Abstract
Kienböck disease is a condition that typically occurs in the “at-risk” patient, in the “at-risk” aspect of the proximal condyle of the “at-risk” lunate. In the active male, repetitive loading causes the stress fracture that commences in the single layer proximal subchondral bone plate. The lunate fracture commences at the point the lunate cantilevers over the edge of the distal radius, and then takes on the shape of the radius. We postulate that the stress fracture violates the parallel veins of the venous subarticular plexus—leading to localized venous hypertension and subsequent ischemia and edema of the fatty marrow. The increased osseous compartment pressure further potentiates the venous obstruction, producing avascular necrosis.
If the fracture remains localized, it can heal or settle into a stable configuration, so that the wrist remains functional. Fractures of the subchondral bone plate produce irregularity of the lunate articular surfaces and secondary “kissing lesions” of the lunate facet and capitate, and subsequent degeneration. The lunate collapses when the fracture is comminuted, or there is disruption of the spanning trabeculae or a coronal fracture.
The secondary effect of the lunate collapse is proximal migration of the capitate between the volar and dorsal fragments, producing collapse of the entire central column. The proximal carpal row is now unstable, and is similar to scapholunate instability, where the capitate migrates between the scaphoid and lunate. The scaphoid is forced into flexion by the trapezium, however, degeneration of the scaphoid and scaphoid facet only occurs in late disease or following failed surgery.
In Kienböck disease, the secondary effects of the collapsing lunate are a “compromised” wrist, including: deformity and collapse of the central column, degeneration of the central column (perilunate) articulations, proximal row instability (i.e., between the central and radial columns), and degeneration of the radial column.
Keywords: Kienböck disease, lunate, etiology, pathogenesis
First described by Kienböck in 1910, lunatomalacia was initially believed to be the result of repetitive trauma to the lunate due to occupational activity.1 This was further supported by the research performed by Müller in 1920.2 Numerous articles have highlighted the “at-risk” patient, as identified genetic,3 4 anatomical, vascular and metabolic associations. It is generally considered that there is a vascular disturbance which occurs in an at-risk lunate.5 6 However, the precise etiological triggers and pathoanatomical events remain unclear. This review article develops the basic science platform; exploring conceptual mechanical and biological models in the development of Kienböck disease (KD).
The “At-Risk” Lunate
The “at-risk” patient is the young, active male, particularly manual laborers who are reported to represent more than 90% in some studies.7 8 Therefore, in this age group, repetitive stress is a likely factor. In addition, a significant asymptomatic older population has been identified.9
The lunate morphology is known to affect the kinematics of the carpus,10 and predisposes to degeneration of the carpus.11 The proximal articular “condyle” is the common site of KD. The convex proximal surface subchondral bone plate is only a single trabecular layer.12 Multiple trabeculae span the subchondral bone plate—transmitting the load and maintaining the height of the lunate.12 This “at-risk” zone in the specimen in Fig. 1 was measured to be 0.1-mm thick.
Fig. 1.
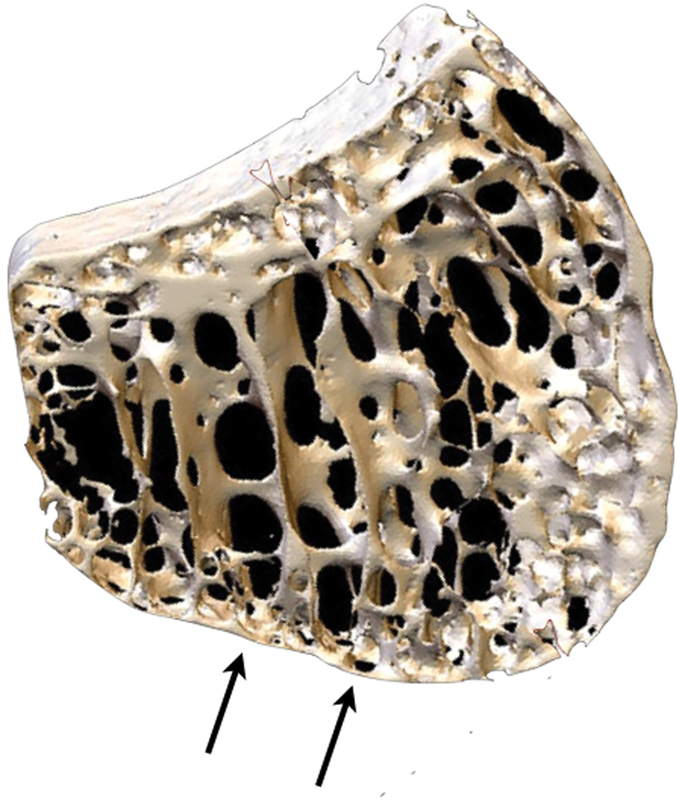
Microanatomy of the lunate. 3D micro-CT scan of the lunate demonstrating the thin proximal single layer of subchondral bone plate, which was measured to be 0.1-mm thick. There are spanning trabeculae principally on the radial aspect.13 3D, three-dimensional; CT, computed tomography. (Image courtesy of Dr. Gregory Bain.)
