Structured Abstract
Background
Individuals with chronic kidney disease, particularly those requiring dialysis, are at high risk of sudden cardiac death (SCD). However, comprehensive data for the full-spectrum of kidney function and SCD risk in the community are sparse. Furthermore, newly developed equations for estimated glomerular filtration rate (eGFR) and novel filtration markers might add further insight to the role of kidney function in SCD.
Methods
We investigated the associations of baseline eGFRs using either serum creatinine, cystatin C, or both (eGFRcr, eGFRcys, and eGFRcr-cys), cystatin C itself, and β2-microglobulin (B2M) with SCD (205 cases through 2001) among 13,070 blacks and whites ARIC participants at baseline during 1990–92 using Cox regression models accounting for potential confounders.
Results
Low eGFR was independently associated with SCD risk: for example, HR for eGFR <45 vs ≥90 ml/min/1.73m2 was 3.71 (95%CI 1.74–7.90) with eGFRcr; 5.40 (2.97–9.83) with eGFRcr-cys; and 5.24 (3.01–9.11) with eGFRcys. When eGFRcr and eGFRcys were included together in a single model, the association was only significant for eGFRcys. When three eGFR, cystatin C, and B2M were divided into quartiles, B2M demonstrated the strongest association with SCD (HR for 4th quartile vs 1st quartile 3.48 (2.03–5.96) vs. ≤2.7 for the other kidney markers).
Conclusions
Kidney function was independently and robustly associated with SCD in the community, particularly when cystatin C or B2M was used. These results suggest the potential value of kidney function as a risk factor for SCD and the advantage of novel filtration markers over eGFRcr in this context.
Index Words: Sudden cardiac death (SCD), estimated glomerular filtration rate (eGFR), kidney function, cystatin C, β2-microglobulin (B2M), β-trace protein (BTP), chronic kidney disease (CKD), Atherosclerosis Risk in Communities (ARIC) Study
Sudden cardiac death (SCD), a sudden and unexpected pulseless condition with cardiac etiology, is a public health issue worldwide.1 In the U.S., 180,000 to 450,000SCD cases are estimated to occur every year2 and account for 7% to 18% of all deaths.3, 4 Since SCD can occur out-of-hospital before the chance of any medical care and 25% of those with out-of-hospital cardiac arrest have no symptoms before the onset2, it is critical to identify individuals at high risk and try to prevent SCD.1
Chronic kidney disease (CKD) is a well-known risk factor of cardiovascular mortality.5 Individuals with CKD have similar mortality risk to those with prior myocardial infarction (MI).6 Kidney dysfunction is associated with risk of SCD in several studies.7–13 However, these studies were conducted in selected populations with coronary artery disease,9, 11 heart failure,7, 8, 10 end-stage renal disease,12 or exclusively older individuals,13 leaving uncertainty as to whether kidney function is associated with SCD in a middle-aged general population.
Recently, new equations for eGFR using serum creatinine and/or cystatin C (eGFRcr, eGFRcys, and eGFRcr-cys) were designed. eGFRcr-cys showed greater accuracy and better prognostication than GFRcr.14–16 However, these equations have not been studied in the context of SCD. Furthermore, several novel markers of kidney function such as β2-microglobulin (B2M) and β-trace protein (BTP) may more accurately estimate kidney function and predict cardiovascular disease and mortality beyond serum creatinine and cystatin C.17–19 Thus, the objective of this study was to comprehensively investigate kidney function assessed with various filtration markers and its relationship to SCD in middle-aged individuals from a community-based cohort, the Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study Participants
The ARIC Study consists of 15,792 individuals aged 45 to 64 years at baseline (1987–1989), from four U.S. communities in North Carolina, Mississippi, Minnesota, and Maryland. Details of the ARIC study have been described elsewhere.20 The current study used visit 2 (1990–92) as baseline, at which 14,348 participants attended and B2M and cystatin C were measured along with serum creatinine. Participants were excluded from the study if they did not have records of B2M (n=975), cystatin C (n=88), follow-up (n=173), or if they were of non-black, non-white ethnicity (n=42), for a final study sample of 13,070 participants. We repeated the analysis using data at visit 4 (1996–98), when BTP was assessed in addition to serum creatinine, cystatin C and B2M. This sensitivity analysis consisted of 10,406 participants out of 11,656 participants at visit 4, after excluding those who did not have data of cystatin C, B2M, or BTP (n=1,069) or follow-up (n=150) or who were non-black and non-white (n=31).
