Abstract
Over the past few decades, MRI-based visualization of demyelinated CNS lesions has become pivotal to the diagnosis and monitoring of multiple sclerosis (MS). In this Review, we outline current efforts to correlate imaging findings with the pathology of lesion development in MS, and the pitfalls that are being encountered in this research. Multimodal imaging at high and ultra-high magnetic field strengths is yielding biologically relevant insights into the pathophysiology of blood–brain barrier dynamics and both active and chronic inflammation, as well as mechanisms of lesion healing and remyelination. Here, we parallel the results in humans with advances in imaging of a primate model of MS — experimental autoimmune encephalomyelitis (EAE) in the common marmoset — in which demyelinated lesions resemble their human counterparts far more closely than do EAE lesions in the rodent. This approach holds promise for the identification of innovative biological markers and next-generation clinical trials that will focus more on tissue protection and repair.
Introduction
More than a third of a century has elapsed since the publication of the first in vivo MRI study of multiple sclerosis (MS) lesions, a period during which the identification of such lesions has become our single most important diagnostic test for MS. Remarkably, the in-depth study of MS lesions by means of contemporary imaging techniques continues to uncover fundamental aspects of the pathophysiology of the disease1. Multimodal imaging at high and ultra-high magnetic field strengths has yielded biologically relevant insights into lesion structure and blood–brain barrier (BBB) dynamics that are gradually narrowing the gap between the macroscopic view of the radiologist and the microscopic view of the pathologist.
In this Review, we explore how imaging findings can mirror the pathological development of the demyelinated MS lesion. We describe the patterns by which gadolinium leaks into the parenchyma, either centrifugally (outward from the central vein) or centripetally (inward from the lesion edge), highlighting the dynamism of the BBB in veins and capillaries as it responds to inflammatory stimuli. The introduction of ultra-high-field 7 T human MRI scanners, and of MRI techniques that are highly sensitive to paramagnetic substances, has enabled visualization of prominent central veins within most demyelinated MS lesions, and of paramagnetic rims at the lesion margin in some cases. We also review progress in the imaging of demyelination and remyelination.
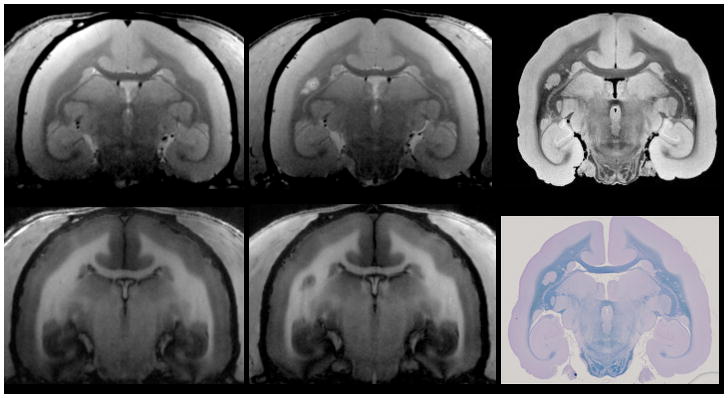
Throughout this article, we parallel the results in humans with recent advances in 7 T imaging of a primate model of MS, experimental autoimmune encephalomyelitis (EAE) in the common marmoset. The demyelinated brain and spinal cord lesions resemble the human counterpart far better in this model than in the rodent EAE model2. Animal models offer the possibility to image at baseline and frequently after disease induction, thereby enabling investigation of early, subtle radiological changes within normal brain tissue before lesion formation3. Another important advantage of animal models is that the pathology underlying the MRI findings can be accessed once the experiment is ended. Lesions in the marmoset brain are often small, so high-resolution (≤100 μm isotropic voxels) scans of the fixed brain, performed prior to processing the tissue for histology, can be very useful (Figure 1). Customized 3D-printed tissue holders and slicers facilitate matching of the histopathology with MRI, which greatly improves the interpretability of the data4. This technology, combined with serial MRI scanning before and after lesion development, can elucidate the cellular basis of development and repair of EAE lesions and, by extension, the histopathologically similar lesions in MS itself.
Figure 1. MRI and histology of the marmoset brain.
A common marmoset housed at the NIH Intramural Research Program facility was studied in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care and the National Institute of Neurological Disorders and Stroke’s Animal Care and Use Committee. a,b | Baseline 7 T MRI showing a healthy brain on proton density-weighted (a) and T1-weighted (b) images. After MRI, the animal was immunized with 200 μg of fresh-frozen human white matter homogenate, and started developing white matter lesions soon after. c,d | These lesions are visible on follow-up proton density-weighted (c) and T1 weighted (d) MRI scans performed before termination of the experiment. Note that a central vein within the lesion (red arrow) can be discerned on the proton density image (c). e,f | 7 T post-mortem 100 μm isotropic MRI after necropsy (e) was used to guide tissue processing for histology (f), allowing precise correlation between MRI and histopathology (red arrows). Complete demyelination, as seen on Luxol fast blue staining (f), matches the radiological appearance of the lesion both in vivo and post mortem (c, d, e).
Taken together, the advances described in this article hold considerable promise for deepening our understanding of the biology of MS while opening up the possibility of next-generation proof-of-concept clinical trials mainly focused on tissue protection and repair.
Initiation of MS lesion development
Immunopathology
Breakdown of immunological tolerance to CNS myelin or myelin-like antigens is generally accepted to be the basis of MS pathogenesis. Routes of physiological immune surveillance have been recognized to be involved early in the pathogenesis of both MS and EAE. Peripheral lymphocytes undergo trafficking, participate in immune surveillance and interact with CNS-resident antigen-presenting cells in the meninges (at the blood–leptomeningeal barrier), along ventricular ependymal surfaces (blood–choroid plexus barrier), within perivascular spaces (BBB), and in cervical lymph nodes.5 Once they escape peripheral tolerance, autoreactive lymphocytes can be further activated and become hostile effectors (CD8+, CD4+ T-helper 1 (TH1), CD4+ TH17, and B lymphocytes),6 thereby precipitating demyelination and axonal injury, which are the typical features of MS.
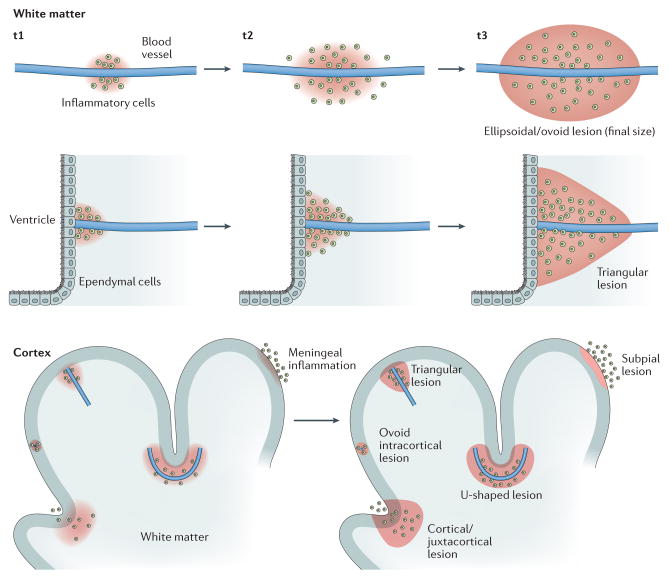
In early disease, the most dramatic immunopathological events leading to focal areas of demyelination — the ‘lesion’ of MS — tend to occur at the level of the BBB in small or medium-size parenchymal veins, although early subpial cortical demyelination has also been recognized.7 As they follow the venous vasculature, demyelinated MS lesions are commonly found around the ventricles (deep venous system, subependymal and medullary veins), at the grey–white matter junction (superficial venous system, especially cortical veins), within the optic nerve (branches of the central retinal vein), and in the brainstem (anteriorly and transversely oriented deep veins) and spinal cord (often posterior deep spinal veins). The spatial predilection and timing of a particular vein’s involvement remain fascinating research topics. In general, however, the process of rapid demyelination, which progressively spreads from and along a prominent parenchymal vein to involve the surrounding tissue, directly influences the macrostructure and shape (ovoid, ellipsoidal, triangular or U-shaped) of MS lesions (Figure 2)
Figure 2. Perivenular topography of demyelinated MS lesions in white and grey matter.
