Abstract
Hepatic repair is directed chiefly by the proliferation of resident mature epithelial cells. Further if predominant injury is to cholangiocytes, the hepatocytes can transdifferentiate to cholangiocytes to assist in the repair and vice versa as shown by various fate-tracing studies. However, the molecular bases of reprograming remain elusive. Using two models of biliary injury where repair occurs via cholangiocyte proliferation and hepatocyte transdifferentiation to cholangiocytes, we identify an important role of Wnt signaling. First we identify upregulation of specific Wnt proteins in the cholangiocytes. Next, using conditional knockouts of Wntless and Wnt co-receptors LRP5/6, transgenic mice expressing stable β-catenin, and in vitro studies, we show a role of Wnt signaling through β-catenin in hepatocyte to biliary transdifferentiation. Lastly, we show that specific Wnts regulate cholangiocyte proliferation but in a β-catenin-independent manner. Conclusion: Wnt signaling regulates hepatobiliary repair after cholestatic injury in both β-catenin dependent and independent manners.
Keywords: cholestasis, transdifferentiation, EpCAM, CK19, DDC, Wntless, ductular reaction, Wnt7A, hepatocytes, cholangiocytes
Chronic cholestasis results from impairment of bile formation or bile flow and can progress to cirrhosis and hepatocellular insufficiency requiring transplantation (1). Mice fed the biliary toxin 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) exhibit characteristic features of chronic cholestasis, such as bile duct injury, cholangitis, and periductal fibrosis (2). DDC feeding also triggers a ductular reaction composed of both proliferating cholangiocytes as well as hepatocytes that acquire a cholangiocyte-like phenotype through a process called transdifferentiation (3). In fact, several high-profile studies in recent years have identified hepatocyte-to-cholangiocyte transdifferentiation, and vice versa, to promote hepatobiliary repair in the context of specific injuries (3–6). However, no studies to date have addressed the molecular mechanisms underlying such repair processes.
The Wnt/β-catenin pathway is a critical regulator of liver development and physiology, and has been investigated in a wide range of pathologies such as hepatocellular carcinoma, alcoholic liver disease, fibrosis, acute liver injury, and others (reviewed in (7)). Wnt proteins are extracellular signaling molecules that trigger activation of the canonical Wnt pathway and nuclear translocation of β-catenin by binding to its cell surface receptor Frizzled and co-receptor low-density lipoprotein related protein (LRP) 5/6. Because the post-translational modification of Wnt proteins required for bioactivation renders them hydrophobic, a molecular chaperone is required for transport of Wnts to the cell surface. This process is mediated by Wntless (Wls), a multipass transmembrane protein that conveys Wnts from the Golgi to the membrane for secretion (8). Cell-specific Wls deletion has divulged important roles for this signaling pathway in brain and bone development (9). In the liver, inhibiting Wnt secretion from Kupffer cells and endothelial cells has revealed that these cell types are an important source of Wnt proteins that in turn activate hepatocyte β-catenin during liver regeneration (10).
Although little is known about the impact of Wnt signaling in chronic liver injury, liver-specific β-catenin knockout (KO) mice exhibited significantly fewer cells staining positive for A6, a ductular and progenitor marker, after 2 weeks of DDC diet, suggesting that Wnt signaling may play a meaningful role in inducing atypical ductular proliferation during cholestasis (11). In order to explore this hypothesis, we performed a thorough characterization of Wnt signaling in mice on control and DDC diet. Through manual microdissection and liver cell isolation, we identify specific Wnts that are upregulated during cholestasis and address the cellular source of these Wnts. Through use of in vitro model systems, we demonstrate the role of these Wnts in hepatocyte-to-biliary transdifferentiation and cholangiocyte proliferation. Finally, we utilize liver-specific KO mice lacking either all Wnt signaling, or lacking canonical Wnt/β-catenin signaling only, to validate the importance of these Wnts in the repair of cholestatic liver disease in mice. Thus, we have identified that Wnt signaling is important for biliary repair in cholestatic liver disease, and that the blockade of Wnt secretion from EpCAM-expressing cells disrupts this process, resulting in increased liver injury and mortality in the setting of cholestatic liver disease.
Materials and Methods
Mice
Liver-specific Wls KO mice and liver-specific LRP5/6 KO mice were generated by as previously described (10). Mice overexpressing a stable form of β-catenin, where serine 45 is mutated to aspartic acid (S45D), have been characterized previously (12). Genotyping on all mice was performed by polymerase chain reaction (PCR) analysis using genomic DNA isolated from a tail clipping, as described previously (9, 10, 13, 14).
Wls KO, S45D mice, and corresponding controls were administered 0.1% DDC diet as described previously (11). Mice were sacrificed after 2 weeks or 1 month of diet, and livers harvested for subsequent analysis. LRP5/6 KO mice and controls were subjected to bile duct ligation (BDL) by double-ligating the common bile duct above the pancreas and cutting it between the ligatures (15). Mice were sacrificed 14 days after surgery. Blood samples were collected from the orbital sinus at the time of sacrifice. Serum biochemical measurements for total bilirubin and alanine aminotransferase (ALT) were performed by the University of Pittsburgh Medical Center Clinical Chemistry laboratory.
