Abstract
The membrane-associated enzyme NAPE-PLD (N-acyl phosphatidylethanolamine specific-phospholipase D) generates the endogenous cannabinoid arachidonylethanolamide and other lipid signaling amides, including oleoylethanolamide and palmitoylethanolamide. These bioactive molecules play important roles in several physiological pathways including stress and pain response, appetite and lifespan. Recently, we reported the crystal structure of human NAPE-PLD and discovered specific binding sites for the bile acid deoxycholic acid. In this study we demonstrate that in the presence of this secondary bile acid, the stiffness of the protein measured by elastic neutron scattering increases, and NAPE-PLD results ~7 times faster to catalyze the hydrolysis of the more unsaturated substrate N-arachidonyl-phosphatidylethanolamine, compared with N-palmitoyl-phosphatidylethanolamine. Chenodeoxycholic acid and glyco- or tauro-dihydroxy conjugates can also bind to NAPE-PLD and drive its activation. The only natural monohydroxy bile acid, lithocholic acid, shows an affinity of ~20 μM and acts instead as a reversible inhibitor (IC50 ≈ 68 μM). Overall, these findings provide important insights into the allosteric regulation of the enzyme mediated by bile acid cofactors, and reveal that NAPE-PLD responds primarily to the number and position of their hydroxyl groups.
Lipid signaling molecules and their enzymes constitute a complex network with multiple nodes of interaction and cross-regulation1. Deciphering these nodes can provide essential clues to their signaling mechanisms. Among bioactive lipidic molecules, the amides of fatty acids with ethanolamine (FAE) promote essential neurological, cyto-protective, and metabolic actions. The endogenous cannabinoid arachidonylethanolamide (anandamide) is known to elicit analgesic and anxiolytic actions in rodents by activating cannabinoid receptors in central and peripheral neurons2. Anandamide contributes to stimulate appetite3–7, addiction8, and brain synaptogenesis9. Oleylethanolamide is another important lipid amide that regulates body weight and lifespan engaging nuclear peroxisome proliferator-activated receptors10–12. Palmitoylethanolamide serves as an early stop signal that contrasts the progress of inflammation13–16. Upon stimulation, the membrane-associated enzyme NAPE-PLD synthesizes all different FAEs from their corresponding N-acyl phosphatidylethanolamine (NAPE)9, 17, 18, a ubiquitous and abundant glycerophospholipid mostly found in tissues and biological fluids that are involved in degenerating processes19.
The crystal structure of human NAPE-PLD obtained recently in our laboratory at 2.65 Å of resolution20 disclosed how the dinuclear zinc active site of the enzyme recognizes NAPE and catalyzes the production of FAEs. Structural and functional analysis unveiled that the secondary bile acid (BA) deoxyxcholic acid (DCA) binds the protein dimer, in a specific cavity close to the active site (Figure 1). Results showed the interaction between NAPE-PLD and the steroid acid was a stimulus for the enzyme hydrolytic process20. This discovery casted an interesting light on NAPE-PLD as a point where FAE signaling and BA physiology converge21. However, despite the relevance of this lipidic cross-talk, the chemical determinants of BAs contributing to turn the enzyme on remain unclear22.
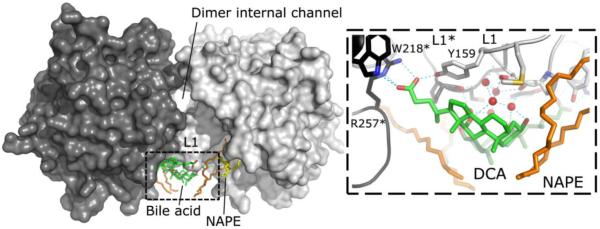
Figure 1.
Structure of human NAPE-PLD and bile acid interactions. Surface representation of the NAPE-PLD protein dimer (PDB code: 4qn9). The two subunits (dark gray and light gray) are partly separated by an internal channel having a diameter of ~9 Å. The subunits interact mainly thought their L1 loops. These loops bind bile acid molecules (carbon atoms in green) and the glycerophospholipidic substrate N-arachydonyl-PE (carbon atoms of the sn-1 and sn-2 fatty acid chain are in orange, while those of the sn-3 in yellow). The parallel orientation of the two monomers suggests that both subunits function concurrently by recruiting NAPE substrates from the membrane. The right panel shows details of the interaction between NAPE-PLD and deoxycholic acid (DCA). The bile acid carboxyl group interacts with residue Y159 of the L1 loop, together with W218 and arginine R257 of the opposite dimer subunit (highlighted by asterisks). The steroid hydroxyls form a network of hydrogen bonds (dotted lines) that involves five L1-bridging water molecules (red spheres). Single-letter abbreviations of amino acids have been used for clarity.
