Abstract
Molecular richness of snake venoms is an important source of proteins and toxins with potent effects on the cardiovascular system. The alteration of the vascular system in the victim after a venomous snake bite is usually expressed by a significant decrease in blood pressure. Therefore, exploring snake venom to extract and characterize its biomolecules is of considerable medical interest, and formed the basis of this study. We assessed the potential of the venom of Montivipera bornmuelleri, a viper from Lebanon, to induce relaxant effect on isolated Wistar rat aorta via several mechanisms of action. The overall hypotensive effect of Montivipera bornmuelleri venom results from its synergetic action on different channels for the reduction of blood pressure. By actions of its metalloproteinases and phospholipase A2, the venom may induce the production of nitric oxide acting accordingly a vasodilator effect. It could act on the voltage-dependent potassium channels and/or the L-type calcium channels, inhibiting angiotensin converting enzyme and/or inhibiting the α1-adrenoceptors. This work demonstrates vasorelaxant effect of the Montivipera bornmuelleri venom acting on different pathways, reducing blood pressure.
Keywords: Montivipera bornmuelleri, snake venom, cardiovascular, blood pressure, aortic rings, vasorelaxant, calcium channels
Introduction
Snake venoms contain a range of biomolecules that can remodel the cardiovascular activity. The site of action of these molecules comprises the cardiac muscle, vascular smooth muscle and vascular capillary bed. Thus, following envenomation, a number of varying intensity symptoms may occur simultaneously and are expressed clinically by hypotension, vasodilation, vasoconstriction, arrhythmia, tachycardia, violent contractions of the heart, a collapse and possible cardiac arrest. The remodelling of the vascular activity in the victim after a venomous snake bite is usually expressed by a significant decrease in blood pressure (Schaeffer et al, 1984; de Mesquita et al, 1991). This hypotension is either due to the direct effects of specific antihypertensive agents present in the venom or indirect effects of pulmonary vascular obstruction and coronary ischemia (Tibballs, 1998). Direct hypotensive agents are of significant interest in the pharmaceutical field because they can form the basis of drug development for the treatment of hypertension. These include: (i) Bradykinin potentiating peptide (BPP); it is endogenous molecule with powerful hypotensive effect due to vasodilation and increased capillary permeability. It is a proteolytic product of the plasma protein kininogen degraded by angiotensin converting enzyme that also cleaves angiotensin I into a potent vasoconstrictor, angiotensin II (Moreau et al, 2005). BPP was detected for the first time in the venom of Bothrops jararaca 1965 (Ferreira, 1965): (ii) Natriuretic peptides (NPs); these are released in response to a myocardial overload and cause a variety of effects, such as diuresis and natriuresis reducing the absorption of water in the kidney and the blood volume. NPs also induce vasodilation and are involved in the balancing of the renin-angiotensin-aldosterone system involved in maintaining blood volume and pressure. Since, the NPs are considered as important regulators of the cardiovascular and urinary systems, they are then explored as potential therapeutic agents for the problems of high blood pressure and heart failure. Snake venom is one of the main sources of exogenous NP. The first NP venom was identified from Eastern green mamba and called Dendroaspis natriuretic peptide (DNP) (Schweitz et al, 1992): (iii) Blockers of L-type Calcium channel; several blockers of L-type calcium channels have been identified from snake venoms, such as calciseptine (de Weille et al, 1991) and FS2 toxins isolated from the venom Dendroaspis polylepispolylepis (Strydom, 1977; Yasuda et al, 1994): (iv) Vascular endothelial growth factors (VEGFs); they are angiogenic and lymphangiogenic regulatory proteins well-known, whose role in the body is to trigger formation of new blood and lymph vessels from existing vessels needed for the growth of tissues and development of the human body. The activity of VEGFs-F, isolated from snake venoms of Vipera ammodytes ammodytes and Daboia russelli, respectively (Yamazaki et al, 2003) showed a strong hypotensive effect in rats by promoting vascular permeability and can improve the proliferation of vascular endothelial cells (Cominetti et al, 2004): (v) Sarafotoxin; it is one of the strongest snake toxins (LD50 of about 15 mg/kg in mice, intravenous route) and highly similar to endothelins which are naturally occurring proteins in very small quantities in mammals and bind to receptors on smooth muscle cells of various vital organs (intestine, uterus, blood vessels, etc.): (vi) Antagonists of α-adrenergic receptors; adrenergic receptors are a class of receptors coupled to G proteins that bind catecholamines, especially norepinephrine and epinephrine. According to their sequence similarity, their pharmacology and signaling mechanisms, these receptors are classified into three groups (Ahlquist, 1948). These three classes are α-1, α-2 and β. The α1-adrenergic receptors (α1-AR) are found in both the central and peripheral nervous system. In the peripheral system, they produce a contraction and are located on the vascular smooth muscles and non-vascular muscles.
