Abstract
Rationale: Obstructive sleep apnea is a state-dependent disease. One of the key factors that triggers upper airway collapse is decreased pharyngeal dilator muscle activity during sleep. To date, there have not been effective methods to reverse pharyngeal hypotonia pharmacologically in sleeping humans.
Objectives: We tested the hypothesis that administration of desipramine 200 mg prevents the state-related reduction in genioglossus activity that occurs during sleep and thereby decreases pharyngeal collapsibility.
Methods: We conducted a placebo-controlled, double-blind, crossover trial with 10 healthy participants. Participants received active treatment or placebo in randomized order 2 hours before sleep in the physiology laboratory.
Measurements and Main Results: Genioglossus activity during wakefulness and sleep, genioglossus muscle responsiveness to negative epiglottic pressure, and upper airway collapsibility during passive and active conditions were compared between on- and off-drug states. Desipramine abolished the normal reduction of genioglossus activity from wakefulness to non-REM sleep that occurred on the placebo night. Specifically, tonic (median, 96% [86–120] vs. 75% [50–92] wakefulness; P = 0.01) but not phasic genioglossus activity was higher with desipramine compared with placebo. Upper airway collapsibility was also reduced with desipramine compared with placebo (−10.0 cm H2O [−15.2 to −5.8] vs. −8.1 cm H2O [−10.4 to −6.3]; P = 0.037).
Conclusions: Desipramine reduces the state-related drop in tonic genioglossus muscle activity that occurs from wakefulness to non-REM sleep and reduces airway collapsibility. These data provide a rationale for a new pharmacologic therapy for obstructive sleep apnea.
Clinical trial registered with www.clinicaltrials.gov (NCT02428478).
Keywords: pharyngeal dilator muscle, norepinephrine reuptake inhibitor, sleep-disordered breathing, pharmacological treatment
At a Glance Commentary
Scientific Knowledge on the Subject
The normal reduction in upper airway muscle activity from wakefulness to sleep is a key factor in obstructive sleep apnea development as it facilitates upper airway collapse.
What This Study Adds to the Field
To our knowledge, the present study represents the first time upper airway muscle activity was able to be increased during sleep to near-wakefulness levels with a drug administered orally (desipramine) in healthy humans. This study provides a rationale for the pharmacologic treatment of obstructive sleep apnea.
Obstructive sleep apnea (OSA) is a state-dependent disease characterized by a normal breathing pattern during wakefulness and intermittent respiration during sleep due to repetitive collapse of the upper airway. Loss of pharyngeal dilator muscle activation during sleep is central to the pathogenesis of OSA (1). However, attempts to pharmacologically reverse pharyngeal hypotonia that occurs in sleeping humans have thus far been ineffective.
Many neuromodulators are sleep–wake dependent. Neurons promoting wakefulness from the ascending reticular activating system or eliciting sleep from the ventrolateral preoptic area of the hypothalamus (2) also project to respiratory motoneurons and modulate their membrane electrical properties, thus affecting the responses of these motoneurons to a given synaptic input (3).
Recent animal research has shed new light on the mechanism of pharyngeal hypotonia that occurs during sleep, namely that noradrenergic (4) and antimuscarinic (5) processes play a primary role. Withdrawal of endogenous serotonin at the hypoglossal nucleus was long thought to be the primary mechanism underlying decreased pharyngeal muscle activity during sleep (6). However, the studies implicating serotonin were conducted in vagotomized animals (7). It is now known that vagal afferents inhibit serotonin at the hypoglossal nucleus and thus vagotomy will exaggerate the influence of endogenous serotonin. Several recent studies have shown that withdrawal of endogenous serotonin has minimal effects on genioglossus electromyography (EMGGG) in animals with an intact vagus nerve (8, 9). Loss of noradrenergic activity is now thought to play the key role in the non-REM (NREM) sleep–related hypotonia of pharyngeal muscles. Chan and colleagues (4) showed that, in rats, the noradrenergic antagonist terazosin substantially reduces genioglossus activity during wakefulness and produces REM-like atonia during NREM sleep. However, alpha-1 receptor stimulation with phenylephrine at the hypoglossus motor nucleus increases genioglossus activity in NREM sleep compared with placebo, corroborating the importance of noradrenergic mechanisms. While noradrenergic withdrawal is thought to be the main cause of pharyngeal hypotonia in NREM sleep, there are additional mechanisms that cause further reductions in muscle activity during REM sleep. For example, Chan and colleagues (4) failed to reverse REM atonia with alpha-1 receptor agonists applied to the hypoglossal nucleus. Recently, Horner and colleagues identified this inhibitory process as muscarinic by demonstrating restoration of EMGGG during REM sleep with the muscarinic antagonist scopolamine (10, 11).
Despite these findings, there has not yet been a systematic attempt to stimulate the pharyngeal muscles with noradrenergic and antimuscarinic drugs in sleeping humans. Accordingly, we tested the hypothesis that administration of desipramine (200 mg), a tricyclic antidepressant (TCA) with strong noradrenergic, mild antimuscarinic, and mild serotonergic effects, before sleep would increase genioglossus activity during NREM and REM sleep by preventing the reduction in muscle activity that occurs as a function of sleep. The primary outcome variable of this study was EMGGG during sleep. EMGGG sleep values were expressed as a percentage of the preceding quiet wakefulness values. As a secondary outcome, we also assessed the impact of desipramine on the collapsibility of the upper airway under both passive and active conditions.
