Abstract
While the pace of discovery of new agents, mechanisms and risk factors involved in drug-induced liver injury (DILI) remains brisk, advances in the treatment of acute DILI seems slow by comparison. In general, the key to treating suspected DILI is to stop using the drug prior to developing irreversible liver failure. However, predicting when to stop is an inexact science, and commonly used ALT monitoring is an ineffective strategy outside of clinical trials. The only specific antidote for acute DILI remains N-acetylcysteine (NAC) for acetaminophen poisoning, although NAC is proving to be beneficial in some cases of non-acetaminophen DILI in adults. Corticosteroids can be effective for DILI associated with autoimmune or systemic hypersensitivity features. Ursodeoxycholic acid, silymarin and glycyrrhizin have been used to treat DILI for decades, but success remains anecdotal. Bile acid washout regimens using cholestyramine appear to be more evidenced based, in particular for lefluonomide toxicity. For drug-induced acute liver failure, the use of liver support systems is still investigational in the United States and emergency liver transplant remains limited by its availability. Primary prevention appears to be the key to avoiding DILI and the need for acute treatment. Pharmacogenomics, including HLA genotyping and the discovery of specific DILI biomarkers offers significant promise for the future. This article describes and summarizes the numerous and diverse treatment and prevention modalities that are currently available to manage DILI.
Keywords: hepatotoxicity, DILI, prevention, treatment
Introduction: the challenges of treating drug-induced liver injury
The field of drug-induced hepatotoxicity has been expanding rapidly in terms of identifying mechanisms of injury, predicting patients at risk and recognizing pharmacologic properties of potential hepatotoxins. By comparison, research aimed specifically at treating DILI has lagged behind.[1] The management of acute (or chronic) drug-induced liver injury (DILI), including herbal and dietary supplement induced liver injury (HDSLI) has not changed appreciably since we reviewed this topic 15 years ago.[2] It still involves a process that begins with the recognition of hepatic injury and assigning causality to a specific agent.[3] After making a diagnosis of DILI, a decision must be made regarding whether or not the suspected drug can be continued with frequent monitoring to determine if tolerance or drug adaptation is present, or if the injury meets criteria where the drug needs to be stopped temporarily or permanently.[3] In addition to applying these stopping rules, the decision to treat the hepatic injury with a specific antidote (e.g. N-acetylcysteine (NAC) in the case of acetaminophen (APAP) overdose), an immunosuppressive agent (e.g corticosteroids for drug-induced hypersensitivity reactions or autoimmune injury), or to utilize non-specific hepatoprotective agents such as ursodiol, silibinin, glycyrrhizic acid , depends on the perceived severity of the hepatotoxicity. Although acute DILI remains a rare event, it can be dramatic when it happens and cases leading to jaundice may have a fatal outcome. Indeed, approximately 50% of all cases of acute liver failure (ALF) are caused by drugs.[4] While most drug induced ALF (DI-ALF) cases are due to APAP overdose, 12% are caused by other idiosyncratic drug reactions (including HDSLI), making DI-ALF more common than ALF attributable to acute viral hepatitis. If acute DI-ALF is present, urgent referral to a liver transplant (LT) center should be initiated without delay.[4] Alternatives to LT such as liver support devices in the form of molecular adsorbent recirculating systems (MARS), plasmapheresis and other modalities remain largely investigational in the U.S.[5], as will be discussed.
Given the significant case-fatality rate (or need for LT) of 10% and higher for acute DI-ALF,[6, 7] , and the relatively few specific medical therapies available for these cases, much attention has appropriately been focused on the primary prevention of DILI and HDSLI. The identification of pharmacogenetic risk factors associated with hepatotoxicity offers the opportunity to engage in a more precise, personalized medicine approach, whereby DILI can be predicted and therefore, avoided. [8, 9] Another tactic to prevent DILI has been through legislation and regulations that restrict the access to agents, most notably paracetamol in the UK and parts of the European Union.[10] While underlying cirrhosis or other chronic liver disease is generally not considered a significant host risk factor for DILI,[11] remaining vigilant to avoid potentially hepatotoxic agents (such as anti-tuberculosis drugs (ATDs) in patients with chronic viral hepatitis B or HIV), seems prudent [12]. Finally, while we await the identification of a biomarker that can accurately predict liver injury prior to its development,[13] we remain reliant on the old standard of alanine aminotransferase (ALT) testing to monitor for DILI. Measuring ALT levels is done in the hope of stopping a particular agent prior to crossing the threshold into ALF.[14] Indeed, certain medications mandate the testing of ALT at frequent intervals as part of a risk evaluation and management strategy (REMS), while others recommend only periodic monitoring. For certain well-recognized hepatotoxins, such as isoniazid (INH), reporting of clinical symptoms of acute hepatitis is given more weight than biochemical monitoring,[15] although this can have dire consequences if stopping criteria are ignored.[16]
In this review, we have endeavored to provide a comprehensive update on the current treatment and prevention of DILI since we last reviewed this topic 15 years ago.[2] While many therapeutic options are available, only acetaminophen has a specific antidote. For the other hundreds of agents that are associated with DILI/HDSLI, treatment options are often nonspecific, or limited by reports of anecdotal success. The shortcomings posed by many of the existing options will be discussed along with the research directions that are poised to produce therapeutic advances for DILI in the future.
Methods
We reviewed PubMed/MEDLINE publications on the treatment and prevention of DILI and HDSLI over the past 15 years through September 2015. We developed a search strategy using terms that included, but were not limited to, liver injury, hepatotoxicity, DILI, drug use in cirrhosis and chronic liver disease, all correlated with the terms treatment and prevention. Based on the publications found, we reviewed those that were specific to treatment or prevention, and cross-checked their reference lists to identify any additional articles that might have been missed in the initial database search to ensure as complete a literature review as possible. We chose to discuss treatment options that have been utilized in humans, including those described in case reports, case-series and clinical trials. We are mindful that while many experimental animal-based studies offer potentially useful therapeutic alternatives, they remain untested in patients.
General management principles of DILI
Recognizing acute drug induced liver injury
The ability to identify the signature of a specific type of DILI given the multitude of agents that are currently in use can take a lifetime of learning. LiverTox[17] and other resources can provide the clinician with the most up to date diagnostic criteria and clinical features of DILI, and provide quick reference in the setting of suspected hepatotoxicity.[18–20]
Causality assessment
The various causality assessment methodologies in use to diagnose DILI have been the subject of several recent publications, and are beyond the scope of this review.[21] [22, 23] While all of the methodologies are imperfect[22, 23] suffice it to say that the most important aspect of establishing causality is having a high index of suspicion that a particular drug or herbal agent is responsible for the injury. A number of detrimental outcomes can follow the misdiagnosis of DILI.[24] These include stopping a beneficial drug where no equivalent alternatives are available, falsely branding a drug as unsafe, opening the prospect of medical-legal consequences, and most importantly, leaving the actual cause of the injury undiagnosed. Historically, causality assessment was based in large part on expert opinion, which remains and important means of establishing the diagnosis. The use of the semi-quantitative Roussel Uclaf Causality Assessment Method (RUCAM) scoring system coupled with expert opinion by the U.S. DILI Network,[21] creates a so-called “medical reasoning” approach to identify DILI.[14] It appears to be the best diagnostic method while a truly accurate and definitive means to identify DILI is awaited. Being able to confidently diagnose DILI is oftentimes hindered by having incomplete patient data and laboratory results.[4, 25, 26] Agarwal and colleagues[27] have suggested a list of specific elements they deem necessary to evaluate potential DILI cases, in an effort to overcome the numerous deficiencies that often negatively impact causality assessment.
LiverTox, a collaborative effort by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Library of Medicine (NLM) was launched in October 2012, and provides the most up-to-date, interactive and searchable information on the diagnosis, cause, frequency, patterns, and management of DILI and HDSLI.[17] As such, it functions as a virtual resource on more than 650 agents (www.livertox.nih.gov).
Drug discontinuation criteria
The cardinal rule of managing suspected acute (or chronic) DILI is to stop the offending drug. [2, 4] However, it is important to distinguish between asymptomatic, low-level elevations in liver associated enzymes (LAEs) where the drug can be safely continued and biochemical changes that may be indicative of more serious hepatic damage. For some drugs causing mild elevations in ALT or other LAEs, the injury either does not progress beyond the asymptomatic, low-level elevation or resolves and returns to normal (or baseline) despite continuation of the medication. This phenomenon is referred to as “drug tolerance” or the “drug adaptive response.”[28] Drug tolerance needs to be recognized in order to prevent early discontinuation of beneficial therapy as these asymptomatic elevations are not associated with significant impairment in hepatic function.
DILI Stopping rules
Published in 2009, stopping rules from the U.S. Food and Drug Administration (FDA) were outlined to prevent hepatotoxicity in clinical trials.[29] This remains an important guidance document in both the clinical trial setting as well as the post-marketing phase for new drugs as discontinuation elements have been incorporated into the clinic setting for approved drugs.[25, 26] The FDA recommends that if a patient’s ALT level rises above 3 times the upper limit of normal (ULN) in the absence of any symptoms or evidence of hepatic impairment [e.g. an increase in bilirubin or international normalized ratio (INR)], the drug can likely be continued safely with frequent LAE monitoring . For ALT values that rise above 5 times the ULN, more intensive monitoring should be performed. If the ALT rises above 8 times the ULN, clinicians should consider stopping the drug at that time, as this is generally accepted as the threshold where DILI may progress to ALF and become irreversible.[3, 29] Table 1 summarizes these FDA stopping rules, which while intended for agents in clinical trials, have also found utility in clinical practice [1].
Table 1.
FDA Stopping Rules to Prevent Serious DILI in Clinical Trials[29]
| For an increase in ALT/AST >3X ULN - repeat testing in 48–72 hr to determine if these enzymes are increasing or decreasing and an inquiry should be made about hepatitis- related symptoms |
| If repeat testing shows ALT/AST remain >3X ULN or if symptoms are present, close observation is appropriate to determine the trend of the injury |
Treatment should be discontinued when:
|
ALT = alanine aminotransferase; AST = aspartate aminotransferase; INR= international normalized ratio; RUQ = right upper quadrant; ULN= upper limit of normal
For any patient who develops an increase in ALT above 3 times the ULN in association with a total serum bilirubin level greater than twice the ULN (indicating impaired liver function from the injury), or any hepatic-related symptoms, the FDA guidance states that Hy’s Law biochemical criteria have been met,[3, 6, 30, 31] implying the patient is at high risk for developing ALF. Named for the clinical observation made by the late Hyman Zimmerman,”Hy’s Law” predicts that patients who develop drug-induced hepatocellular jaundice have a mortality rate of 10–50%.[18] [3, 6, 30–33]
For additional information in the recognition of DILI, its causality assessment and drug-stopping rules, the interested reader is directed to the recent guidelines published by the American College of Gastroenterology [4], as well as resources discussing the regulatory aspects of DILI in drug development.[1, 25, 26, 34]
DILI Desensitization-rechallenge
Deliberate re-exposure (rechallenge) to the same drug suspected as causing acute DILI is generally discouraged [4, 35], owing to the fear of producing an even more severe reaction, including death from ALF.[35–39] For certain life-threatening illness, such as active tuberculosis, where no alternative therapies are available, desensitization-rechallenge strategies have been successfully implemented, whereby the same agents causing DILI can be safely restarted.[37, 38, 40, 41] Table 2 outlines strategies that have been suggested to desensitize patients to INH.
Table 2.
