Abstract
Interleukin (IL)-6 has a variety of biological functions. For example, it stimulates the production of acute-phase reactants (C-reactive protein and serum amyloid A) and hepcidin which interferes with iron recycling and absorption, causing iron-deficient anemia, and augments expression of vascular endothelial growth factor and receptor activator of nuclear factor-κB ligand in synovial cells, leading to neovascularization and osteoclast formation. IL-6 also acts on lymphocytes, not only on B cells to stimulate autoantibody production, but also on naïve T helper cells to promote Th17 cell differentiation. Thus, an imbalance between T cell subsets possibly contributes to development of rheumatoid arthritis. Several clinical studies have demonstrated that a humanized anti-IL-6 receptor antibody, tocilizumab, improves clinical symptoms in rheumatoid arthritis. Tocilizumab prevented radiographic progression of joint destruction by inhibiting cartilage/bone resorption. Tocilizumab also improved hematological abnormalities, including hypergammaglobulinemia, high levels of autoantibodies, and elevation of erythrocyte sedimentation rate and acute-phase proteins. Importantly, tocilizumab improved quality of life by reducing systemic symptoms, including fatigue, anemia, anorexia, and fever. These findings have confirmed that hyperproduction of IL-6 is responsible for the above clinical symptoms, including joint destruction. Many patients treated with tocilizumab achieved clinical remission associated with decreased serum IL-6, suggesting that IL-6 enhances autoimmunity. Tocilizumab is a new therapeutic option for rheumatoid arthritis.
Keywords: interleukin-6, tocilizumab, efficacy, safety, mode of action
Introduction
Rheumatoid arthritis is a chronic, progressive autoimmune inflammatory disease of unknown etiology that particularly affects the joints of the hands and feet. The synovial tissue of affected joints is infiltrated by inflammatory cells, such as macrophages and lymphocytes, leading to hyperplasia with neovascularization, which causes joint swelling, stiffness, and pain. The final results are cartilage destruction and bone resorption in the joints, with some patients suffering permanent disability. Patients with rheumatoid arthritis also develop multiple systemic symptoms, including fever, fatigue, anemia, anorexia, osteoporosis, weight loss, and muscle weakness, and their lungs, skin, and liver may be affected. By five years after diagnosis, about 40% of patients are unable to work, and by 10 years, over 50% are unable to work. Patient lifespan is reduced by up to 10 years because of the effects of chronic inflammation on major organs.1
Although the etiology of rheumatoid arthritis is not fully understood, recent work has demonstrated that proinflammatory cytokines play an important role in its pathogenesis. Recent evidence suggests that a humanized antihuman interleukin (IL)-6 receptor antibody, tocilizumab, may be highly effective in treating patients with rheumatoid arthritis.2,3 In this review, we summarize the results of seven large clinical Phase III studies conducted worldwide, and discuss the possible mechanisms of action of tocilizumab in rheumatoid arthritis.
Tocilizumab
Tocilizumab is a humanized antihuman IL-6 receptor antibody (human IgG1 subclass). It is a κ light chain glycoprotein with a molecular weight of 148 kDa. It was derived from a mouse monoclonal antibody with IL-6-neutralizing activity that was humanized by means of complementarity-determining region grafting techniques.4
Tocilizumab binds to both the soluble IL-6 receptor and membrane-bound IL-6 receptor, with a dissociation constant (Kd value) of 2.5 × 10–9 M, thus completely inhibiting the binding of IL-6 to the IL-6 receptor. Tocilizumab inhibits the proliferation of KPMM2, a human myeloma cell line, in response to IL-6 via the membrane-bound IL-6 receptor. It also inhibits the soluble IL-6 receptor-mediated signal transduction, as examined using the human gp130-transfected mouse pro-B cell line, BAF (BAF-h130).5
Efficacy
In several large-scale clinical Phase III studies conducted in Japan and worldwide, including the US and Europe, tocilizumab has shown consistent efficacy.6–12 The results of clinical trials are summarized in Table 1. In addition, its efficacy has been confirmed recently in everyday clinical practice in Japan.13,14
Table 1.
Brief summary of Phase III clinical trials
| Trial | Groups | Sample size | Study duration | Study population | ACR20 | ACR50 | ACR70 | DAS remission (%) |
|---|---|---|---|---|---|---|---|---|
| Japanese P3 (SATORI) | MTX | 125 | 24 weeks | Active RA, refractory to MTX | 25 | 11 | 6 | 2 |
| TCZ 8 mg/kg | 80 | 49 | 30 | 43 | ||||
| Japanese P3 (SAMURAI) | DMARDs | 306 | 52 weeks | Active RA, refractory to DMARDs, including MTX | 34 | 13 | 6 | 3 |
| TCZ 8 mg/kg | 78 | 64 | 44 | 59 | ||||
| International P3 (OPTION) | Placebo + MTX | 623 | 24 weeks | Active RA, refractory to MTX | 26 | 11 | 2 | 1 |
| TCZ 4 mg/kg + MTX | 48 | 31 | 12 | 13 | ||||
| TCZ 8 mg/kg + MTX | 59 | 44 | 22 | 27 | ||||
| International P3 (TOWARD) | Placebo + MTX | 1216 | 24 weeks | Active RA, refractory to DMARDs | 25 | 9 | 3 | 3 |
| TCZ 8 mg/kg + MTX | 61 | 38 | 21 | 30 | ||||
| International P3 (AMBITION) | MTX | 673 | 24 weeks | Active RA | 53 | 34 | 15 | 12 |
| TCZ 8 mg/kg | 70 | 44 | 28 | 34 | ||||
| International P3 (RADIATE) | Placebo + MTX | 498 | 24 weeks | Active RA, refractory to TNF blockers | 10 | 4 | 1 | 2 |
| TCZ 4 mg/kg + MTX | 30 | 17 | 5 | 8 | ||||
| TCZ 8 mg/kg + MTX | 50 | 29 | 12 | 30 | ||||
| International P3 (LITHE) | Placebo + MTX | 1170 | 52 weeks | Active RA, refractory to MTX | 25 | 10 | 4 | 8 |
| TCZ 4 mg/kg + MTX | 47 | 29 | 16 | 30 | ||||
| TCZ 8 mg/kg + MTX | 56 | 36 | 20 | 47 |
Abbreviations: MTX, methotrexate; TCZ, tocilizumab; DMARDS, disease-modifying antirheumatic drugs; TNF, tumor necrosis factor; RA, rheumatoid arthritis.