Our main work focused on the venom of Montivipera bornmuelleri, venomous viper species, endemic in the Lebanese mountains. In our previous studies, we gave evidence of rich and complex protein content of its venom (Hraoui-Bloquet et al, 2012; Accary et al, 2014) and successfully isolated and partially characterized a phospholipase A2 (PLA2) (Accary et al, 2015), and an L-amino acid oxidase (LAAO) (Rima et al, 2013) enzymes. We have also investigated the biological activities of this venom showing potent antimicrobial action against resistant and non-resistant bacterial strains (Accary et al, 2014a). We also demonstrated its dual effect – as an inhibitor and an activator – on the blood coagulation cascade without having any hemolytic effect (Accary et al, 2014b). Here, we continue the exploration of the biological properties of the venom of Montivipera bornmuelleri, with the aim to study its effect on the isolated aortas and thus evaluate its impact on vascular contractility.
Material and Methods
Vascular reactivity
Wistar rats from either gender weighting 400-550gm were used. Rats were anesthetized with sodium pentobarbital (70mg/kg) intraperitoneally and the thoracic aorta was quickly removed, gently cleaned of adhering fat and connective tissues, taking care not to damage the endothelium and cut in rings. Ring segments (3-5mm) were mounted between two stainless steel triangle wires in 10ml organ bath filled with modified Krebs solution under passive tension of 2gm for about 1hr. The Krebs solution had the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.17, NaHCO3 20 and glucose 11. The rings were maintained at 37°C and gassed with a 95% (v/v) O2 and 5% (v/v) CO2 mixture (pH=7.4). Changes in isometric tension were recorded on a data acquisition system aided with force-displacement transducers (4.1 BIOPAC MP150 system, CEROM, France). In all experiments after 1hr of equilibration period aortic rings were challenged with KCl (80mM) to assure the good contractile condition of the preparations. The functionality of the endothelium was verified by obtaining at least 60% relaxation to acetylcholine (muscarinic receptor agonist: 1μM) in the aortic rings pre-contracted with phenylephrine, a selective agonist α1-adrenergic (0.3μM). During the equilibration period and after the tests KCL and functionality of the endothelium, the rings were washed every 15min with fresh Krebs solution.
Evaluation of the vasorelaxant effect of the Montivipera bornmuelleri venom
After 1hr equilibration, aortic rings precontracted using phenylephrine (1μM) or KCl (80mM) and once the plateau was obtained, a concentration-response curve of venom (0.05ng/ml to 50μg/ml) was established to evaluate the venom vasorelaxant effect. The same protocol was repeated on rings incubated for 30min, separately or in combination, with the following inhibitors: L-nitroarginine methyl ester (L-NAME) (10μM): an inhibitor of nitric oxide synthase or NOS; tetraethyl ammonium (TEA) (10μM): a potassium channel inhibitor; enalaprilate: an inhibitor of angiotensin converting enzyme and NS398 (10μM): inhibitor of cyclo-oxygenase 2. Moreover, this effect was also evaluated on their freed rings endothelium.
Venom effect on the contraction induced by calcium
After 60min equilibration period in the normal Krebs solution, the aortic rings were incubated for 30min in Ca2+ free Krebs solution plus 0.1mM ethylene diamine tetra acetic acid (EDTA). After adding KCl (60mM), dose-response curve to calcium was obtained on control. The same protocol was repeated on the same aortic rings after washing and incubated for 30min with venom (50μg/ml and 5μg/ml) or nicardipine (0.1μM).
Venom effect on the contraction induced by phenylephrine
Once equilibrated in Krebs, the rings were stimulated by phenylephrine (from 1nM to 0.1mM) to obtain a concentration-response curve control. The same procedure was repeated after 30min incubation with the venom (5μg/ml).