Cell lines
Hep3B human hepatoma cells, 293T human embryonic kidney cells, and AML12 mouse hepatocytes were obtained from the American Type Culture Collection (ATCC; Manassas, VA) and cultured as detailed in Supplementary Methods. To directly co-culture 293T cells and AML12 cells (or primary hepatocytes), 293T cells were first transfected with Wnt-expressing plasmids in 6-well plates using Lipofectamine 3000 (Invitrogen, Carlsbad, CA), as per the manufacturer’s instructions. Cells were allowed to incubate for 4 hours, after which an equal number of AML12 cells were added to the culture wells (2.5 × 105 cells of each type). Co-cultures were harvested 48 hours later for RNA analysis.
For analysis of transdifferentiation, AML12 cells and Hep3B cells were treated with recombinant human Wnt7A (abcam, Cambridge, MA), which was added at a concentration of 500 ng/mL. Cells were harvested 48 hours later for RNA analysis or measurement of luciferase activity.
Cell separation
Parenchymal and non-parenchymal cells from mouse liver were isolated by a 2-step collagenase perfusion (16). The parenchymal cell fraction, which consists mostly of hepatocytes, also contains hepatic stellate cells due to their close physical association with hepatocytes in vivo. Positive selection of CD45+ cells and EpCAM+ cells from non-parenchymal cells was performed using the QuadroMACS column separation Kit (Miltenyi Biotech, Cambridge, MA) (10). Cells were then purified using the Dead Cell Removal Kit (Miltenyi Biotech). Anti-mouse CD45 antibody, anti-mouse EpCAM antibody (Biolegend, San Diego, CA) and microbeads were used according to the manufacturer’s instructions. To assess the purity of EpCAM+ cells, low expression of CD68 (Kupffer cells) and Tie2 (endothelial cells) was confirmed by qRT-PCR.
Additional methods are available in the online Supplementary Methods.
Results
Cells in the portal triad express Wnt 7A, 7B, and 10A after DDC diet
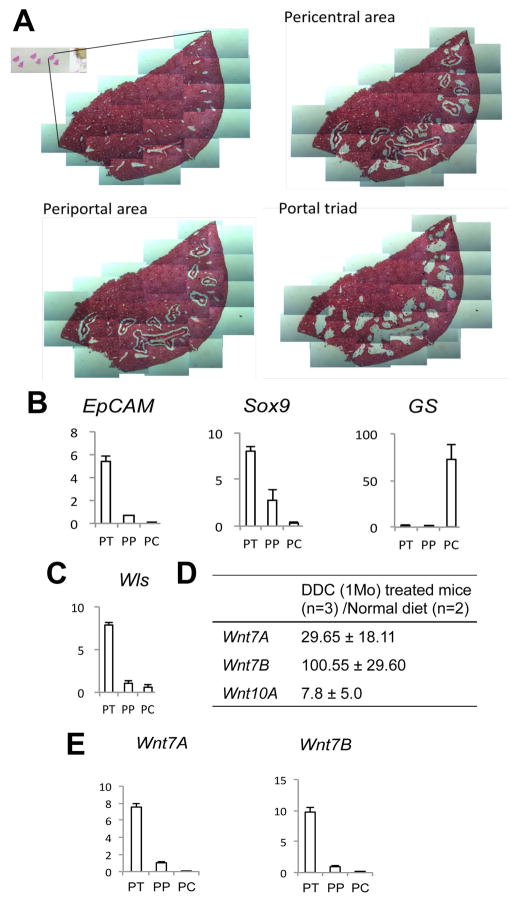
To address the role of Wnt signaling in biliary injury and cholestasis, we subjected WT mice to DDC diet and then sacrificed the mice after 1 month (28 days). The pericentral area (PC), periportal area (PP), and portal triad (PT) regions were then carefully removed from formalin-fixed, paraffin-embedded liver sections by manual microdissection and subjected to real-time PCR for gene expression analysis (Figure 1A). Measurement of glutamine synthetase (GS), which is expressed in pericentral hepatocytes, and EpCAM, which is expressed by normal cholangiocytes and oval cells in the portal triad (17), confirms the specificity of this isolation (Figure 1B). Sox9, another biliary marker, was expressed mainly in the PT; however, its presence in the PP region indicates the acquisition of a biliary-like phenotype by a population of hepatocytes around the PT, which is a hallmark of cholestatic liver disease. Interestingly, Wls expression was also highest in the PT region, indicating that either cholangiocytes or periportal hepatocytes may be the source of Wnts in this model (Figure 1C).
Figure 1. Spatial analysis of Wls and Wnts after DDC diet.
(A) Periportal (PP), pericentral (PC), and portal triad (PT) areas were dissected from H&E-stained frozen liver sections. (B) Expression of periportal and pericentral markers is shown by qRT-PCR. (C) Wls expression was detected mainly in the PT region, as shown by qRT-PCR. (D) Wnts 7A, 7B, and 10A were dramatically upregulated in the livers of DDC-fed mice compared to controls. (E) Expression of Wnt7A and Wnt7B was detected in the PT after DDC diet.