Here we have investigated structure-activity correlations and binding kinetics of different natural BAs, and identified the steroidal hydroxylation pattern as the key element for enzyme recognition and activation. We have also evaluated the effect of DCA on NAPE-PLD dynamics by neutron scattering (NS), to characterize how the enzyme activation occurs. NS experiments provide quantitative measurements of the thermal mean square atomic fluctuations, and information on global protein dynamics in the timescale from picoseconds to nanoseconds23, 24. Results show that, in the presence of DCA, the enzyme limits protein structure fluctuations and enhances its maximum catalytic reaction rate towards the unsatured substrate N-arachydonyl-PE, as determined by lipid chromatography mass spectrometry.
RESULTS AND DISCUSSION
Structure-activity relationships of natural bile acids as NAPE-PLD modulators
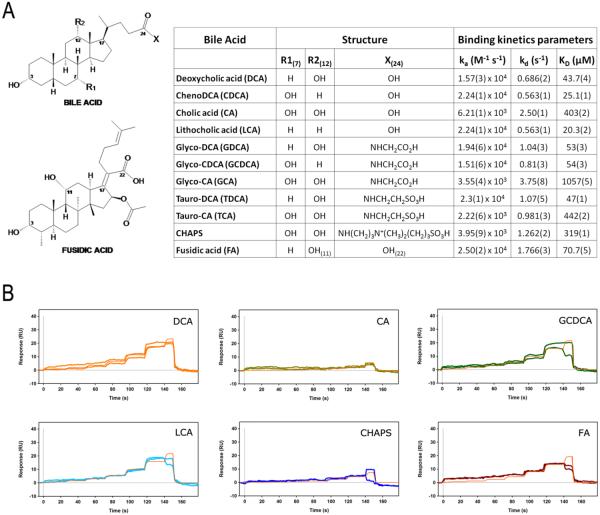
To investigate how NAPE-PLD recognizes major human endogenous BAs, we measured their binding affinity and kinetics using surface plasmon resonance (SPR) with fast-step injections25. For each compound sensorgram, the values of label-free biomolecular interaction expressed as association constant, ka (M−1 s−1), dissociation constant, kd (s−1), and binding constant, KD = kd / ka (μM), are reported in Figure 2A. Interaction kinetics of different BAs showed similar fast association and dissociation rates (Figure 2B), in line with the constitutively exposed binding site on the surface of the protein dimer (Figure 1). The affinities of BAs spanned from ~20 μM to ~1 mM, and BAs had similar dissociation constants (from 0.5 s−1 to 2.5 s−1). Stronger binders showed values of ka in the order of ~104 M−1 s−1, while weaker binders had values of ka in the order of ~103 M−1 s−1, suggesting the kinetics of association mainly influences the affinity of BAs.
Figure 2.
Bile acid recognition by NAPE-PLD. (A) Kinetics parameters of bile acids and compound analogues (CHAPS, Fusidic acid) against NAPE-PLD, determined at 25 °C using surface plasmon resonance. (B) FastStep kinetics response profiles (and their replicates) for selected compounds. The concentration profile of the association phase was created within the biosensor SensìQ Pioneer using a twofold dilution series, and 100 μM as the highest analyte concentration. Red lines show a global fit to the response data used to extract the binding constants reported in the table (A).
The monohydroxy BA lithocholate (LCA, 3α) showed the highest affinity for NAPE-PLD (KD ≈ 20 μM) (Figure 2A). The dihydroxy BAs, chenodeoxycholic acid (CDCA, 3α 7α) and deoxycholate acid (DCA, 3α 12α), had a KD ≈ 25 μM and ≈ 43 μM, respectively. Addition of a third hydroxyl group to the steroid acid moiety reduced of ~tenfold the interaction affinity, as the trihydroxy cholic acid (CA, 3α 7α 12α) bound weakly to the enzyme (KD ≈ 403 μM) (Figure 2A). These results revealed that the enzyme bound preferentially more hydrophobic BAs, and it was sensitive to the presence and position of hydroxyl groups at the steroidal body. The correlation trend between affinities and hydroxyl groups resulted also for natural BAs that were conjugated with glycine and taurine (Figure 2A). For example, glycodeoxycholic acid (GDCA) and taurodeoxycholic acid (TDCA) showed binding affinities that were similar to that observed for DCA, while the affinities of taurocholic acid (TCA) and the synthetic cholic acid-derivative CHAPS resulted ~tenfold lower (Figure 2A).
Interesting, despite a different side chain substitution in position C17 (Figure 2A), the bile acid-analogue fusidic acid (FA) exhibited a KD ≈ 70 μM (Figure 2A). The structure of this natural antibiotic26, 27 had two free hydroxyl groups in α-orientation (3α, 11α) that could contribute to the interaction with the enzyme. These observations indicate that the number and orientation of the free hydroxyls drive the interaction between the bound steroid and the protein dimer, possibly involving water molecules located in between (Figure 1).
