Abstract
Objective
The primary aim of this trial was to assess the feasibility of MIE in a multi-institutional setting.
Background
Esophagectomy is an important, potentially curative treatment for localized esophageal cancer, but is a complex operation. Minimally invasive esophagectomy (MIE) may decrease the morbidity and mortality of resection, and single-institution studies have demonstrated successful outcomes with MIE.
Methods
We conducted a multi-center, phase II, prospective cooperative group study (coordinated by ECOG) to evaluate the feasibility of MIE. Patients with biopsy-proven high-grade-dysplasia or esophageal cancer were enrolled at 17 credentialed sites. Protocol surgery consisted of either 3-stage MIE or Ivor Lewis MIE. The primary end point was 30-day mortality. Secondary end points included adverse events, duration of hospital-stay, and 3-year outcomes.
Results
Protocol surgery was completed in 95 of the 104 patients eligible for the primary analysis (91.3%). The 30-day mortality in eligible patients who underwent MIE was 2.1%; perioperative mortality in all registered patients eligible for primary analysis was 2.9%. Median intensive care unit and hospital stay were 2 and 9 days, respectively. Grade 3 or higher adverse events included anastomotic leak (8.6%), acute respiratory distress syndrome (5.7%), pneumonitis (3.8%), and atrial fibrillation (2.9%). At a median follow-up of 35.8 months, the estimated 3-year overall survival was 58.4% (95% confidence interval: 47.7%–67.6%). Locoregional recurrence occurred in only 7 patients (6.7%).
Conclusions
This prospective multicenter study demonstrated that MIE is feasible and safe with low peri-operative morbidity and mortality and good oncological results. This approach can be adopted by other centers with appropriate expertise in open esophagectomy and minimally invasive surgery.
Introduction
The incidence of esophageal cancer has been increasing dramatically over the past three decades, and esophageal cancer affects more than 450,000 people worldwide.1,2 While squamous cell carcinoma predominates worldwide, in the western world, including the United States, this profound increase has been due to an increase in the incidence of adenocarcinoma of the esophagus. 1,2 Esophagectomy is an important component of curative treatment for localized esophageal cancer. However, esophageal resection is a complex operation and the mortality of esophageal resection has been significant. In a study from the United States, the mortality of esophagectomy ranged from 8-23% and was dependent upon hospital volume.3 The morbidity associated with esophageal resection has also raised concerns about the procedure, and referral for esophagectomy, despite its therapeutic benefit. In an effort to decrease the morbidity associated with esophagectomy, we and others have adopted a minimally invasive approach to esophageal resection.4-6
A minimally invasive approach to esophagectomy was originally described by Cuschieri and DePaula. 7,8 Since then, minimally invasive esophagectomy has been performed with increasing frequency. 4-6 However, the adoption of minimally invasive esophagectomy (MIE) has been slow, in part because of the complexity of esophagectomy, even when performed by an open technique, and the relatively small number of esophageal resections that are undertaken in most centers. Ideally, a successful MIE program should perform a sufficient number of esophageal resections per year to maintain expertise in postoperative management as well with the technical aspects of the procedure. Additionally, centers should have experience in performing other minimally invasive procedures involving the foregut.
The feasibility of minimally invasive esophagectomy has been previously demonstrated in single-institution studies. Until recently, there were no large, prospective multicenter trials investigating minimally invasive esophagectomy. E2202 is a two-stage, phase II National Cancer Institute (NCI) sponsored study that was coordinated by the Eastern Cooperative Oncology Group (ECOG) and also included participation of credentialed surgeons from the Cancer and Leukemia Group B (CALGB) and the American College of Surgeons Oncology Group (ACOSOG). The primary objective of this trial was to evaluate the safety, feasibility, and outcomes following minimally invasive esophagectomy (MIE) in a multi-institutional setting. This was the first prospective multicenter study of MIE to be undertaken.9
Methods
Study Design
This was a prospective, phase II trial, with a two-stage design to evaluate the feasibility and outcomes after MIE in a multi-institutional setting in centers that had experience in both esophageal surgery and minimally invasive surgical techniques. Feasibility was defined as the ability to carry out MIE without significant perioperative mortality. The primary end point was 30-day mortality. The secondary end points included adverse events (recorded using the Common Terminology Criteria for Adverse Events, Version 3), duration of intensive care unit (ICU) stay, number of lymph nodes removed, and clinical outcomes at 3 years. The study was registered in the National Institute of Health (NIH) clinical trials.gov (identifier NCT00063986). The study was approved as required by each institution's Institutional Review Board (IRB).
