Abstract
Background
A group of patients with idiopathic pulmonary fibrosis (IPF) present with disease affecting one lung markedly more than the other. At this time, it is unclear how this population differs from those who present with more symmetric disease. We sought to explain the characteristics of the asymmetric group and how their disease progresses.
Methods
In this retrospective case-control study we accessed an interstitial lung disease (ILD) database and identified 14 asymmetric IPF cases via high-resolution computed tomography (HRCT) scoring of each lung lobe’s disease severity. We identified 28 symmetric IPF controls from the same database using the same methods, and compared the clinical features of each group.
Results
Patients with asymmetric disease exhibited similar demographics as those in the general IPF population; they were predominantly male (64%), elderly (69 years old), and used tobacco (57%). We found a trend toward significantly increased all-cause mortality in the case population two years following diagnosis (p = 0.089). Pulmonary function tests were significantly lower in the case group at the time of diagnosis, then both groups experienced gradual decline. We found no statistically significant differences in number of IPF exacerbations (cases 43%, controls 39%, p = 0.824) and gastro-esophageal reflux (both groups 50%).
Conclusion
Patients with asymmetric IPF resemble patients in the general IPF population but may have a lower overall survival rate. Further systemic factors may be studied to identify reasons for disease asymmetry and clinical decline in this population.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrosing condition involving the lung interstitium. The diagnosis of IPF requires exclusion of plausible explanations for the lung disease and the identification of a pattern of usual interstitial pneumonia (UIP) on either high resolution computed tomography (HRCT) or histopathology from a surgical lung biopsy (SLB) [1].
Recent guidelines describe the HRCT features of UIP as a basilar and subpleural distribution of reticular abnormalities, traction bronchiectasis with (definite UIP) or without honeycombing (possible UIP) [1]. The guidelines describe upper or mid-lung distribution as inconsistent with UIP but remain silent of unilateral or bilateral disease [1].
In this retrospective case-control study we sought to determine the prevalence of asymmetric disease on HRCT from a group of patients seen at the Interstitial Lung Disease Clinic at the University of Michigan and if clinical characteristics and/or disease course differed between patients with symmetric versus asymmetric disease.
METHODS
Patient selection
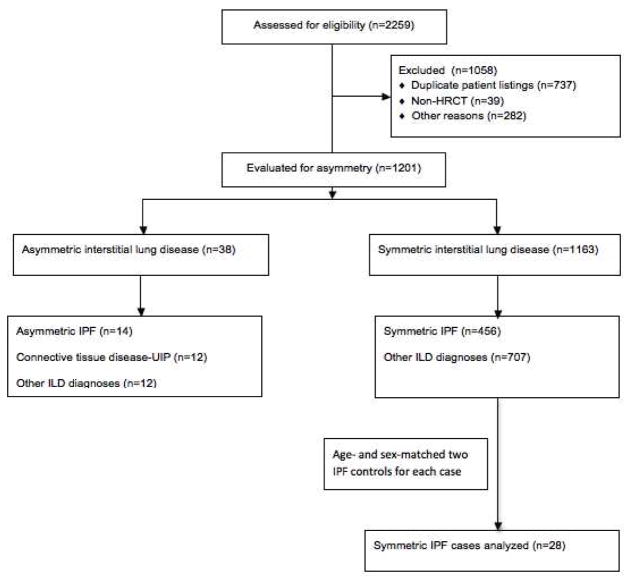
Our study was reviewed and approved by the University of Michigan institutional review board. We selected cases from a database of cases presented at the University of Michigan Interstitial Lung Disease Conference between March 8, 2009 and March 12, 2014. There were 2259 patients present at the time of data retrieval in March 2014. Duplicate submissions to the conference were omitted, as were submissions with CT scans that were not high resolution. Other submissions were excluded for patients whose data were incomplete for review. (Figure 1)
Figure 1.
Flowchart describing selection of asymmetric patients and matched controls
As part of the ILD conference HRCT studies are prospectively scored for the profusion of interstitial and alveolar infiltrates using a previously described IPF radiographic scoring system that has demonstrated clinical applicability as evidenced by high mortality rates correlating with high fibrotic scores on HRCT interpretations [2]. For each CT scan, the radiologists evaluate each of the five lung lobes for disease severity on the basis of ground glass, reticular infiltrates, and honeycombing. Each lung segment’s severity was given a score of 0–5. A score of 0 corresponds to 0% disease activity within a given lobe, while 5 equaled >75% involvement of the lobe [2]. (Table 1)
Table 1.