Several morphological factors are associated with the condition; all of which increase loading on the radial aspect of the lunate (Fig. 2).
Fig. 2.
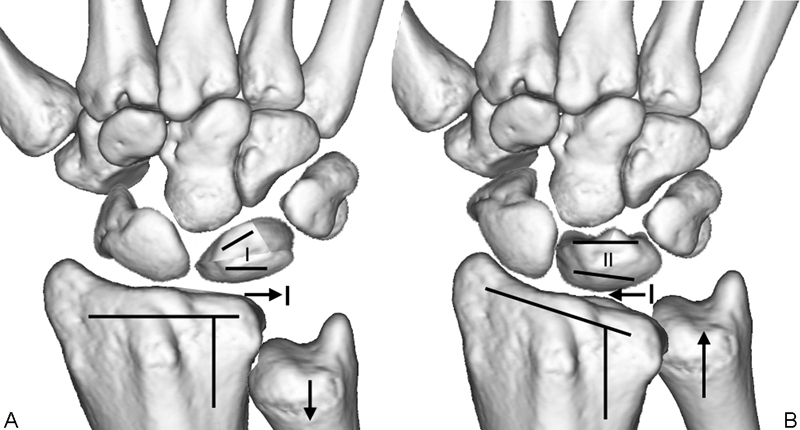
Morphological factors associated with Kienböck disease. (A) The “at-risk” factors increase the load and shear stresses on the radial aspect of the lunate. (B) The “low-risk” factors lead to a wide distribution of the stresses on the lunate.
An “uncovered” lunate overhangs the lunate fossa, and has a point-loading where the condyle articulates on the ulnar lip of the radius (Fig. 3). With ulnar deviation the capitate loads principally onto the lunate, which is then distributed to the proximal lunate, at the point where it articulates with the ulnar lip of the radius. The proximal subchondral bone plate is a cantilever, at the site of the thin proximal subchondral bone plate. The stress fracture occurs, at the point where the fulcrum loads the cantilever. A negative ulnar variance is important, as the ulnar is then unable to disperse the load from the lunate or triquetrum. It also explains why a radial shortening osteotomy is of clinical value.13
Fig. 3.
The uncovered type I lunate. The uncovered type I lunate is positioned on the radial edge of the lunate, and with power grip in ulnar deviation loads on the radial edge of the radius.
A negative ulnar variance leads to increase the loading of the radiocarpal joint—thereby distributing more stress to the lunate than would otherwise occur in an ulnar-neutral wrist.14
A Viegas type 1 lunate has a single articular facet for the capitate and positions the capitate in a more radial direction.15 16 There is preferential loading through the midcarpal articulation and the radiolunate articulation tends to load in the same location.17
Zapico type 1 lunates (occurring in ulnar-negative wrists) preferentially load on the radial aspect of the distal and proximal lunate, thereby creating a shearing force on the lunate.18 19
Stress Fracture of the Lunate
Prior to the stress fracture occurring in the lunate, while the lunate is being abnormally loaded, a physiological response can be seen on imaging. Bone edema and hyperemia can be identified on gadolinium-enhanced magnetic resonance imaging (MRI) and osteoblastic activity can be seen on a single-photon emission computed tomography (SPECT) scan (Fig. 4).
Fig. 4.
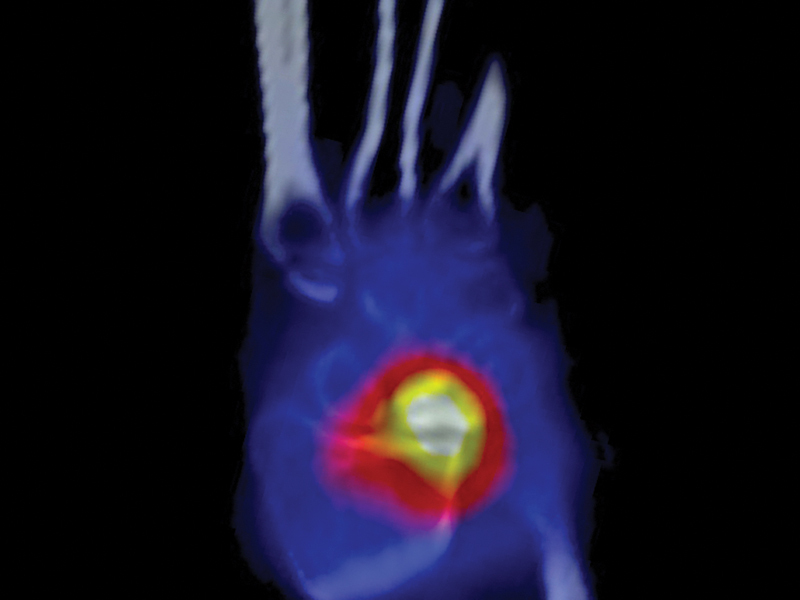
The SPECT scan demonstrates increased uptake within the lunate due to new bone formation and remodeling within the lunate. There is also increased uptake on the radial aspect of the distal radius due to the abnormal loading. SPECT, single-photon emission computed tomography.
