Accumulation of fumaric acid, catalyzed by a cytosolic fumarase, is essential for Arabidopsis thaliana to acclimate photosynthesis to low temperature.
Abstract
Although cold acclimation is a key process in plants from temperate climates, the mechanisms sensing low temperature remain obscure. Here, we show that the accumulation of the organic acid fumaric acid, mediated by the cytosolic fumarase FUM2, is essential for cold acclimation of metabolism in the cold-tolerant model species Arabidopsis (Arabidopsis thaliana). A nontargeted metabolomic approach, using gas chromatography-mass spectrometry, identifies fumarate as a key component of the cold response in this species. Plants of T-DNA insertion mutants, lacking FUM2, show marked differences in their response to cold, with contrasting responses both in terms of metabolite concentrations and gene expression. The fum2 plants accumulated higher concentrations of phosphorylated sugar intermediates and of starch and malate. Transcripts for proteins involved in photosynthesis were markedly down-regulated in fum2.2 but not in wild-type Columbia-0. Plants of fum2 show a complete loss of the ability to acclimate photosynthesis to low temperature. We conclude that fumarate accumulation plays an essential role in low temperature sensing in Arabidopsis, either indirectly modulating metabolic or redox signals or possibly being itself directly involved in cold sensing.
In recent years, there has been a growing recognition that we need to increase photosynthesis in crops if we are to improve yields to match global food demand (Zhu et al., 2010). Fluctuations in climate across the season can represent a significant stress to plants, producing imbalances between photosynthesis and metabolism, limiting overall photosynthesis. Optimizing investment in different processes is an important factor determining the yield and fitness of plants and a target for improving crop yields. Temperature is a key environmental variable experienced by plants (Ruelland et al., 2009).
Plants grown in a particular environment tend to adjust their growth and biochemical composition to suit those conditions, a process termed acclimation. This can be seen both during development, with plant morphology being environmentally sensitive, and dynamically, with tissues formed in one set of conditions altering their cell structure and metabolism if transferred to new conditions (Strand et al., 1999; Savitch et al., 2001; Athanasiou et al., 2010). For example, when plants developed at one temperature are moved to a lower temperature, a process of cold hardening occurs. This dynamic acclimation response is often considered to have two components, although these are difficult to disentangle. Structural changes occurring in the leaf result in an increased ability to tolerate subzero temperatures—freezing tolerance—while metabolic changes allow plants to increase the capacity of biochemical processes to compensate for the lower temperature—metabolic acclimation (Knight and Knight, 2012). The latter is well illustrated by the example of photosynthesis (Strand et al., 1999; Savitch et al., 2001): When a cold-tolerant plant is transferred to low temperature, there is an immediate loss of the effective photosynthetic capacity (i.e. the maximum rate of photosynthesis achievable under the conditions experienced). As the plant acclimates, photosynthetic capacity recovers, such that it may reach a level at low temperature previously achieved at the original higher temperature.
Although cold hardening is known to every gardener from a temperate climate, the mechanisms used to sense changes in temperature are poorly understood (Knight and Knight, 2012). Various possible sensors, including nucleic acid thermometers and thermosensitive proteins, have been described in different organisms (Sengupta and Garrity, 2013), however to date there has been no unequivocal identification of a plant thermosensor involved in cold acclimation (Penfield and MacGregor, 2014). There is some evidence for the involvement of cold-sensitive Ca2+ channels, possibly regulated via membrane fluidity (Knight and Knight, 2012; Penfield and MacGregor, 2014). Nevertheless, the molecular details remain unknown.
Among the potential signals plants could use to sense temperature, metabolites are strong candidates. As temperature falls, enzyme reactions slow. This can vary between individual steps in a pathway giving rise to differences in both absolute and relative concentrations of metabolites, which have the potential to trigger temperature signaling pathways. Acclimation of metabolism to cold may be linked to phosphate starvation (Stitt and Hurry, 2002). A resultant accumulation of sugar phosphates both inhibits photosynthesis directly, preventing recycling of inorganic phosphate, and triggers changes in gene expression similar to those induced in phosphate-starved plants (Strand et al., 2003). Mutants with altered phosphate levels show altered acclimation to low temperature, though only some traits are affected (Hurry et al., 2000).
Here, we examine the low temperature responses of the cold-tolerant model plant Arabidopsis (Arabidopsis thaliana). We show that low temperature induces a change in metabolic fluxes, resulting in an accumulation of the organic acid fumaric acid, predominantly in the anionic (fumarate) form in vivo. Inhibition of fumarate accumulation results in an overall shift in the cold response of leaves, with a complete inhibition of cold acclimation of photosynthesis. These data indicate that fumarate accumulation reflects a novel sensing mechanism playing a major role in cold acclimation.
RESULTS
Fumarate Is an Important Sink for Photosynthesis at Low Temperature
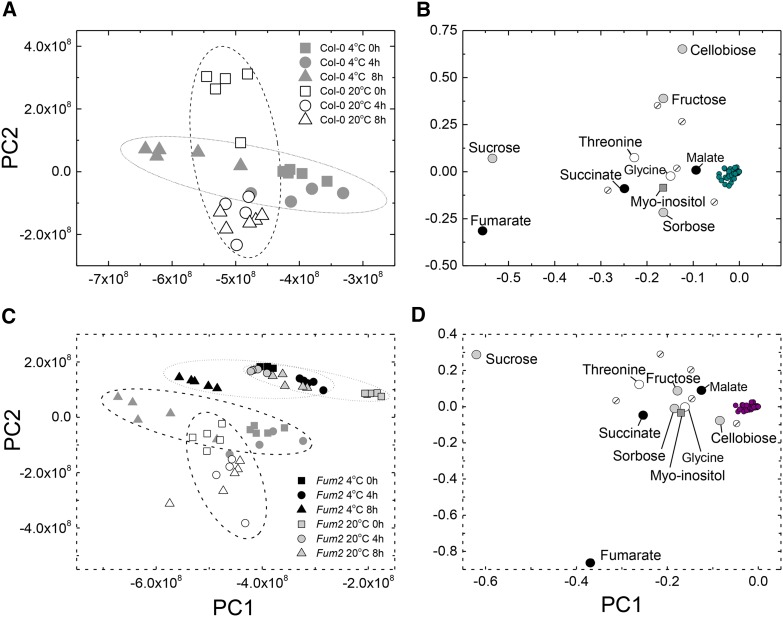
Plants of Arabidopsis, accession Columbia-0 (Col-0), were grown for 8 weeks under control conditions (8-h photoperiod, 20°C/18°C; 100 µmol m−2 s−1) before being transferred to a day/night temperature of 4°C. Plants were harvested after 4 or 8 h, and metabolite content was analyzed using nontargeted gas chromatography-mass spectrometry (GC-MS). Data were analyzed using principal component analysis (PCA; Fig. 1; for a complete dataset, see Supplemental Table S1). The first two principal components (PC1 and PC2) explained 93% of the variation in the data, and the clustering allowed discrimination of the samples, depending on the time of day and temperature. PC2 mainly explained the diurnal variation in leaves at 20°C, while PC1 mostly accounted for variation at 4°C. Examination of the PCA loadings showed that sugars and organic acids were prominent. In particular, Suc and fumarate were notable in their contribution to PC1, which dominates the response at low temperature.