IPF Thin-Section CT Scoring System
| Score | Ground Glass Involvement |
|---|---|
| 0 | No disease |
| 1 | Ground glass opacities (GGOs) involving <5% of lobe |
| 2 | GGOs ≤ 25% of lobe |
| 3 | GGOs 25–49% of lobe |
| 4 | GGOS 50–75% of lobe |
| 5 | GGOs > 75% of lobe |
| Score | Interstitial Involvement |
|---|---|
| 0 | No interstitial disease |
| 1 | Honeycombing (+/− septal thickening) < 5% of lobe |
| 2 | Honeycombing (+/− septal thickening) < 25% of lobe |
| 3 | Honeycombing (+/− septal thickening) 25–49% of lobe |
| 4 | Honeycombing (+/− septal thickening) 50–75% of lobe |
| 5 | Honeycombing (+/− septal thickening) > 75% of lobe |
We identified 1201 patients with ILD after excluding duplicates and chest CT submissions that were not high-resolution (Figure 1). Other reasons for exclusion included submissions lacking chest CT scans, incomplete CT scoring, and absence of any imaging. We determined asymmetry of the 1201 patients by summing the lobe scores for each lung to derive a total involvement score for each lung. We then took the mean score of each lung and calculated the difference in disease activity between the right and left lungs. Prior to database accession and patient identification, we selected a lung involvement difference of >1.0 to be considered relevant for asymmetry, corresponding to roughly 25% more IPF involvement. The patient could meet criteria through either alveolar or reticular criteria. Using the predetermined >1.0 value, we identified 38 patients to have asymmetric ILD, and 1163 with symmetric disease. Fourteen of the 38 asymmetric disease patients had a diagnosis of IPF provided by the ILD conference. The majority of IPF diagnoses (10/14) were made via open lung surgical biopsy.
We selected controls from the remaining 1163 patients in the symmetric group. We identified IPF controls diagnoses listed in the ILD conference. Of the remaining 456 patients, two patients were matched on gender and age (within 3 years) for each asymmetric case. We preferentially chose patients with surgical lung biopsies demonstrating UIP to maximize likelihood patients selected had a true diagnosis of IPF. In the control group, a diagnosis of IPF was made via open lung surgical biopsy in 27 of the 28 patients.
Patient Characteristics
For patients in both the case and control groups we retrieved baseline demographics. These consisted of age, gender, and ethnicity. We also obtained separate diagnoses hypothesized to be related to asymmetric disease, including gastro-esophageal reflux (GER), prior radiation therapy to the chest, smoking history, and cigarette pack-year history. History of radiation therapy considered relevant were treatments implemented for malignancy. GER prevalence was identified through patient self-reporting, and in those patients on regularly-administered proton-pump inhibitors and H2 blockers. Tobacco usage history was collected according to patterns reported to medical care providers.
Longitudinal data retrieved were baseline pulmonary function tests (PFTs) at the time of the patient’s first HRCT and for two subsequent years. PFTs included for analysis were FEV1, FVC, and DLCO – all absolute and percent predicted. We extracted the number of IPF exacerbations, defined as an acute worsening in respiratory status in <30 days not attributable to infection or other identifiable cause [3,4]. We considered acute exacerbations on the basis of formal chart diagnosis, coupled with negative workup for cardiac and thrombotic causes, and negative culture data. Last, we retrieved data for all-cause mortality. Date of death was determined through accession of the publicly available Social Security Death Index.
Statistical Analysis
Analysis of PFT trends over time was adjusted for age, gender, smoking, GER, and number of IPF exacerbations. Demographics and baseline PFTs were summarized and compared between case and control groups with p-values determined by t-tests for continuous variables such as baseline PFTs, radiographic scoring and pack-years smoked, and chi-square tests for categorical values, including GER prevalence and gender. Mixed models with linear spline components at one-year intervals were used to describe and compare average trajectories in PFTs over time for the two groups. Kaplan-Meier survival curves were generated starting from time zero – the time of diagnosis – and following patients to a maximum eight years afterward. Gender-Age-Physiology (GAP) index and stage were calculated for symmetric and asymmetric patients at the time of diagnosis.