With repetitive loading, a stress microfracture will occur, and with further loading, the fracture will propagate. It can be seen to be similar to a “die punch,” caused by the shape of the impacting distal radius. The fracture, typically involves the radial half of the lunate and only in the most extensive cases does it involve the ulnar side (Fig. 5A–C). If not loaded repetitively, the microfracture in the lunate can heal spontaneously. A single traumatic event such as a translunate fracture rarely leads to avascular necrosis.20 21
Fig. 5.
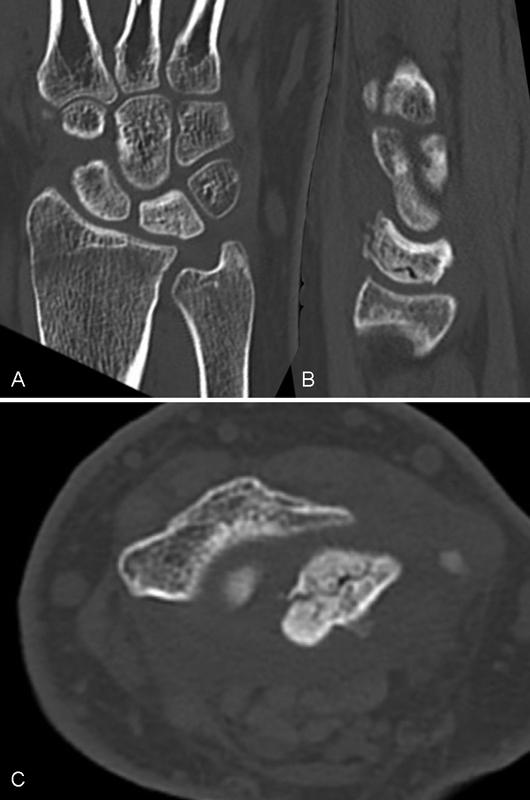
(A–C) Early case of Kienböck disease. The distribution of the fractures corresponds to the shape of the distal radius. Once the subchondral bone is breached, the friable medullary trabeculae are exposed and comminution and collapse occur.
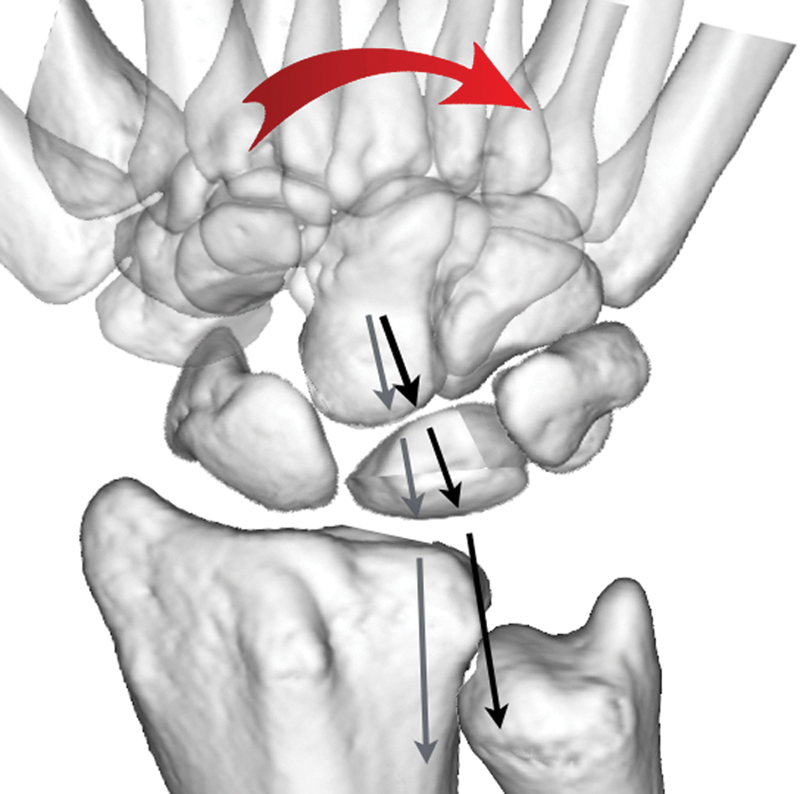
A fracture of the distal lunate can occur due to the “nutcracker effect” of the capitate on the lunate (Figs. 6A, B and 7A, B). With the coronal lunate fracture, the capitate migrates proximally between the volar and dorsal fragments of the lunate.
Fig. 6.