Next, expression of Wnt genes after 1 month of DDC diet was compared to normal liver. We identified three Wnts - Wnt7A, Wnt7B, and Wnt10A - to be remarkably increased after DDC diet (Figure 1D). Further analysis revealed that expression of Wnt7A and 7B, which showed the highest fold increase after DDC, was detected mainly in the PT (Figure 1E). Thus, during DDC-induced cholestasis, a significant upregulation of several specific Wnts occurs in cells of the PT region, which also coincides with the site of Wls expression.
EpCAM expressing cells are the source of Wnts during cholestasis
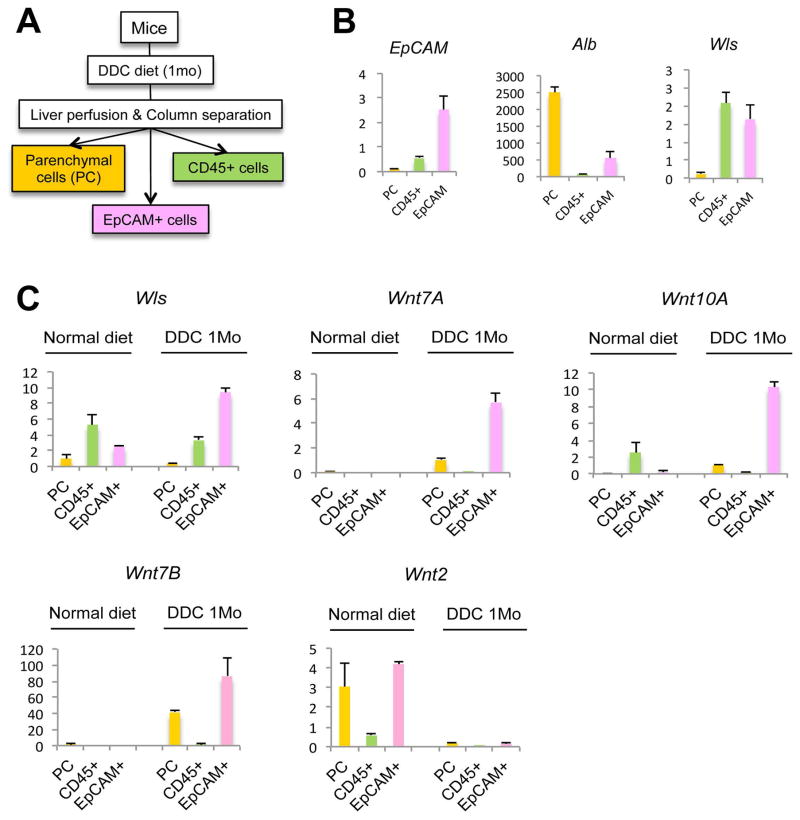
To determine which cell type in the PT is responsible for Wnt production during cholestasis, we isolated different cell populations from mouse livers subjected to DDC diet for 28D. CD45+ cells (macrophages), EpCAM+ cells (cholangiocytes), and parenchymal cells (PC; hepatocytes) were isolated by a two-step liver perfusion and column separation technique (Figure 2A). The purity of the cell populations obtained by isolation was verified by measuring the expression of EpCAM, which was highest in the EpCAM+ enriched cell population, and albumin, which was expressed mainly in the PC fraction (Figure 2B). Measurement of Wls expression in the cell fractions demonstrated that both CD45+ and EpCAM+ cells express Wls and could thus be the source of Wnt production in the PT during cholestasis. Further analysis of these cell populations before and after DDC diet, however, demonstrates a 3-fold increase in Wls expression in the EpCAM+ cells after DDC diet, while expression of Wls in the CD45+ population decreases, implicating EpCAM+ cells as the source of Wnts during cholestasis (Figure 2C). Indeed, measurement of Wnt7A, 7B, and 10A, the three Wnts increased after DDC diet, shows that expression of these genes, which is virtually absent on a normal diet, is dramatically increased in the EpCAM+ cell fraction after DDC, whereas Wnt2, which is expressed in normal liver, is absent after DDC treatment, demonstrating the specificity of Wnt expression during cholestasis (Figure 2C). Immunohistochemistry (IHC) for Wnt7A and Wnt10A also confirms the expression of these two proteins predominantly in cholangiocytes and in a few periportal hepatocytes after DDC injury (Supplementary Figure 1). Thus, EpCAM+ cells are the primary Wnt-producing cells in the PT during DDC-induced liver injury.
Figure 2. Cellular analysis of Wls and Wnts after DDC diet.
(A) Parenchymal cells (PC), EpCAM+ cells, and CD45+ cells were separated from non-parenchymal cells after liver perfusion. (B) Expression of EpCAM, albumin (Alb), and Wls in PC, CD45+ cells, and EpCAM+ cells is shown by qRT-PCR. (C) Wls, Wnt7A, Wnt7B, and Wnt10A expression increases in EpCAM+ cells after DDC treatment, while expression of Wnt2 decreases after DDC, as shown by qRT-PCR.