Venom effect on the contraction induced by angiotensin I (Ang I)
After an equilibration period of 1hr, different concentrations of AngI (from 1nM to 1mM) were added following in the tanks for a curve control concentration-response AngI. The same procedure was repeated after incubating the rings with the venom (5μg/ml) or of enalaprilate (10μM) for 30min.
Chemicals
Sodium pentobarbital was purchased at Ceva Animal Health (France), phenylephrine hydrochloride, acetylcholine chloride, L-Name, TEA, enalaprilate, the NS398 and AngI were obtained from Sigma-Aldrich (France). All products were prepared as stock solutions in distilled water, except for indomethacin and NS398 of enalaprilate that were dissolved in dimethylsulfoxide (DMSO, Sigma-Aldrich). All other reagents such as components of the Krebs and anhydrous calcium chloride are also obtained from Sigma-Aldrich (France).
Statistical Analysis
The cumulative curves of concentration-relaxation and concentration-contraction are compared as a whole, by an ANOVA (Analysis of Variance). In the situation where significant differences between the curves appear, we carry in addition to Dunnett's multiple comparison tests. These statistical studies are performed with the PRISM software. Whatever the statistical model used, a value of p<0.05 was accepted for the differences between the data are considered significant.
Results
Vasorelaxant effect of the venom of Montivipera bornmuelleri
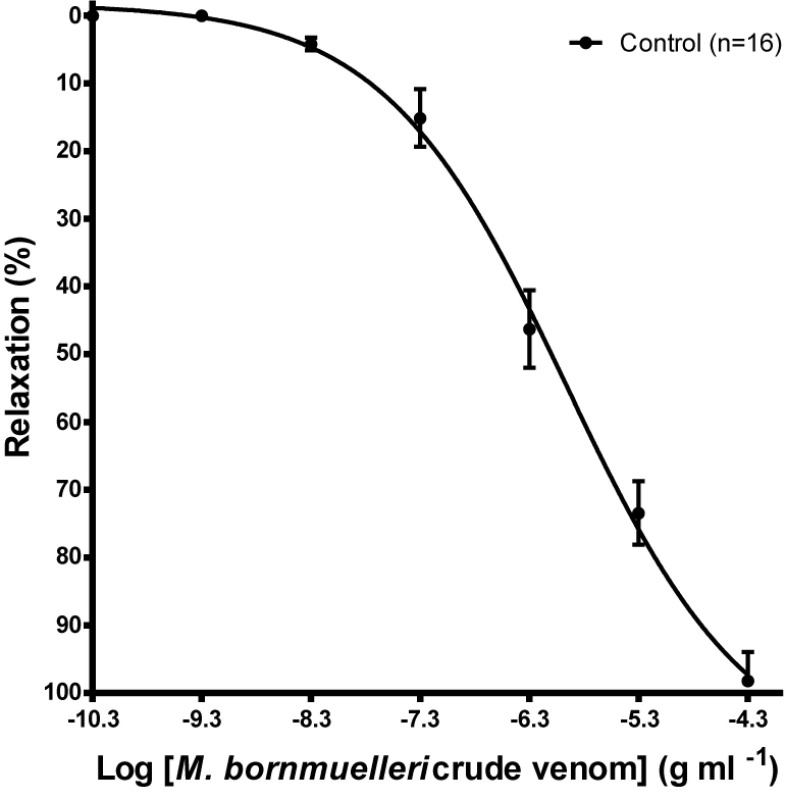
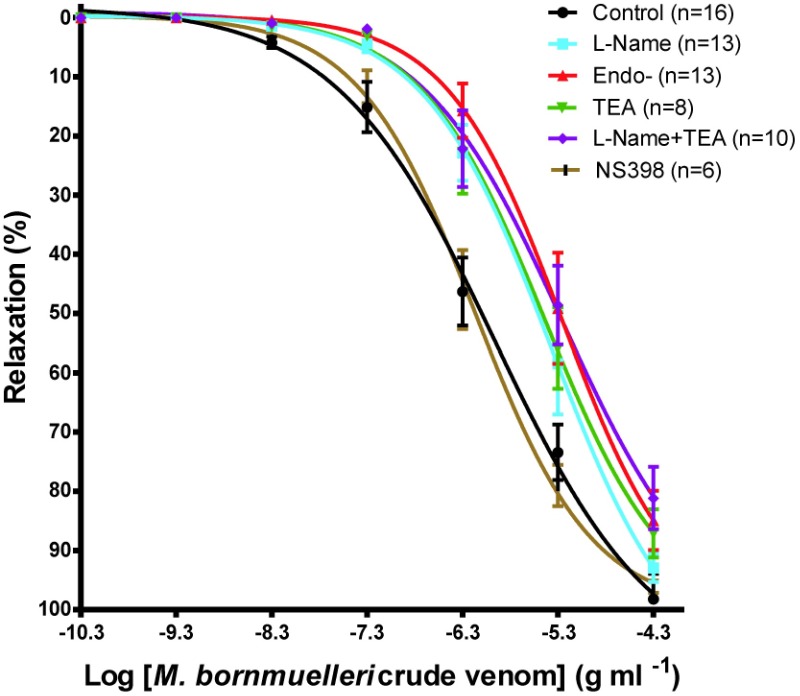