In the newly forming MS lesion, the distribution of myelin-scavenging inflammatory cells, spreading along and outward from an inflamed central vein, largely dictates the final lesion configuration. An ellipsoidal and/or rounded configuration is typical of MS lesions in the white matter. Of note, the shape of MS lesions can be also affected by the macrostructure of the surrounding tissue. For example, both the ependymal wall of the ventricles and the cortex may act as barriers to the spread of myelin-scavenging inflammatory cells. Depending of the trajectory of the vein and its position relative to these structures, lesions might assume a triangular shape, for periventricular and leukocortical–juxtacortical lesions, or a U shape, for leukocortical–juxtacortical lesions. The lower panel shows the various types of cortical demyelination. In addition to vasculocentric demyelination, which occurs in purely intracortical and leukocortical lesions, plaque-like demyelination can affect the subpial layers of the cortex and is thought to be directly triggered by leptomeningeal inflammation. MS, multiple sclerosis; t, time point.
Human imaging
As demyelinated lesions in the brain and spinal cord are easy to detect on long-echo-time MRI pulse sequences, such as T2-weighted and T2-fluid-attenuated inversion recovery (FLAIR), these techniques have been implemented in everyday clinical practice and are integral to current MS diagnostic criteria8,9. Though recognized in pathological studies as early as the 19th century10, the perivenular topography of MS lesion development has only recently been directly visualized in vivo, using high-resolution susceptibility-sensitive imaging at a variety of magnetic field strengths (Supplementary information S1 (movie))11–18. The reasons for the characteristic prominence of the central vein within MS demyelinated lesions in comparison to surrounding brain parenchyma are still open to debate, and include an elevated concentration of deoxyhaemoglobin (higher oxygen extraction at the site of inflammation) and changes in vessel diameter (slower venous flow, postinflammatory scarring processes of the vein wall)19–21.
Far from being a mere morphological feature of MS lesions, the ‘central vein sign’ has potential relevance for the diagnostic work-up9,22, as it is consistently described as atypical in small vessel disease15,16,23,24 and migraine25, and is rarely observed in other immunological conditions characterized by brain lesions (for example, vasculitis and neuromyelitis optica spectrum disorder)26. Large population studies will be required to ascertain the true positive predictive value of the central vein sign.
Marmoset imaging
Various EAE immunization protocols have been described in marmosets27–30, but in this Review we will focus primarily on disease induced by subcutaneous injection of human white matter homogenate with an adjuvant3,27, which in our hands produces a disease that bears strong similarities to relapsing–remitting MS. As in MS, MRI can detect demyelinated lesions that form in the CNS of marmosets with EAE31–33. EAE lesions in the marmoset brain present with similar features to MS lesions, including distribution throughout the CNS with a preferred location in the subcortical white matter3,34,35, uptake of gadolinium contrast agent3,36, and perivenular topography37 (Figure 1). Interestingly, the veins around which most of the lesions form are already visible on scans prior to disease induction, supporting the notion that the inflammation associated with EAE affects previously normal veins. These superficial medullary veins drain the white matter lesions and pass through the cortex into the subarachnoid space. The close relationship between these veins and cerebrospinal fluid within their perivascular spaces is likely to have an important role in lesion development.
Before abrupt opening of the BBB
Immunopathology
A prevalent adaptive immune response to myelin-like antigens triggers an aggressive, scavenger-type response of the innate immune system against CNS myelin. This response is characterized by a classic triad: first, dramatic opening of the BBB; second, blood-derived inflammatory cell (monocyte–macrophage) invasion; and third, macrophage-mediated stripping of myelin sheaths from axons38,39 The temporal sequence of the earliest pathological events has not been completely unravelled, and remains the subject of active investigation in animal studies. Perivascular inflammatory cuffing is the most prominent pathological feature associated with the newly forming lesion, and is characterized by aggregates of lymphocytes and monocytes confined within the perivascular space of the postcapillary venule and not yet breaching the glia limitans of the BBB (parenchymal basement membrane and astrocytic end-feet). Other notable events include variable immunoglobulin and complement deposition (the distinguishing feature of immunopattern II compared with immunopattern I)40; parenchymal microglial and astrocytic activation; perivascular swollen or apoptotic oligodendrocytes with still-intact myelin (the so-called prephagocytic or preactive lesion)41,42; and early axonal and synaptic dysfunction38,39,43,44.
Human imaging
The early pathological findings in both MS and EAE are consistent with the in vivo detection of subtle radiological changes in the normal-appearing white matter days to weeks before the parenchymal gadolinium enhancement that defines the radiological onset of the impending MS lesion45–52. These data, derived mostly from quantitative or semiquantitative MRI studies that are pathologically nonspecific and only statistically significant at the group level, support the concept of a short-term temporal dissociation between the earliest immunological events in lesion development and the dramatic opening of the BBB (captured on MRI as parenchymal leakage of gadolinium) and subsequent demyelination. Recently, taking advantage of high-resolution imaging and frequent scanning, we have directly visualized focal alterations of MRI signal, on T2-FLAIR and T2*-weighted images, around the central vein of the future MS lesion18. Specifically, in some cases, before the overt onset of the radiologically defined demyelinated lesion, the ‘inflamed’ central vein enhances in isolation, perhaps owing to higher BBB permeability with entrapment of the contrast agent within the perivascular space18.
Marmoset imaging
In a study published in 20143, we monitored the status of BBB permeability by mapping the longitudinal relaxation time (‘T1’) before and after injection of a triple dose of gadolinium-based MRI contrast agent. We performed weekly MRI scans, including T1 mapping, as well as proton density-weighted and T2-weighted imaging, after induction of EAE in marmosets. We showed that the BBB becomes focally and increasingly permeable starting approximately 4 weeks prior to lesion appearance. We also demonstrated that areas of abnormal BBB permeability observed at the time of sacrifice corresponded to inflammatory nodules without associated demyelination. Those nodules consisted of small clusters of perivascular and parenchymal, IBA1-positive, activated microglia and macrophages in association with variable numbers of perivascular lymphocytes. Our results suggest that early changes in vascular permeability, associated with perivascular inflammatory cuffing and parenchymal microglial activation, might precede the formation of new lesions.
BBB dynamics in early MS lesions
Immunopathology
The BBB is recognized to be a highly organized structure, composed of endothelial cells, pericytes and astrocytic end-feet that partially isolate the peripheral blood circulation from the CNS parenchyma53. The endothelial and parenchymal basement membranes, consisting of collagen IV, fibronectin and laminin, enclose the ‘perivascular’ space, in which — as stated above — normal and pathological immune surveillance takes place. In early MS lesions, autoreactive and activated lymphocytes directly contribute to impairment of the BBB of the postcapillary venules, characterized initially by moderately increased permeability and eventually by dramatic structural disruption38,39,44,54. Activated CD4+ lymphocytes can secrete the cytokines IFN-γ and tumour necrosis factor (TNF) which upregulate endothelial adhesion molecules (VCAM1 and α1 integrin) and chemokines, thereby inducing massive recruitment of monocytes and allowing transendothelial migration of these cells into the perivascular space and, subsequently, the parenchyma. Structural disruption of the BBB is mediated by the secretion of matrix metalloproteinases that can damage endothelial tight junctions and basement membranes, causing leakage of serum proteins and water (vasogenic oedema) into the white matter55,56. Impaired breakdown of leaking fibrinogen, and its breakdown product fibrin, might also contribute to the inflammatory demyelinating cascade57,58. This highly inflammatory environment eventually leads to disorganization of the BBB’s outer shell, that is, the astrocytic end-feet that comprise the glia limitans.
Human imaging
The striking radiological appearance of newly forming MS lesions is commonly accepted to be related to leakage into the parenchyma (and, therefore, visualization on T1-weighted images) of intravenously injected contrast agents, which are usually gadolinium-based59,60. This leakage reflects the short-term structural opening of the BBB — specifically, damage to tight junctions and basement membranes — that occurs at lesion onset.
Dynamic contrast-enhanced MRI (DCE-MRI) at high magnetic field strength is a technique in which T1-weighted images are quickly acquired before, during, and at various time points after gadolinium injection. Through use of this technique, the spatiotemporal dynamics of BBB opening in active MS lesions can be dissected in vivo, thereby providing complementary insights to the immunopathological studies. In this dynamic view, in essentially all early active demyelinating lesions, gadolinium leaks initially from the central vein and flows outward, filling the lesion within a few minutes. On static images acquired 5–10 min after contrast injection — the typical situation in clinical practice — such lesions would be characterized as having ‘nodular’ enhancement. From a dynamic point of view, this process has been termed centrifugal (outward) contrast enhancement61,62.