Wnt7B and Wnt10A promote proliferation of small cholangiocytes
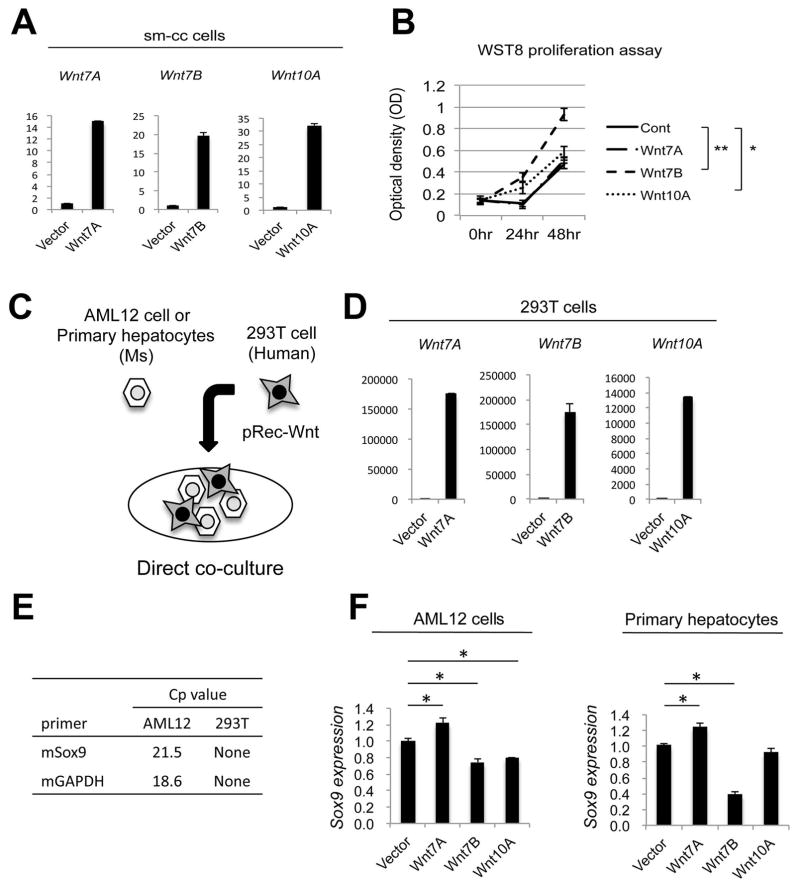
Because Wnts can act in an autocrine manner (18), and because cholangiocyte proliferation is rampant in the PT region after DDC, we then asked whether the Wnts produced by EpCAM+ cholangiocytes or progenitors had a mitogenic impact on cholangiocytes. A small mouse-SV40 cholangiocyte (sm-cc) cell line (19) was transiently transfected with Wnt7A, Wnt7B, or Wnt10A plasmids; successful transfection was confirmed by upregulation of the specific Wnt being expressed (Figure 3A). Interestingly, Wnt7B significantly induced proliferation of sm-cc cells, while Wnt10A had a modest impact on proliferation and Wnt7A had no effect (Figure 3B). Thus, EpCAM+ cells secrete Wnts that facilitate their proliferation in an autocrine fashion.
Figure 3. Wnt7B and Wnt10A induce proliferation of cholangiocytes, while Wnt7A increases Sox9 expression in hepatocytes.
(A) Expression of Wnt7A, Wnt7B, and Wnt10A expression in small cholangiocyte (sm-cc) cell cultures after transfection with Wnt-expressing plasmids. (B) A significant increase in proliferation as determined by Wst-8 assay was evident in sm-cc cells expressing Wnt7B or Wnt10A plasmid, but not Wnt7A. (C) Human 293T cells expressing either Wnt7A, Wnt7B, or Wnt10A were co-cultured with either mouse AML12 cells or primary mouse hepatocytes. (D) Wnt mRNA expression in 293T cells after transfection. (E) Mouse Sox9 and GAPDH mRNA expression in total cell lysates demonstrates the specificity of these primers in identifying gene expression from mouse hepatocytes only. (F) 293T cells expressing Wnt7A induce Sox9 expression in co-cultured AML12 cells and primary hepatocytes, as measured by qRT-PCR. * p < 0.05, ** p < 0.01.
Wnt7A induces Sox9 expression in cultured hepatocytes through activation of β-catenin
Because chronic biliary injury induces the reprogramming of hepatocytes to a biliary phenotype, we next wanted to determine whether the Wnts produced by EpCAM+ cells play any role in this process. We generated a direct co-culture system where 293T human embryonic kidney cells were transfected with Wnts 7A, 7B, or 10A and co-cultured with either mouse-derived AML12 cells or primary mouse hepatocytes (Figure 3C). Transfection of 293T cells with the individual Wnt plasmids induced a robust expression of the corresponding Wnt (Figure 3D). Primers were then designed to specifically detect the expression of mouse Sox9 in the co-cultures, thus excluding the contribution of any endogenous Sox9 expression in human 293T cells (Figure 3E). Sox9 expression was modestly but significantly increased in both AML12 cells and primary mouse hepatocytes co-cultured with Wnt7A-expressing 293T cells. Cells cultured with 293T cells expressing Wnt7B or Wnt10A showed either no effect on Sox9 expression or a decrease in Sox9 expression (Figure 3F).
