Abstract
Objective
To examine the relationship between serum chemokine levels and patient responsiveness in rheumatoid arthritis (RA) patients to etanercept (ETN) and the influence of ETN administration on serum chemokine levels.
Methods
Serum levels of the chemokines CX3CL1, CXCL8, CXCL10, and CCL3 were quantified prior to (at baseline) and after 14 weeks of treatment with ETN in 20 patients using enzyme-linked immunosorbent assay. Disease status was assessed using the Disease Activity Score (DAS28). The response to ETN was classified according to the European League Against Rheumatism (EULAR) response criteria.
Results
By 14 weeks, ETN produced a significant overall reduction in DAS28 among the 20 patients with RA; eight patients achieved a good response, and 10 patients achieved a moderate response based on EULAR response criteria. A significant reduction in CX3CL1 was observed in the responsive group, although ETN treatment had no significant effect on the serum levels of the other three chemokines. In addition, the messenger ribonucleic acid expression of CX3CR1 in peripheral blood mononuclear cells and the cell-surface expression of CX3CR1 protein in peripheral blood CD8+CD3+ T cells were both decreased after ETN treatment.
Conclusions
Our results suggest that the CX3CL1 and CX3CR1 in patients with active RA may be sensitive to antitumor necrosis factor-α therapy and confirm that CX3CL1/CX3CR1 axis plays a crucial role in the pathogenesis of RA.
Keywords: rheumatoid arthritis, chemokine, CX3CL1, CX3CR1, TNF antagonist, etanercept
Introduction
Rheumatoid arthritis (RA), which is characterized by hyperplasia and chronic inflammatory changes within the synovium, is the most common of the inflammatory arthropathies, affecting up to 1% of the population.1 In recent years, research has focused on the involvement of chemokines, which are proinflammatory molecules with potentially important roles in the pathogenesis of inflammatory arthritis.2–4
The chemokines comprise a family of small 8–10 kDa proteins that were first described as chemoattractant cytokines; they are synthesized at the site of inflammation and play a role in leukocyte migration and trafficking.5 Chemokines are named according to their structure and are subdivided into four families (CC, CXC, C, and CX3C) based on the number and spacing of the first two cysteines within a conserved cysteine motif. In RA patients, a number of these molecules have been isolated from the synovium.6,7
Etanercept (ETN) consists of the p75 tumor necrosis factor (TNF) receptor fused to the constant region of the human immunoglobulin G1 (IgG1) antibody (Ab)8 and inhibits the binding of TNF to its cellular receptors, thereby inhibiting TNF-mediated inflammation and the progression of joint abnormalities seen in RA patients.9 Several clinical trials have provided compelling evidence of the clinical efficacy of ETN for both the induction and maintenance of remission in RA patients.10,11 Because ETN often induces rapid clinical improvement in RA patients, it is very important to investigate whether ETN administration has any effects on the serum chemokine concentration with or without the clinical improvement of RA disease activity.
The aim of our study was to examine the relationship between serum chemokine levels and patient responsiveness to ETN as well as the effect of ETN administration on serum chemokine levels.
Materials and methods
Patients
Twenty patients with RA who fulfilled the 1987 American College of Rheumatology (ACR) criteria12 were enrolled in our study between November 2009 and July 2010. All patients had active RA that, according to ACR guidelines,13 failed to respond to treatment with methotrexate or other disease-modifying antirheumatic drugs. Glucocorticoids (<10 mg/day of prednisolone) and nonsteroidal anti-inflammatory drugs had been given at a stable dose for at least 4 weeks prior to enrollment and during the course of treatment in the study. ETN was administered by subcutaneous injection at a dosage of 25 mg twice a week. Disease activity and its clinical improvement were assessed using the Disease Activity Score (DAS28) (erythrocyte sedimentation rate [ESR] 4) with the European League Against Rheumatism (EULAR) response criteria.14 Patients with moderate to good responses to ETN therapy were defined as the responsive group, whereas patients showing no response were defined as the unresponsive group. The improvement rate (%) of DAS28 was defined as follows: ((DAS28 at baseline − after 14 weeks/DAS28 at baseline) × 100). Serum and peripheral blood cells were collected immediately prior to (baseline) and 14 weeks after starting ETN therapy. In addition, control serum was collected from 20 healthy age- and sex-matched volunteers. The serum rheumatoid factor (RF), matrix metal-loproteinase (MMP-3), C-reactive protein levels, and ESR were determined using a latex photometric immunoassay and the Westergren method, respectively. The anticyclic citrullinated protein antibody (anti-CCP Ab) was measured using a commercial double-ligand enzyme-linked immunosorbent assay (ELISA) kit (Diastat Anti-CCP; MBL Tokyo, Japan). All experiments were carried out in accordance with protocols approved by the Human Subjects Research Committee at our institution, and informed consent was obtained from all patients and volunteers.