After a few days to a few weeks, many of the larger lesions exhibit a shift in the pattern of gadolinium enhancement. In such cases, the expanding inflammatory edge enhances first, and gadolinium then flows centripetally (inward) toward the demyelinated lesion centre17,61,62 (Supplementary information S2 and S3 (movies)). When this shift occurs, the appearance of the lesion on static images might be either a nodule or a ring, depending on several factors. Especially important among these factors is the time elapsed between contrast injection and MRI acquisition, but the location of the lesion is also important in determining whether the ring is complete or open: an open ring is typically seen when the lesion abuts grey matter or the ventricles. Centrifugal enhancement clearly supports the centrality of perivenular pathogenic events at lesion onset. The significance of centripetal enhancement is less certain, and we hypothesize that this feature might be part of the physiological — though detrimental if unrestrained — wound-healing inflammatory reaction of the CNS parenchyma to focal demyelination.
Marmoset imaging
The dramatic opening of the BBB can also be visualized in active EAE lesions, using T1-weighted images obtained after intravenous injection of gadolinium-based contrast agents. Like MS lesions, active EAE lesions usually display nodular enhancement on static postcontrast T1-weighted images. To our knowledge, open-ring enhancement has not been reported in marmoset EAE. The spatiotemporal dynamics of the gadolinium leakage in active EAE lesions have not yet been fully characterized, mainly due to the limitations imposed by the smaller dimensions of EAE lesions and the higher cardiac output of marmosets compared with humans. Nonetheless, a recent quantitative MRI study has provided some insights into the dynamics of BBB leakage over the span of a few weeks3. Following the early gradual increase in permeability that occurs prior to lesion development, the BBB reaches its maximum permeability once the lesion becomes visible on all types of images (T1-weighted, T2-weighted, T2*-weighted and proton density-weighted, as well as T2-FLAIR). After the lesion appears, the permeability of the BBB gradually decreases back to near-normal levels, signifying a restored BBB, in approximately 2–4 weeks3.
Macrophage-mediated demyelination
Immunopathology
Stripping of myelin from axons and phagocytosis of myelin debris are mediated by blood-derived macrophages and activated CNS-resident microglia, which together constitute the prevalent cellular population within the active demyelinating lesion38,63–65. These cell types are difficult to distinguish even at the tissue level (although progress has recently been made on this front66), and here we will use the term ‘macrophage’ for simplicity.
The proinflammatory status of macrophages probably contributes to further tissue damage via free radical formation and secretion of proteases and cytokines, including IFN-γ and TNF. Macrophages track the spatiotemporal pattern of BBB opening and demyelination described above. Thus, they initially distribute throughout the lesion, starting from the centre, and they subsequently become more concentrated at the lesion margin (so-called ‘late active’ lesions). Interestingly, the efficiency by which macrophages clear myelin debris seems to decrease with age67. In addition, to limit amplification of oxidative stress and mitochondrial energy failure, an excess of free iron, probably released from oligodendrocytes during the process of demyelination and/or mediated by endothelial transferrin receptor upregulation, is buffered mainly by macrophages and astrocytes68–70 Persistently demyelinated axons are more prone to degenerate in an inflammatory environment71,72, which might explain the varying degrees of axonal injury and loss that coexist with demyelination in MS lesions.
Human imaging
A persistent issue in imaging of MS lesions in vivo is related to the fact that inflammation, demyelination and axonal loss within lesions are not cleanly distinguishable on T2-weighted and T1-weighted images. This lack of specificity has prompted a number of attempts to develop MRI approaches that are more pathologically interpretable, such as quantification of the short T2 component in multi-echo T2 relaxation experiments, which seems to correlate with water selectively enclosed between myelin bilayers (so-called ‘myelin water imaging’)73–76.
Complementary work on the spatiotemporal dynamics of the BBB has shown that the front of demyelination and inflammation can be captured by coupling DCE-MRI with susceptibility-based MRI at 7 T17. These experiments have shown that in centripetally enhancing lesions, the initial opening of the BBB at the lesion edge clearly colocalizes with a paramagnetic rim on phase images — a novel endogenous marker of inflammation17 (Supplementary information S2 (movie) and Figure 3). Other investigations showed that on 3 T phase images, visualization was better overall in young lesions in early disease than in older lesions51,77–79. Properties of the younger lesions that might explain this observation include a greater propensity to demyelination than to axonal loss, and high inflammatory activity with attendant paramagnetic substances, such as free radicals and iron.
Figure 3. Serial MRI evaluations in progressive MS.
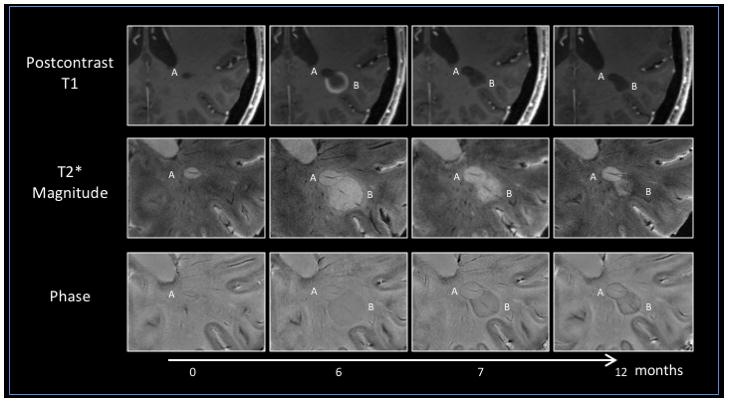
An untreated 49-year-old woman with progressive MS and radiological relapses underwent serial MRI evaluations at 7 T under an institutional review board-approved natural history protocol at the NIH. Lesion A is a pre-existing, non-enhancing lesion with a paramagnetic rim visible on both T2*-weighted magnitude and phase images, unknown onset, lesion A). Lesion B, a new centripetally enhancing MS lesion (open ring on postcontrast T1-weighted images; Supplementary information S2 (movie)), appears adjacent to lesion A at month 6. Both lesions exhibit a central vein on T2*-weighted magnitude and phase images. In lesion B, the peripheral T2*-weighted magnitude and phase rim persists after resolution of contrast enhancement (1 and 6 months after lesion onset). Of note, lesion B shrinks over time after enhancement resolves, whereas lesion A nearly triples in size, even in the absence of visible contrast enhancement on postcontrast T1-weighted images. In both lesions by month 12, the persistent T2*-weighted magnitude and phase rim has become darker and thicker, suggesting accrual of paramagnetic substances at the lesion edge. Pathologically, the rim possibly results from macrophages loaded with iron, and lesions with this radiological feature might, therefore, correspond to the so-called ‘chronic active’ or ‘smouldering’ lesions. MS, multiple sclerosis.
Marmoset imaging
By combining serial conventional imaging and histopathology, we have demonstrated that <1-week-old EAE lesions surround a central inflamed blood vessel that is cuffed with T cells and macrophages3. Perivascular B cells and blood-derived MRP14+ early activated macrophages are also observed, albeit less frequentlyand macrophage–microglia-associated demyelination and disrupted axons are seen throughout the lesion. Lesions 1–5 weeks old continue to enlarge, and active demyelination is observed at the lesion edges. Interestingly, early activated macrophages are only found at the leading edge of demyelination3.
Conventional MRI lacks specificity toward tissue pathology in EAE lesions, as in MS lesions, and inflammation, demyelination and axonal loss cannot be differentiated in vivo31. In one study, quantitative MRI findings correlated with macrophage infiltration, but demyelination was a potential confounder33. Recently, an advanced MRI technique, based on complex analysis of the T2* decay curve, was used for in vivo detection of the myelin water content in white matter of healthy marmosets75. However, the application of this technique to track demyelination and remyelination remains challenging, as T2* decay curves can be strongly affected by oedema in the extracellular space, iron accumulation in macrophages, and the orientation of white matter tracts relative to the magnetic field of the MRI system. To date, a paramagnetic rim on phase images has not been observed in marmoset EAE lesions, and whether this is due to lack of sensitivity of the applied MRI techniques or to biological differences remains uncertain.
Lesion evolution after BBB restoration
Immunopathology
The process of BBB closure and inflammation resolution is recognized as a potential source of new therapeutic targets in MS, but the underlying mechanisms have not been completely unravelled. An emerging idea is that inflammation in MS, while clearly driving demyelination and tissue destruction, can also trigger healing, albeit dysregulated and, hence, either incomplete or ineffective. In this context, an analogy has been drawn between extra-CNS wound healing and the process of repair within MS lesions80. The initiation of healing seems to reside in the extreme plasticity of macrophages, the functional phenotypes of which run the gamut from destructive (‘classically activated’) to restorative (‘alternatively activated’) in the face of environmental stimuli that may vary over time. In the realm of remyelination81–85, recent data indicate that in early lesions, classically activated macrophages (sometimes called ‘proinflammatory’ macrophages) can induce the recruitment of oligodendrocyte precursors, whereas alternative (‘anti-inflammatory’) activation is required for differentiation of these precursor cells into myelinating oligodendrocytes67. Remyelination remains the subject of extensive investigations that are outside the scope of this Review84–89.