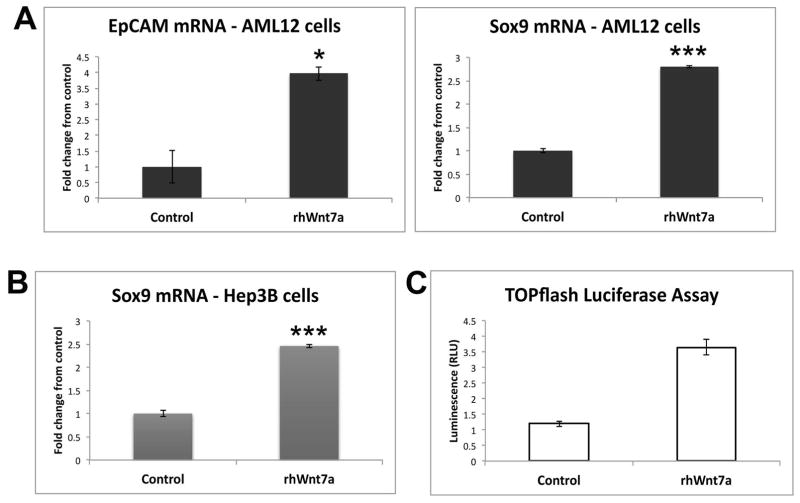
While this experiment replicated in vitro the paracrine effect of Wnt7A on hepatocytes and identified a preliminary role of Wnt7A in hepatocyte transdifferentiation, we wanted to validate these findings by measuring expression of biliary markers in cultured hepatocytes treated with recombinant Wnt7A protein. AML12 cells cultured in the presence of recombinant Wnt7A protein for 2 days showed significantly increased expression of both EpCAM and Sox9 mRNA (Figure 4A), further demonstrating that Wnt7A contributes to hepatocyte reprogramming. To demonstrate that this effect was observed across species, we utilized human hepatoma cells and confirmed that Wnt7A also increases Sox9 expression in Hep3B cells (Figure 4B). Next, we wanted to determine whether Wnt7A acts through the canonical Wnt signaling pathway, culminating in transcriptional activation of β-catenin, in order to activate Sox9 expression. We transfected Hep3B cells with TOPflash luciferase reporter, which measures β-catenin/TCF4-dependent transcriptional activation, in the presence or absence of Wnt7A. Figure 4C shows that Wnt7A significantly increases luciferase activity, suggesting that β-catenin-dependent Wnt signaling promotes reprogramming toward a biliary phenotype.
Figure 4. Recombinant Wnt7A protein induces expression of biliary markers in vitro through activation of β-catenin.
(A) Expression of EpCAM and Sox9 is increased in AML12 cells treated with recombinant human Wnt7A. (B) Sox9 expression is significantly increased in Hep3B cells treated with recombinant human Wnt7A. (C) Treatment of Hep3B cells with recombinant human Wnt7A increases TOPflash reporter activity. *-p<.05; ***-p<0.001
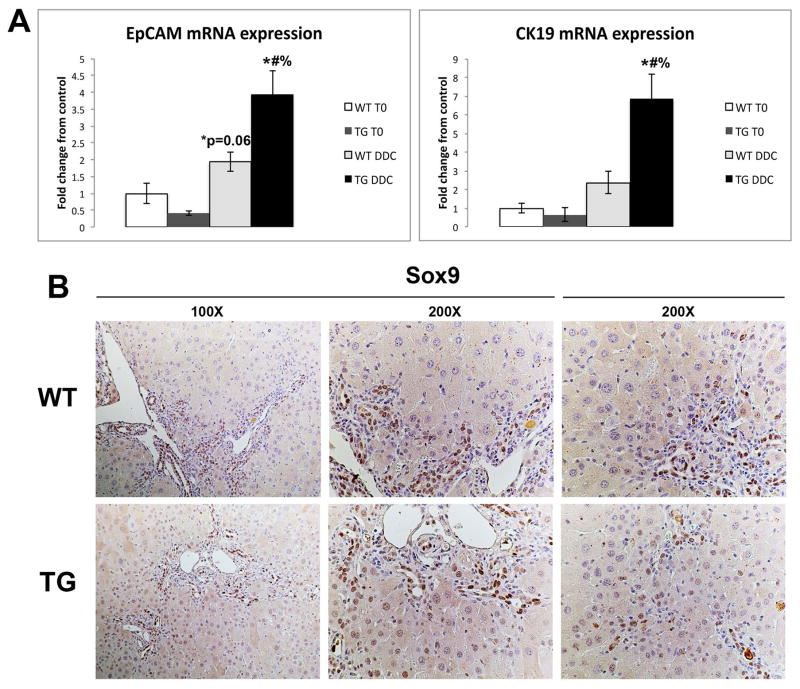
Mice overexpressing Ser-45 mutant β-catenin show increased hepatocyte-to-biliary transdifferentiation after DDC diet
We have previously published that mice expressing a mutated non-degradable form of β-catenin (S45D) in liver have a significant number of hepatocytes expressing cholangiocyte markers after long-term exposure to DDC compared to age-matched WT mice (20). In order to verify the role of Wnt/β-catenin signaling in hepatocyte reprogramming in vivo, we performed a detailed analysis of livers from WT and S45D mice on DDC diet. We observed that increased expression of biliary markers EpCAM and cytokeratin 19 (CK19) occurs as early as 28D after DDC in S45D mice (Figure 5A). Furthermore, S45D hepatocytes in the periportal region also begin to express Sox9, a transcription factor normally restricted to bile ducts, as shown by IHC (Figure 5B) (21). Therefore, activation of β-catenin enhances hepatobiliary repair after DDC injury by inducing transdifferentiation of hepatocytes to cholangiocytes.