Two Desensitization-rechallenge strategies for isoniazid (INH) and other anti-TB medications
| Author(ref) | Recommendations |
|---|---|
| American Thoracic Society (ATS)[15, 38] |
Rechallenged patients who reach a treatment-limiting threshold should have clinical and biochemical monitoring performed at 2- to 4-week intervals. After ALT returns to less than two times the ULN, rifampin may be restarted with or without ethambutol After 3 to 7 days, INH may be reintroduced, subsequently rechecking ALT If symptoms recur or ALT increases, the last drug added should be stopped For those patients who have experienced prolonged or severe hepatotoxicity, but tolerate reintroduction with rifampin and INH, rechallenge with pyrazinamide may be hazardous. In this circumstance, pyrazinamide may be permanently discontinued, with treatment extended to 9 months Although pyrazinamide can be reintroduced in some milder cases of hepatotoxicity, the benefit of a shorter treatment course likely does not outweigh the risk of severe hepatotoxicity from pyrazinamide rechallenge. |
| Senousy et al[41] |
Reintroduction should not be attempted until liver function is normal, liver tests as less than two times the ULN and more than 2 weeks since the disappearance of jaundice Staged reintroduction starting with Rifampin, then INH then pyrazinamide, unless patients had severe liver dysfunction, then pyrazinamde should be avoided Each drug should be given for 3–7 days before the next medication is added Medications should be reintroduced at doses lower than what was used in initial therapy and doses should be gradually up titrated to the therapeutic range |
ALT = alanine aminotransferase; INH= isoniazid
Switching from one drug to another in the same or related class in an attempt to avoid recurrent hepatotoxicity in a patient who has experienced DILI is probably done frequently in clinical practice, but formal studies are lacking. Successful drug substitutions in this setting have been anecdotally reported for thiazolidinediones,[42] statins,[43] apixaban,[44] and non-estolate salts of erythromycin.[45] In the case of penicillin, cephalosporins can generally be given safely, although the semi-synthetic penicillins have actually been more likely to cause DILI.[45] Similarly, telithromycin, is at least as likely to cause liver injury as macrolides.[46]
General treatment recommendations for suspected DILI
Gastrointestinal decontamination
Gastric aspiration (GA) by lavage and administration of activated charcoal (AC) appear useful in certain instances of acute APAP,[47] toxic mushroom,[48, 49] salicylate[50] and other acute overdose or toxic ingestions.[51] There are few apparent downsides to attempting to remove undigested pills after an APAP overdose, although the closer to the time of ingestion the better.[47, 52] [53, 54] Inducing vomiting using syrup of ipecac, however, is more controversial. [47, 52] [53, 54] Doing so is not recommended after salicylate overdose,[50] nor is it recommended if the patient presents for treatment several hours after APAP overdose.[47] In vitro data suggest that AC may deactivate NAC administered orally [55], however this has not been reproduced in vivo, and is considered inconsequential. [56] Activated charcoal reduces peak serum concentrations of APAP, [57] which can potentially reduce the number of patients who require NAC.[58] Table 3 provides a list of hepatotoxins for which AC can be given to mitigate the effects of a toxic ingestion.
Table 3.
Hepatotoxic ingestions for which activated charcoal (AC) can be given [51]
| Acetaminophen* |
| Carbamazepine |
| Dapsone |
| Quinine |
| Salicylic Acid |
| Phenytoin |
| Valproic Acid |
| Aminita phalloides |
When given concurrently with N-acetylcysteine (NAC), activated charcoal may deactivate NAC thus inhibiting its hepatoprotectant effects
Chelation therapy
Chelators have been used in the setting of heavy metal poisoning associated with DILI [Table 4].[59, 60] [61, 62] Chelating agents bind to toxic metal ions to form complex structures that facilitate their removal from both the intracellular and extracellular spaces.[59] 2,3-Dimercaprol has long been the mainstay of chelation therapy for lead or arsenic poisoning, however serious side effects have limited its use in favor of less toxic chelators including , calcium disodium ethylenediamine tetraacetic acid (CaNa2EDTA) , calcium trisodium DTPA, D-penicillamine, British Anti Lewisite (BAL), meso-2,3-dimercaptosuccinic acid (DMSA) and deferoxamine.[59, 60] [61]
Table 4.
| Drug/toxin | Antidote |
|---|---|
| Iron* | Deferoxamine Deferasirox Deferiprone |
| Arsenic | Dimercaprol DMSA |
| Copper | Penicillamine Trientene |
| Lead | Penicillamine Succimer |
| Mercury | Penicillamine |
oral agents have limited data to support their use
Hepatic necrosis from acute iron toxicity is the second leading cause of death related to acute iron ingestion.[59] Particularly problematic in children, acute iron ingestion should be managed with a combination of gastric aspiration and the administration of deferoxamine, the most specific iron chelator. Deferoxamine needs to be given either intramuscularly (90 mg to 1 g/kg) or intravenously at a rate of 15 mg/kg/h, as its gastric absorption is very low. The newer oral iron chelators have not been studied in acute iron intoxication to our knowledge.[62, 63] More study is needed in acute iron toxicity for both deferasirox and desferoxamine.[62]
Specific antidotes to treat DILI
N-acetylcysteine for acetaminophen overdose
NAC remains extremely effective at managing and preventing acute injury to APAP overdose.[64, 65] NAC binds the toxic N-acetyl-p-benzoquinonimine (NAPQI) metabolite that may overwhelm the body’s natural stores of detoxifying glutathione leading to severe necrosis.[66] While it is beyond the scope of this review to provide the specific details of assessing and treating APAP intoxication in all of its various forms (including an acute single overdose In adults and children, repeated supratherapeutic ingestions [RSTI], and an overdose of uncertain dose or duration), Table 5 provides an evidence-based approach to the management of acute acetaminophen poisoning based on several authoritative reviews.[47, 56, 67, 68]
Table 5.
| Clinical Scenario | Recommendation |
|---|---|
| Elements needed in the initial history |
|
| Question of self-harm or inadvertent overdose |
|
| When to give activated charcoal (AC) |
|
| Acute, single, unintentional ingestion | Patient should be referred to an emergency department (ED):
Patients under age 6 years
Patients 6 years of age or older:
If initial contact with poison center occurs at > 36 hours after the ingestion and the patient is well:
|
| Repeated supratherapeutic ingestions (RSTIs) |
Patients under 6 years of age are referred to the ED if the ingestion is:
Patients 6 years of age or older are referred to the ED if:
Patients with increased susceptibility to acetaminophen toxicity (e.g. alcoholism, isoniazid use, prolonged fasting) should be referred to the ED if:
|
| Other considerations |
|
The treatment algorithm for acetaminophen overdose as initially recommended by Rumack and colleagues is well time-tested.[67, 69] [57, 64] Management strategies have been reviewed by others[68, 69] and only the main points will be covered here. In the U.S., Canada, Australia , New Zealand and Hong Kong, treatment with NAC is initiated if the acetaminophen plasma concentration after a single acute overdose exceeds 150 ug/mL at 4 hours. This “150 line” on the Rumack-Matthews nomogram is a level imposed by FDA to ensure as many patients as possible are treated without increasing the risk of overtreatment and the attendant costs of therapy.[68, 70] In other nations, such as the U.K., the threshold plasma concentration was lowered to 100 mg/L in 2012 [71] This change in national guidelines resulted in an increased proportion of patients being admitted and treated with NAC, most of whom presented with concentrations between 100 and 149mg/L. However, the change was not associated with any increase in adverse reactions to NAC.[71] In Hong Kong, Chan et al[70] reported that only 21% of 893 patients presented with an overdose concentration above the 150 treatment line threshold, with 12.5% having serum levels between 100 and 150ug, and 66.5% were below the 100ug treatment line. They cautioned that while lowering the treatment line to 100ug would cut the NAC failure rate from 0.45% to 0.22%, it needs to be balanced against a higher incidence of anaphylactoid reactions and increased costs.[70]
The choice of IV versus oral NAC depends on several factors, including the alertness of the patient and their ability to take medications by mouth. The duration of IV therapy is shorter (i.e. a 20 hour infusion vs an oral loading dose followed by 17 additional oral doses 4 hours apart), and is the preferred route in patients with altered mental status, gastrointestinal hemorrhage or obstruction, the inability to tolerate oral NAC, pregnancy, and a short gut syndrome.[68] [56, 72] The IV formulation may even be effective when given over a shorter infusion time of just 12 hours.[73] Both IV and orally administered NAC have been proven to be equally efficacious in the setting of an APAP overdose.[68] While both formulations appear to be well tolerated,[74] a non-IgE mediated hypersensitivity reaction similar to anaphylaxis with rash, urticaria, pruritus, angioedema, bronchospasm, hypotension is more common with IV administration and can be seen in up to 28.5% of patients[75] making proper patient selection essential [75–78] IV NAC also requires monitoring for arrhythmias and hypotension.[72] If a severe anaphylactoid reaction occurs, the infusion should be stopped immediately and treatment with epinephrine, diphenhydramine, corticosteroids and albuterol begun, with resumption of the IV NAC at a lower dose.[78][68] Risk factors for the anaphylactoid reaction include female gender, a history of asthma or atopy,[77] and a history of a prior NAC reaction.[75] Patients of non-Danish origin have also appear to be more susceptible.[75] Most reactions occur shortly after the start of treatment [77] , and in patients with higher acetaminophen serum levels (> 200 −300mg/L).[76] As NAC reactions appear to be mediated by histamine, pre-treating selected at-risk patients with antihistamines is suggested.[78]
NAC for non-acetaminophen DI-ALF
While NAC has traditionally been used only to treat APAP-induced ALF, new data are emerging that support its use in preventing ALF due to other agents, in particular, anti-TB drugs.[79] Khandewal and colleagues[80] have suggested a role for administering NAC empirically in the setting of indeterminate ALF, particularly in the subset of patients with hyperacute liver failure in whom up to 20% have unrecognized ALF from APAP. Data from the U.S. Acute Liver Failure Study Group have confirmed the potential benefits of NAC use in selected patients with non-APAP ALF, including DILI with autoimmune features and acute hepatitis B .[81] In their prospective, double blind trial, Singh at al randomly assigned 173 ALF patients without evidence of APAP overdose to receive IV NAC or placebo for 72 hours. These investigators found that patients with early encephalopathy (grade I-II) who received with NAC showed significant improvement in bilirubin and ALT levels and improved outcomes compared to those with higher grades (III-IV), in whom NAC was not beneficial [81]. Hu et al[82] performed a meta-analysis of prospective trials of NAC for non-APAP ALF between 2000–2014. In the 4 trials selected for review, (2 in adults and 2 in children), 331 patients received oral (one study) or IV NAC (3 studies) and 285 patients served as controls. No significant difference was found for overall survival (71% vs. 67%), but both transplant-free survival and post-LT survival were significantly higher in the NAC-treated patients (41% vs 30% p =0.01 and 85.7% vs 71.4% p=0.03 respectively). There was also a trend toward a shorter hospital stay (median duration 9 vs. 13 days) in the NAC recipients. A number of experts [83, 84] have suggested that NAC be used in all cases of early DI-ALF in adults regardless of the implicated drug. However, the use of NAC in children with non-APAP ALF has not been found to be efficacious and is not recommended.[85]
Corticosteroids
Historically, corticosteroids have been used predominantly in the setting of treating immune-mediated drug reactions, such as the anticonvulsant hypersensitivity syndrome[86] or other forms of acute DILI accompanied by serious skin injury, eosinophilia and systemic symptoms (DRESS).[87] Not all instances of such hypersensitivity reactions respond to corticosteroids. Lee et al noted that recovery from liver injury and mortality from severe DRESS reactions were not significantly reduced by steroids, with patients having prolonged jaundice being the least likely to respond.[88]
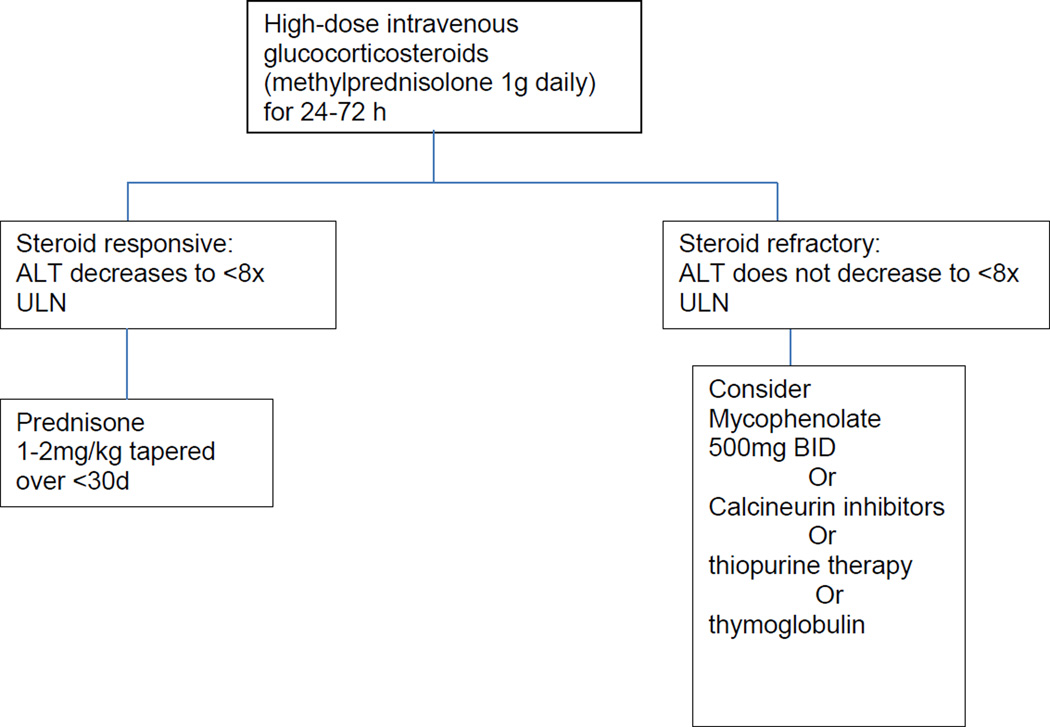
DILI due to tyrosine kinase inhibitors (TKIs) and iplimumab may also be treated with corticosteroids. In general, the management of adverse effects from TKIs (including hepatotoxicity) involves interrupting treatment temporarily, switching to an alternative agent, using lower doses, and administering corticosteroids.[89, 90] [91] Ipilimumab, a monoclonal antibody used to treat metastatic melanoma by inhibiting cyotoxic T-lymphocyte antigen 4 (CTLA-4), has been associated with several immune-type reactions, including hepatitis and colitis. Other reactions include ocular, hypophyseal and thyroid injury that can be seen in up to 15% of patients.[92] Corticosteroids, along with other immunosuppressive agents, have been required to treat these toxicities.[93, 94] [95] An algorithmic approach to managing ipilimumab-induced hepatotoxicity is given in Figure 1.