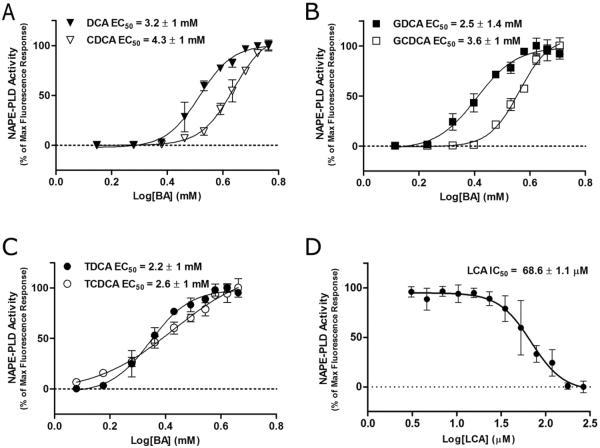
Comparative dose-response curves in the fluorescence-based activity assay showed that both DCA and CDCA activated NAPE-PLD upon binding, and DCA was slightly more efficient (EC50 ≈ 3.2 mM) to enhance the activity of the enzyme (EC50 ≈ 4.3 mM) (Figure 3A). Their glycine and taurine conjugates also promoted the catalysis of NAPE-PLD, supporting the observation that NAPE-PLD was more sensitive to the hydroxylation in position C12α versus C7α (e.g. GDG, EC50 ≈ 2.5 mM; GCDG, EC50 ≈ 3.6 mM) (Figure 3B–C).
Figure 3.
Modulation of NAPE-PLD enzyme activity by different natural bile acids. (A–D) Effect of different bile acids on enzyme activity, expressed as fluorescence spectroscopic changes observed during turn-on/off assay. All graphs were obtained by fluorescence-based assay following the reaction (at 25 °C for 30 min) between the enzyme (25 nM), pre-incubated with increasing concentrations of the bile acids and the substrate PED6. LCA was tested in the presence of DCA (0.2%) to solubilize the substrate (D).
As reported above, the monohydroxy BA LCA bound NAPE-PLD (Figure 2). However, LCA was not able alone to solubilize the substrate and activate the membrane enzyme (not shown). We thus evaluated its effect in the presence of DCA at 0.2% (w/v), where the enzyme had maximum activity20. Contrary to other examined natural BAs, LCA showed to inhibit the DCA-induced activation of the enzyme with an IC50 ≈ 68 μM (Figure 3D). Overall, these findings highlighted the crucial role of the hydroxyl groups in determining (i) BA physicochemical profile, (ii) affinity for NAPE-PLD, as well as (iii) enzyme activation level. We tried to structurally rationalize these results by x-ray crystallography, but unfortunately, all attempts to crystallize NAPE-PLD in complex with different BAs were unsuccessful.
Deoxycholic acid restricts protein structure fluctuations
We next asked whether the interaction between BA and NAPE-PLD (Figure 1) affects protein global flexibility. We performed elastic incoherent neutron scattering (EINS) experiments in the presence of different concentrations of DCA at the high-resolution backscattering spectrometer IN13 of the Institut Laue-Langevin (ILL, Grenoble - France), to spotlight possible protein dynamics transitions occurring in the accessible space-time window (~2 Å in ~0.1 ns)28. The compound DCA was selected for the availability of a three-dimensional description of its complex with the enzyme.
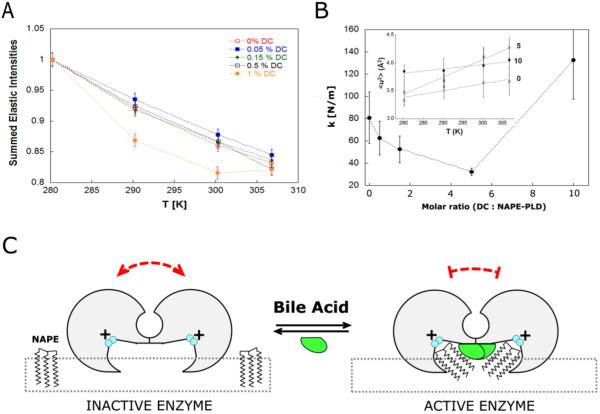
From a first visual inspection of the EINS intensities - summed of the available scattering vector (Q) range (0.3 < Q < 4.9 Å−1) and plotted as a function of temperature (280K, 290K, 300K, and 307K) -, we observed a significant effect of DCA concentration on the dynamics of protein atoms (Figure 4A). The EINS experiments were carried out in D2O buffer, at DCA concentrations of 0.00%, 0.05%, 0.15%, 0.50%, 1.00% (w/v), corresponding to BA / NAPE-PLD ratios of 0.0, 0.5, 1.5, 5.0, and 10.0, respectively. Indeed, in the absence of DCA, the normalized sum of elastic intensities decreased almost linearly with the temperature, and the scattering intensities were almost not affected by the addition of DCA up to a concentration of 0.15% (w/v). On the contrary, the protein atomic fluctuations were not linear when the concentration of DCA exceeded ~5 time that of the enzyme (Figure 4B).