Figure 5. Expression of biliary markers increases in S45D mice after DDC diet.
(A) qRT-PCR shows that expression of EpCAM and CK19 is increased in S45D mice after 1 month of DDC as compared to WT controls. (B) IHC shows the presence of Sox9 in the nuclei of S45D periportal hepatocytes after DDC, which is absent in WT. *-p<.05 vs. WT T0; #-p<.05 vs. S45D T0; %-p<.05 vs. WT DDC
Liver-specific Wls KO mice show decreased ductular response and increased mortality following DDC diet
In order to assess the impact of inhibiting Wnt signaling during cholestasis, we generated liver-specific conditional Wls KO mice and performed a detailed analysis of Wls gene expression in various cell populations at baseline. After confirming deletion of Wls in the KO liver (Supplementary Fig. 2A), we separated non-parenchymal cells - containing Kupffer cells (Kp), endothelial cells (EC), and cholangiocytes, or biliary epithelial cells (BEC) - from parenchymal cells, which contain hepatocytes (HC) as well as hepatic stellate cells (HSC) (Supplementary Fig. 2B), and measured Wls expression in these two cell populations. As shown in Supplementary Fig. 2C, Wls expression is much higher in non-parenchymal cells than in parenchymal cells; however, parenchymal cells from KO livers have significantly less Wls expression than WT livers, confirming efficient knockdown of Wls gene expression in hepatocytes. Given the previous observation that the Alb-Cre system deletes floxed target genes in cholangiocytes as well (11), we looked at Wls expression in EpCAM+ cells. Indeed, Wls expression is remarkably suppressed in EpCAM+ cells isolated from the KO as compared to WT (Supplementary Fig. 3A). This was confirmed by Western blotting showing loss of Wls in EpCAM+ cells, which also express CK19 (Supplementary Fig. 3B).
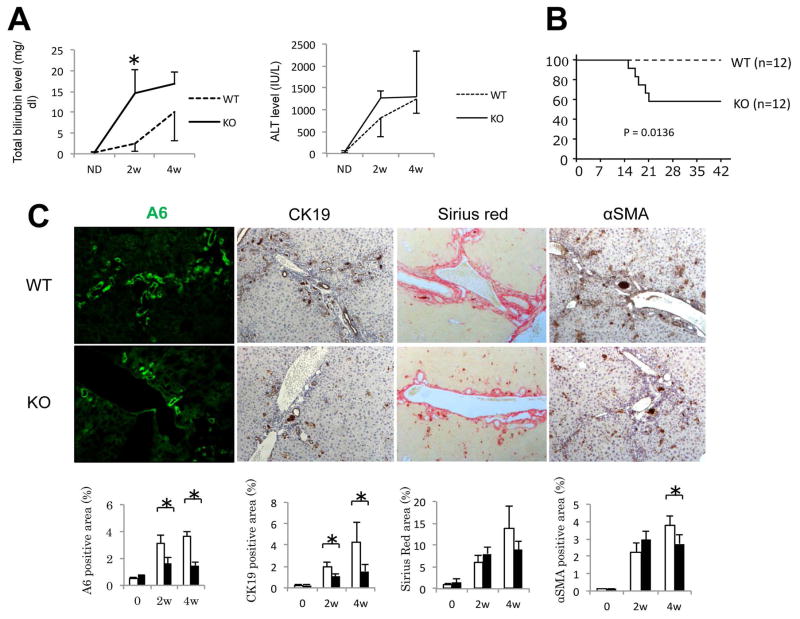
Next, we administered DDC diet to WT and Wls KO mice for 28D. Analysis of serum biochemistry from both groups of animals revealed higher total bilirubin and alanine aminotransferase (ALT) levels in KO as early as 2 weeks after DDC diet (Figure 6A). These findings corresponded with increased mortality in Wls KO mice fed DDC diet (Figure 6B). Histological analysis demonstrated that KO had fewer cells positive for A6, fewer CK19 positive cells, and fewer myofibroblasts, although there was no overall difference in fibrosis between the two groups (Figure 6C). Thus, blocking Wnt secretion from hepatocytes and cholangiocytes resulted in fewer bile ducts, which negatively affected overall survival.
Figure 6. Wls KO have more hepatic damage, less ductular proliferation, and decreased survival after DDC diet.
(A) Serum total bilirubin and ALT levels are higher in Wls KO than WT after DDC diet. * p < 0.05. (B) Kaplan-Meier survival analysis shows a significant decrease in survival of Wls KOs on DDC diet. (C) Quantification of immunohistochemistry for A6 (200×), CK19 (100×), Sirius red (100×), and αSMA (100×) shows a significant decrease in ductular proliferation in Wls KO after DDC. * p < 0.05.