Eligibility
Eligible patients included patients with high-grade dysplasia or esophageal cancer of the mid- or distal esophagus, where esophagectomy was planned. Pathological diagnosis was required prior to registration, and the stomach was required to be available for use as a conduit. Patients with a prior antireflux or gastric operation were excluded. Additional exclusion criteria included patients who had a prior right thoracotomy or a prior major neck operation, other than removal of a superficial skin lesion. Laparoscopic or thoracoscopic staging was allowed on the day of surgery. Intra-operative exclusion was also possible, if endoscopic findings or minimally invasive staging demonstrated unexpected findings that made the patient ineligible or demonstrated that the stomach would not be suitable as a conduit. Patients who were converted to an open procedure but still underwent esophagectomy were included in the “intent-to treat” analysis.
Site and Surgeon Credentialing
A total of 17 sites registered patients in this study. Credentialed surgeons from ECOG, CALGB, and ACOSOG participated (See Table, Supplemental Digital Content 1). Surgeon credentialing criteria included:
Surgeon/surgeon group should have performed at least 5 MIE procedures prior to enrolling in the study
Surgeon/surgeon group should perform at least 8 esophageal resections/year at their site
Surgeons should perform at least ten minimally invasive esophageal cases/year at their site.
A skills videotape reviewing the MIE steps and courses on MIE were also part of the credentialing process.
Operative Technique
The operative technique has previously been described.4,10 The procedure had to be performed with a completely minimally invasive approach in the chest (VATS) and abdomen (laparoscopy). No hybrid, hand–assisted laparoscopic, or robotic procedures were allowed. All VATS procedures were performed in a lateral decubitus position. The location (neck versus high chest) and type (hand-sewn or stapled) of anastomosis was at the discretion of the surgeon. Similarly, the use of a feeding jejunostomy tube and inclusion of a gastric emptying procedure, such as a pyloroplasty, was at the discretion of the surgeon.
Outcomes Measured
Adverse events were recorded using the Common Terminology Criteria for Adverse Events, Version 3.0. Length of hospital stay, ICU stay, and home-status (home versus residential care facility after discharge) were also recorded. The recurrence and survival during longer-term follow-up were recorded.
Statistical Methods
A two–stage design was implemented, where 35 patients were planned to be entered into the first stage. If 4 or more deaths occurred (up to 30 days after surgery), no further accrual would occur. If 3 or fewer deaths occurred, then the study would continue to full accrual after an interim analysis. Allowing for 5% ineligibility, total accrual was planned at 105 patients.
Under the design, there was a 0.02 probability of stopping early if the mortality rate was 3% and at least 0.47 probability of stopping early if the mortality rate was ≥10%. If the mortality rate was excessive (≥16%), the probability of stopping was > 0.83. The regimen would be considered safe and feasible if 93 out of 100 patients survived at least 30 days after operation; there was an overall 95% chance of observing this scenario under the null hypothesis of 3% or lower peri-operative mortality, and a 10.9% chance of observing this scenario under the alternative hypothesis of mortality ≥ 10%.
For overall survival and recurrence, Kaplan–Meier methods were used with log-rank tests for comparing differences based on stage. All p-values were two-sided. An “intent-to-treat” analysis was performed for the primary analysis.
Results
Patients
The study was activated in March 2004. After 35 patients were enrolled in the first stage, an interim analysis was conducted. Next, with low mortality in the interim analysis, the study continued to the second stage, reaching a final accrual of 110 patients. One patient was ineligible at registration; a second patient withdrew consent after registration. Of the remaining 108 patients, 4 met intra-operative exclusion criteria, leaving 104 patients eligible for the primary analysis (Figure 1). One additional patient, who was intraoperatively excluded due to advanced disease, was included in the final intent-to-treat analysis. Of the 4 registered patients who met the intra-operative exclusion criteria, 3 had metastatic disease and did not undergo resection. One patient had non-protocol surgery for a cervical tumor and was included in the safety analysis because he still received surgery (n=105).
