Loss of plant PsaI Photosystem I subunit destabilizes the binding of PsaL and PsaH subunits of this photosystem and causes nonphotochemical dark-reduction of the plastoquinone pool keeping plants in state 2 in the dark.
Abstract
PsaI represents one of three low molecular weight peptides of PSI. Targeted inactivation of the plastid PsaI gene in Nicotiana tabacum has no measurable effect on photosynthetic electron transport around PSI or on accumulation of proteins involved in photosynthesis. Instead, the lack of PsaI destabilizes the association of PsaL and PsaH to PSI, both forming the light-harvesting complex (LHC)II docking site of PSI. These alterations at the LHCII binding site surprisingly did not prevent state transition but led to an increased incidence of PSI-LHCII complexes, coinciding with an elevated phosphorylation level of the LHCII under normal growth light conditions. Remarkably, LHCII was rapidly phosphorylated in ΔpsaI in darkness even after illumination with far-red light. We found that this dark phosphorylation also occurs in previously described mutants impaired in PSI function or state transition. A prompt shift of the plastoquinone (PQ) pool into a more reduced redox state in the dark caused an enhanced LHCII phosphorylation in ΔpsaI. Since the redox status of the PQ pool is functionally connected to a series of physiological, biochemical, and gene expression reactions, we propose that the shift of mutant plants into state 2 in darkness represents a compensatory and/or protective metabolic mechanism. This involves an increased reduction and/or reduced oxidation of the PQ pool, presumably to sustain a balanced excitation of both photosystems upon the onset of light.
PSI is indisputably the most efficient solar energy converter with a potential quantum efficiency close to 100% (Nelson and Yocum, 2006; Nelson, 2009). It mediates the oxidation of plastocyanin and, subsequently, the reduction of ferredoxin resulting in the production of NADPH. The connection between PSII and PSI in the linear electron transport is provided by the cytochrome b6f complex. The electrochemical proton gradient build-up during the light-driven electron transport across the thylakoid membrane provides the proton motive force used for the conversion of ADP to ATP. Besides the linear electron transport, which leads to the production of both ATP and NADPH, PSI is also able to perform cyclic electron transport without the involvement of PSII and resulting solely in the generation of ATP (Yamori and Shikanai, 2016). Two routes of cyclic electron transport exist, the PROTON GRADIENT REGULATION (PGR)5/PGRL1- and the NAD(P)H dehydrogenase (NDH)-dependent pathway providing the possibility to adjust the ATP/NADPH ratio to handle changing metabolic demands and to prevent especially PSI from photoinhibition under challenging light regimes (Munekage et al., 2002; Kramer et al., 2004; Joliot and Johnson, 2011; Suorsa et al., 2012).
Although the catalytic core and the function of PSI in cyanobacteria and plants remained very similar since the endosymbiotic event, its structural organization strikingly changed during plant evolution (Amunts and Nelson, 2008, 2009). The size of the entire plant PSI complex increased compared to the cyanobacterial PSI (Jordan et al., 2001; Amunts et al., 2010; Qin et al., 2015). Moreover, plant PSI gained four unique subunits (PsaG, PsaH, PsaN, and PsaO) while it lost the cyanobacterial subunits PsaM and PsaX (Amunts and Nelson, 2009). In cyanobacteria, phycobilisomes serve as an additional antenna system for the cyanobacterial photosynthetic apparatus while plants have developed highly sophisticated and stably bound light harvesting complexes (Nelson and Yocum, 2006). According to the high-resolution structures, the most fundamental difference is the fact that the cyanobacterial PSI mainly functions as a trimer and, in some species, also as a tetramer, whereas plants contain only monomeric PSI (Watanabe et al., 2011; Nelson and Junge, 2015). The subunits PsaL, PsaI, and PsaM build up the cyanobacterial trimer connecting domain of PSI (Chitnis and Chitnis, 1993; Naithani et al., 2000), whereby the three transmembrane helices of PsaL provide most of the contact sites between the individual PSI monomers (Grotjohann and Fromme, 2005). The small subunit PsaI resides between PsaL and PsaM in the trimer-connecting domain and contributes to the trimerization of PSI mainly by stabilizing the binding of PsaL to the complex (Nakamoto, 1995; Xu et al., 1995; Schluchter et al., 1996; Grotjohann and Fromme, 2005). During evolution, the protein composition and structure of this PSI region has been remodeled completely and gained an entirely new function in green algae and land plants: the binding of the mobile light-harvesting complex (LHC)II trimer in the state transition process (Jansson et al., 1996; Amunts and Nelson, 2008; Amunts et al., 2010; Schöttler et al., 2011; Qin et al., 2015).
For optimal performance of the photosynthetic machinery plants possess highly flexible short-term mechanisms, the so called nonphotochemical quenching (NPQ) of excited states to readjust and fine-tune the balanced excitation of both photosystems in case of fluctuating light qualities and quantities (for review, see Rochaix et al., 2012; Tikkanen and Aro, 2014; Goldschmidt-Clermont and Bassi, 2015). The energy-dependent quenching component of NPQ leads to the dissipation of excess light energy in form of heat to prevent photoinhibition (Horton, 2012; Ruban et al., 2012; Tikkanen and Aro, 2012). State transition (qT) represents the second component of NPQ and enables plants to balance the antenna cross-sections of PSI and PSII in a phosphorylation-dependent manner predominantly under low light conditions (Bonaventura and Myers, 1969; Murata, 1969; Allen et al., 1981; Goldschmidt-Clermont and Bassi, 2015). Preferential excitation of PSII (state 2) results in a more reduced redox state of the PQ pool, leading to the activation of the STN7/STT7 kinase, which phosphorylates the mobile component of LHCII (Depège et al., 2003; Bonardi et al., 2005). According to the traditional state transition theory, the phosphorylated LHCII trimer being composed of LHCb1 and LHCb2 detaches from PSII in the grana and travels to the stroma lamellae where it attaches to PSI thereby, increasing its antenna cross-section (Allen et al., 1981; Larsson et al., 1983; Kyle et al., 1984; Bellafiore et al., 2005). In contrast, state 1 is induced by light conditions that favor PSI excitation (e.g. enrichment in far-red light) and results in an oxidized state of the PQ pool leading to the inactivation of the STN7 kinase, subsequent dephosphorylation of the mobile LHCII by TAP38/PPHI, and consequently, to its remigration and reattachment to PSII in the grana region (Pribil et al., 2010; Shapiguzov et al., 2010).
Nowadays it is assumed that changing light conditions leads to the activation of a highly sophisticated network including state transition, NPQ, PGR5/PGRL1-dependent cyclic electron transport, and phosphorylation of PSII (Tikkanen and Aro, 2012; Rochaix, 2014; Allahverdiyeva et al., 2015; Goldschmidt-Clermont and Bassi, 2015). Moreover, it was shown that LHCII phosphorylation results in an accumulation of P-LHCII-PSII complexes and PSI-LHCI in the “transition zone” where grana and stroma lamellae merge, allowing energy distribution via LHCII between both photosystems residing in the same energetically connected LHCII lake (Tikkanen et al., 2008; Tikkanen et al., 2010; Grieco et al., 2015).
The 4 kD low molecular weight (LMW) PSI subunit PsaI, which is closely located to the LHCII binding domain, is predicted to be composed of a single α-helical transmembrane domain flanked by a few hydrophilic N- and C-terminal amino acids (Supplemental Fig. S1). The PsaI polypeptide sequence is conserved from cyanobacteria to higher plants whereby the hydrophobic domain displays the highest identity indicating that both short extremities exposed to the membrane surface do not represent main functional parts of the protein. In Synechocystis sp. PCC 6803, PsaI is co-transcribed with the upstream-located PsaL gene. During evolution, psaL was transferred into the nuclear genome of plants, whereas the three LMW subunits of PSI PsaC, PsaI, and PsaJ remained encoded in the plastid genome in most plant species. The only exception so far known is provided by some green algae, including Chlamydomonas reinhardtii, where psaI was lost from the chloroplast genome and was integrated as well into the nuclear genome. Interestingly, it also appears that the PsaI gene is absent in the nuclear and the plastid genome of several Lathyrus species (Magee et al., 2010). Besides of these exceptions, the PsaI gene resides in the plastid RbcL-AccD-PsaI-Ycf4-CemA-PetA gene cluster in vascular plants (Walter et al., 2010; Stoppel et al., 2012). The role of PsaI in plants is not known so far. In cyanobacteria, PsaI is important for the structural organization of PsaL in the complex and for the formation of PSI trimers (Nakamoto, 1995; Xu et al., 1995; Schluchter et al., 1996). However, the conclusions drawn from the function of cyanobacterial PsaI may not be transferable to its role in higher plants due to the drastic functional and structural remodeling of the “trimer connecting domain” of PSI into the “LHCII docking site.” In this study, we have inactivated PsaI in tobacco and discovered that it primarily functions in the stable association of PsaL and PsaH to PSI. The absence of PsaI led to a general reversible shift of the plants into state 2 accompanied by increased phosphorylation levels of the LHCII under normal growth-light conditions. This happens as a consequence of a more reduced PQ pool, presumably due to impaired excitation energy transfer from the attached LHCII to PSI in the light. Surprisingly, strong phosphorylation of the LHCII could be observed in darkness even after extended far-red light treatment caused by a fast nonphotochemical reduction of the PQ pool, pointing to a preventive mechanism in the dark to achieve excitation balance between the two photosystems and/or to avoid their photoinhibition when light returns or under fluctuating light conditions (Grieco et al., 2012; Horton 2012).