Human imaging
After the resolution of gadolinium enhancement, usually 2–8 weeks following its emergence, MS lesions are radiologically defined as ‘chronic’. The lesions remain clearly visible on both T2-weighted and T1-weighted images, and the extent of T1 hypointensity seems to correlate with axonal degeneration in the white matter90,91. Unfortunately, as discussed above, clinical MRI sequences are unable to directly image remyelination. After oedema resolves, typically 1–3 months after lesion onset, tracking of longitudinal signal intensity on T1-weighted, T2-weighted, proton density-weighted, and magnetization transfer-weighted sequences, as well as through lesion volume measurement, remains the established strategy to assess short-term lesion repair92,93.
Marmoset imaging
The subacute EAE lesions described above show a decrease in BBB permeability in their core area over approximately 4 weeks3. After 5 weeks, the lesions display markedly reduced BBB permeability and inflammation, and the macrophages disappear. These lesions are still visible on MRI but become smaller over time — a finding that is consistent not only with a reduction in inflammation but also with potential repair, including remyelination. As most marmoset experiments end within weeks to months of lesion development, the natural history and pathology of chronic lesions has not been extensively studied, and is an enticing topic for future research.
Chronic MS lesions
Immunopathology
On histological sections, chronic lesions appear hypocellular and demyelinated, with a varying degree of axonal loss. However, an inflammatory infiltrate, wherein macrophages and activated microglia contain early or late myelin degradation products, characterizes the margins of a variable percentage (20–40%) of demyelinated lesions (variously termed ‘chronic active’, ‘slowly expanding’, or ‘smouldering’)63,64,68,94,95. On the basis of autopsy studies, this lesion category is most common in people at the transition to clinically progressive disease and those with long disease duration, supporting the notion that these lesions contribute to long-term and clinically important tissue damage68,94,95.
Human imaging
Chronic inflammation is trapped behind an intact BBB and, hence, remains invisible to current gadolinium-based MRI. Through the use of high-resolution, noncontrast, susceptibility-based imaging, a paramagnetic rim has recently been detected at the margin of some nonenhancing MS lesions. Initial MRI–pathology investigations suggested that this radiological finding might aid identification of chronic active/smouldering lesions in vivo. Steady accumulation of macrophages at the lesion edge, in association with paramagnetic inflammatory species related to free radical production and/or intracellular iron accumulation, might explain the paramagnetic signal observed in vivo12,17,78,96–100 (Figure 3).
In the context of early lesion evolution, the subset of centripetally enhancing lesions with a phase rim that persists after the closure of the BBB tend to exhibit poor lesion recovery in terms of lesion volume and T1 hypointensity at year 1, suggesting impairment of early repair and/or permanent tissue injury (unpublished data, Figure 3). If confirmed, this feature will constitute a valuable imaging biomarker, not only to better stage MS lesions in vivo, but also to uncover specific components of MS-related hidden inflammation. Similarly, peripheral macrophages experimentally labelled with ultrasmall superparamagnetic iron oxide particles (USPIO) have been tracked at the edges of demyelinated lesions with an apparently intact BBB (that is, lesions with no observable leakage of traditional gadolinium-based contrast agents)101–103. A direct comparison between USPIO-based and susceptibility-based imaging is currently lacking.
Marmoset imaging
Bert ‘t Hart and colleagues applied conventional imaging (T2-weighted and T1-weighted with gadolinium injection) to a group of marmosets reaching the chronic stage of EAE disease31. Among the chronic lesions, late active demyelinating lesions (with macrophages containing myelin proteolipid protein degradation products located at the edge) were observed together with inactive demyelinated and remyelinated lesions, as confirmed using histopathology31. However, the MRI scans performed in this study could not differentiate these chronic active lesions from their inactive counterparts. USPIO-based MRI, which has been successful in detecting infiltrated macrophages in rodent EAE104–106, could prove useful for identifying chronic active lesions in marmoset EAE. Another approach would be to perform serial susceptibility-based imaging to detect phase rims in those chronic lesions. These strategies represent enticing targets for future research.
Caveat
Four different initial immunological patterns are associated with acute myelin damage and BBB opening, supporting the idea that MS is a collection of different pathophysiological entities40,107,108. What has been described in this Review — the staging of white matter MS lesions and its MRI correlates — probably refers to immunological patterns I and II (myelin-autoimmune hypothesis), which together are the most common types of acute MS lesions40. No imaging correlate has yet been shown to definitively identify lesions with immunological pattern III, in which the prime mover is thought to be toxic and/or hypoxic injury to mature oligodendrocytes40.
Cortical and leptomeningeal involvement
Immunopathology
Cortical and leptomeningeal involvement has recently been recognized as a key feature in MS pathogenesis (early disease with clinical relapses and remission)7,109 and related neurodegeneration (thought to be largely responsible for clinical progression)110. Like the white matter, the cortex can display demyelination, but with much less pronounced inflammation and, importantly, without abrupt opening of the BBB7,111–113. Remyelination may be enhanced within the cortex in comparison with the white matter84,114.
Topographically, cortical lesions develop along the pial surface (‘subpial lesions’) or around cortical venules that drain into the subarachnoid space (small intracortical and leukocortical demyelinated lesions) 111,115. This topography supports the hypothesis that leptomeningeal inflammation, involving effector lymphocytes, cytotoxic diffusible mediators and immunoglobulins, is strictly related to cortical lesion development via direct damage of the subpial layers of the cortex and/or inflammation spreading into the parenchyma7. Subpial cortical demyelination has been characterized as specific to MS-related pathological processes116. Of note, aggregates of lymphocytes resembling lymphoid follicles have been recognized in the leptomeninges in patients with progressive MS, and have been judged to reflect an extreme form of compartmentalized, long-lasting inflammation7,117–122. The interplay between leptomeningeal inflammation, cortical demyelination and neurodegeneration, as well as more generally between tissue damage in white and grey matter, is still under investigation110,123,124.
Human imaging
Cortical lesions have been reported in vivo at all stages of MS (though more frequently in progressive MS), but subsequent autopsy findings indicate that their numbers are profoundly underestimated on MRI125–127. Of note, cortical lesions do not enhance after gadolinium administration, which at present precludes their staging in the living brain. Double inversion recovery125, phase-sensitive inversion recovery128, and high-resolution T2*-weighted129–131 and magnetization-transfer-weighted sequences132,133 can detect some of these lesions, especially those with transcortical involvement and/or demyelination of the underlying white matter (‘leukocortical lesions’). Subpial demyelination affecting only the superficial cortical layers, and small intracortical lesions have been visualized more reliably on high-resolution post-mortem images (Figure 4), but are only sporadically reported in vivo129–131. Cortical demyelination shows positive correlations with disability and cognitive impairment scores, thereby underlining the clinical importance of improved detection of this feature130,134–138. Together with periventricular, infratentorial, spinal cord, and optic nerve lesions, cortical/juxtacortical lesions contribute to the recently proposed revised ‘dissemination in space’ MRI criteria for the diagnosis of MS9.
Figure 4. Cortical and leptomeningeal lesions.
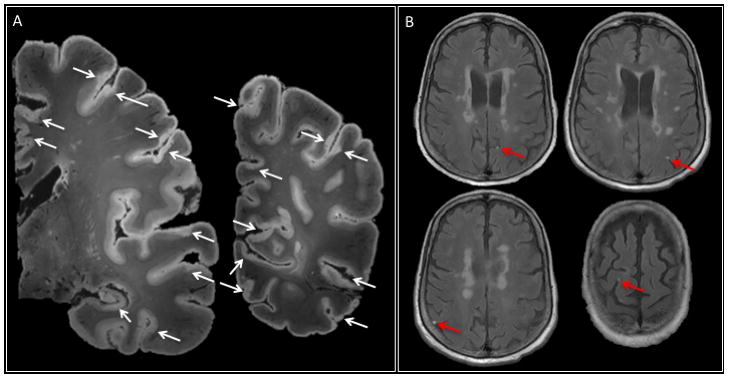
a | 7 T post-mortem MRI (two representative coronal slices of the left hemisphere, T2*-weighted gradient-echo sequence, 64 nl isotropic voxels) in a 66-year-old woman with progressive multiple sclerosis and disease duration (time from symptom onset to death) of 20 years. The arrows indicate widespread involvement of the cortex in the demyelination process, including subpial cortical and leukocortical lesions. Cortical lesions often face each other across sulci, consistent with the idea that leptomeningeal inflammation has a direct pathogenic role in cortical lesion development. b | Several foci of leptomeningeal contrast enhancement (arrows) were apparent in this patient on postcontrast 3 T MRI in vivo.