Kidney Function Markers
eGFR was calculated using the CKD-EPI equations based on serum creatinine, cystatin C, and both (eGFRcr, eGFRcys, and eGFRcr-cys, respectively).14, 15 Creatinine was measured at visit 2 in serum specimens and at visit 4 in plasma specimens by the modified kinetic Jaffé method. Cystatin C was measured using the Gentian immunoassay and B2M was measured using Roche B2M reagent on the Roche Modular P800 Chemistry analyzer in stored serum samples at visit 2. Cystatin C, B2M, and BTP were measured at visit 4 by a particle-enhanced immunonephelometric assay with a BNII nephelometer (Siemens Healthcare Diagnositics). Reliability coefficients after removing outliers (>3 standard deviation differences) for masked replicate samples were ≥0.94 for these filtration markers.21
Covariates
At every visit, participants reported information on smoking and alcohol intake, underwent a physical examination, and provided blood samples. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or treatment for hypertension. Body mass index was calculated as weight in kilograms divided by height in meters squared. Obesity was defined as body mass index (BMI) ≥30 kg/m2. Education was categorized as advanced (completed college or more), intermediate (high school to less than college), and no or basic (less than high school). Diabetes mellitus (DM) was defined as fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, treatment for diabetes mellitus, or a self-reported physician diagnosis of diabetes mellitus. High-density lipoprotein (HDL) cholesterol level was determined using enzymatic methods, and low-density lipoprotein (LDL) cholesterol level was calculated using the Friedewald equation. Prevalent coronary heart disease (CHD) was defined as self-reported CHD or the presence of a previous MI by electrocardiogram at visit 1 or subsequent CHD events prior to the visit of interest. Incident CHD was defined by a definite or probable MI, coronary angioplasty, and coronary artery bypass surgery.22 Cornell voltage for left ventricular hypertrophy (sum of S amplitude in V3 and R amplitude in aVL) and heart rate were obtained from electrocardiogram. Prevalent heart failure (HF) was defined as self-reported use of HF medications within 2 weeks or “manifest” HF by Gothenburg criteria.23 Incident HF was defined as the first occurrence of either a hospitalization that included an International Classification of Disease, 9th Revision (ICD-9) discharge code of 428 (428.0–428.9) among the primary or secondary diagnoses.23
Identification of Sudden Cardiac Death
The ARIC study performs continuous and comprehensive surveillance for all potential cardiovascular-related hospitalizations and deaths in the four communities. A group of physicians reviews medical chart of potential cases and adjudicates CHD cases. Possible fatal CHD in the ARIC Study is intended to broadly capture deaths with any signs or history of cardiovascular disease and is not usually included in the CHD outcome. Of these cases, to identify SCD cases, a sudden pulseless condition presumed to be due to a ventricular tachyarrhythmia, a separate group of physicians classified definite and possible fatal CHD cases into definite sudden arrhythmic death, possible sudden arrhythmic death, not sudden arrhythmic death, or unclassifiable.23, 24 Definite and possible sudden arrhythmic deaths composed SCD outcome for this study.24 Those participants who did not develop SCD were censored at earlier of either death other than SCD or administratively censored at December 31, 2001.
Statistical Analysis
All statistical analyses were performed using Stata 13.1 for Windows (Stata Co., College Station, Texas), and P <0.05 was considered statistically significant. Baseline characteristics were summarized according to the status of SCD during follow-up.
We used Poisson regression models to estimate incidence rates of SCD based on eGFR with linear splines after adjustment for age, sex, and race. Knots at 45, 60, 75, 90, and 105 ml/min/1.73m2 were selected according to eGFR clinical thresholds and previous literature.16 Subsequently, the association of clinical eGFR categories with SCD was quantified using Cox proportional hazards models. eGFR category 3B, 4, and 5 (30–44, 15–29, and <15 ml/min/1.73m2, respectively) were merged due to a relatively small number of participants in these categories. Three models were constructed to evaluate independent associations of kidney function with SCD. Model 1 adjusted for age, sex, race, and field center. Model 2 additionally included education level, CHD, HF, DM, hypertension, heart rate, Cornell voltage, BMI, HDL and LDL cholesterols, current drinking, and current smoking. Model 3 was intended to evaluate the independence across kidney markers and thus further adjusted for eGFRcr in the analysis for eGFRcys, cystatin C, and B2M and eGFRcys in the analysis of eGFRcr. To compare all five kidney function markers, (eGFRcr, eGFRcr-cys, eGFRcys, cystatin C, and B2M), each marker was categorized by quartile, with quartile 1 (best kidney function) serving as reference.
To appreciate any unique aspects of kidney function markers in terms of SCD risk, we also tested their associations with all-cause mortality and non-SCD (all-cause mortality excluding SCD). Seemingly unrelated regression models were used to compare hazard ratios (HRs) of different mortality outcomes according to kidney markers.
We conducted a few sensitivity analyses to assess the robustness of our findings. In Model 2, we further adjusted for corrected QT interval (QT interval divided by squared RR interval) or incident CHD and HF as time-varying covariates. We repeated the main analyses excluding participants on dialysis at baseline (n=12). Stratified analyses were also performed based on age (below vs above median (57 years)), sex, race, CHD, HF, DM, hypertension, and obesity at baseline. To obtain reliable estimates with adequate events in each subgroup, we contrasted top two versus bottom two quartiles of each GFR and kidney function marker. Interaction was assessed using the likelihood ratio test for models with and without interaction terms. Finally, we repeated the analysis using data at visit 4, allowing the additional assessment of BTP. As visit 4 had a shorter follow-up and less SCD cases, we assessed tertiles of kidney measures.
