Abstract
Introduction
Human serum albumin (HSA) is a critical protein in human blood plasma, which can be highly damaged by oxidative stress. The aim of this study was to analyze modifications of this protein after oxidation using a Fenton system.
Methods
In this 2015 experiment, different ratios of Fenton reagent (Fe2+/H2O2) was incubated with one concentration of human serum albumin (1mg/ml). Hence, HSA was incubated 30 min with various combinations of a Fenton system and quantified oxidation products such as carbonyl groups, fragmentations, degradations, and oxidized free thiol group using reliable techniques. Image and data analysis were carried out using ImageJ software and Excel (version 2007), respectively.
Results
An SDS-PAGE profile showed no cross link and aggregation. However, protein band intensity has decreased to 50% in the highest ratio of H2O2/Fe. Carbonylation assay indicated carbonyl/protein (molc/molp) ratio increased linearly in lower ratios and the values plateau at higher levels of H2O2/Fe 2+. The only free sulfhydryl group on HSA was oxidized in all ratios of the Fenton system.
Conclusion
To sum, the structure of HSA has been changed following treatment with Hydroxyl Radical as the main product of Fenton reaction. These data confirm the antioxidant activity of HSA.
Keywords: HSA, Fenton system, Carbonylation assay, SDS-PAGE
1. Introduction
Oxidative stress (ROS) is accompanied by aging and several neurodegenerative diseases and cancers. Indeed, ROS are generated as byproducts of cellular metabolism and have the potential to increase significant biological damage (1). There are several evidences to support the idea that the most important mechanism of protein oxidative damage is metal-catalyzed oxidation (MCO), leading to cleavage of polypeptide backbone, cross linking, and modification of the amino acid side chains leading to the loss of protein function and to structural alteration. Protein oxidation has become a crucial focus of research, as increased levels of oxidized proteins are one of the most important biomarkers of oxidative stress (2, 3). It is almost clear that post-translational modifications (PTMs) generated by oxidative damage from ROS and RNS on protein integrity are responsible for many pathologies and biological aging (3). Albumin is a crucial plasma antioxidant protein, involving several physiological and pharmacological functions. This protein is the most abundant protein in human plasma with a molecular weight of 66 KD. It has been well known that human serum albumin (HSA) is an easy target for ROS (4). Thereby, HSA is continuously exposed to oxidative stress, so that modifications of the conformation and function of HSA could happen and lead to alteration of its properties. By now, it is shown that advanced oxidation protein products (AOPPs) are formed as a main result of interaction between plasma proteins, especially HSA, and hydroxyl radicals (°OH) produced by Fenton reaction, which takes place during ROS production or ischemia events (5). In the present study, we chose a simple oxidation system consisting of H2O2 and FeSO4, namely, Fenton reaction, to induce the formation of hydroxyl radicals from H2O2. It is a common belief that the highly reactive hydroxyl radical (HO°) is a main product generated in Fenton’s reaction (6). The aim of this work is to oxidize human serum albumin (HSA) by Fenton reaction in various levels and quantified different protein damages such as carbonyl groups, protein degradation, fragmentations, aggregations, and thiol groups.
2. Material and Methods
2.1. Research Design and Setting
This experimental research was planned to study the effects of different ratios of a Fenton system, ranging from 0.1 to 20 mM of H2O2 and a fixed concentration of FeSO4 (1mM) on HSA. This study was accomplished at Babol University of Medical Sciences in 2015.
2.2. Chemicals and Reagents
Human serum albumin (HSA) (Sigma, A9511), sodium dodecyl sulphate (Merck, Germany), tetramethylethylenediamide (Applichem, A1148), ammonium persulphate (Merck, Germany), acrylamid for electrophoresis (Merck, Germany), N-N′-methylene diacrylamide (Merck, Germany), sodium carbonate (Merck, Millipore), formaldehyde 37% (Merck, Germany), tris-base, methanol (Merck, Germany), ethanol (Merck, Germany), glycerol (Merck, Germany), acetic acid (Glacial, Merck), 2,4-Dinitrophenylhydrazine (DAEJUNG, South Korea), trichloroacetic acid (TCA) (Merck, Germany), guanidine-Hcl (DAEJUNG, South Korea), ethyl acetate for analysis (Merck, Germany), bromophenol blue (Merck, Germany), sodium thiosulfate pentahydrate (Merck, Germany), hydrogen peroxide 30% (Merck, Millipore), ferrous sulphate (SIGMA, F7002), DTNB(5,5-dithiobis(2-nitrobenzoic acid) 99% (SIGMA), Hcl 37% (Merck, Germany), 1,4-Dithiothreitol (Scharlau Chimie S.A, European Union), Coomassie Brilliant Blue G250 for electrophoresis (Merck, Germany).