Figure 1. Consort Diagram.
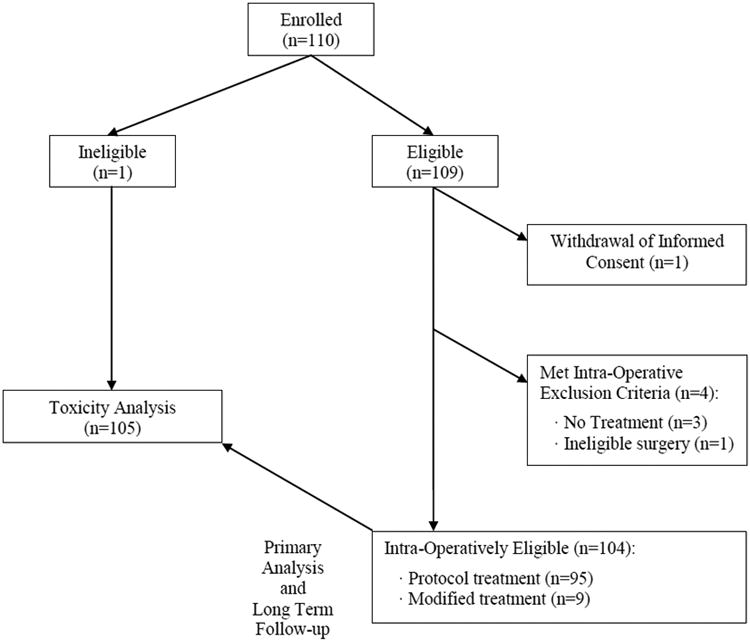
The median age of the 104 patients in the primary analysis was 65 years (range 36–83), with 83 men (79.8%) and 21 (20.2%) women. Patient characteristics are summarized in Table 1. ECOG performance status was 0, 1, and 2 in 71 patients (68.3%), 29 patients (27.9%), and 4 patients (3.8%), respectively. Thirty-five (33.7%) patients received neoadjuvant therapy, consisting of chemotherapy alone in 8 (7.7%) and chemotherapy and radiation in 27 (26%).
Table 1. Patient demographic and disease characteristics.
| Characteristic | Patients in primary analysis; n=104 n (%) (unless noted) |
|---|---|
| Age | |
| Median, years (range) | 65 (36-83) |
| 36-54 years | 22 (21.2%) |
| 55-69 years | 49 (47.1%) |
| 70-83 years | 33 (31.7%) |
| Sex | |
| Male | 83 (79.8%) |
| Female | 21 (20.2%) |
| Primary site | |
| Mid-Thoracic Esophagus | 11 (10.6%) |
| Lower Thoracic Esophagus (Excludes GE Junction) | 38 (36.5%) |
| Gastro-Esophageal Junction | 49 (47.1%) |
| Esophagus, NOS | 4 (3.9%) |
| Other Primary Site | 2 (1.9%) |
| Clinical Staging for Esophageal Cancer | |
| Stage 0 | 9 (9.4%) |
| Stage I | 21 (21.9%) |
| Stage IIa | 22 (22.9%) |
| Stage IIb | 10 (10.4%) |
| Stage III | 32 (33.3%) |
| Unknown Stage | 2 (2.1%) |
| Regional Lymph Node Involvement | |
| Not Involved or No Clinical Evidence of Involvement | 60 (57.7%) |
| Involved | 43 (41.3%) |
| Unknown | 1 (1.0%) |
| Neoadjuvant therapy | 35 (33.7%) |
| Radiation Therapy | 27 (26.0%) |
| Chemotherapy | 35 (33.7%) |
| ECOG performance status* | |
| 0 | 71 (68.3%) |
| 1 | 29 (27.9%) |
| 2 | 4 (3.8%) |
ECOG performance status—0: fully active, able to carry on all pre-disease performance without restriction; 1: restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature; and 2: ambulatory and capable of self-care but unable to carry out any work activity.
GE indicates gastroesophageal; NOS, not otherwise specified.
Operative Outcomes
Protocol surgery was completed in 95 of the 104 patients (91.3%). Non-protocol surgery was performed in 9 patients (8.7%), mostly as a result of elective conversion due to failure to progress; emergent conversion to open surgery for bleeding was required in 2 patients (1 laparotomy, 1 thoracotomy). The use of neoadjuvant therapy did not impact the conversion rate.
