Abstract
Objective
To test the hypothesis that macrophage migration inhibitory factor (MIF) is involved in the disease activity of systemic vasculitis.
Methods
Patients with systemic vasculitis were divided into three groups based on the size of the affected vessels. Microscopic polyangiitis (MPA) was considered as small vessel vasculitis (SVV), polyarteritis nodosa as medium-sized vessel vasculitis (MVV), and giant cell arteritis and Takayasu arteritis as large vessel vasculitis (LVV). Sera from patients with systemic vasculitis and healthy individuals were collected, and MIF levels were measured using an enzyme-linked immunosorbent assay. Disease activity of vasculitis was assessed using the Birmingham Vasculitis Activity Score (BVAS).
Results
Serum MIF levels were significantly higher in the vasculitis patients than in healthy individuals. Among the vasculitis patients, MIF levels were significantly higher in patients in the SVV group (median; 4161.7 pg/ml) than in the other groups (MVV; 1443.2 pg/ml and LVV; 1576.7 pg/ml). In patients with MPA, a positive correlation was observed between serum MIF levels and CRP levels and disease activity (BVAS). Notably, serum MIF levels were significantly diminished after clinical improvement.
Conclusions
Our findings suggest that MIF may have an important role in small vessel vasculopathy and serve as a useful serologic marker of MPA disease activity.
Keywords: macrophage migration inhibitory factor, systemic vasculitis, microscopic polyangiitis, cytokine
Introduction
Although the causes of most vasculitis syndromes remain unclear, advances in molecular and cellular immunology have enabled the elucidation of many of the effector mechanisms that mediate inflammatory vascular damage.1,2 Vascular endothelial dysfunction is seen in a variety of inflammatory diseases. It is therefore not surprising that endothelial cells (ECs) play a pivotal role in the pathogenesis of systemic vasculitis,3,4 mainly by amplifying and perpetuating the inflammatory process through the expression and secretion of various cytokines, chemokines, cell adhesion molecules and other inflammatory molecules. In addition, specific cell–cell interactions, particularly between ECs and invading mononuclear cells, contribute to the progression of vasculitis1 and other autoimmune diseases, including rheumatoid arthritis (RA)5 and systemic lupus erythematosus (SLE).6
Macrophage migration inhibitory factor (MIF) was originally identified as a soluble factor in the culture medium of activated T lymphocytes that inhibited migration of macrophages,7,8 and is recognized as a multipotent cytokine in the regulation of immune and inflammatory responses.9 Several cell populations, such as T cells,10 macrophages/monocytes,11 synovial fibroblasts,12 and endothelial cells,13 have been shown to secrete and express MIF. Furthermore, MIF has been implicated in various inflammatory and immune-mediated diseases, including RA,12,14 SLE,15,16 scleroderma,17 and inflammatory bowel diseases.18 More recently, increased serum MIF levels have also been associated with systemic vasculitis, including Wegener’s granulomatosis and antineutrophil cytoplastic antibody (ANCA)-related angiitis.19,20 However, the relationship between MIF and disease activity, auto-antibodies levels, or the organs affected are not well known. Therefore, the aim of the present study was to further investigate the relationship between serum levels of MIF and various clinical parameters and disease activity in systemic vasculitis syndromes.
Materials and methods
Patients
A cross-sectional study was performed in 31 patients with systemic vasculitis between March 2005 and April 2008. All vasculitis patients were divided into three groups based on the size of the affected vessels following the definitions adopted by the Chapel Hill Consensus Conference.21 Microscopic polyangiitis (MPA) was diagnosed on the basis of the criteria proposed by Sorensen and colleagues22 (n = 14) and was considered small vessel vasculitis (SVV). Polyarteritis nodosa23 (PAN; n = 7) was considered medium-sized vessel vasculitis (MVV). Giant cell arteritis24 (GCA; n = 5) and Takayasu arteritis25 (TA; n = 5) were considered large vessel vasculitis (LVV; n = 10). The patient characteristics are summarized in Table 1. Serum samples were collected during both the active and inactive disease states. In addition, serum samples (n = 22) were also collected from 22 age- and sex-matched healthy individuals (male/female, 9/13, median age, 62 y; 20–89 y) in our hospital who served as controls. Vasculitis, except LVV group, was pathologically defined as fibrinoid necrosis in a vessel wall within a muscle or sural nerve biopsy, or as leucocytoclasis in a skin biopsy. One patient diagnosed with MPA, who had an ulcer in the sclera, would not consent to a tissue biopsy. Levels of ANCA against myeloperoxidase (MPO) were assessed using an enzyme-linked immunosorbent assay (ELISA), obtained from NIPRO Corp. (Tokyo, Japan); in the ELISA, values > 10 units were considered abnormal.