With respect to leptomeningeal inflammation, a recent study, in which in vivo and post-mortem MRI findings were correlated with pathology, reported the prevalence of subarachnoid-space abnormalities in a cohort of ~300 people with MS on both 3 T and 7 T postcontrast T2-FLAIR images139. Spatially confined disruption of the blood–leptomeningeal barrier, manifesting as foci of gadolinium enhancement, was identified in ~35% of individuals with progressive MS and ~25% of all patients studied (Figure 4). The presence of leptomeningeal enhancement correlated with whole-brain and cortical atrophy139. In autopsy cases, these foci colocalized with perivascular inflammatory infiltrates — specifically, lymphocytes and macrophages clustered around leptomeningeal vessels and along intracortical perivascular spaces — and adjacent subpial cortical demyelination139.
Marmoset imaging
Demyelinated cortical lesions similar to those in MS (leukocortical, intracortical and subpial) are known to affect the marmoset EAE brain, as shown in several histopathological studies140–143. Clusters of T lymphocytes and plasma cells have recently been observed in the meninges overlying subpial cortical demyelination144, suggesting that local meningeal lymphocytic and plasma cell infiltration also contributes to the pathogenesis of subpial demyelination in marmoset EAE. However, in vivo MRI visualization of cortical demyelination and meningeal inflammation has not yet been reported in this animal model.
Conclusions
In this Review, we have summarized recent research efforts to narrow the gap between pathology and imaging in MS lesion development and staging. We have also presented parallel efforts in marmoset EAE, an animal model bearing strong immunological, pathological and radiological similarities to MS. In marmoset EAE, the pathological basis of developing and repairing lesions can be investigated using a combination of serial in vivo MRI imaging and histology, and the results of these investigations have strong relevance for our understanding of similar processes in MS. Some observations from human imaging, such as open-ring enhancement, phase rims, meningeal enhancement and cortical lesions, have not been consistently reported in marmoset EAE, and certain observations from marmoset imaging, such as consistently increased permeability of the BBB in the weeks preceding focal demyelination, have not been described in humans. These differences may be attributable in large part to technical factors, but biology could also contribute substantially.
The parallels reviewed here suggest that the combination of human MS and marmoset EAE research is a promising pathway to achieve a deeper understanding of the pathobiology of BBB dynamics, chronic inflammation, and mechanisms of repair and remyelination, together with the development of new MRI techniques to detect and monitor their evolution in vivo. The ability to image both marmosets and humans at the same field strength (7 T) enables ready investigation and translation of new MRI markers from bench to bedside, and vice versa. Finally, this unique approach holds promise for the identification of novel biological targets and next-generation clinical trials that will focus on tissue protection and repair — a major unmet need in MS.
Supplementary Material
Coronal 3 T FLAIR* images (combination of coregistered T2*-weighted magnitude and T2-weighted fluid-attenuation inversion recovery images) from a 33-year-old woman with relapsing–remitting multiple sclerosis. A prominent central vein can be seen in the majority of lesions.
Dynamic contrast enhancement MRI shows a centripetally enhancing lesion (red arrow) in an untreated 49-year-old woman with progressive multiple sclerosis and radiological relapses.
Dynamic contrast enhancement MRI of a new periventricular centripetally enhancing lesion in a 33-year-old man with relapsing–remitting multiple sclerosis. Over a 20 min period, contrast material progressively and completely fills the lesion.
Key points.
Recent research efforts have narrowed the gap between in vivo MRI and pathology with respect to multiple sclerosis (MS) lesion development and staging
Demyelinated lesions in marmoset experimental autoimmune encephalomyelitis (EAE) resemble their human counterparts far better than do lesions in rodent EAE models
The perivenular topography of MS lesions might facilitate the radiological work-up
Smouldering lesions and meningeal inflammation are important features of chronic inflammation that should be targeted for in vivo imaging
Acknowledgments
The Intramural Research Program of the National Institute of Neurological Disorders and Stroke supported this study. D.S.R. has received research support from Vertex Pharmaceuticals and the Myelin Repair Foundation. This funding is not a competing interest in the context of the current article.
Biographies
Martina Absinta earned her medical degree, residency in neurology, and PhD in molecular medicine–experimental neurology (expected April 2016) at Vita-Salute University-San Raffaele Hospital, Milan, Italy, under the supervision of Prof. Massimo Filippi and Prof. Giancarlo Comi. She is currently a postdoctoral fellow in the Translational Neuroradiology Unit (National Institute of Neurological Disorders and Stroke, NIH, Bethesda, Maryland, USA), under the mentorship of Dr Daniel S. Reich. Her research interests include ultra-high-field MRI–pathology correlations in multiple sclerosis, and related identification of imaging biomarkers of disease course.
Pascal Sati earned his PhD in physics in France. After postdoctoral experience at Washington University in St. Louis, Missouri, USA under the supervision of Prof. Anne H. Cross and Prof. Dmitriy A. Yablonskiy, he joined the Translational Neuroradiology Unit (National Institute of Neurological Disorders and Stroke, NIH, Bethesda, Maryland, USA), where he specialized in ultra-high-field MRI in multiple sclerosis (MS) under the supervision of Dr Daniel S. Reich and Dr Jeff H. Duyn. He is currently a staff scientist at the Translational Neuroradiology Unit, developing novel MRI techniques for better understanding, diagnosis and evaluation of MS. He is also interested in preclinical imaging of animals with experimental autoimmune encephalomyelitis, which enables him to perform bench-to-bedside research.
Daniel S. Reich — a neurologist and neuroradiologist — directs the Translational Neuroradiology Unit in the National Institute of Neurological Disorders and Stroke, part of the NIH (Bethesda, Maryland, USA). In his clinical practice, he cares for patients with multiple sclerosis (MS) and other neurological diseases, and he also leads several clinical studies focusing on MS. Research in his laboratory focuses on the use of advanced MRI techniques to understand the sources of disability in MS, and on ways of adapting these approaches for research trials and patient care. He is particularly interested in harnessing noninvasive imaging modalities to dissect biological mechanisms of tissue damage.
Footnotes
Competing interests statement
The authors declare no competing interests.
Author contributions
All authors researched data for the article, made substantial contributions to discussions of the content, wrote the article, and reviewed and/or edited the manuscript before submission.