Source of Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This study was also supported by unrestricted research fund from Kyowa Hakko Kirin and a grant R01DK089174 to Dr. Selvin. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Among 13,070 blacks and whites at the second visit (1990–92) of the ARIC Study, 205 participants developed SCD during a median of 11.2 years of follow-up (incidence rate: 1.4 per 1,000 person-years). Basic characteristics of the cohort are shown in Table 1 based on incidence of SCD during the follow-up. Those who developed SCD were more likely to be older, male, African American, and smokers and have diabetes, hypertension, dyslipidemia, history of CHD and HF, and higher Cornell voltage, compared to those without SCD during follow-up.
Table 1.
≥Baseline Characteristics by SCD status
| SCD | No SCD | |
|---|---|---|
| Number | 205 | 12865 |
| Age | 59.6 (5.5) | 56.9 (5.7) |
| Male (%) | 136 (66) | 5599 (44) |
| African American (%) | 83 (40) | 3160 (25) |
| Education | ||
| Advanced | 55 (27) | 4754 (37) |
| Intermediate | 66 (32) | 5283 (42) |
| No or Basic | 83 (41) | 2709 (21) |
| Current drinking (%) | 96 (47) | 7287 (57) |
| Current smoking (%) | 73 (36) | 2799 (22) |
| Diabetes mellitus (%) | 83 (40) | 1853 (14) |
| Hypertension (%) | 131 (64) | 4520 (35) |
| Coronary heart disease (%) | 72 (36) | 679 (5) |
| Heart failure (%) | 29 (14) | 597 (5) |
| Systolic blood pressure (mmHg) | 132.2 (25.6) | 121.2 (18.6) |
| Diastolic blood pressure (mmHg) | 73.5 (12.5) | 72.2 (10.3) |
| Body mass index (kg/m2) | 28.9 (5.6) | 28.0 (5.4) |
| Heart Rate (bpm) | 68.7 (12.8) | 65.9 (10.0) |
| Cornell Voltage (uV) | 1625 (763) | 1237 (548) |
| LDL cholesterol (mg/dL) | 143.5 (43.4) | 133.2 (36.7) |
| HDL cholesterol (mg/dL) | 41.7 (13.1) | 49.7 (16.8) |
| eGFRcr category (%) | ||
| ≥ 90 | 107 (52) | 9394 (73) |
| 60–89 | 73 (36) | 3231 (25) |
| 45–59 | 16 (8) | 172 (1.3) |
| <45 | 9 (4) | 68 (0.5) |
| eGFRcr (ml/min/1.73 m2) | 86.9 (22.2) | 96.6 (15.6) |
| eGFRcr-cys (ml/min/1.73 m2) | 81.2 (23.1) | 95.4 (16.9) |
| eGFRcys (ml/min/1.73 m2) | 75.0 (23.8) | 91.0 (18.2) |
| Cystatin C (mg/L) | 1.11 (0.51) | 0.88 (0.29) |
| β2-microglobulin (mg/L) | 2.6 (2.0) | 2.0 (1.4) |
Data are presented as mean (SD), n (%). eGFRcr indicates estimated GFR based on serum creatinine; eGFRcr-cys, eGFR based on creatinine and cystatin C; eGFRcys, eGFR based on cystatin C.
eGFR based on Creatinine and Cystatin C and SCD Risk
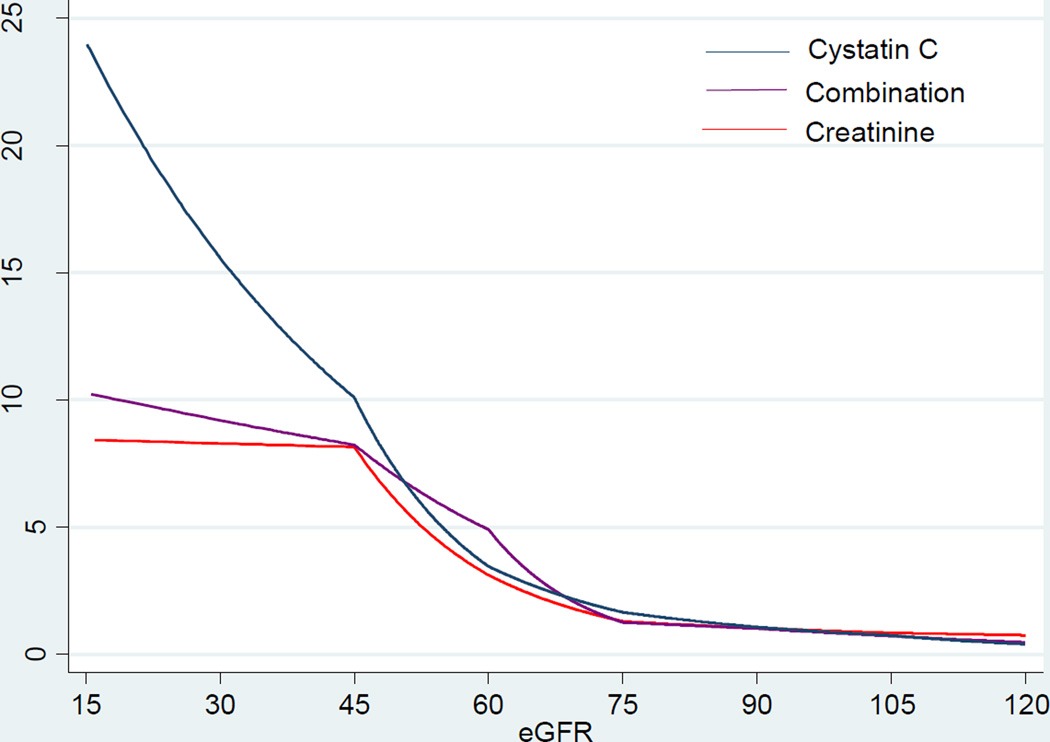
Figure 1 shows demographically-adjusted incidence rates according to eGFR using serum creatinine, cystatin C, and both. Overall, eGFRcys showed the steepest gradient in rate of SCD. In contrast, eGFRcr and eGFRcr-cys demonstrated similar patterns, although the latter had slightly steeper gradient. Unlike the J-shaped associations with total mortality reported in prior studies,19 we did not observe a J-shaped association between any measures of eGFR and SCD in the present study.