RESULTS
Targeted Inactivation of the Plastid PsaI Gene in Tobacco
In order to determine the function of the plastid encoded PsaI protein in plants, transplastomic ΔpsaI knockout plants were generated by transformation of chloroplasts of Nicotiana tabacum cv Petit Havanna. A terminator less amino glycoside 3′ adenyl transferase (aadA) resistance cassette was introduced into the middle part of the PsaI gene in the same transcriptional orientation of the RbcL-AccD-PsaI-Ycf4-CemA-PetA gene cluster to avoid lack of expression of the downstream located genes (Fig. 1A). Seven independently generated ΔpsaI knockout lines, which showed essentially the same phenotype, were regenerated. The homoplastomic state of two selected lines was confirmed by an inheritance assay after being backcrossed with the wild type for five times to eliminate possible interfering somaclonal variations (Supplemental Fig. S2). Wild-type tobacco plants appeared pale when germinated on selection medium. Tobacco lines carrying the aadA selection cassette in a neutral insertion site of the plastome were used in this work as wild-type control plants (Umate et al., 2008). These wild-type control plants and the two independent ΔpsaI mutants showed uniform resistance to spectinomycin and streptomycin (Supplemental Fig. S2).
Figure 1.
Targeted inactivation of the PsaI gene by plastid transformation. A, Physical map of the tobacco RbcL/PetA operon. An aadA selection cassette was inserted into the naturally occurring StuI site of the PsaI gene using the transformation vector pUC19 leading to an interruption of the gene after nucleotide 45. P, 16S rRNA promoter (P); R, ribosome binding site. B, Homoplastomy of the generated ΔpsaI plants and the expression of the genes RbcL, AccD, PsaI, Ycf4, CemA, and PetA were investigated by RNA gel blot analysis using strand-specific probes. The approximate size of transcripts in kb is indicated on the left side. Ten micrograms of total leaf RNA were loaded. MBB, Methylene blue staining of the RNA gel blots (lower part). C, Wild type without an aadA cassette (WT–), wild type carrying an aadA in a neutral insertion side (WT+), and two homoplastomic mutants devoid of PsaI (ΔpsaI-1 and ΔpsaI-2) grown in the climate chamber for seven weeks.
Sequencing of the flanking regions of the aadA cassette confirmed insertion into the PsaI gene. Furthermore, RNA gel blot analysis using a PsaI-specific probe was performed to prove homoplastomy of the ΔpsaI-1 knock out (Fig. 1B). As expected, the monocistronic psaI wild-type transcript of about 0.1 kb was completely absent in the mutant, confirming the insertion of the selection cassette into the PsaI gene and the homoplastomic state of the mutants. Several higher molecular weight bands between 1.0 and 5.0 kb appeared in ΔpsaI-1 due to the insertion of the selection cassette and the activity of its 16S rRNA promoter, which drives expression of the aadA gene. In order to estimate whether transcripts of the genes located in the same operon are negatively affected due to the insertion of the aadA cassette, RNA gel blots were further probed with respective strand-specific probes (Fig. 1B). The expression of the upstream located genes RbcL and AccD remained unchanged in the ΔpsaI-1 mutant, whereas the transcripts of the downstream located genes Ycf4, CemA, and PetA were shifted and appeared in higher amounts in the mutant as compared to the wild type. This can be explained by read-through transcription from the upstream inserted aadA cassette and displays a commonly observed effect in many similar generated knockout mutants (Zoubenko et al., 1994; Krech et al., 2012; Torabi et al., 2014b).
Growth and Photosynthesis of ΔpsaI Tobacco Plants Are Slightly Affected
The homoplastomic ΔpsaI mutants were able to grow photoautotrophically and did not differ significantly in their chlorophyll content (Table I), but exhibited a subtle reduced growth compared to wild-type plants when grown on soil (Fig. 1C). To monitor the effects of the mutation on the photosynthetic performance, in vivo chlorophyll a fluorescence measurements were performed after dark incubation using a pulse amplitude-modulated fluorometer. The Fo level was increased to 0.073 ± 0.004 in the mutant as compared to 0.059 ± 0.008 in the wild type, whereas the maximum quantum yield of PSII (estimated by Fv/Fm) was slightly decreased to 0.79 ± 0.01 versus 0.82 ± 0.01 in the wild-type (Table I). Decreased values were likewise recorded for the effective quantum yield of PSII in ΔpsaI-1 (Table I). Spectroscopic measurements of energy conversion in PSI upon increasing and decreasing light intensities revealed no obvious differences in yield (ΦPSI) or donor side (ΦND) of PSI caused by the lack of PsaI (Supplemental Fig. S3). Photosynthetic electron transport rates and PSI activity of freshly broken chloroplasts measured with a Clark electrode were not altered as well in the mutant (Table I). Furthermore, NADP+ reduction based on equal chlorophyll amount was comparable between WT and ΔpsaI-1 mutant (Table I). This all indicates that the PSI-dependent electron transport rate is not impaired in ΔpsaI-1.
Table I. Photosynthetic parameters of the wild type and ΔpsaI.
| Parameter | Wild Type (n ≥ 5)a | ΔpsaI-1 (n ≥ 5) |
|---|---|---|
| Chlorophyll a+bb | 0.42 ± 0.04 | 0.41 ± 0.06 |
| Foc(dark incubation)d | 0.059 ± 0.008 | 0.073 ± 0.004 |
| ΦPSIIe (dark incubation) | 0.77 ± 0.01 | 0.75 ± 0.01 |
| Fv/Fmf (dark incubation) | 0.82 ± 0.01 | 0.79 ± 0.01 |
| Fv/Fm (far-red incubation)g | 0.81 ± 0.01 | 0.81 ± 0.01 |
| qTh | 0.17 ± 0.02 | 0.17 ± 0.02 |
| t1/2 P700 redi | 1.45 ± 0.17 | 0.99 ± 0.18 |
| Postillumination fluorescence risej | 1.00 ± 0.35 | 2.59 ± 0.76 |
| PSI activityk | 127.3 ± 14.0 | 129.9 ± 17.9 |
| Linear electron transport rate (H2O→MV)l | 144.1 ± 3.2 | 145.8 ± 2.5 |
| NADP+ photoreduction ratem | 0.240 ± 0.027 | 0.234 ± 0.008 |
Number of replicates.
µg Chlorophyll × mm−2.
Dark fluorescence yield.
Plants were dark-adapted for 15 min prior to measurement.
Effective quantum yield of PSII.
Fv/Fm, Maximum quantum yield of PSII.
Plants were adapted to far-red light for 1 h prior to Fv/Fm measurement.
State transition coefficient.
Half-time of nonphotochemical P700 re-reduction in the dark (s).
Nomalized to the wild type, s−1.
Measured as electron flow from N,N,N’,N’-tetramethyl-p-phenylenediamine to methylviologen, µmol O2 mg chlorophyll−1 × h−1.
Measured as electron flow from water to methylviologen, µmol O2 mg chlorophyll−1 × h−1.
mmol NADP+ × h−1 × mg chlorophyll−1.
Impaired Association of PsaL and PsaH to the PSI Complex in ΔpsaI
Thylakoids of mutant and wild-type plants were used for immunological analysis in order to see whether protein levels correlate with the described deficiencies in growth and conspicuities in photosynthesis. Notably, we could not identify any significant changes in the accumulation of proteins of PSI (PsaA, PsaC, PsaL, PsaH, and PsaF), PSII (D1, D2, CP47, PsbO, LHCb1, LHCb2, and LHCb5), the ATP synthase (AtpA/B), the cytochrome b6f complex (cytochrome f, cytochrome b6, and SU IV) or the NADPH-dependent dehydrogenase (NdhH and NdhJ; Fig. 2). Unchanged levels of the cytochrome b6f complex also demonstrates that up-regulation of genes downstream of PsaI, such as PetA (encoding for cytochrome f), had no effect on the accumulation of cytochrome f and other proteins that constitute the same complex, indicating that protein amounts were post-translationally adjusted.
Figure 2.
Accumulation of thylakoid membrane proteins. Immunological analyses of wild-type and ΔpsaI-1 thylakoid membranes were performed using antibodies against representative PSI, PSII, Cytochrome b6f, ATP synthase, and NAD(P)H dehydrogenase (NDH) proteins. Thylakoid membrane proteins corresponding to 5 and 2.5 µg chlorophyll (1 and 1/2, respectively) were loaded. CBB, Coomassie Brilliant Blue staining of the polyvinylidene difluoride (PVDF) membrane.
To address the question whether the protein subunits are properly assembled into the photosynthetic complexes, thylakoids of wild-type and ΔpsaI plants were solubilized with different detergents and subjected to large pore blue native (lpBN)-PAGE (Fig. 3A), which allows a high resolution of large protein complexes, i.e. the megacomplexes (Järvi et al., 2011). The four most prominent chlorophyll-containing supercomplexes of n-dodecyl-β-d-maltoside (β-DM) solubilized thylakoids contained PSII and LHCII proteins and were not altered in abundance in ΔpsaI-1 and ΔpsaI-2 (Fig. 3A; Supplemental Fig. S4, A and B). However, two major differences concerning the integrity of photosynthetic complexes could be determined when β-DM was used for thylakoid solubilization. First, one of the megacomplexes was below limit of detection in the ΔpsaI-1 and ΔpsaI-2 mutants (Fig. 3A, labeled with a triangle, and Supplemental Fig. S4A). This unknown megacomplex is lacking PSII, LHCII, and NDH but contained PSI including its antenna proteins (Supplemental Fig. S4B). It was enriched in the stroma lamellae and the intermittent fraction while only minute amounts could be observed in the grana lamellae after fractionation of thylakoids (Supplemental Fig. S4C). This confirms that the complex represents a PSI megacomplex, which is sterically excluded from the tightly packed grana stacks (Fig. 3).
Figure 3.