2.3. Preparation of Hydrogen Peroxide Standards
The concentration of H2O2 stock solution (0.4M), typically 30% (v/v) H2O2, was determined accurately using a molar extinction coefficient (ɛ) of 39.4M-1 cm-1 at 240 nm. All spectrophotometer analysis was performed by a NanoDrop 2000 UV-VIS Spectrophotometer, Thermo Fisher Scientific Inc.
2.4. Hydroxyl Radical Generating System
Under in vitro conditions, Fe2+ is able to generate the °OH by modification of H2O2 according to the Fenton redox reaction (7). The metal-catalyzed oxidation was launched in 10 mM potassium phosphate buffer pH 7. Oxidation was accomplished by supplementing 500 μL of protein solution (1 mg/ml) with a freshly prepared mixture of FeSO4 (1mM) to increasing concentrations of H2O2 between 0.1 and 20mM. The reaction was incubated for 30 min at room temperature and stopped by addition of cold 10% TCA (8, 9). Subsequently, samples centrifuged at 10000×g for 15 min and washed three times with 10% TCA (8). Finally, supernatant was removed and pellets were dissolved in 10 mM phosphate buffer pH 7. All samples were kept at −80 °C for subsequent analysis. All experiments were carried out in triplicate.
2.5. Gel Electrophoresis
After treating human serum albumin (1 mg/ml) with Fenton reaction, its modifications were analyzed by sodium dodecyl sulphate/polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Before putting samples (4μgr) in each well, they were treated with 10% SDS, 0.5M Tris-base pH 6.8, 2.7mM glycerol and 0.2 M Dithiothreitol (DTT) and resolved by 8% SDS-PAGE according to Laemmli’s method (10). For gel staining, each gel was dyed with Coomassie Blue G-250. Image analysis was carried out using a Labscan machine (powered by Melanie) and analyzed with ImageJ software. The software allowed background subtraction, automatic band detection, and comparative analysis of normalized band optical densities (ODs).
2.6. Carbonyl Content Determination
Protein carbonylation assay was performed by spectrophotometric DNPH assay for detection of protein carbonyl groups (11). Briefly, 200μl of each sample were incubated with 200μL of DNPH 0.2% (prepared in 2N HCl) in the dark for 1 hour at room temperature and vortexed each 10 minutes. The samples were precipitated by an ice cold solution of TCA 20 % and centrifuges at 10000 rpm for 10 minutes. Collected pellets were washed three times with ethanol/ethyl acetate (1/1; v/v) to remove the excess of DNPH reagent. The resulting protein pellet was dissolved in 100 μl of 6M GuHCl, and the absorbance was measured at 370 nm against blank (Hcl treated) as reference. To quantify the level of protein carbonylation groups, we used the molar extinction coefficient (22000 M -1 cm -1) and expressed protein carbonyl groups in mol carbonyl per mol protein. We normalized absorbance to 15μM of protein and calculated carbonyl concentration by Beer–Lambert Law. We calculated carbonyl/protein concentration (molC/molP) by division of carbonyl content to protein concentration. Experiments were repeated at least three times to ensure the reproducibility of data.
2.7. DTNB Assay
The free thiol concentration of protein was measured by Ellman’s reagent (11). A 50 μl of protein solution (2mg/ml) was dispensed into 1ml centrifuge tubes and 50 μl of DTNB (0.5mM), prepared in 100mM phosphate buffer pH 7, was added to each sample. Finally, all solutions were vortexed and incubated in the dark conditions for 30 min at room temperature. A protein solution consisted of 50 μl of 100mM phosphate buffer, plus 50 μl of protein solution was selected as blank for each group. In addition, a DTT (10mM) group was chosen as a positive control group. Absorbance of each solution was measured at 412 nm against blank as follows: Absorbance Change = A412 (Protein+DTNB) - A412 (Protein + Phosphate Buffer) – A412 (DTNB alone blank). A thiol concentration was determined for each solution by dividing the above value by an extinction coefficient at 412 (13600 M-1 cm-1).