Methods
Subjects
Healthy subjects aged 18–60 years were included in the study protocol. Individuals were excluded from the study if they had a clinically diagnosed sleep disorder; were taking medications known to influence breathing or muscle physiology; or had allergies to lidocaine, oxymetazoline HCl, or desipramine. Also excluded were subjects with benign prostatic hyperplasia or urinary retention, which can be exacerbated by TCAs; individuals with underlying cardiac disease (i.e., arrhythmias); and individuals taking psychiatric medications such as TCAs for medical care. The protocol was approved by the Partners Institutional Review Board at Brigham and Women’s Hospital. All subjects provided written informed consent before enrollment in the study. The trial is registered with www.clinicaltrials.gov (NCT02428478).
Measurements and Equipment
Anthropometric data were collected on both study nights. In addition to the standard clinical polysomnography montage, subjects breathed through a sealed nasal mask attached to a pneumotachometer (Hans-Rudolph, Shawnee, KS) and a pressure transducer (Validyne Engineering, Northridge, CA). Mask pressure was monitored with a pressure transducer (Validyne Engineering) referenced to atmosphere.
Epiglottic pressure (Pepi) was determined with a small, flexible, pressure-tipped catheter (Millar Instruments, Houston, TX) that was inserted through the decongested (oxymetazoline HCl) and anesthetized (4% lidocaine) nostril until the tip of the catheter was located 1–2 cm caudally to the base of the tongue. EMGGG was recorded as described in our previous studies (12–14). See the online supplement for further details.
Protocol
Two overnight sleep studies were performed 1 week apart: a placebo night and a desipramine (200 mg by mouth) night. We used a double-blind, randomized control design. Randomization was performed by the investigational pharmacy, and all data analysis and subject exclusions were performed before unblinding of the intervention allocation. For each night, the subjects arrived at the sleep laboratory at approximately 7:00 p.m. The placebo or desipramine was administered approximately 2 hours before lights were turned off. Once the patient had been set up for overnight monitoring, the procedures described below were performed.
Wake versus Sleep EMGGG at Atmospheric Pressure
A period of at least 10 minutes of quiet wakefulness was recorded to quantify the subject’s awake EMGGG activity in the lateral position. We chose the lateral position to minimize airway narrowing and thus the effect of the negative pressure reflex on genioglossus activity. It is known that the supine position is associated with increased upper airway resistance and greater Pepi swings that can reflexively increase EMGGG compared with the lateral position (15). At least 30 minutes of data were then collected during subjects’ NREM sleep in the lateral position before upper airway physiology was assessed.
Upper Airway Physiology Using Continuous Positive Airway Pressure Manipulation
Subjects were placed supine and connected to a positive–negative pressure source (Philips Respironics, Murrysville, PA) to enable rapid switching between pressure levels. When stable sleep was reached, the pressure in the mask was increased to the required level to abolish flow limitation, as determined by the airflow waveform and Pepi signals. Following a baseline recording period of 5 minutes, the continuous positive airway pressure (CPAP) level was reduced to varying suboptimal pressures using two approaches:
-
1.
CPAP was decreased gradually (<1 cm H2O/min) to slowly reduce ventilation and thereby increase Pepi swings and pharyngeal muscle activity. During this procedure, we assessed the EMGGG response to progressively greater negative Pepi swings (genioglossus muscle responsiveness) and the upper airway collapsibility under maximally active conditions.
-
2.
CPAP was decreased acutely to subtherapeutic levels to assess upper airway collapsibility under eupneic (passive) conditions.
See the Data Analysis section for further details. After subjects had approximately 2 hours of sleep in these conditions, CPAP was removed and subjects returned to the lateral position so that we could collect additional EMGGG data at atmospheric pressure.
Data Analysis
The EMGGG was quantified in arbitrary units derived from signal processing of the raw signal and as a percentage of wakefulness (%wake) for between-nights comparison of baseline sleep EMGGG activity. EMGGG analysis was performed on a breath-by-breath basis to identify a maximum value and a minimum value during inspiration and expiration, respectively (EMGGG peak and tonic). The difference between peak and tonic values was used to estimate respiratory-related phasic activity.
Wakefulness EMGGG values were obtained from a minimum of 10 epochs (30 s each) with the subject lying in the lateral position. Criteria for breath selection during wakefulness were (1) stable breathing (constant Pepi swings) and (2) absence of movement artifacts (i.e., swallowing, speech, yawns).
As genioglossus respiratory activity has been shown to be highly related to Pepi (16), EMGGG values during sleep were matched for the same Pepi swing range recorded during wakefulness. Criteria for breath selection during sleep were (1) Pepi swings within ±0.5 cm H2O from median Pepi swing during wakefulness, (2) absence of arousal- or postarousal-related genioglossus hyperactivation (arousal length +10 s) (17), and (3) absence of other obvious artifacts (i.e., swallowing, electrical interference). Apneas and hypopneas were scored using standard American Academy of Sleep Medicine guidelines (18), and the arousal index and apnea–hypopnea index (AHI) reported are the values recorded while the subject slept without pressure support.