Determination of serum chemokine levels
Serum levels of the chemokines, including CXCL8 (interleukin 8 [IL-8]), CXCL10 (interferon-inducible protein 10), CCL3 (macrophage inflammatory protein 1α), and CX3CL1 (fractalkine), were quantified using commercial ELISA kits according to the manufactures’ instructions (R&D Systems, Minneapolis, MN, USA).
Flow cytometry
Flow cytometric analysis of CX3CR1 expression on peripheral blood mononuclear cells (PBMCs) was carried out as described previously.15 PBMCs from heparinized venous blood collected from patients and eight healthy age- and sex-matched volunteers were labeled first with anti-CD3-FITC, anti-CD4-PE, anti-CD8-PE, anti-CD14 (monocyte)-FITC (all from BD PharMingen, San Diego, CA, USA), or rabbit anti-CX3CR1 Ab (ProSci Inc, Poway, CA) and then with a secondary Ab (biotin-conjugated antirabbit IgG) and tertiary reagent (CyChrome-conjugated streptavidin; BD PharMingen). The fluorescence intensity and percentages of positive cells were measured on a three-color FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Isolation of total RNA and real-time polymerase chain reaction
Total cellular RNA was isolated from the PBMCs, which were collected from patients and seven healthy age- and sex-matched volunteers, and the real-time polymerase chain reactions (PCRs) were all performed as described previously.16 One-microgram samples of total RNA were reverse transcribed into cDNA using TaqMan RT reagents (Applied Biosystems, Foster City, CA), after which real-time PCR was conducted using an ABI Prism® 7900 sequence detection system (Applied Biosystems). Detection of CX3CR1 and ribosomal RNA expression was accomplished using appropriate Assays-on-Demand™ primers and probes (Applied Biosystems). To make quantitative comparisons between different samples, a dilution series of pooled cDNA from unstimulated PBMCs from healthy volunteers was used as the internal standard for CX3CR1 and was loaded in each analysis. The maximum levels were assigned a value of 100 units.
Statistical analysis
Data were expressed as means ± standard error of the mean. The differences between groups were evaluated using the Mann–Whitney U test. Follow-up data were evaluated using Wilcoxon’s test. The relationship between serum chemokine levels and the RA disease activity and indicated measures was evaluated using Spearman’s rank correlation. Values of P < 0.05 were considered significant.
Results
Kinetic study of serum chemokine levels in patients treated with ETN
The patient characteristics are summarized in Table 1. At the start of therapy, the mean age of the patients was 60.1 years, disease duration was 10.4 years, and the baseline DAS28 was 5.9. At baseline, there was no significant correlation between RA disease activity (DAS28) and RF and MMP-3 levels. There was also no significant correlation between the serum levels of any of the chemokines tested and either RA disease activity or serum measures, including RF and MMP-3 (data not shown). Fourteen weeks after the start of ETN treatment, there was a significant overall reduction of RA disease activity (DAS28) from 5.84 ± 0.25 to 3.47 ± 0.23 (P < 0.001) among the patients. In all RA patients, 18 patients treated with ETN achieved moderate and good responses, and two showed no clinical improvement. However, there were no significant differences in patient characteristics and laboratory parameters at baseline between the responder and non-responder groups (Table 1). Next, we examined the serum levels of chemokines between RA patients and healthy individuals. As shown in Table 2, the serum levels of both CX3CL1 and CCL3 in RA patients were significantly higher than those in healthy individuals, although the serum levels of two other chemokines (CXCL8 and CXCL10) showed no significant differences between RA patients and healthy controls. In addition, there was a significant reduction in the serum levels of CX3CL1 (Figure 1A; CX3CL1: baseline, 3314.8 ± 530.6 pg/mL vs 14 weeks, 1996.5 ± 471.9 pg/mL; P < 0.05) among responsive patients, although the ETN treatment had no significant effect on the serum levels of the other three chemokines tested (CCL3, CXCL8, and CXCL10) (Figure 1B–D). Moreover, no significant reduction in the serum levels of RF and MMP-3 was observed after 14 weeks of ETN treatment (data not shown).