Figure 1.
Age-, Sex-, and Race-adjusted Incidence Rate of SCD based on eGFR
The associations of eGFR with SCD remained significant even after adjusting for other risk factors, particularly for eGFR categories below 60 ml/min/1.73m2 (Model 2 in Table 2). Specifically, adjusted HRs for <45 ml/min/1.73m2 compared with eGFR category of ≥90 ml/min/1.73m2 were 3.71 [95% CI 1.74–7.90] for eGFRcr, 5.40 [2.97–9.83] for eGFRcr-cys, and 5.24 [3.01–9.11] for eGFRcys. With eGFR ≥90 ml/min/1.73m2 as a reference, eGFR 60–89 ml/min/1.73m2 was significantly associated with SCD risk in all eGFR in Model 1 with demographic adjustment, but only in eGFRcys in Model 2. When incident CHD and HF were adjusted for as a time-varying covariate, the associations were attenuated, but remain similar (e.g., HR for eGFRcr <45 ml/min/1.73m2 was 2.19 [1.03–4.67]). The further adjustment for corrected QT interval and exclusion of those on dialysis at baseline did not alter the results (data not shown). Of note, when eGFRcr and eGFRcys were modeled together (Model 3), eGFRcys, but not eGFRcr, remained significant. When contrasting the association with SCD vs. all-cause mortality or non-SCD, eGFRs (particularly when cystatin C was used) tended to be more strongly associated with SCD than all-cause mortality or non-SCD (Tables S1 and S2).
Table 2.
Hazard Ratios and 95% Confidence Intervals of SCD based on Level of eGFR
| eGFR (ml/min/1.73 m2) | |||||
|---|---|---|---|---|---|
| Range | ≥ 90 | 60–89 | 45–59 | <45 | |
| N | 9,501 | 3,304 | 188 | 77 | |
| eGFRcr | Model 1 | Reference | 1.55 (1.14–2.11) | 6.12 (3.57–10.50) | 8.64 (4.32–17.27) |
| Model 2 | Reference | 1.33 (0.95–1.87) | 3.94 (2.25–6.89) | 3.71 (1.74–7.90) | |
| Model 3 | Reference | 0.94 (0.64–1.36) | 1.75 (0.90–3.44) | 1.01 (0.39–2.67) | |
| N | 8,536 | 4,155 | 262 | 117 | |
| eGFRcr-cys | Model 1 | Reference | 1.81 (1.32–2.49) | 6.54 (3.93–10.87) | 12.67 (7.31–21.95) |
| Model 2 | Reference | 1.35 (0.96–1.90) | 3.38 (1.95–5.86) | 5.40 (2.97–9.83) | |
| Model 3* | - | - | - | - | |
| N | 7,303 | 4,990 | 574 | 203 | |
| eGFRcys | Model 1 | Reference | 1.90 (1.36–2.66) | 5.39 (3.40–8.52) | 14.28 (8.77–23.35) |
| Model 2 | Reference | 1.48 (1.03–2.12) | 2.76 (1.50–4.56) | 5.24 (3.01–9.11) | |
| Model 3 | Reference | 1.43 (0.98–2.11) | 2.59 (1.46–4.59) | 4.60 (2.10–10.05) | |
Model 1: adjusted for age, sex, race, and field center
Model 2: Model 1 plus education, CHD, HF, DM, hypertension, heart rate, Cornel voltage, BMI, HDL and LDL cholesterols, current drinking, and current smoking
Model 3: Model 2 plus eGFRcys for eGFRcr; Model 2 plus eGFRcr for eGFRcys
When both serum creatinine and cystatin C are available, it is most likely that integrated eGFRcr-cys or both of eGFRcr and eGFRcys would be used for clinical decision making.