Table 1.
Patient characteristics and classifications of systemic vasculitis
| Vasculitis classification | SVV | MVV | LVV | |
|---|---|---|---|---|
|
| ||||
| Diseases | MPA | PAN | GCA | TA |
| N (male/female) | 14 (4/10) | 7 (2/5) | 5 (2/3) | 5 (0/5) |
| Age (mean) | 73 (53–87) | 59 (48–67) | 73 (60–92) | 21 (19–25) |
| CRP (mg/dl) | 2.6 (0.2–21.0) | 3.6 (0–10.4) | 1.2 (0.1–14.2) | 8.9 (0.1–26.4) |
| ESR (mm/h) | 43 (11–150) | 43.3 (16–97) | 95 (7–101) | 70 (13–140) |
| ANCA titers (units) | ||||
| MPO | 87.5 (0–640) | ND | ND | ND |
Abbreviations: MPA, microscopic polyangiitis; SVV, small vessel vasculitis; PAN, polyarteritis nodosa; MVV, medium-sized vessel vasculitis; GCA, giant cell arteritis; TA, Takayasu arteritis; LVV, large vessel vasculitis; ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; ND, Not detected.
Notes: All parameters in patients with vasculitis are statistically insignificant.
Serum C-reactive protein (CRP) levels and the erythrocyte sedimentation rate (ESR) were determined using a latex photometric immunoassay and the Westergren method, respectively. Vasculitis disease activity, except LVV group, was assessed using the Birmingham Vasculitis Activity Scores (BVAS).26 Clinical and biochemical parameters and serum cytokine in the patients were prospectively recorded. This study was carried out in accordance with protocols approved by the Human Subjects Research Committee at our institution, and informed consent was obtained from all patients and volunteers.
ELISA
MIF in serum was quantified using a commercial ELISA kit according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). The sensitivity limit of this ELISA was ~10 pg/ml.
Statistical analysis
Data are expressed as the median (ranges). The differences between groups were evaluated using the Mann–Whitney U test. Follow-up data were evaluated using Wilcoxon’s signed-rank test. The relationship between MIF levels and the indicated parameters were evaluated using the Spearman rank correlation. P-values < 0.05 were considered significant.
Results
Serum MIF levels
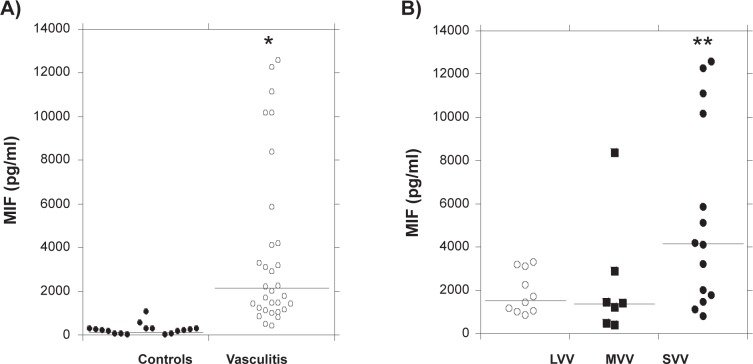
We initially used an ELISA to compare the MIF levels in serum samples from all systemic vasculitis patients (n = 31) with those in samples from healthy individuals (n = 22). We found that MIF levels were markedly higher in the vasculitis patients as compared with the healthy group (2031.7 [447.0–12600.4] pg/ml vs 181.5 [0–1097.0] pg/ml; Figure 1A; p < 0.0001). We then examined serum MIF levels in the three vasculitis subgroups, categorized based on the size of the affected vessels. We found that MIF levels were relatively higher in patients in the SVV (MPA) group (4161.7 [816.9–12600.4] pg/ml, p < 0.05) than in the other two groups (MVV, 1443.2 [447.0–8408.6] pg/ml and LVV, 1576.7 [856.1–3288.9] pg/ml; Figure 1B).