Figure 1.
PCA of GC-EI-TOF/MS data from low-temperature-treated plants. Plants of Col-0 and fum2.2 were grown under 100 µmol m2 s−1 light, 8 h light/16 h dark, 500 µL L−1 CO2, and 20°C light/18°C dark conditions for 8 weeks, before being transferred to 4°C for a single photoperiod. Fully expanded leaves were flash frozen and excised at 0 h, 4 h, and 8 h after the photoperiod began. Circles are indicative only and have no statistical significance. A, PCA constructed from the GC-EI-TOF/MS data from the Col-0 wild-type line during the first photoperiod after transfer to 4°C. Ten principal components were used in the calculation, accounting for 98.9% of the total explained variance (TEV). PC1 explained 65.9% TEV, with PC2 explaining a further 27.1% TEV. B, Loading plot of PCA data, indicating metabolites responsible for the variance in the data. C, PCA constructed from the GC-EI-TOF/MS data from both Col-0 wild-type and fum2.2 lines during the first photoperiod after transfer to 4°C. Ten principal components were used in the calculation, accounting for 96.4% TEV. PC1 explained 58.1% TEV, with PC2 explaining a further 31.4% TEV. D, Loading plot of PCA data, indicating metabolites responsible for the variance in the data. In B and D, named metabolites are those judged not to cluster with the majority of metabolites: organic acids are represented by black circles, sugars by gray circles, amino acids by white circles, sugar alcohols by gray squares, and unknown metabolites by striped circles.
Diurnal accumulation of Suc occurs in many plants, with this nonreducing disaccharide being a predominant transport molecule. Suc is recognized to play an important role in cold tolerance (Stitt and Hurry, 2002). The accumulation of fumarate is less common but has been observed in phylogenetically distant species (Chia et al., 2000). Fumarate accumulation depends on the presence of a cytosolic fumarase, FUM2 (At5g50950; Pracharoenwattana et al., 2010). Plants lacking FUM2 do not accumulate fumarate but show no visible growth phenotype, with no significant difference in leaf area or dry weight after 8 weeks under standard (i.e. nonchilling) conditions (data not shown). We examined the response of a fum2 knockout mutant line (fum2.2) during the first photoperiod following transfer to 4°C. PCA of GC-MS data clearly separated the fum2.2 mutant from wild-type Col-0 (Fig. 1, C and D). In fum2.2 plants, a response was seen through the day that was explained mainly by PC1, however there was only weak separation of plants at 20°C or 4°C. PCA loadings of the whole dataset were again dominated by Suc and fumarate (Fig. 1). In PCA of fum2.2 alone, fumarate did not make an important contribution (see Supplemental Fig. S1).
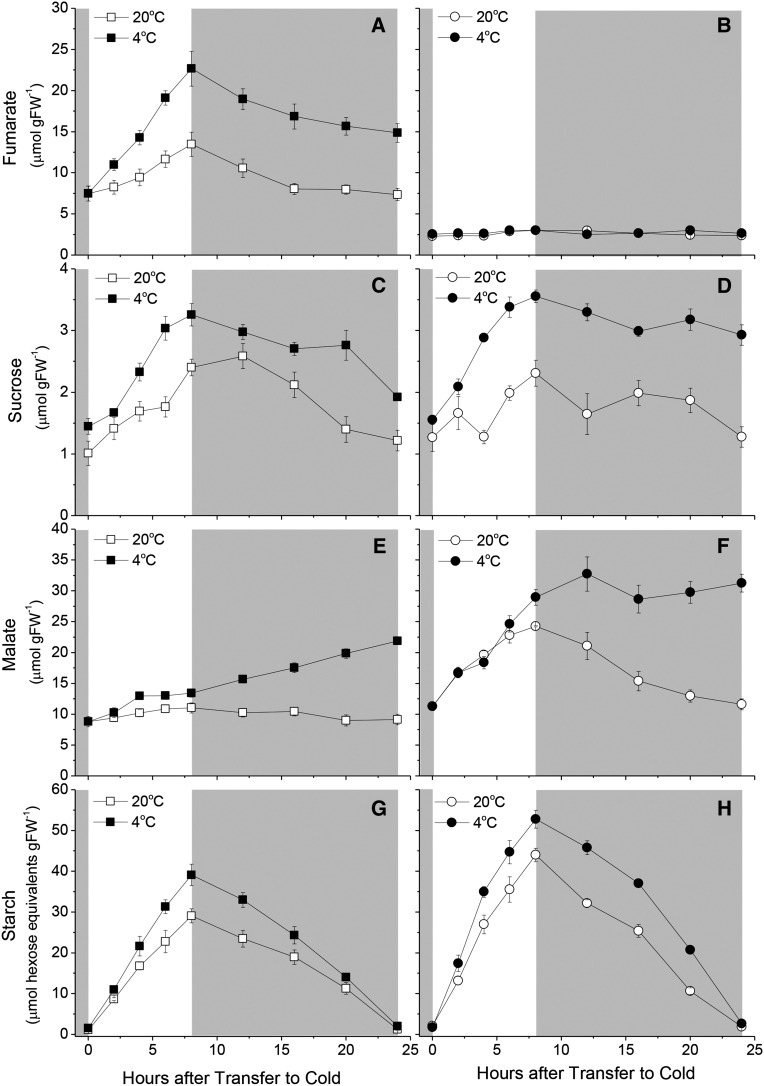
We used enzyme-linked quantitation to carry out a detailed analysis of changes in Suc and fumarate through the day-night cycle (Fig. 2). We also assayed malate, the substrate for FUM2, and starch, the major leaf storage carbohydrate in Arabidopsis. At 20°C, both Suc and fumarate accumulated in an approximately linear manner over the photoperiod, mirroring accumulation of starch, quantitatively the most important carbon store assayed. The concentration of all these compounds fell overnight. In terms of organic carbon, Suc and fumarate were of a similar magnitude, while starch represented substantially more. In plants transferred to cold, all three molecules accumulated to higher concentrations, consistent with a reduction in the leaf metabolic rate and carbon export, without a substantial reduction in the gross photosynthesis. As a proportion of the accumulation under control conditions, the increase in starch (∼25%) was smaller than that seen in either Suc (∼75% increase in diurnal fluctuation) or fumarate (∼200%). At 20°C, malate showed a small diurnal fluctuation. The accumulation of malate was marginally increased at 4°C, however, strikingly, during the subsequent night, malate concentration rose such that by dawn its concentration was similar to that of fumarate at the end of the day.