The median time for the thoracic component of the MIE surgery was 135 minutes (range 50-528 min) and for the abdominal component was 210 minutes (range 30-527). The median total procedure time (thoracic plus abdominal) was 330 minutes (range 120-813 minutes) and median “skin incision-to-application of the wound dressing” time was 424 minutes (range 148-813). Pyloroplasty was undertaken in 66 patients (64.1%) and a feeding jejunostomy in 100 (96.2%). The median number of lymph nodes removed was 19 (range 2-55). In patients with node-positive disease, the median number of positive lymph nodes was 2 (range 1-12). The resection was complete with negative margins (R0) in 96% of patients. These are summarized in Table 2.
Table 2. Surgery and Pathology Data.
| Characteristic | Patients in primary analysis; n=104 n (%) (unless noted) |
|---|---|
| Primary tumor resection statusa | |
| Complete (R0) | 99 (96.1%) |
| R1 | 2 (1.9%) |
| R2 | 2 (1.9%) |
| Type of anastomosisa | |
| Handsewn | 10 (9.7%) |
| Stapled-EEA | 59 (57.3%) |
| Stapled-GIA | 34 (33.0%) |
| Pylorus procedurea | |
| None | 27 (26.2%) |
| Pyloroplasty | 66 (64.1%) |
| Other | 10 (9.7%) |
| Feeding jejunostomya | |
| None | 3 (2.9%) |
| Needle | 64 (62.1%) |
| Standard | 36 (35.0%) |
| Protocol surgical treatment- MIE | 95 (91.3%) |
| Non-Protocol Surgical Treatment | 9 (8.7%) |
| Length of the operation (min)b | |
| Thoracic component, median (range) | 135.0 (50.0-528.0) |
| Abdominal component, median (range) | 210.0 (30.0-527.0) |
| Total of thoracic and abdominal components, median (range) | 330.0 (120.0-813.0) |
| Skin incision to wound dressing time, median (range) | 423.5 (148.0-813.0) |
| Duration of intensive care stayc | |
| Number of post-op days in ICU, median (range) | 2 (0-39) |
| Effectiveness of the lymph node dissection by MIE | |
| Number of lymph nodes removed, median (range)a | 19 (2-55) |
| Number of positive lymph nodes, median (range)a | 2 (1-12) |
EEA, end-to-end anastomosis; GIA, gastrointestinal anastomosis; ICU, intensive care unit; MIE, minimally invasive esophagectomy.
data missing for 1 patient
data missing for 11 patients
data missing for 3 patients
Peri-operative Outcomes
Median hospital stay was 9 days (range 4-138 days), and median ICU stay was 2 days (range 0-39 days). Three deaths (2.9%) occurred within 30 days after surgery. The 30-day mortality rate in eligible patients who received MIE (n=95) was 2.1%. Of the 105 patients included in the safety analysis, grade 3 or grade 4 adverse events occurred in 52 patients (49.5%) within 30 days after surgery. The incidence of peri-operative complications, which have traditionally been of concern with esophagectomy, are reported in Table 3. Grade 3 or higher complications included pneumonitis/pulmonary infiltrates (3.8%), anastomotic leak (8.6%), acute respiratory distress syndrome (ARDS; 5.7%) and atrial fibrillation (2.9%).
Table 3. Grade 1- 4 Adverse Events Typically Associated With Esophagectomy within 30 Days After Surgery.