Table 1.
Patient characteristics
| All | Responders | Nonresponders | P | |
|---|---|---|---|---|
| Patients (male/female) | 20 (1/19) | 18 (1/17) | 2 (0/2) | |
| Age (years) | 60.1 ± 3.2 | 60.2 ± 3.3 | 59.0 ± 1.8 | NS |
| Disease duration (years) | 10.4 ± 1.9 | 10.7 ± 2.1 | 7.5 ± 1.5 | NS |
| Dosage of MTX (mg/week) | 2.9 ± 0.8 | 3.0 ± 0.8 | 2.0 ± 2.0 | NS |
| Prednisolone (mg/day) | 4.0 ± 0.9 | 3.6 ± 0.9 | 7.5 ± 2.5 | NS |
| ESR (mm/h) | 70.0 ± 7.3 | 72.2 ± 7.2 | 50.5 ± 41.5 | NS |
| CRP (mg/dL) | 4.2 ± 1.1 | 4.6 ± 1.2 | 1.7 ± 1.4 | NS |
| TJC | 10.9 ± 1.8 | 9.8 ± 1.8 | 20.5 ± 2.5 | NS |
| SJC | 6.2 ± 0.8 | 6.3 ± 0.9 | 5.5 ± 0.7 | NS |
| DAS28 (ESR-4) | 5.9 ± 0.2 | 5.8 ± 0.3 | 6.5 ± 0.8 | NS |
| RF (IU/mL) | 175.0 ± 36.7 | 177.0 ± 36.8 | 86.0 ± 53.1 | NS |
| MMP-3 (ng/mL) | 214.0 ± 41.1 | 230.0 ± 45.0 | 97.5 ± 29.5 | NS |
Abbreviations: CRP, C-reactive protein; DAS28, Disease Activity Score 28; ESR, erythrocyte sedimentation rate; MMP-3, matrix metalloproteinase-3; MTX, methotrexate; NS, not significant; RF, rheumatoid factor; SJC, swollen joint counts; TJC, tender joint counts.
Table 2.
Serum concentration of chemokines in patients with RA and healthy controls
| RA (n = 20) |
Healthy controls (n = 20) |
P | |
|---|---|---|---|
| CX3CL1 (pg/mL) | 6440.0 ± 2601.0 | 506.8 ± 251.3 | <0.0001 |
| CCL3 (pg/mL) | 663.0 ± 234.3 | 61.5 ± 24.5 | <0.0005 |
| CXCL10 (pg/mL) | 286.6 ± 151.1 | 382.1 ± 152.5 | NS |
| CXCL8 (pg/mL) | 5.1 ± 1.4 | 17.8 ± 6.7 | NS |
Abbreviations: NS, not significant; RA, rheumatoid arthritis.
Figure 1.