Figure 1.
Drug-induced autoimmune like hepatitis (DI-AIH)
A form of DILI resembling chronic AIH (DI-AIH) and has been reported in the setting of nitrofurantoin, minocycline, methyldopa, diclofenac, statin, ciproterone acetate and dapsone use.[18, 96–103] Seventy to eighty percent of these cases of DI-AIH are associated with antinuclear and/or smooth muscle antibodies. Bjornsson and colleagues [100] found that of 261 AIH patients seen during a 10 year period at the Mayo Clinic, 24 (9.2%) were due to drug-related causes, most commonly minocycline or nitrofurantoin . The role of corticosteroids in managing these DI-AIH patients is usually reserved for the subset who do not resolve promptly after withdrawal of the offending agent.[102] Bjornsson and colleagues found that while corticosteroids induced remission equally in both the drug-induced and idiopathic AIH patients, the prognosis was better for the DI-AIH group.[100] Being able to discontinue immunosuppressive therapy was achieved in all 14 DI-AIH cases with no relapses, whereas 65% of the idiopathic AIH patients relapsed after corticosteroids were stopped.[100] Such a response to corticosteroid withdrawal can serve as a practical means to differentiate DI-AIH from idiopathic AIH. [100, 102][103]
Recent observations also suggest that various biologic and immunomodulatory drugs are linked to DI-AIH, including adalimumab,[104] imatinib[105, 106] and infliximab.[107, 108] The majority of these patients have been treated with corticosteroids for the autoimmune features found on liver biopsy (e.g. plasma cells) with resolution of liver injury. Pentoxyfylline and other tumor necrosis factor-alpha blockers offer an alternative to steroids in this setting, but with fewer data.[109]
Ursodeoxycholic acid
Acute drug-induced cholestatic jaundice is less often fatal compared to cases of hepatocellular jaundice[1, 110–112] and there is a lack of compelling evidence that therapeutic intervention significantly shortens the time to resolution.[2, 113] Nevertheless, anecdotal case reports suggest ursodeoxycholic acid (UDCA) may decrease the duration of acute injury from amoxicillin-clavulanate DILI[114] or help prevent the development of the vanishing bile duct syndrome.[114–116] Uraz et al[117] suggest a role for UDCA in preventing hepatocellular injury from methotrexate(MTX) based on animal studies, although human studies with MTX are lacking. Wree et al[118] reported the successful use of a 3-day course of pulsed steroids (prednisone 15–20mg/kg/day) or a tapered steroid regimen over several weeks (prednisone 2–5mg/kg/day with weekly reductions) combined with oral UDCA in a dose of 750–1500mg/day in 15 patients with severe DILI. In their series, patients with autoimmune features had the slowest improvement, with some requiring long-term immunosuppressive therapy.
Cholestyramine
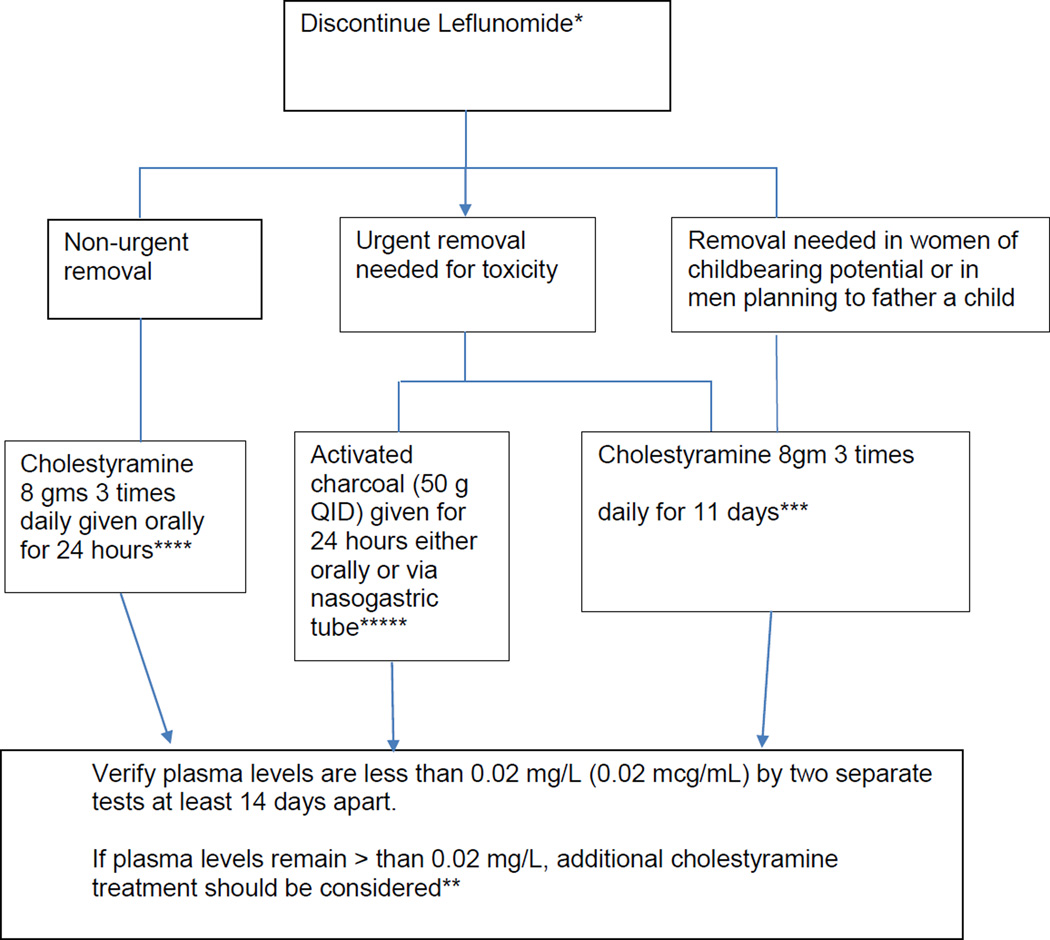
Cholestyramine may offer some relief as monotherapy for the pruritus associated with chronic cholestatic DILI[2] or when combined with antihistamines such as hydroxyzine or diphenhydramine if an immunoallergic reaction is present.[119] A novel treatment strategy utilizing cholestryramine as a form of bile acid washout has been used to manage the various toxicities associated with leflunomide, including DILI.[120] While injury is usually self-limited once leflunomide is stopped, the long half-life and enterohepatic circulation have made it amenable to treatment using activated charcoal (50g every 6 hours for 24 hours) or a bile acid binding resin, such as cholestyramine, to increase the clearance of the drug and reduce plasma concentrations more rapidly. Cholesytramine given orally in a dose of 8 grams three times a day for 24 hr has decreased plasma leflunomide levels by 40% at 24 hr and as much as 65% by 48 hr in healthy volunteers.[121] Without this drug elimination procedure, it may take up to 2 years to reach plasma M1 metabolite levels less than 0.02 mg/L due to individual variations in drug clearance.[121] The algorithm for administering cholestyramine in this setting is given in Figure 2.
Figure 2.
Suggested Algorithm for bile acid washout with cholestyramine for leflunamide toxicity [121]
*The goal of elimination is to achieve non-detectable plasma levels (less than 0.02 mg/L or 0.02 mcg/mL) after stopping treatment with leflunomide.
** Plasma concentrations of the active metabolite of leflunomide (A77 1726 or teriflunomide) may be detectable in plasma for up to 2 years following discontinuance of the drug
***The 11 days do not need to be consecutive unless there is a need to lower the plasma level rapidly.
**** reduces plasma concentrations of A77 1726 by approximately 40% in 24 hours and 49–65% in 48 hours
*****reduces plasma concentrations of A77 1726 by approximately 37% in 24 hours and 48% in 48 hours
Silymarin
The active component of milk thistle (Silybum marianum) is composed of three flavonoids (silybin, silydianin and silychristin) collectively referred to as silymarin [122]. Silybin (silibinin) accounts for 50–70% of the biological activity of the compound. When given IV, silibinin has been used successfully in Aminita phalloides poisoning and in various animal studies of liver injury caused by APAP, carbon tetrachloride, phenylhydrazine, and alcohol.[122] A number of investigators have evaluated silymarin to prevent DILI from anti-TB drugs (ATDs). Gu et al[123]performed a prospective, multicenter randomized trial in 565 patients receiving ATDs. They compared silibinin capsules (70mg three times a day for 8 weeks) given to 277 individuals to 291 who served as controls. Neither the incidence of liver injury, the number of patients diagnosed with DILI, nor the number of patients in whom ATD treatment was suspended were statistically different between the groups after up to 8 weeks of therapy, although the frequency of anorexia and nausea was lower in the silibinin group (p<0.05) . In contrast, Luangchosiri and colleagues [124] found that silymarin was able to reduce the incidence of ATD DILI at week 4 compared to placebo (3.7% vs 32.1%), albeit in a relatively small trial of 55 patients. They attributed the lower risk of liver injury to a smaller decline in superoxide dismutase levels in the silymarin-treated patients than was seen in controls.
Glycyrrhiza glabra
This licorice derivative has anti-hepatitis C properties and has been shown to be hepatoprotective by inducing indigenous interferon.[125]. It is currently used as monotherapy or in combination with cysteine and glycine, particularly in India and Japan.[125] A commonly used combination of monoammonium glycyrrhizinate - glycine-L-cysteine HCl (Monofit 20ml/day) is routinely used to treat acute DILI in Japan[126]. Data for DILI prevention by glycyrrhizic acid is drawn largely from animal models demonstrating a protective effect in the setting of carbon tetrachloride hepatotoxicity.[127] We are unaware of any cases where glycrrhiza glabra was prospectively given to prevent DILI.
L-Carnitine
Valproic acid (VPA) overdose leads to L-carnitine depletion resulting in impaired transport of long-chain fatty acids into the mitochondrial matrix and a subsequent decrease in ATP production, resulting in excessive production and accumulation of toxic products.[128] Chronic VPA use also has been shown to deplete L-carnitine stores. Supplementing patients with L-Carnitine has been shown to be effective in treating valproic acid (VPA) liver injury in children and adults. [128] [129] In a case series of 4 children aged 1–9 years, Xiong et al [130] reported that IV injections of L-carnitine (in addition to prompt discontinuation of valproate) led to resolution of liver injury and an earlier return to normal liver function.