Figure 4.
The interaction of bile acids restricts NAPE-PLD structure fluctuations. (A) Elastically scattered intensity binned over the explored range of momentum transfer (Q) as a function of temperature for NAPE-PLD at different concentrations of the bile acid DCA (left panel). (B) Dependence of the force constant k (resilience) upon the molar ratio [DCA : NAPE-PLD]. Inset: temperature dependence of the mean square displacements (< u2 >) for the complex between NAPE-PLD and DCA, at molar ratio 0, 5 and 10. (C) Hypothetical mechanism of bile acid-mediated restriction of protein dynamics. Functional, structural and dynamics measurements are consistent with a model where bile acids (green) promote the assembly of inactive NAPE-PLD subunits (gray) into an active dimer, which has a reduced dynamics. The resulting lipid-protein complex can recognize the substrate NAPE at the membrane interface (square dots) to promote its hydrolysis. The positively charged binuclear zinc center of the active site is colored in cyan.
We further quantified how much the protein flexibility was affected by the increase of DCA concentration, measuring the temperature dependence of the atomic mean square displacement <u2> (Å2) (Figure 4B), which was calculated using equation (2) (Methods). The slope of the scan represented the protein dynamics (pseudo-)force constant k (N/m) - equation (3) (Methods) -, or “resilience” of the protein, as introduced by Zaccai in Science23. The moderate reduction of the k value to the increase of the molar ratio between DCA and protein (Figure 4B, inset) suggested an initial slightly higher protein mobility acquired by NAPE-PLD, possibly due to the presence of the BA detergent molecules in solution. On the other hand, at higher concentration of DCA (~10 times that of the enzyme) the system lost flexibility, and the DCA-mediated restriction of protein dynamics fluctuations became evident by the slope of the scan (Figure 4B, inset). Interestingly, the reported crystal structure of NAPE-PLD20 showed coordinates for eleven molecules of DCA bound to the protein dimer (Figure 1), suggesting how these molecules might contribute to the stiffness of the enzyme (Figure 4C).
Bile acids regulate the biosynthesis of FAEs
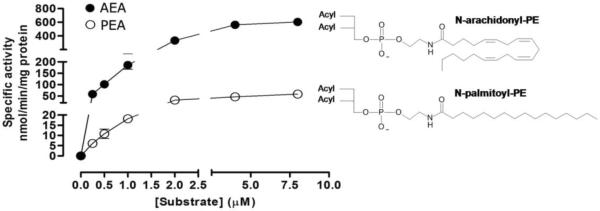
To study whether bound BA molecules regulate enzyme substrate specificity, we measured the enzyme kinetic parameters against N-arachidonoyl-PE and N-palmitoyl-PE in the presence of DCA (0.2% w/v) (Figure 5). Lipid mass spectrometry electrospray ionization results showed Km values of ~9 μM for both substrates, suggesting a similar affinity for NAPE-PLD. These values were consistent with those obtained previously using a radioactivity-based assay (~3 μM), where the enzymatic reactions were performed in a buffer solution containing the synthetic detergent Triton X-100 (0.1%) for substrate solubilization and enzyme activation18.
Figure 5.
Rate of the bile-acid activated enzyme versus NAPE. Human NAPE-PLD (0.3 μg of protein) were allowed to react with various concentrations of the substrates N-arachidonoyl-PE and N-palmitoyl-PE in the presence of the cofactor DCA (0.2%, corresponding to the maximum enzyme activation), at 37 °C for 15 min. The graph shows actual data points for the products arachidonylethanolamide (AEA, solid symbols) and palmitoylethanolamide (PEA, open symbols), their best fit line (Prism software). Km values for the two substrates were 9.6 (± 4.2) μM and 9.2 (± 1.9) μM, respectively. The maximum velocity (Vmax) resulted 1131 (± 124.4) nmol/min/mg protein for N-arachidonoyl-PE and 155.8 (± 38.36) nmol/min/mg protein for N-palmitoyl-PE. UPLC-MS/MS assays were repeated three times for each substrate.