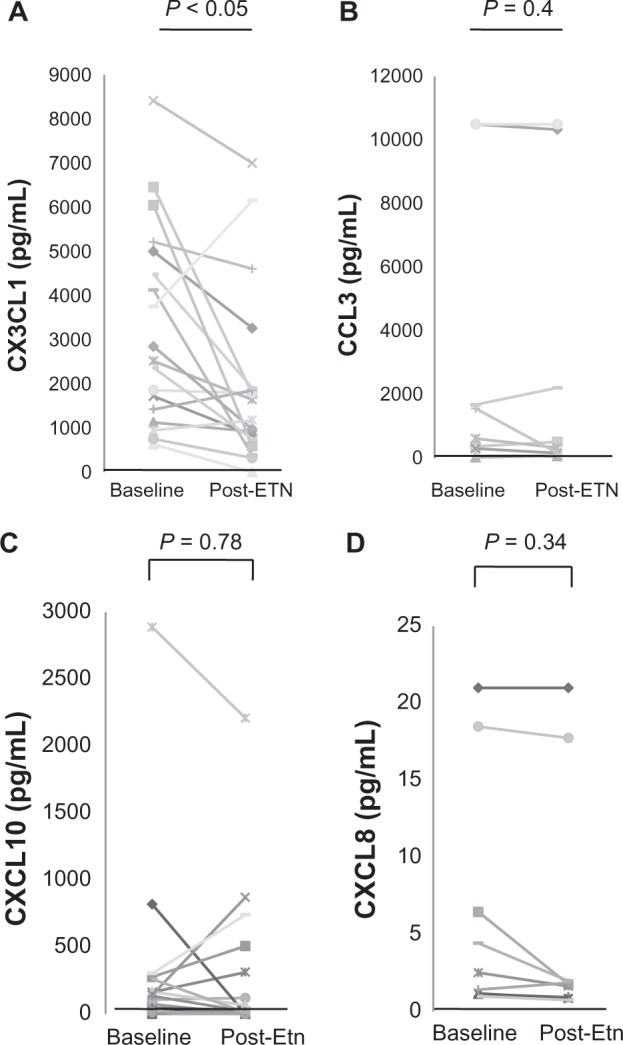
Changes in serum chemokine levels after ETN administration. Paired serum samples from 18 RA patients with moderate to good responses were collected at baseline and 14 weeks after starting ETN treatment. Each line represents an individual patient. A) CX3CL1, B) CCL3, C) CXCL10, and D) CXCL8. A significant reduction of serum CX3CL1 was seen after ETN treatment (*P < 0.05).
Abbreviations: ETN, etanercept; RA, rheumatoid arthritis.
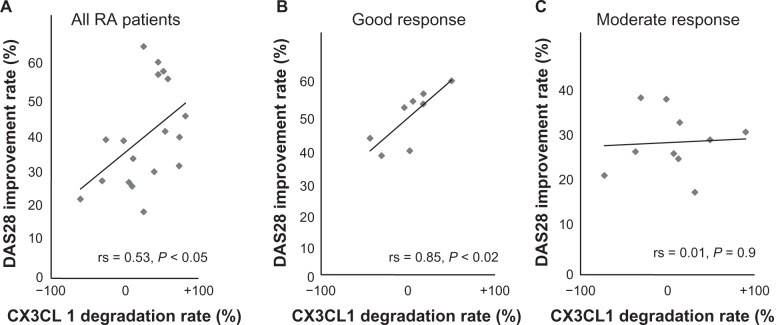
We next analyzed the correlation among the serum levels of CX3CL1 and clinical laboratory parameters. The degradation rate of the serum CX3CL1 levels showed a significant correlation with the DAS28 improvement rate (r = 0.53; P < 0.05; Figure 2A) but not with any of the clinical laboratory parameters. Furthermore, patients who achieved a good response had a significant correlation with the improvement rate of disease activity (Figure 2B), whereas there were no significant correlations in the moderate response group (Figure 2C). No significant correlation was observed between the DAS28 improvement rate and the levels of CCL3, CXCL10, and CXCL8 and clinical laboratory parameters (data not shown).
Figure 2.
Effect of ETN treatment on the improvement rate of RA disease activity (DAS28) and CX3CL1 levels in RA patients with basal CX3CL1 levels. The correlation between the DAS28 improvement rate (%) and the degradation rate of CX3CL1 (%) to ETN was statistically examined. The CX3CL1 degradation rate was defined as follows: ((CX3CL1 at baseline − CX3CL1 after ETN treatment/CX3CL1 at baseline) × 100). A) All RA patients (n = 20), B) patients with good responses (n = 8), and C) patients with moderate responses (n = 10). Each point represents an individual patient sample from RA patients.
Abbreviations: DAS28, Disease Activity Score 28; ETN, etanercept; RA, rheumatoid arthritis.