Improved signs and symptoms of rheumatoid arthritis
In two Japanese studies (SATORI and SAMURAI), adult patients with moderate-to-severe active rheumatoid arthritis refractory to low-dose methotrexate or disease-modifying antirheumatic drugs (DMARDs) were given tocilizumab every four weeks as monotherapy. Excellent clinical benefit (American College of Rheumatology [ACR] responses and remission rates) was observed at weeks 24 and 52.6,7 AMBITION, one of several international studies, demonstrated the superiority of tocilizumab 8 mg/kg as monotherapy over methotrexate in patients who had not failed previous methotrexate or biologic treatment.8 The superiority of tocilizumab was apparent from as early as week 2, with between-group differences increasing over time.
Similarly, tocilizumab in combination with methotrexate proved highly effective in patients with rheumatoid arthritis who had experienced inadequate clinical response to previous therapy with at least one traditional DMARD including methotrexate (TOWARD study), methotrexate (OPTION and LITHE studies), or at least one anti-tumor necrosis factor (TNF) agent (RADIATE study).9–12 Tocilizumab significantly improved ACR response rates and Disease Activity Score using 28 joint counts (DAS28), as well as rates of remission. The efficacy of tocilizumab is similar in all kinds of patients. Of note, tocilizumab 8 mg/kg produced greater clinical benefit than tocilizumab 4 mg/kg.
Recently, the efficacy of tocilizumab in patients with rheumatoid arthritis seen in daily clinical practice in Japan was reported (REACTION study).13 Among 229 patients, 55% concomitantly received methotrexate and 63% had previously received anti-TNF therapy. The DAS28 of all 229 patients significantly decreased after 24 weeks of therapy. Tocilizumab was discontinued in 47 cases (20.5%) due to lack of efficacy (5.2%), adverse events (11.4%), and other reasons (3.9%). The overall retention rate at 24 weeks was 79.5%. This study suggests that tocilizumab therapy in daily clinical practice is highly efficacious in patients with active rheumatoid arthritis, including the population refractory to anti-TNF therapy. Tocilizumab infusion is therefore applicable not only as an alternative approach for patients resistant to anti-TNF therapy, but also as primary biologic therapy for patients with active rheumatoid arthritis.
Prevention of joint destruction
Tocilizumab 8 mg/kg as monotherapy significantly prevented joint damage in terms of total Sharp score, erosion score, and joint space narrowing score, compared with conventional DMARDs and methotrexate (SAMURAI study).7 Moreover, tocilizumab 4 or 8 mg/kg combined with methotrexate significantly inhibited the progression of radiographic damage compared with methotrexate alone (LITHE study). In that study, tocilizumab 8 mg/kg showed greater clinical benefit than 4 mg/kg.11
Improvement of quality of life scores
In several trials, tocilizumab significantly improved health-related quality of life and physical function in patients with rheumatoid arthritis, as assessed by Modified Health Assessment Questionnaire scores, Health Assessment Questionnaire Disease Index, Functional Assessment of Chronic Illness Therapy-Fatigue score, and The Short-Form 36 Health Survey scores.6,7,15
Tocilizumab also apparently improved anemia in patients with rheumatoid arthritis. Hemoglobin levels increased as early as week 2, and normalized by week 6 (AMBITION study).8 This efficacy was maintained through 24 weeks, but methotrexate did not show any apparent effect on anemia in that study. Improvement of anemia may contribute, in part, to the amelioration of health-related quality of life and physical function in patients with rheumatoid arthritis.
Long-term efficacy
The results of a long-term efficacy study in Japan (STREAM) have recently been published.15 In that study, 94/143 patients (66%) received tocilizumab 8 mg/kg monotherapy for five years. There were 32 withdrawals due to adverse events (22%), one withdrawal due to poor response (0.7%), and 14 withdrawals due to patient request or other reasons. Of 88 patients receiving concomitant steroids, 78 (88.6%) had the dose decreased and 28 (31.8%) were able to discontinue steroid use altogether. At five years, ACR20/50/70 response rates were 84.0%, 69.1%, and 43.6%, respectively, and the DAS28 remission (DAS28 < 2.6) rate was 55.3%.
In summary, tocilizumab as monotherapy or in combination with traditional DMARDs can lead to rapid and clinically relevant improvements in the signs and symptoms of rheumatoid arthritis, and prevent joint damage in biologics-naïve patients, as well as in patients with longstanding disease who fail to respond to traditional DMARDs or anti-TNF agents.
With respect to the efficacy of tocilizumab, it is reported that results differ depending on whether disease activity is evaluated by methods that use inflammatory markers (DAS28) or by methods that do not use inflammatory markers (clinical disease activity index, CDAI).15–17 However, such differences are not limited to tocilizumab, and are similarly observed in clinical studies for other biologics.17 This means that CDAI is a stricter criterion than DAS28. The weighting given to inflammatory markers when calculating the DAS28 score is high, and tocilizumab rapidly reduces levels of inflammatory markers. Therefore, joint symptoms may not improve in concert with the reduction in DAS28 score in the very early stages of treatment when improvement of inflammatory markers is a main factor. For long-term therapy, DAS28 score correlates with CDAI.15,16
Safety
Infections
Infections are the most frequent adverse events during tocilizumab therapy, with upper respiratory infection, urinary tract infection, bronchitis, gastroenteritis, and pneumonia being the most common.19 Although with long-term tocilizumab the incidence of adverse events is similar to that with other biologics, tocilizumab significantly reduces inflammatory markers. Therefore, when administering tocilizumab, it is important to pay attention not only to abnormal test values associated with infection but also to the emergence of symptoms of infection in patients. It is also important to educate patients who are going to be treated with tocilizumab that they should come in promptly for treatment if they notice any physical abnormalities, such as cough, sputum, or skin abnormalities.
Gastrointestinal perforation
Nonfatal gastrointestinal perforation occurred in five (0.8%) patients enrolled in the Japanese clinical studies, and a causal relationship with tocilizumab could not be ruled out in three cases.19 In international clinical studies, 26 (0.65%) patients experienced gastrointestinal perforation and three died of this complication.20
Recent Japanese postmarketing surveillance of tocilizumab in 6424 patients showed that 10 (0.2%) patients experienced gastrointestinal perforations (unpublished data). Most cases (n = 8) involved the lower gastrointestinal tract, and six patients had complications of the diverticulum (n = 2), amyloidosis (n = 2), and anamnestic ulcer (n = 2). All patients were taking corticosteroids, and five patients were taking nonsteroidal anti-inflammatory drugs that may have contributed to gastrointestinal perforation.