3. Results and discussion
3.1. Gel Electrophoresis of HSA
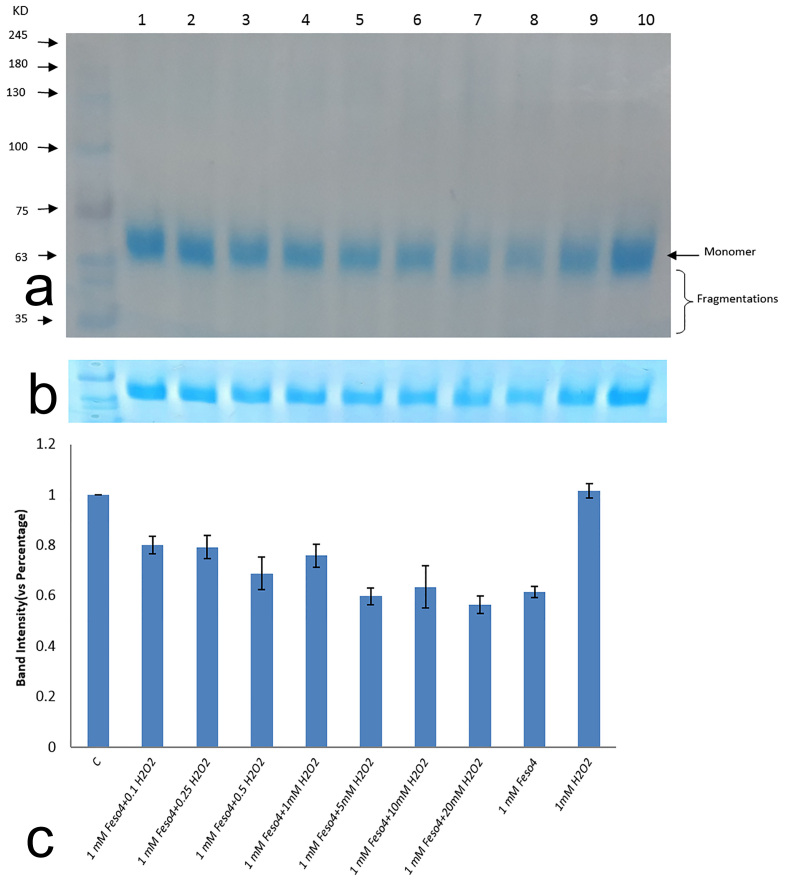
SDS-PAGE is a practical technique to analyze protein damages that were produced by Fenton reaction. A Fenton reaction may give rise to breakage of polypeptide chains through oxidative damage of amino acids in protein, and these damages can be visualized by the creation of cross links, fragments, and smearing of the gel. Figure 1 shows different damages that were produced by Fenton reaction. Densitometry of bands showed the loss of protein in higher ratios of a Fenton system. Fenton products caused breakage of covalent bonds, the effects of which are illustrated in the SDS-PAGE portrait by progressive disappearance of monomer band (Figure 1b). The results demonstrated that, by increasing the hydrogen peroxide concentration, the rate of protein removal was increased. This is owing to the formation of more hydroxyl radicals. As can be seen in the SDS-PAGE profile (Figure 1a), there is no aggregation or cross link due to formation of covalent bands between specific amino acids such as tyrosine leading to dimer and oligomer. Considering the SDS-PAGE profile, protein fragmentations are increasing in higher ratios of Fenton reaction (Figure 1b). It is noteworthy that, in the highest ratio of the Fenton system, about 50% of native protein has broken down. It can be obviously seen that the sample treated with H2O2 has approximately the same band intensity compared with non-oxidized protein, but the Fe2+ treated group showed a 40% drop in its band densitometry against native counterpart (Figure 1c).
Figure 1.