Blocking Wnt secretion from cholangiocytes decreased cholangiocyte proliferation and abrogated transdifferentiation of hepatocytes after DDC diet
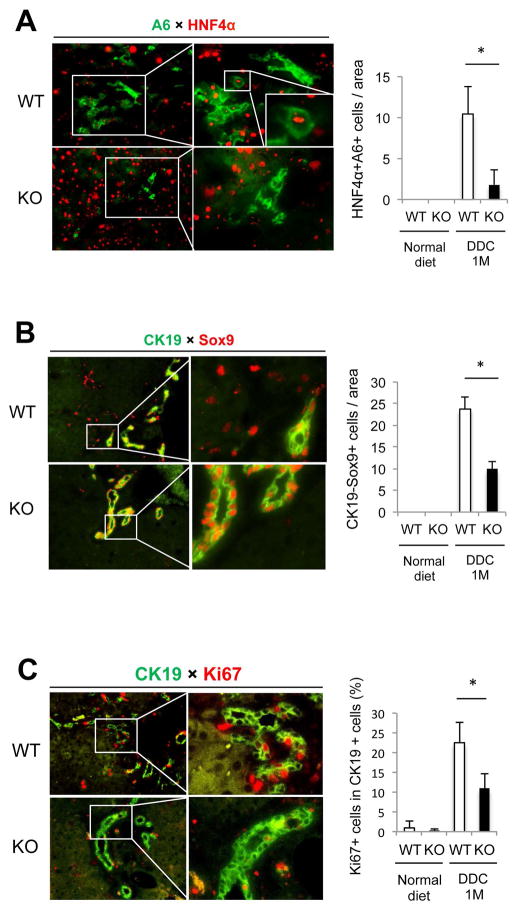
To determine which functions of Wnt signaling were impaired in Wls KO mice during cholestasis, we performed double immunofluorescence for hepatocyte and biliary markers, as well as markers of proliferation, on WT and KO livers after DDC diet. There was a significant decrease in the numbers of transdifferentiating hepatocytes, which stained for both A6 and HNF4α, in Wls KO compared to the control (Figure 7A). The number of Sox9+CK19- cells, which also represents the subset of hepatocytes acquiring a biliary phenotype, is likewise decreased in KO (Figure 7B). Finally, the number of actively proliferating cholangiocytes, as determined by dual labeling for CK19 and Ki67, is decreased as well in KO, further substantiating the mitogenic role of Wnts in inducing biliary proliferation (Figure 7C).
Figure 7. Cholangiocyte proliferation and hepatocyte-to-cholangiocyte transdifferentiation are disrupted in Wls KO after DDC diet.
(A) Representative images of WT and KO livers stained for biliary marker A6 (green) and hepatocyte marker HNF4α (red) are shown. Many large hepatocytes expressing A6 are seen in WT after DDC treatment, whereas the number of A6+HNF4α+ cells was significantly decreased in KO. (B) Representative images of WT and KO livers stained for biliary marker CK19 (green) and Sox9 (red) are shown. The presence of CK19 negative cells expressing Sox9, which is indicative of incompletely differentiated cholangiocytes derived from transdifferentiating hepatocytes, is significantly higher in WT than in KO after DDC treatment. (C) Representative images of WT and KO livers stained for CK19 (green) and proliferation marker Ki67 (red) are shown. The number of proliferating cholangiocytes is higher in WT after DDC treatment than in KO. * p < 0.05. Images taken at 100×; magnifications are 400×.
Wnt7A, 7B, and 10A are overexpressed in multiple cholestatic injury models across species
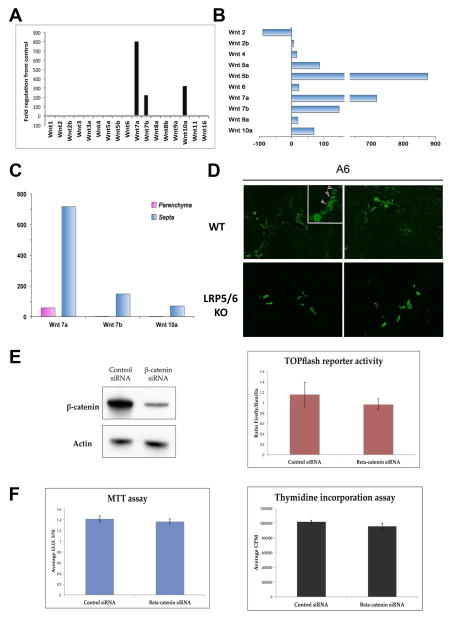
We next validated the role of Wnt signaling in BDL, a surgical obstruction that results in cholestasis, intrahepatic accumulation of toxic bile acids, and proliferation of cholangiocytes. Expression of various Wnts was measured by gene array 14D after BDL in WT mice, which is comparable to 4 weeks of DDC diet. Intriguingly, Figure 8A shows that expression of Wnt7A, Wnt7B, and Wnt10A – the same three Wnts upregulated after 28D of DDC diet – were also dramatically increased in whole livers after BDL. Moreover, we also analyzed Wnt gene expression in a rat model of biliary fibrosis induced by BDL. RNA was isolated from laser capture microdissected fibrotic septa and surrounding parenchymal tissue regions, as described previously (22). A notable increase in Wnts7A, 7B, and 10A, in addition to Wnts 5A and B, was evident in fibrotic septa as compared to the parenchyma (Figure 8B and 8C), demonstrating the relevance of these three Wnt genes in cholestatic injury across species.