Thus, we did not run a model including any other eGFR in the analysis of eGFRcr-cys (“−” in Model 3).
eGFR, Cystatin C, B2M and SCD Risk
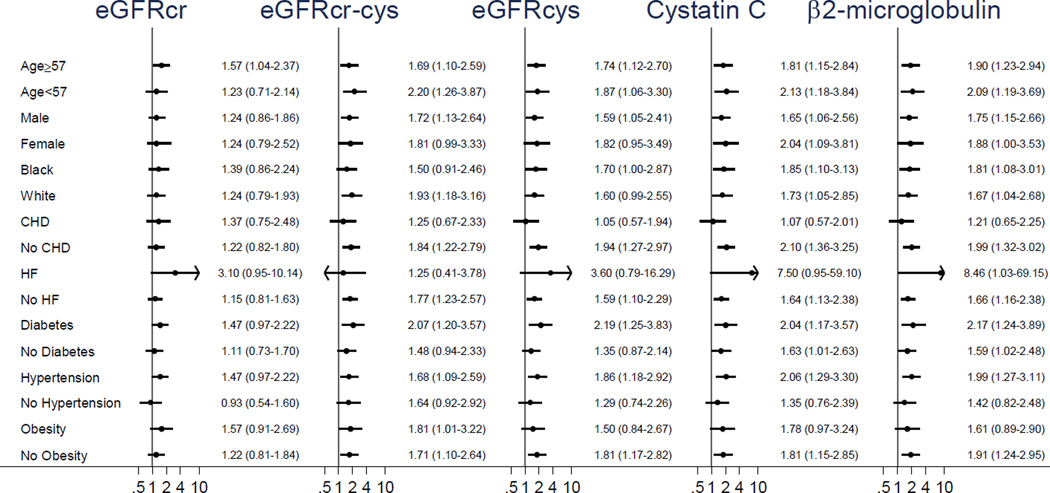
Subsequently, we contrasted associations of SCD with quartiles of cystatin C and B2M alone with the associations of SCD with quartiles of eGFRcr, eGFRcr-cys, and eGFRcys (Table 3). The 4th quartile of every kidney measure was significantly associated with SCD risk in Model 2. Of note, the adjusted HR for the 4th quartile was highest for B2M followed by cystatin C and eGFRcys. Again, we observed similar results after the adjustment for incident CHD and HF as time-varying covariates or corrected QT interval and the exclusion of those on dialysis at baseline (data not shown). The further adjustment by eGFRcr slightly attenuated but did not materially alter the results for cystatin C and B2M (Model 3 in Table 3). These associations for SCD tended to be stronger than those for all-cause mortality and non-SCD (Tables S3 and S4). The associations of the kidney filtration markers with SCD were qualitatively consistent across all subgroups (Figure 2 and Table S5).
Table 3.
Hazard Ratios and 95% Confidence Intervals of SCD by eGFR, Cystatin C, and β2-microglobulin
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | ||
|---|---|---|---|---|---|
| eGFRcr (ml/min/1.73 m2) |
Range | 105.7–160.8 | 97.5–105.7 | 88.7–97.5 | 4.31–88.7 |
| Model 1 | Reference | 1.23 (0.76–2.00) | 1.25 (0.76–2.05) | 2.24 (1.46–3.44) | |
| Model 2 | Reference | 1.23 (0.75–2.02) | 1.04 (0.62–1.75) | 1.72 (1.09–2.71) | |
| Model 3 | Reference | 1.02 (0.62–1.69) | 0.73 (0.43–1.25) | 0.81 (0.47–1.38) | |
| eGFRcr-cys (ml/min/1.73 m2) |
Range | 106.6–160.1 | 96.6–106.6 | 85.0–96.6 | 3.68–85.0 |
| Model 1 | Reference | 1.08 (0.63–1.85) | 1.61 (0.98–2.64) | 3.50 (2.21–5.53) | |
| Model 2 | Reference | 0.98 (0.56–1.71) | 1.26 (0.75–2.14) | 2.07 (1.26–3.38) | |
| Model 3 | - | - | - | - | |
| eGFRcys (ml/min/1.73 m2) |
Range | 104.8–163.2 | 93.5–104.8 | 78.7–93.5 | 3.75–78.7 |
| Model 1 | Reference | 1.54 (0.89–2.69) | 1.89 (1.11–3.20) | 4.54 (2.79–7.41) | |
| Model 2 | Reference | 1.34 (0.76–2.37) | 1.46 (0.85–2.52) | 2.43 (1.45–4.09) | |
| Model 3 | Reference | 1.26 (0.71–2.23) | 1.28 (0.74–2.22) | 1.78 (1.01–3.14) | |
| Cystatin C (mg/L) |
Range | 0.34–0.76 | 0.76–0.85 | 0.85–0.97 | 0.97–9.49 |
| Model 1 | Reference | 1.43 (0.79–2.60) | 1.90 (1.09–3.31) | 4.77 (2.85–7.99) | |
| Model 2 | Reference | 1.29 (0.70–2.39) | 1.47 (0.82–2.64) | 2.64 (1.52–4.61) | |
| Model 3 | Reference | 1.20 (0.65–2.23) | 1.30 (0.72–2.34) | 1.99 (1.09–3.63) | |
| β2-microglobulin (mg/L) |
Range | 0.81–1.62 | 1.62–1.83 | 1.83–2.11 | 2.11–57.74 |
| Model 1 | Reference | 2.10 (1.19–3.70) | 2.36 (1.35–4.13) | 5.60 (3.34–9.40) | |
| Model 2 | Reference | 1.92 (1.08–3.42) | 1.78 (1.001–3.17) | 3.48 (2.03–5.96) | |
| Model 3 | Reference | 1.81 (1.01–3.23) | 1.62 (0.90–2.90) | 2.77 (1.55–4.94) | |
Model 1: adjusted for age, sex, race, and field center
Model 2: Model 1 plus education, CHD, HF, DM, hypertension, heart rate, Cornell voltage, BMI, HDL and LDL cholesterols, current smoking, and current drinking
Model 3: Model 2 plus eGFRcys for eGFRcr; Model 2 plus eGFRcr for eGFRcys, cystatin C, and β2-microglobulin
Figure 2.