The Montivipera bornmuelleri venom produced a concentration-dependent relaxing effect on K+ depolarized smooth muscle. The maximum effect (Emax) obtained for the highest concentration (50μg/ml) is 98.22% ±4.232 (Figure 1). Except for NS398, endothelium suppression and preincubation of the K+ depolarized tissues with L-NAME, TEA, L-NAME / TEA resulted in a non parallel shift to the right of the dose response curves (Figure 2) with significant reduction in the global relaxation response to the venom. The relaxation percentage obtained for the highest concentration of venom were, respectively, 92.97% ±2.360 (n=13), 87.132% ±4.07 (n=8), 81.150% ±5.252 (n=10), 99.99% ±0.006 (n=6), 84.937 ±5.021% (n=13).
Figure 1.
Relaxant effect of the venom of Montivipera bornmuelleri in isolated aortic rings from Wistar rats precontracted with phenylephrine (1μM). Data are expressed as mean±SEM (n=16).
Figure 2.
Cumulative concentration-relaxation curves to the venom of Montivipera bornmuelleri in isolated aortic rings from Wistar rat pre-incubated with various antagonists. Data are presented as mean±SEM, p<0.05 for each curve versus control except for the presence of NS398.
Effect of the Montivipera bornmuelleri venom on the contraction induced by CaCl2
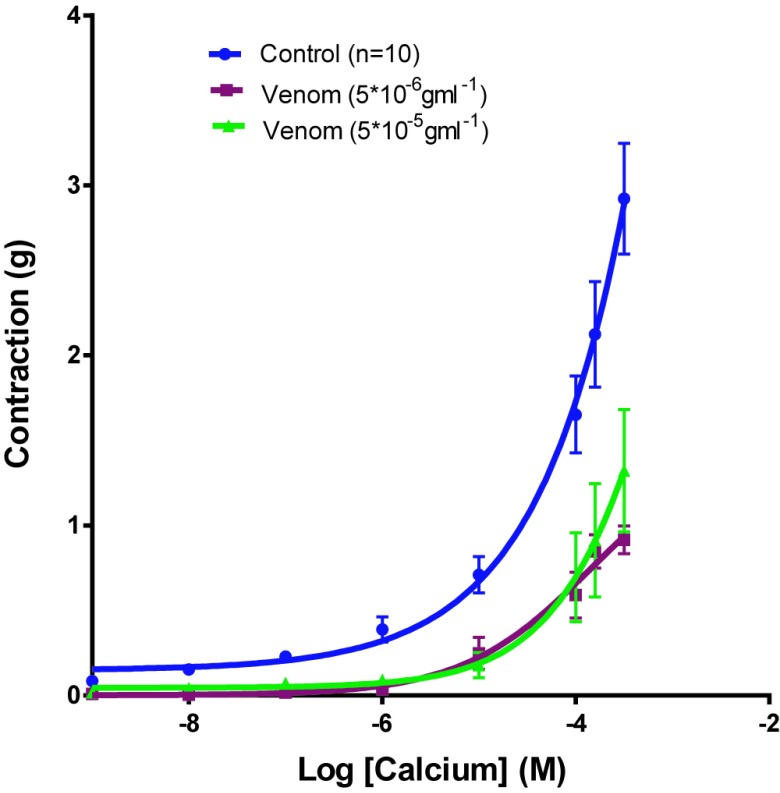
CaCl2 induced contractile concentration-dependent effect on vascular smooth muscle depolarized by an enhanced K+ solution. The pre-incubation of these tissues with the venom and nicardipine caused deviation to the right of the concentration-response curves CaCl2 (Figure 3) and a significant reduction of the contraction with an Emax of 0,917gm±0.081 with a venom concentration of 5x10-6gm/ml (n=6, p<0.0001 vs. control), 1,323g±0.36 with a venom concentration of 5x10-5gm/ml (n=6, p<0.0001 vs. control) and 0,203gm±0.053 for nicardipine (n=6, p<0.0001 vs control).