Despite similar Km values, in the presence of the cofactor DCA the values of Vmax were ~1131 nmol/min/mg protein for N-arachidonoyl-PE and ~156 nmol/min/mg protein for N-palmitoyl-PE (Figure 5), revealing the enzyme was not equally active against NAPE with different N-acyl groups. Hence, the DCA-complexed NAPE-PLD resulted much faster in hydrolyzing the more unsatured substrate N-arachidonoyl-PE with respect to N-palmitoyl-PE. Indeed, when the specific interaction of BAs for NAPE-PLD had not been discovered yet, the values of Vmax were reported to be similar (~73 nmol/min/mg and ~98 nmol/min/mg, respectively)18. Consistent with our results, measurements obtained recently by brain lipidomics analysis showed that all FAEs were significantly reduced in both the “Luquet line” and “Deutsch line” of NAPE-PLD(−/−) knockout mice29. However, in the “Luquet line” the magnitude of the effect was stronger for N-arachidonoyl ethanolamine and other unsatured long N-fatty acid chains (N-docosahexaenoyl ethanolamine and N-linoleoyl ethanolamine), compared with N-oleoyl ethanolamine and N-palmitoyl ethanolamine29.
Conclusions
In the present study we have identified the structural determinants of BAs responsible for recognition and modulation of NAPE-PLD, and investigated how this process occurs. Our analyses demonstrate that the enzyme recognizes the cofactors for the number and position of hydroxyl groups at the steroidal body. The hydroxylation pattern of BAs anchors the BA to the L1 loops that interconnect the two protein subunits, and contributes to cluster a small number of water molecules at the BA-enzyme interface (Figure 1). Structure20 and protein dynamics show that when molecules of BA are bound to their allosteric binding sites, the amplitudes of protein thermal motions are restricted, facilitating the active site pocket to accommodate different NAPEs and stimulating the enzyme to promote the catalysis (Figure 4C). Importantly, the complex BA-enzyme enhances the reaction rate with the substrate N-arachidonoyl-PE, suggesting the interaction between NAPE-PLD and BAs, and the stiffening of the enzyme dynamics might favor the selective production of the endocannabinoid anandamide and other unsatured long FAEs. Contrary to DCA, the secondary BA LCA inhibits the enzyme activation in the low μM range (~68 μM). We attempted to investigate by X-ray crystallography how LCA might promote this effect, but high concentrations of DCA (> 2.0 mM) were indispensable to efficiently solubilize and crystallize the membrane protein, thus preventing LCA binding. However, this observation supports the hypothesis that the ratio between the hydrophobic and cytotoxic LCA30 and DCA might affect the regulation of FAE biosynthesis. Major free and conjugated dihydroxy BAs function similarly as cofactors to activate NAPE-PLD, providing information that may guide the design of specific BA-based allosteric enzyme modulators. In vitro, the BA concentration required for a half-maximal response spans from ~2 to ~4 mM. Consequently, dihydroxy BAs bind and activate NAPE-PLD at concentrations that can be found in the gut during food digestion, where the total mean postprandial concentrations of BAs span from ~10 mM in the upper ileum to ~2 mM in the lower ileum31. On the contrary, we expect that the trihydroxy bile acid CA might result in poorly associated to NAPE-PLD, considering that it has a ~tenfold lower binding affinity for the enzyme (this work), the two primary BAs CDCA and CA show a similar content in the human BA pool (~40%), and dihydroxy conjugates comprised 76% of the primary BAs in the serum32−35. In vivo studies are required to assess the physiological meaning of our findings.
METHODS
Protein expression and purification
Protein expression and purification was performed as described by20. pMAL c5x vector harboring the gene of nape-pld (Δ47) was transformed into Escherichia coli RosettagamiB (DE3) pLysS cells (Novagen) to overexpress the encoded recombinant protein with an N-terminus Maltose binding protein (MBP) tag and a C-terminus hexahistidine tag. The cells were first grown at 37 °C to an absorbance at 600 nm of 0.7, and protein expression was induced with 250 μM isopropyl-β-D-thiogalactoside (IPTG) (Sigma) at 28 °C for 16 h. The cells were then harvested by centrifugation (4,500 g, 20 min, 4 °C) treated with 1mg/ml of lysozyme and frozen at −20 °C.
Cells were then resuspended in ice cold lysis buffer 0.02M HEPES pH 7.8, 0.2M NaCl, EDTA free protease inhibitor cocktail (Sigma) and lysed by sonication on ice. After removal of the debris by centrifugation (12,000 g, 1h, 4 °C), the supernatant was mixed with 5 mL of Amilose Sepharose resin (New England Biolabs) and incubated on a rotor for 4h at 4 °C. The MBP-tagged protein was then eluted using lysis buffer without protease inhibitors, and digested with recombinant factor Xa protease (Sigma-aldrich) according to the manufacturer's suggestions.