Figure 2.
Diurnal metabolite levels under low temperature conditions. Fumarate (A and B), Suc (C and D), malate (E and F), and starch (G and H) levels were determined throughout a 24-h period in plants exposed to low temperature using enzyme-linked assays. Plants of Col-0 (A, C, E, and G) and fum2.2 (B, D, F, and H) were grown under 100 µmol m2 s−1 light, 8 h light/16 h dark, 500 µL L−1 CO2, and 20°C light/18°C dark conditions for 8 weeks, then transferred to low temperature (4°C) for a 24-h period. Fully expanded leaves were excised and flash frozen from individual plants at 2-h intervals in the light and 4-h intervals in the dark.
In fum2 plants, the accumulation of fumarate was abolished at both 20°C and 4°C (Fig. 2 for data from Col-0 and fum2.2; see Supplemental Fig. S2 for supporting data from an alternative knockout line, fum2.1). At 20°C, there was an increase in leaf malate accumulation, however, when plants were exposed to 4°C, malate concentrations did not significantly increase. The diurnal fluctuation in malate was largely lost. Compared with Col-0, Suc accumulation was not significantly changed in the fum2 mutants at either 20°C or 4°C, but starch accumulation was higher in both conditions.
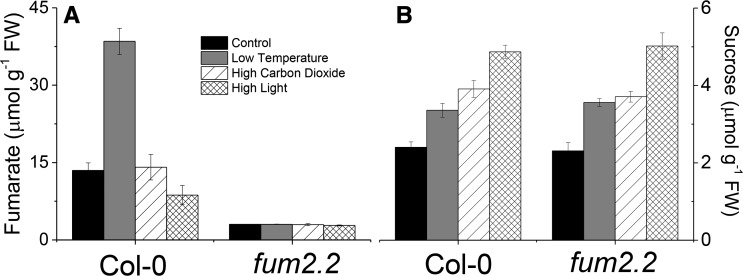
To determine whether the accumulation of fumarate is specific to low temperature, we examined plant responses to alternative conditions resulting in increased leaf carbohydrate—high CO2 and high light. Plants were grown as before and then transferred to either 1500 µL L−1 CO2 or 400 µmol m−2 s−1 light. Both treatments resulted in an increase in the end-of-day Suc (Fig. 3) and starch (Supplemental Fig. S3). Neither increased fumarate content. Plants of fum2 accumulated similar amounts of Suc to Col-0 but higher amounts of starch across all conditions.
Figure 3.
Accumulation of fumarate and Suc in different environmental conditions. Fumarate (A) and Suc (B) levels were determined by enzyme-linked assays following exposure for one photoperiod to different environmental conditions. Plants of Col-0 and fum2.2 were grown under 100 µmol m2 s−1 light, 8 h light/16 h dark, 500 µL L−1 CO2, and 20°C light/18°C dark conditions for 8 weeks. Plants were then transferred to control (identical) conditions, low temperature (4°C), high carbon dioxide (1500 µL L−1), or high light (400 µmol m2 s−1 light) for one photoperiod. Measurements were taken at the end of the photoperiod (8 h) from liquid N2 flash-frozen, fully expanded leaves, as in Figure 2.
Fumarate Accumulation Reduces the Accumulation of Phosphorylated Sugars
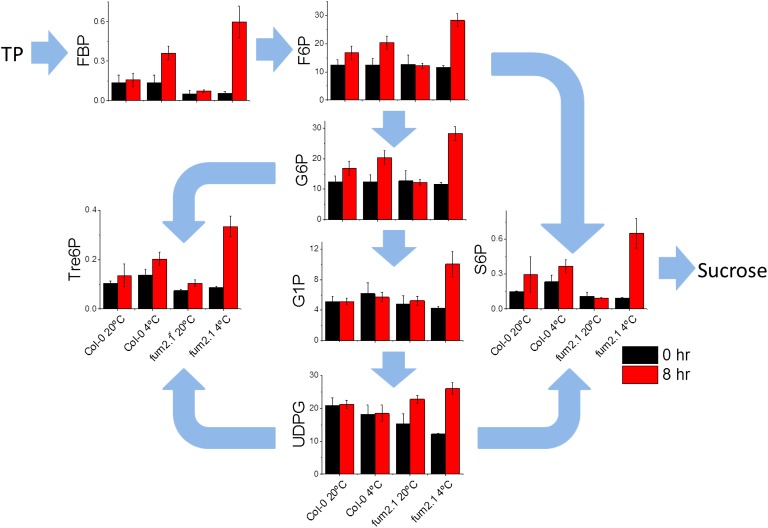
Fumarate accumulation is an important component of the diurnal leaf metabolome and is specifically increased at low temperature. The absence of fumarate accumulation may have important effects on other primary metabolites. We therefore performed a targeted analysis of metabolites using anion-exchange liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS; Fig. 4). We could quantify many primary metabolites leading to starch or Suc, as well as those involved in glycolysis and the tricarboxylic acid cycle (Fig. 4; Supplemental Table S2). The primary chloroplast export is triose phosphate, which can be a substrate for Suc synthesis or feed into glycolysis, providing substrates respiration in the mitochondria or fumarate synthesis in the cytosol. When Col-0 plants were exposed to cold, only modest changes in leaf metabolite concentrations were seen. In the pathways to Suc and fumarate, only Fru 1,6-bisphosphate increased significantly at 4°C, relative to 20°C, while phosphoenolpyruvate decreased in concentration (Supplemental Table S2). There were modest changes in metabolites not directly involved in these pathways, including trehalose 6-phosphate (Tre6P), which has previously been implicated in sugar signaling (Lunn et al., 2006) and was about 2-fold higher at 4°C. In contrast, in fum2.2, marked changes were seen in the end-of-day concentrations of several primary metabolites, including quantitatively important molecules such as Glc 6-phosphate and Fru 6-phosphate (Fig. 4). In all cases (except UDP-Glc), end-of-day levels in the fum2.2 mutant were significantly higher at 4°C than in all other conditions (ANOVA, P < 0.05). Changes were also seen in the concentration of various organic acids, including a marked increase in pyruvate (Supplemental Table S2). These data are consistent with fumarate being an important sink for carbon upon transfer to low temperature, allowing the leaf to buffer metabolism in response to changes in the environment.