| Adverse Event | Grade 1 or 2 (n) | Grade 3 (n) | Grade 4 (n) | Total Patients With Grade 3 or Higher Complications (n = 105), n (%) |
|---|---|---|---|---|
| Atrial fibrillation | 14 | 2 | 1 | 3 (2.9%) |
| Anastomotic leak | 3 | 6 | 3 | 9 (8.6%) |
| Pneumonitis/pulmonary infiltrates | 1 | 3 | 1 | 4 (3.8%) |
| ARDS | 0 | 0 | 6 | 6 (5.7%) |
| Chylothorax | 2 | 1 | 0 | 1 (1%) |
| Dysphagia | 8 | 12 | 2 | 14 (13.3%) |
| Esophageal Stenosis | 0 | 6 | 0 | 6 (5.7%) |
ARDS, acute respiratory distress syndrome
Long-term outcomes
Survival
At a median follow-up of 35.8 months, 64 patients were alive. The estimated 1-year overall survival was 80.5% [95% confidence interval (CI): 71.4%–86.9%]; estimated 2-year overall survival was 68.0% (95% CI: 57.9%–76.2%), and estimated 3-year overall survival was 58.4% (95% CI: 47.7%–67.6%) (Fig. 2A). Survival was stratified as per pathological staging (AJCC 6th Edition). With regard to stage II subsets, there was no significant difference in overall survival between stages IIa (n = 18) and IIb (n = 14) with widely overlapping confidence intervals; the stratified log-rank test did not show any significant difference with P value of 0.31. Neither of 95% CIs (2-sided) of median overall survival for stages IIa and IIb was available due to the high survival rates. Ten of 18 patients with stage IIa disease and 2 of 14 patients with stage IIb disease received neoadjuvant therapy. We were not able to make an inference about the impact of neoadjuvant therapy on overall survival in patients with stage IIa or IIb esophageal cancer (due to too small sample size). The median overall survival for all patients has not been reached. Survival by pathological stage is demonstrated in Figure 2B.
Figure 2.
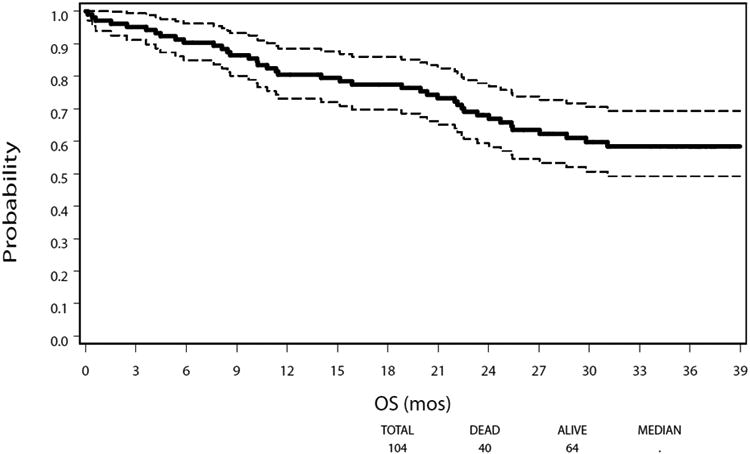
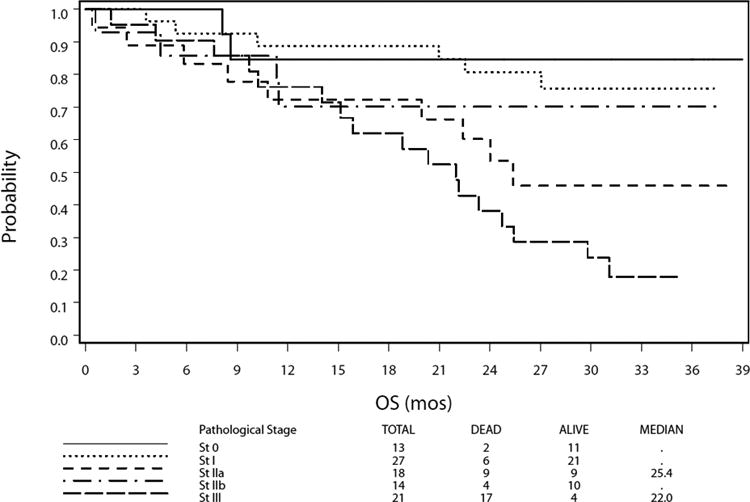
Overall Survival After Minimally Invasive Esophagectomy. A. Kaplan-Meier plot of estimated overall survival for the study cohort. Dotted lines denote a 95% confidence band for the probability of survival. The median overall survival was not reached. B. Kaplan-Meier plot of estimated overall survival stratified by pathological stage. NR indicates not reached; OS, overall survival.
Recurrence
During follow-up, recurrence occurred in 29 of the 102 patients for whom disease follow-up data was available (28.4%). The estimated 3-year recurrence rate was 33.8% (95% CI 24.5%- 55.3%). Loco-regional recurrence occurred in 7 of 102 patients (6.9%), distant recurrence in 19 (18.6%), and both loco-regional and distant recurrence in 2 patients (2.0%). The site of recurrence was not determined in one patient.