HSA oxidation as a function of Fenton reaction in different ratios. A) HSA was oxidized by increasing ratio of H2O2 to FeSo4 and analyzed by SDS-PAGE (10% Poly acrylamide). Lane 1: control group, Lane 2: H2O2 (0.1mM) + FeSo4 (1mM); Lane 3: H2O2 (0.25mM) + FeSo4 (1mM); Lane 4: H2O2 (0.5mM) + FeSo4 (1mM); Lane 5: H2O2 (1mM) + FeSo4 (1mM), Lane 6: H2O2 (5mM) + FeSo4 (1mM), Lane 7: H2O2 (10mM) + FeSo4 (1mM), Lane 8: H2O2 (20mM) + FeSo4 (1mM), Lane 9: FeSo4 (1mM), Lane 10: H2O2 (1mM). B) SDS-PAGE profile of HSA disappearance treated with different ratios of Fenton reaction. C) The average percentage of band intensity of HSA (4μg) as a function of Fenton reaction in different levels. Calculations were performed to similarly treated samples from triplicate experiments
3.2. Protein Carbonylation
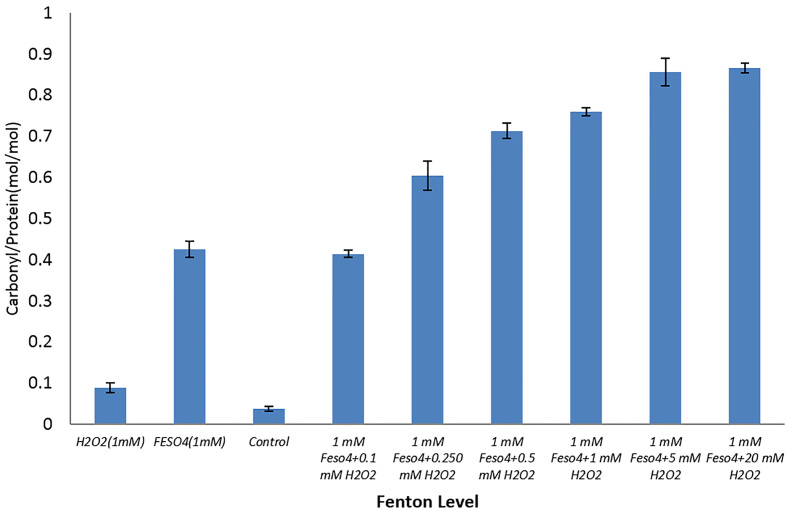
Protein carbonylation is the most deteriorating irreversible modification as a result of oxidative stress (12). According to Figure 2, a carbonyl/protein (mol/mol) ratio is increasing linearly in lower Fenton ratios and the values plateau at higher levels of H2O2/Fe 2+. It is noteworthy that reaction of HSA with H2O2 alone gave a low level of carbonyl groups bound to protein (0.088±0.012) while Fe2+ treatment at a fixed concentration (1mM) could produce a significant level of carbonyl on protein by itself (0.42±0.019). This result is in agreement with another study performed by Henrietta et al. (13). These results indicate that the type of oxidizing agent can play an important role in protein carbonylation and are consistent with former results presented by Madian et al. (14). As previous studies showed, conversion of proline, lysine, arginine, and hystidine residues (RKPT) into carbonyl derivatives is the most important precursor of carbonylated compounds, which increase the susceptibility and sensitivity of HSA to irreversible modification owing to oxidative stress (12). As there are several RKPT-enriched regions on this protein, this increased the sensitivity of this protein to damage by oxidants. Nevertheless, metal catalyzed oxidation (MCO) of a protein may be a site-specific process, in which Fe (II) makes a complex with protein and react with H2O2 to yield °OH. It was shown that hydroxyl radicals are able to generate structural changes in the N-terminal side of HSA (5). These results revealed that hydroxyl radicals which have been formed through Fenton reaction in in vivo conditions can present a new source of numerous ROS and RNS producers in the animal’s body.
Figure 2.
Carbonyl/protein ratio after incubation with different concentrations of H2O2, in the range of 0.1, 0.250, 0.5, 1, 5, 10, 20 mM, and a fixed concentration of FeSo4 (1mM) in phosphate buffer, pH 7
3.3. DTNB Assay
HSA is a great antioxidant in blood owing to its free thiol at Cys-3, which accounts for about 80% of the total plasma thiol component (15). In the present study, we investigated the possible effect of Fenton reaction on HSA (1 mg/ml) in various levels of oxidation. Our results showed that the ratio of thiol/protein was 0.26 ±0.05 (molt/molp) for native protein. After oxidation of samples through Fenton reaction, the ratios have dipped to zero in each sample. Although the thiol content for a FeSO4 (1mM) treated sample, alone, was 0.23 ± 0.02 (molt/molp), it is important to note that the thiol ratio also has dropped to zero in H2O2 exposed solely. These results are in accordance with the former investigation, which has indicated the H2O2-mediated and Fenton reaction oxidation of thiol groups (16). As for the positive control group (DTT group), the figure of thiol concentration was 504 ± 7.5 μM.