As described in previous studies (19, 20), we also measured the following for each subject:
-
1.
Genioglossus responsiveness to negative pressure. We calculated the increase in peak EMGGG per change in nadir Pepi across each pressure drop.
-
2.
Passive upper airway collapsibility. We calculated the critical pressure level (passive Pcrit) yielding zero airflow through a passive airway (x-intercept of a linear regression between peak inspiratory flow vs. mask pressure on the third to fifth breaths following CPAP drop, if breaths were flow limited).
-
3.
Active upper airway collapsibility. We calculated the Pcrit yielding zero flow when upper airway muscles were maximally activated without arousal (active Pcrit). After maximally raising pharyngeal muscle activity (via gradual CPAP reduction), CPAP was switched acutely to 0 cm H2O (20) and various other CPAP levels, and the first breath was assessed if flow limited. Similarly to passive Pcrit, the active Pcrit was taken as the x-intercept of the linear flow–pressure relationship.
Statistical Analysis
On the basis of unpublished pilot data, the study was powered to detect an increase in EMGGG by 47 ± 20%wake on desipramine compared with placebo (80% power and 5% level of significance). To reduce the impact of outliers in this small group of subjects, variables were compared using a Wilcoxon matched-pairs signed-rank test, with P < 0.05 considered statistically significant. Data are expressed as median (25th–75th percentile range). Statistical analyses were performed using Prism 6.0 software (GraphPad Software, La Jolla, CA).
Results
Subjects
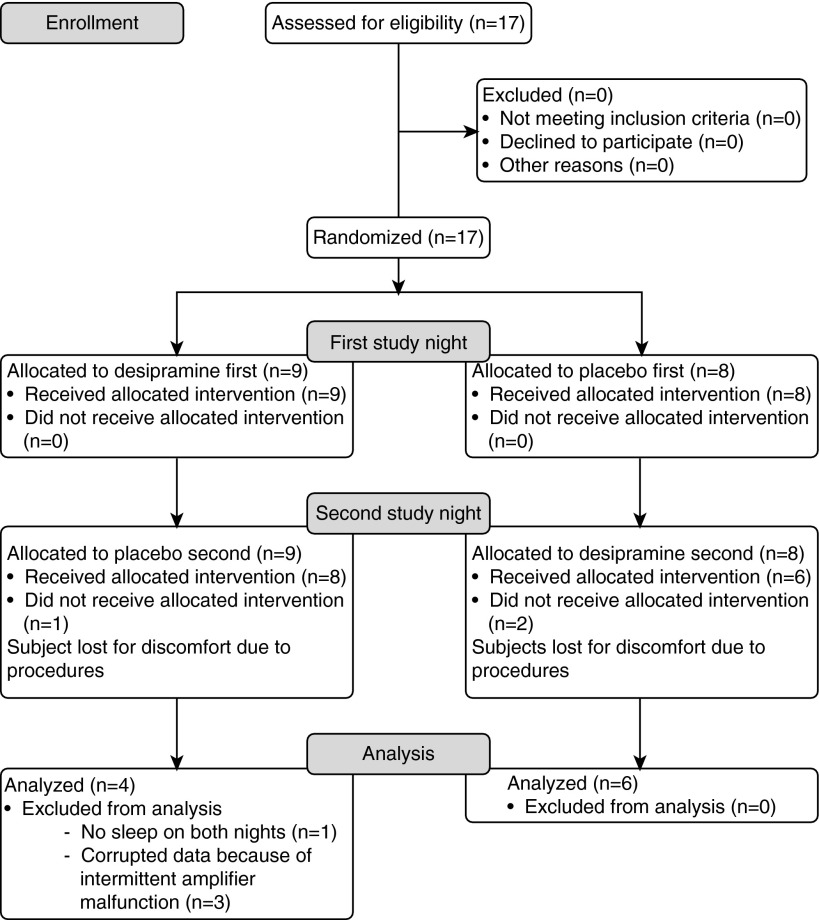
A total of 17 subjects were enrolled in the study. Three subjects did not come back for the second visit of the study protocol; three subjects were excluded due to intermittent electromyographic amplifier malfunction; and one subject did not sleep on both study nights. Thus, data from 10 subjects were analyzed. A diagram of the clinical trial is shown in Figure 1.
Figure 1.
Diagram of the clinical trial.
Anthropometric Data
Table 1 describes the subjects’ characteristics. None of the subjects were taking medications at the time of the study, with the exception of subject 5, who was taking pantoprazole 20 mg for gastroesophageal reflux disease, and subject 10, who was taking iron supplements for anemia. Subject 6 (24 yr old) reported a history of allergic asthma and rheumatoid arthritis; none of these conditions were active at the time of the study.
Table 1.
Anthropometric Characteristics (n = 10 Subjects)
| Characteristic | Value |
|---|---|
| Female sex, % | 60 |
| Age, yr | 27.5 (24.0–39.5) |
| Height, cm | 169.8 (161.3–173.9) |
| Weight, kg | 66.2 (63.3–73.3) |
| BMI, kg/m2 | 23.3 (22.5–26.8) |
| Neck circumference, cm | 35.7 (31.9–37.0) |
| Waist circumference, cm | 81.0 (71.3–83.4) |
Definition of abbreviation: BMI = body mass index.