References
- 1.Filippi M, et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2012;11:349–360. doi: 10.1016/S1474-4422(12)70003-0. [DOI] [PubMed] [Google Scholar]
- 2.‘t Hart BA, van Kooyk Y, Geurts JJ, Gran B. The primate autoimmune encephalomyelitis model; a bridge between mouse and man. Ann Clin Transl Neurol. 2015;2:581–593. doi: 10.1002/acn3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maggi P, et al. The formation of inflammatory demyelinated lesions in cerebral white matter. Ann Neurol. 2014;76:594–608. doi: 10.1002/ana.24242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guy JR, et al. Custom fit 3D-printed brain holders for comparison of histology with MRI in marmosets. J Neurosci Methods. 2016;257:55–63. doi: 10.1016/j.jneumeth.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 6.Bartholomäus I, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 7.Lucchinetti CF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filippi M, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016;15:292–303. doi: 10.1016/S1474-4422(15)00393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rae-Grant AD, Wong C, Bernatowicz R, Fox RJ. Observations on the brain vasculature in multiple sclerosis: a historical perspective. Mult Scler Relat Disord. 2014;3:156–162. doi: 10.1016/j.msard.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Tan IL, et al. MR venography of multiple sclerosis. AJNR Am J Neuroradiol. 2000;21:1039–1042. [PMC free article] [PubMed] [Google Scholar]
- 12.Hammond KE, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol. 2008;64:707–713. doi: 10.1002/ana.21582. [DOI] [PubMed] [Google Scholar]
- 13.Tallantyre EC, et al. Demonstrating the perivascular distribution of MS lesions in vivo with 7-Tesla MRI. Neurology. 2008;70:2076–2078. doi: 10.1212/01.wnl.0000313377.49555.2e. [DOI] [PubMed] [Google Scholar]
- 14.Sati P, George IC, Shea CD, Gaitan MI, Reich DS. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology. 2012;265:926–932. doi: 10.1148/radiol.12120208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tallantyre EC, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology. 2011;76:534–539. doi: 10.1212/WNL.0b013e31820b7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kau T, et al. The “central vein sign”: is there a place for susceptibility weighted imaging in possible multiple sclerosis? Eur Radiol. 2013;23:1956–1962. doi: 10.1007/s00330-013-2791-4. [DOI] [PubMed] [Google Scholar]
- 17.Absinta M, et al. Seven-tesla phase imaging of acute multiple sclerosis lesions: a new window into the inflammatory process. Ann Neurol. 2013;74:669–678. doi: 10.1002/ana.23959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Absinta M, et al. Direct MRI detection of impending plaque development in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2015;2:e145. doi: 10.1212/NXI.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaitan MI, de Alwis MP, Sati P, Nair G, Reich DS. Multiple sclerosis shrinks intralesional, and enlarges extralesional, brain parenchymal veins. Neurology. 2013;80:145–151. doi: 10.1212/WNL.0b013e31827b916f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller K, et al. Detailing intra-lesional venous lumen shrinking in multiple sclerosis investigated by sFLAIR MRI at 7-T. J Neurol. 2014;261:2032–2036. doi: 10.1007/s00415-014-7460-2. [DOI] [PubMed] [Google Scholar]
- 21.Dal-Bianco A, et al. Veins in plaques of multiple sclerosis patients — a longitudinal magnetic resonance imaging study at 7 Tesla. Eur Radiol. 2015;25:2913–2920. doi: 10.1007/s00330-015-3719-y. [DOI] [PubMed] [Google Scholar]
- 22.George IC, et al. Clinical 3-tesla FLAIR* MRI improves diagnostic accuracy in multiple sclerosis. Mult Scler. 2016 doi: 10.1177/1352458515624975. http://dx.doi.org/10.1177/1352458515624975. [DOI] [PMC free article] [PubMed]
- 23.Lummel N, et al. Presence of a central vein within white matter lesions on susceptibility weighted imaging: a specific finding for multiple sclerosis? Neuroradiology. 2011;53:311–317. doi: 10.1007/s00234-010-0736-z. [DOI] [PubMed] [Google Scholar]
- 24.Kilsdonk ID, et al. Improved differentiation between MS and vascular brain lesions using FLAIR* at 7 Tesla. Eur Radiol. 2014;24:841–849. doi: 10.1007/s00330-013-3080-y. [DOI] [PubMed] [Google Scholar]
- 25.Solomon AJ, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol. 2015;3:82–87. doi: 10.1002/acn3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wuerfel J, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler. 2012;18:1592–1599. doi: 10.1177/1352458512441270. [DOI] [PubMed] [Google Scholar]
- 27.Massacesi L, et al. Active and passively induced experimental autoimmune encephalomyelitis in common marmosets: a new model for multiple sclerosis. Ann Neurol. 1995;37:519–530. doi: 10.1002/ana.410370415. [DOI] [PubMed] [Google Scholar]
- 28.Genain CP, Hauser SL. Experimental allergic encephalomyelitis in the New World monkey Callithrix jacchus. Immunol Rev. 2001;183:159–172. doi: 10.1034/j.1600-065x.2001.1830113.x. [DOI] [PubMed] [Google Scholar]
- 29.Jagessar SA, et al. Autoimmunity against myelin oligodendrocyte glycoprotein is dispensable for the initiation although essential for the progression of chronic encephalomyelitis in common marmosets. J Neuropathol Exp Neurol. 2008;67:326–340. doi: 10.1097/NEN.0b013e31816a6851. [DOI] [PubMed] [Google Scholar]
- 30.Jagessar SA, Dijkman K, Dunham J, ‘t Hart BA, Kap YS. Experimental autoimmune encephalomyelitis in marmosets. Methods Mol Biol. 2016;1304:171–186. doi: 10.1007/7651_2014_113. [DOI] [PubMed] [Google Scholar]
- 31.Hart BA, et al. Histopathological characterization of magnetic resonance imaging-detectable brain white matter lesions in a primate model of multiple sclerosis: a correlative study in the experimental autoimmune encephalomyelitis model in common marmosets (Callithrix jacchus) Am J Pathol. 1998;153:649–663. doi: 10.1016/s0002-9440(10)65606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.‘t Hart BA, et al. Modelling of multiple sclerosis: lessons learned in a non-human primate. Lancet Neurol. 2004;3:588–597. doi: 10.1016/S1474-4422(04)00879-8. [DOI] [PubMed] [Google Scholar]
- 33.Blezer EL, Bauer J, Brok HP, Nicolay K, t Hart BA. Quantitative MRI-pathology correlations of brain white matter lesions developing in a non-human primate model of multiple sclerosis. NMR Biomed. 2007;20:90–103. doi: 10.1002/nbm.1085. [DOI] [PubMed] [Google Scholar]
- 34.Jordan EK, et al. Serial MR imaging of experimental autoimmune encephalomyelitis induced by human white matter or by chimeric myelin-basic and proteolipid protein in the common marmoset. AJNR Am J Neuroradiol. 1999;20:965–976. [PMC free article] [PubMed] [Google Scholar]
- 35.Diem R, et al. Autoimmune optic neuritis in the common marmoset monkey: comparison of visual evoked potentials with MRI and histopathology. Invest Ophthalmol Vis Sci. 2008;49:3707–3714. doi: 10.1167/iovs.08-1896. [DOI] [PubMed] [Google Scholar]
- 36.Boretius S, et al. Monitoring of EAE onset and progression in the common marmoset monkey by sequential high-resolution 3D MRI. NMR Biomed. 2006;19:41–49. doi: 10.1002/nbm.999. [DOI] [PubMed] [Google Scholar]
- 37.Gaitan MI, et al. Perivenular brain lesions in a primate multiple sclerosis model at 7-tesla magnetic resonance imaging. Mult Scler. 2014;20:64–71. doi: 10.1177/1352458513492244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–753. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- 39.Prineas JW, Parratt JD. Oligodendrocytes and the early multiple sclerosis lesion. Ann Neurol. 2012;72:18–31. doi: 10.1002/ana.23634. [DOI] [PubMed] [Google Scholar]
- 40.Lucchinetti C, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 41.Singh S, et al. Microglial nodules in early multiple sclerosis white matter are associated with degenerating axons. Acta Neuropathol. 2013;125:595–608. doi: 10.1007/s00401-013-1082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peferoen LA, et al. Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2015;74:48–63. doi: 10.1097/NEN.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 43.Nikic I, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011;17:495–499. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez JI, et al. Focal disturbances in the blood–brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis. 2014;74:14–24. doi: 10.1016/j.nbd.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 45.Filippi M, Rocca MA, Martino G, Horsfield MA, Comi G. Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol. 1998;43:809–814. doi: 10.1002/ana.410430616. [DOI] [PubMed] [Google Scholar]
- 46.Goodkin DE, et al. A serial study of new MS lesions and the white matter from which they arise. Neurology. 1998;51:1689–1697. doi: 10.1212/wnl.51.6.1689. [DOI] [PubMed] [Google Scholar]
- 47.Werring DJ, et al. The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: a serial diffusion MRI study. Brain. 2000;123:1667–1676. doi: 10.1093/brain/123.8.1667. [DOI] [PubMed] [Google Scholar]
- 48.Eisele P, et al. Reduced diffusion in a subset of acute MS lesions: a serial multiparametric MRI study. AJNR Am J Neuroradiol. 2012;33:1369–1373. doi: 10.3174/ajnr.A2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tartaglia MC, et al. Choline is increased in pre-lesional normal appearing white matter in multiple sclerosis. J Neurol. 2002;249:1382–1390. doi: 10.1007/s00415-002-0846-6. [DOI] [PubMed] [Google Scholar]