Miscellaneous agents to prevent DILI
Folic acid supplementation is routinely given to reduce methotrexate (MTX) toxicity.[131] Methionine, a glutathione precursor, given with APAP tablets was considered a possible preventive measure in the past, owing largely to the antioxidant properties of methionine.[132] Preliminary pharmacokinetic data showed that acute toxicity (LD50) of APAP was reduced when used in this combination. [132] However, concerns over cost, mutagenic and carcinogenic properties and the pro-atherosclerotic features of methionine prevented this formulation from moving forward. [133, 134] Animal models have demonstrated a potential role of bosentan, an endothelin-1 receptor antagonist, to protect against damage from APAP, but human trials are lacking.[135] Alpha-lipoic acid may also offer a potential role in the cytoprotection of DILI.[136]
Direct toxin removal with liver support machines
The use of liver assist devices, such as molecular adsorbent recirculating systems (MARS), or plasma exchange, have proven effective as a bridge to LT, but remain largely investigational in the U.S.[5, 137, 138] The main reason cited as to why such liver support systems have not been widely adopted is the lack of a demonstrable survival benefit. Nevertheless, data from the RELIEF trial that utilized albumin dialysis with MARS, has shown a decrease in retained toxins, and improved hemodynamics and hepatic encephalopathy (HE).[137] In that trial, 189 patients (most of whom had liver disease from causes other than DILI), were randomized to MARS versus standard medical therapy. While the investigators were unable to demonstrate a difference in 28-day survival rate [odds ratio (OR) 0.87 with 95% CI 0.44–1.72], they did show an improvement in HE by day 4 (grade II-IV to grade 0–1 in 62.5% versus 38.2%). In addition there was a statistically significant decrease in creatinine and bilirubin levels. Despite the lack of survival benefit at 28 days, MARS may offer a potential bridge for those patients awaiting LT, particularly those patients with DI-ALF.[5]
Liver transplantation
Ideally, referral for DI-ALF should be made before the onset of more severe grades of HE, coma, bleeding from coagulopathy, sepsis or severe multisystem organ failure including hepatorenal syndrome. Outcomes are generally better at a tertiary care centers where supportive measures can be quickly implemented, including treatment of cerebral edema, prevention of hypoglycemia and the correction of acidosis. While DI-ALF accounts for a relatively small proportion of all liver transplants, the use of emergency LT is limited by its availability.[7, 139] As a result, efforts aimed at identifying the clinical and biochemical factors that predict the need for emergency LT are continually being explored.[140]
Strategies for the prevention of DILI
Avoiding inappropriate use of potentially hepatotoxic drugs
Zimmerman frequently remarked that while chronic liver disease (CLD) by itself was not a significant risk factor for the vast majority of drugs being taken, he provided an important caveat that recognized if acute hepatic injury were to develop in a patient with significant CLD, the consequences could be dire.[18] Many clinicians are appropriately reticent to prescribe medications that are potentially hepatotoxic to individuals with underlying liver disease. However, relatively few drug examples can be found where the risk of DILI is proven to be increased in the setting of CLD. These include the ATDs and some antiretroviral medications in patients with chronic hepatitis B or C.[1, 3, 11, 141–143] In addition, leflunomide, especially when given in combination with MTX, appears to be at higher risk of causing DILI.[144] Table 6 lists several potential hepatotoxic agents whose use is restricted or contraindicated in patients with underlying CLD. [145–149] While many medications carry recommendations to reduce their dose in patients with hepatic impairment,[150, 151] it is unclear whether or not that strategy is, in fact, protective against developing acute DILI.[11] Most medications can be taken by patients with cirrhosis safely without the added risk of acute DILI.[11, 152] It often comes as a surprise to many patients and clinicians alike that APAP taken in doses ≤ 2 g a day is considered a relatively safe alternative to non-steroidal anti-inflammatory drugs (NSAIDs), even in the face of cirrhosis,[11, 141, 153–155] or chronic alcohol use.[156]
Table 6.
Some potentially hepatotoxic agents whose use is restricted or contraindicated in patients with underlying chronic liver disease.[145–149]
| Agomelatine |
|---|
| Anti-tuberculosis drugs |
| Ketoconazole |
| Methotrexate |
| Telithromycin |
| Trovafloxacin |
| Tolvaptan |
| Valproic Acid |
About 10% of patients in the U.S. DILIN registry had pre-existing CLD (mostly non-alcoholic fatty liver disease or chronic hepatitis C).[110] In general, these patients had similar clinical features and demographics to patients without CLD, and there were no significant differences in the classes of agents causing DILI, suggesting that CLD in general, did not increase the risk of hepatotoxicity. Among the 5 leading causes of injury in DILIN (antimicrobials, herbals, cardiovascular drugs, anti-neoplastic agents, and centrally-acting drugs), there were no significant differences between patients with and without CLD. The one exception was azithromycin, which was found to be a statistically higher cause of DILI in the CLD group (6.7% vs 1.5%).
HMG CoA reductase inhibitors (statins) are one of the best studied drug classes in CLD and appear safe in this setting.[157] Both retrospective analyses and prospective clinical trials have demonstrated the hepatic safety of these agents in this setting.[158, 159] In particular, a prospective, randomized, placebo-controlled trial of high dose pravastatin was designed to assess its safety and efficacy in hypercholesterolemic patients with predominantly NAFLD or chronic hepatitis C who had baseline elevations of ALT up to five times the ULN.[159] The safety endpoint was a doubling of the baseline ALT levels, which, in fact, occurred less frequently in the pravastatin group compared to the placebo recipients at all time points, suggesting that lowering of lipids also had a saluatory effect on the underlying liver disease. These data, coupled with finding that statins may reduce the risk of hepatocellular carcinoma and other malignancies, have helped remove most concerns about prescribing statins in CLD.[160–162]
Clinical monitoring of isoniazid for symptoms of acute hepatitis
The American Thoracic Society (ATS) recommends self-monitoring of symptoms by patients suggestive of hepatotoxicity for ATDs, and incorporates face-to-face questionnaires.[15] However, the U.S. DILIN has shown that many patients inappropriately continued INH despite meeting clear-cut stopping criteria.[16] Adherence to the ATS guidelines was poor in patients who developed acute DILI, with 55% continuing INH for more than 7 days despite meeting stopping criteria.[16] Such data may support a “belt and suspenders” approach to prevent serious injury from occurring, where ALT testing is combined with clinical symptom monitoring.
Blood level monitoring to prevent hepatotoxicity
While measuring plasma and serum levels of medications to help direct further therapy in cases of APAP overdose is routinely performed,[69] monitoring blood levels of other potential hepatotoxins to avoid reaching a toxic threshold is recommended for only a select number of agents. For example, in Japan, sustained high trough levels of voriconazole (>4–5 ug/ml) have been associated with an increased risk of liver injury, and decreasing the dose to remain below this threshold is part of the therapeutic drug monitoring that is recommended.[163, 164]
Restricting medication availability to reduce acute liver failure
Limiting the availability of known hepatotoxic drugs has also been shown to prevent DILI. In 2009, the U.S. FDA advisory committee proposed that combination APAP-narcotic products be eliminated to reduce unnecessary exposure to APAP.[165, 166] In addition, the FDA announced a collaborative initiative with the National Board of Pharmacy that sought to eliminate the use of APAP abbreviations on prescription products in order to reduce the possibility of inadvertent overdoses. This was followed by the requirement that a boxed warning be added to all APAP products to highlight the potential risk for severe liver injury. In 2011, the FDA limited the strength of APAP in combination prescription drug products to 325 mg per tablet, and placed a boxed warning on the label highlighting the potential for severe liver injury from all prescription drug products that contain APAP.[167]
Legislation in the U.K. and France that limited the sale of APAP to 16 gm in pharmacies and up to 12 gm in supermarkets in blister packs of 500mg tablets has led to significant reductions in all overdose cases and serious liver injury.[10, 168] It is estimated that as many as 765 deaths have been prevented by this change in availability.[169] Similarly, the American Thoracic Society recommends that pharmacies limit the dispensing of anti-TB drugs to a one-month supply at a time.[15] Just how well such interventions work may be in the eye of the beholder. For example, King and colleagues[170] examined how the label of active ingredients and the warnings for APAP-containing products were conveyed and interpreted by patients discharged from emergency rooms in Chicago and an out-patient hospital pharmacy in Atlanta. They found that fewer than 7% of prescriptions referred to APAP by its full name rather than an abbreviation, and fewer than 30% of patients were able to identify APAP as the active ingredient. while nearly 90% of bottles warned about maximum daily APAP doses, only 11.5% warned about the risks of taking other APAP-containing products concomitantly. The authors concluded that such difficulties in identifying APAP and its warnings likely are contributing to the ongoing burden of APAP-related hepatotoxicity. Indeed, a study by Civan and colleagues found that the use of multiple APAP -containing medications contributes to excessive dosing.[171]
Liver associated enzyme monitoring
Despite the recommendations to perform aminotransferase (in particular ALT) testing for numerous drugs suspected of causing DILI, [1, 14] [18] this method of monitoring is often considered ineffective, costly, inconvenient and unproven to provide a large clinical benefit.[172] As a case in point, Graham et al[173] found that fewer than 5% of patients were being appropriately monitoring after just 3 months of being on troglitazone, despite several “Dear Doctor” letters and other dire warnings about its potential fatal hepatotoxicity. Even when a REMS program mandates ALT monitoring, as is the case with bosentan,[174] results have been less than optimal.[175] Addressing the inconvenience of testing, a new home point-of-care ALT test similar to glucose fingerstick testing is in the process of being developed,[176] and may offer a cost-effective alternative across all liver diseases, including DILI.
Pharmacogenomics and DILI
As Benjamin Franklin pragmatically stated, “an ounce of prevention is worth a pound of cure,” and such an expression was never more true than when it applies to DILI. Given the relatively limited therapeutic armamentarium currently available to treat specific causes of acute DILI, the ability to predict who is at risk of hepatotoxicity is the subject of active research efforts across the globe. While it is true in 2015 that the FDA has not had to withdraw any drugs in their post-marketing periods specifically for hepatotoxicity since the late 1990s,[14] a number of drugs have seen restrictions placed on their use due to the risk of hepatotoxicity.(Table 6) In addition, DILI/HDSLI still accounts for more than 50% of all causes of ALF in the US and other western nations.[7, 177] Since no one antidote is available to treat all forms and causes of DILI, the best treatment is indeed likely to be its primary prevention. To that end, the discovery of accurate predictive biomarkers that can be measured prior to the onset of DILI in its earliest form remains the quest of numerous researchers.[1, 8, 13, 14, 175] ALT measures injury that has already occurred, and while useful in defining stopping rules, it is neither sensitive nor specific when it comes to predicting the severity of hepatotoxicity.[3, 14]
It is anticipated that the discovery of specific proteomic and genomic biomarkers will be expanded to many more agents causing severe DILI, and their predictive values will rise to the point where clinical decisions regarding withholding the drug can be made with increasing confidence.[8] At present, only abacavir has a recommendation to test all potential recipients for the presence of HLA-B*5701, and to avoid prescribing the drug to those harboring the at-risk allele.[178] While genome-wide association studies (GWAS) have detected specific HLA genotypes predictive of DILI,[179, 180] and are of major significance in our understanding of susceptibility to DILI, such genetic markers have only been found for a limited number of agents (Table 7). Although specific HLA genotypes confer an increased risk of DILI for agents such as flucloxacillin and amoxicillin-clavulanate , the positive predictive value of these genetic markers remains well below their negative predictive value, and no recommendations have been made that would ban the use of such medications in an at-risk patient at this time.[1, 8] 181]
Table 7.
Specific HLA polymorphisms associated with an increased (or decreased) risk of DILI [180]
| Drug | Risk allele | Odds Ratio |
|---|---|---|
| Anti-TB agents (isoniazid, rifampicin, pyrazinamide) |
HLA-DQB1*02:01 HLA-DQA1*01:02 |
1.9 0.2* |
| Amoxicillin-clavulanate | DRB1*15:01 DQB1*06:02 DRB1*07 |
2.3–10 0.18* |
| Flucloxacillin | DRB1*07:01-DQB1*03:03 DRB1*15 |
7 |
| Lapatinib | DRB1*07:01-DQA1*02:01 | 2.6–9 |
| Lumiracoxib | DRB1*15:01-DQB1*06:02- DRB5*01:01-DQA1*01:02 |
5 |
| Nevirapine | DRB1*01:02 | 4.72 |
| Ximelagatran | DRB1*07-DQA1*02 | 4.4 |
risk decreased
Further refinement of such pharmacogenomic research, perhaps combined with the elucidation of a specific serum or urinary biomarker that more accurately predicts liver injury, may bring the field of hepatotoxicity closer to a truly preventative approach using precision medicine. The presence of certain metabolic polymorphisms associated with hepatic injury related to INH and other anti-TB medications may offer a solution to which patients require biochemical as well as clinical symptom monitoring to prevent severe DILI. For example, slow acetylator status reflected in NAT2 and CYP2E1 polymorphisms, appear to convey the greatest susceptibility to severe DILI.[182, 183].[9]
DILI-specific biomarkers
Proteomic-based biomarkers, such as apolipoprotein E, from stored sera in the U.S. DILI Network have been identified as potential candidates to identify DILI. [184] Although apolipoprotein E is involved with acute phase injury and inflammatory responses and holds potential promise as a biomarker to identify patients who develop acute DILI, these investigators were unable to determine whether there were any specific differences in the protein expression for the DILI caused by any of the specific medications studied. Given the large number of different causative agents and the small number of patients exposed to any individual drug, the identification of a biomarker that accurately identifies the risk of DILI from one or more specific agents causing idiosyncratic DILI will be eagerly awaited.