Figure 3.
Cumulative concentration-contraction curves to CaCl2 in isolated aortic rings from Wistar rat or pre-incubated with two different concentrations of the Montivipera bornmuelleri venom and with nicardipine. Data are presented as mean±SEM ****p<0.0001 vs venom control.
Effect of the Montivipera bornmuelleri venom on cumulative contraction induced by phenylephrine
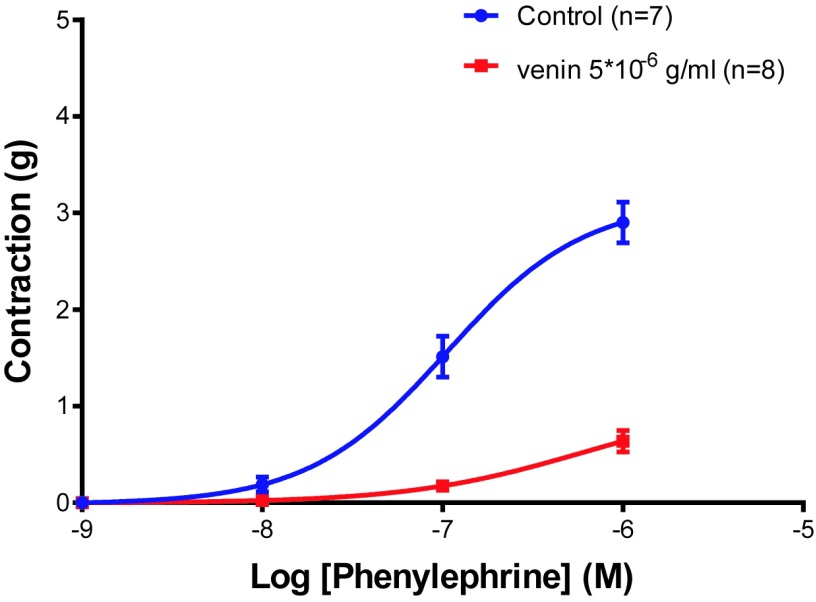
The phenylephrine induced a dose-dependent contractile effect (Emax=2.902±0.211gm, n=7). The contraction was significantly decreased (Figure 4) in rings incubated with a concentration of 5x10-6gm/ml venom (Emax =0.638±0.111, n=8, p<0.0001).
Figure 4.
Cumulative concentration-contraction curves to phenylephrine in aortic rings isolated from Wistar rats (control) or preincubated with two different concentrations of the Montivipera bornmuelleri venom. ****p<0.0001 vs venom control.