Liver function disorder
Liver function disorders, such as transient elevations in alanine aminotransferase or aspartate aminotransferase, were commonly observed in tocilizumab-treated patients, and the incidence of these elevations increased when tocilizumab was combined with potentially hepatotoxic drugs, such as methotrexate.21,22 No clinically apparent hepatitis or hepatic impairment was observed in either the international23 or Japanese studies.19 Abnormal increases in bilirubin levels occurred rarely in patients treated with tocilizumab.19
Altered lipid profile
Changes in serum lipid profiles were commonly observed in patients treated with tocilizumab.19 These increases occurred soon after initiation of treatment and stabilized during long-term therapy. Serum total cholesterol increased in 38% of patients on tocilizumab monotherapy in one Japanese study7 and in 23% of the patients given a combination of tocilizumab and nonbiologic DMARDs in a European study.10 However, total cholesterol levels stabilized in the upper normal range in most cases. If required, lipid-lowering agents allowed lipid abnormalities to be controlled. Due to a concomitant increase in high-density lipoprotein cholesterol levels, the atherogenic index ([total cholesterol − high-density lipoprotein cholesterol]/high-density lipoprotein cholesterol) remained unchanged in most patients.
The lipid increase may be related to the mechanism of action of tocilizumab, because IL-6 decreases serum cholesterol in cancer patients.24,25 We have recently found that IL-6 induces expression of the very low density lipoprotein receptor on cell surfaces, leading to increased consumption of low-density lipoprotein cholesterol.26 Decreased total cholesterol and high-density lipoprotein cholesterol levels are often seen in patients with severe rheumatoid arthritis, possibly because of cytokine-induced activation of the reticuloendothelial system.27 In fact, the suppression of TNF-α by infliximab has similar effects on lipids.28,29 TNF-α is a potent inducer of IL-6, and its blockade rapidly reduces serum IL-6 levels in patients with rheumatoid arthritis.30 It is likely that suppression of IL-6 production partly contributes to the lipid profile changes following TNF-α blockade.
Adverse hematological events
Tocilizumab produced rapid, transient, and dose-dependent decreases in neutrophils by inhibiting the biological effects of IL-6 on recruitment of neutrophils into peripheral blood. An anomalous decrease in neutrophil counts (<1000/mm3) occurred in 6% of patients on tocilizumab 8 mg/kg.19 However, there was no clear association between decreases in neutrophils and the occurrence of serious infections.
Antirheumatic mechanism
Overproduction of IL-6 has been found in the synovial fluid and blood of patients with rheumatoid arthritis, and IL-6 levels correlate with disease activity.31,32 Some of the clinical aspects of rheumatoid arthritis, such as the production of acute-phase proteins, increase in platelet counts and neutrophil counts, induction of osteoclasts, production of autoantibodies, and decrease in albumin, can be explained by the activities of IL-6.33 However, the precise mechanism by which IL-6 blockade leads to improvement of rheumatoid arthritis is not well understood. In this part of the review, we discuss the antirheumatic effect of tocilizumab based on our recent research.
Protection against synovitis
Changes in the synovium are marked by neovascularization, infiltration of inflammatory cells, and synoviocyte hyperplasia that produce a pannus (inflamed vascular tissue). Newly formed blood vessels are thought to be involved in the development and maintenance of synovitis because they support the infiltration of inflammatory cells, and the growth and survival of synovial cells.
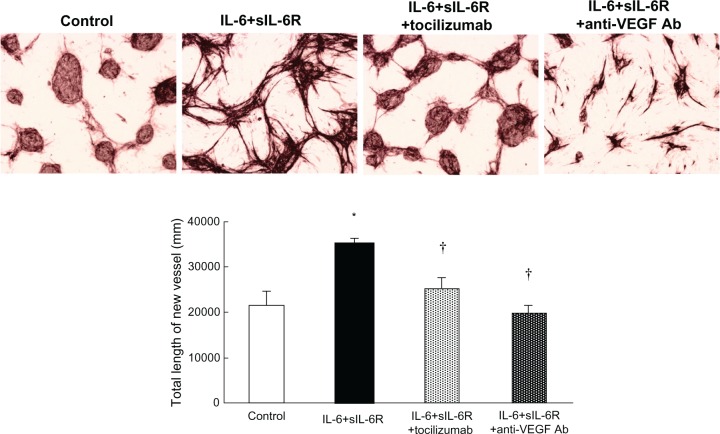
A number of growth factors and cytokines have angiogenic activity.34 Among these, vascular endothelial growth factor (VEGF) is thought to be the most important angiogenic factor in the pathogenesis of rheumatoid arthritis. VEGF production is induced by IL-6 stimulation of fibroblast-like synoviocytes from patients with rheumatoid arthritis (RA-FLS), and treatment with tocilizumab significantly lowers serum VEGF levels in patients with the disease.35 We found that IL-6 induced angiogenesis in a coculture system of human umbilical vascular endothelial cells and RA-FLS.36 Moreover, this angiogenesis was completely inhibited by anti-VEGF antibody, indicating that VEGF plays a crucial role in IL-6-induced angiogenesis (Figure 1).
Figure 1.
Tubule formation in coculture of rheumatoid arthritis fibroblast-like synovial cells and human vascular endothelial cells.35 Human vascular endothelial cells and rheumatoid arthritis fibroblast-like synovial cells were cocultured for 21 days alone (control), with IL-6 + soluble IL-6 receptor), with IL-6 + soluble IL-6 receptor + tocilizumab, or with IL-6 + soluble IL-6 receptor + anti-VEGF antibody. The medium (with cytokine and antibody) was replaced every two days. After culture, the formed tubules were detected by anti-CD31 antibody.
Notes: *P < 0.05 by unpaired t-test (versus control); †P < 0.05 by Dunnett’s multiple comparison test (versus IL-6 + soluble IL-6 receptor).
Abbreviations: Ab, antibody; IL, interleukin; VEGF, vascular endothelial growth factor; sIL-6R, soluble interleukin-6 receptor.
With collagen-induced arthritis in monkeys, we found that tocilizumab treatment significantly reduced joint swelling and infiltration of inflammatory cells into inflamed joints when tocilizumab was injected after the onset of arthritis.37 We found that IL-6 augmented production of chemokines, such as monocyte chemotactic protein-1 and IL-8 from endothelial cells, mononuclear cells, and RA-FLS.38 Moreover, IL-6 induced adhesion molecules, such as intracellular adhesion molecule-1, in endothelial cells and increased adhesion of monocytes to endothelial cells.38 These lines of evidence strongly support the idea that IL-6 aggravates the local inflammatory reaction by amplifying inflammatory cell infiltration. Suppression of angiogenesis may also reduce cell migration, because newly formed blood vessels are conduits for the infiltration of inflammatory cells.