Figure 4.
Analysis of sugar phosphates using targeted anion-exchange LC-MS/MS. Plants of Col-0 and fum2.2 were grown under 100 µmol m2 s−1 light, 8 h light/16 h dark, 500 µL L−1 CO2, and 20°C light/18°C dark conditions for 8 weeks. Tissue from mature leaves was flash frozen and excised from plants at the beginning (0 h; black bars) and end (8 h; red bars) of the first photoperiod after transfer to low temperature and in control plants. LC-MS/MS was carried out as done by Lisec et al. (2006). y axis scales are metabolite levels in µmol g−1 fresh weight (FW). Abbreviations not defined in the text: F6P, Fru 6-phosphate; FBP, Fru 1,6-bisphosphate; G1P, Glc 1-phosphate; G6P, Glc 6-phosphate; S6P, Suc 6-phosphate; TP, triose phosphate; UDPG, UDP-Glc.
Inhibiting Fumarate Accumulation Changes the Transcriptome Response to Low Temperature
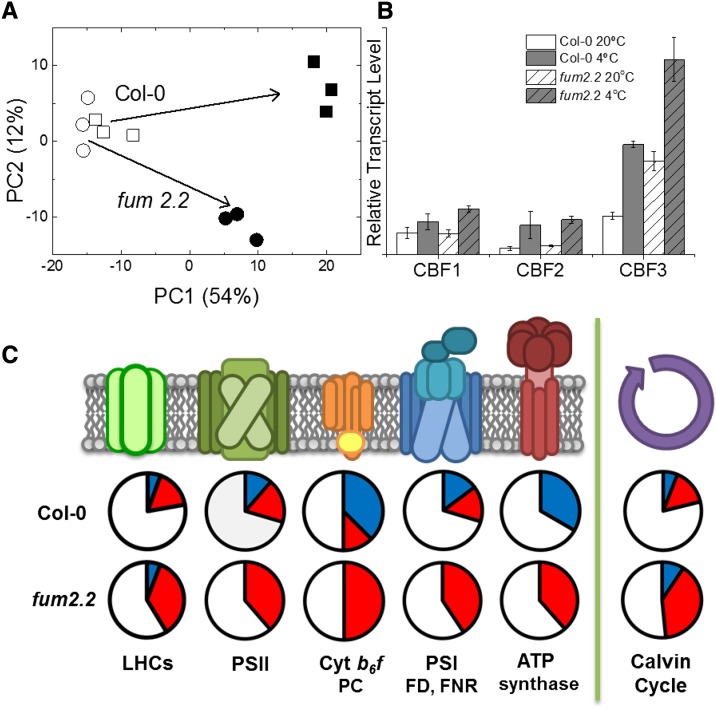
Inhibiting fumarate accumulation results in widespread changes in the metabolome, including altering various molecules previously implicated in regulating gene expression. We used microarray analysis to examine the responses of the transcriptome to knocking out FUM2 and to cold (see Supplemental Table S2 for a complete dataset). Leaves were flash frozen under growth conditions at the end of the photoperiod. Preliminary analysis, using PCA, showed that, at 20°C, genotypes were only weakly separated. However, at 4°C, there is a marked separation of the genotypes, showing a clear divergence in their response (Fig. 5).
Figure 5.
Microarray analysis of transcript changes in response to cold. Microarray analysis was carried out on total RNA extracted from Col-0 and fum2.2 lines at the end of the first photoperiod after transfer to low temperature. A, PCA model of differences in transcripts between Col-0 (squares) and fum2.2 (circles) under control (white symbols) and cold-treated (black symbols) conditions. B, Relative transcript levels of CBF transcription factors during cold treatment. C, Diagram showing relative changes in transcript level of genes associated with various photosynthetic processes (P < 0.001, fold change > 1.5). Blue sections represent up-regulated genes, red sections represent down-regulated genes, and white sections represent unchanged genes. Abbreviations not defined in the text: FD, ferredoxin; FNR, ferredoxin NADP reductase; LHC, light-harvesting complex; PC, plastocyanin.
Genes were considered to have significantly changed in abundance when P < 0.001 was observed, with a fold change > 1.5. Using these constraints, 2604 transcripts responded significantly in Col-0, with 1340 being up-regulated and 1264 being down-regulated in response to low temperature. In fum2.2, 4080 transcripts responded significantly, with 1772 being up-regulated and 2307 being down-regulated. In total, 851 genes were up-regulated in both genotypes, with 489 being up-regulated in only the Col-0 and 921 only in fum2.2. A similar pattern was seen among down-regulated genes, with 777 in common, 487 down-regulated only in the Col-0, and 1503 only in fum2.2. This indicates a stronger effect of cold on transcript levels in fum2.2 and a clear divergence in the response to cold.
To understand in more detail how FUM2 expression affects transcriptomic response to cold, we performed cluster analysis (Supplemental Fig. S4). The main clusters identified in this way showed exaggerated responses to low temperature, either up or down, in fum2.2. Overrepresented gene ontology groups identified using DAVID (the Database for Annotation, Visualization and Integrated Discovery; Huang et al., 2009) included genes associated with auxin, ethylene, abscisic acid, and brassinosteroid signaling. A substantial number of transcription factors showed different expression patterns.
