Cold-regulated VRN1 and VRN3 expression is consistent with an early origin of vernalization responsiveness in the temperate Pooideae grasses.
Abstract
The ability of plants to match their reproductive output with favorable environmental conditions has major consequences both for lifetime fitness and geographic patterns of diversity. In temperate ecosystems, some plant species have evolved the ability to use winter nonfreezing cold (vernalization) as a cue to ready them for spring flowering. However, it is unknown how important the evolution of vernalization responsiveness has been for the colonization and subsequent diversification of taxa within the northern and southern temperate zones. Grasses of subfamily Pooideae, including several important crops, such as wheat (Triticum aestivum), barley (Hordeum vulgare), and oats (Avena sativa), predominate in the northern temperate zone, and it is hypothesized that their radiation was facilitated by the early evolution of vernalization responsiveness. Predictions of this early origin hypothesis are that a response to vernalization is widespread within the subfamily and that the genetic basis of this trait is conserved. To test these predictions, we determined and reconstructed vernalization responsiveness across Pooideae and compared expression of wheat vernalization gene orthologs VERNALIZATION1 (VRN1) and VRN3 in phylogenetically representative taxa under cold and control conditions. Our results demonstrate that vernalization responsive Pooideae species are widespread, suggesting that this trait evolved early in the lineage and that at least part of the vernalization gene network is conserved throughout the subfamily. These results are consistent with the hypothesis that the evolution of vernalization responsiveness was important for the initial transition of Pooideae out of the tropics and into the temperate zone.
The ability of plants to match reproductive output with favorable environmental conditions is a key component of individual fitness and likely contributes to current distributional patterns of biodiversity (Rathcke and Lacey, 1985; Chuine and Beaubien, 2008; Matsuoka et al., 2008). A key aspect of reproductive output is flowering time, a major developmental transition that is controlled by exogenous and endogenous factors, including temperature seasonality in temperate plants (Bäurle and Dean, 2006; Dalchau et al., 2010; McClung and Davis, 2010; Tsuji et al., 2011). In “winter” annuals and perennials, the flowering transition is induced by an extended period of cold that results in a physiological switch allowing plants to flower when secondarily induced by specific day lengths and/or warm temperatures. Rapid flowering following exposure to cold temperatures is termed vernalization responsiveness (Chouard, 1960) and is postulated to be an evolutionary strategy to ready temperate plants for spring flowering (Wollenberg and Amasino, 2012; Preston and Sandve, 2013; Fjellheim et al., 2014). However, in regions experiencing little cold or temperature seasonality, a response to vernalization can substantially delay flowering, resulting in reduced fitness. The latter mismatch between seasonal cues and flowering has been postulated to be one of the negative impacts of global warming on plant fitness (Franks et al., 2007; Cook et al., 2012). Thus, understanding the relative lability and genetic architecture underlying gains/losses of vernalization responsiveness will facilitate our understanding of local adaptation and plant responses to climate change.
An exemplar lineage in which to study the evolution of vernalization responsiveness is the ecologically diverse grass family (Poaceae), which originated 51 to 82 million years ago, probably in tropical forests (Kellogg, 2001; Prasad et al., 2011; Christin and Osborne, 2013; Christin et al., 2014; Magallón et al., 2015). Despite its tropical origins, a few grass clades have successfully diversified in temperate environments (Edwards et al., 2010; Edwards and Smith, 2010; Humphreys and Linder, 2013). In particular, the diversification of Pooideae grasses in the northern hemisphere coincides with a period of major global cooling that peaked during the Eocene-Oligocene boundary 34 to 33.5 million years ago (Zachos et al., 2001; Mannion et al., 2014). During this time, it is hypothesized that Pooideae became more cold tolerant (Sandve et al., 2008, 2011; Sandve and Fjellheim, 2010; Preston and Sandve, 2013; Vigeland et al., 2013) and evolved a response to vernalization (Preston and Kellogg, 2008; Ream et al., 2012). Indeed, vernalization responsiveness has been identified in several so-called core group Pooideae (Davis and Soreng, 1993), such as wheat (Triticum aestivum), barley (Hordeum vulgare), oats (Avena sativa), and ryegrass (Lolium perenne), and a species sister to core Pooideae, Brachypodium distachyon (Brachypodieae; Takahashi and Yasuda, 1971; Heide, 1994; Trevaskis et al., 2003; Yan et al., 2003; Preston and Kellogg, 2008; Greenup et al., 2011; Ream et al., 2014). However, it is unknown whether vernalization responsiveness evolved at the base of the entire Pooideae, concomitant with the tropical to temperate zone transition.
The genetic basis of vernalization responsiveness has been extensively studied in a few economically important core Pooideae species (Ream et al., 2012), revealing two key epistatic vernalization genes, VERNALIZATION1 (VRN1; = FRUITFULL1 [FUL1]) and VRN2, and their downstream floral integrator VRN3 (= FLOWERING LOCUS T [FT]; Danyluk et al., 2003; Trevaskis et al., 2003; Yan et al., 2003; Amasino, 2004; Preston and Kellogg, 2006, 2008; Distelfeld et al., 2009; Greenup et al., 2009; Ream et al., 2012). In winter wheat and barley, cold nonfreezing temperatures of the fall/winter cause an increase in leaf and shoot apical meristem transcription of the flowering promoter VRN1, leading to repression of the floral repressor VRN2 and the consequent derepression of VRN3 (Yan et al., 2003; Yan et al., 2004a; Dubcovsky et al., 2006; Trevaskis et al., 2007; Preston and Kellogg, 2008; Distelfeld et al., 2009). The activation of VRN1 expression during cold occurs in response to histone modifications, resulting in the achievement of flowering competency, which can be visualized by the transition of the shoot apical meristem to the double-ridge stage (Preston and Kellogg, 2008; Oliver et al., 2009, 2013; Trevaskis, 2010). Once competent, vernalized plants are able to respond to inductive warm temperatures and long days of spring, resulting in the up-regulation of VRN3 in leaves, and VRN1 in shoot apices, followed by a rapid transition to flowering.
Allelic variation in VRN1, VRN2, and VRN3 has been shown to correlate with population differences in cold-mediated flowering in barley, wheat, ryegrass, and B. distachyon (Danyluk et al., 2003; Yan et al., 2003, 2004a, 2004b, 2006; Fu et al., 2005; von Zitzewitz et al., 2005; Szűcs et al., 2007; Kippes et al., 2015). For example, disruption of the regulatory site in the first intron or CArG-like motif of the wheat and barley VRN1 promoter results in constitutive expression of VRN1, leading to early flowering in the absence of cold (Yan et al., 2003, 2004b; von Zitzewitz et al., 2005; Cockram et al., 2007; Pidal et al., 2009). The extent to which Pooideae species outside the core group plus Brachypodieae are responsive to vernalization is unclear (but see Woods et al., 2016). Indeed, although evidence suggests the late incorporation of VRN2 into the vernalization network at the base of core Pooideae (Woods et al., 2016), it remains to be determined whether other components of this gene network trace back earlier to the ancestor of Pooideae.
Here, we test predictions of the hypothesis that VRN1-mediated vernalization responsiveness evolved early in the diversification of Pooideae. Although we find no evidence of positive selection along branches leading to the Pooideae or core Pooideae VRN1 lineages, our data demonstrate that VRN1 and/or VRN3 expression is directly (VRN1) or indirectly (VRN3) upregulated by cold in several phylogenetically widespread species and that this correlates with the reconstructed early origin of vernalization-controlled flowering. Taken together, these results are consistent with recruitment of flowering time genes into the vernalization network at or around the base of Pooideae.