Data are expressed as median (25th–75th percentile) unless otherwise specified.
Effect of Desipramine on EMGGG Activity during Sleep
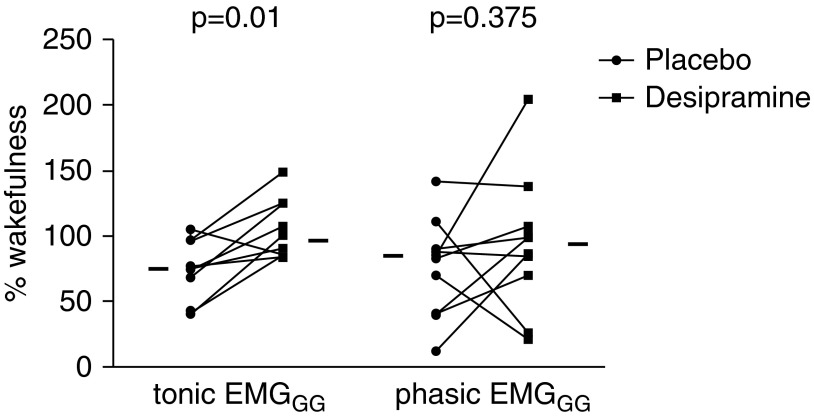
The breaths analyzed during sleep (off CPAP) were 220 ± 66 during placebo nights and 215 ± 67 during desipramine nights. Individual data comparing EMGGG (expressed as a percentage of the awake EMGGG) during NREM sleep between nights are presented in Figure 2. Electromyography recordings from a representative subject are presented in Figure 3. Tonic EMGGG activity was higher during NREM sleep while subjects were on desipramine compared with placebo (96%wake [86–120] vs. 75%wake [50–92]; P = 0.01). Phasic EMGGG activity (peak minus tonic) was not consistently altered by desipramine (84%wake [49–90] on placebo vs. 93%wake [74–106] on desipramine; P = 0.38). Compared with wakefulness, during NREM sleep on the placebo night there was a significant decrease in tonic (P = 0.006) and phasic (P = 0.048) EMGGG activity that did not occur on the desipramine night (P > 0.5 for both tonic and phasic EMGGG).
Figure 2.
Desipramine increases tonic but not phasic genioglossus electromyographic (EMGGG) activity during non-REM sleep. Individual data are shown for EMGGG activity in non-REM sleep during placebo night versus desipramine night. Horizontal lines indicate median percentage of wake values. During placebo nights, there was a median reduction of tonic EMGGG activity of 25.4% of wakefulness value, while on desipramine nights the reduction was only 4.2% of wakefulness value. In contrast, desipramine increased phasic EMGGG activity in only six subjects.
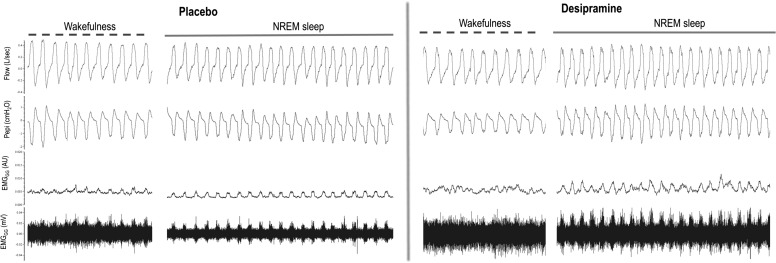
Figure 3.
Sample electromyogram showing the effects of desipramine on genioglossus activity. In this subject, desipramine, compared with placebo, increased tonic and phasic genioglossus activity in non-REM sleep for the same range of epiglottic pressures. AU = arbitrary units; EMGGG = genioglossus electromyography; NREM = non-REM; Pepi = epiglottic pressure.
There was no apparent difference in REM EMGGG activity in the three subjects who had REM sleep on both nights (tonic EMGGG, 92%wake [72–93] on placebo vs. 84%wake [68–91] on desipramine [P > 0.5]; phasic EMGGG, 71%wake [63–123] on placebo vs. 81%wake [53–122] on desipramine [P > 0.5]). A small number of REM epochs while these three subjects were off CPAP were available for EMGGG analysis. These epochs were characterized by periods of intense genioglossus activity interspersed with periods of atonia, and the values of EMGGG activity reported reflect the contribution of both of these patterns of activation during REM sleep.
Muscle responsiveness to progressively greater Pepi swings was unchanged between the placebo and desipramine nights (P > 0.5) (see online supplement). Wakefulness EMGGG tonic and phasic values were similar between nights (see online supplement).