Results
Study Population
Our study population included 14 patients with asymmetric disease and 28 controls (Table 2). This equated to 1.17% (14/1201) of the total submissions to the database, and 2.98% (14/470) of the total IPF population. The asymmetric group had a male predominance (64.3%) and was of advanced age (mean 69.3 years old). There was no difference in smoking history, smoking pack years, and GER prevalence. Only one of the 14 cases had a history of radiation therapy to the chest for malignancy. Of the patients with asymmetric disease, 9/14 patients (64.3%) had left lung-dominant disease activity. Nine patients (64.3%) met asymmetry criteria by alveolar disease patterns, and five patients (35.7%) met asymmetry criteria by interstitial disease patterns. At the time of diagnosis, radiographic scoring revealed a greater degree of disease involvement in the case population, though this did not meet statistical significance (alveolar pattern p = 0.16, interstitial pattern p = 0.85). (Table 2) The mean GAP index and stage at the time of diagnosis for the asymmetric patients were 4.36 and 1.93; the mean GAP index and stage for the symmetric patients were comparable at 3.72 and 1.68. Neither the GAP index nor stage met statistical significance.
Table 2.
Demographics and baseline PFTs
| All | Case | Control | p value | |
|---|---|---|---|---|
| No. | 42 | 14 | 28 | |
| Male, No. (%) | 27 (64%) | 9 (64%) | 18 (64%) | 1.0000 |
| Ever Smoking, No. (%) | 25 (60%) | 8 (57%) | 17 (61%) | 0.8241 |
| GER, No. (%) | 21 (50%) | 7 (50%) | 14 (50%) | 1.0000 |
| Exacerbation, No. (%) | 17 (40%) | 6 (43%) | 11(39%) | 0.8241 |
| Age, Yrs (mean±sd) | 69 ± 7 | 69 ± 8 | 69 ± 7 | 0.9300 |
| Pack Years, Yrs (mean±sd) | 17 ± 18 | 16 ± 18 | 17 ± 19 | 0.8497 |
| FEV1 Liters (mean±sd) | 2.07 ± 0.69 | 1.88 ± 0.68 | 2.17 ± 0.68 | 0.2119 |
| FEV1 %Pred (mean±sd) | 74 ± 23 | 60 ± 28 | 81 ± 16 | 0.0152 |
| FVC Liters (mean±sd) | 2.45 ± 0.82 | 2.17 ± 0.79 | 2.58 ± 0.81 | 0.1272 |
| FVC %Pred (mean±sd) | 64 ± 17 | 56 ± 19 | 68 ± 14 | 0.0217 |
| DLCO mL/min/mmHg (mean±sd) | 11.54 ± 6.17 | 11.47 ± 6.27 | 11.56 ± 6.27 | 0.9668 |
| DLCO, %Pred (mean±sd) | 44 ± 20 | 43 ± 21 | 45 ± 19 | 0.7590 |
| Alveolar | 1.42 ± 1.08 | 1.76 ± 1.19 | 1.26 ± 1.00 | 0.160 |
| Interstitial | 1.23 ± 0.69 | 1.26 ± 0.94 | 1.21 ± 0.54 | 0.851 |
Disease Course
The overall trend of PFT trajectory was similar between cases and controls. Baseline case predicted values for FEV1 and FVC were all lower in the asymmetric case population at the time of IPF diagnosis (Table 3). Over the subsequent two years, each group experienced fluctuations in pulmonary function testing, mostly not meeting significance. IPF exacerbation prevalence was monitored for both groups. The case group suffered six exacerbations (42.9%) and the control group experienced 11 exacerbations (39.3%), which did not meet statistical significance.
Table 3.