Analysis of photosynthetic complex integrity by lpBN-PAGE using different detergents. A, Native thylakoid membrane complexes of wild-type and ΔpsaI-1 were solubilized using 1% β-DM, 1% Triton X-100, or 1.5% digitonin and subsequently separated by lpBN-PAGE. The autofluorescence of the complexes in the gel was imaged using a Typhoon laser scanner. Prominent protein complexes solubilized with β-DM or digitonin are indicated on the left and on the right, respectively. B, Thylakoid membrane complexes solubilized with β-DM and subjected to BN-PAGE (top part) were subsequently separated by SDS-PAGE, transferred to a PVDF membrane, and probed with antisera against PsaD, PsaL, and PsaH, consecutively (bottom part). Six-week-old wild-type and ΔpsaI-1 plants were dark-incubated for 16 h and subsequently transferred to growth light for 30 min before thylakoid isolation. Native thylakoid membrane complexes corresponding to 30 µg chlorophyll were used for solubilization. The mutant PSI monomer complex lacking PsaH, PsaL, and PsaI proteins is labeled with an arrow and the megacomplex missing in ΔpsaI-1 is indicated with a triangle. PSI-M, PSI monomer; PSII-D, PSII dimer; LHCII-T, LHCII trimer.
Second, the lpBN gel of β-DM solubilized thylakoid membrane complexes in ΔpsaI mutants also revealed a highly abundant smaller protein complex (Fig. 3; Supplemental Fig. S4A, labeled with an arrow), which was not present in the wild type but migrated just below the PSI monomer/PSII dimer complexes (Fig. 3A; Supplemental Fig. S4A). Its enrichment in the stroma lamellae and the intermittent fraction (Supplemental Fig. S4C), as well as silver staining of the complex subunits separated in the second dimension indicate that this lower migrating additional mutant band represents an incomplete PSI complex in ΔpsaI mutants (Supplemental Fig. S4D). To reveal the precise composition of this complex, immunological analysis was performed using specific antisera raised against PsaD, PsaL, and PsaH (Fig. 3B). Our data clearly revealed that the additional complex in the ΔpsaI mutants represents photosystem I, which comprises PsaD but misses the subunits PsaL and PsaH (Fig. 3B, lower part). Notably, PsaL and PsaH were predominantly bound to the prominent monomeric PSI complex in the wild type, whereas the majority of these two proteins were migrating in the free protein fraction in ΔpsaI-1 (Fig. 3B, lower part). In sharp contrast to ΔpsaI, the proteins PsaH and PsaL were missing in the free protein fraction in wild-type thylakoids (Fig. 3B).
Solubilization of the thylakoid membrane complexes with Triton X-100 led to a different pattern of the thylakoid protein complexes as compared to the solubilization with β-DM in both the wild type and the mutant. However, the described shift to lower molecular weight of the PSI complex, which arises after treating with β-DM, was even reinforced when Triton X-100 was used as a detergent in ΔpsaI-1 (Fig. 3A). It appeared that the entire PSI monomer band was shifted toward lower molecular weight in ΔpsaI-1. Otherwise, no differences were observed between wild-type and ΔpsaI-1 mutants. In order to investigate whether PsaH and PsaL are associated to PSI in the mutant in vivo but easily dissociate from the complex during solubilization, we used digitonin, which represents a milder detergent than Triton X-100 and β-DM. Indeed, no obvious differences in the complex pattern between wild type and ΔpsaI-1 were visible (Fig. 3A). This indicates that the detachment of PsaH and PsaL from PSI complexes represents a solubilization effect caused by a less stable connectivity of PsaH and PsaL to the PSI complex in the absence of PsaI. A similar shift of the PSI monomer complex to a lower molecular weight could also be observed in Arabidopsis (Arabidopsis thaliana) mutants lacking PsaL (Pesaresi et al., 2009). This confirms that an affected LHCII docking site caused a faster migration of PSI in BN-PAGE analysis. Interestingly, it also appeared that in contrast to the wild type, the ΔpsaI-1 mutant accumulated higher levels of LHCII trimers, which are not tightly bound to PSII and PSI complexes (therefore also called extra LHCII; Fig. 3A). This extra LHCII is supposed to be connected to photosystems in vivo and forms an energetically connected antenna lake serving as shared antenna for both photosystems (Grieco et al., 2015). Recently, it could be shown that functional megacomplexes containing both PSI and PSII exist in Arabidopsis (Järvi et al., 2011; Grieco et al., 2015; Yokono et al., 2015), and our results indicate that they obviously also occur in Nicotiana tabacum as visible in thylakoids treated with digitonin (Fig. 3A).
Impaired Stability of PsaL and PsaH in the LHCII Docking Site Impacts State Transition
Localization of PsaI close to the LHCII docking site of PSI and its apparent role in the stable association of PsaH and PsaL raised the question whether the lack of PsaI changes the state transition process. Commonly, the state transitions are measured by exposing dark-incubated leaves successively for a certain time span to state 1 and state 2 favoring light conditions while monitoring the changes in chlorophyll fluorescence yield caused by the reversible dissociation of the mobile LHCII from PSII. The qT value given by qT = (Fm1−Fm2)/Fm2 is a measure for the functionality of state transition (Lunde et al., 2000). In order to monitor state transition noninvasively and to record qT, leaves of wild type and ΔpsaI-1 were dark-adapted for 15 min before illumination with blue light for 20 min to determine the maximum fluorescence in state 2 (Fm2). Subsequently, Fm1 was recorded after illumination with actinic light supplemented with far-red light for another 20 min. Consequently, a qT value of 0.170 ± 0.02 could be calculated for both wild type and ΔpsaI-1 (Table I), indicating that the state transition process can be induced by changes in light quality even though PsaL and PsaH are only loosely bound in the absence of PsaI.
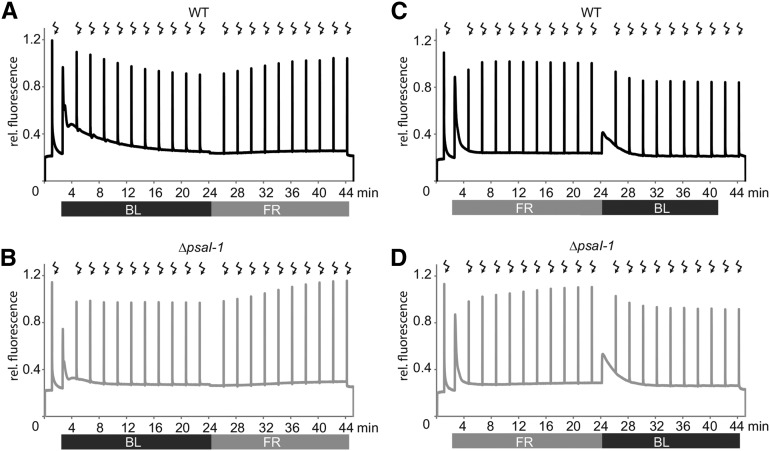
However, when investigating the state transition kinetics in more detail by applying saturating light pulses every 2 min during the light treatments, striking differences in the chlorophyll fluorescence curves of wild type and ΔpsaI-1 were noticed (Fig. 4, A and B). The maximum fluorescence (Fm’) during induction of state 2 was characterized in the wild type by a typical time-dependent decrease presumably caused by enhanced excitation of PSII by blue light and consequent detachment of LHCII from PSII (Fig. 4A). Strikingly, the maximum fluorescence remained at a more or less constant level during blue light illumination in ΔpsaI-1, suggesting that the mobile LHCII antenna either does not detach from PSII or was already detached at the starting point of the measurement (Fig. 4B). However, after the onset of PSI favoring light, both genotypes displayed the same time-dependent increase of the maximum fluorescence levels (Fig. 4, A and B), showing that the reattachment of LHCII to PSII is not impaired in ΔpsaI-1 and suggesting that the mobile LHCII tends to be linked to PSI in the dark in the mutant, in contrast to the tobacco wild type where it is usually attached to PSII in the absence of light (Umate et al., 2008). To prove this hypothesis, the described state transition measurements were performed exactly the other way round. Leaves were first illuminated with PSI favoring light after dark-incubation followed by blue light illumination (Fig. 4, C and D). As expected, the maximum fluorescence stayed at a constant level during treatment with far-red supplemented light in the wild type (Fig. 4C), as the mobile LHCII trimer is already connected to PSII after dark incubation. In contrast, the course of Fm’ was characterized by a constant increase of the maximum fluorescence during far-red light treatment in the mutant (Fig. 4D). In the second phase of the measurement, after the onset of blue light, both genotypes exhibited a comparable decrease in Fm’ (Fig. 4, C and D). In summary, these experiments provide evidence that state transition can still be performed by using different light qualities in the absence of PsaI; however, unlike the wild type, the mutant plants are obviously forced into state 2 even in the absence of light.
Figure 4.
Chlorophyll a fluorescence kinetics of state transition. A and B, State 2 was induced by illuminating dark-incubated leaves (15 min) of wild type (A) and ΔpsaI-1 (B) with actinic blue light (BL) for 20 min. Afterward, state 1 was initiated by illumination with actinic light supplemented with far-red (FR) light. C and D, Dark-incubated leaves (15 min) of wild type (C) and ΔpsaI-1 (D) were treated with actinic light supplemented with far-red light (FR) for 20 min followed by induction of state 2 by blue light (BL) illumination for another 20 min. After dark-incubation, Fv/Fm value was determined before actinic light was switched on and saturating light pulses (flashes) were applied every 2 min to follow the kinetics of Fm' during actinic light treatment. The duration of light treatment of the leaves with blue light or far-red light is displayed with a black or gray bar, respectively. Representative curves of single measurements are depicted.