Discharge Status
Most patients (92/102, 90.2%) were living at home and only 8 (7.8%) required admission or readmission to a care facility during follow-up. It is not known whether admission was related to the protocol therapy, the diagnosis of esophageal cancer, or for other reasons.
Discussion
In this Phase II, multicenter trial investigating MIE, with participation from ECOG, CALGB and ACOSOG centers, we have shown that MIE is safe and feasible, with acceptable perioperative and oncological outcomes. The optimal approach to esophagectomy remains controversial. Previously, a randomized study was performed in the Netherlands comparing an extended transthoracic approach (with en bloc lymphadenectomy) with a transhiatal approach in 220 patients.11 No significant difference in survival was noted between the two groups of patients. The overall mortality in that series was 3%, again with no difference noted between the two groups. The authors then published a follow-up report with complete 5-year survival data.12 Although there was no difference in 5-year survival results (34 versus 36%), sub-group analysis suggested an advantage to the transthoracic approach in patients with tumors that were confined to the esophagus without involvement of the cardia.12 In this subgroup, a superior overall survival of 51% compared with 37% (p=0.033) was seen when a transthoracic esophagectomy was performed. An improvement in locoregional disease-free survival was also seen with patients who had less than 8 involved lymph nodes when a transthoracic esophagectomy was performed. There was no significant differences in survival between these approaches in patients with N0 disease, or in those with 8 or more involved nodes. These data suggest that outcomes may be better with a transthoracic approach in the subset of patients with limited nodal involvement. A VATS approach could, in theory, achieve these same oncological goals, but hopefully with decreased morbidity compared with thoracotomy.
The morbidity and mortality after open esophagectomy has been a major concern. In a series of 1777 patients undergoing esophagectomy in the prospective United States Department of Veterans Affairs database, peri-operative mortality was 10% and morbidity was 50%.13 Other studies have also demonstrated high mortality, particularly when operations are performed in low-volume centers - where mortality as high as 23% has been reported.3,14 However, the results in more recent series, particularly from experienced centers and surgeons appear to be better than those reported above. The Society of Thoracic Surgeons recently published results from their database. A total of 2315 esophagectomies were performed at 73 centers.15 Hospital mortality was only 2.7% in this report. Interestingly, a study using national Medicare data, looked at trends in esophagectomy, as one of 8 different cancer or cardiovascular operations.16 From 1999 to 2008, there was a shift of ∼32% of esophagectomy cases to high-volume hospitals. This has resulted in an 11% decrease in operative mortality.
For patients who are symptomatic with dysphagia, or those with more locally advanced disease, the risks of esophagectomy may be acceptable, but for patients who are relatively asymptomatic with high-grade dysplasia or intramucosal cancer, nonoperative approaches, such as endoscopic resection and ablative techniques, are becoming increasingly popular.17 However, the risk of missing occult invasive cancer and nodal disease remains a concern after endoscopic therapy.18-20 Minimally invasive esophagectomy may be more acceptable as patients decide between resection of the esophagus, which provides an oncologically sound option, or an esophagus-sparing approach, which requires commitment to multiple, routine follow-up endoscopies.