RESULTS
Identification of Vernalization-Responsive Species
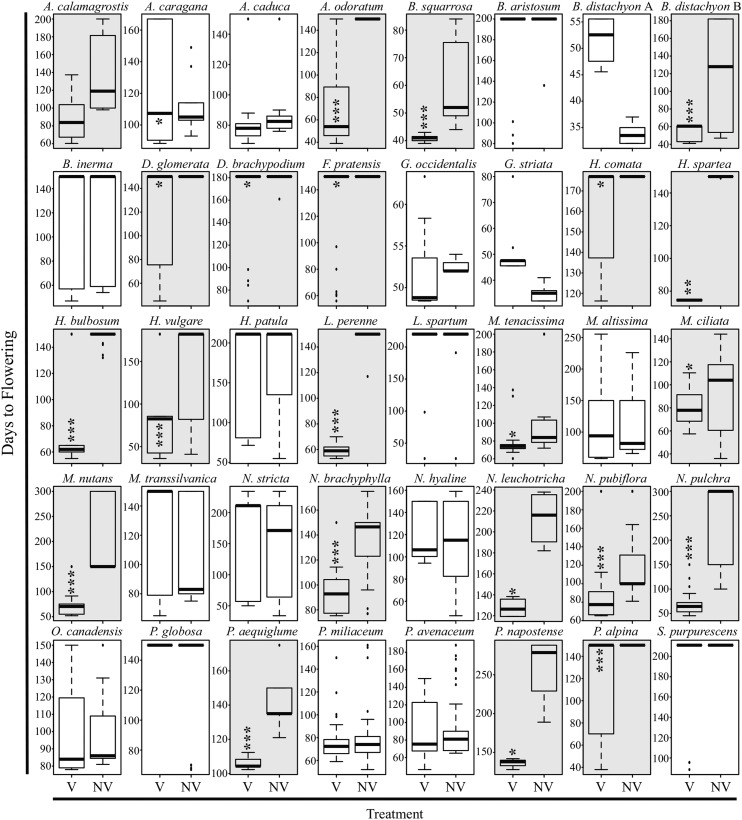
In order to determine if species across Pooideae are responsive to vernalization, temperature adjusted days to flowering, tiller number at flowering, and leaf number at flowering were measured and compared between vernalized and unvernalized plants of phylogenetically representative species. Adjusted days to flowering takes into account the difference in accumulated heat units between warm and cold conditions during the 6-week vernalization period (33.6 d) and attempts to correct for the potential negative impact of reduced temperature on growth (Kirby et al., 1989; Baloch et al., 2003). Of the 61 focal taxa that germinated under growth chamber conditions (Supplemental Table S1), 40 had at least three flowering individuals in one or both treatments, including 10 core and 30 noncore Pooideae (Fig. 1). Within the flowering core Pooideae that were not previously known to be responsive to vernalization (i.e. excluding the controls barley, wheat, Festuca pratensis, and ryegrass) five out of six (83%) flowered significantly earlier with versus without vernalization, whereas this ratio (removing the two B. distachyon controls) was only 13 out of 28 (46%) for taxa outside the core group (Fig. 2). Vernalization-responsive species also showed reduced tiller number at flowering with versus without cold treatment, except for Achnatherum calamagrostis, Duthiea brachypodium, Hesperostipa spartea, Macrochloa tenacissima, Nassella pubiflora, and Piptatherum aequiglume (Supplemental Fig. S1). However, in the case of H. spartea and M. tenacissima, total number of leaves at flowering was significantly (P < 0.05) lower with versus without vernalization (Supplemental Fig. S2).
Figure 1.
Box plots of days to flowering under vernalization (V) and control (NV) conditions. Days to flowering are adjusted by heat units relative to control plants. Data shown are from two to three experimental replicates for species that had at least three flowering individuals per replicate. Days to flowering for nonflowering individuals was scored as the number of days at which experiments were terminated, i.e. between 150 and 300 d. Gray boxes denote responsive taxa; white boxes unresponsive taxa. *P < 0.05, **P < 0.01, and ***P < 0.001.
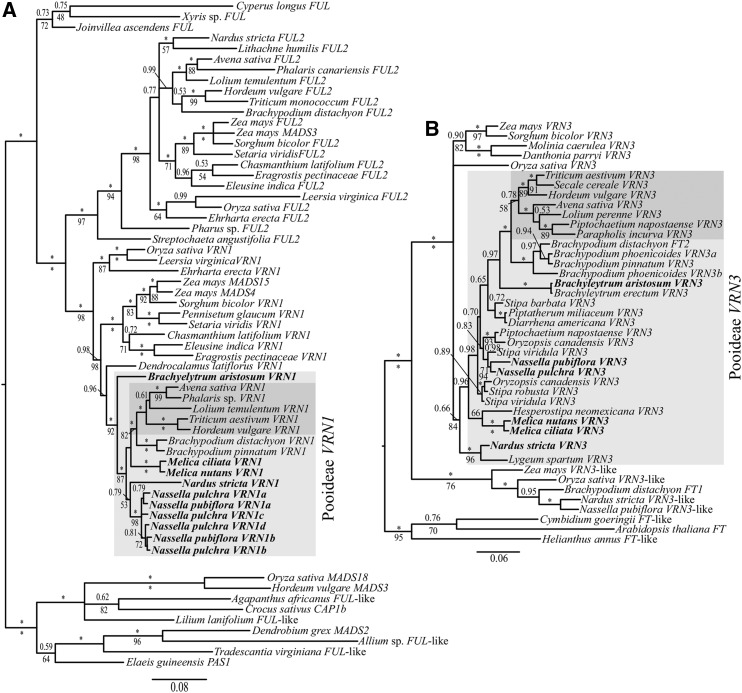
Figure 2.
VRN1 (A) and VRN3 (B) Bayesian 50% majority rule tree with PPs > 0.5 (above branches) and MLB support values > 50% of 1,000 bootstrap replicates (below branches). Bars indicate substitutions per site. Focal genes are indicated in bold, focal clades are denoted with gray boxes, and focal core Pooideae genes are indicated with dark-gray boxes. Asterisks denote PP values of 1.0 and MLB values of 100%.
Evolutionary History of VRN1- and VRN3-Like Genes
Bayesian and maximum likelihood (ML) analyses support two major clades within the grass VRN1/FUL2 gene tree, consistent with gene duplication at the base of Poaceae (Preston and Kellogg, 2006; Fig. 2A). The VRN1 clade is strongly supported by a Bayesian posterior probability (PP) value of 1.0 and a ML bootstrap support value (MLB) of 98%, whereas the FUL2 clade is strongly supported by a PP value of 1.0 and a MLB value of 97%. Within each clade, topologies largely track relationships found in the Poaceae species tree (Grass Phylogeny Working Group II, 2012). One major exception in the VRN1 clade is the position of Ehrhartoideae sequences (e.g. rice [Oryza sativa] OsMADS14) sister to all other sequences from other major grass subfamilies. Within the well-supported Pooideae clades (1.0 PP and 92% MLB for VRN1; 0.99 PP for FUL2), relationships adhere to the tribal-level species tree, except for the position of Nardus stricta VRN1 and FUL2. Similar to the VRN1/FUL2 tree, Bayesian and ML analyses suggest a gene duplication event at or before the base of grasses, giving rise to two major VRN3/FT clades (Fig. 2B). The VRN3 clade is strongly supported by PP and MLB values of 1.0 and 100%, respectively, whereas the VRN3-like clade is supported by PP values of 1.0 and MLB values of 76%. Tribal-level relationships within Pooideae VRN3 tree are weakly supported.
To test for evidence of positive selection that might indicate novel protein-binding domains within Pooideae VRN1 genes, PAML analyses were conducted by comparing two competing branch-site models. The likelihood ratio test estimated that it was not significantly likely that positive selection occurred on the branch leading to Pooideae (ω = 1.88; P = 0.81; Fig. 2) nor on the branch leading to the core Pooideae (ω = 1.00; P = 1.00; green box in Fig. 2). This suggests that VRN1 evolved under purifying or neutral selection along these branches.