Synovial fibroblastic cells produce large amounts of IL-6 when stimulated by inflammatory cytokines, such as IL-1, TNF, and IL-17, and we found that IL-6 augmented the proliferation of synovial fibroblastic cells in the presence of soluble IL-6 receptor.39,40 Tocilizumab may exert its anti-synovitis effect via inhibition of the biological activities of IL-6. In fact, semiquantitative assessment by ultrasonography indicated that tocilizumab significantly improves synovitis in patients with rheumatoid arthritis.41
Protection against joint destruction
Irreversible joint destruction is a characteristic feature of rheumatoid arthritis. Tocilizumab monotherapy for 52 weeks showed significantly less radiographic change in total Sharp score (bone erosion and joint space narrowing) than DMARD treatment. Interestingly, tocilizumab halted the progression of both bone erosion and joint space narrowing.7
As a pathogenic mechanism of bone destruction, osteoclasts activated by inflammatory cytokines are thought to be responsible for focal bone erosion. In fact, osteoclasts are often seen in the synovium at sites of cartilage destruction in patients with rheumatoid arthritis.42,43 The receptor activator of NF-κB (RANK) and its ligand (RANKL) are essential factors for osteoclastogenesis.44,45 Osteoclast precursor cells express RANK and differentiate into mature osteoclasts following RANKL stimulation. RANKL also stimulates osteoclast migration, fusion, activation, and survival, so acts at all stages of osteoclast generation and activity. It has been shown that RANKL is expressed in rheumatoid arthritis synovial membranes at sites of bone erosion.42,46
We found that IL-6 and soluble IL-6 receptor, but not IL-6 alone, induced RANKL expression in RA-FLS. On the other hand, neither TNF-α nor IL-17 induced RANKL expression, although each stimulates cell growth and IL-6 production. Interestingly, TNF-α and IL-17 each induced RANKL expression in the presence of soluble IL-6 receptor. In cocultures of RA-FLS and the osteoclast precursor cell line, RAW 264.7, IL-6 and soluble IL-6 receptor induced NFATc1 and TRAP5b mRNA expression in osteoclast precursor cells. IL-6 and the soluble IL-6 receptor directly induced osteoclastogenesis by inducing RANKL expression in RA-FLS.40
Cartilage degeneration is also observed in rheumatoid arthritis joints. RANKL inhibition clearly halted the progression of bone erosion, but did not improve joint space narrowing in patients with rheumatoid arthritis, strongly suggesting that RANKL/RANK signaling does not participate in cartilage degeneration in patients with rheumatoid arthritis.47 Matrix metalloproteinase (MMP) and a disintegrin and metalloproteinase with thrombospondin (ADAMTS) are thought to play crucial roles in cartilage matrix degeneration. We found that IL-6 induced MMP-1, MMP-3, and MMP-13 production from chondrocytes and synovial cells.48,49
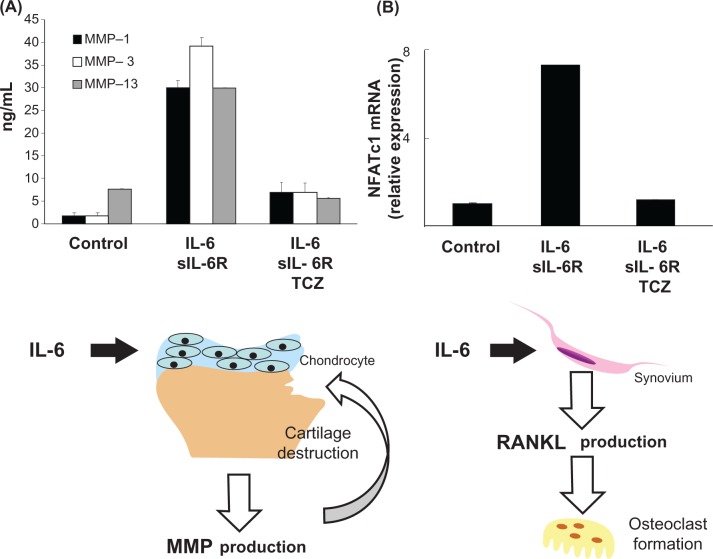
From these data, we suggest that the preventive effect of tocilizumab on joint destruction is mediated by the inhibition of IL-6-induced RANKL induction, followed by osteoclastogenesis and suppression of IL-6-induced production of MMPs (Figure 2). In a subanalysis of the SAMURAI and OPTION studies, improvements in bone resorption markers (such as C-terminal crosslinking telopeptide of type I collagen and deoxypyridinoline) and cartilage turnover markers (such as C-terminal crosslinking telopeptide of type II collagen, N-terminal propeptide of type IIA, and type II collagen helical peptide) were seen in the tocilizumab group.50,51 Moreover, tocilizumab decreased serum MMP-3 levels in several clinical trials.17,52
Figure 2.
Induction of matrix metalloproteinase and RANKL mRNA expression by IL-6.39,47 A) Chondrocytes were cultured with IL-6 + soluble IL-6 receptor or with IL-6 + soluble IL-6 receptor + TCZ for 24 hours. After culture, cell-free supernatants were collected and production of MMPs was measured by enzyme-linked immunosorbent assay. B) Rheumatoid arthritis fibroblast-like synovial cells were cultured with IL-6 + soluble IL-6 receptor or with IL-6 + soluble IL-6 receptor + TCZ for 24 hours. After culture, expression of RANKL mRNA relative to the control was measured by real time polymerase chain reaction. Each column and vertical line indicates mean and standard deviation of triplicate culture.
Notes: *P < 0.05 by unpaired t-test (versus control); †P < 0.05 by unpaired t-test (versus IL-6 + soluble interleukin-6 receptor).
Abbreviations: IL, interleukin; TCZ, tocilizumab; MMP, matrix metalloproteinase.