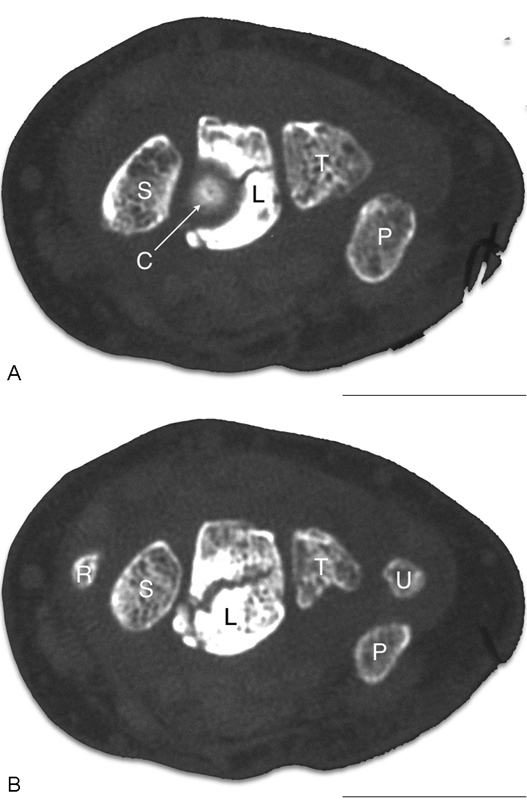
Capitate nutcracker. (A) The capitate is seen at the center of the “target sign” on the radial aspect of the lunate. (B) A coronal fracture propagates across the lunate.
Fig. 7.
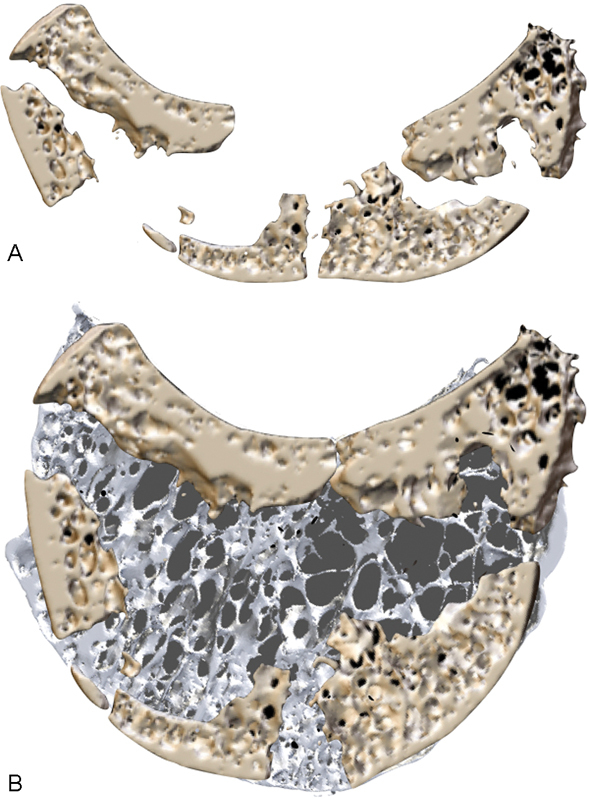
Lunate fragmentation. Micro-CT sagittal images of Kienböck lunate: (A) Fragmentation and collapse of the lunate. (B) Reformatted image of the same lunate, superimposed on a normal lunate. Note that most of the cortex is still present but fragmented. However, there is an extensive void, due to resorption of much of the medullary lunate. CT, computed tomography. (Image courtesy of Dr. Gregory Bain.)
Compartment Syndrome of Bone
Avascular necrosis (AVN) is considered to be as a result of “compartment syndrome of the bone” (Fig. 8). The factors associated with AVN are different depending upon the patients' age, sex, race, comorbidities, and are also anatomically specific. The factors that impact on the osseous compartment pressure include the arterial supply, emboli, venous drainage, and the intraosseous marrow.
Fig. 8.
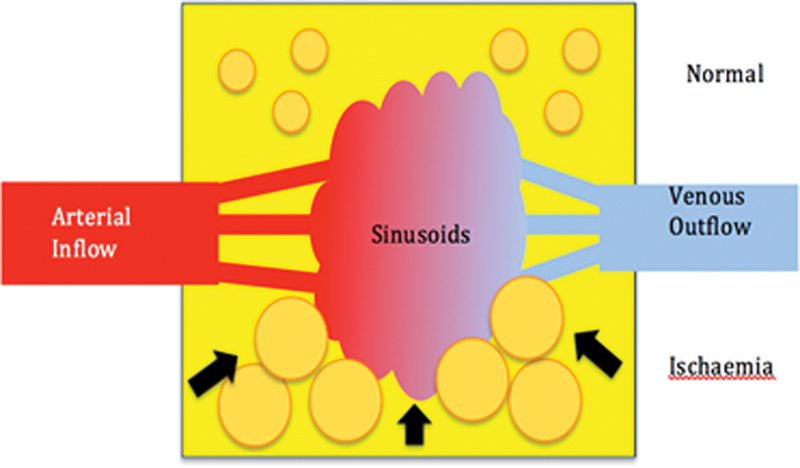
Compartment syndrome of bone. Diagrammatic representation of the normal vascularization of the lunate (superior half) with the normal fat cell and venous drainage. With ischemia (inferior half), there is interstitial edema and the marrow fat cells become swollen. This leads to tamponade of the sinusoids, thus decreasing the venous outflow. This further increases the intraosseous pressure, reduced arterial inflow, and produces necrosis. (Image courtesy of Dr. Gregory Bain.)