VRN1 and VRN3 Gene Expression in Response to Vernalization
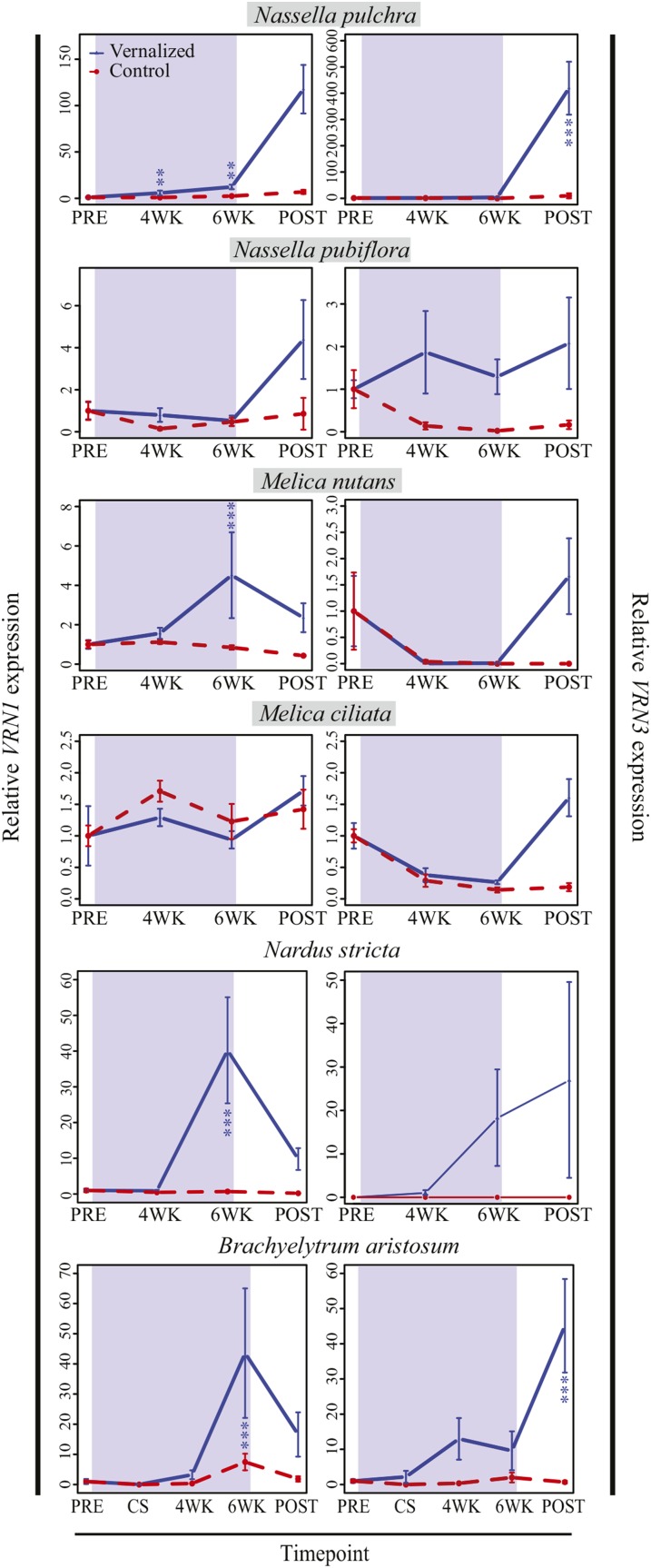
In order to test predictions that VRN genes will increase in expression during (VRN1) and shortly following (VRN3) vernalization, VRN1 and VRN3 expression profiles were determined for six phylogenetically representative species outside core Pooideae. As predicted, VRN1 transcript levels increased significantly in the vernalization requiring species Nassella pulchra (Stipeae; P < 0.001) and Melica nutans (Meliceae; P < 0.001) during vernalization (Fig. 3), at which stage apical meristems of both species had clearly transitioned to flowering (Fig. 4, B and G). Furthermore, there was a significant interaction between time point and treatment (N. pulchra, P < 0.001; M. nutans, P < 0.01), with VRN1 leaf mRNA transcripts increasing more sharply from pretreatment to “4wk” or “6wk” cold time points compared with pretreatment to “4wk” or “6wk” warm time points (Fig. 3; Supplemental Table S2). The inability of warm-treated N. pulchra and M. nutans to up-regulate VRN1 expression correlated with the delay in shoot apical meristem development relative to vernalized plants (Fig. 4, B versus C, and F versus G).
Figure 3.
Relative VRN1 and VRN3 expression in vernalization responsive (gray boxed taxa) and unresponsive noncore Pooideae species. Blue background indicates the timing of cold treatment when relevant; color-coded asterisks indicate level of significance (*P < 0.05, **P < 0.01, and ***P < 0.001) for differences between samples collected after 6 weeks cold (blue) or warm (red; VRN1 only) or posttreatment cold (blue) or warm (red; VRN3 only) versus pretreatment samples. Error bars indicate se. Data shown represents one to three experimental replicates with three to four biological replicates each. PRE, after 4 weeks at 20°C; CS, 1 d cold shock at 4°C (cold treatment only); 4WK, 4 weeks at 4 or 20°C; 6WK, 6 weeks at 4 or 20°C; POST, 2 weeks after treatment at 20°C.
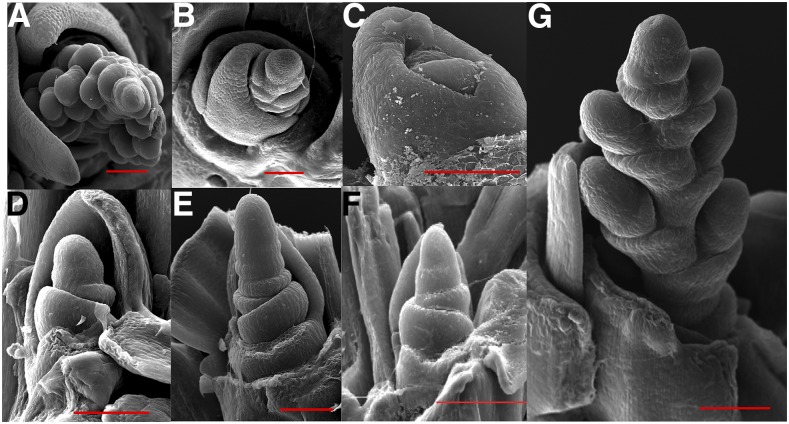
Figure 4.
Shoot apical meristem (SAM) developmental transition in Nassella and Melica with and without vernalization treatment. A, N. pubiflora SAM has transitioned to an inflorescence meristem after 56 d (4 week time point) in the warm (control) treatment. B, N. pulchra SAM has transitioned to a floral meristem after 70 d (6 week time point) in the cold treatment. C, N. pulchra vegetative SAM after 84 d (post time point) in the warm treatment. D, M. ciliata SAM after 70 d in the cold treatment. E, M. ciliata SAM elongating and transitioning to a floral meristem after 70 d in the warm treatment. F, M. nutans SAM remains in the vegetative state after 84 d in the warm treatment. G, M. nutans SAM has transitioned to an inflorescence meristem after 84 d in the cold treatment. Bar = 100 μm.
In contrast to their close relatives, leaf VRN1 expression in weakly vernalization responsive N. pubiflora and Melica ciliata did not increase with vernalization (Fig. 3) and showed no difference in response between warm and cold treatments (interaction term in Supplemental Table S2), and both species transitioned to the double ridge stage of flowering faster (in terms of uncorrected days) in warm versus cold treatments (Fig. 4, A and D versus E). However, in the distantly related nonresponsive species N. stricta (Nardeae) and Brachyelytrum aristosum (Brachyelytreae), VRN1 was significantly upregulated during vernalization (N. stricta, P < 0.001; B. aristosum, P < 0.001), and relative transcript levels showed a strong time point by treatment interaction (N. stricta, P < 0.05; B. aristosum, P < 0.05; Fig. 3). This lack of correlation between flowering time and VRN1 expression in N. stricta and B. aristosum is suggestive of independent losses of vernalization responsiveness through a VRN1-independent mechanism, as has been observed in spring cereals. Alternatively, vernalization responsiveness evolved after the VRN1-VRN3 regulon was in place, after the divergence of Brachyelytreae and Nardeae from the rest of Pooideae.
In species where VRN1 was upregulated during cold, we predicted that VRN3 transcription would rapidly increase postcold. In line with this prediction, N. pulchra, M. nutans, and B. aristosum showed a significant time point by treatment interaction for VRN3 expression (Supplemental Table S2). Transcript levels were significantly higher postvernalization relative to prevernalization in N. pulchra and B. aristosum (Fig. 3; Supplemental Table S2). Furthermore, VRN3 expression in M. nutans at the “post” time point was significantly higher in cold than warm (P < 0.001), despite only marginal up-regulation from pre- to postvernalization time points (Fig. 3). In the case of N. stricta, although there was a trend toward increased VRN3 expression following cold (Fig. 3), this was not significant (Supplemental Table S2).