The protein was finally purified by using Ni-affinity (eluting with 0.02M HEPES pH 7.8, 0.2M NaCl and 500mM imidazole) and. size-exclusion chromatography with Superdex 16/600 200pg (GE Healthcare) column pre-equilibrated with buffer 0.02M HEPES pH 7.8, 0.2M NaCl in the absence or presence of 0.1% sodium deoxycholate acid (DCA).
Binding kinetics
Kinetic parameters of the interaction between NAPE-PLD and bile acids were measured by SPR (Surface Plasmon Resonance) using FastStep injections25 at 25 °C (Biosensor SensíQ Pioneer, ICX Technologies). The protein sample was minimally biotinylated on ice for 3h using an equimolar concentration of sulfo-NHS-LCA-biotin, and passed through a Superdex-200 gel filtration column (buffer 0.02M HEPES pH 7.8, 0.2M NaCl) to remove the excess of free biotin. Streptavidin was immobilized onto a COOH5 sensor chip using a standard amine-coupling method and HBS as running buffer. This coupling method resulted in a density ~15,000 RU (resonance units) of neutravidin on all flow cells. Biotinylated NAPE-PLD (Δ47) was then captured to densities of ~3000 RU. The kinetic profile of BA binding was obtained using 100μM as the highest concentration and Faststep injections at a flow rate of 30μL/min. The response data were processed with QDAT program of the biosensor using a reference surface to correct for any bulk refractive index changes and blank injections for double referencing. The binding profiles were fit globally to a 1:1 interaction model.
Neutron scattering
Incoherent neutron scattering of protein samples is dominated by hydrogen atoms23, because hydrogen comprises approximately 50% of all protein atoms, and the incoherent scattering cross sections of hydrogen (80.27 barn) is significantly larger than that of other atoms in biological macromolecules (nitrogen 0.5 barn, carbon and oxygen 0.001 barn). Hydrogen atoms are homogeneously distributed in biological macromolecules, thus neutron scattering can probe average molecular dynamics. In order to enhance the scattering contribution of NAPE-PLD atoms and neglect that of the buffer, the purified protein was concentrated and re-suspended five times against a 100Xvolume of D2O (deuterium 2.08 barn).
Temperature-dependent EINS scans were performed on the thermal neutron high resolution backscattering spectrometers IN13 of the Institut Laue-Langevin (ILL), Grenoble, France. With a nearly Q-independent energy resolution of δE = 8 μeV (full width at half maximum) and an accessible momentum transfer range of 0.2 < Q < 4.9 Å−1. IN13 allows the investigation of molecular motions on a time scale up to 100 ps and with an amplitude from 1.3 Å to ~31 Å. Elastic neutron scattering spectra of NAPE-PLD (100 mg/mL) were collected in the temperature range 280 – 310 K, with ~3 hours of acquisition time per point to optimize the signal to noise ratio.
The program LAMP36 was used for data reduction, consisting in removal of the empty cell contribution and normalization with respect to a vanadium scan (a totally incoherent sample) to compensate for differences in detector efficiency and geometry. In order to avoid corrections from multiple scattering events, cell thickness and geometry were properly chosen to minimize neutron absorption from the sample. A typical transmission of ~95% was guaranteed using standard flat aluminum sample holder with a thickness of 0.4 mm.
EINS data analysis
The scattered elastic incoherent intensity can be described in a Gaussian approximation by the dynamic structure factor at zero energy exchange28, 37.
| (1) |
where ΔE is instrumental energy resolution, related to the time window through Heisenberg's uncertainty principle, and <u2> is the average time-dependent atomic mean square displacement in the limit defined by ΔE38.
Similarly, to the Guinier approximation in small angle scattering, the Q range of validity for the Gaussian approximation depends on the geometry of the motion and could go as far as <u2> Q2 ≈ 439. For each temperature the <u2> can be obtained by the slope of the semi-logarithmic plot of the incoherent scattering function through
| (2) |
An effective average force constant for sample dynamics, < k >, can be calculated from the slope of <u2> as a function of temperature, by applying a quasi-harmonic approximation23:
| (3) |
Enzyme activity assays
The activity of NAPE-PLD in the presence of different BAs was measured by fluorescence assay as described previously20. Briefly, the enzyme was diluted to a final concentration of 25 nM in assay buffer (50 mM Tris-HCl, pH 8), pre-incubated for 1h at room temperature with varying concentrations of bile acids (0–5 mM). The substrate PED6 [N-((6-(2,4-Dinitrophenyl)amino)hexanoyl)-2-(4,4-Difluoro-5,7-Dimethyl-4-Bora-3a,4a-Diaza-s-Indacene-3-Pentanoyl)-1-Hexadecanoyl-sn-Glycero-3-Phosphoethanolamine, Triethylammonium Salt] in 2.5% DMSO/Ethyl acetate (1:4 v/v) was added to a final concentration of 10μM in the reaction buffer (1% DMSO/Ethyl acetate mixture), and the fluorescence measured after 30 minutes of incubation in the dark at 25 °C, using emission filter at 485 nm and excitation filter at 535 nm. The effect on activity of LCA was measured in the presence of 0.2% w/v DCA in the reaction buffer to solubilize the substrate and activate the enzyme. Plates were finally incubated for 30 min at 25 °C in the dark. Reaction fluorescence was measured using the EnVision 2104 Multilabel Plate Reader and results analyzed by Prism software.