PFT Progression
| Baseline | 1-year | 2-year | ||
|---|---|---|---|---|
| FVC % predicted | Control | 68.25 | 71.28 (+3.03) | 67.23 (−4.05) |
| Case | 56.46 | 55.36 (−1.10) | 53.39 (−1.97) | |
| P value | 0.3150 | 0.2494 | ||
| FEV1 % predicted | Control | 80.71 | 87.43 (+6.73) | 82.89 (−4.54) |
| Case | 68.86 | 68.66 (−0.20) | 66.66 (−2.00) | |
| P value | 0.2006 | 0.2825 | ||
| DLCO % predicted | Control | 46.65 | 45.93 (−0.72) | 41.39 (−4.55) |
| Case | 48.85 | 43.54 (−5.32) | 39.40 (−4.13) | |
| P value | 0.4946 | 0.9416 |
Values listed as percent predicted, calculated using patient’s height, age, and gender. Values in parentheses represent percentage improved or declined since the prior time point. p values compares change from the prior time point between case and control.
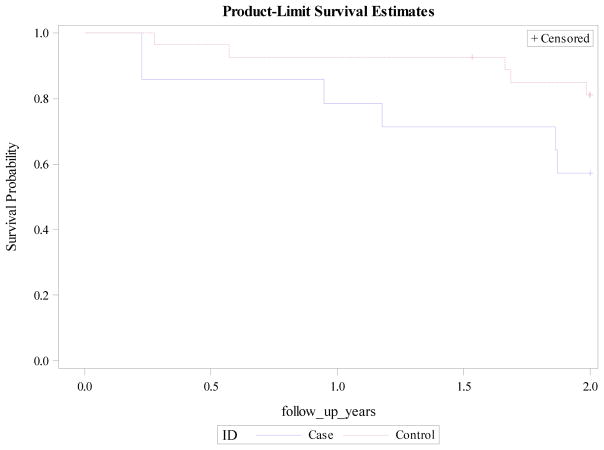
Case mortality diverged from controls at two years following diagnosis. Though this did not meet statistical significance, there was a trend toward significance (p = 0.0892). Similar divergence with higher case mortality was seen at the end of the follow-up period, but again lacked statistical significance (p = 0.2220). (Figure 2)
Figure 2.
Survival plot two years following IPF diagnosis
Discussion
We present a collection of patients with objectively different patterns of IPF as evidenced by asymmetric disease distributions on HRCT. It was suspected that these patients would have underlying similarities (e.g. particular demographic characteristics or comorbidities) to explain their discrepancies in IPF presentation, though we found no statistically significant evidence of this with respect to the factors we examined. Our findings imply that patients with asymmetric IPF cannot, at this time, be assumed to have alternative provocations of disease activity, such as increased tobacco use, radiation exposure, or aspiration events, which have links to the general IPF population [6,7,8,9,10]. Patients with asymmetric disease cannot be screened from a general IPF pool on the basis of gender or ethnicity, since a compelling segment of this group is constituted of white males – findings seen in the general IPF population [11]. Tcherakian et al. described IPF subjects with similarly asymmetric disease with similar advanced age and male predominance, though a substantially greater percentage of patients with asymmetric disease had GER [12]. This finding, in conjunction with right-sided disease predominance and higher frequency of acute IPF exacerbations compelled this team to suggest GER as a causative mechanism [12]. Our study was underpowered to detect a difference in population prevalence of GER, though we hesitate to embrace this mechanism for multiple reasons. First, the percentages of those afflicted with GER in cases and controls were identical. Second, the majority of asymmetric patients had greater disease severity involving the left side, which would oppose recurrent microaspiration as a mechanism for disease progression given the predilection for aspiration events to favor the right lung [13]. Third, only three of the fourteen cases had strict unilateral involvement, which would favor a systemic process.
The small sample size in our study limited our ability to detect significance in all-cause mortality. In our population we observed a trend toward significantly higher all-cause mortality at two years in the asymmetric group. This finding proves discouraging, as one would expect an unaffected lung to provide reserve for disease progression. Reasons for such a trend are unclear, but may include 1) IPF progression to include the contralateral lung – a derivation that could not be assessed by this study, since repeat HRCT scorings were not routinely applied following diagnosis, 2) unidentified pro-fibrotic stimuli which may yield more profound disease in the asymmetric population, and 3) clinical practice may be to treat both phenotypes similarly, so it remains possible that asymmetric IPF patients are treated less aggressively in terms of referral for lung transplantation and newer therapies.