Although VRN1 transcript levels were not significantly upregulated by cold in N. pubiflora or M. ciliata, VRN3 expression showed a significant time point by treatment interaction for both species (P < 0.05 and P < 0.01, respectively), with higher mean transcript levels in vernalization versus control conditions (Supplemental Table S2). However, the fact that both species had transitioned to flowering well before the posttreatment time point (56 d or earlier) under warm conditions (Fig. 4, A and E) suggested that the peak of VRN3 expression in warm conditions might have been missed. To test this interpretation, follow-up experiments were run, with RNA samples taken after only 1, 2, and 3 weeks of treatment (Supplemental Fig. S3; Supplemental Table S3). As expected, N. pubiflora VRN3 expression significantly increased in warm, but not cold treatment (Supplemental Table S2; Supplemental Fig. S3). Interestingly, in M. ciliata, VRN3 expression did not significantly differ between cold and warm treatments, as transcript levels in both treatments steadily decreased across the seven week experiment (Supplemental Table S3; Supplemental Fig. S3) and did not correlate with the transitional apical meristem morphology (Fig. 4, D and E).
Ancestral State Reconstruction of Vernalization-Mediated Flowering and VRN1 Expression
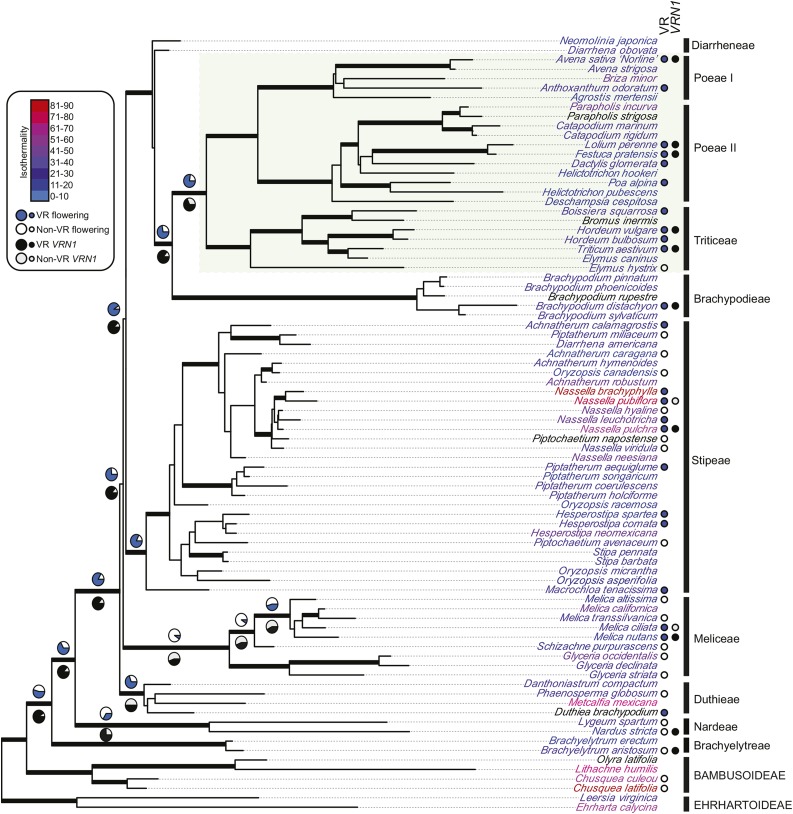
To formally test the hypothesis that vernalization responsiveness in both flowering and VRN1 expression evolved at the base of Pooideae, we carried out ancestral trait reconstruction using a rooted chloroplast phylogeny that included all focal taxa (Fig. 5). Bayesian MCMC analyses supported a one-rate/symmetrical model of evolution with an early origin of vernalization responsiveness in flowering, at least in the ancestor of Duthieae-Meliceae-Stipeae-Diarrheneae-Brachypodieae-core Pooideae (PP = 0.83 ± 0.09). The ancestor of this group plus Nardeae was also reconstructed as vernalization responsive in flowering, but with less support (PP = 0.67 ± 0.07), whereas the ancestral state of all Pooideae was largely equivocal (PP = 0.55 ± 0.05; Fig. 5). Although most other ancestors of major clades in Pooideae were reconstructed as either vernalization responsive (e.g. Brachypodieae-core Poodieae, PP = 0.72 ± 0.12) or equivocal, the ancestor of Meliceae was estimated to flower without a response to vernalization (PP = 0.90 ± 0.07; Fig. 5). According to this scenario, the ancestor of Meliceae lost a flowering response to vernalization, meaning that vernalization responsiveness in Melica is derived within Pooideae. A similar reconstruction of gene expression strongly supported the ancestor of Pooideae VRN1 (PP = 0.90 ± 0.12) and that of most other major clades as being responsive to vernalization (Fig. 5). The exception again was the ancestor of Meliceae, although in this case the ancestral state was equivocal (PP = 0.58 ± 0.06 for nonresponsiveness).
Figure 5.
Best Pooideae Bayesian tree topology based on partitioned matK and ndhF sequences. Thick black branches denote > 0.95 Bayesian posterior probability. Green box defines core Pooideae. Taxon label colors show average isothermality (ratio of diurnal to seasonal temperature variation) based on Worldclim bio3 data extracted from GBIF locality points. Vernalization-responsive (VR) flowering and VRN1 expression are indicated at branch tips where available. Probabilities of responsiveness are shown as pie charts at selected branches of interest.
DISCUSSION
Vernalization responsiveness has evolved multiple times independently within the angiosperms and is hypothesized to have played a key role in repeated plant diversification within the cold temperate zone (Ream et al., 2012; Preston and Sandve, 2013; Preston et al., 2016; Woods et al., 2016). One prediction of this hypothesis is the early origin of a vernalization-induced flowering gene network in predominantly temperate clades, facilitating their niche transition. To test this prediction, we generated flowering data for representative taxa of cool-season Pooideae grasses and reconstructed vernalization responsiveness across the subfamily. Additionally, we leveraged genetic data on economically important crop species (Takahashi and Yasuda, 1971; Heide, 1994; Trevaskis et al., 2003; Yan et al., 2003; Preston and Kellogg, 2008; Greenup et al., 2011; Ream et al., 2014) to determine if orthologs of the vernalization gene VRN1, and the floral integrator VRN3, are cold responsive across the diversity of Pooideae. Our findings demonstrate that vernalization responsiveness is widespread in Pooideae and probably evolved early in the subfamily. Moreover, the increase in VRN1 transcription and that of its positively correlated target VRN3 in response to cold is reconstructed as ancestral to all Pooideae. Potential caveats to our study are the lack of population-level sampling and restrictions to the number of flowering accessions we were able to work with. However, we attempted to control for bias toward vernalization responsive or nonresponsive taxa by selecting accessions from across the Pooideae phylogeny and those that span the northern (e.g. Nassella viridula) to southern temperate (e.g. Nassella hyaline), to highland tropical (e.g. Nassella brachyphylla) zones. Taken together, our data support the early evolution of vernalization responsiveness in Pooideae. This could have coincided with a major niche shift from the tropics to the temperate zone.
It was previously demonstrated in wheat and barley that loss of VRN1 function occurs readily via mutations in cis-regulatory sequences within the promoter and first intron (Yan et al., 2003, 2004b; von Zitzewitz et al., 2005; Cockram et al., 2007; Pidal et al., 2009; Kippes et al., 2015). Furthermore, loss of the VRN3 repressor VRN2 and mutations in cis-regulatory regions of the VRN3 promoter and first intron have been implicated in transitions from winter to spring flowering phenotypes (Yan et al., 2004b, 2006; Trevaskis et al., 2007; Kippes et al., 2016). Although not supported by the ancestral trait reconstruction analysis, which favored a symmetric model of evolution, these functional data together suggest that vernalization responsiveness is easier to lose than gain, lending further weight to the early origin hypothesis for the evolution of vernalization responsiveness in Pooideae. To our knowledge, no members of Bambusoideae have been explicitly tested for a response to vernalization. Regardless, we assume it unlikely that the ancestor of Bambusoideae and Pooideae used cold as a flowering cue. This is largely based on the tropical reconstruction of the Bambusoideae-Ehrhartoideae-Pooideae clade and the largely unpredictable timing of flowering in many bamboos (Nadguada et al., 1990; Edwards and Smith, 2010; Guerreiro 2014; Veller et al., 2015). Furthermore, although some highland rice (Ehrhartoideae) varieties are chilling tolerant (Chawade et al., 2013), there are no examples of vernalization-responsive rice populations.