The effect of DCA on enzyme kinetics was measured in multiple reaction monitoring by ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS). The protein (630 ng) was incubated with different substrate concentrations, N-arachidonoyl-PE or N-palmitoyl-PE, in [0.3 ml of 0.05 M Tris-HCl buffer pH 7.4, 0.2% w/v DCA], for 15 min at 37 °C. Reactions were stopped by addition of cold acetonitrile containing either PEA-d4 or AEA-d4 as internal standard, assuming that a steady state was reached (Michaelis Menten condition). After centrifugation (3000×g at 4 °C for 20 min), the organic layers were collected and used for the UPLC-MS/MS acquisition. LC-MS/MS analyses were carried out on an Acquity UPLC system coupled with a Xevo TQ-MS triple quadrupole mass spectrometer (Waters Inc. Milford, MA, USA). Chromatographic separation was achieved using a BEH C18 column and a flow rate elution of 0.5 mL/min. Analytes were quantified by multiple reaction monitoring (MRM) in the positive ESI mode. Data were acquired by MassLynx software and quantified by TargetLynx software. Calibration curves were obtained by plotting the analyte to IS peak areas ratio versus the corresponding analyte concentration using weighted (1/x) least square regression analysis. Each experiment was run in triplicate. Prism software was used to fit the velocity/concentration profiles and determine the Michaelis Menten kinetic parameters (Vmax and Km) for the products arachidonylethanolamide and palmitoylethanolamide.
ACKNOWLEDGMENTS
The financial support of Marie Curie Action IRG (FP7-PEOPLE-2010-RG, contract no PIRG07-GA-2010-268385 to P. Magotti) and the National Institute on Drug Abuse (grants DK073955 and DA012413 to D. Piomelli) is gratefully acknowledged. We thank A. Armirotti, L. Bono, and M. Pini for assistance with systems of mass spectrometry and liquid handling. E. Margheritis, S. Peruzzi, and F. Natali performed and analyzed neutron scattering studies; P. Magotti designed and performed SPR assays; B. Castellani, P. Magotti, and E. Romeo performed fluorescence and LC-MS assays; S. Mostarda analyzed bile acid purity; G. Garau directed the project, designed experiments, analyzed and interpreted the results, and wrote the manuscript, together with A. Gioiello and D. Piomelli.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Wymann MP, Schneiter R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- (2).Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- (3).Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- (4).Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- (5).Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- (6).Hansen HS. Role of anorectic N-acylethanolamines in intestinal physiology and satiety control with respect to dietary fat. Pharmacol. Res. 2014;86:18–25. doi: 10.1016/j.phrs.2014.03.006. [DOI] [PubMed] [Google Scholar]
- (7).Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- (9).Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316:1212–1216. doi: 10.1126/science.1137406. [DOI] [PubMed] [Google Scholar]
- (10).Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- (11).Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, Cuomo V, Piomelli D. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Folick A, Oakley HD, Yu Y, Armstrong EH, Kumari M, Sanor L, Moore DD, Ortlund EA, Zechner R, Wang MC. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science. 2015;347:83–86. doi: 10.1126/science.1258857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, Piomelli D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- (14).Solorzano C, Zhu C, Battista N, Astarita G, Lodola A, Rivara S, Mor M, Russo R, Maccarrone M, Antonietti F, Duranti A, Tontini A, Cuzzocrea S, Tarzia G, Piomelli D. Selective N-acylethanolamine-hydrolyzing acid amidase inhibition reveals a key role for endogenous palmitoylethanolamide in inflammation. Proc. Natl. Acad. Sci. U.S.A. 2009;106:20966–20971. doi: 10.1073/pnas.0907417106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Zhu C, Solorzano C, Sahar S, Realini N, Fung E, Sassone-Corsi P, Piomelli D. Proinflammatory stimuli control N-acylphosphatidylethanolamine-specific phospholipase D expression in macrophages. Mol. Pharmacol. 2011;79:786–792. doi: 10.1124/mol.110.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Alhouayek M, Muccioli GG. Harnessing the anti-inflammatory potential of palmitoylethanolamide. Drug Discovery Today. 2014;19:1632–1639. doi: 10.1016/j.drudis.2014.06.007. [DOI] [PubMed] [Google Scholar]