Light-Dark Transition Rapidly Induced State 2 in ΔpsaI Mutants
In order to get further insight into the unexpected and unusual processes happening in the dark in the mutant, which obviously differ from the situation in the wild type, further chlorophyll a fluorescence measurements were performed (Fig. 5A). This time, dark-adapted wild type and mutant plants were first illuminated with far-red supplemented light for 40 min in order to fully oxidize the PQ pool in the plants and to reach state 1. This light was superimposed by saturating light pulses in a time interval of 2 min. Afterward, the actinic light was turned off for 30 min before far-red supplemented light was again applied for another 20 min. Fm’ values were recorded during the two light periods from wild-type and ΔpsaI-1 plants (Fig. 5A). In the wild type, there was not much difference between the Fm’ value recorded after 40 min of the first far-red light period and the Fm’ values during the second far-red light period after dark incubation (Fig. 5A). This indicates that wild-type plants remained fixed in state 1 in the dark after far-red light treatment. In sharp contrast to that, in the mutant there was a vast difference between the Fm’ value at the endpoint of the far-red illumination and at the starting point of the second far-red light period (Fig. 5A). Furthermore, the Fm’ curve in the second light period displayed the same increase over time as in the first far-red light period in the mutant, whereas the Fm’ in the wild type stayed at a constant level after approximately 6 min. This proves that the decrease in the antenna cross-section of PSII is an active process, which happens already within few minutes after light-dark switches in the absence of PsaI.
Figure 5.
Nonphotochemical and light-driven kinetics of state transition and LHCII phosphorylation. A, Wild-type and ΔpsaI-1 leaves were illuminated with far-red supplemented light for 40 min (indicated by gray bar labeled with FR) to reach a steady-state level of Fm'. Subsequently, the actinic light was turned off for 30 min (indicated by black bar labeled with D) before it was switched on again for 20 min. Saturating light pulses were applied every 2 min during illumination with far-red supplemented light to determine Fm'. Each data point displays the mean value of at least three biological replicates. Error bars indicate the corresponding standard derivations. B and C, Wild-type and ΔpsaI-1 plants were dark-incubated for 16 h (16 h D) before being transferred to growth light for 2 h (2 h GL). Subsequently, they were exposed to far-red light for 1 h (1 h FR), followed by 30 min of darkness (30 min). Thylakoid membrane complexes were isolated immediately after each light or dark treatment in the presence of sodium fluoride. lpBN-PAGE was performed after solubilization of the thylakoids with 1.5% digitonin (B). Prominent complexes are indicated on the left. Thylakoid membrane complexes corresponding to 4 µg chlorophyll were used for solubilization. Furthermore, thylakoids were subjected to SDS-PAGE, and immunological analysis using P-LHCb2 antisera were performed (C). Thylakoids corresponding to 0.5 µg chlorophyll were loaded. CBB, Coomassie Brilliant Blue staining of the PVDF membrane.
Putative changes in the dark state 1 are also reflected in the higher Fo and lower Fv/Fm values in the ΔpsaI-1 mutant after 15 min dark incubation (Table I). In contrast, after 1 h far-red light illumination, the Fv/Fm value of ΔpsaI-1 did not differ from the wild type (Table I).
To reveal whether nonphotochemical dark reduction of PQ is responsible for the fast transfer of mutant plants into state 2 in the dark, we measured the net re-reduction rate of P700 in wild-type and ΔpsaI-1 leaves in the dark. After oxidation of P700 with far-red light, its re-reduction was displayed by an exponential decay. The mean value of the half-time of P700 re-reduction (t1/2P700 red) was significantly lower in the ΔpsaI-1 mutant (0.99 ± 0.18) compared to the wild type (1.45 ± 0.17), indicating that light-independent reduction of the PQ pool via nonphotochemical processes is indeed severely increased in the mutant (Table I). We also measured the post-illumination fluorescence rise, which was about 2.5 times faster in the mutant as compared to the wild type. This increase also reflects an elevated nonphotochemical reduction of the PQ pool.
Phosphorylation and Association of the Mobile LHCII Trimer to PSI in the Dark
In order to investigate the phosphorylation status and the dynamics of the mobile LHCII antenna, thylakoid membrane complexes were isolated in the presence of the phosphatase inhibitor sodium fluoride followed by lpBN-PAGE analyses. Solubilization of the thylakoid membrane protein complexes was performed using digitonin, which predominantly solubilizes the complexes located in the stroma lamellae and the adjacent transition zone to the grana stacks, whereas access to the complexes residing in the tightly appressed grana core region is largely limited in contrast to thylakoids treated with the detergents β-DM and Triton X-100 (Krause, 2006; Järvi et al., 2011). Moreover, unlike β-DM and Triton X-100, digitonin has been reported to retain even relatively weak protein-protein interactions during solubilization (Krause, 2006; Järvi et al., 2011). Thus, during solubilization of the thylakoid complexes, the state transition-specific PSI-LHCII complex remains preserved and is visible in native gels (Pesaresi et al., 2009; Järvi et al., 2011).
To investigate whether the “dark state 2” in the mutants goes along with the attachment of LHCII to PSI and whether the lack of PsaI effects the assembly of this PSI complex, wild-type and ΔpsaI-1 plants were exposed to a consecutive series of different light conditions followed by isolation of native thylakoids and subsequent lpBN-PAGE. Wild-type and ΔpsaI-1 plants were first dark-adapted for 16 h and subsequently illuminated with growth light for 2 h. Afterward, they were subjected to far-red light for 1 h before being incubated in darkness for 30 min. Native thylakoid membrane complexes were isolated after each treatment, solubilized with digitonin, and subjected to lpBN-PAGE (Fig. 5B). After 16 h of darkness, only a very faint PSI-LHCII band could be observed in the wild type, consistent with the assumption that the largest portion of the mobile LHCII is bound to PSII in the absence of light. Compared to wild type, the PSI-LHCII supercomplex was clearly more abundant in the ΔpsaI-1 plants after 16 h dark incubation (Fig. 5B). An increased abundance of the PSI-LHCII complex could be observed after illuminating the wild-type and ΔpsaI-1 plants with growth light for 2 h (Fig. 5B); nevertheless, the relative abundance of the complex still being higher in the mutant than in wild type (Fig. 5B). After far-red light treatment, the PSI-LHCII complex completely disappeared in both lines due to the preferential excitation of PSI (Fig. 5B). When the plants were subsequently incubated in the dark for 30 min, a relatively weak PSI-LHCII complex band could be observed in the wild type, indicating that the plants were shifted mostly to state 1. In sharp contrast to that, the PSI-LHCII complex accumulated to much higher amounts in the mutant compared to the wild type and notably also exhibited a higher abundance than observed under growth light conditions (Fig. 5B). The extra LHCII described above (Fig. 3A) also over-accumulated under all light conditions tested (Fig. 5B).
To see whether the accumulation of the PSI-LHCII complex in the mutant under normal growth light and darkness is linked to the phosphorylation status of the LHCII proteins, the same thylakoids that were used for the above-described lpBN-PAGE analyses were also subjected to SDS-PAGE, followed by immunodetection of the phosphorylated LHCb2 (P-LHCb2). P-LHCb2 was chosen due to reports showing that although LHCb1 is important for state transition to occur, it mainly plays a role in the remodeling of the thylakoid membrane (Pietrzykowska et al., 2014), which is typical for state transition (Dietzel et al., 2011). In contrast, LHCb2 is more rapidly phosphorylated than LHCb1, and the state transition-specific PSI-LHCII complex contains only P-LHCb2, but not P-LHCb1 (Leoni et al., 2013). P-LHCb2 provides the surface for the interaction with PSI, and phosphorylation of LHCb2 is essential for rapid induction of state transition (Pietrzykowska et al., 2014). Expectedly, after 16 h darkness, only minute traces of phosphorylated LHCb2 could be detected in wild-type thylakoids in agreement with the weak abundance of the PSI-LHCII complex (Fig. 5, B and C). In contrast, in ΔpsaI-1 large amounts of P-LHCb2 were detectable (Fig. 5C). Also, in accordance with the higher abundance of the PSI-LHCII complex in growth light, the phosphorylation status of LHCb2 was higher in both wild type and ΔpsaI-1 compared to the situation after 16 h darkness (Fig. 5, B and C). However, the mutant accumulated slightly higher amounts of P-LHCb2 compared to the wild type in growth light (Fig. 5C). As expected from the spectroscopic measurements (Fig. 4) and lpBN analyses (Fig. 5B), the phosphorylation of LHCb2 could be reversed in both lines by far-red light illumination (Fig. 5C). Strikingly, the mutant thylakoids exhibited the highest amounts of P-LHCb2 after 30 min dark incubation compared to all other light conditions, and its abundance was also extremely enhanced compared to the wild type after 30 min darkness (Fig. 5C), congruent with the observed accumulation of the PSI-LHCII complex (Fig. 5B) and the faster nonphotochemical re-reduction rate of the PQ pool (Table I). In summary, it can be concluded that the absence of PsaI leads to a more reduced redox state of the PQ and, consequently, an elevated phosphorylation level of the LHCb2, not only in growth light but especially in darkness, resulting in an enhanced attachment of the mobile LHCII to PSI.
Energy Transfer from LHCII to PSI Is Diminished in ΔpsaI
To prove, whether LHCII attachment to PSI redistributes the energy transfer between both photosystems, i.e. increases the transfer of energy to PSI, 77K measurements were performed. Wild-type and ΔpsaI-1 plants kept for 16 h in the dark showed no differences in the relative excitation of PSII (685 nm) and PSI (735 nm; Fig. 6A), although considerably more LHCII is phosphorylated and attached to PSI in the mutant (Fig. 5B). This indicates that the energy is not transferred efficiently from LHCII to PSI in ΔpsaI-1. Transferring plants in growth light for 2 h increased the PSI signal to the same extent in mutant and wild type (Fig. 6B), confirming an increased antenna cross-section of PSI under these conditions (Fig. 5B). Again, no differences in the relative excitation of PSII and PSI could be detected between wild type and mutants despite the larger amounts of PSI-LHCII complexes in ΔpsaI (Fig. 6B). Thylakoids illuminated with far-red light and subsequent 30 min dark incubation were also investigated (Fig. 6, C and D). As expected, there were no differences in the obtained emission spectra between wild type and ΔpsaI-1 after far-red illumination (Fig. 6C) in accordance with a loss of PSI-LHCII complexes in both lines (Fig. 5B). In contrast, a slightly enhanced relative excitation of PSI in ΔpsaI-1 compared to the wild type could be observed after 30 min dark incubation (Fig. 6D), consistent with a much higher amount of PSI-LHCII complexes in the mutant (Fig. 5B). Thus, it can be concluded that the alterations at the LHCII docking site in PSI (Fig. 3) caused by the lack of PsaI do not hinder state transition (Fig. 4) and the binding of the LHCII to PSI per se (Fig. 5B). However, it affects the stable association of its direct neighbors PsaH and PsaL in the PSI complex (Fig. 3), which in turn hinders an efficient transfer of energy from LHCII to PSI (Fig. 6). This might result in a more reduced PQ pool causing hyper-phosphorylation of LHCII and consequently its increased connectivity with PSI under growth light conditions to balance the disequilibrium (Figs. 5B and 6).