In addition to supporting an early origin of vernalization responsiveness in Pooideae, our data suggest multiple independent losses, and at least one derived gain, of this trait. Based on our reconstruction, vernalization responsiveness in flowering has been lost at least once in the Duthieae and Stipeae, similar to multiple losses documented in cereal cultivars of the core Pooideae (Jensen et al., 2005; Kippes et al., 2016) and Brachypodieae (Higgins et al., 2010). Since cold-activated VRN1 expression is present in the nonvernalization responsive sister tribes to most Pooideae (i.e. Brachyleytreae and Nardeae), we suggest that cold regulation of VRN1 evolved at the base of Pooideae but was inadequate to induce flowering in its early inception, as suggested by N. stricta VRN3 expression. Indeed, in addition to the vernalization response, cold-regulated VRN1 expression has been implicated in regulating the cold acclimation response, through a negative interaction with dehydration responsive element-binding/C-repeat Binding Factor (DREB1/CBF) genes (Dhillon et al., 2010; Deng et al., 2015). If this interaction is present in B. aristosum and N. stricta, it might explain the initial evolution of cold responsiveness in VRN1. Alternatively, if vernalization responsive flowering is actually ancestral to Pooideae, it might be that it was lost independently in Brachyleytreae and Nardeae due to a dampening of VRN1-mediated VRN3 regulation or the evolution of a parallel flowering pathway.
Another example of an inferred loss of vernalization responsiveness is in the ancestor of Meliceae. If this scenario holds, we would hypothesize one or more derived origins for vernalization responsiveness in Melica using a conserved or derived VRN1-VRN3 pathway, as observed in M. nutans. One prediction of the derived VRN1-VRN3 pathway hypothesis is novel VRN1 cis-regulatory mutations, or VRN1 regulatory modules, in M. nutans relative to other vernalization responsive Pooideae species such as B. distachyon or N. pulchra. It would also be of interest to determine whether temperate grasses outside Pooideae flower in response to vernalization, such as Danthonia californica (Danthoniodeae) and Themeda triandra (Panicoideae), and if these independent origins involved the repeated cooption of VRN1.
Despite evidence for an early origin of vernalization responsiveness in Pooideae (this study), data suggest modifications to the underlying genetic pathway, at least at the base of the core Pooideae (Woods et al., 2016). In barley, VRN1 directly binds to the promoter of the flowering repressor VRN2, resulting in the down-regulation of VRN2 during winter cold (Deng et al., 2015). However, although it likely functions as a flowering repressor across grasses (Yan et al., 2004a; Weng et al., 2014; Nemoto et al., 2016; Woods et al., 2016), noncore Pooideae VRN2 transcripts are not down-regulated in response to cold or VRN1 up-regulation (Woods et al., 2016). The co-option of VRN2 into the vernalization network might therefore have occurred through changes in the DNA-binding domain of VRN1 or through novel cis-regulatory changes in the VRN2 promoter.
In the case of VRN1, further steps should be taken to understand the underlying mechanism for its regulation by cold. Were discrete cis-regulatory changes needed in order to accomplish both inhibition of VRN1 during warm conditions and induction during prolonged cold? Did protein-coding changes occur in upstream regulators of the VRN1 locus? Is the epigenetic cold regulation of VRN1 conserved across Pooideae? Understanding the conservation of these mechanisms will help elucidate the complex evolution that has facilitated the emergence of seasonally driven flowering.
MATERIALS AND METHODS
Growth Conditions and Experimental Design
Controlled growth chamber experiments were performed to determine absence or presence of vernalization responsiveness in 79 Pooideae species. Species were selected to represent all major tribes and geographic localities of the subfamily, as outlined in Supplemental Table S3. For each experimental replicate conducted at the University of Vermont (UVM), 40 to 100 seeds of each accession were germinated on 1% agar plates in the dark, planted in soil, and randomly assigned to either a vernalization or control treatment, both of which were conducted under long days (16 h light:8 h dark). Seedlings in the vernalization treatment were initially exposed to four weeks of 20°C, followed by 6 weeks of 4°C, and then 2 weeks at 20°C. Seedlings assigned to the control treatment were maintained at 20°C for 12 weeks. Plants in both treatments were then moved to a 20 to 22°C greenhouse until flowering, death, or termination of the experiment. Experiments were replicated after switching chambers at the University of Vermont or by running similar experiments twice at the Norwegian University of Life Sciences (NMBU). In the latter case, control temperatures were set to 18°C instead of 20°C, and the period of vernalization was increased to 8 weeks under 8-h short days. Since the basal Pooideae Brachyelytrum aristosum and Nardus stricta failed to flower within 2 years of seed germination, several fruiting plants were collected from a single population in Vermont (B. aristosum) and Norway (N. stricta) and grown in a long-day greenhouse at 18 to 22°C for 6 months. Plants were then randomly assigned to a treatment, and flowering time experiments were conducted as previously described.
Follow-up growth chamber experiments with Melica ciliata and Nassella pubiflora were performed as previously described with two replicates, except that after seedlings were initially exposed to 4 weeks at 20°C they spent only 3 weeks with or without vernalization. Rather than subtracting vernalization time completely from days to flowering (e.g. Woods et al., 2016), heading was calculated in temperature/light-adjusted days (Kirby et al., 1989; Baloch et al., 2003), i.e. total days to flowering minus 33.6 (UVM) or 55.2 (NMBU) days in the vernalization treatment. This correction was calculated by assuming that the control plants (18–20°C) accumulated 4.5 to 5 times more heat units than the vernalized individuals (4°C) across the 42 to 56 d of vernalization, based on a baseline of growth succession at 0°C (Baloch et al., 2003), and that short-day vernalized plants (NMBU only) received half as much light units. However, since some plants experience nonlinear growth rates in response to temperature, we also counted number of leaves and tillers at flowering and made comparisons between treatments. Days to flowering, leaf number at flowering, and tiller number at flowering were calculated when the first inflorescence overtopped the flag leaf.
RNA Extraction and cDNA Synthesis
In order to test predictions for VRN1 and VRN3 expression in response to vernalization across the Pooideae phylogeny, obligately and facultatively responsive pairs were chosen from the two largest noncore Pooideae tribes (Melica nutans and M. ciliata in Meliceae, and Nassella pulchra and N. pubiflora in Stipeae, respectively). Further species were also chosen to represent the Pooideae lineage sister to remaining Pooideae (N. stricta in Nardeae and B. aristosum in Brachyelytreae). During the course of each growth chamber experiment (see above), RNA was extracted from the youngest expanded leaf for each of four randomly selected individuals from each focal species per treatment and experimental replicate without repeated measures. Time points for tissue collection were after 4 weeks at 20°C (4-week pretreatment), 4 weeks at 20°C followed by 4 weeks cold or warm (8 weeks total), 4 weeks at 20°C followed by 6 weeks cold or warm (10 weeks total), and 2 weeks at 20°C after the previous 6 weeks cold or warm (12 weeks total, posttreatment). Time points for tissue collection in the M. ciliata and N. pubiflora follow-up experiment were after 2 weeks at 20°C (2 weeks pretreatment), 4 weeks at 20°C (4 weeks pretreatment), followed by 1 (5 weeks total), 2 (6 weeks total), and 3 weeks (7 weeks total) of 4°C vernalization or 20°C control conditions. Leaf tissue was frozen in liquid nitrogen and RNA was extracted using TRI Reagent (Ambion) followed by DNase treatment with TURBO DNA-free DNase (Ambion) according to the manufacturer’s instructions. cDNA was synthesized using 0.5 μg of RNA in an iScript cDNA synthesis reaction (Bio-Rad).