Improvement of anemia in rheumatoid arthritis
Anemia is the most common extra-articular manifestation of rheumatoid arthritis, and is estimated to occur in 30%–60% of patients.53,54 There are two primary types of anemia in rheumatoid arthritis, ie, anemia of chronic disease and iron-deficiency anemia. Anemia of chronic disease is characterized by hypoferremia in the presence of adequate iron stores. It is an inflammatory anemia, and inflammatory cytokines are thought to play important roles in anemia in rheumatoid arthritis.55,56 In fact, tocilizumab therapy in patients with rheumatoid arthritis rapidly improves anemia.10,11
With collagen-induced arthritis in monkeys, we found that anemia was also induced after collagen immunization. Anemia in monkeys with collagen-induced arthritis is characterized by decreased serum iron and transferrin saturation and by elevated serum ferritin, and its severity is correlated with serum IL-6 levels. Therefore, anemia in monkeys with collagen-induced arthritis is very similar to human anemia in inflammatory diseases, at least with respect to the changes in serum parameters.57 Hepcidin is a master regulator of iron homeostasis in humans and other mammals.58 It inhibits the absorption of iron in the small intestine and the release of recycled iron from macrophages, effectively decreasing the delivery of iron to maturing erythrocytes in the bone marrow.59 In fact, mice genetically engineered to overproduce hepcidin died of severe iron deficiency shortly after birth.60 Interestingly, IL-6 induces hepcidin production in liver cells.61
We found that administration of tocilizumab to monkeys with collagen-induced arthritis rapidly improved anemia and induced a rapid but transient reduction in serum hepcidin. Hepcidin mRNA expression was more potently induced by serum from arthritic monkeys, and this was inhibited by the addition of tocilizumab.62 From these lines of evidence, we propose that tocilizumab improves anemia in monkey arthritis via the inhibition of IL-6-induced hepcidin production.
Immunological improvement
There is no doubt that T cells play important roles in the onset of rheumatoid arthritis. CD4+ T helper cells have been classified as Th1 and Th2 cells on the basis of their cytokine production profiles, and recently, Th17 cells which produce IL-17 in autoimmune pathology have become recognized as a separate subset, which is interesting in the context of events that were previously thought to be Th1-mediated. In vitro studies utilizing mouse cells have shown that the costimulation of IL-6 and transforming growth factor-β is essential for the differentiation of Th17 cells from naïve CD4+ T cells.63 Furthermore, studies suggesting an involvement of IL-6 in the induction of Th17 cells in arthritis models have been reported. We found that the antihuman IL-6 receptor antibody suppressed the onset of arthritis in glucose-6-phosphate isomerase-induced and collagen-induced arthritis models, and concomitantly inhibited the appearance of Th17 cells (Figure 3).64–66 Interestingly, anti-TNF antibody conversely augmented the induction of Th17 cells, although it significantly suppressed the onset of arthritis.67 The involvement of Th17 cells in rheumatoid arthritis is therefore still controversial. However, the involvement of CD4+CD161+ T cells in autoimmune diseases has been attracting attention recently.68 These T cells produce large amounts of IL-17, and are increased in psoriasis and Crohn’s disease. It is also reported that peripheral blood mononuclear cells from patients with rheumatoid arthritis produced higher levels of IL-17 than peripheral blood mono-nuclear cells from healthy subjects when stimulated with anti-CD3 and anti-CD28 antibodies.69 We anticipate that the role of IL-17-producing T cells in rheumatoid arthritis will be clarified in the near future. From our and others results, we have summarized the mode of action of tocilizumab in Figure 4.
Figure 3.
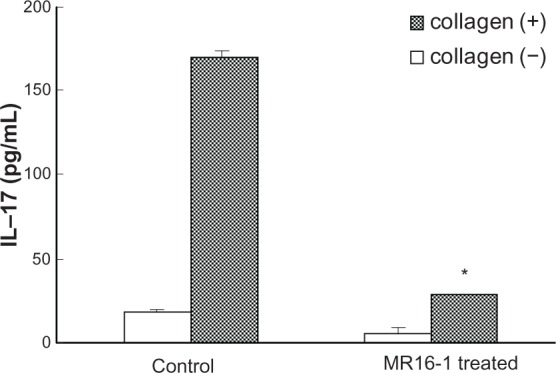
Production of IL-17 by splenic CD4+ T cells from collagen-induced arthritis in mice.66 A mouse collagen-induced arthritis model was established with anti-IL-6R antibody (MR16–1) or control administered on the day of first collagen immunization. CD4+ T cells purified from the spleen of the mice (21 days after first immunization) were cultured with collagen and anti-CD28 antibody for 48 hours. After culture, IL-17 production was measured by enzyme-linked immunosorbent assay. Each column and vertical line indicates mean and standard deviation of triplicate culture.
Note: *P < 0.05 versus control, by unpaired t-test.
Abbreviation: IL, interleukin.
Figure 4.
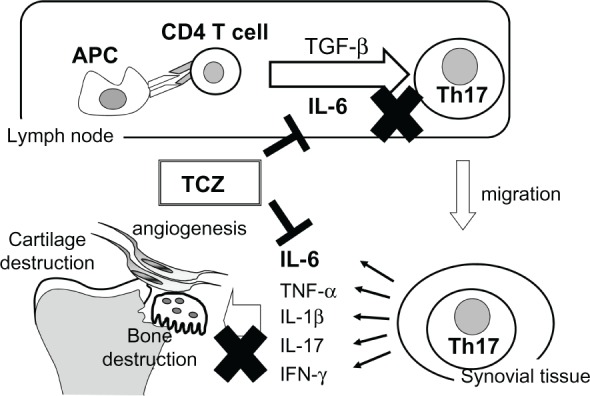
Immunological and anti-inflammatory effects of tocilizumab in rheumatoid arthritis.
Abbreviations: APC, antigen-presenting cells; IL, interleukin; TCZ, tocilizumab; TGF-β, transforming growth factor-beta; TNF, tumor necrosis factor.
Place of tocilizumab in disease management
Thanks to the recent deployment of biologic agents, disease remission is now an achievable goal in many patients with rheumatoid arthritis. In fact, biologics targeting TNF have markedly increased the disease remission rate. However, up to 50% of patients treated with such TNF blockers still fail to achieve remission.70 Around 14%–38% of patients do not respond to first-line anti-TNF treatment, and as many as 40% discontinue treatment within a year, and 50% within two years.70 The reasons for lack of treatment efficacy are not clearly understood. A possible mechanism is the development of antibodies that target and neutralize infliximab and adalimumab. It was shown recently that patients treated with infliximab or adalimumab who had anti-TNF-α antibodies had lower serum trough levels of the drug and poorer response to treatment.71 Moreover, TNF blockers are associated with a significantly increased risk of infection.72 TNF blockers may also increase the risk of certain malignancies, although this is controversial.73
Tocilizumab can also achieve a high rate of clinical remission. For example, in the SATORI study, 59% of patients achieved DAS28 remission (DAS28 < 2.6) at week 52.6 Similar results were reported in a long-term study (STREAM),15 and the rate of DAS28 remission increased as the duration of treatment increased (55.3% at five years). Interestingly, anti-tocilizumab antibodies were detected in only 3%–5% of patients, even though no immunosuppressants, such as methotrexate, were used. This strongly suggests that tocilizumab shows sustained long-term efficacy. In fact, the STREAM study demonstrated good sustained efficacy and a generally good safety profile over the five years of the study. Of particular note, only one of 143 patients withdrew as a result of an unsatisfactory response, indicating that no general loss of response occurred during long-term treatment.