Lee and Gelberman have both studied the arterial supply of the lunate, and reported that a single volar artery can exist, and report that this is likely to be a risk factor for KD.5 6 However, it is interesting to note that lunate avascular necrosis as a result of translunate fracture dislocation is a rare event. Primary arterial (Raynaud disease and systemic sclerosis) and embolic disorders (atrial fibrillation)22 are rarely associated with KD.23 24
Conditions which increase the fat content in bone marrow (e.g., alcohol and Gaucher disease), are rarely associated with KD.22 However, when the intraosseous fat is ischemic, it becomes edematous and potentiates the “compartment syndrome” within the lunate.
Increased intraosseous pressure in the lunate is seen in KD, and rises significantly on wrist extension.25 In some cases this may exceed arterial blood pressure. Jensen hypothesized this impairment in venous outflow may be a factor in the development of KD.25 26
Crock studied the venous osseous drainage of the lunate, describing the extraosseous veins accompany the arteries. He also described the subarticular venous plexus, which are parallel veins positioned just below the subchondral bone plate (Fig. 9A, B).27
Fig. 9.
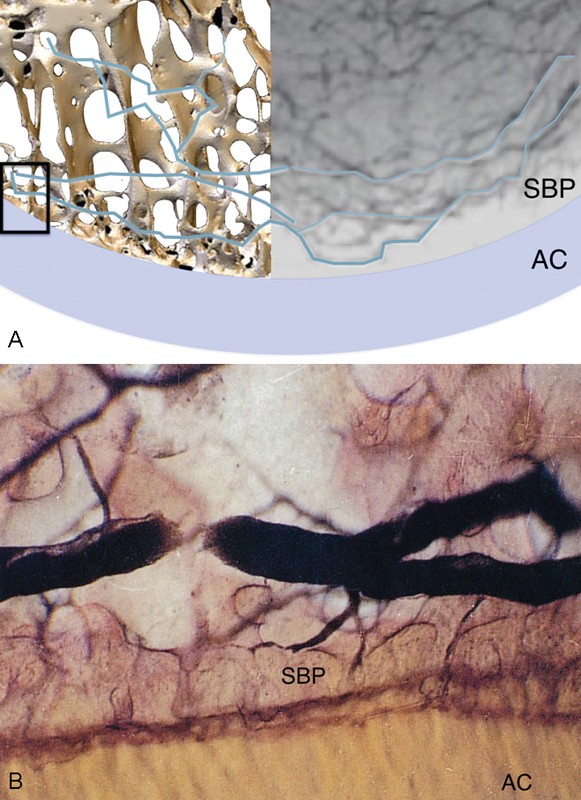
Subarticular venous plexus. (A) A composite image of subarticular venous plexus and 3D micro-CT of the subchondral bone plate. The small box on the left of image depicts the area demonstrated in the magnified views show in (B), where the venule within the subarticular plexus seen in profile. The subarticular venous plexus is directly adjacent, making it particularly at risk, even with a stress fracture. The calcified zone of the AC, and SBP are well seen. 3D, three-dimensional; AC, articular cartilage; CT, computed tomography; SBP, subchondral bone plate. (Image courtesy of Crock AO.)17
The literature supports impairment of venous drainage as critical in the development of AVN in the lunate and femoral head.22 25 26
We propose that two mechanisms exist which may lead to AVN of the lunate; either as a localized or global lunate phenomenon. Obstruction of the single vein that accompanies the single volar artery, will produce a global hypertension of the lunate.
A stress fracture of the proximal lunate will violate the subarticular venous plexus and produce localized venous hypertension. The venous obstruction produces edema of the fat cells, which potentiates the hypertension.
The global AVN corresponds to Lichtman stage 1, and has a positive enhancement with gadolinium-enhanced MRI scanning (Fig. 10).28
Fig. 10.