Gene Isolation, Cloning, and Phylogenetic Analyses
To determine their evolutionary history, VRN1- and VRN3-like genes were amplified from RNA-derived cDNA synthesized from vernalized Parapholis incurva, Brachypodium pinnatum, Brachypodium phoenicoides, Stipa barbata, Stipa robusta, N. pulchra, N. pubiflora, Nassella viridula, Piptochaetium napostense, Piptatherum miliaceum, Oryzopsis canadensis, Hesperostipa neomexicana, M. nutans, M. ciliata, Diarrhena americana, N. stricta, Lygeum spartum, Brachyelytrum erectum, and B. aristosum. Each amplicon was cloned and sequenced using degenerate PCR primers designed on previously available grass sequences (Supplemental Table S4). Cloning was done using the pGEM-T kit (Promega) and 8 to 10 clones were sequenced per amplicon using the T7 forward primer by Beckman Coulter Genomics. Newly generated nucleotide sequences were initially aligned with VRN1- and VRN3-like genes downloaded from GenBank using MAFFT (Katoh and Standley, 2013), followed by manual adjustment in Mesquite (Maddison and Maddison, 2011). Bayesian analyses were done using MrBayes 3.2.2 in XSEDE (Miller et al., 2010) on the Cipres Science Gateway server using 10 million generations, with 25% of saved trees discarded as burn-in. The VRN1 and VRN3 analyses were run under the GTR + Γ and GTR + I + Γ models of evolution, respectively, based on results of MrModeltest 2.3 (Nylander, 2004). ML analyses were done using RAxML-HPC BlackBox on the Cipres Science Gateway (Miller et al., 2010).
Ancestral State Reconstructions for Vernalization Traits
A Pooideae phylogeny was estimated using a partitioned dataset of the chloroplast markers matK and ndhF. Gene sequences were obtained from GenBank or were generated with gene-specific primers as previously described (Liang and Hilu, 1996; Davis and Soreng, 2007). Nucleotides were initially aligned using MAFFT (Katoh and Standley, 2013), followed by manual alignment, and subjected to Bayesian analyses in MrBayes on the Cipres Science Gateway (Miller et al., 2010). To obtain support for tree topologies, Bayesian posterior probabilities were generated by running MrBayes for 10 million replicates with two independent runs, four chains, and 25% of trees discarded as burn-in.
A Bayesian (MCMC) approach was used to reconstruct the evolutionary history of discrete vernalization response traits (flowering and VRN1 expression) across Pooideae using the Multistate function in BayesTraits V2 (Pagel et al., 2004; Pagel and Meade, 2006). As outgroups, the two bamboo species Chusquea culeou and Chusquea latifolia were coded as nonresponsive to vernalization in flowering based on previous studies (Guerreiro, 2014). To account for phylogenetic uncertainty, 200 rooted trees with branch lengths were selected from two independent runs of Bayesian post-burn-in trees and used to assess different models of character evolution. Comparison of marginal likelihoods based on stepping stone estimation identified a one-rate/symmetrical, rather than a two-rate/asymmetrical, model as a better fit for reconstruction of both traits. MCMC analyses were carried out using 10 million generations, sampling every 1,000th tree, with a burn-in of 25%. Means of the posterior probability distributions were calculated for ancestral state estimates at specific nodes based on the Bayesian majority-rule consensus tree.
Georeference data for each species were downloaded from the Global Biodiversity Information Facility (www.gbif.org; GBIF Secretariat), purged of duplicate samples, and used to extract isothermality (bio3; ratio of diurnal to yearly temperature variation) and minimum temperature of the coldest month (bio6) downloaded from Worldclim (http://www.worldclim.org/; Hijmans et al., 2005) with the raster package in R version 3.1.2. Bioclim variables were averaged across all unique locality points for each species.
Tests for Positive Selection
To estimate the ratio (ω) between nonsynonymous (dN) and synonymous (dS) substitution rates of protein-coding VRN1 sequences, we used the codeml branch-site model (Zhang et al., 2005) in PAML (Yang, 2007). Codeml calculates the likelihood of the data (VRN1 alignment and gene tree) under two competing models. The null model allows codons to evolve under either purifying (0 < ω < 1) or neutral (ω = 1) selection along all branches. The alternative model differs by allowing codons on specified branches to evolve under positive selection (ω > 1). To ensure convergence of the likelihoods, tests for positive selection were run four times independently using different ω-seeds (0.5, 1, 1.5, and 2). A likelihood ratio test implemented in PAML was used to test if ω values were significantly higher under the alternative model.
qRT-PCR
Gene-specific qRT-PCR primers were designed in Primer3 (Rozen and Skaletsky, 2000) based on results of phylogenetic analyses (Supplemental Table S4). For each primer pair, amplification efficiencies were determined using a dilution series (Scoville et al., 2011), and amplicon identity was confirmed by sequencing. Following correction for primer efficiency, target gene critical threshold values were normalized against the geomean of two housekeeping genes, UBIQUITIN5 (UBQ5) and ELONGATION FACTOR1a (EF1α; Supplemental Table S4), as previously described (Scoville et al., 2011). To minimize plate effects, full 96-well plates were arrayed per primer set containing all samples per species using the Fast SYBR Green Master Mix (Applied Biosystems) on a StepOne real-time PCR machine (Life Technologies). Three technical replicates were averaged per biological replicate, and two to three experimental replicates each with three biological replicates were assayed per time point/treatment.
Statistical Analyses
To determine whether to classify a species as vernalization responsive or unresponsive, a one-tailed t test was performed using R version 3.1.2 comparing corrected days to flowering, leaf number at flowering, and tiller number at flowering for plants subjected to warm versus cold treatments. We classified a species as vernalization responsive if it flowered in significantly less time, with significantly less leaves or tillers (P < 0.05) with versus without vernalization.
To test for the fixed effects of temperature, time point, and their interaction on gene expression, linear mixed effects models were employed in R version 3.1.2 (R Development Core Team, 2008) using the multcomp (Hothorn et al., 2008) and nlme (Pinheiro et al., 2016) packages, treating replicate and time as random effects where applicable. To reduce heteroscedasticity, data that had no bearing on a priori predictions (posttreatment for VRN1; 4 weeks and 6 weeks for VRN3) were excluded from analyses; remaining data were subjected to log transformation as required to increase normality. When interaction terms were significant for VRN1, pairwise contrasts were conducted on the difference in expression in vernalization versus control treatments between 4 week “pre” and 8 or 10 week time points. When no interaction between temperature and time point was found, models were rerun without an interaction term, and pairwise comparisons were conducted only across time points. The same statistical test for the interaction of time point and treatment was conducted on data from the M. ciliata and N. pubiflora follow-up experiment. When interaction terms were significant for VRN3, contrasts were conducted on the difference in expression between vernalization and control treatments from the 2- and 4-week pretreatment time points to the 1-, 2-, and 3-week vernalization and control time points.
Scanning Electron Microscopy
Approximately four shoot apical meristems were dissected at each RNA extraction time point, fixed in formalin acetic acid (50% ethanol, 5% glacial acetic acid, and 10% of 37% formaldehyde) solution for 8 to 12 h, and progressively transitioned from 50 to 100% ethanol over a 3-h time period before critical point drying. Samples were placed on stubs and further dissection was conducted before sputter coating with gold particles. Imaging was done using a JEOL 6060 scanning electron microscope using an accelerating voltage of 25 kV.
Accession Numbers
New VRN1 and VRN3 gene sequences were deposited in GenBank under accession numbers KX588675-KX588711, matK under KX601224-KX601244, and ndhF under KX601245-KX601267. Aligned matrices are available on request.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Box plots of tiller number at flowering under vernalization and control conditions.
Supplemental Figure S2. Box plots of total leaf number at flowering under vernalization and control conditions for a subset of plants grown at the University of VT.
Supplemental Figure S3. VRN3 expression during three weeks of vernalization in Melica ciliata and Nassella pubiflora.
Supplemental Table S1. Accessions used for vernalization experiments.
Supplemental Table S2. Summary statistics for linear mixed effects models calculating the influence of time point and temperature on VRN1 and VRN3 expression.