Tocilizumab not only improves local signs and symptoms, but also systemic ones, such as anemia, anorexia, fever, and fatigue, thereby potentially improving patient quality of life. This is the great advantage of tocilizumab because systemic efficacy is difficult to achieve with other therapies, including anti-TNF therapies. Tocilizumab also improves hypoalbuminemia and erythrocyte sedimentation rate, which may also improve patient quality of life.
Tocilizumab almost completely normalizes serum amyloid and C-reactive protein levels, which may improve secondary amyloidosis74 (seen in 5% of patients with rheumatoid arthritis in Europe75) and may counter the increased risk of cardiovascular disease caused by high C-reactive protein in patients with rheumatoid arthritis.76 These effects may be related to IL-6 receptor blockade because IL-6 is intimately involved in the regulation of gene expression for serum amyloid and C-reactive protein synthesis.77 To improve secondary amyloidosis, serum amyloid needs to be kept below 10 μg/mL, and tocilizumab treatment is the only rheumatoid arthritis therapy that can decrease serum amyloid to this extent.
A clear advantage of IL-6 receptor blockade over TNF blockade in tuberculosis infection has been reported.78 In TNF inhibitor therapy, screening for tuberculosis infection and medication for tuberculosis are necessary before treatment. Ogata et al demonstrated that interferon synthesis by tuberculosis antigens was inhibited by TNF inhibitors but not by tocilizumab.79 Moreover, in collaboration with Dr Okada, we found that after inoculation with Mycobacterium tuberculosis, mice treated with antimouse TNF antibody all died much earlier than control mice, whereas those treated with an antimouse IL-6 receptor antibody did not. TNF is well known to play a critical role in the formation of granulomas that keep mycobacteria inside macrophages, whereas IL-6 has no such activity (unpublished data). Such differences may explain the reduced risk of tuberculosis during tocilizumab therapy.
The US Food and Drug Administration has approved tocilizumab for moderate to severe rheumatoid arthritis in adults who have not achieved an adequate response with one or more TNF antagonist therapies, and in the UK, the National Institute of Clinical Excellence recommends tocilizumab, in combination with methotrexate, for the treatment of moderate to severe active rheumatoid arthritis in people whose disease has responded inadequately to one or more TNF inhibitors, those whose rheumatoid arthritis has responded inadequately to rituximab or in whom rituximab is contraindicated, or when rituximab is withdrawn because of an adverse effect.1 However, in Japan and in European countries other than the UK, tocilizumab can be used for patients with rheumatoid arthritis who show an inadequate response to DMARDs. In the clinical trials and in actual clinical practice carried out in Japan, many patients treated with tocilizumab have achieved complete remission (DAS28 < 2.6). Additionally, as we have described, basic research suggests that tocilizumab ameliorates autoimmune phenomena as well as inflammation. Moreover, the earlier tocilizumab treatment is started, the better the clinical response80 in terms of both stopping disease progression and achieving complete remission. All of these findings strongly suggest that tocilizumab can be considered as a first-line drug for treatment of DMARD-resistant patients with rheumatoid arthritis.
Footnotes
Disclosure
M Mihara and Y Ohsugi are employees of Chugai Pharmaceutical Co, Ltd. T Kishimoto is a patent holder of tocilizumab.
References
- 1.NICE Rheumatoid Arthritis Consultation Document [Internet] [Accessed January 26, 2010]. Available at: www.nice.org.uk.
- 2.Ohsugi Y, Kishimoto T. The recombinant humanized anti-IL-6 receptor antibody tocilizumab, an innovative drug for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2008;8:669–681. doi: 10.1517/14712598.8.5.669. [DOI] [PubMed] [Google Scholar]
- 3.Ohsugi Y. Recent advances in immunopathophysiology of interleukin-6: An innovative therapeutic drug, tocilizumab (recombinant humanized anti-human interleukin-6 receptor antibody), unveils the mysterious etiology of immune-mediated inflammatory diseases. Biol Pharm Bull. 2007;30:2001–2006. doi: 10.1248/bpb.30.2001. [DOI] [PubMed] [Google Scholar]
- 4.Sato K, Tsuchiya M, Saldanha J, et al. Reshaping a human antibody to inhibit the interleukin 6-dependent tumor cell growth. Cancer Res. 1993;53:851–856. [PubMed] [Google Scholar]
- 5.Mihara M, Kasutani K, Okazaki M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and soluble interleukin-6 receptor, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol. 2005;5:1731–1740. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Nishimoto N, Miyasaka N, Yamamoto K, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): Significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol. 2009;19:12–19. doi: 10.1007/s10165-008-0125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): Evidence of clinical and radiographic benefit from an x ray reader-blinded randomised, controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–1167. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: Tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 10.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): A double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 11.Kremer JL, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate at 1 year: The LITHE study. Arthritis Rheum. 2010 doi: 10.1002/art.30158. November 19. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: Results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamanaka H, Tanaka Y, Inoue E, et al. Efficacy and tolerability of tocilizumab in rheumatoid arthritis patients seen in daily clinical practice in Japan: Results from a retrospective study (REACTION study) Mod Rheumatol. 2010 doi: 10.1007/s10165-010-0366-7. October 16. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirabayashi Y, Ishii T, Harigae H. Clinical efficacy of tocilizumab in patients with active rheumatoid arthritis in real clinical practice. Rheumatol Int. 2010;30:1041–1048. doi: 10.1007/s00296-009-1095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishimoto N, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Azuma J. Long-term safety and efficacy of tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): Evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. 2009;68:1580–1584. doi: 10.1136/ard.2008.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishimoto N, Takagi N. Assessment of the validity of the 28-joint disease activity score using erythrocyte sedimentation rate (DAS28-ESR) as a disease activity index of rheumatoid arthritis in the efficacy evaluation of 24-week treatment with tocilizumab: Subanalysis of the SATORI study. Mod Rheumatol. 2010;20:539–547. doi: 10.1007/s10165-010-0328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funahashi K, Koyano S, Miura T, Hagiwara T, Okuda K, Matsubara T. Efficacy of tocilizumab and evaluation of clinical remission as determined by CDAI and MMP-3 level. Mod Rheumatol. 2009;19:507–512. doi: 10.1007/s10165-009-0203-z. [DOI] [PubMed] [Google Scholar]