Figure 6.
77K fluorescence emission spectra. Fluorescence emission spectra were recorded from isolated and unsolubilized wild-type and ΔpsaI-1 thylakoid membrane complexes preincubated in the dark for 16 h (16 h D; A) followed by 2 h growth light (2 h GL; B), and subsequent far-red light treatment for 1 h (1 h FR; C), followed by 30 min incubation in darkness (30 min D; D). The samples were excited at 440 nm at 77K, and the resulting light emission spectra were recorded between 650 and 800 nm. Spectra from six measurements were normalized with respect to the PSII peak at 685 nm and were averaged.
Phosphorylation of LHCII as Compensatory Mechanism in the Dark
The question arose whether dark phosphorylation of LHCII in ΔpsaI mutants represents a specific effect of the mutation. To address this question, we investigated the dark phosphorylation in the PSI mutants ΔpsaJ (Hansson et al., 2007) and ycf4 (Krech et al., 2012) in tobacco. The latter encodes an important but nonessential assembly factor of PSI. In addition, we investigated the ΔpsaL mutant in Arabidopsis (Pesaresi et al., 2009). We first shifted the plants into state 1 by far-red light illumination for 1 h and afterward kept them in the dark for 30 min. Comparable to wild type and ΔpsaI, LHCb2 was not phosphorylated in state 1 in ΔpsaJ (Supplemental Fig. S5). In contrast, in Δycf4 and ΔpsaL LHCb2 was already phosphorylated in state 1 (Supplemental Fig. S5). Unlike a minor detectable LHCb2 phosphorylation in the wild type, all three mutants showed a high LHCb2 phosphorylation level after 30 min dark incubation with the highest levels in Δycf4 and ΔpsaL (Supplemental Fig. S5). This all indicates that dark phosphorylation is not solely related to a function of PsaI but rather reflects a response due to deficiencies in PSI performance regardless whether PSI assembly (Δycf4), stability and electron transfer within PSI (ΔpsaJ), the LHCII docking site of PSI, or state transitions (ΔpsaI and ΔpsaL) are impaired in plants.
DISCUSSION
Diverged Function of Plant PsaI in Stabilizing the LHCII Docking Site of PSI
PsaI of plants is a conserved LMW component of PSI. The peptide shares around 45% sequence identity with that of cyanobacteria. In cyanobacteria, PsaI has been shown to maintain hydrophobic interactions with carotenoids, build up connections with bordering PSI monomers, stabilize PsaL in the complex, and consequently, prove necessary for the preservation of the trimeric PSI complex (Nakamoto, 1995; Xu et al., 1995; Schluchter et al., 1996). However, this “trimer connecting domain” being composed of PsaL, PsaI, and PsaM in cyanobacteria has been remodeled in plants since the endosymbiotic event. PsaM as well as the C-terminal domain of PsaL got lost, and the newly acquired subunit PsaH interacts with PsaI and encircles PsaL, thus hindering the trimerization of the PSI complex in plants (Jansson et al., 1996; Ben-Shem et al., 2003; Qin et al., 2015). The nose-shaped region of PSI formed by PsaH, PsaL, PsaO, and PsaI, which is located opposite to the half-moon shaped LHCI belt in the plant complex, gained a novel task compared to its bacterial counterpart: the binding of the mobile LHCII trimer, which is crucial for the transfer of excitation energy to PSI during state transition (Lunde et al., 2000; Jensen et al., 2004; Amunts et al., 2010; Schöttler et al., 2011).
Inactivation of PsaI allows photoautotrophic growth and normal accumulation of subunits of both photosystems as well as normal function of PSI-dependent electron transport as revealed by unchanged ΦPSI and ΦND values, PSI activity, as well as NADP+ photoreduction (Figs. 1C and 2; Table I; Supplemental Fig. S3). Treatment with the mild detergent digitonin did not result in any disassembly of the PSI complex, indicating that PsaH and PsaL are associated with PSI in vivo in ΔpsaI lines. This stands in contrast to the situation in the Arabidopsis ΔpsaL mutant, where the entire PSI complex is shifted toward lower molecular weight when being solubilized with digitonin due to the complete absence of PsaL, which hinders the association of PsaH to the PSI (Pesaresi et al., 2009). However, unlike in wild type, treatment of ΔpsaI thylakoids with β-DM or Triton led to dissociation of both PsaL and PsaH to some extent from the complex, visibly by a partial shift of the PSI complex (Fig. 3). This demonstrates that in the absence of PsaI, the subunits PsaH and PsaL are presumably attached to PSI in vivo but their connectivity is looser. Destabilized association of PsaH and PsaL to PSI did not affect the capability of the plants to perform state transition nor electron transport within PSI (Fig. 4; Table I). Illumination of plants with PSII favoring light led to a functional dissociation of the mobile LHCII from PSII (visualized by fluorescent transients) in both wild type and ΔpsaI. In contrast, Arabidopsis plants devoid of PsaL and PsaH are severely impaired in their ability to perform state transition (Lunde et al., 2000, 2003). These observations were made using artificial light quality changes, which provoke an extreme response of the whole system but do not occur to such an extent under natural light conditions when plants are especially subjected to varying light intensities and the LHCII almost always serves as an antenna for both photosystems (Wientjes et al., 2013).
As indicated by the presence of the PSI-LHCII complex in native gels, in the absence of PsaI, the mobile LHCII was not only able to functionally detach from PSII but could also stably bind to PSI under normal light conditions. Surprisingly, this “state transition” complex accumulated in higher amounts in the ΔpsaI mutant in normal growth light and even in the dark despite the instability of PsaL and PsaH (Fig. 5B). In contrast, when PsaL is absent, Arabidopsis plants are not able to build up this PSI-LHCII complex at all and, therefore, have difficulties to regulate their photosynthetic machinery upon changing light conditions (Lunde et al., 2000, 2003; Pesaresi et al., 2009). Although we detected an increased association of LHCII to PSI in the ΔpsaI mutant as compared to the wild type under growth light conditions and after long-term (16 h) dark exposition, the transfer of energy from LHCII to PSI was unexpectedly unchanged (Fig. 6, A and B). An even larger proportion of LHCII attaches to PSI only after 30 min darkness. This coincided with a slightly increased excitation of PSI (Fig. 6D). Thus, we conclude that the lack of PsaI does not hinder the binding of the LHCII to PSI but causes an inefficient energy transfer from LHCII to PSI. This further enhances LHCII phosphorylation and association of P-LHCII to PSI, which can be interpreted as a compensatory mechanism to balance the excitation of both photosystems. Recently, it was shown that the LHCII is not just able to supply PSI with excitation energy by its binding to the LHCII docking site. Additional LHCII trimers can energetically interact with the PSI-LHCII complex via the LHCI antenna (Benson et al., 2015). Moreover, excitation energy can be transferred efficiently within physically connected PSI-PSII megacomplexes, which build up depending on the present light conditions (Järvi et al., 2011; Suorsa et al., 2015; Yokono et al., 2015; Tiwari et al., 2016). Therefore, balanced excitation of PSI in ΔpsaI and previously described PSI mutants could involve to an elevated degree alternative pathways, but not necessarily the “classical” LHCII docking site of PSI.
Nonphotochemical Dark-Reduction of PQ as a Compensatory Mechanism
The redox status of the PQ pool is well known to trigger signals for phosphorylation of proteins, chlorophyll biosynthesis, changes in chloroplast gene expression, phytopathogenic attack, and various retrograde signaling pathways (Pesaresi et al., 2010; Puthiyaveetil et al., 2012; Nosek et al., 2015; Steccanella et al., 2015). Most present studies were restricted to effects of the PQ redox state in the light, but far less is known about its regulation in the dark. Phosphorylation and association of LHCII with PSI in the dark triggered by a reduced PQ pool is one of the striking and surprising differences between the different PSI and LHCII docking site mutants and the wild type. Although an increased reduction of PQ can be explained by a diminished excitation or function of PSI in light exposed mutant plants, the question remains how they regulate the dark-reduction of PQ.
In the dark, the redox state of the PQ is poised by reducing agents such as ascorbate (Hou et al., 2003) and the chlororespiratory pathway involving electron transfer from reducing equivalents to PQ and the NDH complex and reoxidation of PQ by the plastid terminal oxidase (PTOX), which transfers the electrons to oxygen (for review, see Nawrocki et al., 2015). Consequently, these light-independent processes probably help to keep up a proton gradient across the thylakoid membrane, which in turn might be necessary for various processes that depend on a proton motive force (for review, see Nawrocki et al., 2015). Changes in nonphotochemical dark reduction/oxidation of PQ were previously shown to occur under certain stress conditions, such as high light, heat, cold, and CO2 deprivation, but the exact mechanism and original electron sources remained rather elusive (Rumeau et al., 2007; Trouillard et al., 2012). Thus, the nonphotochemical dark reduction of PQ in ΔpsaI could arise from an increased reduction and/or a decreased oxidation rate and relies on the availability of oxygen and reducing equivalents.