Previous work on cold acclimation, in particular in relation to freezing, has identified a group of C-REPEAT BINDING FACTOR1 to 3 (CBF1–CBF3; also called DREB1A–DREB1C) as playing an important role, including triggering acclimation of the metabolome (Cook et al., 2004). All of these transcription factors were up-regulated in fum2.2 to a similar or greater extent than in Col-0. CBF3 (DREB1A) was up-regulated in fum2.2 under control conditions, suggesting that, while FUM2 activity is not essential for this cold-sensing pathway, FUM2 expression does modulate its activity. We also saw up-regulation of previously described cold-responsive (COR) proteins, with no marked difference between phenotypes (Supplemental Table S3). For example, among 16 COR genes identified by Hannah et al. (2005), only two (At5g06760 and At4g15910) showed significantly different responses between genotypes, both showing higher expression in fum2.2 than in Col-0 at 4°C and at 20°C.
Among the gene families showing differential expression between Col-0 and fum2.2, a notable group was genes encoding components of the photosynthetic apparatus. In Col-0, there was no clear pattern of response to cold, with genes from the same complexes showing both up- and down-regulation (Fig. 5). There was however an indication of an up-regulation of genes encoding components of the light-using reactions, with subunits encoding the cytochrome b6f and ATPase increasing significantly, along with the Rubisco large subunit RbcL. By contrast, in fum2.2, there was a clear down-regulation of many genes involved in photosynthesis, including complexes showing a tendency to increase in Col-0 (Fig. 5).
Plants Lacking FUM2 Protein Are Unable to Acclimate Photosynthesis in Response to Cold
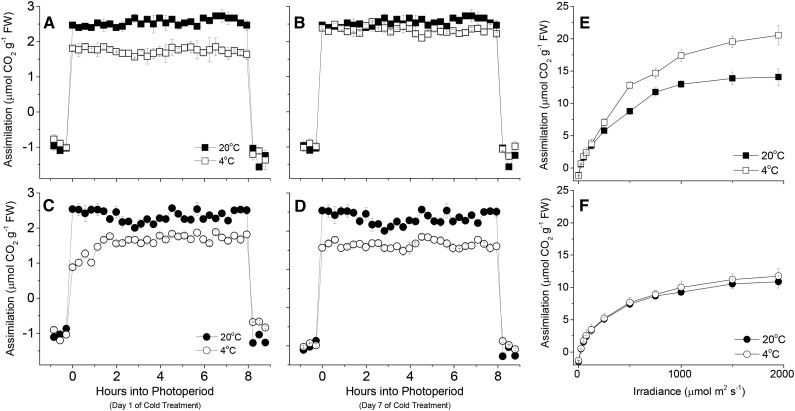
Previous work has suggested that in the short term (minutes), cold results in inhibition of photosynthesis, due to slowed phosphate recycling (Stitt and Hurry, 2002). In the medium term (hours), posttranslation activation of enzymes such as Suc-phosphate synthase alleviates this. In the longer term (days), acclimation results in changes in the capacity for photosynthesis. Changes in phosphate availability have been suggested to play a key role in this process, with various genes being sensitive to both cold and phosphate starvation (Hurry et al., 2000). Given the contrasting responses of photosynthetic genes to low temperature in the genotypes studied, we examined the responses of plants in terms of photosynthesis (Fig. 6; see Supplemental Fig. S5 for fum2.1 mutant). Measurements of photosynthesis were made on Col-0 and fum2.2 through the photoperiod under growth conditions at 20°C. No significant difference could be discerned between genotypes. In the first photoperiod after transfer to 4°C, photosynthesis was inhibited in both genotypes to a similar degree. In Col-0, photosynthesis was approximately constant through the first photoperiod, while fum2 mutants showed a clear suppression of photosynthesis early in the photoperiod, which was alleviated as the day progressed. In both genotypes, the rate of photosynthesis was lower at 4°C than at 20°C. When plants were examined after 7 d at 4°C, a clear difference could be seen between the genotypes. The rate of photosynthesis in Col-0 recovered to the values seen at 20°C. In contrast, in fum2 mutants, no photosynthetic acclimation occurred, with steady-state photosynthesis on day 7 still being lower at 4°C than at 20°C. Measurements of the light response of photosynthesis confirmed that there was no acclimation of photosynthetic capacity in fum2.2 (Fig. 6). Already at 20°C, the fum2.2 mutant had a significantly lower light-saturated rate of photosynthesis, consistent with FUM2 expression playing a role in determining the composition of the photosynthetic apparatus, even under control conditions. Acclimation of photosynthetic capacity did not occur in either fum2 mutants (Supplemental Fig. S5).
Figure 6.
Assimilation rates in cold-treated plants. Plants of Col-0 and fum2.2 were grown under 100 µmol m2 s−1 light, 8 h light/16 h dark, ambient CO2, and 20°C light/18°C dark conditions for 8 weeks. Assimilation was measured using a LiCor LI-6400 gas analyzer fitted with an Arabidopsis long-reach leaf chamber. Assimilation rates were measured through the first (A) and seventh (B) photoperiod in Col-0 plants and the first (C) and seventh (D) photoperiod in fum2.2 plants subject to control (20°C) or low (4°C) temperatures. A to D, Measurements were taken under growth conditions, with growth light and CO2 levels. E and F, Measurements were taken at 20°C, at 2000 µL L−1 CO2.
DISCUSSION
Although responses to low temperature have been studied extensively and some progress has been made toward unraveling the signaling pathways involved, the primary sensing of cold remains uncertain (Ruelland et al., 2009; Knight and Knight, 2012). Candidate cold sensors include Ca2+ channels and membrane rigidity (Knight and Knight, 2012; Schulze et al., 2012). Metabolic signals, although widely recognized to be important in controlling gene expression, for example in the regulation of sugar metabolism and photosynthesis (Smeekens et al., 2010), are often not discussed in the context of cold sensing. This is perhaps surprising given that changes in the absolute and relative concentrations of metabolites occur rapidly in response to cold and that the accumulation of metabolites plays an important role in freezing tolerance (Strand et al., 2003). There is evidence that cytosolic phosphate concentration is involved in low temperature limitation of photosynthesis and also, directly or indirectly, regulates gene expression (Hurry et al., 2000). Data presented here point to a role for metabolic flux to fumarate, or the FUM2 enzyme, acting in sensing cold and triggering metabolic acclimation.