- 18.Smolen JS, Aletaha D. Interleukin-6 receptor inhibition with tocilizumab and attainment of disease remission in rheumatoid arthritis: The role of acute-phase reactants. Arthritis Rheum. 2011;63:43–52. doi: 10.1002/art.27740. [DOI] [PubMed] [Google Scholar]
- 19.Nishimoto N, Ito K, Takagi N. Safety and efficacy profiles of tocilizumab monotherapy in Japanese patients with rheumatoid arthritis: Meta-analysis of six initial trials and five long-term extensions. Mod Rheumatol. 2010;20:222–232. doi: 10.1007/s10165-010-0279-5. [DOI] [PubMed] [Google Scholar]
- 20.Van Vollenhoven RF, Keystone EC, Furie R, Blesch A, Wang C, Curtis JR. Gastrointestinal safety in patients with rheumatoid arthritis treated with tocilizumab: Data from Roche clinical trials. Abstr 1613. Arthritis Rheum. 2009;60(Suppl):S602. [Google Scholar]
- 21.Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54:2817–2829. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield V, Dhillon S, Plosker GL. Tocilizumab: A review of its use in the management of rheumatoid arthritis. Drugs. 2009;69:609–632. doi: 10.2165/00003495-200969050-00007. [DOI] [PubMed] [Google Scholar]
- 23.Van Vollenhoven RF, Scali JJ, Curtis JR, et al. Safety of tocilizumab in patients with rheumatoid arthritis: Analysis of median of 3.1 years of treatment in long-term extension studies; Poster SAT0172 presented at the European League against Rheumatism Annual European Congress of Rheumatology; Rome, Italy. June 16–19, 2010. [Google Scholar]
- 24.Van Gameren MM, Willemse PH, Mulder NH, et al. Effects of recombinant human interleukin-6 in cancer patients: A phase I-II study. Blood. 1994;84:1434–1441. [PubMed] [Google Scholar]
- 25.Weber J, Yang JC, Topalian SL, et al. Phase I trial of subcutaneous interleukin-6 in patients with advanced malignancies. J Clin Oncol. 1993;11:499–506. doi: 10.1200/JCO.1993.11.3.499. [DOI] [PubMed] [Google Scholar]
- 26.Vis M, Nurmohamed MT, Wolbink G, et al. Short term effects of infliximab on the lipid profile in patients with rheumatoid arthritis. J Rheumatol. 2005;32:252–255. [PubMed] [Google Scholar]
- 27.Dursunoğlu D, Evrengül H, Polat B, et al. Lp(a) lipoprotein and lipids in patients with rheumatoid arthritis: Serum levels and relationship to inflammation. Rheumatol Int. 2005;25:241–245. doi: 10.1007/s00296-004-0438-0. [DOI] [PubMed] [Google Scholar]
- 28.Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: A challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68:460–469. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- 29.Hashizume M, Yoshida H, Koike N, Suzuki M, Mihara M. Overproduced interleukin 6 decreases blood lipid levels via upregulation of very- low-density lipoprotein receptor. Ann Rheum Dis. 2010;69:741–746. doi: 10.1136/ard.2008.104844. [DOI] [PubMed] [Google Scholar]
- 30.Popa C, Netea MG, Radstake T, et al. Influence of anti-tumour necrosis factor therapy on cardiovascular risk factors in patients with active rheumatoid arthritis. Ann Rheum Dis. 2005;64:303–305. doi: 10.1136/ard.2004.023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 32.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: Correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–234. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 34.Maruotti N, Cantatore FP, Crivellato E, Vacca A, Ribatti D. Angiogenesis in rheumatoid arthritis. Histol Histopathol. 2006;21:557–566. doi: 10.14670/HH-21.557. [DOI] [PubMed] [Google Scholar]
- 35.Nakahara H, Song J, Sugimoto M, et al. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48:1521–1529. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 36.Hashizume M, Hayakawa N, Suzuki M, Mihara M. IL-6/soluble interleukin-6 receptor trans-signalling, but not TNF-alpha induced angiogenesis in a HUVEC and synovial cell co-culture system. Rheumatol Int. 2009;29:1449–1454. doi: 10.1007/s00296-009-0885-8. [DOI] [PubMed] [Google Scholar]
- 37.Uchiyama Y, Yorozu K, Hashizume M, Moriya Y, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, ameliorates joint swelling in established monkey collagen-induced arthritis. Biol Pharm Bull. 2008;31:1159–1163. doi: 10.1248/bpb.31.1159. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki M, Hashizume M, Yoshida H, Mihara M. Anti-inflammatory mechanism of tocilizumab, a humanized anti-IL-6R antibody: Effect on the expression of chemokine and adhesion molecule. Rheumatol Int. 2010;30:309–315. doi: 10.1007/s00296-009-0953-0. [DOI] [PubMed] [Google Scholar]
- 39.Mihara M, Moriya Y, Kishimoto T, Ohsugi Y. Interleukin-6 (IL-6) induces the proliferation of synovial fibroblastic cells in the presence of soluble IL-6 receptor. Br J Rheumatol. 1995;34:321–325. doi: 10.1093/rheumatology/34.4.321. [DOI] [PubMed] [Google Scholar]
- 40.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 2008;47:1635–1640. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 41.Sagawa A. The effect of short term treatment of anti-IL-6 receptor antibody tocilizumab on signs and symptoms and synovial inflammation in patients with active rheumatoid arthritis. Ann Rheum Dis. 2010;69(Suppl 3):539. [Google Scholar]
- 42.Fujikawa Y, Shingu M, Torisu T, Itonaga I, Masumi S. Bone resorption by tartrate-resistant acid phosphatase-positive multinuclear cells isolated from rheumatoid synovium. Br J Rheumatol. 1996;35:213–217. doi: 10.1093/rheumatology/35.3.213. [DOI] [PubMed] [Google Scholar]