Increased CX3CR1 mRNA and cell-surface expression of CX3CR1 protein in patients with RA and their reduction by ETN therapy
CX3CR1, a specific CX3CL1 receptor, is also crucially involved in the dysregulation of CX3CL1 expression under conditions of inflammation.17 To examine the effects of the expression of CX3CR1 messenger ribonucleic acid (mRNA) and the protein, we used real-time PCR and flow cytometry to analyze their expression in peripheral blood-specific cell populations from patients with RA who had been randomly selected at the start of ETN therapy and from healthy controls.
As shown in Figure 3A, basal expression of CX3CR1 mRNA in PBMCs was significantly (P < 0.01) higher in RA patients (4500 ± 2179 units) than in the healthy control group (194.7 ± 114.0 units). In addition, there was no correlation between RA disease activity (DAS28 score) and basal levels of CX3CR1 mRNA in RA patients (Figure 3A). Notably, the expression of CX3CR1 mRNA was significantly reduced after ETN treatment (P < 0.05; Figure 3B).
Figure 3.
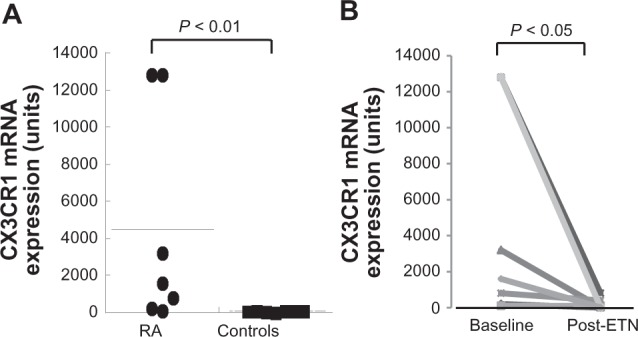
Expression of CX3CR1 mRNA in peripheral blood lymphocytes from RA patients. The expression of CX3CR1 mRNA in peripheral blood lymphocytes from seven RA patients and seven healthy individuals randomly selected at the start of ETN therapy. Data are expressed in arbitrary units. A) Comparison of CX3CR1 mRNA levels between RA patients and healthy individuals (controls). B) Significant reductions in CX3CR1 mRNA were found after ETN therapy; P < 0.05 vs baseline.
Abbreviations: ETN, etanercept; mRNA, messenger ribonucleic acid; RA, rheumatoid arthritis.
Consistent with the reduced expression of CX3CR1 mRNA in those responsive to ETN, the percentage of CX3CR1-positive CD8+CD3+ lymphocytes was increased in RA patients compared with the healthy controls (Figure 4A, B). Flow cytometric analysis of CX3CR1 expression was examined in four of seven patients whose mRNA expression was examined, and there was a positive correlation between mRNA expression and cell-surface expression of CX3CR1, although this was statistically insignificant (data not shown). Furthermore, the percentage of CX3CR1-positive CD8+CD3+ T cells tended to be lower after ETN treatment in the responsive group (14.3%–7.3%), albeit the difference was not statistically significant (Figure 4C, D). In addition, there was no significant alteration in the CX3CR1 surface expression on either CD4+CD3+ lymphocytes or monocytes after ETN treatment (data not shown).
Figure 4.
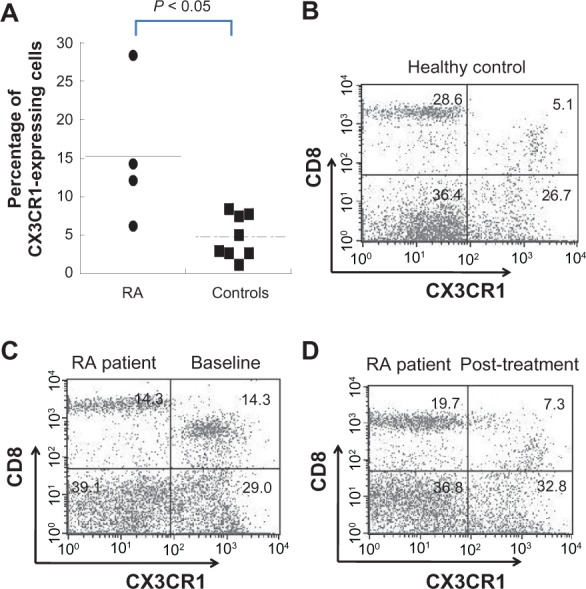
Cell-surface expression of CX3CR1 protein in PBMCs from RA patients and its reduction after ETN treatment. PBMCs were obtained from four RA patients and eight healthy controls and labeled with anti-CD3+, anti-CD4+, anti-CD8+, anti-CD14+, and anti-CX3CR1 Ab. The CX3CR1 expression on gated cells was then assayed by three-color flow cytometry. A) Percentage of CX3CR1-positive CD8+CD3+ lymphocytes from patients with active RA was significantly higher than in healthy controls. Representative dot plot graph of CX3CR1 expression on CD8+CD3+ T cells from B) healthy controls, C) RA patient at baseline, and D) RA patient after ETN treatment.