We are hesitant to attribute the mortality trend to high rates of immunosuppression; while 10/14 asymmetric patients were diagnosed prior to the publication of the PANTHER-IPF study in 2012, which showed increased mortality in IPF patients treated with prednisone, azathioprine, and N-Acetylcysteine, only 5/14 received immunosuppression (5 patients received prednisone and 1 patient was treated with mycophenolate mofetil) [14]. We also do not presume the trend toward higher mortality to reflect changes in IPF criteria, since our patient population was comprised of patients identified before and after diagnostic criteria changes in 2011 [1]. Our findings contradict that of previous findings [12], which found no mortality difference between these two presentations, despite cases and controls being similar between our study and comparisons.
We found a pattern of oscillating improvement and decline in pulmonary function in both the asymmetric and symmetric groups, though not meeting statistical significance. To our knowledge, this marks the first study of pulmonary function testing in this unique set of patients despite its clinical relevance and prognostic ability in IPF [15,16,17,18]. Our cases exhibited uniformly worse pulmonary function testing at the time of diagnosis, though they possessed similar GAP stages, and experienced decline over the following two years. The severity of deterioration we observed for both groups was unexpectedly mild given the prognosis of IPF. We hypothesize the decline was secondary to our small cohort in conjunction with the deaths of patients with more extreme PFT deteriorations, allowing for survival of patients with more stable IPF. One possible explanation for the oscillations in pulmonary function may relate to intermittent IPF exacerbations, which cause abrupt and usually irreversible decline in respiratory status [3,4]. IPF exacerbations in either group could cause large swings in PFTs, owing to a small cohort. Another possibility may relate to the retrospective nature of the collection of PFT data that were obtained as part of clinical care. A larger, prospective study would be required to confirm any differences in PFT course over time.
Asymmetric IPF patients may have demonstrated worse survival in our study owing to their advanced disease at presentation. At the time of first pulmonary function testing, their FVC % predicted and FEV1 % predicted were both significantly lower than that of the controls’. Though not meeting statistical significance, radiographic scoring at presentation revealed more infiltrates, especially for alveolar patterns, which trended toward significance, supporting this hypothesis. Why these patients present with more advanced disease is unclear, but may represent a later referral to pulmonologists owing to the uniqueness and relative uncommonness of their CT findings.
Limitations
A major limitation of our study was the lack of power owing to small sample size. Only 3.07% of our database’s IPF population met inclusion criteria for asymmetry, which limited the number of subjects for study, though this still represents a compelling fraction of the IPF population. Moving forward, combining populations from various ILD databases with similar radiographic criteria would help afford statistical significance to important patient characteristics, which we could not. These would include, but are not limited to, GER, aspiration events, history of radiation and occupational exposures.
The retrospective nature of this study limits observation of various clinical factors. Aspiration events, radiation exposure, and GER documentation were driven by patient reporting, as opposed to objective findings such as swallow evaluations and pH monitoring. It was infeasible to obtain further testing and acquire outside data to corroborate these self-reported diagnoses due to the high mortality rate in our patient populations and time lapsed in many patients between date of diagnosis and date of study. Lastly, there were no data available regarding diaphragmatic paralysis, which may be linked to the development of unilateral disease or exaggerate HRCT findings.
Conclusion
We studied a group of patients with IPF with a distinct pattern on HRCT whose trajectory and attributes to date have not been thoroughly described. Asymmetric IPF patients resemble those with more classic disease patterns in regard to their age, gender, and smoking history. At the time of presentation, their pulmonary function may be suppressed, though their decline mirrors those with symmetric disease. Of particular concern to the clinician, however, is that these patients may experience higher mortality. Moving forward, this suggests a more aggressive approach to treatment of this population.
Highlights.
IPF associations such as GER and smoking cannot identify patients with asymmetric IPF
Asymmetric disease resembles classic IPF patterns in terms of age, gender, and race
Patients with asymmetry present with worse pulmonary function at the time of diagnosis
Asymmetric IPF is associated with a trend toward increased two-year mortality
Pulmonary decline in patients with asymmetric IPF mirrors those with symmetric disease
Acknowledgments
Funding Information: National Institutes of Health NHLBI grants R01 HL91743, T32 HL00749 and K24 HL111316
Role of Sponsor: The NIH provided grant support for effort.