Recent work with biomarkers of hepatocyte necrosis and apoptosis have identified microRNAs,[185, 186] keratin-18 and high mobility group box 1 (HMGB-1)[187] as useful markers of APAP injury. These indicators may predict which patients (both adults and children)[188] will progress to a more severe injury, and thus be able to direct where more intensive treatment to the appropriate at-risk indivuidual. Similarly, early measurement of circulating kidney injury molecule 1 (KIM-1) appears to be an independent predictor of patient outcome in APAP poisoning, outperforming serum creatinine and other biomarkers to identify both nephrotoxicity as well as hepatotoxicity.[189]
Expert Commentary
At present, while there are several well-accepted treatment options for the management of acute severe DILI, they are limited in number. Outside of NAC for APAP overdose, no other specific antidotes exist for the hundreds of additional agents capable of causing severe hepatic injury. NAC for non-APAP related ALF has limited efficacy. Corticosteroids and other immunosuppressive agents play an important role in the treatment of DILI with autoimmune and hypersensitivity features, but they are not always effective. Silymarin, ursodeoxycholic acid and a few other “cytoprotective” agents have been described as having anecdotal success in protecting against or ameliorating acute DILI, but would not be expected to be able to reverse DI-ALF. While liver transplantation is available for those fortunate enough to be able to receive this life-saving therapy, demand far exceeds supply. Liver assist devices may be the best hope to reverse DI-ALF, and research in this area is continuing. However, until such time as more effective therapies for acute DILI are developed, prevention may be the best treatment of all. The identification of specific biomarkers, such as micro-RNAs, that predict which patients are at risk of developing severe liver injury after intentional or accidental acetaminophen overdose, are poised to find utility in the clinical arena. These and other markers seem capable of ushering in a new wave of biochemical injury predictors that may soon relegate the role of ALT to that of a supporting player.
Five Year View
Our predictions for the prevention and treatment of DILI in the near future include the identification of additional HLA associations and other pharmacogenetic risk factors for specific drugs. The discovery of such predictors should permit pre-prescription testing of patients , and support the clinical decision to withhold (or more intensively monitor) a particular agent in at-risk individuals. It is expected that advances in identifying predictive DILI biomarkers (such as microRNAs) that can be measured either prior to, or at the start of subclinical acute liver injury, will replace the current reliance on ALT measurements (as a marker of hepatic injury that has already occurred). Such knowledge will allow for more accurate management of APAP overdoses, or provide sufficiently accurate information to make a decision whether or not to continue a particular drug based on the presence of these new biomarkers. It is anticipated that given the ongoing shortage of available organs for liver transplant (LT), artificial liver support devices will become more efficient and routinely employed for cases of DI-ALF, as either a bridge to LT , or of sufficient usefulness as stand-alone therapy that the need for LT is obviated.
Exciting work in the field of nanotechnology holds the promise of developing novel treatment and prevention approaches for DILI in the not too distant future. The ability to deliver inhibitors of inflammatory cytokines, apoptosis and other cellular events leading to liver cell necrosis and death directly to hepatocytes or other substructures to prevent DILI is an attractive possibility for the future, and is being actively pursued.[190, 191, 192, 193]
The role of the gut microbiome in protecting against (or permitting) DILI will likely continue to be defined, offering new treatment approaches to be devised. It is currently being explored for its potential to protect against acetaminophen-induced and other other forms of acute DILI.[194]
Finally, several drug safety initiatives and international consortia composed of both academic, industry and regulatory stakeholders are actively engaged in identifying genetic and other biomarkers that offer the promise of transforming the era of personalized medicine into one where hepatic injury from drugs can be detected at the earliest juncture, if not completed prevented.[195] [196, 197]
Table 8.
side effects of NAC [73]
Most common
|
Other
|
Key Issues.
Medical treatment of acute DILI is currently limited by the relatively few specific therapies and antidotes that are available
Stopping the suspected offending agent causing DILI remains the key treatment approach.
NAC administered within 4–16 hours of an acute acetaminophen OD is highly effective in preventing hepatotoxicity
NAC for non-acetaminophen acute liver failure is less effective; only benefitting adults with early stage encephalopathy
Corticosteroids have been successfully used to treat drug-induced autoimmune hepatitis from a number of agents, and the clinical response can help distinguish the drug-induced variety from idiopathic autoimmune hepatitis as relapse is much more likely to occur with the latter form after steroids are withdrawn.
Corticosteroids are often given in cases of severe drug-induced hypersensitivity reactions that involve the liver (including DRESS and other severe skin injury), although successful reversal of such immunoallergic reactions is not assured.
Intentional re-exposure to a drug causing severe DILI is rarely attempted for fear of causing an even more serious reaction, but a rechallenge-desensitization strategy is usually successful in instances where hepatotoxicity is caused by anti-tuberculosis drugs
While herbal products are widely used, their specific hepatoprotective properties appear limited in the treatment of acute DILI or its prevention. Silymarin and other agents have been given with only anecdotal success. Similarly, ursodeoxycholic acid used to prevent cholestatic DILI from becoming chronic lacks a substantial database.
Avoiding the use of certain potentially hepatotoxic drugs in patients with underlying chronic liver disease, or who are identified as having a genetic or other risk factor for developing DILI is a first line approach to prevention, as is the monitoring of ALT and other liver associated enzymes to detect hepatotoxicity early for specific agents.
Restricting the behind- (or over) -the-counter availability of potentially toxic doses of acetaminophen through legislation has been successful in preventing overdoses in UK and European nations. However, where access is unrestricted (as in the US), better labeling and patient education is still needed to protect against both intentional and unintentional overdoses.
Acknowledgments
The paper was supported by a grant from the National Institute of Health (grant number: T32DK007769).
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Lewis JH. The Art and Science of Diagnosing and Managing Drug-induced Liver Injury in 2015 and Beyond. Clin Gastroenterol Hepatol. 2015 doi: 10.1016/j.cgh.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Marino G, Zimmerman HJ, Lewis JH. Management of drug-induced liver disease. Current gastroenterology reports. 2001;3(1):38–48. doi: 10.1007/s11894-001-0039-y. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JH. Drug-Induced Liver Injury Throughout the Drug Development Life Cycle: Where We Have Been, Where We are Now, and Where We are Headed. Perspectives of a Clinical Hepatologist. Pharm Med. 2013;27:165–191. [Google Scholar]
- 4.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–966. doi: 10.1038/ajg.2014.131. quiz 967. [DOI] [PubMed] [Google Scholar]
- 5.Saliba F, Samuel D. Artificial liver support: a real step forward. Minerva medica. 2015;106(1):35–43. [PubMed] [Google Scholar]
- 6.Senior JR. How can ‘Hy’s law’ help the clinician? Pharmacoepidemiology and drug safety. 2006;15(4):235–239. doi: 10.1002/pds.1210. [DOI] [PubMed] [Google Scholar]
- 7.Reuben A, Koch DG, Lee WM. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52(6):2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aithal GP. Pharmacogenetic testing in idiosyncratic drug-induced liver injury: current role in clinical practice. Liver international : official journal of the International Association for the Study of the Liver. 2015;35(7):1801–1808. doi: 10.1111/liv.12836. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto T, Ohno M, Azuma J. Future of pharmacogenetics-based therapy for tuberculosis. Pharmacogenomics. 2014;15(5):601–607. doi: 10.2217/pgs.14.38. [DOI] [PubMed] [Google Scholar]
- 10.Gunnell D, Hawton K, Murray V, et al. Use of paracetamol for suicide and non-fatal poisoning in the UK and France: are restrictions on availability justified? Journal of epidemiology and community health. 1997;51(2):175–179. doi: 10.1136/jech.51.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JH, Stine JG. Review article: prescribing medications in patients with cirrhosis - a practical guide. Aliment Pharmacol Ther. 2013;37(12):1132–1156. doi: 10.1111/apt.12324. [DOI] [PubMed] [Google Scholar]
- 12.Devarbhavi H, Singh R, Patil M, Sheth K, Adarsh CK, Balaraju G. Outcome and determinants of mortality in 269 patients with combination anti-tuberculosis drug-induced liver injury. J Gastroenterol Hepatol. 2013;28(1):161–167. doi: 10.1111/j.1440-1746.2012.07279.x. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins MT, Lewis JH. Latest advances in predicting DILI in human subjects: focus on biomarkers. Expert opinion on drug metabolism & toxicology. 2012;8(12):1521–1530. doi: 10.1517/17425255.2012.724060. [DOI] [PubMed] [Google Scholar]
- 14.Senior JR. New biomarkers for drug-induced liver injury: are they really better? What do they diagnose? Liver international : official journal of the International Association for the Study of the Liver. 2014;34(3):325–327. doi: 10.1111/liv.12384. [DOI] [PubMed] [Google Scholar]
- 15.Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. American journal of respiratory and critical care medicine. 2006;174(8):935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi PH, Fontana RJ, Chalasani NP, et al. Under-reporting and Poor Adherence to Monitoring Guidelines for Severe Cases of Isoniazid Hepatotoxicity. Clin Gastroenterol Hepatol. 2015;13(9):1676–1682. doi: 10.1016/j.cgh.2015.02.024. e1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoofnagle JH, Serrano J, Knoben JE, Navarro VJ. LiverTox: a website on drug-induced liver injury. Hepatology. 2013;57(3):873–874. doi: 10.1002/hep.26175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman HJ. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. Philadelphia: Lippincott-Williams & Wilkins; 1999. [Google Scholar]
- 19.Nikolaos P. Drug hepatotoxicity. Clin Liver Dis. 2013;17(4):507–786. [Google Scholar]
- 20.Kaplowitz N, DeLeve LD, editors. Drug-induced liver disease. 3rd. London: Elsevier/Academic Press; 2013. p. 746. [Google Scholar]
- 21.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51(6):2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro MA, Lewis JH. Causality assessment of drug-induced hepatotoxicity: promises and pitfalls. Clin Liver Dis. 2007;11(3):477–505. doi: 10.1016/j.cld.2007.06.003. v. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Cortes M, Stephens C, Lucena MI, Fernandez-Castaner A, Andrade RJ. Causality assessment methods in drug induced liver injury: strengths and weaknesses. J Hepatol. 2011;55(3):683–691. doi: 10.1016/j.jhep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Aithal GP, Rawlins MD, Day CP. Accuracy of hepatic adverse drug reaction reporting in one English health region. Bmj. 1999;319(7224):1541. doi: 10.1136/bmj.319.7224.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regev A, Seeff LB, Merz M, et al. Causality assessment for suspected DILI during clinical phases of drug development. Drug safety. 2014;37(Suppl 1):S47–S56. doi: 10.1007/s40264-014-0185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avigan MI, Bjornsson ES, Pasanen M, et al. Liver safety assessment: required data elements and best practices for data collection and standardization in clinical trials. Drug safety. 2014;37(Suppl 1):S19–S31. doi: 10.1007/s40264-014-0183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal VK, McHutchison JG, Hoofnagle JH. Important elements for the diagnosis of drug-induced liver injury. Clin Gastroenterol Hepatol. 2010;8(5):463–470. doi: 10.1016/j.cgh.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis JH. The adaptive response (drug tolerance) helps to prevent drug-induced liver injury. Gastroenterology & hepatology. 2012;8(5):333–336. [PMC free article] [PubMed] [Google Scholar]
- 29.FDA Drug Guidance. http://www.fda.gov/downloads/Drugs/…/Guidances/UCM174090.pdf.