Swanstrom and colleagues in an important study published the first series of MIE in North America, and this was performed with a transhiatal approach.21 Luketich and colleagues then published an initial series of MIE that included a VATS mobilization of the esophagus with lymph node dissection.22 In 2003, this experience was updated to include 222 patients from a single center.4 Operative mortality in this larger series was 1.4%. Around the time of this publication, several other sites in the USA were beginning to implement MIE programs. This led to the development of the E2202 study discussed in this manuscript. Similarly, other centers around the world have now started to develop MIE programs. A center from Europe recently reported a case-controlled series comparing open esophagectomy and MIE.23 However, some of the patients in the MIE group in their series underwent laparotomy rather than laparoscopy. Despite this, morbidity was significantly less in the MIE group (25% versus 74%; p=0.014). Respiratory complications were also lower in patients who had VATS rather than thoracotomy (9.7% versus 38.7%; p=0.008). Another group from Australia also compared their experience with MIE and open esophagectomy.24 Similar to the European study, the abdominal portion of the procedure in the MIE group did not consistently include laparoscopy, although VATS was routinely performed. As with the European Study, the use of MIE led to a lower incidence of respiratory complications, which has been shown in other studies to be a factor predictive of mortality.25 The overall mortality in the Australian study was 3.5%. The specific mortality for each group was not provided. Recently, the outcomes in 1011 patients who underwent minimally invasive esophagectomy from a single institution were reported.5 The median number of lymph nodes resected was 21. The operative mortality was 1.68%, median ICU stay was 2 days, and length of hospital stay was 8 days, suggesting that MIE can be performed safely, with good results in an experienced center. In one systematic review of over 1000 patients, MIE was found to be associated with lesser overall complication rates, and a shorter ICU and hospital stay.26 Another meta-analysis comparing MIE with an open approach to esophageal resection showed comparable lymph node dissection, and no differences in 30-day mortality or 3-year survival.27
Recently, the early postoperative results of a randomized trial comparing open esophagectomy and MIE were reported.28 In this study, the primary end point was a difference in respiratory complications. A total of 5 institutions participated; 59 patients were randomly assigned to the MIE group and 56 underwent an open esophagectomy. The hospital length of stay was shorter in patients who underwent MIE (11 days vs. 14 days), and there were fewer pulmonary infections in the MIE group (9% within 2 weeks of MIE vs. 29% after open esophagectomy). In our series, grade 3 and higher pneumonitis occurred in 3.8% of patients, which is comparable with the MIE arm of the trial.
Further, the oncological outcomes in the current ECOG trial were good with an estimated 3-year overall survival of 58.4%, and a low loco-regional recurrence of 6.9%. The median survival was not reached. The estimated overall survival of 58.4% at 3 years is acceptable when compared with published series of open esophagectomy. In Hulscher's randomized study of transthoracic esophagectomy compared with transhiatal esophagectomy, 5-year survival was 36% and median survival was just over 2 years in the transthoracic group.5 Another report of en-bloc esophagectomy included 40 patients with a median follow-up of 34.1 months.29 Overall survival was 51% at 5 years in this study. These results suggest that MIE can provide equivalent oncological outcomes as compared with open transthoracic esophagectomy.
Conclusions
In conclusion, this prospective cooperative group study demonstrates that MIE is feasible, and can be undertaken with low mortality in a multicenter setting. The study is the largest and the first prospective multicenter trial designed to investigate the use of MIE. Unlike some previous studies, a completely minimally invasive approach in both the chest and abdomen was used in all patients. Our 30-day mortality was low, morbidity was acceptable and this study demonstrates the feasibility of this approach in a multicenter setting. However, it should also be emphasized that the procedures were performed by credentialed surgeons with demonstrated experience in esophageal surgery and minimally invasive techniques. In addition, at a median follow-up of 36 months, oncological outcomes are acceptable. This approach can be adopted by other centers, provided that appropriate expertise with both open esophagectomy and minimally invasive techniques is available in those centers.
Supplementary Material
Supplementary Digital Content 1: ECOG 2202 Minimally Invasive Esophagectomy Trial: Accrual by Group and Institution (with number of surgeons in each institution)
Acknowledgments
Source of Funding: This study was coordinated by the Eastern Cooperative Oncology Group and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA39229, CA32291, CA17145 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services.
Footnotes
Conflicts of Interests: The authors have no relevant conflicts of interest related to this manuscript.