3.4. More discussion
HSA (66KD) is a heart-shape protein containing 585 residues (17). It contains 35 cysteine amino acids, in which all of these residues are engaged in the formation of 17 disulfide bonds except Cys-34, containing the only free thiol group. Several studies have shown the modification of free SH group on Cys to different oxidized products such as sulfenic acid (−SOH), sulfinic acid (−SO2H), sulfonic acid (−SO3H) through different ROS (18). Moreover, it has shown that °OH radicals, which are produced through gamma radiolysis and Fenton reaction, have an important role in protein oxidation and totally oxidative stress (19). In this report, we propose an antioxidant activity of HSA owing to simple reduction of sulfhydryl groups on Cys-34 exposed with increasing amounts of OH radicals produced by Fenton system. The results are in agreement with the former report concerning the radical scavenger properties of HSA through the free thiol group on Cys-34 residue (20, 21). Despite this, we need to analyze the structure of oxidized HSA precisely to identify the actual modifications of sulfhydryl side chain of this residue. Our results indicated that HSA has a significant propensity to protein carbonylation. Regarding the fact that carbonylation is site selective, this phenomenon may be due to its structure or conformation. These findings confirmed the results of Guilherme Vargas Bochi et al., indicating advanced oxidation products (AOPPs) formation as a result of Fenton reaction (4). It would be of interest to say that HSA has no propensity to the formation of cross link, as we have no protein aggregation in the SDS-PAGE profile. However, the band’s densitometry indicates the loss of protein in a higher level of the Fenton system. Similar results were obtained in gamma irradiation of BSA (22). It is important to point out that protein carbonylation (Figure 2) and band densitometry disappearance (Figure 1a) are increasing in higher levels of Fenton reaction, concomitantly. Considering the SDS-PAGE profile, breakdown of the polypeptide chain results in the formation of destroyed low-molecular-weight subunits. Schuessler and Schilling showed that proline subunits are the main target for chain sequestering in BSA (22). For quantification of thiol and carbonyl groups, the DTNB and DNPH assays were used as sensitive methods, respectively. As these techniques were not qualified to identify the types of oxidation products on the protein structure, we could not report on the type of oxidation byproducts that has been formed on protein throughout this study.
4. Conclusions
In conclusion, our data propose that Fenton reaction may be an important pathway to the harmful effect on human serum albumin. To scrutinize the structural and conformational damages created through oxidation, other techniques are underway. In addition, the modification of protein arrangement after oxidation will be later investigated using TEM.
Acknowledgments
We wish to express our sincere gratitude to the staff at the Cellular and Molecular Biology Research Center in Babol University of Medical Sciences for providing facilities necessary to achieve the desired goals of this project. We also thank Dr. Cecile Sicard-Roselli (Laboratoire de Chimie Physique d’Orsay, University of Paris SUD 11, France), Mr. V. Fatahi and Mrs. M. Golpour (Babol University of Medical Sciences, Iran) for their helpful discussions on the project. Moreover, the authors gratefully acknowledge Mr. S. Kazemi and Dr. A. Mostafazadeh for generously providing their materials.
Footnotes
iThenticate screening: May 05, 2016, English editing: July 12, 2016, Quality control: August 06, 2016
Conflict of Interest:
There is no conflict of interest to be declared.
Authors’ contributions:
All authors contributed to this project and article equally. All authors read and approved the final manuscript.