Abbreviations: ETN, etanercept; PBMCs, peripheral blood mononuclear cells; RA, rheumatoid arthritis.
Discussion
ETN possibly affects the production of a variety of cytokines, chemokines, mitogens, and proteases that exist downstream from the TNF-α cascade and promote the inflammatory process of RA.18–21 We have shown that although the serum levels of CXCL8, CXCL10, and CCL3 were unaffected by ETN therapy, the CX3CL1 levels declined in patients who showed a clinical response to ETN treatment. These results indicate that CX3CL1, but not other chemokines, may be sensitive to the suppression of disease activity by a TNF antagonist such as ETN.
CX3CL1 is the sole member of the unique family of the CX3C chemokine in the largest of the chemokine families, consisting of 373 amino acids.22,23 CX3CL1 has been implicated in several inflammatory disorders, including glomerulonephritis, systemic sclerosis, systemic lupus erythematosus, and systemic vasculitis, by several groups, including our laboratory.24–28 Importantly, CX3CL1 has a crucial involvement in the pathogenesis of RA, consistent with recent findings, including our report that the expression of CX3CL1 is upregulated in inflamed synovial RA lesions and that RA disease activity is positively correlated with CX3CL1 levels in the serum and synovial fluid, and the serum CX3CL1 was upregulated in systemic vasculitis found in RA (rheumatoid vasculitis [RV]) and was also correlated with disease activity.27,29–32 As shown in the present study, both CX3CL1 and CX3CR1 were upregulated in patients with RA compared with normal individuals, suggesting that the CX3CL1/CX3CR1 axis has a crucial role in the pathogenesis of RA. Furthermore, in the present study, we found that ETN treatment reduces the serum CX3CL1 in RA patients. Moreover, we revealed that the serum concentrations of CX3CL1 correlated with the improvement of the DAS28. Therefore, CX3CL1 might be useful markers of the RA activity in patients with anti-TNF-α therapy.
The expression of a variety of cytokines and chemokines, including CXCL10, CCL3, CXCL8, RANTES, and CCL2, is reportedly blocked or diminished after ETN therapy.21,33,34 However, we found that ETN had no significant effect on serum CCL3, CXCL8, and CXCL10 levels. The explanation for this difference in the response to ETN is not clear. These results may elucidate the hypothesis that CCL3, CXCL8, or CXCL10 production not only depends on TNF-α stimulation but also is affected by other mediators. In future, the effect of blocking these important chemokines combined with the blocking of TNF-α in an arthritic animal model needs to be studied.
Our previous report indicates that CX3CL1/CX3CR1 systems are upregulated in RA and RV in accordance with the activity of the disease.27 In the present study, the expression of CX3CL1 and its specific receptor CX3CR1 was suppressed following ETN therapy. Our result may reflect a diminished interaction between the endothelium and synovium, resulting in the reduced infiltration of leukocytes (especially CD8+ T-lymphocyte cells) into the rheumatoid synovium, which in turn likely reflects diminished CX3CL1/CX3CR1-mediated chemotactic effects on monocytes, natural killer cells, and T lymphocytes.
Taken together, our results suggest that the CX3CL1/CX3CR1 system in patients with active RA may be sensitive to anti-TNF-α therapy and confirm that CX3CL1 plays a crucial role in the pathogenesis of RA. The CX3CL1/CX3CR1 system might contribute to the pathogenesis of RA.