- 30.Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiology and drug safety. 2006;15(4):241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 31.Kaplowitz N. Rules and laws of drug hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15(4):231–233. doi: 10.1002/pds.1212. [DOI] [PubMed] [Google Scholar]
- 32.Lewis JH. ‘Hy’s law,’ the ‘Rezulin Rule,’ and other predictors of severe drug-induced hepatotoxicity: putting risk-benefit into perspective. Pharmacoepidemiology and drug safety. 2006;15(4):221–229. doi: 10.1002/pds.1209. [DOI] [PubMed] [Google Scholar]
- 33.Andrade RJ, Lucena MI, Kaplowitz N, et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44(6):1581–1588. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- 34.Senior JR. Evolution of the Food and Drug Administration approach to liver safety assessment for new drugs: current status and challenges. Drug safety. 2014;37(Suppl 1):S9–S17. doi: 10.1007/s40264-014-0182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papay JI, Clines D, Rafi R, et al. Drug-induced liver injury following positive drug rechallenge. Regulatory toxicology and pharmacology : RTP. 2009;54(1):84–90. doi: 10.1016/j.yrtph.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Hunt CM, Papay JI, Rich DS, Abissi CJ, Russo MW. The evaluation of drug rechallenge: the casopitant Phase III program. Regulatory toxicology and pharmacology : RTP. 2010;58(3):539–543. doi: 10.1016/j.yrtph.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Sharma SK, Singla R, Sarda P, et al. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(6):833–839. doi: 10.1086/650576. [DOI] [PubMed] [Google Scholar]
- 38.Saukkonen J. Challenges in reintroducing tuberculosis medications after hepatotoxicity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(6):840–842. doi: 10.1086/650577. [DOI] [PubMed] [Google Scholar]
- 39.Andrade RJ, Robles M, Lucena MI. Rechallenge in drug-induced liver injury: the attractive hazard. Expert opinion on drug safety. 2009;8(6):709–714. doi: 10.1517/14740330903397378. [DOI] [PubMed] [Google Scholar]
- 40.Thongraung W, Lertphongpiroon W, Pungrassami P, Ratanajamit C. Physicians’ practices regarding management of antituberculosis drug-induced hepatotoxicity. The Southeast Asian journal of tropical medicine and public health. 2012;43(3):724–734. [PubMed] [Google Scholar]
- 41.Senousy BE, Belal SI, Draganov PV. Hepatotoxic effects of therapies for tuberculosis. Nature reviews. Gastroenterology & hepatology. 2010;7(10):543–556. doi: 10.1038/nrgastro.2010.134. [DOI] [PubMed] [Google Scholar]
- 42.Lebovitz HE. Differentiating members of the thiazolidinedione class: a focus on safety. Diabetes/metabolism research and reviews. 2002;18(Suppl 2):S23–S29. doi: 10.1002/dmrr.252. [DOI] [PubMed] [Google Scholar]
- 43.Charles EC, Olson KL, Sandhoff BG, McClure DL, Merenich JA. Evaluation of cases of severe statin-related transaminitis within a large health maintenance organization. Am J Med. 2005;118(6):618–624. doi: 10.1016/j.amjmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Anastasia EJ, Rosenstein RS, Bergsman JA, Parra D. Use of apixaban after development of suspected rivaroxaban-induced hepatic steatosis; a case report. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2015;26(6):699–702. doi: 10.1097/MBC.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 45.Stine JG, Lewis JH. Hepatotoxicity of antibiotics: a review and update for the clinician. Clin Liver Dis. 2013;17(4):609–642. doi: 10.1016/j.cld.2013.07.008. ix. [DOI] [PubMed] [Google Scholar]
- 46.Brinker AD, Wassel RT, Lyndly J, et al. Telithromycin-associated hepatotoxicity: Clinical spectrum and causality assessment of 42 cases. Hepatology. 2009;49(1):250–257. doi: 10.1002/hep.22620. [DOI] [PubMed] [Google Scholar]
- 47.Dart RC, Erdman AR, Olson KR, et al. Acetaminophen poisoning: an evidence-based consensus guideline for out-of-hospital management. Clinical toxicology (Philadelphia, Pa.) 2006;44(1):1–18. doi: 10.1080/15563650500394571. [DOI] [PubMed] [Google Scholar]
- 48.Ahishali E, Boynuegri B, Ozpolat E, et al. Approach to mushroom intoxication and treatment: can we decrease mortality? Clinics and research in hepatology and gastroenterology. 2012;36(2):139–145. doi: 10.1016/j.clinre.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Colak S, Kandis H, Afacan MA, et al. Assessment of patients who presented to the emergency department with mushroom poisoning. Human & experimental toxicology. 2015;34(7):725–731. doi: 10.1177/0960327114557902. [DOI] [PubMed] [Google Scholar]
- 50.Chyka PA, Erdman AR, Christianson G, et al. Salicylate poisoning: an evidence-based consensus guideline for out-of-hospital management. Clinical toxicology (Philadelphia, Pa.) 2007;45(2):95–131. doi: 10.1080/15563650600907140. [DOI] [PubMed] [Google Scholar]
- 51.Isbister GK, Kumar VV. Indications for single-dose activated charcoal administration in acute overdose. Current opinion in critical care. 2011;17(4):351–357. doi: 10.1097/MCC.0b013e328348bf59. [DOI] [PubMed] [Google Scholar]
- 52.McNamara RM, Aaron CK, Gemborys M, Davidheiser S. Efficacy of charcoal cathartic versus ipecac in reducing serum acetaminophen in a simulated overdose. Annals of emergency medicine. 1989;18(9):934–938. doi: 10.1016/s0196-0644(89)80456-1. [DOI] [PubMed] [Google Scholar]
- 53.Saincher A, Sitar DS, Tenenbein M. Efficacy of ipecac during the first hour after drug ingestion in human volunteers. Journal of toxicology. Clinical toxicology. 1997;35(6):609–615. doi: 10.3109/15563659709001241. [DOI] [PubMed] [Google Scholar]
- 54.Christophersen AB, Levin D, Hoegberg LC, Angelo HR, Kampmann JP. Activated charcoal alone or after gastric lavage: a simulated large paracetamol intoxication. British journal of clinical pharmacology. 2002;53(3):312–317. doi: 10.1046/j.0306-5251.2001.01568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tenenbein PK, Sitar DS, Tenenbein M. Interaction between N-acetylcysteine and activated charcoal: implications for the treatment of acetaminophen poisoning. Pharmacotherapy. 2001;21(11):1331–1336. doi: 10.1592/phco.21.17.1331.34427. [DOI] [PubMed] [Google Scholar]
- 56.Farrell SEDG, Tarabar A, et al. Acetaminophen toxicity treatment & management. http://emedicine.medscape.com/article/820200-treatment#d12.
- 57.Wolf SJ, Heard K, Sloan EP, Jagoda AS. Clinical policy: critical issues in the management of patients presenting to the emergency department with acetaminophen overdose. Annals of emergency medicine. 2007;50(3):292–313. doi: 10.1016/j.annemergmed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 58.Buckley NA, Whyte IM, O’Connell DL, Dawson AH. Activated charcoal reduces the need for N-acetylcysteine treatment after acetaminophen (paracetamol) overdose. Journal of toxicology. Clinical toxicology. 1999;37(6):753–757. doi: 10.1081/clt-100102452. [DOI] [PubMed] [Google Scholar]
- 59.Flora SJ, Pachauri V. Chelation in metal intoxication. International journal of environmental research and public health. 2010;7(7):2745–2788. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blanusa M, Varnai VM, Piasek M, Kostial K. Chelators as antidotes of metal toxicity: therapeutic and experimental aspects. Curr Med Chem. 2005;12(23):2771–2794. doi: 10.2174/092986705774462987. [DOI] [PubMed] [Google Scholar]
- 61.Aaseth J, Skaug MA, Cao Y, Andersen O. Chelation in metal intoxication--Principles and paradigms. Journal of trace elements in medicine and biology : organ of the Society for Minerals and Trace Elements (GMS) 2015;31:260–266. doi: 10.1016/j.jtemb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 62.Cappellini MD, Pattoneri P. Oral iron chelators. Annual review of medicine. 2009;60:25–38. doi: 10.1146/annurev.med.60.041807.123243. [DOI] [PubMed] [Google Scholar]
- 63.Xia S, Zhang W, Huang L, Jiang H. Comparative efficacy and safety of deferoxamine, deferiprone and deferasirox on severe thalassemia: a meta-analysis of 16 randomized controlled trials. PloS one. 2013;8(12):e82662. doi: 10.1371/journal.pone.0082662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Green JL, Heard KJ, Reynolds KM, Albert D. Oral and Intravenous Acetylcysteine for Treatment of Acetaminophen Toxicity: A Systematic Review and Meta-analysis. The western journal of emergency medicine. 2013;14(3):218–226. doi: 10.5811/westjem.2012.4.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. Journal of toxicology. Clinical toxicology. 2002;40(1):3–20. doi: 10.1081/clt-120002882. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187(1):185–194. [PubMed] [Google Scholar]
- 67.Daly FF, Fountain JS, Murray L, Graudins A, Buckley NA. Guidelines for the management of paracetamol poisoning in Australia and New Zealand--explanation and elaboration. A consensus statement from clinical toxicologists consulting to the Australasian poisons information centres. The Medical journal of Australia. 2008;188(5):296–301. doi: 10.5694/j.1326-5377.2008.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 68.Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013;17(4):587–607. doi: 10.1016/j.cld.2013.07.005. viii. [DOI] [PubMed] [Google Scholar]
- 69.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319(24):1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 70.Chan ST, Chan CK, Tse ML. Paracetamol overdose in Hong Kong: is the 150-treatment line good enough to cover patients with paracetamol-induced liver injury? Hong Kong medical journal = Xianggang yi xue za zhi / Hong Kong Academy of Medicine. 2015 doi: 10.12809/hkmj144481. [DOI] [PubMed] [Google Scholar]
- 71.Bateman DN, Dear JW, Carroll R, et al. Impact of reducing the threshold for acetylcysteine treatment in acute paracetamol poisoning: the recent United Kingdom experience. Clinical toxicology (Philadelphia, Pa.) 2014;52(8):868–872. doi: 10.3109/15563650.2014.954125. [DOI] [PubMed] [Google Scholar]
- 72.Bari KFR. Acetaminophen overdose: what practitioners need to know. AASLD Clinical Liver Disease. 2014;4(1):17–21. doi: 10.1002/cld.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bateman DN, Dear JW, Thanacoody HK, et al. Reduction of adverse effects from intravenous acetylcysteine treatment for paracetamol poisoning: a randomised controlled trial. Lancet. 2014;383(9918):697–704. doi: 10.1016/S0140-6736(13)62062-0. [DOI] [PubMed] [Google Scholar]
- 74.Bebarta VS, Kao L, Froberg B, et al. A multicenter comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clinical toxicology (Philadelphia, Pa.) 2010;48(5):424–430. doi: 10.3109/15563650.2010.486381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt LE. Identification of patients at risk of anaphylactoid reactions to N-acetylcysteine in the treatment of paracetamol overdose. Clinical toxicology (Philadelphia, Pa.) 2013;51(6):467–472. doi: 10.3109/15563650.2013.799677. [DOI] [PubMed] [Google Scholar]
- 76.Waring WS, Stephen AF, Robinson OD, Dow MA, Pettie JM. Lower incidence of anaphylactoid reactions to N-acetylcysteine in patients with high acetaminophen concentrations after overdose. Clinical toxicology (Philadelphia, Pa.) 2008;46(6):496–500. doi: 10.1080/15563650701864760. [DOI] [PubMed] [Google Scholar]
- 77.Sandilands EA, Bateman DN. Adverse reactions associated with acetylcysteine. Clinical toxicology (Philadelphia, Pa.) 2009;47(2):81–88. doi: 10.1080/15563650802665587. [DOI] [PubMed] [Google Scholar]
- 78.Bailey B, McGuigan MA. Management of anaphylactoid reactions to intravenous N-acetylcysteine. Annals of emergency medicine. 1998;31(6):710–715. doi: 10.1016/s0196-0644(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 79.Baniasadi S, Eftekhari P, Tabarsi P, et al. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur J Gastroenterol Hepatol. 2010;22(10):1235–1238. doi: 10.1097/MEG.0b013e32833aa11b. [DOI] [PubMed] [Google Scholar]
- 80.Khandelwal N, James LP, Sanders C, Larson AM, Lee WM. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology. 2011;53(2):567–576. doi: 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh S, Hynan LS, Lee WM. Improvements in hepatic serological biomarkers are associated with clinical benefit of intravenous N-acetylcysteine in early stage non-acetaminophen acute liver failure. Dig Dis Sci. 2013;58(5):1397–1402. doi: 10.1007/s10620-012-2512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu J, Zhang Q, Ren X, Sun Z, Quan Q. Efficacy and safety of acetylcysteine in “non-acetaminophen” acute liver failure: A meta-analysis of prospective clinical trials. Clinics and research in hepatology and gastroenterology. 2015 doi: 10.1016/j.clinre.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Sundaram V, Shaikh OS. Acute liver failure: current practice and recent advances. Gastroenterology clinics of North America. 2011;40(3):523–539. doi: 10.1016/j.gtc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 84.Lee WM, Hynan LS, Rossaro L, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856–864. doi: 10.1053/j.gastro.2009.06.006. 864.e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Squires RH, Dhawan A, Alonso E, et al. Intravenous N-acetylcysteine in pediatric patients with nonacetaminophen acute liver failure: a placebo-controlled clinical trial. Hepatology. 2013;57(4):1542–1549. doi: 10.1002/hep.26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knowles SR, Shapiro LE, Shear NH. Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug safety. 1999;21(6):489–501. doi: 10.2165/00002018-199921060-00005. [DOI] [PubMed] [Google Scholar]
- 87.Avancini J, Maragno L, Santi CG, Criado PR. Drug reaction with eosinophilia and systemic symptoms/drug-induced hypersensitivity syndrome: clinical features of 27 patients. Clin Exp Dermatol. 2015 doi: 10.1111/ced.12682. [DOI] [PubMed] [Google Scholar]