Effect of the Montivipera bornmuelleri venom on the cumulative concentration-contraction curve obtained with AngI
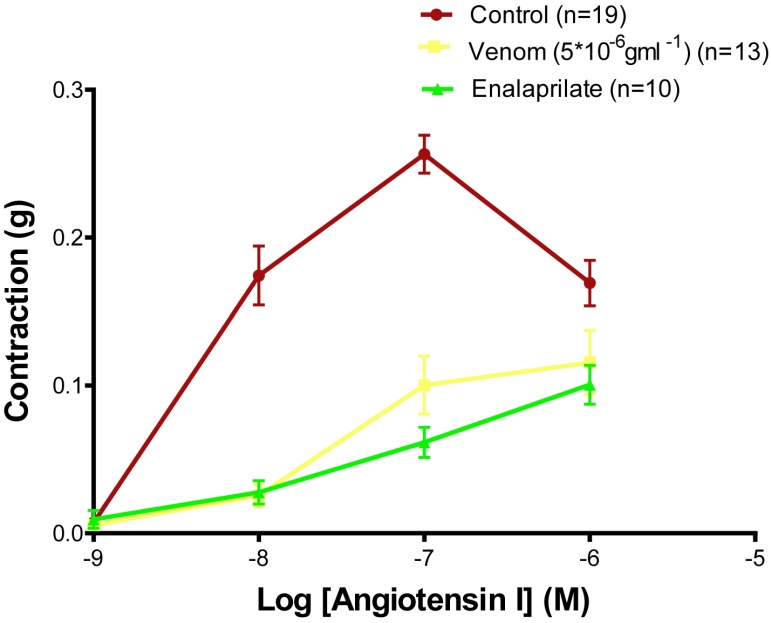
The effect of the Montivipera bornmuelleri venom and enalaprilate – an angiotensin converting enzyme (ACE) inhibitor – on the vascular contraction induced by AngI was evaluated by performing a cumulative concentration-contraction curve on rat aortic rings. The contractile response to AngI was significantly reduced after incubation with the venom of the rings and of enalaprilate (Figure 5). The maximum effect of AngI (Emax=0.256±0.014gm, n=13) is reduced in the presence of venom (Emax=0.116±0.022gm, n=13, p<0.0001), and enalaprilate (Emax = 0.104±0.01gm, n=10, p<0.0001). However, the difference between the effect of the venom and enalaprilate on the contractility induced by AngI is not significant.
Figure 5.
Cumulative concentration-contraction curves to AngI in aortic rings isolated from Wistar rats (Control) or preincubated with the Montivipera bornmuelleri venom and enalaprilate. Data are presented as mean±SEM ****p<0.0001 vs venom and enalaprilate control.
Discussion
The Montivipera bornmuelleri venom shows a concentration-dependent decrease in vascular contractility. It affects aortic rings producing a depressing effect of the contraction induced by phenylephrine. This effect may involve one or more mechanisms of action by acting either directly on the receptors themselves, or on signaling pathway of NO and angiotensin II.
The endothelium plays an important role in the control of vascular tone, and acts as a source of many chemical mediators. Endothelial cells have a particular ability to secrete and to synthesize vasoactive substances which control blood flow such as nitric oxide (NO), prostacyclin and endothelin. The nitric oxide synthase (NOS) is an enzyme protein which synthesizes NO and is involved in the control of the vasomotor in inhibiting smooth muscle contraction. The action of the Montivipera bornmuelleri venom on this pathway was therefore investigated on rings preincubated with L-Name, a blocker of NOS and on rings without endothelium. A deviation to the right of cumulative concentration-relaxation curves is observed with a significant difference in the vasorelaxant response showing the effect of the venom to induce the release of chemical mediators in particular vasodilators NO. However, the Montivipera bornmuelleiri venom does not affect prostacyclin production since the inhibition of cyclooxygenase-2 by the NS398 had not effect on the vasorelaxant response. The action of the venom, altered by L-NAME and the removal of the endothelium, correlates with its induced inflammatory activity mainly through interaction of metalloproteinases and PLA2 with endothelial cells (Teixeira et al, 2003; Schattner et al, 2005; Accary et al, 2015). In addition, the comparison of the maximum response obtained in the different conditions compared to control shows no significant change. This fact may suggest the intervention of other pathways through which the venom exerts its vasorelaxant effect. The involvement of potassium channels in the vasorelaxant action of the venom was also evaluated using an antagonist of these channels, the TEA. The opening of potassium channels in smooth muscle cells results in an increase in K+ permeability and thereafter a K+ efflux. The efflux of K+ from cells induced hyperpolarization of the membrane and therefore the closure of voltage gated calcium channels, resulting in vasorelaxation. Pre-incubation with TEA could effectively oppose the effect of the Montivipera bornmuelleri venom whereas simultaneous blockage of NOS and potassium channels result in a more effective inhibition of the relaxation response induced by the venom when compared versus blocking them separately. This observation reinforces the fact that the venom exerts its action by acting on several targets.