Acknowledgments
This work was supported, in part, by a grant from the Ministry of Health and Welfare of Japan.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Murphy G, Caplice N, Molloy M. Fractalkine in rheumatoid arthritis: a review to date. Rheumatology (Oxford) 2008;47(10):1446–1451. doi: 10.1093/rheumatology/ken197. [DOI] [PubMed] [Google Scholar]
- 2.Vergunst CE, van de Sande MG, Lebre MC, Tak PP. The role of chemokines in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 2005;34(6):415–425. doi: 10.1080/03009740500439159. [DOI] [PubMed] [Google Scholar]
- 3.Tarrant TK, Patel DD. Chemokines and leukocyte trafficking in rheumatoid arthritis. Pathophysiology. 2006;13(1):1–14. doi: 10.1016/j.pathophys.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Brennan F, Beech J. Update on cytokines in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19(3):296–301. doi: 10.1097/BOR.0b013e32805e87f1. [DOI] [PubMed] [Google Scholar]
- 5.Wells TN, Proudfoot AE. Chemokine receptors and their antagonists in allergic lung disease. Inflamm Res. 1999;48(7):353–362. doi: 10.1007/s000110050472. [DOI] [PubMed] [Google Scholar]
- 6.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 7.Szekanecz Z, Szücs G, Szántó S, Koch AE. Chemokines in rheumatic diseases. Curr Drug Targets. 2006;7(1):91–102. doi: 10.2174/138945006775270231. [DOI] [PubMed] [Google Scholar]
- 8.Goldbach-Mansky R, Lipsky PE. New concepts in the treatment of rheumatoid arthritis. Annu Rev Med. 2003;54:197–216. doi: 10.1146/annurev.med.54.101601.152342. [DOI] [PubMed] [Google Scholar]
- 9.Alldred A. Etanercept in rheumatoid arthritis. Expert Opin Pharmacother. 2001;2(7):1137–1148. doi: 10.1517/14656566.2.7.1137. [DOI] [PubMed] [Google Scholar]
- 10.Bathon JM, Martin RW, Fleischmann RM, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343(22):1586–1593. doi: 10.1056/NEJM200011303432201. [DOI] [PubMed] [Google Scholar]
- 11.Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355(7):704–712. doi: 10.1056/NEJMct055183. [DOI] [PubMed] [Google Scholar]
- 12.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 13.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46(2):328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 14.Van der Heijde DM, van’t Hof MA, van Riel PL, et al. Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis. 1990;49(11):916–920. doi: 10.1136/ard.49.11.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Odai T, Matsunawa M, Takahashi R, et al. Correlation of CX3CL1 and CX3CR1 levels with response to infliximab therapy in patients with rheumatoid arthritis. J Rheumatol. 2009;36(6):1158–1165. doi: 10.3899/jrheum.081074. [DOI] [PubMed] [Google Scholar]
- 16.Kasama T, Isozaki T, Odai T, et al. Expression of angiopoietin-1 in osteoblasts and its inhibition by tumor necrosis factor-alpha and interferon-gamma. Transl Res. 2007;149(5):265–273. doi: 10.1016/j.trsl.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Oppenheim JJ, Howard OM. Chemokines and chemokine receptors as novel therapeutic targets in rheumatoid arthritis (RA): inhibitory effects of traditional Chinese medicinal components. Cell Mol Immunol. 2004;1(5):336–342. [PubMed] [Google Scholar]
- 18.Feldmann M, Brennan FM, Williams RO, Elliott MJ, Maini RN. Cytokine expression and networks in rheumatoid arthritis: rationale for anti-TNF alpha antibody therapy and its mechanism of action. J Inflamm. 1995–1996;47(1–2):90–96. [PubMed] [Google Scholar]
- 19.Charles P, Elliott MJ, Davis D, et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999;163(3):1521–1528. [PubMed] [Google Scholar]