Effects on Upper Airway Collapsibility
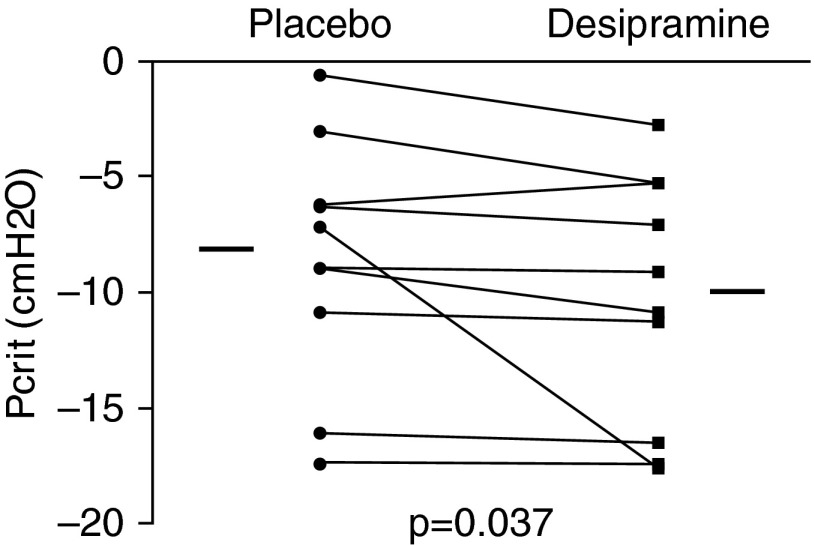
There was a significant reduction in passive Pcrit (less collapsible airway) during the desipramine night compared with the placebo night (Figure 4). With the exception of subject 4, who showed a change of −11 cm H2O in Pcrit while on desipramine, the other subjects showed small drops in Pcrit. The median change in passive Pcrit between placebo and desipramine was −0.6 cm H2O (−2.1 to −0.1). Of note, the CPAP level required to collapse the airway was consistently below zero in these healthy individuals. A post hoc analysis revealed that, during passive Pcrit drops, tonic EMGGG activity (but not phasic activity) was greater while subjects were on desipramine (see online supplement), suggesting that desipramine increased upper airway stiffness by enhancing tonic activity of upper airway muscles at this time. Active Pcrit was unchanged between nights (−10.5 cm H2O [−15.6 to −8.6] on placebo vs. −14.9 cm H2O [−18.5 to −6.8] on desipramine; P > 0.5).
Figure 4.
Individual data showing the effect of desipramine on the passive critical closing pressure (Pcrit). With the exception of one subject who showed a change in Pcrit from −6 cm H2O on placebo to −17.5 cm H2O on desipramine, the other subjects had consistent but small changes in collapsibility, with a median reduction of −0.6 cm H2O on desipramine compared with placebo. Horizontal lines indicate median values.
Effects on Sleep
Noradrenergic stimulation of the central nervous system could have led to sleep disruption. To investigate this possibility, we compared sleep architecture between the placebo and drug nights. The results are shown in Table 2. Desipramine had no significant effect on sleep architecture. The arousal index was also similar between nights, and there was a trend for increased sleep efficiency during the desipramine night compared with the placebo night. However, there were also nonsignificant trends for increased NREM stage 2 sleep and decreased NREM stage 3 and REM sleep with desipramine compared with placebo. Of note, eight subjects reached REM sleep while on placebo, whereas only three subjects reached REM sleep while on desipramine. All the subjects had an AHI less than 1 on both nights, except for subject 5, who had an AHI of 3.2 while on placebo versus 0 events per hour while on desipramine.
Table 2.
Sleep Parameters
| Parameter | Placebo | Desipramine | P Value |
|---|---|---|---|
| TST, min | 292.5 (260.3–347.0) | 320.5 (278.3–366.5) | 0.375 |
| SE, % | 64.8 (58.9–78.9) | 78.1 (68.5–84.9) | 0.065 |
| NREM1, % TST | 17.2 (10.8–23.4) | 21.2 (16.2–27.4) | 0.254 |
| NREM2, % TST | 54.0 (46.8–64.7) | 60.9 (53.7–70.8) | 0.084 |
| NREM3, % TST | 12.1 (9.9–18.0) | 8.0 (3.7–14.9) | 0.131 |
| REM, % TST | 11.5 (6.7–15.3) | 1.0 (0.0–3.1) | 0.071 |
| AHI, events/h | 0.0 (0–0) | 0.0 (0–0) | 0.999 |
| ArI, events/h | 17.0 (12.6–23.4) | 20.1 (11.8–23.5) | 0.275 |
Definition of abbreviations: AHI = apnea–hypopnea index; ArI = arousal index; NREM1 = non-REM sleep stage 1; NREM2 = non-REM sleep stage 2; NREM3 = non-REM sleep stage 3; SE = sleep efficiency; TST = total sleep time.
Data are expressed as median (25th–75th percentile).
Discussion
The main findings of this study are as follows. Compared with placebo, in our healthy adult subjects desipramine (1) significantly increased baseline tonic EMGGG but not phasic activity during NREM sleep, (2) reduced passive upper airway collapsibility, (3) did not change muscle responsiveness to intrapharyngeal negative pressure, and (4) did not significantly alter sleep architecture or sleep efficiency. Taken together, these results have important implications for the pharmacologic treatment of patients with OSA. Indeed, they suggest that raising noradrenergic activity in the central nervous system can restore EMGGG activity to near-wakefulness levels with only relatively minor effects on sleep architecture.