Subgroup Comparisons by Kidney Filtration Markers (above vs. below median) across Demographic and Clinical Subgroups.*
Every P value for interaction was ≥0.05
*adjusted for age, sex, race, field center, education, CHD, HF, DM, hypertension, heart rate, Cornell voltage, BMI, HDL and LDL cholesterols, current drinking, and current smoking
Analysis with Visit 4 Data Including BTP
When we used visit 4 data as baseline, there were 56 SCD cases during a median of 5.4 year-follow-up among 10,406 participants (incidence rate: 1.0 per 1000 person-years). Basic characteristics of the cohort at visit 4 based on incidence of SCD during the follow-up are shown in Table S6. The results were largely consistent with the primary analysis using visit 2 as baseline (Table S7). BTP was independently associated with SCD (HR for the third tertile: 26.6 [3.45–204.8]). Again, we observed more robust associations for cystatin C and B2M compared to eGFRcr.
Discussion
In this community-based study, reduced kidney function, as assessed by three eGFR equations, and each of cystatin C, B2M, and BTP, was associated with increased risk of SCD, independently of traditional risk factors at baseline and incident CHD and HF during follow-up. eGFR below 60 ml/min/1.73m2 was consistently associated with higher SCD risk compared to eGFR ≥90 ml/min/1.73m2. The association was more evident when kidney dysfunction was assessed with the novel filtration markers cystatin C and B2M, than with serum creatinine. In the primary analysis with visit 2 data, B2M demonstrated slightly stronger association over cystatin C. Although it was exploratory due to the small number of SCD cases after visit 4, BTP demonstrated a significant association with SCD as well.
Our results are consistent with previous findings in highly selected populations7–13 and extend these findings in several respects. First, we found the association of kidney dysfunction and SCD in a middle-aged general population (48–67 years of age). This is important since years of life lost due to SCD peaks in this age range in the US.4 Of importance, the associations were qualitatively consistent across key subgroups. Second, we confirmed the robust association of eGFR based on new cystatin C equations with SCD, as demonstrated for other cardiovascular outcomes.16 Finally, to our knowledge, this is the first study reporting the independent associations of B2M and BTP with SCD.
There are several potential mechanisms linking kidney dysfunction to SCD beyond well-studied relationship of kidney function to CHD and HF.25–30 Electrolyte abnormalities as a result of impaired kidney function may decrease myocardium membrane stability and trigger ventricular tachyarrhythmia leading to SCD.25 Kidney dysfunction is associated with prolonged QT interval, and arrhythmias such as Torsades de Pointes could be initiated by early afterdepolarizations.26 Reduced kidney function is also related to inflammation27 and sympathetic over-activity.28 Inflammation could be a trigger for SCD through direct effects on myocardium (i.e., tissue damage).27 Sympathetic over-activity due to renal dysfunction might lead to left ventricular hypertrophy.28 Indeed, left ventricular hypertrophy often coexists in patients with kidney dysfunction29 and is a known substrate for lethal ventricular arrhythmia.30
In consistent with the previous report of the association between cystatin C and SCD risk in older adults,13 eGFRcys showed a stronger association with SCD as compared with eGFRcr or eGFRcr-cys. This finding is consistent with previous studies of CHD and mortality.16 To what extent the stronger association of eGFRcys over the other two eGFR equations is due to a better estimation of kidney function or non-eGFR determinants is still under debate.16 For estimating measured GFR, eGFRcys and eGFRcr have been shown to be similar.15 When eGFRcr and eGFRcys were modeled together for SCD risk in our study, only eGFRcys remained significant, potentially suggesting the involvement of non-GFR determinant such as inflammation. Given that some investigators recommend the assessment of cystatin C to confirm CKD among those mildly reduced eGFRcr,18 our findings suggest that in such a clinical scenario, healthcare providers should focus on eGFRcys for SCD risk evaluation.