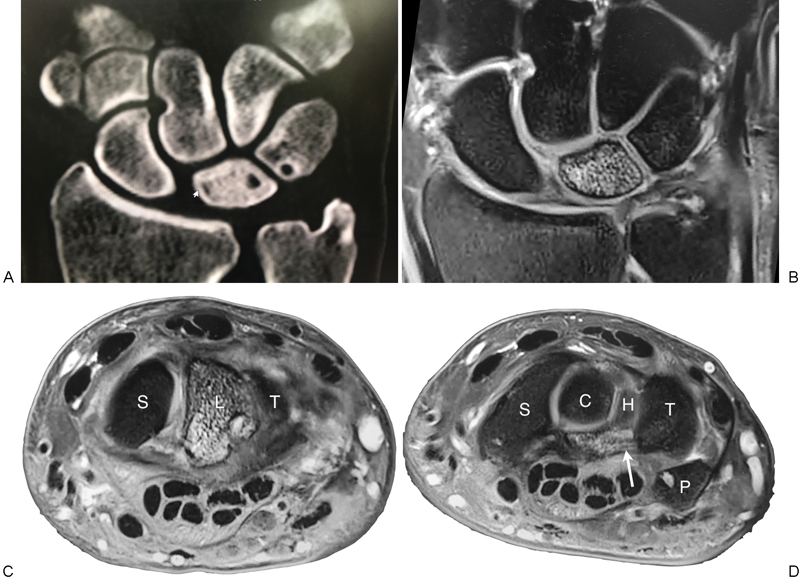
Global AVN of the lunate. (A and B) Global sclerosis of the lunate, with large volar perforations of the lunate and triquetrum. We believe this is in part related to the venous hypertension. There is also some localized sclerosis around the venous perforation of the triquetrum. (C and D) Note the changes on the MRI, including the edema of the volar ligaments at the lunotriquetral articulation (white arrow). AVN, avascular necrosis; MRI, magnetic resonance imaging.
How Does the Disease Affect the Wrist?
In the normal wrist the radial, central, and ulnar columns maintain the height of the carpus and transmit the load of the carpus. The articular surfaces are the interfaces that allow motion and transmit the load from their column.
The proximal row is the intercalated segment between the forearm and distal carpal row, which links the three columns. It enables the three columns to change their orientation, and thereby change the direction of the hand, but at the same time, share the load across the three columns.
In KD, the secondary effects of the collapsing lunate are a “compromised” wrist, including:
Deformity and collapse of the central column
Degeneration of the central column (perilunate) articulations
Proximal row instability (i.e., between the central and radial columns)
Degeneration of the radial column
Reviewing each of these in turn, helps understand the total effect of KD on the wrist.
Deformity and Collapse of the Central Column
The loss of height of the lunate and degeneration of the perilunate articulations produces a loss of height of the central column. Lunate comminution, disruption of the spanning trabeculae or a coronal lunate fracture will result in the collapse of the lunate. This will allow proximal migration of the capitate, which collapses the entire central column.
Degeneration of the Central Column (Perilunate) Articulations
Fractures of the lunate subchondral bone plate produce irregularity of the lunate articular surface. If the fracture settles and heals in a stable configuration, then the articulation will remain functional. Irregularity of the lunate articular surface produces secondary “kissing lesions” of the lunate facet and capitate. With time this leads to degenerative arthritis of both the radiolunate and lunocapitate joints.29 30 As with all joints, degenerative arthritis and collapse will lead to a secondary shortening of the soft tissues. This creates a static deformity, which in time becomes a fixed deformity.
Proximal Row Instability
Proximal row instability is due to disruption of the linkage between the three columns. Therefore, changes in the central column will have a secondary effect on the radial column. Dynamic changes occur with loss of height of the lunate, which changes the fine balance of the scapholunate ligament, so that the capitate becomes wedged into the scapholunate interval. With loss of height of the central column, the trapezium forces the scaphoid into flexion, thereby compromising the radial column.31
A static deformity is created with lunate collapse, as the capitate will disrupt and diastase the proximal carpal row, as seen in scapholunate instability.32 At this point, the scaphoid collapses into full flexion.
Initially, the radial column deformity is correctable, so lunate and central column reconstructive procedure could be effective. However, once there is a fixed deformity and/or central column articular degeneration, a limited wrist fusion is required (scaphocapitate fusion).
Degeneration of the Radial Column
Degeneration of the radioscaphoid articulation is unusual in KD, and only seen in late disease of following failed surgery (Kienböck disease advanced collapse [KDAC]–Lichtman IV). Once the radioscaphoid articulation is also compromised, the wrist is now unreconstructable (failed), and a salvage procedure is then required.
Analogy with Scapholunate Instability
As noted there are several similarities between KD and scapholunate instability. Scapholunate instability occurs following disruption of the scapholunate ligaments and is accompanied by the proximal capitate migration into the scapholunate interval. The proximal carpal row becomes unstable, as unbalanced forces push the scaphoid into flexion and lunate into extension (dorsal intercalated segmental instability [DISI]). There is a progression from dynamic, to static, and then becoming a fixed deformity, especially once articular degeneration occurs.