In addition, inhibition of the vascular contraction induced by Ca2+ suggests that the Montivipera bornmuelleri venom reduced the Ca2+ influx through voltage-dependent calcium channels in smooth muscle of the isolated aorta. However, the Ca2+ influx through the L-type calcium channels is not the only source which increases the concentration of intracellular calcium. It can also be released from intracellular stores of the sacroplasmic reticulum and/or other types of calcium channels. We have also found that the venom may interfere with α1-adrenergic receptors by significantly inhibiting the vasoconstrictor effect of phenylephrine. Since α-Adrenergic receptors antagonists have been already isolated from many snake venoms, we could suggest the presence of some proteins in the Montivipera bornmuellei venom that interacts with phenylephrine at the level of the α1-adrenoceptors.
Factors potentiating the bradykinin effect have been detected for the first time in the venom of Bothrops jararaca (Ferreira, 1965). These proteins inhibit the ACE allowing a reduction in blood pressure (Ferreira et al, 1970; Lopes et al, 2014). The presence of such a homologue in the Montivipera bornmuelleri venom was investigated by performing cumulative concentration-contraction curves to AngI in aortic rings preincubated with venom and enalaprilate. Compared to the control, the venom significantly inhibits the contraction induced by AngI by acting on the converting enzyme. Such result must be confirmed via cumulative concentration-contraction curves to angiotensin II in aortic rings preincubated with venom of Montivipera bornmuelleri.
Conclusions
Overall most Viperidae venoms lower blood pressure after envenomation. Intravenous injection of venoms of Vipera palaestinae, Crotalus durissus Daboia russelli and cascavella also cause an immediate drop in blood pressure (Bicher et al, 1966; Evangelista et al, 2011). Our work demonstrates the vasorelaxant effect of the Montivipera bornmuelleri venom. Via its metalloproteinases and its PLA2, the venom may induce the production of nitric oxide acting accordingly as vasodilator agent. It could act on the voltage-dependent potassium channels and/or the L-type calcium channels, and may inhibit the ACE and/or the α1-adrenoceptors. Our data demands further studies on the isolation and identification of substances responsible for the vasorelaxant action of venom in particular those involved in the inhibition of calcium channel and the phenylephrine antagonism. These biomolecules can be potential candidates for the development of new pharmacological tools and the design of vasorelaxant new drugs.
Acknowledgments
We would like to thank the National Council for Scientific Research (CNRS-Lebanon) and Francophone University Agency (AUF) for the financial support and the funding of our work.
Abbreviations
- ACE
angiotensin converting enzyme
- α1-AR
α1-Adrenergic receptors
- AngI
Angiotensin I
- BPP
Bradykinin potentiating peptide
- DMSO
Dimethylsulfoxide
- DNP
Dendroaspis natriuretic peptide
- EDTA
Ethylene diamine tetra acetic acid
- LAAO
L-amino acid oxidase
- LD50
Lethal dose at 50%
- NPs
Natriuretic peptides
- L-NAME
L-Nitroarginine methyl ester
- NOS
Nitric oxide synthase
- PLA2
Phospholipases A2
- TEA
Tetraethyl ammonium
- VEGFs
Vascular endothelial growth factors
Competing Interest
None declared.
References
- Accary C, Hraoui-Bloquet S, Hamze M, et al. Protein content analysis and antimicrobial activity of the venom of Montivipera bornmuelleri, a viper from Lebanon. Inf Disord Drugs Targ. 2014a;14:49–55. doi: 10.2174/1871526514666140522114754. [DOI] [PubMed] [Google Scholar]
- Accary C, Rima M, Kouzayha A, et al. Effect of Montivipera bornmuelleri snake venom on human blood: coagulation disorders and hemolytic activities. Open J Hematol. 2014b;5:4. [Google Scholar]
- Accary C, Mantash A, Mallem Y, Fajloun Z, Elkak A. Separation and Biological Activities of Phospholipase A2 (Mb-PLA2) from the Venom of Montivipera bornmuelleri, a Lebanese Viper. J Liq Chroma Relat Technol. 2015;38:833–839. [Google Scholar]
- Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- Bicher HI, Roth M, Gitter S. Neurotoxic activity of Vipera palestinae venom. Depression of central autonomic vasoregulatory mechanisms. Med Pharmacol Exp Int J Exp Med. 1966;14:349–59. doi: 10.1159/000135805. [DOI] [PubMed] [Google Scholar]
- Cominetti MR, Terruggi CH, Ramos OH, et al. Alternagin-C, a disintegrin-like protein, induces vascular endothelial cell growth factor (VEGF) expression and endothelial cell proliferation in vitro. J Biol Chem. 2004;279:18247–18255. doi: 10.1074/jbc.M311771200. [DOI] [PubMed] [Google Scholar]
- de Mesquita LC, Selistre HS, Giglio JR. The hypotensive activity of Crotalus atrox (western diamondback rattlesnake) venom: identification of its origin. Am J Trop Med Hyg. 1991;44:345–353. doi: 10.4269/ajtmh.1991.44.345. [DOI] [PubMed] [Google Scholar]
- de Weille JR, Schweitz H, Maes P, Tartar A and Lazdunski M. Calciseptine, a peptide isolated from black mamba venom, is a specific blocker of the L-type calcium channel. Proc Natl Acad Sci USA. 1991;88:2437–2440. doi: 10.1073/pnas.88.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista JS, Evangelista JJ, Evangelista IL, et al. Hypotensive effects of the Crotalus durissus cascavella venom: involvement of NO. Nat Prod Commun. 2011;6:871–874. [PubMed] [Google Scholar]
- Ferreira SH, Bartelt DC, Greene LJ. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry. 1970;9:2583–2593. doi: 10.1021/bi00815a005. [DOI] [PubMed] [Google Scholar]
- Ferreira SH. A Bradykinin-Potentiating Factor (BPF) Present in the venom of Bothrops jararaca. Br J of Pharmacol and Chemother. 1965;24:163–169. doi: 10.1111/j.1476-5381.1965.tb02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hraoui-Bloquet S, Sadek R, Accary C, Hleihel W and Fajloun Z. An ecological study of the Lebanon Mountain viper Montivipera Bornmuelleri (Werner, 1898) with a preliminary biochemical characterisition of its venom. Leb Sci J. 2012;13:89–101. [Google Scholar]
- Lopes D, Junior N, Costa P, et al. A new structurally atypical bradykinin-potentiating peptide isolated from Crotalus durissus cascavella venom (South American rattlesnake) Toxicon. 2014;90:36–44. doi: 10.1016/j.toxicon.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- Rima M, Accary C, Haddad K, et al. Identification of L-Amino Acid Oxydase (Mb-LAAO) with antibacterial activity in the venom of Montivipera bornmueleri, a viper from Lebanon. Inf Disord and Drugs Targ. 2013;13:337–343. doi: 10.2174/187152651305140403122334. [DOI] [PubMed] [Google Scholar]
- Schaeffer RC, Jr, Briston C, Chilton SM, Carlson RW. Hypotensive and hemostatic properties of rattlesnake (Crotalus viridis helleri) venom and venom fractions in dogs. J Pharmacol Exp Ther. 1984;230:393–398. [PubMed] [Google Scholar]
- Schattner M, Fritzen M, Ventura JS, et al. The snake venom metalloproteases berythractivase and jararhagin activate endothelial cells. Biol Chem. 2005;386:369–374. doi: 10.1515/BC.2005.044. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps) J Biol Chem. 1992;267:13928–13932. [PubMed] [Google Scholar]
- Strydom DJ. Snake venom toxins. The amino-acid sequence of a short-neurotoxin homologue from Dendroaspis polylepis polylepis (black mamba) venom. Eur J Biochem. 1977;76:99–106. doi: 10.1111/j.1432-1033.1977.tb11574.x. [DOI] [PubMed] [Google Scholar]
- Tibballs J. The cardiovascular, coagulation and haematological effects of tiger snake (Notechis scutatus) prothrombin activator and investigation of release of vasoactive substances. Anaesth Intensive Care. 1998;26:536–547. [PubMed] [Google Scholar]
- Teixeira CFP, Landucci ECT, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon. 2003;42:947–962. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Takani K, Atoda H, Morita T. Snake venom vascular endothelial growth factors (VEGFs) exhibit potent activity through their specific recognition of KDR (VEGF receptor 2) J Biol Chem. 2003;278:51985–51988. doi: 10.1074/jbc.C300454200. [DOI] [PubMed] [Google Scholar]
- Yasuda O, Morimoto S, Jiang B, et al. FS2 a mamba venom toxin, is a specific blocker of the L-type calcium channels. Artery. 1994;21:287–302. [PubMed] [Google Scholar]