Figure 1.
Serum MIF levels in the normal control and vasculitis groups. Serum was obtained from all systemic vasculitis patients while their disease was active (newly diagnosed, untreated) and normal individuals. A) Serum MIF was assayed by ELISA. *p < 0.0001 vs healthy individuals (controls). B) Serum MIF levels in the three vasculitis groups categorized based on the size of the affected vessels. Among the vasculitis patients, MIF levels were relatively higher in patients in the SVV group than in the other two groups (MVV and LVV). **p < 0.05. Each bar in the figure indicates the median of MIF levels.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; LVV, large vessel vasculitis; MIF, migration inhibitory factor; MVV, medium-sized vessel vasculitis; SVV, small vessel vasculitis.
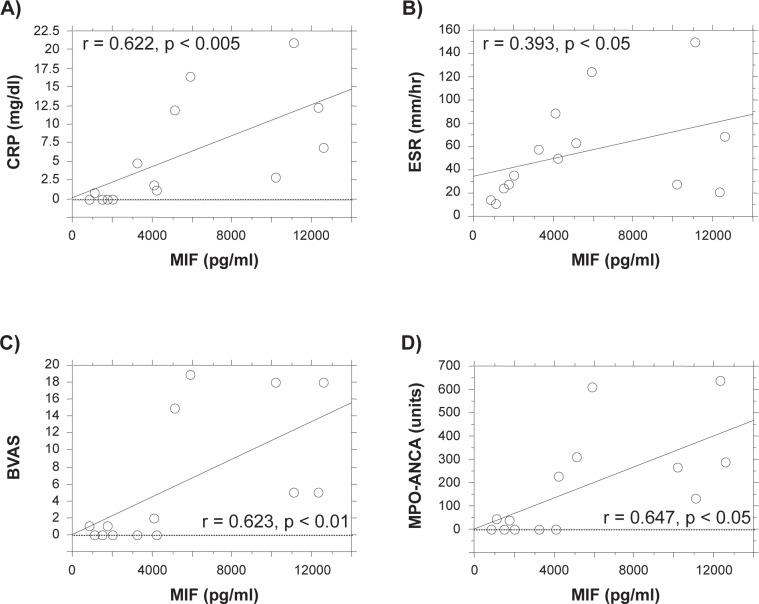
Because the results summarized above suggest that serum MIF levels may be involved in the pathophysiology of SVV (MPA), we assessed the correlation between serum MIF and serum inflammatory parameters and vasculitis disease activity. The individual data including serum levels of MIF and organ involvements of MPA patient are summarized in Table 2. As shown in Figure 2, there was a positive correlation between MIF levels in the MPA patients and either CRP levels (Figure 2A, r = 0.622, p < 0.005) or ESR levels (Figure 2B, r = 0.393, p < 0.05). Importantly, MIF levels in MPA patients also correlated significantly with disease activity, as measured by the BVAS (r = 0.623, p < 0.01) (Figure 2C). Furthermore, a positive correlation was found between MIF levels and serum MPO-ANCA titers in MPA patients (r = 0.647, p < 0.05) (Figure 2D).
Table 2.