We observed stronger associations of B2M with SCD, as compared to GFR equations incorporating cystatin C. Similar patterns have been observed for other cardiovascular and kidney outcomes.21 This may indicate that B2M is a better filtration marker than serum creatinine or cystatin C. Indeed, B2M has several advantages as a kidney filtration maker. B2M is a 100-amino acid single polypeptide chain and a part of the major histocompatibility class I molecule on the surface of human cells,31 which is not dependent on muscle mass. B2M does not undergo renal tubular excretion like creatinine. B2M also has comparatively low within-person variability.32 Similarly to cystatin C, non-kidney determinants may still contribute to the strong associations between B2M and SCD. B2M can be elevated due to immune response, inflammation, and malignancy, conditions which may increase the risk of SCD.17, 33, 34 In our analysis, further adjustment by high-sensitivity C-reactive protein did not alter the association (results not shown).
BTP also showed a significant association with SCD, comparable to cystatin C and B2M. BTP is one of the most prominent proteins in human cerebrospinal fluid and functions as a prostaglandin D synthase.35 BTP has been shown to be a good marker of GFR.36 Further evaluation of BTP with a longer follow-up and more SCD cases would be required to estimate the association between BTP and SCD more precisely.
Our results have significant clinical and public health implications. Although currently low ejection fraction is the key indication of implantable cardioverter-defibrillator (ICD), “risk stratification approach” has been proposed for SCD prevention.37 In this context, our results suggest kidney function as a candidate predictor. Preventing or delaying kidney disease progression is already a clinically important task, since end-stage renal disease is a devastating condition with high mortality risk, poor quality of life, and high medical cost.38 Our results suggest that the efforts to preserve kidney function may result in low SCD risk. This is particularly important since some studies question the benefit of ICD in patients with severe kidney dysfunction.39
Limitations of the study merit consideration. The adjudication of SCD in the ARIC Study has been done only in cases occurring before December 31, 2001, providing limited number of SCD, particularly after visit 4. Also, this may raise a concern whether our findings are applicable to the current clinical practice where more intensive primary and secondary prevention strategies are implemented than 1990’s. Thus, confirmation in contemporary data would be warranted, although there is no clear evidence suggesting distortion of the association between kidney function and SCD risk over time. Nonetheless, we have a median of 11.2 years of follow-up for our main analysis, long and large enough to detect an appropriate number of SCD cases (>200 cases) for our study question. For both the primary (visit 2 as baseline) and secondary (visit 4 as baseline) analysis, we relied on a single measurement of kidney function markers; thus, there could be misclassification due to short-term variability. However, it is reassuring that the results were consistent between the primary and secondary analyses. In addition, there remains a possibility of residual confounding although we adjusted for various variables known to be associated with SCD.
In conclusion, kidney function assessed by serum creatinine, cystatin C, and novel filtration markers, B2M and BTP, was consistently associated with SCD in the community, independent of traditional risk factors and intermediate CHD and HF events. These results provide evidence that persons with kidney dysfunction are at high risk of SCD and suggest the potential usefulness of both traditional and novel kidney filtration markers in SCD risk assessment when risk-centered approach is implemented for SCD prevention. Our results also suggest the value of novel filtration markers beyond and above serum-creatinine based eGFR.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. Reagents for the beta 2 microglobulin assays at visit 2 were donated by the manufacturer (Roche Diagnostics).
Funding Sources
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This grant was also supported by grant R01DK089174 to Dr. Selvin.
Dr. Sotoodehnia was supported by HL111089, HL116747, and the Laughlin Family. Dr. Selvin reports grants from NIH/NIDDK, during the conduct of the study; personal fees from Roche Diagnostics, outside the submitted work. Dr. Calkins reports personal fees from Medtronic, St. Jude Medical, Atricure, Abbott, Boerringer Inglheim, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The other authors declare that they have no relevant financial interests.
