated from air-grown, midlog-phase C. reinhardtii (total of ∼3 mg chlorophyll). Samples were withdrawn directly from the cultivation flasks and kept at 4°C to prevent further protein synthesis. Cells at 2.5 µg (chl)/mL were precipitated (3,200g, 5 min, 4°C) and resuspended in 600 µL of buffer A (50 mm Tris-HCl, pH 8.5, 20 mm sodium dithionite, 60 mm NaCl, and 1 mm protease inhibitor cocktail [Sigma]). The cell suspension was lysed using a Minilys tissue lyser (Bertin Technologies) at two 5000 rpm cycles of 45 s each in the presence of glass beads (Sigma). The soluble proteins were separated by a 10-min (14,000g, 4°C) centrifugation and further concentrated using Vivaspin 500 (Sartorius) to yield 180 µL at protein concentration of ∼1µg/µL. Increasing amounts (3–10 µL corresponding to 30–100 µg chl) of soluble proteins were loaded onto 4-12% Bis-Tris Plus PAGE gels (Novex by Life Technologies) and analyzed by immunoblotting using rabbit polyclonal HydA1/HydA2 antibodies (Agrisera). A known standard of purified HydA1 (5 ng) was mixed with a lysate of the hydA1-hydA2 double mutant, to mimic protein interference. The HydA1/2 bands were analyzed by DNR imager and quantified using GelQuant software. The minimal sensitivity was 1 ng of HydA1/2 per lane.
Chemical Determination of [FeFe]-Hydrogenase Hydrogen Production Rate
Samples were withdrawn directly from the cultivation flasks and pelleted by centrifugation for 2 min at 4000 rpm. Then they were perforated by Triton/MV mix as described previously (Meuser et al., 2012). Dissolved hydrogen production was analyzed using membrane inlet mass spectrometry (MIMS).
Gas-Exchange Profiles of H2 and O2
Samples of cell suspensions at 2.5 µg (chl)/mL were centrifuged for 2 min at 4000 rpm at room temperature, then resuspended in fresh TAP + 50 mm HEPES pH 7.8 (NaOH) to yield a final concentration of 40 µg chl mL−1. For analyses, 5 mL of a concentrated sample was introduced to a sealed quartz cuvette (Starna Cells). A quadrupole mass spectrometer (QMS 200 M1; Pfeiffer Vacuum) was connected to the inlet probe by a vacuum line. The cuvette construct was then fitted into a metabolic chamber (optical unit ED-101US/MD; Walz), which kept the sample thermostated at 24.5°C during the experiment. Light was guided through the light ports of the chamber with a Schott light (KL1500-T) covered with light-reducing filters to attain final light intensities of 77 to 2000 µE. Masses of H2, N, 16O2, 18O2, and Ar were repeatedly measured using a 0.5-s dwelling time per mass. The measured traces of oxygen were corrected for the continuous removal of the measured gas by the vacuum line as described by Luz and Barkan (2005). H2 trace was analyzed as described by Mus et al. (2005).
Stable Isotope Analysis
Oxygen unidirectional flux rates were determined as described previously (Radmer et al., 1978), with a slight modification. A small volume of 99% 18O2 (Sigma-Aldrich) saturated TAP solution was injected at time points of interest. The trace of the isotope was then corrected for the constant MIMS consumption. Absolute rates were calculated as follows:
 |
 |
 |
 |
 |
R is the linear regression of mass 32 or 36 in light or dark (L or D, respectively); F is the enrichment factor taken as the ratio between masses 32 and 36 at the maximum peak obtained after injection; and K is an additional correction to the assumption that respiration in the dark should be the same for both masses (Radmer et al., 1978; Kana, 1990).
Dissection of Chloroplast Oxygen Uptake
Cells at a concentration of 40 µg chl mL−1 were kept in a thermostated (24.5°C) MIMS cuvette under irradiance of 77 µE with constant stirring while the cuvette was open to air. DBMIB was added to yield a final concentration of 20 µm. The cells were incubated for 2 min and then the cuvette was sealed for the MIMS measurement. A small volume of 99% 18O2 (Sigma-Aldrich) solution was injected, and after reaching a recorded mass spectrometer peak of 18O2, additional injections of DCMU (final concentration 10 µm) or n-PG (final concentration 400 µm) were followed. Then, the sample was incubated for an additional 3 min in light, after which irradiance was increased to 1200 µE for an additional 3 min.