Individual laboratory data, disease activity and organ involvements in patients with MPA
| Patient# | age | sex | CRP (mg/dl) | ESR (mm/h) | MIF (pg/ml) | BVAS | MPO-ANCA (units/ml) | Organ involvements | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Kidney | Pulmonary | Neuritis | ||||||||
| 1 | 87 | female | 11.9 | 63 | 5180.0 | 15 | 611.0 | |||
| 2 | 77 | female | 0 | 28 | 1779.2 | 1 | 0.0 | |||
| 3 | 85 | male | 0.8 | 11 | 1122.6 | 0 | 230.0 | + | ||
| 4 | 61 | female | 1.9 | 89 | 4104.8 | 2 | 0.0 | + | + | |
| 5 | 68 | female | 0 | 24 | 1494.6 | 0 | 310.0 | + | ||
| 6 | 76 | female | 16.5 | 124 | 5881.5 | 19 | 44.0 | + | ||
| 7 | 53 | female | 1.2 | 50 | 4218.6 | 0 | 265.0 | + | + | |
| 8 | 73 | male | 2.8 | 28 | 10200.0 | 18 | 38.0 | + | ||
| 9 | 60 | male | 21 | 150 | 11169.3 | 5 | 0.0 | + | + | + |
| 10 | 72 | female | 0 | 14 | 816.9 | 1 | 131.0 | + | ||
| 11 | 72 | female | 0 | 36 | 2031.7 | 0 | 0.0 | + | ||
| 12 | 69 | male | 12.3 | 21 | 12300.0 | 5 | 640.0 | + | + | |
| 13 | 80 | female | 4.8 | 58 | 3219.0 | 0 | 0.0 | + | + | |
| 14 | 77 | female | 6.8 | 69 | 12600.4 | 18 | 288.0 | + | + | |
Notes: Laboratory data including C-reactive protein (CRP) levels, erythrocyte sedimentation rate (ESR), macrophage migration inhibitory factor (MIF), disease activity; Birmingham Vasculitis Activity Scores (BVAS), myeloperoxidase–antineutrophil cytoplasmic antibody; (MPO-ANCA) and organ involvements from each MPA patient (n = 14) were indicated. Main manifestations were listed in organ involvements.
Figure 2.
Positive correlation between serum MIF levels and levels of the inflammatory markers CRP (A), ESR (B), disease activity (BVAS, C), and serum MPO-ANCA titers (D) in patients with MPA. Serum samples were collected from patients with MPA during both the active and inactive disease states. Serum MIF was assayed by ELISA. Each point represents a sample collected from a different MPA patient.
Abbreviations: BVAS, Birmingham Vasculitis Activity Scores; CRP, C-reactive protein; ELISA, enzyme-linked immunosorbent assay; ESR, erythrocyte sedimentation rate; MIF, migration inhibitory factor; MPA, microscopic polyangiitis; MPO-ANCA, myeloperoxidase–antineutrophil cytoplastic antibody.
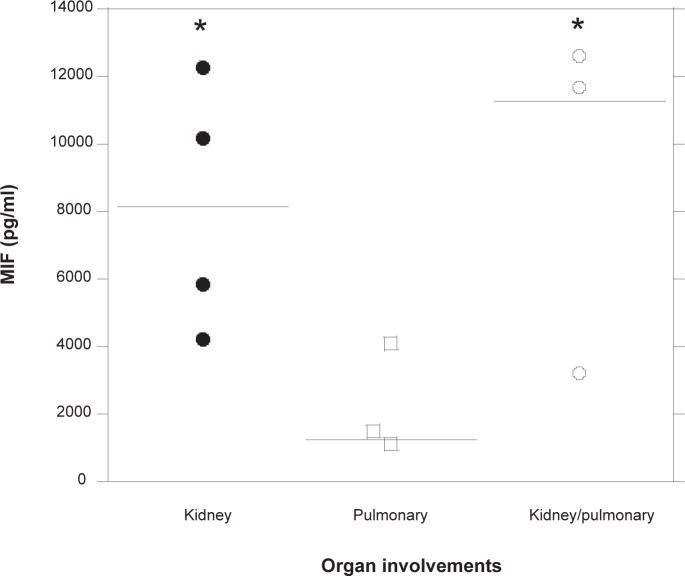
Next, we examined whether the increased levels of serum MIF are involved in the specific organ manifestations in patients with MPA. Among the major involved organs of MPA patients, four patients had kidney involvements, three patients had pulmonary involvements, and three other patients had the coexistence of both kidney and pulmonary involvements. Other involvements were skin in one patient, muscle in one patient, peripheral neuritis in two patients, and ulcer in the sclera in one patient. Because kidney and pulmonary involvements are serious and important organ manifestations in MPA, serum MIF levels were compared between patients with kidney and pulmonary involvements. As shown in Figure 3, serum MIF levels were significantly increased in MPA patients with both kidney (5882.0 [4219.7–10200.4], p < 0.05) and kidney/pulmonary involvements (11690.5 [3219.2–12600.4], p < 0.05), as compared with those complicated with only pulmonary manifestations (1495.7 [1123.0–4105.9]). There were no significant differences between kidney alone and kidney/pulmonary manifestations in MPA patients.