Reference List
- 1.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 2010;122(22):2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Kong MH, Fonarow GC, Peterson ED, Curtis AB, Hernandez AF, Sanders GD, et al. Systematic review of the incidence of sudden cardiac death in the United States. Journal of the American College of Cardiology. 2011;57(7):794–801. doi: 10.1016/j.jacc.2010.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stecker EC, Reinier K, Marijon E, Narayanan K, Teodorescu C, Uy-Evanado A, et al. Public health burden of sudden cardiac death in the United States. Circulation Arrhythmia and electrophysiology. 2014;7(2):212–217. doi: 10.1161/CIRCEP.113.001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–814. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 7.Hreybe H, Ezzeddine R, Bedi M, Barrington W, Bazaz R, Ganz LI, et al. Renal insufficiency predicts the time to first appropriate defibrillator shock. American heart journal. 2006;151(4):852–856. doi: 10.1016/j.ahj.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. The American journal of cardiology. 2006;98(4):485–490. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Deo R, Lin F, Vittinghoff E, Tseng ZH, Hulley SB, Shlipak MG. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension. 2008;51(6):1578–1582. doi: 10.1161/HYPERTENSIONAHA.107.103804. [DOI] [PubMed] [Google Scholar]
- 10.Saxon LA, Bristow MR, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation. 2006;114(25):2766–2772. doi: 10.1161/CIRCULATIONAHA.106.642892. [DOI] [PubMed] [Google Scholar]
- 11.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney international. 2009;76(6):652–658. doi: 10.1038/ki.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green D, Roberts PR, New DI, Kalra PA. Sudden cardiac death in hemodialysis patients: an in-depth review. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57(6):921–929. doi: 10.1053/j.ajkd.2011.02.376. [DOI] [PubMed] [Google Scholar]
- 13.Deo R, Sotoodehnia N, Katz R, Sarnak MJ, Fried LF, Chonchol M, et al. Cystatin C and sudden cardiac death risk in the elderly. Circulation Cardiovascular quality and outcomes. 2010;3(2):159–164. doi: 10.1161/CIRCOUTCOMES.109.875369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. The New England journal of medicine. 2013;369(10):932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinkai S, Chaves PH, Fujiwara Y, Watanabe S, Shibata H, Yoshida H, et al. Beta2-microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C-reactive protein. Archives of internal medicine. 2008;168(2):200–206. doi: 10.1001/archinternmed.2007.64. [DOI] [PubMed] [Google Scholar]
- 18.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA : the journal of the American Medical Association. 2011;305(15):1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. Journal of the American Society of Nephrology : JASN. 2009;20(10):2214–2222. doi: 10.1681/ASN.2008090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 21.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;59(5):653–662. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. Journal of the American College of Cardiology. 2011;57(16):1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) The American journal of cardiology. 2008;101(7):1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 24.Peacock JM, Ohira T, Post W, Sotoodehnia N, Rosamond W, Folsom AR. Serum magnesium and risk of sudden cardiac death in the Atherosclerosis Risk in Communities (ARIC) Study. American heart journal. 2010;160(3):464–470. doi: 10.1016/j.ahj.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, et al. Cardiac arrest and sudden death in dialysis units. Kidney international. 2001;60(1):350–357. doi: 10.1046/j.1523-1755.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Zipes DP. Early afterdepolarizations, U waves, and torsades de pointes. Circulation. 2002;105(6):675–676. [PubMed] [Google Scholar]
- 27.Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney international. 2008;74(10):1335–1342. doi: 10.1038/ki.2008.449. [DOI] [PubMed] [Google Scholar]
- 28.Rump LC, Amann K, Orth S, Ritz E. Sympathetic overactivity in renal disease: a window to understand progression and cardiovascular complications of uraemia? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2000;15(11):1735–1738. doi: 10.1093/ndt/15.11.1735. [DOI] [PubMed] [Google Scholar]
- 29.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. Journal of the American Society of Nephrology : JASN. 2012;23(10):1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinier K, Dervan C, Singh T, Uy-Evanado A, Lai S, Gunson K, et al. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8(8):1177–1182. doi: 10.1016/j.hrthm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyata T, Jadoul M, Kurokawa K, Van Ypersele de Strihou C. Beta-2 microglobulin in renal disease. Journal of the American Society of Nephrology : JASN. 1998;9(9):1723–1735. doi: 10.1681/ASN.V991723. [DOI] [PubMed] [Google Scholar]
- 32.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J. Within-person variability in kidney measures. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(5):716–722. doi: 10.1053/j.ajkd.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinic proceedings. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 34.Morel P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, McCoy J, et al. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood. 2009;113(18):4163–4170. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann A, Nimtz M, Conradt HS. Molecular characterization of beta-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology. 1997;7(4):499–506. doi: 10.1093/glycob/7.4.499. [DOI] [PubMed] [Google Scholar]
- 36.White CA, Akbari A, Doucette S, Fergusson D, Hussain N, Dinh L, et al. A novel equation to estimate glomerular filtration rate using beta-trace protein. Clinical chemistry. 2007;53(11):1965–1968. doi: 10.1373/clinchem.2007.090126. [DOI] [PubMed] [Google Scholar]
- 37.Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM, Jr, et al. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014;129(4):516–526. doi: 10.1161/CIRCULATIONAHA.113.007149. [DOI] [PubMed] [Google Scholar]
- 38.Mittal SK, Ahern L, Flaster E, Maesaka JK, Fishbane S. Self-assessed physical and mental function of haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2001;16(7):1387–1394. doi: 10.1093/ndt/16.7.1387. [DOI] [PubMed] [Google Scholar]
- 39.Pun PH, Al-Khatib SM, Han JY, Edwards R, Bardy GH, Bigger JT, et al. Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death in CKD: a meta-analysis of patient-level data from 3 randomized trials. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(1):32–39. doi: 10.1053/j.ajkd.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.