Physiological Insights
To our knowledge, this is the first study to demonstrate that EMGGG activity during sleep in humans can be restored to near-wakefulness levels with a systemically administered drug. From 1982 to 1991, the TCA protriptyline was administered to patients with OSA in several small controlled trials (21–24) in an effort to attenuate OSA severity based on previous anecdotal clinical findings (21). While some patients with OSA in these trials showed an important reduction in their AHI, the mechanisms underlying TCA effects on OSA pathophysiology were poorly understood until Bonora and colleagues showed that, in cats, administration of protriptyline led to a significant increase in hypoglossal and recurrent laryngeal nerve activities compared with baseline (25). TCAs cause an increase in the central nervous system concentration of several monoamines involved in promoting wakefulness, such as noradrenaline, serotonin, and dopamine. In addition, they have antimuscarinic and antihistaminic properties. Desipramine has the highest affinity for the norepinephrine reuptake receptor in this pharmaceutical class, and this affinity correlates with its noradrenergic potency in vivo, which (even at a dose of 100 mg) is superior to that of the other TCAs (26). Consequently, it is reasonable to believe that a single dose of desipramine 200 mg administered before bedtime can cause an increase in norepinephrine concentration at the synaptic level, thereby limiting the physiologic reduction in genioglossus activity associated with NREM sleep. If this is the case, our results would confirm the findings of Chan and colleagues (4) in rat models, where the injection at the hypoglossal motor nucleus of the alpha-1 receptor agonist phenylephrine increased genioglossus activity in NREM sleep compared with placebo. Notably, Chan and coworkers also found that the increase in EMGGG tonic activity was five times higher than the increase in inspiratory-related phasic activity after infusion of phenylephrine. In contrast to Chan’s study, our present study did not reveal an increase in genioglossus activity during wakefulness. This might be related to the longer time needed to reach a minimal effective concentration after administering the drug orally.
Hypoglossal motoneurons receive both inspiratory (phasic) drive and a continuous tonic drive that persists in expiration when inspiratory activation is withdrawn. Consistent with previous reports in the literature (27, 28), our data show a reduction in phasic and tonic EMGGG in healthy individuals during NREM sleep compared with wakefulness. The three major sources of neural input to the hypoglossal motor nucleus are (1) the upper airway mechanoreceptor reflex (negative pressure), which is mediated by the superior laryngeal nerve through the solitary tract nucleus; (2) phasic respiratory inputs from the pre-Botzinger complex, which are modulated by PaCO2 and PaO2 levels; and (3) the wakefulness stimulus provided by the state-dependent monoaminergic system (norepinephrine, serotonin, acetylcholine, histamine, orexin) in the central nervous system (27, 29). While the first two mechanisms contribute mainly to genioglossus inspiratory phasic activation, the wakefulness stimulus is believed to provide primarily the tonic drive to the pharyngeal muscles and thus is responsible for baseline airway size and stiffness (1, 27, 30, 31). The wakefulness stimulus is obviously reduced during sleep as the firing of monoaminergic neurons decreases. Thus, tonic activity should fall. However, this notion has been challenged recently by EMGGG recordings of single motor units (SMUs) in healthy subjects. In these studies, SMUs that fire more rapidly or only during inspiration commonly stop firing completely during the transition from wakefulness to NREM sleep, while tonic units actually increase their firing frequency during NREM sleep (32). The explanation for the clear decrement in tonic activity from multiunit recordings during NREM sleep and the maintenance of activity in tonic SMUs remains elusive at this time. However, only a relatively small proportion of the overall muscle activity can be obtained by using SMU approaches, and quantifying recruitment of additional motor units using standard SMU techniques remains challenging.
Our findings support the notion that changes in the tonic drive to the respiratory motoneurons may be a principal mechanism by which sleep affects EMGGG activity. We observed that inhibition of norepinephrine reuptake at the presynaptic level in the central nervous system, mediated by desipramine, led to maintenance of tonic genioglossus activity close to wakefulness levels, whereas this was not the case for inspiratory phasic activity.
In addition to the increase in baseline tonic EMGGG activity observed in this study, desipramine also reduced passive upper airway collapsibility. We speculate that these two findings are linked and that the passive Pcrit is importantly related to tonic activity of the genioglossus as well as the other pharyngeal dilator muscles on which desipramine might have the same effect, increasing the overall upper airway stiffness. The modest but significant change in EMGGG activity and Pcrit in this group of healthy subjects suggests that this drug (or drugs with a similar profile) could potentially play a role in OSA therapy, depending on how much the Pcrit needs to be changed. It is notable that Younes and colleagues (33) showed that in most patients with OSA the genioglossus activity required to open the airway is minimal, suggesting that even small changes might potentially be clinically important. The lack of a statistically significant effect on phasic EMGGG activity might result in a limited effect of the drug in maintaining upper airway patency in patients with OSA, in whom the increase in phasic activity mediated by upper airway negative pressure reflexes is an important compensatory mechanism capable of reducing OSA severity. However, as shown in Figure 2, 6 of 10 subjects in the present study had an increase in both phasic and tonic EMGGG activity, and it is possible that in patients with OSA desipramine might be able to increase phasic activity more consistently, given the greater magnitude of the Pepi swings these patients experience during sleep.