Before the development of degenerative changes, a proximal row carpectomy can be performed. Secondary articular degenerative changes are common at the time of presentation, but fortunately the radiolunate articulation is usually preserved. The four corner fusion is a good surgical option for late scapholunate advanced collapse (SLAC) wrist disease, as it bypasses the diseased radial column and mobilizes the wrist through the stabilized the central column. In very late disease or failed surgery, the radiolunate articulation is compromised, in which case a salvage procedure is required.
KD is due to structural compromise and the collapse of the lunate. The capitate migrates proximally usually between the volar and dorsal aspects of the lunate. The proximal carpal row becomes unstable, as unbalanced forces push the scaphoid into flexion and dorsal lunate into extension. As emphasized above with SLAC wrist disease, there is progression from dynamic, to static, and then fixed deformity. Degeneration of the articular cartilage occurs concurrently and can accelerate this process.
Before the development of degenerative changes, a proximal row carpectomy can be performed. Secondary articular degenerative changes are common at the time of presentation, but fortunately the radioscaphoid articulation is usually preserved. The scaphocapitate fusion is a good surgical option for late KD, as it bypasses the diseased central column and mobilizes the wrist through the stabilized radial column. In very late disease or failed surgery, the radioscaphoid articulation is compromised, in which case a salvage procedure is required.
Conclusion
In the above paragraphs we have separated the various aspects of KD, and how it affects the lunate and the wrist. However, all of these aspects are interrelated, and vary with time. For this reason, it is important to assess the osseous, vascular, cartilaginous, and instability components of KD in each case with quality imaging before making clinical decisions.33
Footnotes
Location The Department of Orthopaedic Surgery, Flinders University, Adelaide, South Australia, Australia. Conflict of Interest None.
Erratum: David M. Lichtman's name in the article has been corrected as per erratum published on May 18, 2016. DOI of the erratum is 10.1055/s-0036-1584237.
References
- 1.Kienböck R. Concerning traumatic malacia of the lunate and its consequences: joint degeneration and compression. Fortsch Geb Roentgen. 1910;16:77–103. [Google Scholar]
- 2.Müller W. Über die Erweichung und Verdichtung des Os lunatum, eine typische Erkrankung des Handgelenks. Beitr Klin Chir. 1920;119:664. [Google Scholar]
- 3.Ringsted A. Kienböck bei 2 Br ü dern. Acta Chir Scand. 1932;xx:185–196. [Google Scholar]
- 4.Templeman D, Engber W. Kienbock's Disease—Case Report of Familial Occurrence. Iowa Orthop J. 1985;5:107–109. [Google Scholar]
- 5.Gelberman R H, Bauman T D, Menon J, Akeson W H. The vascularity of the lunate bone and Kienböck's disease. J Hand Surg Am. 1980;5(3):272–278. doi: 10.1016/s0363-5023(80)80013-x. [DOI] [PubMed] [Google Scholar]
- 6.Lee M L. The intraosseus arterial pattern of the carpal lunate bone and its relation to avascular necrosis. Acta Orthop Scand. 1963;33:43–55. doi: 10.3109/17453676308999833. [DOI] [PubMed] [Google Scholar]
- 7.Therkelsen F, Andersen K. Lunatomalacia. Acta Chir Scand. 1949;97(6):503–526. [PubMed] [Google Scholar]
- 8.Stahl S, Stahl A S, Meisner C, Rahmanian-Schwarz A, Schaller H-E, Lotter O. A systematic review of the etiopathogenesis of Kienböck's disease and a critical appraisal of its recognition as an occupational disease related to hand-arm vibration. BMC Musculoskelet Disord. 2012;13(1):225. doi: 10.1186/1471-2474-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Leeuwen W F, Janssen S J, Ter Meulen D P, Ring D. What Is the Radiographic Prevalence of Incidental Kienböck Disease? Clin Orthop Relat Res. 2016;474(3):808–813. doi: 10.1007/s11999-015-4541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain G I, Yeo C J, Morse L P. Kienböck Disease: Recent Advances in the Basic Science, Assessment and Treatment. Hand Surg. 2015;20(3):352–365. doi: 10.1142/S0218810415400079. [DOI] [PubMed] [Google Scholar]
- 11.McLean J M, Turner P C, Bain G I, Rezaian N, Field J, Fogg Q. An association between lunate morphology and scaphoid-trapezium-trapezoid arthritis. J Hand Surg Eur Vol. 2009;34(6):778–782. doi: 10.1177/1753193409345201. [DOI] [PubMed] [Google Scholar]