- (17).Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- (18).Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- (19).Schmid HH. Pathways and mechanisms of N-acylethanolamine biosynthesis: can anandamide be generated selectively? Chem. Phys. Lipids. 2000;108:71–87. doi: 10.1016/s0009-3084(00)00188-2. [DOI] [PubMed] [Google Scholar]
- (20).Magotti P, Bauer I, Igarashi M, Babagoli M, Marotta R, Piomelli D, Garau G. Structure of human N-acylphosphatidylethanolamine-hydrolyzing phospholipase D: regulation of fatty acid ethanolamide biosynthesis by bile acids. Structure. 2015;23:598–604. doi: 10.1016/j.str.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Kostic M. Bile Acids, Chemical Libraries, Ebola Virus, and Bacterial Uracil. Chemistry & Biology. 2015;22:427–428. [Google Scholar]
- (22).Zorn JA, Wells JA. Turning enzymes ON with small molecules. Nat. Chem. Biol. 2010;6:179–188. doi: 10.1038/nchembio.318. [DOI] [PubMed] [Google Scholar]
- (23).Zaccai G. How soft is a protein? A protein dynamics force constant measured by neutron scattering. Science. 2000;288:1604–1607. doi: 10.1126/science.288.5471.1604. [DOI] [PubMed] [Google Scholar]
- (24).Frauenfelder H, Mezei F. Neutron scattering and protein dynamics. Acta Crystallogr., Sect. D: Biol. Crystallogr. 2010;66:1229–1231. doi: 10.1107/S0907444910022985. [DOI] [PubMed] [Google Scholar]
- (25).Rich RL, Quinn JG, Morton T, Stepp JD, Myszka DG. Biosensor-based fragment screening using FastStep injections. Anal. Biochem. 2010;407:270–277. doi: 10.1016/j.ab.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Godtfredsen WO, Jahnsen S, Lorck H, Roholt K, Tybring L. Fusidic acid: a new antibiotic. Nature. 1962;193:987. doi: 10.1038/193987a0. [DOI] [PubMed] [Google Scholar]
- (27).Fernandes P, Pereira D. Efforts to support the development of fusidic acid in the United States. Clin. Infect. Dis. 2011;52(Suppl 7):S542–546. doi: 10.1093/cid/cir170. [DOI] [PubMed] [Google Scholar]
- (28).Zaccai G. Neutron scattering perspectives for protein dynamics. J. Non-Cryst. Solids. 2011;357:615–621. [Google Scholar]
- (29).Leishman E, Mackie K, Luquet S, Bradshaw HB. Lipidomics profile of a NAPE-PLD KO mouse provides evidence of a broader role of this enzyme in lipid metabolism in the brain. Biochim. Biophys. Acta. 2016;1861:491–500. doi: 10.1016/j.bbalip.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab. Rev. 2004;36:703–722. doi: 10.1081/dmr-200033475. [DOI] [PubMed] [Google Scholar]
- (31).Northfield TC, McColl I. Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut. 1973;14:513–518. doi: 10.1136/gut.14.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ahlberg J, Angelin B, Bjorkhem I, Einarsson K. Individual bile acids in portal venous and systemic blood serum of fasting man. Gastroenterology. 1977;73:1377–1382. [PubMed] [Google Scholar]
- (34).Hofmann AF. Enterohepatic circulation of bile acids. Comprehensive Physiology. 2011 [Google Scholar]
- (35).Angelin B, Björkhem I, Einarsson K, Ewerth S. Hepatic Uptake of Bile Acids in Man: FASTING AND POSTPRANDIAL CONCENTRATIONS OF INDIVIDUAL BILE ACIDS IN PORTAL VENOUS AND SYSTEMIC BLOOD SERUM. J. Clin. Invest. 1982;70:724–731. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Richard D, Ferrand M, Kearley G. Analysis and visualisation of neutron-scattering data. J. Neutron Res. 1996;4:33–39. [Google Scholar]
- (37).Rahman A, Singwi K, Sjölander A. Theory of slow neutron scattering by liquids. I. Phys. Rev. 1962;126:986. [Google Scholar]
- (38).Vural D, Hong L, Smith JC, Glyde HR. Motional displacements in proteins: The origin of wave-vector-dependent values. Phys. Rev. E. 2015;91:052705. doi: 10.1103/PhysRevE.91.052705. [DOI] [PubMed] [Google Scholar]
- (39).Réat V, Zaccai G, Ferrand M, Pfister C. Functional dynamics in purple membrane. Biol. macromol. dyn. 1997:117–122. [Google Scholar]