References
- 1.Seyfizadeh N, Mahjoub S, Zabihi E, Moghadamnia AA, Pouramir M, Mir H, et al. Cytoprotective Effects of Arbutin Against Tert-Butyl Hydroperoxid Induced Toxicity in Hep-G2 Cell Line. World Applied Sciences Journal. 2012;19(2):163–7. [Google Scholar]
- 2.Gaber MH. Effect of gamma-Irradiation on the Molecular Properties of Bovine Serum Albumin. J Biosci Bioeng. 2005;100(2):203–6. doi: 10.1263/jbb.100.203. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen AT, Donaldson RP. Metal-catalyzed oxidation induces carbonylation of peroxisomal proteins and loss of enzymatic activities. Arch Biochem Biophys. 2005;439(1):25–31. doi: 10.1016/j.abb.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Bochi GV, Torbitz VD, Cargnin LP, de Carvalho JA, Gomes P, Moresco RN. An Alternative Pathway Through the Fenton Reaction for the Formation of Advanced Oxidation Protein Products, a New Class of Inflammatory Mediators. Inflammation. 2014;37(2):512–21. doi: 10.1007/s10753-013-9765-1. [DOI] [PubMed] [Google Scholar]
- 5.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10(8):1343–74. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbehenn R, Dodick T, Poopat U, Spencer B. Fenton-Type Reactions and Iron Concentrations in the Midgut Fluids of Tree-Feeding Caterpillars. Arch Insect Biochem Physiol. 2005;60(1):32–43. doi: 10.1002/arch.20079. [DOI] [PubMed] [Google Scholar]
- 7.Kanti Das T, Wati MR, Fatima-Shad K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association With Alzheimer’s Disease. Arch Neurosci. 2015;2(2):20078. doi: 10.5812/archneurosci.20078. [DOI] [Google Scholar]
- 8.Lenarczyk M, Cohen EP, Fish BL, Irving AA, Sharma M, Driscoll CD, et al. Chronic Oxidative Stress as a Mechanism for Radiation Nephropathy. Radiat Res. 2009;171(2):164–72. doi: 10.1667/RR1454.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guedes S, Vitorino R, Domingues R, Amado F, Domingues P. Oxidation of bovine serum albumin: identification of oxidation products and structural modifications. Rapid Commun Mass Spectrom. 2009;23(15):2307–15. doi: 10.1002/rcm.4149. [DOI] [PubMed] [Google Scholar]
- 10.He F. Laemmli-SDS-PAGE. Bio-protocol Bio. 2011;101(80) [Google Scholar]
- 11.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1–2):23–38. doi: 10.1016/S0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 12.Castro-Acosta RM, Rodriguez-Limas WA, Valderrama B, Ramirez OT, Palomares LA. Effect of metal catalyzed oxidation in recombinant viral protein assemblies. Microb Cell Fact. 2014;13(1):25. doi: 10.1186/1475-2859-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Headlam HA, Davies MJ. Markers Of Protein xideased Carbonyl Products. Free Radic Biol Med. 2004;36(9):1175–84. doi: 10.1016/j.freeradbiomed.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 14.Madian AG, Regnier FE. Proteomic Identification Of Carbonylated Proteins And Their Oxidation Sites. J Proteome Res. 2010;9(8):3766–80. doi: 10.1021/pr1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turell L, Radi R, Alvarez B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic Biol Med. 2013;65:244–53. doi: 10.1016/j.freeradbiomed.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taverna M, Marie AL, Mira JP, Guidet B. Specific antioxidant properties of human serum albumin. Ann Intensive Care. 2013;3(1):4. doi: 10.1186/2110-5820-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami A, Kubota K, Yamada N, Tagami U, Takehana K, Sonaka I, et al. Identification and characterization of oxidized human serum albumin. A slight structural change impairs its ligand-binding and antioxidant functions. Febs j. 2006;273(14):3346–57. doi: 10.1111/j.1742-4658.2006.05341.x. [DOI] [PubMed] [Google Scholar]
- 18.Blouquit Y, Duchambon P, Brun E, Marco S, Rusconi F, Sicard-Roselli C. High sensitivity of human centrin 2 toward radiolytical oxidation: C-terminal tyrosinyl residue as the main target. Free Radic Biol Med. 2007;43(2):216–28. doi: 10.1016/j.freeradbiomed.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. Febs Letters. 2008;582(13):1783–7. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 20.Wang Yumin, Xiong Huayu, Xiuhua Zhang Wang S. Electrochemical study of bovine serum albumin damage induced by Fenton reaction using tris (2,2-bipyridyl) cobalt (III) perchlorate as the electroactive indicator. Electrochimica Act. 2012;67:147–51. doi: 10.1016/j.electacta.2012.02.010. [DOI] [Google Scholar]
- 21.Mishra K, Ojha H, Kallepalli S, Alok A, Kumar Chaudhury N. Protective effect of ferulic acid on ionizing radiation induced damage in bovine serum albumin. Internatuinal Journal of Radiation Research. 2014;12(2):113–21. [Google Scholar]
- 22.Schuessler H, Schilling K. Oxygen effect in the radiolysis of proteins Part 2 Bovine serum albumin. Int J Radiat Biol Relat Stud Phys Chem Med. 1984;45(3):267–81. doi: 10.1080/09553008414550381. [DOI] [PubMed] [Google Scholar]