CO Inhibition
N2 or CO saturated solution (200 µL) of TAP + 50 mm HEPES pH 7.8 (NaOH) was injected into the MIMS cuvette, which contained cells at a concentration of 40 µg chl mL−1 under irradiance of 77 µE, 24.5°C with constant stirring. To monitor the injection of CO, mass 12 (corresponding to carbon) was measured. After 8 min of incubation, the light intensity was increased to 1200 µE, and H2 and 16O2 concentrations were measured simultaneously as described above.
Statistics
Each group within the charts is represented as value ± se. Comparison between rates was examined by Kruscal-Wallis a-parametric ANOVA. Repeated-measurement ANOVA was used when the dependent variable was measured three times on the same sample and the same experiment (Fig. 2D). Significance was set at P < 0.05.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Quantification of oxygen in cell cultures cultivated aerobically under 77, 155, and 600 µE.
Supplemental Figure S2. Quantification of [FeFe]-hydrogenase in cells cultivated aerobically under 77, 155, and 600 µE.
Supplemental Figure S3. Net measured oxygen kinetics.
Supplemental Figure S4. Prolonged H2 production under 1200 µE.
Supplemental Figure S5. Modulation of dark respiration by chloroplast inhibitors.
Supplemental Table S1. Net oxygen production in phase III under intermittent light.
Supplementary Material
Acknowledgments
We thank Prof. Michael Gurevitch (Tel Aviv University), Alexandra Dubini (National Renewable Energy Laboratory), and Dr. Yuval Mazor (Tel Aviv University) for helpful discussion and critical reading of the manuscript. The MIMS equipment was bought using the generous donation of the Australian Friends of Tel Aviv University.
Glossary
- MIMS
membrane inlet mass spectrometry
- LEF
linear electron flow
- DCMU
dichlorophenyl dimethyl urea
- MV
methyl viologen
- DBMIB
2,5-dibromo-6-isopropyl-3-methyl-1,4-benzoquinone
- n-PG
n-propyl gallate
- TAP
Tris-Acetate-Phosphate
Footnotes
This work was supported by Israel Science Foundation-iCORE (757/12, “Comprehensive understanding and modeling of plant responses to multiple abrupt abiotic stresses and to prolonged climatic changes”).
Articles can be viewed without a subscription.
References
- Adamska-Venkatesh A, Krawietz D, Siebel J, Weber K, Happe T, Reijerse E, Lubitz W (2014) New redox states observed in [FeFe] hydrogenases reveal redox coupling within the H-cluster. J Am Chem Soc 136: 11339–11346 [DOI] [PubMed] [Google Scholar]
- Asada K. (2000) The water-water cycle as alternative photon and electron sinks. Philos Trans R Soc Lond B Biol Sci 355: 1419–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, Kaplan A, Berry JA (1980) Internal inorganic carbon pool of Chlamydomonas reinhardtii: evidence for a carbon dioxide-concentrating mechanism. Plant Physiol 66: 407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chader S, Hacene H, Agathos SN (2009) Study of hydrogen production by three strains of Chlorella isolated from the soil in the Algerian Sahara. Int J Hydrogen Energy 34: 4941–4946 [Google Scholar]