- 20.Berg L, Lampa J, Rogberg S, van Vollenhoven R, Klareskog L. Increased peripheral T cell reactivity to microbial antigens and collagen type II in rheumatoid arthritis after treatment with soluble TNFalpha receptors. Ann Rheum Dis. 2001;60(2):133–139. doi: 10.1136/ard.60.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klimiuk PA, Sierakowski S, Domyslawska I, Chwiecko J. Serum chemokines in patients with rheumatoid arthritis treated with etanercept. Rheumatol Int. 2009 Dec 19; doi: 10.1007/s00296-009-1299-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y, Lloyd C, Zhou H, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387(6633):611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 23.Umehara H, Bloom ET, Okazaki T, Nagano Y, Yoshie O, Imai T. Fractalkine in vascular biology: from basic research to clinical disease. Arterioscler Thromb Vasc Biol. 2004;24(1):34–40. doi: 10.1161/01.ATV.0000095360.62479.1F. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Bacon KB, Li L, et al. In vivo inhibition of CC and CX3C chemokine-induced leukocyte infiltration and attenuation of glomerulonephritis in Wistar-Kyoto (WKY) rats by vMIP-II. J Exp Med. 1998;188(1):193–198. doi: 10.1084/jem.188.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa M, Sato S, Echigo T, Hamaguchi Y, Yasui M, Takehara K. Up regulated expression of fractalkine/CX3CL1 and CX3CR1 in patients with systemic sclerosis. Ann Rheum Dis. 2005;64(1):21–28. doi: 10.1136/ard.2003.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yajima N, Kasama T, Isozaki T, et al. Elevated levels of soluble fractalkine in active systemic lupus erythematosus: potential involvement in neuropsychiatric manifestations. Arthritis Rheum. 2005;52(6):1670–1675. doi: 10.1002/art.21042. [DOI] [PubMed] [Google Scholar]
- 27.Matsunawa M, Isozaki T, Odai T, et al. Increased serum levels of soluble fractalkine (CX3CL1) correlate with disease activity in rheumatoid vasculitis. Arthritis Rheum. 2006;54(11):3408–3416. doi: 10.1002/art.22208. [DOI] [PubMed] [Google Scholar]
- 28.Matsunawa M, Odai T, Wakabayashi K, et al. Elevated serum levels of soluble CX3CL1 in patients with microscopic polyangiitis. Clin Exp Rheumatol. 2009;27(1):72–78. [PubMed] [Google Scholar]
- 29.Ruth JH, Rottman JB, Katschke KJ, Jr, et al. Selective lymphocyte chemokine receptor expression in the rheumatoid joint. Arthritis Rheum. 2001;44(12):2750–2760. doi: 10.1002/1529-0131(200112)44:12<2750::aid-art462>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 30.Ruth JH, Volin MV, Haines GK, 3rd, et al. Fractalkine, a novel chemokine in rheumatoid arthritis and in rat adjuvant-induced arthritis. Arthritis Rheum. 2001;44(7):1568–1581. doi: 10.1002/1529-0131(200107)44:7<1568::AID-ART280>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Volin MV, Woods JM, Amin MA, Connors MA, Harlow LA, Koch AE. Fractalkine: a novel angiogenic chemokine in rheumatoid arthritis. Am J Pathol. 2001;159(4):1521–1530. doi: 10.1016/S0002-9440(10)62537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blaschke S, Koziolek M, Schwarz A, et al. Proinflammatory role of fractalkine (CX3CL1) in rheumatoid arthritis. J Rheumatol. 2003;30(9):1918–1927. [PubMed] [Google Scholar]
- 33.Kageyama Y, Ichikawa T, Nagafusa T, Torikai E, Shimazu M, Nagano A. Etanercept reduces the serum levels of interleukin-23 and macrophage inflammatory protein-3 alpha in patients with rheumatoid arthritis. Rheumatol Int. 2007;28(2):137–143. doi: 10.1007/s00296-007-0388-4. [DOI] [PubMed] [Google Scholar]
- 34.Ichikawa T, Kageyama Y, Kobayashi H, Kato N, Tsujimura K, Koide Y. Etanercept treatment reduces the serum levels of interleukin-15 and interferon-gamma inducible protein-10 in patients with rheumatoid arthritis. Rheumatol Int. 2010;30(6):725–730. doi: 10.1007/s00296-009-1356-y. [DOI] [PubMed] [Google Scholar]