As seen previously (Chia et al., 2000; Pracharoenwattana et al., 2010), we observed a substantial diurnal accumulation of fumarate in Arabidopsis, even under control conditions. Plants lacking FUM2, which show a similar rate of photosynthesis to Col-0 under control conditions (Fig. 6), accumulate more starch (Fig. 2). This could suggest that fumarate acts as a simple sink for fixed carbon, complementing other transient carbon stores. However, the observation that cold leads to a disproportionate increase in fumarate accumulation (Fig. 3) points to a specific role for this at low temperatures. Other conditions increasing accumulation of carbon storage molecules do not result in increased fumarate (Fig. 3). Also, at 20°C, the accumulation of malate in the fum2 mutants approximates the accumulation of fumarate in Col-0. This implies that fumarate is not simply a sink for carbon, rather its accumulation has wider effects of leaf metabolism. In the fum2 mutants, which achieve similar rates of photosynthesis to Col-0 at 20°C, the total measured carbon stores are greater, implying either that the wild type is partitioning more carbon into compounds that we are not measuring or that export from the leaf is greater. We do not see differences in aboveground biomass between these genotypes (B.C. Dyson and G.N. Johnson, unpublished data).
Measurements of intermediates of Suc synthesis (Fig. 4) are consistent with fumarate acting as “safety valve” at low temperature, helping to recycle phosphate when Suc synthesis is impaired. At 20°C, flux to fumarate in fum2 is replaced by malate, keeping the total pool of these acids approximately constant. However, at low temperature there is no further increase in malate, implying that this cannot replace fumarate storage. As a result, sugar phosphates accumulate. Previously it has been suggested that phosphate deficiency provides a signal triggering photosynthetic acclimation to cold (Hurry et al., 2000). In this context, we would expect phosphate to result in a stronger acclimation signal in fum2 than in Col-0. Consistent with this, we do see significantly larger changes in the transcriptome in fum2.2, though notably this includes repression rather than activation of many transcripts relative to Col-0. A substantial number of genes encoding proteins required for photosynthesis are repressed by cold in fum2.2 (Fig. 5), consistent with previous observations that sink limitation of photosynthesis results in repression of photosynthetic genes (Krapp et al., 1993; Van Oosten and Besford, 1996). These changes do not however give rise to corresponding changes in photosynthetic capacity, which is either increased or unaltered at low temperature (Fig. 6), supporting the previous suggestion that expression of photosynthetic proteins is controlled posttranscriptionally (Walters, 2005; Piippo et al., 2006; Athanasiou et al., 2010). We conclude that phosphate availability is not the sensor controlling photosynthetic acclimation to cold. It is responding to cold however and may play a significant role in regulating other responses to the environment.
If fumarate accumulation is not acting simply as a sink for photosynthate, how else might it regulate gene expression? One possibility would be that fum2 activity alters the redox balance of the cell. It is well known that organic acids play a role in shuttling reducing equivalents between cellular compartments, via oxaloacetate-malate shunts (Foyer et al., 1990). Of particular relevance here, this shunt allows the transfer of reductant out of the chloroplast under conditions where NADPH production by photosynthetic electron transport exceeds demand for CO2 fixation. It could be envisaged that this pathway is operating at low temperature. Malate, synthesized by malate dehydrogenase in the chloroplast, may be exported from the chloroplast and converted to fumarate. This would still require a source of carbon skeletons, however, since malate has to be exchanged for oxaloacetate. These presumably still originate from CO2 fixation. Nevertheless, the redox state of different compartments could be altered in this way. We have seen that mutants lacking chloroplastic malate dehydrogenase do still accumulate fumarate and are still able to acclimate to low temperature, speaking against this pathway as being important at low temperature (B.C. Dyson and G.N. Johnson, unpublished data). Nevertheless, fumarase activity may contribute to preventing an overreduced state in the cytosol, so providing the necessary conditions for low temperature acclimation of photosynthesis.
An alternative hypothesis would be that FUM2 itself, either alone or in combination with a metabolite, could act as a cold sensor. There are precedents for enzymes acting as sensors, independent of their metabolic function. For example, hexokinase acts as a Glc sensor and can function even when its catalytic activity is impaired (Moore et al., 2003). Alternatively, the concentrations of fumarate and malate or the balance between the two are candidate signals (Scott et al., 2014). Partitioning of organic acids between cellular compartments (cytosol, mitochondria, vacuole) may also be important (Schulze et al., 2012). Given the specific up-regulation of fumarate accumulation at low temperature, which is not simply associated with the accumulation of fixed carbon, a specific role for this pathway in cold sensing seems possible.
Flux to fumarate or direct effects of cold on FUM2 protein are candidates to operate as primary sensors of cold. We can conclude that there is not however a single, simple signaling pathway for sensing cold. CBF transcription factors and known COR genes still respond to cold in fum2. There is an indication that FUM2- and CBF-dependent pathways interact, given the constitutive up-regulation of CBF3 in the fum2 mutant. If this is the case, flux to FUM2 acts to dampen the CBF response, not activate it.
In conclusion, our results show that metabolic fluxes provide important primary signals of cold that are rapidly induced and readily sensed to control cold acclimation. It is probable that a metabolic step close to FUM2 represents a novel cold sensor that plays an essential role in aspects of cold acclimation.
MATERIALS AND METHODS
Plant Material
Col-0 seeds were obtained from the Nottingham Arabidopsis Stock Centre. Seeds possessing insertions in the gene At5g50950 encoding cytosolic FUM2 protein were kindly provided by Professor Steven Smith (University of Western Australia). Plants were grown in a SGC120 growth cabinet (Weiss Technik) with an 8-h photoperiod, temperature of 20°C day/16°C night, and irradiance of 100 µmol m−2 s−1 provided by warm white fluorescent tubes for 8 weeks in 3-inch pots containing peat-based compost. After 8 weeks, plants had reached growth stage 1.10 (Boyes et al., 2001), with more than 10 leaves > 1 mm and persisted without development of flowers for 10 to 12 weeks. Plants for cold treatment were transferred to a similar cabinet at 4°C day/night 1 h prior to the start of the photoperiod. Plants for high light analysis were transferred to a similar cabinet with 400 µmol m−2 s−1 and for high carbon dioxide analysis were transferred to a similar cabinet set at 1500 µL L−1 CO2. Leaves for metabolite and transcript measurements were harvested by removing leaves from plants under growth conditions and immediately flash freezing in liquid nitrogen.