Abbreviation List
- GER
gastro-esophageal reflux
- HRCT
high-resolution computed tomography
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- PFT
pulmonary function test
- GAP
Gender-Age-Physiology
- SLB
surgical lung biopsy
- UIP
usual interstitial pneumonia
Footnotes
Author Contributions: S.J.C. and K.R.F. take responsibility for the content of the manuscript, for the integrity of the data and its analysis. S.J.C. and K.R.F. contributed to the study design, and S.J.C., M.X., S.M., and K.R.F. contributed to data analysis, interpretation, writing and editing of the manuscript.
Financial/nonfinancial disclosures: S.J.C., M.X., S.M., and K.R.F have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Thoracic Society. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gay SE, Kazerooni EA, Toews GB, et al. Idiopathic Pulmonary Fibrosis: Predicting Response to Therapy and Survival. Am J Respir Crit Care Med. 1998;157(4):1063–1072. doi: 10.1164/ajrccm.157.4.9703022. [DOI] [PubMed] [Google Scholar]
- 3.Idiopathic Pulmonary Fibrosis Clinical Research Network Investigators. Acute Exacerbations of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2007;176(7):636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papiris SA, Manali ED, Kolilekas L, et al. Clinical Review: Idiopathic Pulmonary Fibrosis Acute Exacerbations – Unraveling Ariadne’s Thread. Crit Care. 2010;14(6):246. doi: 10.1186/cc9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley B, Ryerson CJ, Vittinghoff E, et al. A Multidimensional Index and Staging System for Idiopathic Pulmonary Fibrosis. Ann Intern Med. 2012;156(10):684–691. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 6.Cardasis JJ, MacMahon H, Husain AN. The Spectrum of Lung Disease due to Chronic Occult Aspiration. Ann Am Thorac Soc. 2014;11(6):865–873. doi: 10.1513/AnnalsATS.201310-360OC. [DOI] [PubMed] [Google Scholar]
- 7.Raghu G, Freudenberger TD, Yang S, et al. High Prevalence of Abnormal Acid Gastro-oesophageal Reflux in Idiopathic Pulmonary Fibrosis. Eur Respir J. 2006;27(1):136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette Smoking: A Risk Factor for Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 1997;155(1):242–248. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 9.Ryu JH, Colby TV, Hartman TE, Vassallo R. Smoking-Related Interstitial Lung Diseases: A Concise Review. Eur Respir J. 2001;17(1):122–132. doi: 10.1183/09031936.01.17101220. [DOI] [PubMed] [Google Scholar]
- 10.Ding N, Li JJ, Sun L. Molecular Mechanisms and Treatment of Radiation-Induced Lung Fibrosis. Curr Drug Targets. 2013;14(11):1247–1356. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ley B, Collard HR, King TE. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–440. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 12.Tcherakian C, Cottin V, Brillet P, et al. Progression of Idiopathic Pulmonary Fibrosis: Lessons from Asymmetrical Disease. Thorax. 2011;66(3):226–231. doi: 10.1136/thx.2010.137190. [DOI] [PubMed] [Google Scholar]
- 13.Light R. Chapter 51. Pneumonia. In: Hall JB, Schmidt GA, Wood LDH, editors. Principles of Critical Care. 3. New York, NY: McGraw-Hill; 2005. [Google Scholar]
- 14.The Idiopathic Pulmonary Fibrosis Clinical Research Network. Prednisone, Azathioprine, and N-Acetylcysteine for Pulmonary Fibrosis. N Engl J Med. 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flaherty KR, Mumford JA, Murray S, et al. Prognostic Implications of Physiologic and Radiographic Changes in Idiopathic Interstitial Pneumonia. Am J Respir Crit Care Med. 2003;168(5):543–548. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 16.Collard HR, King TE, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in Clinical and Physiologic Variables Predict Survival in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2003;168(5):538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 17.Jegal Y, Kim DS, Shim TS, et al. Physiology Is a Stronger Predictor of Survival than Pathology in Fibrotic Interstitial Pneumonia. Am J Respir Crit Care Med. 2005;171(6):639–644. doi: 10.1164/rccm.200403-331OC. [DOI] [PubMed] [Google Scholar]
- 18.Lama VN, Flaherty KR, Toews GB, et al. Prognostic Value of Desaturation During a 6-Minute Walk Test in Idiopathic Interstitial Pneumonia. Am J Respir Crit Care Med. 2003;168(9):1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]