Limitations
This study has several limitations. First, although the study was powered to determine the statistical significance of our primary outcome variable, EMGGG activity, the sample size is still relatively small. Moreover, additional studies are needed to extend the generalizability of these findings to the OSA population. For this reason, we have undertaken a separate trial in subjects with OSA (registered with www.clinicaltrials.gov [NCT02436031]) to establish whether the findings observed in healthy control subjects are applicable to and can have a meaningful impact on OSA pathophysiology.
Second, we did not measure the plasma concentration of desipramine in our subjects. The rate of metabolism of TCAs varies widely from individual to individual, chiefly on a genetically determined basis. High differences in plasma levels may be noted among individuals taking the same oral dose of desipramine (34). Moreover, desipramine has been shown to have different times to peak concentration between individuals, generally between 4 and 6 hours (35). However, we believe that this limitation is mitigated by the fact that desipramine has a very high affinity for the norepinephrine reuptake receptor, and thus even a small plasma concentration would be expected to saturate the reuptake receptors (26). In this study, we measured the acute effects of desipramine, and thus the long-term effects in humans remain unclear. Nevertheless, in animal studies, chronic administration of desipramine leads to downregulation of the norepinephrine transporter on the presynaptic membrane, which would tend to further increase EMGGG activity (36).
Third, we measured only the activity of the genioglossus muscle and not the other muscles of the upper airway. While this muscle is probably representative of upper airway muscles in general, it has both phasic and tonic activity. Measuring the activity of a tonic muscle, such as the tensor palatini, would help confirm our findings.
Fourth, our results did not allow us to draw any firm conclusions regarding the effects of desipramine on genioglossus activity during REM sleep, as only three subjects had REM sleep on both nights. This phenomenon can be ascribed to both the REM-suppressing effect of TCAs (37) and the sleep disruption induced by our experimental design. Thus, more work is needed to address this question.
Conclusions
In this study, we assessed the effect of the TCA desipramine on genioglossus activity and showed maintenance of tonic EMGGG activity at near-wakefulness levels during NREM sleep and a reduction in upper airway collapsibility. This trial is the first, to our knowledge, to demonstrate that it may be possible to activate pharyngeal muscles with systemically administered drugs in sleeping humans. These findings offer new insights that can be used for the development of pharmacologic treatments for OSA.
Supplementary Material
Footnotes
This research project received generous funding from Fan Hongbing, president of OMPA Corporation, Kaifeng, China. This work was also supported by National Institutes of Health grants R01 HL102321 and P01 HL095491 (A.W.), as well as by Harvard Catalyst Clinical Research Center grant UL1 RR 025758-01. L.T.-M. is supported by the American Heart Association (15POST25480003). B.A.E. is supported by the National Health and Medical Research Council (NHMRC) CJ Martin Overseas Biomedical Fellowship (1035115). S.A.S. was supported by the NHMRC and the Menzies Foundation (1053201, 1035115) and is currently supported by the American Heart Association (15SDG25890059). M.M. is supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Foundation, Ministry of Education of Brazil. D.J.E. is supported by an NHMRC RD Wright fellowship (1049814).
Author Contributions: L.T.-M.: contributed to data collection, data analysis and interpretation, and drafting and review of the manuscript for important intellectual content; B.A.E.: contributed to study design, data collection and interpretation, and review of the manuscript for important intellectual content; S.A.S.: contributed to data collection, data analysis, and review of the manuscript; M.M.: contributed to data collection and review of the manuscript; D.J.E.: contributed to data analysis and review of the manuscript for important intellectual content; D.P.W.: contributed to data analysis and interpretation and review of the manuscript for important intellectual content; and A.W.: contributed to study design, data analysis and interpretation, and drafting and review of the manuscript for important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201511-2172OC on March 14, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Horner RL. Respiratory physiology: central neural control of respiratory neurons and motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. St. Louis, MO: Saunders Elsevier; 2011. pp. 237–249. [Google Scholar]
- 2.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 3.Fung SJ, Chase MH. Postsynaptic inhibition of hypoglossal motoneurons produces atonia of the genioglossal muscle during rapid eye movement sleep. Sleep. 2015;38:139–146. doi: 10.5665/sleep.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan E, Steenland HW, Liu H, Horner RL. Endogenous excitatory drive modulating respiratory muscle activity across sleep–wake states. Am J Respir Crit Care Med. 2006;174:1264–1273. doi: 10.1164/rccm.200605-597OC. [DOI] [PubMed] [Google Scholar]
- 5.Torontali ZA, Grace KP, Horner RL, Peever JH. Cholinergic involvement in control of REM sleep paralysis. J Physiol. 2014;592:1425–1426. doi: 10.1113/jphysiol.2014.271304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubin L, Davies RO, Pack AI. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:91–97. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- 7.Sood S, Liu X, Liu H, Nolan P, Horner RL. 5-HT at hypoglossal motor nucleus and respiratory control of genioglossus muscle in anesthetized rats. Respir Physiol Neurobiol. 2003;138:205–221. doi: 10.1016/j.resp.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med. 2005;172:1338–1347. doi: 10.1164/rccm.200502-258OC. [DOI] [PubMed] [Google Scholar]