Figure 3.
Comparison of serum MIF levels between groups of patients with MPA with different specific organ manifestations. Serum MIF levels in MPA patients with complications in either the kidney (n = 4), lungs (n = 3), or both organs (n = 3) were assayed. Each point represents a sample collected from a different MPA patient and each bar in the figure indicates the median of MIF levels. *p < 0.05 versus the patients with pulmonary complications.
Abbreviations: MIF, migration inhibitory factor; MPA, microscopic polyangiitis.
Follow-up studies of the effect of treatment on serum MIF levels
In all MPA patients, serum at both baseline and after clinical improvement was available from 12 MPA patients. Notably, serum MIF levels were significantly diminished in patients following treatment and clinical improvement (p < 0.05), defined as the disappearance or attenuation of clinical and biologic signs of vasculitis, as reflected by a ≥ 50% decrease in the BVAS score (Figure 4). No significant differences in the course of the changes in serum MIF levels were found among the medications. Furthermore, there was no significant correlation between the reduction of serum MIF levels and MPO-ANCA levels after the treatments.
Figure 4.
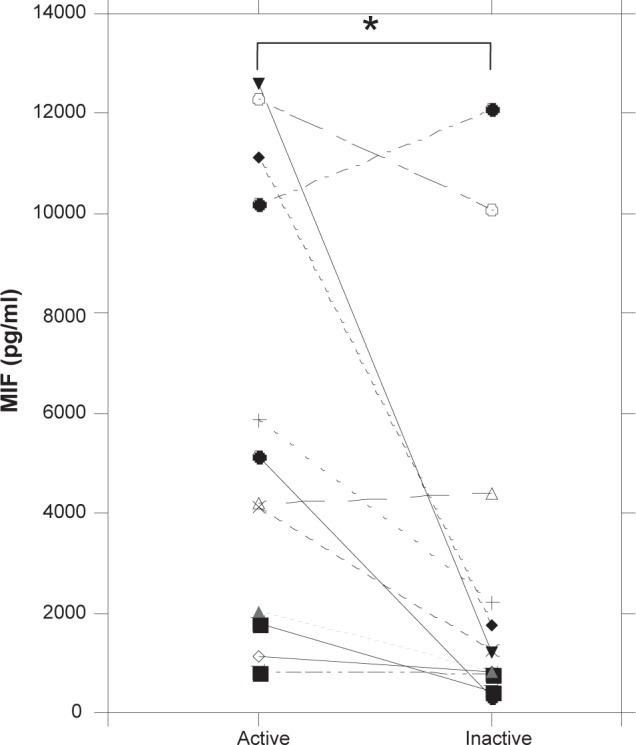
Follow-up measurements of serum MIF levels in MPA patients following treatment. Serum was obtained from 12 MPA patients first while their disease was active (newly diagnosed, untreated) and again when it was inactive (after 3–6 months from first treatment). Serum MIF concentrations were assayed by ELISA. Each point represents a sample collected from a different MPA patient; a significant reduction of MIF levels was seen after clinical improvement. *p < 0.05 vs active.
Abbreviations: ELISA, enzyme-linked immunosorbent assay; MIF, migration inhibitory factor; MPA, microscopic polyangiitis.
Discussion
In the present study, we showed that serum MIF levels are increased in patients with systemic vasculitis as compared with normal controls. Of interest, significantly increased levels of serum MIF were seen in patients with MPA as compared with the levels seen in patients of the other two vasculitis groups: MVV and LVV. The elevated MIF levels seen in MPA patients correlated positively with BVAS, as well as with CRP levels and ESR, and also with serum MPO-ANCA titers. Notably, levels of MIF were significantly diminished in MPA patients in clinical improvement after treatment.