References
- 1.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 4.Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238:486–94. doi: 10.1097/01.sla.0000089858.40725.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luketich JD, Pennathur A, Awais O, et al. Outcomes After Minimally Invasive Esophagectomy: Review of Over 1000 Patients. Ann Surg. 2012;256:95–103. doi: 10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palanivelu C, Prakash A, Rangaswamy S, et al. Minimally invasive esophagectomy: Thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position. An experience of 130 patients J Am Coll Surg. 2006;203:7–16. doi: 10.1016/j.jamcollsurg.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Cuschieri A, Shimi S, Banting S. Endoscopic oesophagectomy through a right thoracoscopic approach. J R Coll Surg Edinb. 1992;37:7–11. [PubMed] [Google Scholar]
- 8.Depaula AL, Hashiba K, Ferreira EA, et al. Laparoscopic transhiatal esophagectomy with esophagogastroplasty. Surg Laparosc Endosc Percut Tech. 1995;5:1–5. [PubMed] [Google Scholar]
- 9.Luketich JD, Pennathur A, Catalano PJ, et al. Results of a phase II multicenter study of MIE (Eastern Cooperative Oncology Group Study E2202) J Clin Oncol. 2009;27:15s. suppl; abstr 4516. [Google Scholar]
- 10.Pennathur A, Awais O, Luketich JD. Technique of Minimally Invasive Ivor Lewis Esophagectomy. Ann Thorac Surg. 2010;89:S2159–S2162. doi: 10.1016/j.athoracsur.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 11.Hulscher JB, Van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Eng J Med. 2002;347:1662–9. doi: 10.1056/NEJMoa022343. [DOI] [PubMed] [Google Scholar]
- 12.Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhital resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg. 2007;246:992–1000. doi: 10.1097/SLA.0b013e31815c4037. [DOI] [PubMed] [Google Scholar]
- 13.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75:217–22. doi: 10.1016/s0003-4975(02)04368-0. [DOI] [PubMed] [Google Scholar]
- 14.Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119:1126–32. doi: 10.1067/mtc.2000.105644. [DOI] [PubMed] [Google Scholar]
- 15.Wright CD, Kucharczuk JC, O'Brien SM, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic database risk adjustment model. J Thorac Cardiovasc Surg. 2009;137:587–95. doi: 10.1016/j.jtcvs.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Eng J Med. 2011;364:2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Eng J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 18.Fernando HC, Murthy SC, Hofstetter W, et al. The Society of Thoracic Surgeons practice guideline series: guidelines for the management of Barrett's esophagus with high-grade dysplasia. Ann Thorac Surg. 2009;87:1993–2202. doi: 10.1016/j.athoracsur.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Pennathur A, Farkas A, Krasinskas AM, et al. Esophagectomy for T1 esophageal cancer: outcomes in 100 patients and implications for endoscopic therapy. Ann Thorac Surg. 2009;87:1048–55. doi: 10.1016/j.athoracsur.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altorki NK, Lee PC, Liss Y, et al. Multifocal neoplasia and nodal metastases in T1 esophageal carcinoma: implications for endoscopic treatment. Ann Surg. 2008;247:434–9. doi: 10.1097/SLA.0b013e318163a2ff. [DOI] [PubMed] [Google Scholar]
- 21.Swanstrom LL, Hanson P. Laparoscopic total esophagectomy. Arch Surg. 1997;132:943–949. doi: 10.1001/archsurg.1997.01430330009001. [DOI] [PubMed] [Google Scholar]
- 22.Luketich JD, Schauer PR, Christie NA, et al. Minimally invasive esophagectomy Ann Thorac Surg. 2000;70:906–11. doi: 10.1016/s0003-4975(00)01711-2. [DOI] [PubMed] [Google Scholar]
- 23.Schoppmann, Prager G, Langer FB, et al. Open versus minimally invasive esophagectomy: a single-center case controlled study. Surg Endosc. 2010;24:3044–3053. doi: 10.1007/s00464-010-1083-1. [DOI] [PubMed] [Google Scholar]
- 24.Zingg U, Smithers BM, Gotley DC, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol. 2011;18:1460–8. doi: 10.1245/s10434-010-1474-5. [DOI] [PubMed] [Google Scholar]
- 25.Atkins BZ, Shah AS, Hutceson KA, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg. 2004;78:1170–6. doi: 10.1016/j.athoracsur.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir. 2009;64:135–46. [PubMed] [Google Scholar]
- 27.Sgourakis G, Gockel I, Radtke A, et al. Minimally invasive versus open esophagectomy: meta-analysis of outcomes. Dig Dis Sci. 2010;55:3031–40. doi: 10.1007/s10620-010-1153-1. [DOI] [PubMed] [Google Scholar]
- 28.Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–92. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 29.Rizetto C, DeMeester SR, Hagen JA, et al. En bloc esophagectomy reduces local recurrence and improves survival compared with transhiatal resection after neoadjuvant therapy for esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2008;135:1288–3. doi: 10.1016/j.jtcvs.2007.10.082. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Digital Content 1: ECOG 2202 Minimally Invasive Esophagectomy Trial: Accrual by Group and Institution (with number of surgeons in each institution)