- 50.Wuerfel J, et al. Changes in cerebral perfusion precede plaque formation in multiple sclerosis: a longitudinal perfusion MRI study. Brain. 2004;127:111–119. doi: 10.1093/brain/awh007. [DOI] [PubMed] [Google Scholar]
- 51.Wiggermann V, et al. Magnetic resonance frequency shifts during acute MS lesion formation. Neurology. 2013;81:211–218. doi: 10.1212/WNL.0b013e31829bfd63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guttmann CR, et al. Multiple sclerosis lesion formation and early evolution revisited: a weekly high-resolution magnetic resonance imaging study. Mult Scler. 2015 doi: 10.1177/1352458515600247. http://dx.doi.org/10.1177/1352458515600247. [DOI] [PubMed]
- 53.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood–brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vos CM, et al. Blood–brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis. 2005;20:953–960. doi: 10.1016/j.nbd.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 55.Gay D, Esiri M. Blood–brain barrier damage in acute multiple sclerosis plaques. An immunocytological study. Brain. 1991;114:557–572. doi: 10.1093/brain/114.1.557. [DOI] [PubMed] [Google Scholar]
- 56.Agrawal SM, et al. Extracellular matrix metalloproteinase inducer shows active perivascular cuffs in multiple sclerosis. Brain. 2013;136:1760–1777. doi: 10.1093/brain/awt093. [DOI] [PubMed] [Google Scholar]
- 57.Davalos D, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ryu JK, et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun. 2015;6:8164. doi: 10.1038/ncomms9164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McFarland HF, et al. Using gadolinium-enhanced magnetic resonance imaging lesions to monitor disease activity in multiple sclerosis. Ann Neurol. 1992;32:758–766. doi: 10.1002/ana.410320609. [DOI] [PubMed] [Google Scholar]
- 60.Frank JA, et al. Serial contrast-enhanced magnetic resonance imaging in patients with early relapsing–remitting multiple sclerosis: implications for treatment trials. Ann Neurol. 1994;36(Suppl):S86–S90. doi: 10.1002/ana.410360719. [DOI] [PubMed] [Google Scholar]
- 61.Gaitan MI, et al. Evolution of the blood–brain barrier in newly forming multiple sclerosis lesions. Ann Neurol. 2011;70:22–29. doi: 10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaitan MI, Sati P, Inati SJ, Reich DS. Initial investigation of the blood–brain barrier in MS lesions at 7 tesla. Mult Scler. 2013;19:1068–1073. doi: 10.1177/1352458512471093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lassmann H, Raine CS, Antel J, Prineas JW. Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J Neuroimmunol. 1998;86:213–217. doi: 10.1016/s0165-5728(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 64.van der Valk P, De Groot CJ. Staging of multiple sclerosis (MS) lesions: pathology of the time frame of MS. Neuropathol Appl Neurobiol. 2000;26:2–10. doi: 10.1046/j.1365-2990.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 65.Yamasaki R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butovsky O, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8:647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 69.Hametner S, et al. Iron and neurodegeneration in the multiple sclerosis brain. Ann Neurol. 2013;74:848–861. doi: 10.1002/ana.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stephenson E, Nathoo N, Mahjoub Y, Dunn JF, Yong VW. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat Rev Neurol. 2014;10:459–468. doi: 10.1038/nrneurol.2014.118. [DOI] [PubMed] [Google Scholar]
- 71.Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 72.Dutta R, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- 73.Laule C, et al. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler. 2006;12:747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- 74.Levesque IR, et al. Reproducibility of quantitative magnetization-transfer imaging parameters from repeated measurements. Magn Reson Med. 2010;64:391–400. doi: 10.1002/mrm.22350. [DOI] [PubMed] [Google Scholar]
- 75.Sati P, et al. Micro-compartment specific T2* relaxation in the brain. Neuroimage. 2013;77:268–278. doi: 10.1016/j.neuroimage.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alonso-Ortiz E, Levesque IR, Pike GB. MRI-based myelin water imaging: a technical review. Magn Reson Med. 2015;73:70–81. doi: 10.1002/mrm.25198. [DOI] [PubMed] [Google Scholar]
- 77.Yablonskiy DA, Luo J, Sukstanskii AL, Iyer A, Cross AH. Biophysical mechanisms of MRI signal frequency contrast in multiple sclerosis. Proc Natl Acad Sci USA. 2012;109:14212–14217. doi: 10.1073/pnas.1206037109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mehta V, et al. Iron is a sensitive biomarker for inflammation in multiple sclerosis lesions. PLoS ONE. 2013;8:e57573. doi: 10.1371/journal.pone.0057573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen W, et al. Quantitative susceptibility mapping of multiple sclerosis lesions at various ages. Radiology. 2014;271:183–192. doi: 10.1148/radiol.13130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shechter R, Schwartz M. CNS sterile injury: just another wound healing? Trends Mol Med. 2013;19:135–143. doi: 10.1016/j.molmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33:137–151. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- 82.Raine CS, Wu E. Multiple sclerosis: remyelination in acute lesions. J Neuropathol Exp Neurol. 1993;52:199–204. [PubMed] [Google Scholar]
- 83.Patrikios P, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 84.Albert M, Antel J, Bruck W, Stadelmann C. Extensive cortical remyelination in patients with chronic multiple sclerosis. Brain Pathol. 2007;17:129–138. doi: 10.1111/j.1750-3639.2006.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bramow S, et al. Demyelination versus remyelination in progressive multiple sclerosis. Brain. 2010;133:2983–2998. doi: 10.1093/brain/awq250. [DOI] [PubMed] [Google Scholar]
- 86.Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346:165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- 87.Kuhlmann T, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131:1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 88.Goldschmidt T, Antel J, König FB, Bruck W, Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology. 2009;72:1914–1921. doi: 10.1212/WNL.0b013e3181a8260a. [DOI] [PubMed] [Google Scholar]
- 89.Franklin RJ, Gallo V. The translational biology of remyelination: past, present, and future. Glia. 2014;62:1905–1915. doi: 10.1002/glia.22622. [DOI] [PubMed] [Google Scholar]
- 90.van Walderveen MA, et al. Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology. 1998;50:1282–1288. doi: 10.1212/wnl.50.5.1282. [DOI] [PubMed] [Google Scholar]
- 91.van Waesberghe JH, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol. 1999;46:747–754. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 92.Chen JT, et al. Magnetization transfer ratio evolution with demyelination and remyelination in multiple sclerosis lesions. Ann Neurol. 2008;63:254–262. doi: 10.1002/ana.21302. [DOI] [PubMed] [Google Scholar]
- 93.Reich DS, et al. Sample-size calculations for short-term proof-of-concept studies of tissue protection and repair in multiple sclerosis lesions via conventional clinical imaging. Mult Scler. 2015;21:1693–1704. doi: 10.1177/1352458515569098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lassmann H. Review: the architecture of inflammatory demyelinating lesions: implications for studies on pathogenesis. Neuropathol Appl Neurobiol. 2011;37:698–710. doi: 10.1111/j.1365-2990.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- 95.Frischer JM, et al. Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque. Ann Neurol. 2015;78:710–721. doi: 10.1002/ana.24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pitt D, et al. Imaging cortical lesions in multiple sclerosis with ultra-high-field magnetic resonance imaging. Arch Neurol. 2010;67:812–818. doi: 10.1001/archneurol.2010.148. [DOI] [PubMed] [Google Scholar]
- 97.Bagnato F, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134:3602–3615. doi: 10.1093/brain/awr278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bian W, et al. A serial in vivo 7T magnetic resonance phase imaging study of white matter lesions in multiple sclerosis. Mult Scler. 2012;19:69–75. doi: 10.1177/1352458512447870. [DOI] [PubMed] [Google Scholar]
- 99.Yao B, et al. Chronic multiple sclerosis lesions: characterization with high-field-strength MR imaging. Radiology. 2012;262:206–215. doi: 10.1148/radiol.11110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hagemeier J, et al. Iron deposition in multiple sclerosis lesions measured by susceptibility-weighted imaging filtered phase: a case control study. J Magn Reson Imaging. 2012;36:73–83. doi: 10.1002/jmri.23603. [DOI] [PubMed] [Google Scholar]
- 101.Vellinga MM, et al. Pluriformity of inflammation in multiple sclerosis shown by ultra-small iron oxide particle enhancement. Brain. 2008;131:800–807. doi: 10.1093/brain/awn009. [DOI] [PubMed] [Google Scholar]
- 102.Tourdias T, et al. Assessment of disease activity in multiple sclerosis phenotypes with combined gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology. 2012;264:225–233. doi: 10.1148/radiol.12111416. [DOI] [PubMed] [Google Scholar]