Supplemental Table S3. Summary statistics for linear mixed effects models calculating the influence of early time point and temperature on VRN3 expression.
Supplemental Table S4. Primers used for VRN1 and VRN3 cloning and qRT-PCR.
Supplementary Material
Acknowledgments
We thank Jeanne Harris, Mary Tierney, and Neil Sarkar for useful discussions, Stacy Jorgensen and Eliszabeth Graves for chloroplast gene sequencing, Iván Jiménez for help with statistical analyses, and two anonymous reviewers for comments on an earlier draft.
Glossary
- ML
maximum likelihood
- MLB
ML bootstrap
- PP
posterior probability
Footnotes
Articles can be viewed without a subscription.
This work was supported by the National Science Foundation (grant no. IOS 1353056 to J.C.P.) and the Norway Research Council (grant no. 231009 to S.F.).
References
- Amasino R. (2004) Vernalization, competence, and the epigenetic memory of winter. Plant Cell 16: 2553–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloch DM, Karow RS, Marx E, Kling JG, Witt MD (2003) Vernalization studies with pacific northwest wheat. Agron J 95: 1201–1208 [Google Scholar]
- Bäurle I, Dean C (2006) The timing of developmental transitions in plants. Cell 125: 655–664 [DOI] [PubMed] [Google Scholar]
- Chawade A, Lindlöf A, Olsson B, Olsson O (2013) Global expression profiling of low temperature induced genes in the chilling tolerant japonica rice Jumli Marshi. PLoS One 8: e81729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouard P. (1960) Vernalization and its relations to dormancy. Annu Rev Plant Physiol 11: 191–238 [Google Scholar]
- Christin PA, Osborne CP (2013) The recurrent assembly of C4 photosynthesis, an evolutionary tale. Photosynth Res 117: 163–175 [DOI] [PubMed] [Google Scholar]
- Christin PA, Spriggs E, Osborne CP, Strömberg CAE, Salamin N, Edwards EJ (2014) Molecular dating, evolutionary rates, and the age of the grasses. Syst Biol 63: 153–165 [DOI] [PubMed] [Google Scholar]
- Chuine I, Beaubien EG (2008) Phenology is a major determinant of tree species range. Ecol Lett 4: 500–510 [Google Scholar]
- Cockram J, Mackay IJ, O’Sullivan DM (2007) The role of double-stranded break repair in the creation of phenotypic diversity at cereal VRN1 loci. Genetics 177: 2535–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook BI, Wolkovich EM, Davies TJ, Ault TR, Betancourt JL, Allen JM, Bolmgren K, Cleland EE, et al. (2012) Sensitivity of spring phenology to warming across temporal and spatial climate gradients in two independent databases. Ecosystems (NY) 15: 1283–1294 [Google Scholar]
- Dalchau N, Hubbard KE, Robertson FC, Hotta CT, Briggs HM, Stan G-B, Gonçalves JM, Webb AA (2010) Correct biological timing in Arabidopsis requires multiple light-signaling pathways. Proc Natl Acad Sci USA 107: 13171–13176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danyluk J, Kane NA, Breton G, Limin AE, Fowler DB, Sarhan F (2003) TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiol 132: 1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JI, Soreng RJ (1993) Phylogenetic structure in the grass family (Poaceae) as inferred from chloroplast DNA restriction site variation. Am J Bot 80: 1444–1454 [Google Scholar]
- Davis JI, Soreng RJ (2007) A preliminary phylogenetic analysis of the grass subfamily Pooideae (Poaceae), with attention to structural features of the plastid and nuclear genomes, including an intron loss in GBSSI. Aliso 23: 335–348 [Google Scholar]
- Deng W, Casao MC, Wang P, Sato K, Hayes PM, Finnegan EJ, Trevaskis B (2015) Direct links between the vernalization response and other key traits of cereal crops. Nat Commun 6: 5882. [DOI] [PubMed] [Google Scholar]
- Dhillon T, Pearce SP, Stockinger EJ, Distelfeld A, Li C, Knox AK, Vashegyi I, Vágújfalvi A, Galiba G, Dubcovsky J (2010) Regulation of freezing tolerance and flowering in temperate cereals: the VRN-1 connection. Plant Physiol 153: 1846–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A, Li C, Dubcovsky J (2009) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12: 178–184 [DOI] [PubMed] [Google Scholar]
- Dubcovsky J, Loukoianov A, Fu D, Valarik M, Sanchez A, Yan L (2006) Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Mol Biol 60: 469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Osborne CP, Strömberg CAE, Smith SA, Bond WJ, Christin PA, Cousins AB, Duvall MR, Fox DL, Freckleton RP, et al. ; C4 Grasses Consortium (2010) The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328: 587–591 [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Smith SA (2010) Phylogenetic analyses reveal the shady history of C4 grasses. Proc Natl Acad Sci USA 107: 2532–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjellheim S, Boden S, Trevaskis B (2014) The role of seasonal flowering responses in adaptation of grasses to temperate climates. Front Plant Sci 5: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks SJ, Sim S, Weis AE (2007) Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc Natl Acad Sci USA 104: 1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Szűcs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Genet Genomics 273: 54–65 [DOI] [PubMed] [Google Scholar]
- Grass Phylogeny Working Group II (2012) New grass phylogeny resolves deep evolutionary relationships and discovers C4 origins. New Phytol 193: 304–312 [DOI] [PubMed]
- Greenup A, Peacock WJ, Dennis ES, Trevaskis B (2009) The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann Bot (Lond) 103: 1165–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenup AG, Sasani S, Oliver SN, Walford SA, Millar AA, Trevaskis B (2011) Transcriptome analysis of the vernalization response in barley (Hordeum vulgare) seedlings. PLoS One 6: e17900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro C. (2014) Flowering cycles of woody bamboos native to southern South America. J Plant Res 127: 307–313 [DOI] [PubMed] [Google Scholar]
- Heide OM. (1994) Control of flowering and reproduction in temperate grasses. New Phytol 128: 347–362 [DOI] [PubMed] [Google Scholar]
- Higgins JA, Bailey PC, Laurie DA (2010) Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS One 5: e10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron WE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25: 1965–1978 [Google Scholar]
- Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50: 346–363 [DOI] [PubMed] [Google Scholar]
- Humphreys AM, Linder HP (2013) Evidence for recent evolution of cold tolerance in grasses suggests current distribution is not limited by (low) temperature. New Phytol 198: 1261–1273 [DOI] [PubMed] [Google Scholar]
- Jensen LB, Andersen JR, Frei U, Xing Y, Taylor C, Holm PB, Lübberstedt T (2005) QTL mapping of vernalization response in perennial ryegrass (Lolium perenne L.) reveals co-location with an orthologue of wheat VRN1. Theor Appl Genet 110: 527–536 [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30: 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA. (2001) Evolutionary history of the grasses. Plant Physiol 125: 1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippes N, Chen A, Zhang X, Lukaszewski AJ, Dubcovsky J (2016) Development and characterization of a spring hexaploid wheat line with no functional VRN2 genes. Theor Appl Genet 129: 1417–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippes N, Debernardi JM, Vasquez-Gross HA, Akpinar BA, Budak H, Kato K, Chao S, Akhunov E, Dubcovsky J (2015) Identification of the VERNALIZATION 4 gene reveals the origin of spring growth habit in ancient wheats from South Asia. Proc Natl Acad Sci USA 112: E5401–E5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby EJM, Siddique KHM, Perry MW, Kaesehagen D, Stern WR (1989) Variation in spikelet initiation and ear development of old and modern Australian wheat varieties. Field Crops Res 20: 113–128 [Google Scholar]
- Liang H, Hilu KW (1996) Application of the matK gene sequences to grass systematics. Can J Bot 74: 125–134 [Google Scholar]
- Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org [Google Scholar]
- Magallón S, Gómez-Acevedo S, Sánchez-Reyes LL, Hernández-Hernández T (2015) A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytol 207: 437–453 [DOI] [PubMed] [Google Scholar]