Recently, Becker and colleagues showed that serum MIF levels were elevated in patients with ANCA-related vasculitis.20 Similarly, we also clearly demonstrated that MIF levels were higher in the SVV group as compared with the MVV or LVV group, and the MIF levels were positively correlated with systemic inflammation and vasculitis disease activity. It has been reported that serum levels of endothelial molecules such as adhesion molecules and EC-derived cytokines were elevated in patients with vasculitis.27–29 Furthermore, vasculitis-affected small vessels seen in MPA may have dysregulated endothelial cell function.30 In patients with MPA, the origin of the increased serum MIF levels seems to be cells that are capable of secreting MIF;11,13,31 specifically, the endothelial cells and/or inflammatory cells such as monocytes and neutrophils. The secreted MIF would in turn regulate the proliferation of EC.32 Furthermore, a positive correlation was found between serum MIF levels and MPO-ANCA titers in MPA patients. We gathered no data on the stimulating capacity of MPO-ANCA on the secretion of cytokines, including MIF, but this is likely to be related to disease activity and MIF levels, given the positive relation between MPO-ANCA titers and disease activity of vasculitis.33 Conversely, MIF upregulates ICAM-1 on endothelial cells34 and stimulates the expression and secretion of other inflammatory cytokines, including tumor necrosis factor alpha and interleukin-8 (IL-8).12,35 The recruitment of leukocytes to sites of inflammation involves adhesion molecule-dependent interactions with EC. Thus, collectively, the dysregulated orchestration of MIF from EC and/or leukocytes and the adhesion molecules and cytokines induced by MIF may have crucial roles in the development of SVV such as MPA.
Another interesting observation in this study was that serum MIF levels were much more pronounced in MPA patients with renal involvements than in MPA patients with pulmonary involvements, as shown in Figure 3. It has been previously shown that MIF protein and mRNA were detected in intrinsic renal cells and glomerular ECs and they were markedly upregulated in the more severe forms of glomerulonephritis, such as crescentic glomerulonephritis.36–38 In glomerulonephritis, mesangial cells and tubular epithelial cells, as well as glomerular capillary endothelium were identified as the major source of MIF expression.36,37 Conversely, MIF promotes macrophage activation and secretion of macrophage-derived cytokines, including IL-1 and fibroblast growth factor, which may induce mesangial cell proliferation.11,39 Collectively, these findings suggest that, combined with other factors, the MIF expressed in the inflamed kidney may be potentially involved in the development of the renal damage seen in MPA.
We showed that in patients with MPA, the serum levels of MIF were reduced after clinical remission. A unique characteristic of MIF is its relationship with glucocorticoids: low/physiological concentrations of glucocorticoids induce the synthesis and release of MIF, while high doses of glucocorticoids can suppress MIF and its promoter activity,12,40 as they do with other proinflammatory cytokines. In all of our patients with MPA, the initial therapies were the administration of high doses of glucocorticoids; therefore, the increased serum MIF levels may be dependent upon the disease activity of vasculitis inflammation, but not the effects of glucocorticoid therapy. In this regard, we observed no significant correlation between the reduction of serum MIF levels and MPO-ANCA levels after the treatments, although the elevated MIF levels seen in untreated MPA patients correlated positively with serum MPO-ANCA titers. These observations may be depended upon the expressional mechanisms and responsiveness of MIF and MPO-ANCA against the immunosuppressive therapies, because MIF would be expressed mainly in ECs and ANCA would be produced from B cells.
Taken together, the present study suggest that increased MIF appears to be involved in the pathogenesis of systemic vasculitis, especially small vessel vasculopathy seen in MPA, and may serve as a useful serologic marker of disease activity in vasculitis.
Acknowledgments
This study was supported in part by a grant from the Ministry of Health and Welfare of Japan. We thank Mrs Hiroko T Takeuchi and Kyoko Nohtomi for their excellent help with the experiments.