- 103.Maarouf A, et al. Ultra-small superparamagnetic iron oxide enhancement is associated with higher loss of brain tissue structure in clinically isolated syndrome. Mult Scler. 2015 doi: 10.1177/1352458515607649. http://dx.doi.org/10.1177/1352458515607649. [DOI] [PubMed]
- 104.Dousset V, et al. Comparison of ultrasmall particles of iron oxide (USPIO)-enhanced T2-weighted, conventional T2-weighted, and gadolinium-enhanced T1-weighted MR images in rats with experimental autoimmune encephalomyelitis. AJNR Am J Neuroradiol. 1999;20:223–227. [PMC free article] [PubMed] [Google Scholar]
- 105.Berger C, Hiestand P, Kindler-Baumann D, Rudin M, Rausch M. Analysis of lesion development during acute inflammation and remission in a rat model of experimental autoimmune encephalomyelitis by visualization of macrophage infiltration, demyelination and blood-brain barrier damage. NMR Biomed. 2006;19:101–107. doi: 10.1002/nbm.1007. [DOI] [PubMed] [Google Scholar]
- 106.Baeten K, et al. Visualisation of the kinetics of macrophage infiltration during experimental autoimmune encephalomyelitis by magnetic resonance imaging. J Neuroimmunol. 2008;195:1–6. doi: 10.1016/j.jneuroim.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 107.Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 1996;6:259–274. doi: 10.1111/j.1750-3639.1996.tb00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Metz I, et al. Pathologic heterogeneity persists in early active multiple sclerosis lesions. Ann Neurol. 2014;75:728–738. doi: 10.1002/ana.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Popescu BF, Bunyan RF, Parisi JE, Ransohoff RM, Lucchinetti CF. A case of multiple sclerosis presenting with inflammatory cortical demyelination. Neurology. 2011;76:1705–1710. doi: 10.1212/WNL.0b013e31821a44f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kutzelnigg A, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 111.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 112.Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 113.Kooi EJ, Strijbis EM, van der Valk P, Geurts JJ. Heterogeneity of cortical lesions in multiple sclerosis: clinical and pathologic implications. Neurology. 2012;79:1369–1376. doi: 10.1212/WNL.0b013e31826c1b1c. [DOI] [PubMed] [Google Scholar]
- 114.Chang A, et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;72:918–926. doi: 10.1002/ana.23693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kidd D, et al. Cortical lesions in multiple sclerosis. Brain. 1999;122:17–26. doi: 10.1093/brain/122.1.17. [DOI] [PubMed] [Google Scholar]
- 116.Fischer MT, et al. Disease-specific molecular events in cortical multiple sclerosis lesions. Brain. 2013;136:1799–1815. doi: 10.1093/brain/awt110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Magliozzi R, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130:1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 118.Magliozzi R, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68:477–493. doi: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- 119.Howell OW, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134:2755–2771. doi: 10.1093/brain/awr182. [DOI] [PubMed] [Google Scholar]
- 120.Choi SR, et al. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain. 2012;135:2925–2937. doi: 10.1093/brain/aws189. [DOI] [PubMed] [Google Scholar]
- 121.Kuerten S, et al. Tertiary lymphoid organ development coincides with determinant spreading of the myelin-specific T cell response. Acta Neuropathol. 2012;124:861–873. doi: 10.1007/s00401-012-1023-3. [DOI] [PubMed] [Google Scholar]
- 122.Magliozzi R, et al. B-cell enrichment and Epstein–Barr virus infection in inflammatory cortical lesions in secondary progressive multiple sclerosis. J Neuropathol Exp Neurol. 2013;72:29–41. doi: 10.1097/NEN.0b013e31827bfc62. [DOI] [PubMed] [Google Scholar]
- 123.Bo L, Geurts JJ, van der Valk P, Polman C, Barkhof F. Lack of correlation between cortical demyelination and white matter pathologic changes in multiple sclerosis. Arch Neurol. 2007;64:76–80. doi: 10.1001/archneur.64.1.76. [DOI] [PubMed] [Google Scholar]
- 124.Calabrese M, et al. Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci. 2015;16:147–158. doi: 10.1038/nrn3900. [DOI] [PubMed] [Google Scholar]
- 125.Geurts JJ, et al. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol. 2005;26:572–577. [PMC free article] [PubMed] [Google Scholar]
- 126.Schmierer K, et al. High field (9.4 Tesla) magnetic resonance imaging of cortical grey matter lesions in multiple sclerosis. Brain. 2010;133:858–867. doi: 10.1093/brain/awp335. [DOI] [PubMed] [Google Scholar]
- 127.Seewann A, et al. Postmortem verification of MS cortical lesion detection with 3D DIR. Neurology. 2012;78:302–308. doi: 10.1212/WNL.0b013e31824528a0. [DOI] [PubMed] [Google Scholar]
- 128.Sethi V, et al. Improved detection of cortical MS lesions with phase-sensitive inversion recovery MRI. J Neurol Neurosurg Psychiatry. 2012;83:877–882. doi: 10.1136/jnnp-2012-303023. [DOI] [PubMed] [Google Scholar]
- 129.Mainero C, et al. In vivo imaging of cortical pathology in multiple sclerosis using ultra-high field MRI. Neurology. 2009;73:941–948. doi: 10.1212/WNL.0b013e3181b64bf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nielsen AS, et al. Contribution of cortical lesion subtypes at 7T MRI to physical and cognitive performance in MS. Neurology. 2013;81:641–649. doi: 10.1212/WNL.0b013e3182a08ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mainero C, et al. A gradient in cortical pathology in multiple sclerosis by in vivo quantitative 7 T imaging. Brain. 2015;138:932–945. doi: 10.1093/brain/awv011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abdel-Fahim R, et al. Improved detection of focal cortical lesions using 7T magnetisation transfer imaging in patients with multiple sclerosis. Mult Scler Relat Disord. 2014;3:258–265. doi: 10.1016/j.msard.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 133.Jonkman LE, et al. Ultra-high field MTR and qR2* differentiates subpial cortical lesions from normal-appearing gray matter in multiple sclerosis. Mult Scler. 2015 doi: 10.1177/1352458515620499. http://dx.doi.org/10.1177/1352458515620499. [DOI] [PubMed]
- 134.Calabrese M, et al. Cortical lesions in primary progressive multiple sclerosis: a 2-year longitudinal MR study. Neurology. 2009;72:1330–1336. doi: 10.1212/WNL.0b013e3181a0fee5. [DOI] [PubMed] [Google Scholar]
- 135.Calabrese M, et al. Cortical lesions and atrophy associated with cognitive impairment in relapsing–remitting multiple sclerosis. Arch Neurol. 2009;66:1144–1150. doi: 10.1001/archneurol.2009.174. [DOI] [PubMed] [Google Scholar]
- 136.Roosendaal SD, et al. Accumulation of cortical lesions in MS: relation with cognitive impairment. Mult Scler. 2009;15:708–714. doi: 10.1177/1352458509102907. [DOI] [PubMed] [Google Scholar]
- 137.Calabrese M, et al. A 3-year magnetic resonance imaging study of cortical lesions in relapse-onset multiple sclerosis. Ann Neurol. 2010;67:376–383. doi: 10.1002/ana.21906. [DOI] [PubMed] [Google Scholar]
- 138.Calabrese M, et al. Cortical lesion load associates with progression of disability in multiple sclerosis. Brain. 2012;135:2952–2961. doi: 10.1093/brain/aws246. [DOI] [PubMed] [Google Scholar]
- 139.Absinta M, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology. 2015;85:18–28. doi: 10.1212/WNL.0000000000001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pomeroy IM, Matthews PM, Frank JA, Jordan EK, Esiri MM. Demyelinated neocortical lesions in marmoset autoimmune encephalomyelitis mimic those in multiple sclerosis. Brain. 2005;128:2713–2721. doi: 10.1093/brain/awh626. [DOI] [PubMed] [Google Scholar]
- 141.Merkler D, et al. Differential macrophage/microglia activation in neocortical EAE lesions in the marmoset monkey. Brain Pathol. 2006;16:117–123. doi: 10.1111/j.1750-3639.2006.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pomeroy IM, Jordan EK, Frank JA, Matthews PM, Esiri MM. Diffuse cortical atrophy in a marmoset model of multiple sclerosis. Neurosci Lett. 2008;437:121–124. doi: 10.1016/j.neulet.2008.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pomeroy IM, Jordan EK, Frank JA, Matthews PM, Esiri MM. Focal and diffuse cortical degenerative changes in a marmoset model of multiple sclerosis. Mult Scler. 2010;16:537–548. doi: 10.1177/1352458509360362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kramann N, et al. Increased meningeal T and plasma cell infiltration is associated with early subpial cortical demyelination in common marmosets with experimental autoimmune encephalomyelitis. Brain Pathol. 2015;25:276–286. doi: 10.1111/bpa.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Coronal 3 T FLAIR* images (combination of coregistered T2*-weighted magnitude and T2-weighted fluid-attenuation inversion recovery images) from a 33-year-old woman with relapsing–remitting multiple sclerosis. A prominent central vein can be seen in the majority of lesions.
Dynamic contrast enhancement MRI shows a centripetally enhancing lesion (red arrow) in an untreated 49-year-old woman with progressive multiple sclerosis and radiological relapses.
Dynamic contrast enhancement MRI of a new periventricular centripetally enhancing lesion in a 33-year-old man with relapsing–remitting multiple sclerosis. Over a 20 min period, contrast material progressively and completely fills the lesion.