- 43.Gravallese EM, Manning C, Tsay A, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–258. doi: 10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 44.Lacey DL, Timms E, Tan HL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 45.Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 46.Takayanagi H, Iizuka H, Juji T, et al. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–269. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 47.Cohen SB, Dore RK, Lane NE, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: A twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–1309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 48.Hashizume M, Mihara M. Desirable effect of combination therapy with high molecular weight hyaluronate and nonsteroidal anti-inflammatory drugs on MMP production. Osteoarth Cartil. 2009;17:1513–1518. doi: 10.1016/j.joca.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki M, Hashizume M, Yoshida H, Shiina M, Mihara M. IL-6 and IL-1 synergistically enhanced the production of MMPs from synovial cells by up-regulating IL-6 production and IL-1 receptor I expression. Cytokine. 2010;51:178–183. doi: 10.1016/j.cyto.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto J, Garnero P, van der Heijde D, et al. Humanized anti-interleukin-6-receptor antibody (tocilizumab) monotherapy is more effective in slowing radiographic progression in patients with rheumatoid arthritis at high baseline risk for structural damage evaluated with levels of biomarkers, radiography, and BMI: Data from the SAMURAI study. Mod Rheumatol. 2010 Jun 24; doi: 10.1007/s10165-010-0325-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garnero P, Thompson E, Woodworth T, Smolen JS. Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate. Arthritis Rheum. 2010;62:33–43. doi: 10.1002/art.25053. [DOI] [PubMed] [Google Scholar]
- 52.Kawashiri SY, Kawakami A, Iwamoto N, et al. Switching to the anti-interleukin-6 receptor antibody tocilizumab in rheumatoid arthritis patients refractory to antitumor necrosis factor biologics. Mod Rheumatol. 2010;20:40–45. doi: 10.1007/s10165-009-0235-4. [DOI] [PubMed] [Google Scholar]
- 53.Baer AN, Dessypris EN, Goldwasser E, Krantz SB. Blunted erythropoietin response to anemia in rheumatoid arthritis. Br J Haematol. 1987;66:559–564. doi: 10.1111/j.1365-2141.1987.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 54.Hochberg MC, Arnold CM, Hogans BB, Spivak JL. Serum immuno-reactive erythropoietin in rheumatoid arthritis: Impaired response to anemia. Arthritis Rheum. 1988;31:1318–1321. doi: 10.1002/art.1780311016. [DOI] [PubMed] [Google Scholar]
- 55.Jongen-Lavrencic M, Peeters HR, Wognum A, Vreugdenhil G, Breedveld FC, Swaak AJ. Elevated levels of inflammatory cytokines in bone marrow of patients with rheumatoid arthritis and anemia of chronic disease. J Rheumatol. 1997;24:1504–1509. [PubMed] [Google Scholar]
- 56.Voulgari PV, Kolios G, Papadopoulos GK, Katsaraki A, Seferiadis K, Drosos AA. Role of cytokines in the pathogenesis of anemia of chronic disease in rheumatoid arthritis. Clin Immunol. 1999;92:153–160. doi: 10.1006/clim.1999.4736. [DOI] [PubMed] [Google Scholar]
- 57.Uchiyama Y, Koike N, Mihara M. Anemia in monkey collagen-induced arthritis is correlated with serum IL-6, but not TNF alpha. Rheumatol Int. 2008;28:879–883. doi: 10.1007/s00296-008-0547-2. [DOI] [PubMed] [Google Scholar]
- 58.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 59.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106:2196–2199. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci U S A. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hashizume M, Uchiyama Y, Horai N, Tomosugi N, Mihara M. Tocilizumab, a humanized anti-interleukin-6 receptor antibody, improved anemia in monkey arthritis by suppressing IL-6-induced hepcidin production. Rheumatol Int. 2010;30:917–923. doi: 10.1007/s00296-009-1075-4. [DOI] [PubMed] [Google Scholar]
- 63.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 64.Iwanami K, Matsumoto I, Tanaka-Watanabe Y, et al. Crucial role of the interleukin-6/interleukin-17 cytokine axis in the induction of arthritis by glucose-6-phosphate isomerase. Arthritis Rheum. 2008;58:754–763. doi: 10.1002/art.23222. [DOI] [PubMed] [Google Scholar]
- 65.Fujimoto M, Serada S, Mihara M, et al. Interleukin-6 blockade suppresses autoimmune arthritis in mice by the inhibition of inflammatory Th17 responses. Arthritis Rheum. 2008;58:3710–3719. doi: 10.1002/art.24126. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida H, Hashizume M, Mihara M. IL-6 blockade preferentially inhibits Th17 differentiation in collagen-induced arthritis. Rheumatol Int. 2011;31:127–131. doi: 10.1007/s00296-010-1552-9. [DOI] [PubMed] [Google Scholar]
- 67.Notley CA, Inglis JJ, Alzabin S, McCann FE, McNamee KE, Williams RO. Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for Th1 and Th17 cells. J Exp Med. 2008;205:2491–2497. doi: 10.1084/jem.20072707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008;20:1361–1368. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
- 69.Zhang C, Zhang J, Yang B, Wu C. Cyclosporin A inhibits the production of IL-17 by memory Th17 cells from healthy individuals and patients with rheumatoid arthritis. Cytokine. 2008;42:345–352. doi: 10.1016/j.cyto.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Papagoras C, Voulgari CV, Drosos AA. Strategies after the failure of the first anti-tumor necrosis factor α agent in rheumatoid arthritis. Autoimmun Rev. 2010;9:574–582. doi: 10.1016/j.autrev.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Radstake TR, Svenson M, Eijsbouts AM, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009;68:1739–1745. doi: 10.1136/ard.2008.092833. [DOI] [PubMed] [Google Scholar]
- 72.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. J Am Med Assoc. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 73.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328–346. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 74.Okuda Y, Takasugi K. Successful use of a humanized anti-interleukin-6 receptor antibody, tocilizumab, to treat amyloid A amyloidosis complicating juvenile idiopathic arthritis. Arthritis Rheum. 2006;54:2997–3000. doi: 10.1002/art.22118. [DOI] [PubMed] [Google Scholar]
- 75.Husby G. Treatment of amyloidosis and the rheumatologist. State of the art and perspectives for the future. Scand J Rheumatol. 1998;27:161–165. doi: 10.1080/030097498440750. [DOI] [PubMed] [Google Scholar]
- 76.Del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 77.Hagihara K, Nishikawa T, Isobe T, Song J, Sugamata Y, Yoshizaki K. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Commun. 2004;314:363–369. doi: 10.1016/j.bbrc.2003.12.096. [DOI] [PubMed] [Google Scholar]
- 78.Van Vollenhoven RF, Nishimoto N, Yamanaka H, et al. Experience with mycobacterium tuberculosis infection reported in the tocilizumab worldwide RA safety database. Ann Rheum Dis. 2009;68(Suppl 3):557. [Google Scholar]
- 79.Ogata A, Mori M, Hashimoto S, et al. Minimal influence of tocilizumab on IFN-gamma synthesis by tuberculosis antigens. Mod Rheumatol. 2010;20:130–133. doi: 10.1007/s10165-009-0243-4. [DOI] [PubMed] [Google Scholar]
- 80.Ohsugi Y, Kishimoto T. The recombinant humanized anti-IL-6 receptor antibody tocilizumab, an innovative drug for the treatment of rheumatoid arthritis. Expert Opin Biol Ther. 2008;8:669–681. doi: 10.1517/14712598.8.5.669. [DOI] [PubMed] [Google Scholar]