References
- 1.Kallenberg CG, Heeringa P, Stegeman CA. Mechanisms of Disease: pathogenesis and treatment of ANCA-associated vasculitides. Nat Clin Pract Rheumatol. 2006;2:661–670. doi: 10.1038/ncprheum0355. [DOI] [PubMed] [Google Scholar]
- 2.Guillevin L, Dorner T. Vasculitis: mechanisms involved and clinical manifestations. Arthritis Res Ther. 2007;9(Suppl 2):S9. doi: 10.1186/ar2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley CD, Rainger GE, Nash GB, Raza K. Endothelial cells, fibroblasts and vasculitis. Rheumatology (Oxford) 2005;44:860–863. doi: 10.1093/rheumatology/keh542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneider NC, Leger AJ, Kuliopulos A. Therapeutic targeting of molecules involved in leukocyte-endothelial cell interactions. FEBS J. 2006;273:4416–4424. doi: 10.1111/j.1742-4658.2006.05441.x. [DOI] [PubMed] [Google Scholar]
- 5.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 6.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- 8.Bloom BR, Shevach E. Requirement for T cells in the production of migration inhibitory factor. J Exp Med. 1975;142:1306–1311. doi: 10.1084/jem.142.5.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacher M, Metz CN, Calandra T, et al. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calandra T, Bernhagen J, Mitchell RA, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leech M, Metz C, Hall P, et al. Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum. 1999;42:1601–1608. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 13.Nishihira J, Koyama Y, Mizue Y. Identification of macrophage migration inhibitory factor (MIF) in human vascular endothelial cells and its induction by lipopolysaccharide. Cytokine. 1998;10:199–205. doi: 10.1006/cyto.1997.0276. [DOI] [PubMed] [Google Scholar]
- 14.Ayoub S, Hickey MJ, Morand EF. Mechanisms of disease: macrophage migration inhibitory factor in SLE, RA and atherosclerosis. Nat Clin Pract Rheumatol. 2008;4:98–105. doi: 10.1038/ncprheum0701. [DOI] [PubMed] [Google Scholar]
- 15.Foote A, Briganti EM, Kipen Y, Santos L, Leech M, Morand EF. Macrophage migration inhibitory factor in systemic lupus erythematosus. J Rheumatol. 2004;31:268–273. [PubMed] [Google Scholar]
- 16.Hoi AY, Morand EF, Leech M. Is macrophage migration inhibitory factor a therapeutic target in systemic lupus erythematosus? Immunol Cell Biol. 2003;81:367–373. doi: 10.1046/j.1440-1711.2003.01183.x. [DOI] [PubMed] [Google Scholar]
- 17.Selvi E, Tripodi SA, Catenaccio M, et al. Expression of macrophage migration inhibitory factor in diffuse systemic sclerosis. Ann Rheum Dis. 2003;62:460–464. doi: 10.1136/ard.62.5.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong YP, Abadia-Molina AC, Satoskar AR, et al. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061–1066. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]
- 19.Ohwatari R, Fukuda S, Iwabuchi K, Inuyama Y, Onoe K, Nishihira J. Serum level of macrophage migration inhibitory factor as a useful parameter of clinical course in patients with Wegener’s granulomatosis and relapsing polychondritis. Ann Otol Rhinol Laryngol. 2001;110:1035–1040. doi: 10.1177/000348940111001108. [DOI] [PubMed] [Google Scholar]
- 20.Becker H, Maaser C, Mickholz E, Dyong A, Domschke W, Gaubitz M. Relationship between serum levels of macrophage migration inhibitory factor and the activity of antineutrophil cytoplasmic antibody-associated vasculitides. Clin Rheumatol. 2006;25:368–372. doi: 10.1007/s10067-005-0045-9. [DOI] [PubMed] [Google Scholar]
- 21.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 22.Sorensen SF, Slot O, Tvede N, Petersen J. A prospective study of vasculitis patients collected in a five year period: evaluation of the Chapel Hill nomenclature. Ann Rheum Dis. 2000;59:478–482. doi: 10.1136/ard.59.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lightfoot RW, Jr, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33:1088–1093. doi: 10.1002/art.1780330805. [DOI] [PubMed] [Google Scholar]