Additionally, we show that enhanced nonphotochemical reduction of the PQ pool generally happens in mutant plants, which are impaired in various aspects of PSI function (Supplemental Fig. S5). In the case of ΔpsaI, dark-reduction of the PQ pool and concomitant attachment of the mobile LHCII to PSI might be a mechanism to prepare the mutated photosynthetic machinery for optimal balanced activity upon the onset of light to avoid excess of electrons generated by PSII. It is assumed that the LHCII transfers excitation energy to PSI under most natural light conditions and that steady-state phosphorylation of LHCII fulfills a photoprotective role for PSI upon fluctuating light conditions (Grieco et al., 2012; Wientjes et al., 2013). Since the energy transfer from the mobile LHCII bound to the docking site in the ΔpsaI mutant is likely impaired, mutant plants tend to regain balanced excitation by increasing the amounts of PSI-LHCII complexes in the light. Although the majority of the mobile LHCII antenna is bound to PSII in the dark-induced “state1” in wild-type plants, part of it is also bound to PSI (Fig. 5B). The increased amount of PSI-LHCII complexes of ΔpsaI and PSI mutants in the dark possibly reflects a nonphotochemical mechanism to balance excitation of both photosystems and/or to protect the photosystems from photoinhibition upon the onset of light or under fluctuating light conditions (Grieco et al., 2012; Horton 2012). Further analysis of the different PSI mutants will be necessary to address the nature of the redox carriers (Fisher and Cramer 2014), the regulation of nonphotochemical dark reduction/oxidation of PQ, and its contribution to multiple physiological processes, such as redox sensing, retrograde signaling, lumenal import of proteins, metabolic processes, and ΔpH-dependent nonphotochemical quenching upon dark-light switches (Pfalz et al., 2012; Puthiyaveetil et al., 2012; Nawrocki et al., 2015).
MATERIALS AND METHODS
Generation of ΔpsaI Knockouts in Tobacco
Using PCR a 1,511-bp fragment containing about 700 bp up- and downstream of the PsaI gene was generated and cloned into the SacI and BamHI sites of the pUC19 vector. The 108-bp coding region of PsaI was then dissected after nucleotide 45 by insertion of a terminatorless chimeric aadA cassette equipped with the tobacco 16S rRNA promoter into the naturally occurring StuI site of PsaI. This clone containing the aadA gene in reading frame orientation of PsaI was used for transformation by particle bombardement of tobacco (Nicotiana tabacum cv Petit Havanna) leaves as described (Svab et al., 1990). The transformed leaf pieces were regenerated, selected, and cultured on medium containing spectinomycin as described (Svab and Maliga, 1993). Two homoplastomic lines were then backcrossed five times using wild-type pollen as donor. Homoplastomy was investigated by inheritance assays (Krech et al., 2012) using solidified Murashige and Skoog medium (Murashige and Skoog, 1962) supplemented with spectinomycin and streptomycin (500 mg L−1 each) and using RNA gel blot analysis.
Plant Growth Conditions and Light Treatments
Nicotiana tabacum cv Petit Havanna mutants ΔpsaI, ΔpsaJ, Δycf4, and wild-type plants carrying the aadA cassette in a neutral insertion site in their plastome (Ohad et al., 2004), and tobacco wild-type plants without aadA cassette as well as Arabidopsis (Arabidopsis thaliana) ΔpsaL mutant (accession Col-0; SALK_000637; Pesaresi et al., 2009) and Arabidopsis wild type (Col-0) were grown on soil under long day conditions (16 h light/8 h dark cycles; 125 µmol m−2 s−1) for 6 to 8 weeks at 25°C (tobacco) or 4 weeks at 22°C (Arabidopsis) in growth chambers. Short-term acclimation of plants was achieved by illumination with far-red light (737 nm; estimated 180 μmol m−2 s−1; Heliospectra L4A Series 10 600W programmable LED light source) or red light (663 nm; 70 μmol m−2 s−1 Heliospectra L4A Series 10 600-W programmable LED light source).
RNA Blot Analysis
Isolation of total RNA and following gel blot analysis were carried out as described (Meurer et al., 2002) using radioactive end labeling of strand-specific 80mer oligonucleotides (Supplemental Table S1) labeled with T4 polynucloetide kinase (New England Biolabs).
In Vivo Chlorophyll a Fluorescence Measurements and Light-Induced PSI Absorbance Changes
A pulse amplitude-modulated fluorometer (Dual-PAM-100; Walz) was used to study chlorophyll a fluorescence parameters and kinetics, such as Fv/Fm, Fm’, and the effective quantum yield of PSII (Schreiber et al., 1986), as well as PSI absorbance changes at 820 nm, ΦPSI, and ΦND (Klughammer and Schreiber, 2008) of mutant and wild-type leaves of the same age. P700+ re-reduction kinetics in the dark were recorded after oxidation of P700 by short illumination with far-red light and the mean values of the half-time of the decay were calculated. If not otherwise indicated, leaves were dark-adapted for 15 min before measuring chlorophyll a fluorescence parameters. For state transition measurements, dark-incubated leaves (15 min) were exposed to actinic blue light (44 μmol m−2 s−1) for 20 min to induce state 2 accompanied by saturating light pulses every 2 min (0.8 s; 10,000 μmol m−2 s−1). Subsequently, induction of state 1 was achieved by illumination with actinic far-red light (Value 258) in addition to blue light for 20 min. Measurements were also performed by inducing first state 1 and then state 2. The values for the maximum fluorescence in state 2 (Fm2) and the maximum fluorescence in state 1 (Fm1) were determined after 20 min, respectively, to calculate qT = (Fm1-Fm2)/Fm2 (Jensen et al., 2000). The transient rise of chlorophyll fluorescence in darkness after exposure of plants to actinic blue light (35 µmol photons m−2 s−1) for 5 min was measured using the imaging PAM system as described (Shikanai et al., 1998).
77K Fluorescence Measurements
The 77K fluorescence emission spectra were obtained from frozen intact thylakoids (50 µg chlorophyll/mL) using an Ocean Optics S2000 spectrometer as described (Grieco et al., 2015). Six technical replicates were averaged and the curves were normalized to the maximum PSII signal at 685 nm. In total, three biological replicates were measured.
Photosynthetic Electron Transport Measurements
Linear and PSI-dependent electron transport was determined using a Clark-type oxygen electrode as electron flow from water and N,N,N’,N’-tetramethyl-p-phenylenediamine to methylviologen, respectively (Allen and Holmes, 1986). Measurements were performed under saturating light conditions with freshly broken chloroplasts.
NADP+ Photoreduction Measurements
NADP+ photoreduction activity was determined from the absorbance change at 340 nm in a reaction mixture (total volume 1000 μL) containing TNM buffer (20 mm Tricine [pH 7.5], 40 mm NaCl, 8 mm MgCl2, 0.1% [v/v] n-dodecyl-β-d-maltopyranoside, 2 mm sodium ascorbate, 0.06 mm 2,6-dichlorophenolindophenol [DCPIP]), 0.5 mm NADP+, 3 μm spinach ferredoxin, 2 μm spinach plastocyanin, 5 nm spinach FNR, and thylakoids equivalent to 10 μg of chlorophyll. The light-induced production of NADPH was measured at 340 nm in a Shimadzu UV-2550 spectrophotometer. The samples were kept at 25°C and irradiated from the side with a Schott KL 1500 light source fitted with two red filters (Schott RG660 and Corning 2-58). NADPH production was quantified using the molar extinction coefficient of 6.2 mm−1 cm−1 at 340 nm.
Isolation of Thylakoids and Separation of Solubilized Thylakoid Membrane Complexes by Blue Native-PAGE
In general, thylakoids were isolated from fresh leaves of dark-adapted (16 h dark) or light-treated plants. The isolation was performed immediately after the light treatments under dim light at 4°C in the presence of 10 mm sodium fluoride in all buffers as described (Järvi et al., 2011). Fractionation of thylakoid membranes into grana lamellae, stroma lamellae, and intermittent fraction was performed as described (Torabi et al., 2014a). Solubilization of thylakoid membrane complexes and further sample preparation for lpBN were essentially performed as described (Järvi et al., 2011). Thylakoids were either solubilized using 1% β-DM or Triton X-100 without shaking the samples for 5 min on ice whereas when using 1.5% digitonin, thylakoids were shaken for 10 min at 20°C. Solubilized thylakoid membranes were separated by BN- or lpBN-PAGE using an acrylamide gradient of 3.5–12.5% (w/v) T (total gel concentration) and 3.0% (w/v) C (cross-linking) in the separation gel combined with a stacking gel composed of either 4% (w/v) T and 2.6% (w/v) C for BN or 3% (w/v) T and 20% (w/v) C for lpBN (Järvi et al., 2011). lpBN-PAGE gels were excited at 633 nm, and autofluorescence was recorded through a 670 nm band-pass filter using a Typhoon laser scanner from Amersham Biosciences/GE Healthcare.
Protein Analysis
Isolated thylakoid membrane proteins were solubilized and subjected to SDS-PAGE and blotted onto polyvinylidene difluoride membranes as described (Schwenkert et al., 2006). Native stripes of the first native dimension were excised, denatured, and run in the second dimension of SDS-PAGE 15% (w/v) T and 4 m urea (Schwenkert et al., 2006). Equal loading was confirmed by Coomassie Brilliant Blue staining prior to immunodecoration. Antibodies specific for PsaA, PsaC, PsaD, PsaL, PsaH, PsaF, PsbH, PsbI, LHCb1, LHCb2, LHCb4, LHCb5, LHCa1, LHCa2, LHCa3, and P-LHCB2 were purchased from Agrisera. Other antibodies used were described elsewhere (Kubicki et al., 1996; Torabi et al., 2014b). The putative structure of PsaI was calculated using I-Tasser (http://zhanglab.ccmb.med.umich.edu/; Yang and Zhang, 2015).
Supplemental Data
The following supplemental materials are available.
Supplemental Table S1. Strand-Specific 80mer Oligonucleotides Used as Hybridization Probes.
Supplemental Figure S1. Predicted Structure of PsaI.
Supplemental Figure S2. Inheritance Assay.