- 9.Sood S, Raddatz E, Liu X, Liu H, Horner RL. Inhibition of serotonergic medullary raphe obscurus neurons suppresses genioglossus and diaphragm activities in anesthetized but not conscious rats. J Appl Physiol (1985) 2006;100:1807–1821. doi: 10.1152/japplphysiol.01508.2005. [DOI] [PubMed] [Google Scholar]
- 10.Grace KP, Hughes SW, Shahabi S, Horner RLKK. K+ channel modulation causes genioglossus inhibition in REM sleep and is a strategy for reactivation. Respir Physiol Neurobiol. 2013;188:277–288. doi: 10.1016/j.resp.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Grace KP, Hughes SW, Horner RL. Identification of the mechanism mediating genioglossus muscle suppression in REM sleep. Am J Respir Crit Care Med. 2013;187:311–319. doi: 10.1164/rccm.201209-1654OC. [DOI] [PubMed] [Google Scholar]
- 12.Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–368. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan AS, White DP, Owens RL, Eckert DJ, Rahangdale S, Yim-Yeh S, Malhotra A. The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. J Appl Physiol (1985) 2010;109:469–475. doi: 10.1152/japplphysiol.00373.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 16.Malhotra A, Pillar G, Fogel RB, Edwards JK, Ayas N, Akahoshi T, Hess D, White DP. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–77. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- 17.Jordan AS, Cori JM, Dawson A, Nicholas CL, O’Donoghue FJ, Catcheside PG, Eckert DJ, McEvoy RD, Trinder J. Arousal from sleep does not lead to reduced dilator muscle activity or elevated upper airway resistance on return to sleep in healthy individuals. Sleep. 2015;38:53–59. doi: 10.5665/sleep.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, Schwab RJ, Loring SH, Malhotra A, White DP, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190:930–937. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, Passaglia CL, Jackson AC, Malhotra A, White DP. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985) 2013;114:911–922. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conway WA, Zorick F, Piccione P, Roth T. Protriptyline in the treatment of sleep apnoea. Thorax. 1982;37:49–53. doi: 10.1136/thx.37.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanzel DA, Proia NG, Hudgel DW. Response of obstructive sleep apnea to fluoxetine and protriptyline. Chest. 1991;100:416–421. doi: 10.1378/chest.100.2.416. [DOI] [PubMed] [Google Scholar]
- 23.Brownell LG, West P, Sweatman P, Acres JC, Kryger MH. Protriptyline in obstructive sleep apnea: a double-blind trial. N Engl J Med. 1982;307:1037–1042. doi: 10.1056/NEJM198210213071701. [DOI] [PubMed] [Google Scholar]
- 24.Smith PL, Haponik EF, Allen RP, Bleecker ER. The effects of protriptyline in sleep-disordered breathing. Am Rev Respir Dis. 1983;127:8–13. doi: 10.1164/arrd.1983.127.1.8. [DOI] [PubMed] [Google Scholar]
- 25.Bonora M, St John WM, Bledsoe TA. Differential elevation by protriptyline and depression by diazepam of upper airway respiratory motor activity. Am Rev Respir Dis. 1985;131:41–45. doi: 10.1164/arrd.1985.131.1.41. [DOI] [PubMed] [Google Scholar]
- 26.Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151:737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RA, Eikermann M, Schory K, Dover L, White DP. Influence of wakefulness on pharyngeal airway muscle activity. Thorax. 2007;62:799–805. doi: 10.1136/thx.2006.072488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The influence of obstructive sleep apnea and gender on genioglossus activity during rapid eye movement sleep. Chest. 2009;135:957–964. doi: 10.1378/chest.08-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan AS, White DP. Pharyngeal motor control and the pathogenesis of obstructive sleep apnea. Respir Physiol Neurobiol. 2008;160:1–7. doi: 10.1016/j.resp.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol (1985) 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- 31.Orem J, Osorio I, Brooks E, Dick T. Activity of respiratory neurons during NREM sleep. J Neurophysiol. 1985;54:1144–1156. doi: 10.1152/jn.1985.54.5.1144. [DOI] [PubMed] [Google Scholar]
- 32.Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31:525–533. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Younes M, Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ. Genioglossus activity available via non-arousal mechanisms vs. that required for opening the airway in obstructive apnea patients. J Appl Physiol (1985) 2012;112:249–258. doi: 10.1152/japplphysiol.00312.2011. [DOI] [PubMed] [Google Scholar]
- 34.Tomalik-Scharte D, Lazar A, Fuhr U, Kirchheiner J. The clinical role of genetic polymorphisms in drug-metabolizing enzymes. Pharmacogenomics J. 2008;8:4–15. doi: 10.1038/sj.tpj.6500462. [DOI] [PubMed] [Google Scholar]
- 35.Weiner D, Garteiz D, Cawein M, Dusebout T, Wright G, Okerholm R. Pharmacokinetic linearity of desipramine hydrochloride. J Pharm Sci. 1981;70:1079–1080. doi: 10.1002/jps.2600700929. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Z, Baros AM, Zhang HT, Lapiz MD, Bondi CO, Morilak DA, O’Donnell JM. Norepinephrine transporter regulation mediates the long-term behavioral effects of the antidepressant desipramine. Neuropsychopharmacology. 2008;33:3190–3200. doi: 10.1038/npp.2008.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayers AG, Baldwin DS. Antidepressants and their effect on sleep. Hum Psychopharmacol. 2005;20:533–559. doi: 10.1002/hup.726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.