- Mannion PD, Upchurch P, Benson RBJ, Goswami A (2014) The latitudinal biodiversity gradient through deep time. Trends Ecol Evol 29: 42–50 [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Takumi S, Kawahara T (2008) Flowering time diversification and dispersal in central Eurasian wild wheat Aegilops tauschii Coss.: genealogical and ecological framework. PLoS One 3: e3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR, Davis SJ (2010) Ambient thermometers in plants: from physiological outputs towards mechanisms of thermal sensing. Curr Biol 20: R1086–R1092 [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), November 14, 2010, New Orleans, LA, pp 1–8 [Google Scholar]
- Nadguada RS, Parasharami VA, Mascarenhas AF (1990) Precocious flowering and seedling behaviour in tissue-cultured bamboos. Nature 344: 335–336 [Google Scholar]
- Nemoto Y, Nonoue Y, Yano M, Izawa T (2016) Hd1,a CONSTANS ortholog in rice, functions as an Ehd1 repressor through interaction with monocot-specific CCT-domain protein Ghd7. Plant J 86: 221–233 [DOI] [PubMed] [Google Scholar]
- Nylander JAA. (2004) Mr Modeltest v2. Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden [Google Scholar]
- Oliver SN, Deng W, Casao MC, Trevaskis B (2013) Low temperatures induce rapid changes in chromatin state and transcript levels of the cereal VERNALIZATION1 gene. J Exp Bot 64: 2413–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver SN, Finnegan EJ, Dennis ES, Peacock WJ, Trevaskis B (2009) Vernalization-induced flowering in cereals is associated with changes in histone methylation at the VERNALIZATION1 gene. Proc Natl Acad Sci USA 106: 8386–8391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M, Meade A (2006) Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am Nat 167: 808–825 [DOI] [PubMed] [Google Scholar]
- Pagel M, Meade A, Barker D (2004) Bayesian estimation of ancestral character states on phylogenies. Syst Biol 53: 673–684 [DOI] [PubMed] [Google Scholar]
- Pidal B, Yan L, Fu D, Zhang F, Tranquilli G, Dubcovsky J (2009) The CArG-box located upstream from the transcriptional start of wheat vernalization gene VRN1 is not necessary for the vernalization response. J Hered 100: 355–364 [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2016) Nlme: linear and nonlinear mixed effects model. R package version 3.1-124. http://CRAN.R-project.org/package=nlme
- Prasad V, Strömberg CAE, Leaché AD, Samant B, Patnaik R, Tang L, Mohabey DM, Ge S, Sahni A (2011) Late Cretaceous origin of the rice tribe provides evidence for early diversification in Poaceae. Nat Commun 2: 480. [DOI] [PubMed] [Google Scholar]
- Preston JC, Kellogg EA (2006) Reconstructing the evolutionary history of paralogous APETALA1/FRUITFULL-like genes in grasses (Poaceae). Genetics 174: 421–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JC, Kellogg EA (2008) Discrete developmental roles for temperate cereal grass VERNALIZATION1/FRUITFULL-like genes in flowering competency and the transition to flowering. Plant Physiol 146: 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JC, Sandve SR (2013) Adaptation to seasonality and the winter freeze. Front Plant Sci 4: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston JC, Zhong J, McKeown M, den Bakker M, Friedman J (2016) Comparative transcriptomics indicates a role for SHORT VEGETATIVE PHASE (SVP) genes in Mimulus guttatus vernalization response. G3 (Bethesda) 6: 1239–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2008) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
- Rathcke B, Lacey EP (1985) Phenological patterns of terrestrial plants. Annu Rev Ecol Syst 16: 179–214 [Google Scholar]
- Ream TS, Woods DP, Amasino RM (2012) The molecular basis of vernalization in different plant groups. Cold Spring Harb Symp Quant Biol 77: 105–115 [DOI] [PubMed] [Google Scholar]
- Ream TS, Woods DP, Schwartz CJ, Sanabria CP, Mahoy JA, Walters EM, Kaeppler HF, Amasino RM (2014) Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon. Plant Physiol 164: 694–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386 [DOI] [PubMed] [Google Scholar]
- Sandve SR, Fjellheim S (2010) Did gene family expansions during the Eocene-Oligocene boundary climate cooling play a role in Pooideae adaptation to cool climates? Mol Ecol 19: 2075–2088 [DOI] [PubMed] [Google Scholar]
- Sandve SR, Kosmala A, Rudi H, Fjellheim S, Rapacz M, Yamada T, Rognli OA (2011) Molecular mechanisms underlying frost tolerance in perennial grasses adapted to cold climates. Plant Sci 180: 69–77 [DOI] [PubMed] [Google Scholar]
- Sandve SR, Rudi H, Asp T, Rognli OA (2008) Tracking the evolution of a cold stress associated gene family in cold tolerant grasses. BMC Evol Biol 8: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville AG, Barnett LL, Bodbyl-Roels S, Kelly JK, Hileman LC (2011) Differential regulation of a MYB transcription factor is correlated with transgenic inheritance of trichome density in Mimulus guttatus. New Phytol 191: 251–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szűcs P, Skinner JS, Karsai I, Cuesta-Marcos A, Haggard KG, Corey AE, Chen THH, Hayes PM (2007) Validation of the VRN-H2/VRN-H1 epistatic model in barley reveals that intron length variation in VRN-H1 may account for a continuum of vernalization sensitivity. Mol Genet Genomics 277: 249–261 [DOI] [PubMed] [Google Scholar]
- Takahashi R, Yasuda S (1971) Genetics of earliness and growth habit in barley. In RA Nilan, Barley Genetics II Proceedings of the 2nd International Barley Genetics Symposium, CAB International, Oxford, UK, pp 388–408 [Google Scholar]
- Trevaskis B. (2010) Goldacre Paper: The central role of the VERNALIZATION1 gene in the vernalization response of cereals. Funct Plant Biol 37: 479 [Google Scholar]
- Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES (2003) MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci USA 100: 13099–13104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Dennis ES, Peacock WJ (2007) The molecular basis of vernalization-induced flowering in cereals. Trends Plant Sci 12: 352–357 [DOI] [PubMed] [Google Scholar]
- Tsuji H, Taoka K, Shimamoto K (2011) Regulation of flowering in rice: two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol 14: 45–52 [DOI] [PubMed] [Google Scholar]
- Veller C, Nowak MA, Davis CC (2015) Extended flowering intervals of bamboos evolved by discrete multiplication. Ecol Lett 18: 653–659 [DOI] [PubMed] [Google Scholar]
- Vigeland MD, Spannagl M, Asp T, Paina C, Rudi H, Rognli OA, Fjellheim S, Sandve SR (2013) Evidence for adaptive evolution of low-temperature stress response genes in a Pooideae grass ancestor. New Phytol 199: 1060–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zitzewitz J, Szűcs P, Dubcovsky J, Yan L, Francia E, Pecchioni N, Casas A, Chen THH, Hayes PM, Skinner JS (2005) Molecular and structural characterization of barley vernalization genes. Plant Mol Biol 59: 449–467 [DOI] [PubMed] [Google Scholar]
- Weng X, Wang L, Wang J, Hu Y, Du H, Xu C, Xing Y, Li X, Xiao J, Zhang Q (2014) Grain number, plant height, and heading date7 is a central regulator of growth, development, and stress response. Plant Physiol 164: 735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg AC, Amasino RM (2012) Natural variation in the temperature range permissive for vernalization in accessions of Arabidopsis thaliana. Plant Cell Environ 35: 2181–2191 [DOI] [PubMed] [Google Scholar]
- Woods DP, McKeown MA, Dong Y, Preston JC, Amasino RM (2016) Evolution of VRN2/GhD7-like genes in vernalization-mediated repression of grass flowering. Plant Physiol 170: 2124–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Yasuda S, Dubcovsky J (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA 103: 19581–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Helguera M, Kato K, Fukuyama S, Sherman J, Dubcovsky J (2004b) Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor Appl Genet 109: 1677–1686 [DOI] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J (2004a) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA 100: 6263–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24: 1586–1591 [DOI] [PubMed] [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292: 686–693 [DOI] [PubMed] [Google Scholar]
- Zhang J, Nielsen R, Yang Z (2005) Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol Biol Evol 22: 2472–2479 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.