- 88.Lee T, Lee YS, Yoon SY, et al. Characteristics of liver injury in drug-induced systemic hypersensitivity reactions. Journal of the American Academy of Dermatology. 2013;69(3):407–415. doi: 10.1016/j.jaad.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 89.Teo YL, Ho HK, Chan A. Formation of reactive metabolites and management of tyrosine kinase inhibitor-induced hepatotoxicity: a literature review. Expert opinion on drug metabolism & toxicology. 2015;11(2):231–242. doi: 10.1517/17425255.2015.983075. [DOI] [PubMed] [Google Scholar]
- 90.Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug safety. 2013;36(7):491–503. doi: 10.1007/s40264-013-0048-4. [DOI] [PubMed] [Google Scholar]
- 91.Valle JW, Faivre S, Hubner RA, Grande E, Raymond E. Practical management of sunitinib toxicities in the treatment of pancreatic neuroendocrine tumors. Cancer treatment reviews. 2014;40(10):1230–1238. doi: 10.1016/j.ctrv.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 92.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 94.Ahmed T, Pandey R, Shah B, Black J. Resolution of ipilimumab induced severe hepatotoxicity with triple immunosuppressants therapy. BMJ case reports. 2015:2015. doi: 10.1136/bcr-2014-208102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng R, Cooper A, Kench J, et al. Ipilimumab-induced toxicities and the gastroenterologist. J Gastroenterol Hepatol. 2015;30(4):657–666. doi: 10.1111/jgh.12888. [DOI] [PubMed] [Google Scholar]
- 96.Alla V, Abraham J, Siddiqui J, et al. Autoimmune hepatitis triggered by statins. Journal of clinical gastroenterology. 2006;40(8):757–761. doi: 10.1097/00004836-200609000-00018. [DOI] [PubMed] [Google Scholar]
- 97.Prussick R, Shear NH. Dapsone hypersensitivity syndrome. Journal of the American Academy of Dermatology. 1996;35(2 Pt 2):346–349. doi: 10.1016/s0190-9622(96)90667-2. [DOI] [PubMed] [Google Scholar]
- 98.Lee WM, Denton WT. Chronic hepatitis and indolent cirrhosis due to methyldopa: the bottom of the iceberg? J S C Med Assoc. 1989;85(2):75–79. [PubMed] [Google Scholar]
- 99.Russo MW, Hoofnagle JH, Gu J, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 2014;60(2):679–686. doi: 10.1002/hep.27157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bjornsson E, Talwalkar J, Treeprasertsuk S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51(6):2040–2048. doi: 10.1002/hep.23588. [DOI] [PubMed] [Google Scholar]
- 101.Stine JG, Chalasani N. Chronic liver injury induced by drugs: A systematic review. Liver international : official journal of the International Association for the Study of the Liver. 2015 doi: 10.1111/liv.12958. [DOI] [PubMed] [Google Scholar]
- 102.Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci. 2011;56(4):958–976. doi: 10.1007/s10620-011-1611-4. [DOI] [PubMed] [Google Scholar]
- 103.Bessone F, Lucena MI, Roma MG, et al. Cyproterone acetate induces a wide spectrum of acute liver damage including corticosteroid-responsive hepatitis: report of 22 cases. Liver international : official journal of the International Association for the Study of the Liver. 2015 doi: 10.1111/liv.12899. [DOI] [PubMed] [Google Scholar]
- 104.Adar T, Mizrahi M, Pappo O, Scheiman-Elazary A, Shibolet O. Adalimumab-induced autoimmune hepatitis. Journal of clinical gastroenterology. 2010;44(1):e20–e22. doi: 10.1097/MCG.0b013e3181a745e7. [DOI] [PubMed] [Google Scholar]
- 105.Aliberti S, Grignani G, Allione P, et al. An acute hepatitis resembling autoimmune hepatitis occurring during imatinib therapy in a gastrointestinal stromal tumor patient. American journal of clinical oncology. 2009;32(6):640–641. doi: 10.1097/COC.0b013e31802b4ef7. [DOI] [PubMed] [Google Scholar]
- 106.Bilgi N, Bell K, Ananthakrishnan AN, Atallah E. Imatinib and Panax ginseng: a potential interaction resulting in liver toxicity. The Annals of pharmacotherapy. 2010;44(5):926–928. doi: 10.1345/aph.1M715. [DOI] [PubMed] [Google Scholar]
- 107.Mancini S, Amorotti E, Vecchio S, Ponz de Leon M, Roncucci L. Infliximab-related hepatitis: discussion of a case and review of the literature. Intern Emerg Med. 2010;5(3):193–200. doi: 10.1007/s11739-009-0342-4. [DOI] [PubMed] [Google Scholar]
- 108.Poulin Y, Therien G. Drug-induced hepatitis and lupus during infliximab treatment for psoriasis: case report and literature review. Journal of cutaneous medicine and surgery. 2010;14(2):100–104. doi: 10.2310/7750.2009.09007. [DOI] [PubMed] [Google Scholar]
- 109.Prandota J. Important role of proinflammatory cytokines/other endogenous substances in drug-induced hepatotoxicity: depression of drug metabolism during infections/inflammation states, and genetic polymorphisms of drug-metabolizing enzymes/cytokines may markedly contribute to this pathology. American journal of therapeutics. 2005;12(3):254–261. [PubMed] [Google Scholar]
- 110.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 889 Patients with Drug-induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129(2):512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 112.Bjornsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scandinavian journal of gastroenterology. 2005;40(9):1095–1101. doi: 10.1080/00365520510023846. [DOI] [PubMed] [Google Scholar]
- 113.Mohi-ud-din R, Lewis JH. Drug- and chemical-induced cholestasis. Clin Liver Dis. 2004;8(1):95–132. doi: 10.1016/S1089-3261(03)00124-7. vii. [DOI] [PubMed] [Google Scholar]
- 114.O’Brien CB, Shields DS, Saul SH, Reddy KR. Drug-induced vanishing bile duct syndrome: response to ursodiol. Am J Gastroenterol. 1996;91(7):1456–1457. [PubMed] [Google Scholar]
- 115.Mork H, al-Taie O, Klinge O, Scheurlen M. [Successful therapy of persistent androgen-induced cholestasis with ursodeoxycholic acid] Zeitschrift fur Gastroenterologie. 1997;35(12):1087–1091. [PubMed] [Google Scholar]
- 116.Katsinelos P, Vasiliadis T, Xiarchos P, et al. Ursodeoxycholic acid (UDCA) for the treatment of amoxycillin-clavulanate potassium (Augmentin)-induced intra-hepatic cholestasis: report of two cases. Eur J Gastroenterol Hepatol. 2000;12(3):365–368. doi: 10.1097/00042737-200012030-00017. [DOI] [PubMed] [Google Scholar]
- 117.Uraz S, Tahan V, Aygun C, et al. Role of ursodeoxycholic acid in prevention of methotrexate-induced liver toxicity. Dig Dis Sci. 2008;53(4):1071–1077. doi: 10.1007/s10620-007-9949-3. [DOI] [PubMed] [Google Scholar]
- 118.Wree A, Dechene A, Herzer K, et al. Steroid and ursodesoxycholic Acid combination therapy in severe drug-induced liver injury. Digestion. 2011;84(1):54–59. doi: 10.1159/000322298. [DOI] [PubMed] [Google Scholar]
- 119.Tani M, Hayashi Y, Okamoto S, et al. Rapid improvement of icterus and pruritus by the oral administration of colestimide in two cases of drug-induced hepatitis. Internal medicine (Tokyo, Japan) 2001;40(11):1098–1103. doi: 10.2169/internalmedicine.40.1098. [DOI] [PubMed] [Google Scholar]
- 120.Wong SP, Chu CM, Kan CH, Tsui HS, Ng WL. Successful treatment of leflunomide-induced acute pneumonitis with cholestyramine wash-out therapy. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2009;15(8):389–392. doi: 10.1097/RHU.0b013e3181c3f87e. [DOI] [PubMed] [Google Scholar]
- 121.Leflunomide Monograph for Professionals) http://www.drugs.com/monograph/leflunomide.html.
- 122.Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: past, present, future. Phytotherapy research : PTR. 2010;24(10):1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- 123.Gu J, Tang SJ, Tan SY, et al. An open-label, randomized and multi-center clinical trial to evaluate the efficacy of Silibinin in preventing drug-induced liver injury. International journal of clinical and experimental medicine. 2015;8(3):4320–4327. [PMC free article] [PubMed] [Google Scholar]
- 124.Luangchosiri C, Thakkinstian A, Chitphuk S, et al. A double-blinded randomized controlled trial of silymarin for the prevention of antituberculosis drug-induced liver injury. BMC Complement Altern Med. 2015;15(1):334. doi: 10.1186/s12906-015-0861-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thyagarajan SP, Jayaram S, Gopalakrishnan V, Hari R, Jeyakumar P, Sripathi MS. Herbal medicines for liver diseases in India. J Gastroenterol Hepatol. 2002;17(Suppl 3):S370–S376. doi: 10.1046/j.1440-1746.17.s3.30.x. [DOI] [PubMed] [Google Scholar]
- 126.Koga K, Kawashima S, Shibata N, Takada K. [Novel formulations of a liver protection drug glycyrrhizin] Yakugaku zasshi : Journal of the Pharmaceutical Society of Japan. 2007;127(7):1103–1114. doi: 10.1248/yakushi.127.1103. [DOI] [PubMed] [Google Scholar]
- 127.Wang GS, Han ZW. [The protective action of glycyrrhiza flavonoids against carbon tetrachloride hepatotoxicity in mice] Yao xue xue bao = Acta pharmaceutica Sinica. 1993;28(8):572–576. [PubMed] [Google Scholar]
- 128.Papaseit E, Farre M, Lopez MJ, Clemente C, Campodarve I. A case of acute valproic acid poisoning treated successfully with L-carnitine. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 2012;19(1):57–58. doi: 10.1097/MEJ.0b013e328345d67e. [DOI] [PubMed] [Google Scholar]
- 129.Russell S. Carnitine as an antidote for acute valproate toxicity in children. Current opinion in pediatrics. 2007;19(2):206–210. doi: 10.1097/MOP.0b013e32805e879a. [DOI] [PubMed] [Google Scholar]
- 130.Xiong H, Liu CT, Zhang YH, et al. [Valproic acid-induced idiosyncratic liver injury in 4 cases] Zhonghua er ke za zhi. Chinese journal of pediatrics. 2012;50(12):890–894. [PubMed] [Google Scholar]
- 131.Shea B, Swinden MV, Ghogomu ET, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. The Journal of rheumatology. 2014;41(6):1049–1060. doi: 10.3899/jrheum.130738. [DOI] [PubMed] [Google Scholar]
- 132.Neuvonen PJ, Tokola O, Toivonen ML, Simell O. Methionine in paracetamol tablets, a tool to reduce paracetamol toxicity. International journal of clinical pharmacology, therapy, and toxicology. 1985;23(9):497–500. [PubMed] [Google Scholar]
- 133.Poirier LA. The role of methionine in carcinogenesis in vivo. Advances in experimental medicine and biology. 1986;206:269–282. doi: 10.1007/978-1-4613-1835-4_20. [DOI] [PubMed] [Google Scholar]
- 134.Jones AL, Hayes PC, Proudfoot AT, Vale JA, Prescott LF, Krenzelok EP. Should methionine be added to every paracetamol tablet? Bmj. 1997;315(7103):301–304. doi: 10.1136/bmj.315.7103.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yayla M, Halici Z, Unal B, Bayir Y, Akpinar E, Gocer F. Protective effect of Et-1 receptor antagonist bosentan on paracetamol induced acute liver toxicity in rats. European journal of pharmacology. 2014;726:87–95. doi: 10.1016/j.ejphar.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 136.Elshazly SM, El-Moselhy MA, Barakat W. Insights in the mechanism underlying the protective effect of alpha-lipoic acid against acetaminophen-hepatotoxicity. European journal of pharmacology. 2014;726:116–123. doi: 10.1016/j.ejphar.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 137.Banares R, Nevens F, Larsen FS, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57(3):1153–1162. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 138.Liu CT, Chen TH, Cheng CY. Successful treatment of drug-induced acute liver failure with high-volume plasma exchange. Journal of clinical apheresis. 2013;28(6):430–434. doi: 10.1002/jca.21291. [DOI] [PubMed] [Google Scholar]
- 139.Mindikoglu AL, Magder LS, Regev A. Outcome of liver transplantation for drug-induced acute liver failure in the United States: analysis of the United Network for Organ Sharing database. Liver Transpl. 2009;15(7):719–729. doi: 10.1002/lt.21692. [DOI] [PubMed] [Google Scholar]
- 140.Tillmann HL, Rockey DC. Improved prediction of the need for liver transplantation in patients with drug-induced liver injury? Gastroenterology. 2014;147(6):1441. doi: 10.1053/j.gastro.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 141.Gupta NK, Lewis JH. Review article: The use of potentially hepatotoxic drugs in patients with liver disease. Aliment Pharmacol Ther. 2008;28(9):1021–1041. doi: 10.1111/j.1365-2036.2008.03822.x. [DOI] [PubMed] [Google Scholar]
- 142.Kwon YS, Koh WJ, Suh GY, Chung MP, Kim H, Kwon OJ. Hepatitis C virus infection and hepatotoxicity during antituberculosis chemotherapy. Chest. 2007;131(3):803–808. doi: 10.1378/chest.06-2042. [DOI] [PubMed] [Google Scholar]
- 143.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35(1):182–189. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 144.Lee SW, Park HJ, Kim BK, et al. Leflunomide increases the risk of silent liver fibrosis in patients with rheumatoid arthritis receiving methotrexate. Arthritis research & therapy. 2012;14(5) doi: 10.1186/ar4075. R232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Watkins PB, Lewis JH, Kaplowitz N, et al. Clinical Pattern of Tolvaptan-Associated Liver Injury in Subjects with Autosomal Dominant Polycystic Kidney Disease: Analysis of Clinical Trials Database. Drug safety. 2015 doi: 10.1007/s40264-015-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gahr M, Kratzer W, Fuchs M, Connemann BJ. Safety and tolerability of agomelatine: focus on hepatotoxicity. Current drug metabolism. 2014;15(7):694–702. doi: 10.2174/1389200215666140926155041. [DOI] [PubMed] [Google Scholar]
- 147.Podcast: FDS. FDA limits usage of nizoral (ketoconazole) oral tablets due to potentially fatal liver injury and risk of drug interactions and adrenal glad problems. http://www.fda.gov/drugs/drugsafety/drugsafetypodcasts/ucm362820.htm. [PubMed]
- 148.Mandell L, Tillotson G. Safety of fluoroquinolones: An update. The Canadian journal of infectious diseases = Journal canadien des maladies infectieuses. 2002;13(1):54–61. doi: 10.1155/2002/864789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Telithromycin. (marketed as Ketek) information. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107824.htm.