- 12.Low S C, Bain G I, Findlay D M, Eng K, Perilli E. External and internal bone micro-architecture in normal and Kienböck's lunates: a whole-bone micro-computed tomography study. J Orthop Res. 2014;32(6):826–833. doi: 10.1002/jor.22611. [DOI] [PubMed] [Google Scholar]
- 13.Rock M G, Roth J H, Martin L. Radial shortening osteotomy for treatment of Kienböck's disease. J Hand Surg Am. 1991;16(3):454–460. doi: 10.1016/0363-5023(91)90013-2. [DOI] [PubMed] [Google Scholar]
- 14.Hultén O. Uber anatomische variationen der handgelenkknochen. Acta Radiol. 1928;9(2):155–168. [Google Scholar]
- 15.Viegas S F, Wagner K, Patterson R, Peterson P. Medial (hamate) facet of the lunate. J Hand Surg Am. 1990;15(4):564–571. doi: 10.1016/s0363-5023(09)90016-8. [DOI] [PubMed] [Google Scholar]
- 16.Rhee P C, Moran S L, Shin A Y. Association between lunate morphology and carpal collapse in cases of scapholunate dissociation. J Hand Surg Am. 2009;34(9):1633–1639. doi: 10.1016/j.jhsa.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Bain G I, Clitherow H D, Millar S. et al. The effect of lunate morphology on the 3-dimensional kinematics of the carpus. J Hand Surg Am. 2015;40(1):81–90. doi: 10.1016/j.jhsa.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Zapico J. Valladolid, Spain: Universidad de Valladolid, Servicio de Publicaciones; 1966. Malacia del semilunar. [Google Scholar]
- 19.Zapico J A Enfermedad de Kienböck Rev Ortop Traumatol (B Aires) 199337 IB(Supl. I):100–113. [Google Scholar]
- 20.Nakanishi A, Yajima H, Kisanuki O. Post-traumatic osteonecrosis of the lunate after fracture of the distal radius. J Plast Surg Hand Surg. 2014;48(6):434–436. doi: 10.3109/2000656X.2013.812763. [DOI] [PubMed] [Google Scholar]
- 21.Wilke B, Kakar S. Delayed Avascular Necrosis and Fragmentation of the Lunate Following Perilunate Dislocation. Orthopedics. 2015;38(6):e539–e542. doi: 10.3928/01477447-20150603-92. [DOI] [PubMed] [Google Scholar]
- 22.Solomon L. Idiopathic necrosis of the femoral head: pathogenesis and treatment. Can J Surg. 1981;24(6):573–578. [PubMed] [Google Scholar]
- 23.Frerix M, Kröger K, Szalay G, Müller-Ladner U, Tarner I H. Is osteonecrosis of the lunate bone an underestimated feature of systemic sclerosis? A case series of nine patients and review of literature. Semin Arthritis Rheum. 2016;45(4):446–454. doi: 10.1016/j.semarthrit.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Rennie C, Britton J, Prouse P. Bilateral Avascular Necrosis of the Lunate in a Patient with Severe Raynaud's Phenomenon and Scleroderma. J Clin Rheumatol. 1999;5(3):165–168. doi: 10.1097/00124743-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Jensen C H. Intraosseous pressure in Kienböck's disease. J Hand Surg Am. 1993;18(2):355–359. doi: 10.1016/0363-5023(93)90375-D. [DOI] [PubMed] [Google Scholar]
- 26.Schiltenwolf M, Martini A K, Mau H C, Eversheim S, Brocai D R, Jensen C H. Further investigations of the intraosseous pressure characteristics in necrotic lunates (Kienböck's disease) J Hand Surg Am. 1996;21(5):754–758. doi: 10.1016/S0363-5023(96)80187-0. [DOI] [PubMed] [Google Scholar]
- 27.Crock H V. Martin Dunitz; 1996. An Atlas of Vascular Anatomy of the Skeleton and Spinal Cord. 1st ed. [Google Scholar]
- 28.Lichtman D M, Mack G R, MacDonald R I, Gunther S F, Wilson J N. Kienböck's disease: the role of silicone replacement arthroplasty. J Bone Joint Surg Am. 1977;59(7):899–908. [PubMed] [Google Scholar]
- 29.Bain G I, Smith M L, Watts A C. Arthroscopic core decompression of the lunate in early stage Kienbock disease of the lunate. Tech Hand Up Extrem Surg. 2011;15(1):66–69. doi: 10.1097/BTH.0b013e3181e1d2b4. [DOI] [PubMed] [Google Scholar]
- 30.Bain G I, Begg M. Arthroscopic assessment and classification of Kienbock's disease. Tech Hand Up Extrem Surg. 2006;10(1):8–13. doi: 10.1097/00130911-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lichtman D M, Degnan G G. Staging and its use in the determination of treatment modalities for Kienböck's disease. Hand Clin. 1993;9(3):409–416. [PubMed] [Google Scholar]
- 32.Watson H K, Ballet F L. The SLAC wrist: scapholunate advanced collapse pattern of degenerative arthritis. J Hand Surg Am. 1984;9(3):358–365. doi: 10.1016/s0363-5023(84)80223-3. [DOI] [PubMed] [Google Scholar]
- 33.Lichtman D M, Bain G I. Berlin, Germany: Springer; 2016. Kienbock's Disease: Advances in Diagnosis and Treatment. [Google Scholar]