Supplemental Figure S3. Spectroscopic Measurement of Energy Conversion in PSI.
Supplemental Figure S4. lpBN-PAGE, Silver Staining, and Immunoblotting.
Supplemental Figure S5. Dark-Phosphorylation of LHCII in PSI Mutants.
Supplementary Material
Acknowledgments
The authors wish to thank Martina Reimers for help with the tissue culture, Dario Leister for offering laboratory space and ΔpsaL seeds, and Ralph Bock for providing Δycf4 seeds.
Glossary
- LHC
light-harvesting complex
- PQ
plastoquinone
- NPQ
nonphotochemical quenching
- LMW
low molecular weight
References
- Allahverdiyeva Y, Suorsa M, Tikkanen M, Aro EM (2015) Photoprotection of photosystems in fluctuating light intensities. J Exp Bot 66: 2427–2436 [DOI] [PubMed] [Google Scholar]
- Allen JF, Bennett J, Steinback KE, Arntzen CJ (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291: 25–29 [Google Scholar]
- Allen JF, Holmes NG (1986) Electron transport and redox titration. In Hipkins MF, Baker NR. eds, Photosynthesis: Energy transduction: A Practical Approach. IRL Press, Oxford, UK, pp 103-141 [Google Scholar]
- Amunts A, Nelson N (2008) Functional organization of a plant Photosystem I: evolution of a highly efficient photochemical machine. Plant Physiol Biochem 46: 228–237 [DOI] [PubMed] [Google Scholar]
- Amunts A, Nelson N (2009) Plant photosystem I design in the light of evolution. Structure 17: 637–650 [DOI] [PubMed] [Google Scholar]
- Amunts A, Toporik H, Borovikova A, Nelson N (2010) Structure determination and improved model of plant photosystem I. J Biol Chem 285: 3478–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892–895 [DOI] [PubMed] [Google Scholar]
- Ben-Shem A, Frolow F, Nelson N (2003) Crystal structure of plant photosystem I. Nature 426: 630–635 [DOI] [PubMed] [Google Scholar]
- Benson SL, Maheswaran P, Ware MA, Hunter CN, Horton P, Jansson S, Ruban AV, Johnson MP (2015) An intact light harvesting complex I antenna system is required for complete state transitions in Arabidopsis. Nat Plants 1: 15176. [DOI] [PubMed] [Google Scholar]
- Bonardi V, Pesaresi P, Becker T, Schleiff E, Wagner R, Pfannschmidt T, Jahns P, Leister D (2005) Photosystem II core phosphorylation and photosynthetic acclimation require two different protein kinases. Nature 437: 1179–1182 [DOI] [PubMed] [Google Scholar]
- Bonaventura C, Myers J (1969) Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta 189: 366–383 [DOI] [PubMed] [Google Scholar]
- Chitnis VP, Chitnis PR (1993) PsaL subunit is required for the formation of photosystem I trimers in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett 336: 330–334 [DOI] [PubMed] [Google Scholar]
- Depège N, Bellafiore S, Rochaix JD (2003) Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299: 1572–1575 [DOI] [PubMed] [Google Scholar]
- Dietzel L, Bräutigam K, Steiner S, Schüffler K, Lepetit B, Grimm B, Schöttler MA, Pfannschmidt T (2011) Photosystem II supercomplex remodeling serves as an entry mechanism for state transitions in Arabidopsis. Plant Cell 23: 2964–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher N, Kramer DM (2014) Non-photochemical reduction of thylakoid photosynthetic redox carriers in vitro: relevance to cyclic electron flow around photosystem I? Biochim Biophys Acta 1837: 1944–1954 [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M, Bassi R (2015) Sharing light between two photosystems: mechanism of state transitions. Curr Opin Plant Biol 25: 71–78 [DOI] [PubMed] [Google Scholar]
- Grieco M, Suorsa M, Jajoo A, Tikkanen M, Aro EM (2015) Light-harvesting II antenna trimers connect energetically the entire photosynthetic machinery - including both photosystems II and I. Biochim Biophys Acta 1847: 607–619 [DOI] [PubMed] [Google Scholar]
- Grieco M, Tikkanen M, Paakkarinen V, Kangasjärvi S, Aro EM (2012) Steady-state phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol 160: 1896–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotjohann I, Fromme P (2005) Structure of cyanobacterial photosystem I. Photosynth Res 85: 51–72 [DOI] [PubMed] [Google Scholar]
- Hansson A, Amann K, Zygadlo A, Meurer J, Scheller HV, Jensen PE (2007) Knock-out of the chloroplast-encoded PSI-J subunit of photosystem I in Nicotiana tabacum. FEBS J 274: 1734–1746 [DOI] [PubMed] [Google Scholar]
- Horton P. (2012) Optimization of light harvesting and photoprotection: molecular mechanisms and physiological consequences. Philos Trans R Soc Lond B Biol Sci 367: 3455–3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou CX, Rintamäki E, Aro EM (2003) Ascorbate-mediated LHCII protein phosphorylation--LHCII kinase regulation in light and in darkness. Biochemistry 42: 5828–5836 [DOI] [PubMed] [Google Scholar]
- Jansson S, Andersen B, Scheller HV (1996) Nearest-neighbor analysis of higher-plant photosystem I holocomplex. Plant Physiol 112: 409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvi S, Suorsa M, Paakkarinen V, Aro EM (2011) Optimized native gel systems for separation of thylakoid protein complexes: novel super- and mega-complexes. Biochem J 439: 207–214 [DOI] [PubMed] [Google Scholar]
- Jensen PE, Gilpin M, Knoetzel J, Scheller HV (2000) The PSI-K subunit of photosystem I is involved in the interaction between light-harvesting complex I and the photosystem I reaction center core. J Biol Chem 275: 24701–24708 [DOI] [PubMed] [Google Scholar]
- Jensen PE, Haldrup A, Zhang S, Scheller HV (2004) The PSI-O subunit of plant photosystem I is involved in balancing the excitation pressure between the two photosystems. J Biol Chem 279: 24212–24217 [DOI] [PubMed] [Google Scholar]
- Joliot P, Johnson GN (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108: 13317–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P, Fromme P, Witt HT, Klukas O, Saenger W, Krauss N (2001) Three-dimensional structure of cyanobacterial photosystem I at 2.5 A resolution. Nature 411: 909–917 [DOI] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (2008) Saturation pulse method for assessment of energy conversion in PS I. PAM Application Notes 1: 11–14 [Google Scholar]
- Kramer DM, Avenson TJ, Edwards GE (2004) Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci 9: 349–357 [DOI] [PubMed] [Google Scholar]
- Krause F. (2006) Detection and analysis of protein-protein interactions in organellar and prokaryotic proteomes by native gel electrophoresis: (Membrane) protein complexes and supercomplexes. Electrophoresis 27: 2759–2781 [DOI] [PubMed] [Google Scholar]
- Krech K, Ruf S, Masduki FF, Thiele W, Bednarczyk D, Albus CA, Tiller N, Hasse C, Schöttler MA, Bock R (2012) The plastid genome-encoded Ycf4 protein functions as a nonessential assembly factor for photosystem I in higher plants. Plant Physiol 159: 579–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki A, Funk E, Westhoff P, Steinmüller K (1996) Differential expression of plastome-encoded ndh genes in mesophyll and bundle-sheath chloroplasts of the C4 plant Sorghum bicolor indicates that the complex I-homologous NAD(P)H-plastoquinone oxidoreductase is involved in cyclic electron transport. Planta 199: 276–281 [Google Scholar]
- Kyle DJ, Kuang T-Y, Watson JL, Arntzen CJ (1984) Movement of a sub-population of the light harvesting complex (LHCII) from grana to stroma lamellae as a consequence of its phosphorylation. Biochim Biophys Acta 765: 89–96 [Google Scholar]
- Larsson UK, Jergil B, Andersson B (1983) Changes in the lateral distribution of the light-harvesting chlorophyll-a/b--protein complex induced by its phosphorylation. Eur J Biochem 136: 25–29 [DOI] [PubMed] [Google Scholar]
- Leoni C, Pietrzykowska M, Kiss AZ, Suorsa M, Ceci LR, Aro EM, Jansson S (2013) Very rapid phosphorylation kinetics suggest a unique role for Lhcb2 during state transitions in Arabidopsis. Plant J 76: 236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde C, Jensen PE, Haldrup A, Knoetzel J, Scheller HV (2000) The PSI-H subunit of photosystem I is essential for state transitions in plant photosynthesis. Nature 408: 613–615 [DOI] [PubMed] [Google Scholar]
- Lunde C, Jensen PE, Rosgaard L, Haldrup A, Gilpin MJ, Scheller HV (2003) Plants impaired in state transitions can to a large degree compensate for their defect. Plant Cell Physiol 44: 44–54 [DOI] [PubMed] [Google Scholar]
- Magee AM, Aspinall S, Rice DW, Cusack BP, Sémon M, Perry AS, Stefanović S, Milbourne D, Barth S, Palmer JD, et al. (2010) Localized hypermutation and associated gene losses in legume chloroplast genomes. Genome Res 20: 1700–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurer J, Lezhneva L, Amann K, Gödel M, Bezhani S, Sherameti I, Oelmüller R (2002) A peptide chain release factor 2 affects the stability of UGA-containing transcripts in Arabidopsis chloroplasts. Plant Cell 14: 3255–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Murata N. (1969) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta 172: 242–251 [DOI] [PubMed] [Google Scholar]
- Naithani S, Hou JM, Chitnis PR (2000) Targeted inactivation of the psaK1, psaK2 and psaM genes encoding subunits of Photosystem I in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth Res 63: 225–236 [DOI] [PubMed] [Google Scholar]
- Nakamoto H. (1995) Targeted inactivation of the gene psaI encoding a subunit of photosystem I of the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 36: 1579–1587 [PubMed] [Google Scholar]
- Nawrocki WJ, Tourasse NJ, Taly A, Rappaport F, Wollman FA (2015) The plastid terminal oxidase: its elusive function points to multiple contributions to plastid physiology. Annu Rev Plant Biol 66: 49–74 [DOI] [PubMed] [Google Scholar]