- 150.Delco F, Tchambaz L, Schlienger R, Drewe J, Krahenbuhl S. Dose adjustment in patients with liver disease. Drug safety. 2005;28(6):529–545. doi: 10.2165/00002018-200528060-00005. [DOI] [PubMed] [Google Scholar]
- 151.Bjornsson ES, Jacobsen EI, Einarsdottir R, Chalasani N. Discrepancies in liver disease labeling in the package inserts of commonly prescribed medications. Gastroenterology. 2015;148(2):269–273. doi: 10.1053/j.gastro.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 152.Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, Sanchez de la Cuesta F. Drug use for non-hepatic associated conditions in patients with liver cirrhosis. European journal of clinical pharmacology. 2003;59(1):71–76. doi: 10.1007/s00228-003-0586-2. [DOI] [PubMed] [Google Scholar]
- 153.Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. American journal of therapeutics. 2005;12(2):133–141. doi: 10.1097/01.mjt.0000140216.40700.95. [DOI] [PubMed] [Google Scholar]
- 154.Rossi S, Assis DN, Awsare M, et al. Use of over-the-counter analgesics in patients with chronic liver disease: physicians’ recommendations. Drug safety. 2008;31(3):261–270. doi: 10.2165/00002018-200831030-00007. [DOI] [PubMed] [Google Scholar]
- 155.Nguyen DBN, Abdelaziz D, Lewis JH. Trainees’ Attitudes and Preferences towards the Use of Over the Counter Analgesics in Patients with Chronic Liver Disease. Adv Pharmacoepidemiol Drug Saf. 2014;3:167. [Google Scholar]
- 156.Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology. 1995;22(3):767–773. [PubMed] [Google Scholar]
- 157.Lewis JH. Clinical perspective: statins and the liver--harmful or helpful? Dig Dis Sci. 2012;57(7):1754–1763. doi: 10.1007/s10620-012-2207-3. [DOI] [PubMed] [Google Scholar]
- 158.Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. The American journal of the medical sciences. 2005;329(2):62–65. doi: 10.1097/00000441-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 159.Lewis JH, Mortensen ME, Zweig S, Fusco MJ, Medoff JR, Belder R. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology. 2007;46(5):1453–1463. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]
- 160.Kumar S, Grace ND, Qamar AA. Statin use in patients with cirrhosis: a retrospective cohort study. Dig Dis Sci. 2014;59(8):1958–1965. doi: 10.1007/s10620-014-3179-2. [DOI] [PubMed] [Google Scholar]
- 161.Lewis JH. Prescribing a statin to a cirrhotic patient to reduce hepatic decompensation and improve survival: impossible you say? Dig Dis Sci. 2014;59(8):1684–1687. doi: 10.1007/s10620-014-3228-x. [DOI] [PubMed] [Google Scholar]
- 162.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):323–332. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 163.Suzuki Y, Tokimatsu I, Sato Y, et al. Association of sustained high plasma trough concentration of voriconazole with the incidence of hepatotoxicity. Clinica chimica acta; international journal of clinical chemistry. 2013;424:119–122. doi: 10.1016/j.cca.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 164.Matsumoto K, Abematsu K, Shigemi A, et al. Therapeutic drug monitoring of voriconazole in Japanese patients: analysis based on clinical practice data. Journal of chemotherapy (Florence, Italy) 2015 doi: 10.1179/1973947815Y.0000000057. 1973947815y0000000057. [DOI] [PubMed] [Google Scholar]
- 165.Graham GG, Day RO, Graudins A, Mohamudally A. FDA proposals to limit the hepatotoxicity of paracetamol (acetaminophen): are they reasonable? Inflammopharmacology. 2010;18(2):47–55. doi: 10.1007/s10787-010-0036-6. [DOI] [PubMed] [Google Scholar]
- 166.Michna E, Duh MS, Korves C, Dahl JL. Removal of opioid/acetaminophen combination prescription pain medications: assessing the evidence for hepatotoxicity and consequences of removal of these medications. Pain medicine (Malden, Mass.) 2010;11(3):369–378. doi: 10.1111/j.1526-4637.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- 167.King JP, Davis TC, Bailey SC, et al. Developing consumer-centered, nonprescription drug labeling a study in acetaminophen. American journal of preventive medicine. 2011;40(6):593–598. doi: 10.1016/j.amepre.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 168.Hawton K, Bergen H, Simkin S, et al. Impact of different pack sizes of paracetamol in the United Kingdom and Ireland on intentional overdoses: a comparative study. BMC public health. 2011;11:460. doi: 10.1186/1471-2458-11-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hawton K, Bergen H, Simkin S, et al. Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyses. Bmj. 2013;346:f403. doi: 10.1136/bmj.f403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.King JP, McCarthy DM, Serper M, et al. Variability in Acetaminophen Labeling Practices: a Missed Opportunity to Enhance Patient Safety. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2015 doi: 10.1007/s13181-015-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Civan JM, Navarro V, Herrine SK, Riggio JM, Adams P, Rossi S. Patterns of acetaminophen use exceeding 4 grams daily in a hospitalized population at a tertiary care center. Gastroenterology & hepatology. 2014;10(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- 172.Lewis JH. Drug-induced liver disease. Med Clin North Am. 2000;84(5):1275–1311. doi: 10.1016/s0025-7125(05)70287-x. x. [DOI] [PubMed] [Google Scholar]
- 173.Graham DJ, Drinkard CR, Shatin D, Tsong Y, Burgess MJ. Liver enzyme monitoring in patients treated with troglitazone. JAMA : the journal of the American Medical Association. 2001;286(7):831–833. doi: 10.1001/jama.286.7.831. [DOI] [PubMed] [Google Scholar]
- 174.Watkins PB. Managing the risk of drug-induced liver injury. Clinical pharmacology and therapeutics. 2013;94(6):629–631. doi: 10.1038/clpt.2013.182. [DOI] [PubMed] [Google Scholar]
- 175.Blanchette CM, Nunes AP, Lin ND, et al. Adherence to risk evaluation and mitigation strategies (REMS) requirements for monthly testing of liver function. Drugs in context. 2015;4 doi: 10.7573/dic.212272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pollock NR, Colby D, Rolland JP. A point-of-care paper-based fingerstick transaminase test: toward low-cost “lab-on-a-chip” technology for the developing world. Clin Gastroenterol Hepatol. 2013;11(5):478–482. doi: 10.1016/j.cgh.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17(4):575–586. doi: 10.1016/j.cld.2013.07.001. viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Phillips E, Mallal S. Successful translation of pharmacogenetics into the clinic: the abacavir example. Molecular diagnosis & therapy. 2009;13(1):1–9. doi: 10.1007/BF03256308. [DOI] [PubMed] [Google Scholar]
- 179.Daly AK, Day CP. Genetic association studies in drug-induced liver injury. Drug metabolism reviews. 2012;44(1):116–126. doi: 10.3109/03602532.2011.605790. [DOI] [PubMed] [Google Scholar]
- 180.Urban TJ, Daly AK, Aithal GP. Genetic basis of drug-induced liver injury: present and future. Semin Liver Dis. 2014;34(2):123–133. doi: 10.1055/s-0034-1375954. [DOI] [PubMed] [Google Scholar]
- 181.Urban TJ, Shen Y, Stolz A, et al. Limited contribution of common genetic variants to risk for liver injury due to a variety of drugs. Pharmacogenetics and genomics. 2012;22(11):784–795. doi: 10.1097/FPC.0b013e3283589a76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee SW, Chung LS, Huang HH, Chuang TY, Liou YH, Wu LS. NAT2 and CYP2E1 polymorphisms and susceptibility to first-line anti-tuberculosis drug-induced hepatitis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2010;14(5):622–626. [PubMed] [Google Scholar]
- 183.Tang SW, Lv XZ, Zhang Y, et al. CYP2E1, GSTM1 and GSTT1 genetic polymorphisms and susceptibility to antituberculosis drug-induced hepatotoxicity: a nested case-control study. Journal of clinical pharmacy and therapeutics. 2012;37(5):588–593. doi: 10.1111/j.1365-2710.2012.01334.x. [DOI] [PubMed] [Google Scholar]
- 184.Bell LN, Vuppalanchi R, Watkins PB, et al. Serum proteomic profiling in patients with drug-induced liver injury. Aliment Pharmacol Ther. 2012;35(5):600–612. doi: 10.1111/j.1365-2036.2011.04982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Starkey Lewis PJ, Dear J, Platt V, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54(5):1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 186.Roderburg C, Benz F, Vargas Cardenas D, et al. Elevated miR-122 serum levels are an independent marker of liver injury in inflammatory diseases. Liver international : official journal of the International Association for the Study of the Liver. 2015;35(4):1172–1184. doi: 10.1111/liv.12627. [DOI] [PubMed] [Google Scholar]
- 187.Antoine DJ, Dear JW, Lewis PS, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58(2):777–787. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yang X, Salminen WF, Shi Q, et al. Potential of extracellular microRNAs as biomarkers of acetaminophen toxicity in children. Toxicol Appl Pharmacol. 2015;284(2):180–187. doi: 10.1016/j.taap.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Antoine DJ, Sabbisetti VS, Francis B, et al. Circulating Kidney Injury Molecule 1 Predicts Prognosis and Poor Outcome in Patients With Acetaminophen-Induced Liver Injury. Hepatology. 2015;62(2):591–599. doi: 10.1002/hep.27857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine : nanotechnology, biology, and medicine. 2014;10(7):1517–1527. doi: 10.1016/j.nano.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reddy LH, Couvreur P. Nanotechnology for therapy and imaging of liver diseases. J Hepatol. 2011;55:1461–1466. doi: 10.1016/j.jhep.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 192.Cui Y, Liu H, Ze Y, et al. Gene expression in liver injury caused by long-term exposure to titanium dioxide nanoparticles in mice. Toxicol Sci. 2012;128(1):171–185. doi: 10.1093/toxsci/kfs153. [DOI] [PubMed] [Google Scholar]
- 193.Isoda K, Hasezaki T, Kondoh M, et al. Effect of surface charge on nano-sized silica particles-induced liver injury. Pharmazie. 2011;66(4):278–281. [PubMed] [Google Scholar]
- 194.Possamai LA, McPhail MJ, Khamri W, et al. The role of intestinal microbiota in murine models of acetaminophen-induced hepatotoxicity. Liver international : official journal of the International Association for the Study of the Liver. 2015;35(3):764–773. doi: 10.1111/liv.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Goodsaid FMFF, Matters W. The Predictive Safety Testing Consortium: a synthesis of the goals, challenges and accomplishments of the Critical Path. Drug Disc Today Technol. 2007;4(2):47–50. doi: 10.1016/j.ddtec.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 196.(iSAEC). The international serious adverse event consortium. www.saeconsortium.org.
- 197.The DILI-sim initiative and DILIsym software. http://dilisym.com.