- Clowez S, Godaux D, Cardol P, Wollman FA, Rappaport F (2015) The involvement of hydrogen-producing and ATP-dependent NADPH-consuming pathways in setting the redox poise in the chloroplast of Chlamydomonas reinhardtii in anoxia. J Biol Chem 290: 8666–8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac L, Latouche G, Cerovic Z, Redding K, Ravenel J, Peltier G (2002) In vivo interactions between photosynthesis, mitorespiration, and chlororespiration in Chlamydomonas reinhardtii. Plant Physiol 129: 1921–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubini A, Mus F, Seibert M, Grossman AR, Posewitz MC (2009) Flexibility in anaerobic metabolism as revealed in a mutant of Chlamydomonas reinhardtii lacking hydrogenase activity. J Biol Chem 284: 7201–7213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermakova M, Battchikova N, Richaud P, Leino H, Kosourov S, Isojärvi J, Peltier G, Flores E, Cournac L, Allahverdiyeva Y, et al. (2014) Heterocyst-specific flavodiiron protein Flv3B enables oxic diazotrophic growth of the filamentous cyanobacterium Anabaena sp. PCC 7120. Proc Natl Acad Sci USA 111: 11205–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier M, King P, Zhang L, Posewitz M, Schwarzer S, Happe T, Ghirardi ML, Seibert M (2003) Expression of two [Fe]-hydrogenases in Chlamydomonas reinhardtii under anaerobic conditions. Eur J Biochem 270: 2750–2758 [DOI] [PubMed] [Google Scholar]
- Ghirardi ML, Posewitz MC, Maness PC, Dubini A, Yu J, Seibert M (2007) Hydrogenases and hydrogen photoproduction in oxygenic photosynthetic organisms. Annu Rev Plant Biol 58: 71–91 [DOI] [PubMed] [Google Scholar]
- Ghirardi ML, Togasaki RK, Seibert M (1997) Oxygen sensitivity of algal H2-production. Appl Biochem Biotechnol 63-65: 141–151 [DOI] [PubMed] [Google Scholar]
- Godaux D, Bailleul B, Berne N, Cardol P (2015) Induction of photosynthetic carbon fixation in anoxia relies on hydrogenase activity and Proton-Gradient Regulation-Like1-mediated cyclic electron flow in Chlamydomonas reinhardtii. Plant Physiol 168: 648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ballester D, Casero D, Cokus S, Pellegrini M, Merchant SS, Grossman AR (2010) RNA-seq analysis of sulfur-deprived Chlamydomonas cells reveals aspects of acclimation critical for cell survival. Plant Cell 22: 2058–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe T, Mosler B, Naber JD (1994) Induction, localization and metal content of hydrogenase in the green alga Chlamydomonas reinhardtii. Eur J Biochem 222: 769–774 [DOI] [PubMed] [Google Scholar]
- Hemschemeier A, Fouchard S, Cournac L, Peltier G, Happe T (2008) Hydrogen production by Chlamydomonas reinhardtii: an elaborate interplay of electron sources and sinks. Planta 227: 397–407 [DOI] [PubMed] [Google Scholar]
- Houille-Vernes L, Rappaport F, Wollman FA, Alric J, Johnson X (2011) Plastid terminal oxidase 2 (PTOX2) is the major oxidase involved in chlororespiration in Chlamydomonas. Proc Natl Acad Sci USA 108: 20820–20825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Kim HC, Choi JA, Abou-Shanab RA, Dempsey BA, Regan JM, Kim JR, Song H, Nam IH, Kim SN, et al. (2014) Photoautotrophic hydrogen production by eukaryotic microalgae under aerobic conditions. Nat Commun 5: 3234. [DOI] [PubMed] [Google Scholar]
- Jans F, Mignolet E, Houyoux PA, Cardol P, Ghysels B, Cuiné S, Cournac L, Peltier G, Remacle C, Franck F (2008) A type II NAD(P)H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc Natl Acad Sci USA 105: 20546–20551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey St, Humphrey G (1975) New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167: 191-194 [Google Scholar]
- Jokel M, Kosourov S, Battchikova N, Tsygankov AA, Aro EM, Allahverdiyeva Y (2015) Chlamydomonas flavodiiron proteins facilitate acclimation to anoxia during sulfur deprivation. Plant Cell Physiol 56: 1598–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana TM. (1990) Light-dependent oxygen cycling measured by an oxygen-18 isotope dilution technique. Mar Ecol Prog Ser 64: 293–300 [Google Scholar]
- Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC (1994) Membrane inlet mass-spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal Chem 66: 4166–4170 [Google Scholar]
- Kosourov SN, Seibert M (2009) Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol Bioeng 102: 50–58 [DOI] [PubMed] [Google Scholar]
- Lubitz W, Ogata H, Rüdiger O, Reijerse E (2014) Hydrogenases. Chem Rev 114: 4081–4148 [DOI] [PubMed] [Google Scholar]
- Luz B, Barkan E (2005) The isotopic ratios 17 O/16 O and 18 O/16 O in molecular oxygen and their significance in biogeochemistry. Geochim Cosmochim Acta 69: 1099–1110 [Google Scholar]
- Mehler AH. (1951a) Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys 33: 65–77 [DOI] [PubMed] [Google Scholar]
- Mehler AH. (1951b) Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch Biochem Biophys 34: 339–351 [DOI] [PubMed] [Google Scholar]
- Meuser JE, D’Adamo S, Jinkerson RE, Mus F, Yang W, Ghirardi ML, Seibert M, Grossman AR, Posewitz MC (2012) Genetic disruption of both Chlamydomonas reinhardtii [FeFe]-hydrogenases: insight into the role of HYDA2 in H2 production. Biochem Biophys Res Commun 417: 704–709 [DOI] [PubMed] [Google Scholar]
- Mus F, Cournac L, Cardettini V, Caruana A, Peltier G (2005) Inhibitor studies on non-photochemical plastoquinone reduction and H(2) photoproduction in Chlamydomonas reinhardtii. Biochim Biophys Acta 1708: 322–332 [DOI] [PubMed] [Google Scholar]
- Prince RC, Kheshgi HS (2005) The photobiological production of hydrogen: potential efficiency and effectiveness as a renewable fuel. Crit Rev Microbiol 31: 19–31 [DOI] [PubMed] [Google Scholar]
- Radmer RJ, Kok B (1976) Photoreduction of O(2) primes and replaces CO(2) assimilation. Plant Physiol 58: 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R, Kok B, Ollinger O (1978) Kinetics and apparent K(m) of oxygen cycle under conditions of limiting carbon dioxide fixation. Plant Physiol 61: 915–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiebritz MT, Reiher M (2012) Hydrogenases and oxygen. Chem Sci 3: 1739–1751 [Google Scholar]
- Stripp ST, Goldet G, Brandmayr C, Sanganas O, Vincent KA, Haumann M, Armstrong FA, Happe T (2009) How oxygen attacks [FeFe] hydrogenases from photosynthetic organisms. Proc Natl Acad Sci USA 106: 17331–17336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernov D, Hassidim M, Luz B, Sukenik A, Reinhold L, Kaplan A (1997) Sustained net CO2 evolution during photosynthesis by marine microorganisms. Curr Biol 7: 723–728 [DOI] [PubMed] [Google Scholar]
- Vincent KA, Parkin A, Lenz O, Albracht SPJ, Fontecilla-Camps JC, Cammack R, Friedrich B, Armstrong FA (2005) Electrochemical definitions of O2 sensitivity and oxidative inactivation in hydrogenases. J Am Chem Soc 127: 18179–18189 [DOI] [PubMed] [Google Scholar]
- White AL, Melis A (2006) Biochemistry of hydrogen metabolism in Chlamydomonas reinhardtii wild type and a Rubisco-less mutant. Int J Hydrogen Energy 31: 455–464 [Google Scholar]
- Winkler LW. (1888) Die bestimmung des im wasser gelösten sauerstoffes. Ber Dtsch Chem Ges 21: 2843–2854 [Google Scholar]
- Winkler M, Hemschemeier A, Gotor C, Melis A, Happe T (2002) [Fe]-hydrogenases in green algae: photo-fermentation and hydrogen evolution under sulfur deprivation. Int J Hydrogen Energy 27: 1431–1439 [Google Scholar]
- Winkler M, Hemschemeier A, Jacobs J, Stripp S, Happe T (2010) Multiple ferredoxin isoforms in Chlamydomonas reinhardtii - their role under stress conditions and biotechnological implications. Eur J Cell Biol 89: 998–1004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.