Enzyme-Linked Assays of Metabolites
Enzymatic assays were carried out on extracts from three fully expanded leaves from three separate plants. Tissue was excised and flash frozen in liquid nitrogen every 2 to 4 h throughout the photoperiod and stored at −80°C before analysis. Starch, Suc, and malate were measured using total starch (Method E), Suc, and malate assay kits (Megazyme), respectively. Malate was measured using a malate assay kit (Megazyme) To measure fumarate, the malate kit was modified to include an extra independent reaction step using 2 units fumarate hydratase enzyme (Sigma). This was added to independent reactions after the malate forming reaction had proceeded to completion. Five biological replicates were assayed at each time point, and each measurement was replicated five times. Technical replicates were averaged and a two-way ANOVA performed on the data (P < 0.05) using SPSS (IBM).
Gas Chromatography Electron Ionization Time-of-Flight Mass Spectrometry Analysis
GC-MS analysis was performed as described previously (Dyson et al., 2015). Briefly, flash-frozen leaves were lyophilized and stored at −80°C. Tissue (30 mg dry weight) was extracted into methanol/water as described previously (Lisec et al., 2006; Allwood et al., 2009). Polar phase extracts were dried and spiked with 100 µL of an internal standard solution containing 200 µg/mL succinic-d4 acid, Gly-d5, and malonic-d2 acid before analysis. Quality control samples were prepared and analyzed as described by Dunn et al. (2011). To enable sample volatility and stability, a two-step derivatization procedure was used (Allwood et al., 2009). Analyses were carried out with a Leco Pegasus III (4D) GC × GC/MS in GC/MS mode (Leco Corporation), with a Gerstel MPS-2 autosampler and an Agilent 6890N gas chromatograph, according to the method of Begley et al. (2009).
Anion-Exchange LC-MS/MS
Mature leaf tissue was harvested as above. Frozen tissue powder was extracted using chloroform-methanol as described by Lunn et al. (2006). Phosphorylated intermediates and organic acids were quantified by high-performance anion-exchange LC-MS/MS, operating in negative ion mode as described by Lunn et al. (2006), with modifications as described by Figueroa et al. (2016). Metabolites were quantified by comparison against authentic standards. Measurements of Tre6P were corrected for ion suppression using a deuterated Tre6P internal standard. Technical replicates were averaged and a two-way ANOVA performed on the data (P < 0.05) using SPSS.
RNA Extraction and Microarray Analysis
RNA was extracted using an RNeasy Plant Mini Kit (Qiagen). cDNA was produced from total RNA, biotinylated, and hybridized to an Arabidopsis (Arabidopsis thaliana) ATH1-121501 oligonucleotide array (Affymetrix) according to the manufacturer’s instructions. The arrays were read using an Agilent GeneArray scanner 3000 7G using Affymetrix GeneChip Operating Software, Version 1.4. dChip software was used for quality control to check for the presence of outliers (Li and Wong, 2001). Expression analysis and normalization were carried out using Robust Multichip Average (Bolstad et al., 2003). PCA was performed using R (https://www.r-project.org/) on log2 scaled data. Differential expression was assessed with a modified t test on logarithmically scaled data using Cyber-T (Baldi and Long, 2001). Since there were three conditions, three Cyber-T tests were performed to cover all three two-way comparisons. Gene lists of differentially expressed genes were generated using criteria of Cyber-T P value less than 0.001, mean fold change greater than 1.5. The gene annotation used was derived from The Arabidopsis Information Resource (http://www.arabidopsis.org/). The Affymetrix chip analysis was performed at the Microarray Facility of the Faculty of Life Sciences at the University of Manchester (Manchester, UK).
Gas-Exchange Measurements
For measurements of photosynthesis under growth conditions, a LiCor LI-6400 gas analyzer, fitted with an Arabidopsis long-reach leaf chamber, was placed in the cabinet and allowed to equilibrate with cabinet conditions. Photosynthesis was monitored over the photoperiod in three separate plants for each treatment using cabinet air and light. For measurements of photosynthetic capacity, plants were removed from the growth cabinet and allowed to equilibrate in lab conditions for at least 1 h. Leaves were clamped into the LI-6400 leaf chamber and illuminated for 30 min at an irradiance of 2000 µmol m−2 s−1 provided by a warm white LED (color temperature 2800–3200 K). The CO2 concentration was maintained at 2000 µL L−1.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. PCA of gas chromatography electron ionization time-of-flight mass spectrometry (GC-EI-TOF/MS) data from leaf material of fum2.2 at 20°C or 4°C.
Supplemental Figure S2. Diurnal metabolite levels under low temperature conditions in fum2.1.
Supplemental Figure S3. Starch levels determined by enzyme-linked assays under different environmental conditions.
Supplemental Figure S4. Cluster analysis of changes in gene expression during acclimation to low temperature in the wild type and fum2.
Supplemental Figure S5. Photosynthetic capacity in plants of Col-0, fum2.1, and fum2.2 grown at 20°C for 8 weeks and in plants then acclimated to 4°C for 7 d.
Supplemental Table S1. Metabolite levels were obtained using GC-EI-TOF/MS on tissue from plants under low temperature conditions.
Supplemental Table S2. Metabolite levels were obtained using LC-MS/MS on tissue from plants under acclimation conditions.
Supplemental Table S3. Microarray analysis of plants grown for 8 weeks at 20°C day/18°C night.
Supplementary Material
Acknowledgments
We thank Leo Zeef and Andrew Hayes (Faculty of Life Sciences, University of Manchester) for their help with the microarray analysis.
Glossary
- PCA
principal component analysis
- T6P
trehalose 6-phosphate
Footnotes
This work was supported by a grant from the UK Biotechnology and Biological Sciences Research Council (BB/J04103/1).
Articles can be viewed without a subscription.