- 24.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 25.Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129–1134. doi: 10.1002/art.1780330811. [DOI] [PubMed] [Google Scholar]
- 26.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. Qjm. 1994;87:671–678. [PubMed] [Google Scholar]
- 27.Bradley JR, Lockwood CM, Thiru S. Endothelial cell activation in patients with systemic vasculitis. Qjm. 1994;87:741–745. doi: 10.1093/oxfordjournals.qjmed.a068892. [DOI] [PubMed] [Google Scholar]
- 28.Johnson PA, Alexander HD, McMillan SA, Maxwell AP. Up-regulation of the endothelial cell adhesion molecule intercellular adhesion molecule-1 (ICAM-1) by autoantibodies in autoimmune vasculitis. Clin Exp Immunol. 1997;108:234–242. doi: 10.1046/j.1365-2249.1997.3741271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundy JS, Haynes BF. Cytokines and adhesion molecules in the pathogenesis of vasculitis. Curr Rheumatol Rep. 2000;2:402–410. doi: 10.1007/s11926-000-0040-8. [DOI] [PubMed] [Google Scholar]
- 30.Filer AD, Gardner-Medwin JM, Thambyrajah J, et al. Diffuse endothelial dysfunction is common to ANCA associated systemic vasculitis and polyarteritis nodosa. Ann Rheum Dis. 2003;62:162–167. doi: 10.1136/ard.62.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riedemann NC, Guo RF, Gao H, et al. Regulatory role of C5a on macrophage migration inhibitory factor release from neutrophils. J Immunol. 2004;173:1355–1359. doi: 10.4049/jimmunol.173.2.1355. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Degranpre P, Kharfi A, Akoum A. Identification of macrophage migration inhibitory factor as a potent endothelial cell growth-promoting agent released by ectopic human endometrial cells. J Clin Endocrinol Metab. 2000;85:4721–4727. doi: 10.1210/jcem.85.12.7003. [DOI] [PubMed] [Google Scholar]
- 33.Sinico RA, Radice A, Corace C, L DIT, Sabadini E. Value of a new automated fluorescence immunoassay (EliA) for PR3 and MPO-ANCA in monitoring disease activity in ANCA-associated systemic vasculitis. Ann N Y Acad Sci. 2005;1050:185–192. doi: 10.1196/annals.1313.019. [DOI] [PubMed] [Google Scholar]
- 34.Lin SG, Yu XY, Chen YX, et al. De novo expression of macrophage migration inhibitory factor in atherogenesis in rabbits. Circ Res. 2000;87:1202–1208. doi: 10.1161/01.res.87.12.1202. [DOI] [PubMed] [Google Scholar]
- 35.Onodera S, Nishihira J, Koyama Y, et al. Macrophage migration inhibitory factor up-regulates the expression of interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid arthritis patients: common transcriptional regulatory mechanism between interleukin-8 and interleukin-1beta. Arthritis Rheum. 2004;50:1437–1447. doi: 10.1002/art.20190. [DOI] [PubMed] [Google Scholar]
- 36.Lan HY, Mu W, Yang N, et al. De Novo renal expression of macrophage migration inhibitory factor during the development of rat crescentic glomerulonephritis. Am J Pathol. 1996;149:1119–1127. [PMC free article] [PubMed] [Google Scholar]
- 37.Tesch GH, Nikolic-Paterson DJ, Metz CN, et al. Rat mesangial cells express macrophage migration inhibitory factor in vitro and in vivo. J Am Soc Nephrol. 1998;9:417–424. doi: 10.1681/ASN.V93417. [DOI] [PubMed] [Google Scholar]
- 38.Lan HY, Yang N, Nikolic-Paterson DJ, et al. Expression of macrophage migration inhibitory factor in human glomerulonephritis. Kidney Int. 2000;57:499–509. doi: 10.1046/j.1523-1755.2000.00869.x. [DOI] [PubMed] [Google Scholar]
- 39.Lovett DH, Ryan JL, Sterzel RB. A thymocyte-activating factor derived from glomerular mesangial cells. J Immunol. 1983;130:1796–1801. [PubMed] [Google Scholar]
- 40.Alourfi Z, Donn RP, Stevens A, Berry A, McMaster A, Ray DW. Glucocorticoids suppress macrophage migration inhibitory factor (MIF) expression in a cell-type-specific manner. J Mol Endocrinol. 2005;34:583–595. doi: 10.1677/jme.1.01647. [DOI] [PubMed] [Google Scholar]