- Nelson N. (2009) Plant photosystem I--the most efficient nano-photochemical machine. J Nanosci Nanotechnol 9: 1709–1713 [DOI] [PubMed] [Google Scholar]
- Nelson N, Junge W (2015) Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu Rev Biochem 84: 659–683 [DOI] [PubMed] [Google Scholar]
- Nelson N, Yocum CF (2006) Structure and function of photosystems I and II. Annu Rev Plant Biol 57: 521–565 [DOI] [PubMed] [Google Scholar]
- Nosek M, Kornaś A, Kuźniak E, Miszalski Z (2015) Plastoquinone redox state modifies plant response to pathogen. Plant Physiol Biochem 96: 163–170 [DOI] [PubMed] [Google Scholar]
- Ohad I, Dal Bosco C, Herrmann RG, Meurer J (2004) Photosystem II proteins PsbL and PsbJ regulate electron flow to the plastoquinone pool. Biochemistry 43: 2297–2308 [DOI] [PubMed] [Google Scholar]
- Pesaresi P, Hertle A, Pribil M, Kleine T, Wagner R, Strissel H, Ihnatowicz A, Bonardi V, Scharfenberg M, Schneider A, Pfannschmidt T, Leister D (2009) Arabidopsis STN7 kinase provides a link between short- and long-term photosynthetic acclimation. Plant Cell 21: 2402–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesaresi P, Hertle A, Pribil M, Schneider A, Kleine T, Leister D (2010) Optimizing photosynthesis under fluctuating light: the role of the Arabidopsis STN7 kinase. Plant Signal Behav 5: 21–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz J, Liebers M, Hirth M, Grübler B, Holtzegel U, Schröter Y, Dietzel L, Pfannschmidt T (2012) Environmental control of plant nuclear gene expression by chloroplast redox signals. Front Plant Sci 3: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowska M, Suorsa M, Semchonok DA, Tikkanen M, Boekema EJ, Aro EM, Jansson S (2014) The light-harvesting chlorophyll a/b binding proteins Lhcb1 and Lhcb2 play complementary roles during state transitions in Arabidopsis. Plant Cell 26: 3646–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribil M, Pesaresi P, Hertle A, Barbato R, Leister D (2010) Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol 8: e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthiyaveetil S, Ibrahim IM, Allen JF (2012) Oxidation-reduction signalling components in regulatory pathways of state transitions and photosystem stoichiometry adjustment in chloroplasts. Plant Cell Environ 35: 347–359 [DOI] [PubMed] [Google Scholar]
- Qin X, Suga M, Kuang T, Shen JR (2015) Photosynthesis. Structural basis for energy transfer pathways in the plant PSI-LHCI supercomplex. Science 348: 989–995 [DOI] [PubMed] [Google Scholar]
- Rochaix JD. (2014) Regulation and dynamics of the light-harvesting system. Annu Rev Plant Biol 65: 287–309 [DOI] [PubMed] [Google Scholar]
- Rochaix JD, Lemeille S, Shapiguzov A, Samol I, Fucile G, Willig A, Goldschmidt-Clermont M (2012) Protein kinases and phosphatases involved in the acclimation of the photosynthetic apparatus to a changing light environment. Philos Trans R Soc Lond B Biol Sci 367: 3466–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruban AV, Johnson MP, Duffy CDP (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817: 167–181 [DOI] [PubMed] [Google Scholar]
- Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ 30: 1041–1051 [DOI] [PubMed] [Google Scholar]
- Schluchter WM, Shen G, Zhao J, Bryant DA (1996) Characterization of psaI and psaL mutants of Synechococcus sp. strain PCC 7002: a new model for state transitions in cyanobacteria. Photochem Photobiol 64: 53–66 [DOI] [PubMed] [Google Scholar]
- Schöttler MA, Albus CA, Bock R (2011) Photosystem I: its biogenesis and function in higher plants. J Plant Physiol 168: 1452–1461 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10: 51–62 [DOI] [PubMed] [Google Scholar]
- Schwenkert S, Umate P, Dal Bosco C, Volz S, Mlçochová L, Zoryan M, Eichacker LA, Ohad I, Herrmann RG, Meurer J (2006) PsbI affects the stability, function, and phosphorylation patterns of photosystem II assemblies in tobacco. J Biol Chem 281: 34227–34238 [DOI] [PubMed] [Google Scholar]
- Shapiguzov A, Ingelsson B, Samol I, Andres C, Kessler F, Rochaix JD, Vener AV, Goldschmidt-Clermont M (2010) The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc Natl Acad Sci USA 107: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikanai T, Endo T, Hashimoto T, Yamada Y, Asada K, Yokota A (1998) Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc Natl Acad Sci USA 95: 9705–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steccanella V, Hansson M, Jensen PE (2015) Linking chlorophyll biosynthesis to a dynamic plastoquinone pool. Plant Physiol Biochem 97: 207–216 [DOI] [PubMed] [Google Scholar]
- Stoppel R, Manavski N, Schein A, Schuster G, Teubner M, Schmitz-Linneweber C, Meurer J (2012) RHON1 is a novel ribonucleic acid-binding protein that supports RNase E function in the Arabidopsis chloroplast. Nucleic Acids Res 40: 8593–8606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, Aro EM (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24: 2934–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa M, Rantala M, Mamedov F, Lespinasse M, Trotta A, Grieco M, Vuorio E, Tikkanen M, Järvi S, Aro EM (2015) Light acclimation involves dynamic re-organization of the pigment-protein megacomplexes in non-appressed thylakoid domains. Plant J 84: 360–373 [DOI] [PubMed] [Google Scholar]
- Svab Z, Hajdukiewicz P, Maliga P (1990) Stable transformation of plastids in higher plants. Proc Natl Acad Sci USA 87: 8526–8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svab Z, Maliga P (1993) High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA 90: 913–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M, Aro EM (2012) Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim Biophys Acta 1817: 232–238 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Aro EM (2014) Integrative regulatory network of plant thylakoid energy transduction. Trends Plant Sci 19: 10–17 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Grieco M, Kangasjärvi S, Aro EM (2010) Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol 152: 723–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikkanen M, Nurmi M, Suorsa M, Danielsson R, Mamedov F, Styring S, Aro EM (2008) Phosphorylation-dependent regulation of excitation energy distribution between the two photosystems in higher plants. Biochim Biophys Acta 1777: 425–432 [DOI] [PubMed] [Google Scholar]
- Tiwari A, Mamedov F, Grieco M, Suorsa M, Jajoo A, Styring S, Tikkanen M, Aro E-M (2016) Photodamage of iron-sulphur clusters in photosystem I induces non-photochemical energy dissipation. Nat Plants 2: 16035. [DOI] [PubMed] [Google Scholar]
- Torabi S, Plöchinger M, Meurer J (2014a) Localization and Topology of Thylakoid Membrane Proteins in Land Plants. Bio Protoc 4: e1363 [Google Scholar]
- Torabi S, Umate P, Manavski N, Plöchinger M, Kleinknecht L, Bogireddi H, Herrmann RG, Wanner G, Schröder WP, Meurer J (2014b) PsbN is required for assembly of the photosystem II reaction center in Nicotiana tabacum. Plant Cell 26: 1183–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillard M, Shahbazi M, Moyet L, Rappaport F, Joliot P, Kuntz M, Finazzi G (2012) Kinetic properties and physiological role of the plastoquinone terminal oxidase (PTOX) in a vascular plant. Biochim Biophys Acta 1817: 2140–2148 [DOI] [PubMed] [Google Scholar]
- Umate P, Fellerer C, Schwenkert S, Zoryan M, Eichacker LA, Sadanandam A, Ohad I, Herrmann RG, Meurer J (2008) Impact of PsbTc on forward and back electron flow, assembly, and phosphorylation patterns of photosystem II in tobacco. Plant Physiol 148: 1342–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Piepenburg K, Schöttler MA, Petersen K, Kahlau S, Tiller N, Drechsel O, Weingartner M, Kudla J, Bock R (2010) Knockout of the plastid RNase E leads to defective RNA processing and chloroplast ribosome deficiency. Plant J 64: 851–863 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kubota H, Wada H, Narikawa R, Ikeuchi M (2011) Novel supercomplex organization of photosystem I in Anabaena and Cyanophora paradoxa. Plant Cell Physiol 52: 162–168 [DOI] [PubMed] [Google Scholar]
- Wientjes E, van Amerongen H, Croce R (2013) LHCII is an antenna of both photosystems after long-term acclimation. Biochim Biophys Acta 1827: 420–426 [DOI] [PubMed] [Google Scholar]
- Xu Q, Hoppe D, Chitnis VP, Odom WR, Guikema JA, Chitnis PR (1995) Mutational analysis of photosystem I polypeptides in the cyanobacterium Synechocystis sp. PCC 6803. Targeted inactivation of PsaI reveals the function of PsaI in the structural organization of PsaL. J Biol Chem 270: 16243–16250 [DOI] [PubMed] [Google Scholar]
- Yamori W, Shikanai T (2016) Physiological Functions of Cyclic Electron Transport Around Photosystem I in Sustaining Photosynthesis and Plant Growth. Annu Rev Plant Biol 67: 81–106 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang Y (2015) Protein Structure and Function Prediction Using I-TASSER. Curr Protoc Bioinformatics 52: 5.8.1–5.8.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokono M, Takabayashi A, Akimoto S, Tanaka A (2015) A megacomplex composed of both photosystem reaction centres in higher plants. Nat Commun 6: 6675. [DOI] [PubMed] [Google Scholar]
- Zoubenko OV, Allison LA, Svab Z, Maliga P (1994) Efficient targeting of foreign genes into the tobacco plastid genome. Nucleic Acids Res 22: 3819–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.