References
- Allwood JW, Erban A, de Koning S, Dunn WB, Luedemann A, Lommen A, Kay L, Löscher R, Kopka J, Goodacre R (2009) Inter-laboratory reproducibility of fast gas chromatography-electron impact-time of flight mass spectrometry (GC-EI-TOF/MS) based plant metabolomics. Metabolomics 5: 479–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou K, Dyson BC, Webster RE, Johnson GN (2010) Dynamic acclimation of photosynthesis increases plant fitness in changing environments. Plant Physiol 152: 366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi P, Long AD (2001) A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics 17: 509–519 [DOI] [PubMed] [Google Scholar]
- Begley P, Francis-McIntyre S, Dunn WB, Broadhurst DI, Halsall A, Tseng A, Knowles J, Goodacre R, Kell DB; HUSERMET Consortium (2009) Development and performance of a gas chromatography-time-of-flight mass spectrometry analysis for large-scale nontargeted metabolomic studies of human serum. Anal Chem 81: 7038–7046 [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193 [DOI] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J (2001) Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia DW, Yoder TJ, Reiter WD, Gibson SI (2000) Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211: 743–751 [DOI] [PubMed] [Google Scholar]
- Cook D, Fowler S, Fiehn O, Thomashow MF (2004) A prominent role for the CBF cold response pathway in configuring the low-temperature metabolome of Arabidopsis. Proc Natl Acad Sci USA 101: 15243–15248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WB, Broadhurst D, Begley P, Zelena E, Francis-McIntyre S, Anderson N, Brown M, Knowles JD, Halsall A, Haselden JN, et al. ; Human Serum Metabolome (HUSERMET) Consortium (2011) Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc 6: 1060–1083 [DOI] [PubMed] [Google Scholar]
- Dyson BC, Allwood JW, Feil R, Xu Y, Miller M, Bowsher CG, Goodacre R, Lunn JE, Johnson GN (2015) Acclimation of metabolism to light in Arabidopsis thaliana: the glucose 6-phosphate/phosphate translocator GPT2 directs metabolic acclimation. Plant Cell Environ 38: 1404–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa CM, Feil R, Ishihara H, Watanabe M, Kölling K, Krause U, Höhne M, Encke B, Plaxton WC, Zeeman SC, et al. (2016) Trehalose 6-phosphate coordinates organic and amino acid metabolism with carbon availability. Plant J 85: 410–423 [DOI] [PubMed] [Google Scholar]
- Foyer C, Furbank R, Harbinson J, Horton P (1990) The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynth Res 25: 83–100 [DOI] [PubMed] [Google Scholar]
- Hannah MA, Heyer AG, Hincha DK (2005) A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet 1: e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57 [DOI] [PubMed] [Google Scholar]
- Hurry V, Strand A, Furbank R, Stitt M (2000) The role of inorganic phosphate in the development of freezing tolerance and the acclimatization of photosynthesis to low temperature is revealed by the pho mutants of Arabidopsis thaliana. Plant J 24: 383–396 [DOI] [PubMed] [Google Scholar]
- Knight MR, Knight H (2012) Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol 195: 737–751 [DOI] [PubMed] [Google Scholar]
- Krapp A, Hofmann B, Schäfer C, Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the ‘sink regulation’ of photosynthesis? Plant J 3: 817–828 [Google Scholar]
- Li C, Wong WH (2001) Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci USA 98: 31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1: 387–396 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M (2006) Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana. Biochem J 397: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Penfield S, MacGregor D (2014) Temperature sensing in plants. In Franklin KA, Wigge PA, eds, Temperature and Plant Development. John Wiley and Sons, London, pp 1–18 [Google Scholar]
- Piippo M, Allahverdiyeva Y, Paakkarinen V, Suoranta UM, Battchikova N, Aro EM (2006) Chloroplast-mediated regulation of nuclear genes in Arabidopsis thaliana in the absence of light stress. Physiol Genomics 25: 142–152 [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana I, Zhou W, Keech O, Francisco PB, Udomchalothorn T, Tschoep H, Stitt M, Gibon Y, Smith SM (2010) Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. Plant J 62: 785–795 [DOI] [PubMed] [Google Scholar]
- Ruelland E, Vaultier M-N, Zachowski A, Hurry V (2009) Cold signalling and cold acclimation in plants. In Kader JC, Delseny M, eds, Advances in Botanical Research, Vol 49 Academic Press, Cambridge, MA, pp 35–150 [Google Scholar]
- Savitch LV, Barker-Astrom J, Ivanov AG, Hurry V, Oquist G, Huner NPA, Gardeström P (2001) Cold acclimation of Arabidopsis thaliana results in incomplete recovery of photosynthetic capacity, associated with an increased reduction of the chloroplast stroma. Planta 214: 295–303 [DOI] [PubMed] [Google Scholar]
- Schulze WX, Schneider T, Starck S, Martinoia E, Trentmann O (2012) Cold acclimation induces changes in Arabidopsis tonoplast protein abundance and activity and alters phosphorylation of tonoplast monosaccharide transporters. Plant J 69: 529–541 [DOI] [PubMed] [Google Scholar]
- Scott IM, Ward JL, Miller SJ, Beale MH (2014) Opposite variations in fumarate and malate dominate metabolic phenotypes of Arabidopsis salicylate mutants with abnormal biomass under chilling. Physiol Plant 152: 660–674 [DOI] [PubMed] [Google Scholar]
- Sengupta P, Garrity P (2013) Sensing temperature. Curr Biol 23: R304–R307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13: 274–279 [DOI] [PubMed] [Google Scholar]
- Stitt M, Hurry V (2002) A plant for all seasons: alterations in photosynthetic carbon metabolism during cold acclimation in Arabidopsis. Curr Opin Plant Biol 5: 199–206 [DOI] [PubMed] [Google Scholar]
- Strand A, Foyer CH, Gustafsson P, Gardestrom P, Hurry V (2003) Altering flux through the sucrose biosynthesis pathway in transgenic Arabidopsis thaliana modifies photosynthetic acclimation at low temperatures and the development of freezing tolerance. Plant Cell Environ 26: 523–535 [Google Scholar]
- Strand A, Hurry V, Henkes S, Huner N, Gustafsson P, Gardeström P, Stitt M (1999) Acclimation of Arabidopsis leaves developing at low temperatures. Increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol 119: 1387–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oosten J-J, Besford RT (1996) Acclimation of photosynthesis to elevated CO2 through feedback regulation of gene expression: Climate of opinion. Photosynth Res 48: 353–365 [DOI] [PubMed] [Google Scholar]
- Walters RG. (2005) Towards an understanding of photosynthetic acclimation. J Exp Bot 56: 435–447 [DOI] [PubMed] [Google Scholar]
- Zhu X-G, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61: 235–261 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.