Figure 8. Like DDC, BDL-induced Wnts regulate β-catenin dependent hepatobiliary transdifferentiation and β-catenin independent cholangiocyte proliferation.
(A) Expression of Wnt7A, Wnt7B, and Wnt10A increases in WT mouse livers after BDL. (B) Wnt7A, Wnt7B, and Wnt10A are also among the top Wnt genes expressed in fibrotic septa of bile duct ligated rats compared to age-matched controls. (C) Wnt7A, Wnt7B, and Wnt10A are primarily expressed in the fibrotic septa (corresponding to the periportal compartment in DDC) after rat BDL. (D) LRP5/6 KO have fewer hepatocytes (arrows) expressing A6 after BDL compared to WT controls. Images taken at 200× magnification. (E) β-catenin siRNA significantly decreases β-catenin protein in sm-cc cells; however, transcriptional activity of β-catenin, which is minimal under normal growth conditions, is not further decreased by β-catenin siRNA. (F) Inhibiting β-catenin does not decrease survival or proliferation of sm-cc cells.
Because of the commonality in Wnt gene signatures, we hypothesized that specific signaling pathways were activated during cholestasis that would differentially induce expression of these Wnts. The ChIP-X database was queried for transcription factors that are predicted to regulate Wnt gene expression (23). Although we identified 6 transcription factors that bind upstream of Wnts 7A, 7B, and 10A, none are exclusive to these three alone (Supplementary Fig. 4), suggesting that there is a complex interaction between transcription factor binding and the existing chromatin landscape. Thus, additional studies would be required to further characterize the mechanism of Wnt upregulation during cholestasis.
Wnt-induced transdifferentiation of hepatocytes to cholangiocytes after BDL is β-catenin dependent, while proliferation of cholangiocytes is β-catenin independent
To determine if Wnt signaling plays a similar role in BDL as in DDC diet, we then performed BDL on liver-specific LRP5/6 KO mice, in which Wnt signaling is disrupted but β-catenin expression is intact (10), as well as littermate controls. Although we found no difference in the total number of A6-positive atypical ductules between the two groups, we did detect the presence of A6-positive hepatocytes in WT after 14D of BDL, which were absent in LRP5/6 KO (Figure 8D). Thus, as in DDC, Wnt/β-catenin signaling in the context of BDL may facilitate the transdifferentiation of hepatocytes to cholangiocytes.
It was intriguing to note that the number of ductular structures was equivalent in WT and LRP5/6 KO. Because deletion of LRP5/6 only inhibits Wnt/β-catenin signaling, leaving non-canonical Wnt signaling intact, we hypothesized that Wnt-stimulated cholangiocyte proliferation was independent of β-catenin. To test this, we transfected sm-cc cells with siRNA against β-catenin, which effectively suppressed protein expression (Figure 8E). Interestingly, TOPflash assay revealed that there is minimal β-catenin transcriptional activity in sm-cc cells at baseline, which is not further decreased by inhibition of β-catenin (Figure 8E). Furthermore, both viability and proliferation of sm-cc cells is unchanged in the presence of β-catenin siRNA, suggesting that Wnt-induced proliferation in cholestasis may be independent of β-catenin activation (Figure 8F).
Discussion
Our lab has recently described the critical role of Wnt signaling in regulation of β-catenin activity during liver regeneration. Utilizing genetic mouse models, we deleted Wls in epithelial cells, hepatic stellate cells, macrophages, and endothelial cells. Disruption of Wnt signaling in these hepatic cell types revealed that Kupffer cells and endothelial cells are required for activation of β-catenin and progression of the cell cycle in hepatocytes, a finding that has also been described by others (10, 24). The development of these model systems has afforded us the opportunity to extend our knowledge of Wnt signaling and β-catenin activation to other liver injury models. Previous work in our laboratory has revealed an important role for β-catenin in repair of DDC-induced liver injury (11, 20). However, because β-catenin can be activated by multiple signaling pathways, including protein kinase A and receptor tyrosine kinases, the regulatory mechanism remained unknown. In this study, we sought to address whether β-catenin activation is Wnt-signaling dependent by characterizing the source and role of Wnts in cholestasis.
Tissue microdissection revealed a dramatic upregulation of Wnt7A, Wnt7B, and Wnt10A in the periportal region of mouse livers subjected to DDC diet for 28D. The expression of these Wnts is very low in the normal liver, suggesting that they are necessary only under specific circumstances such as cholestatic liver disease. Intriguingly, expression of these same 3 Wnts were also increased in livers of mice and rats subjected to BDL, indicating a common signaling mechanism in cholestatic liver injury regardless of origin. Our findings were consistent with previous reports that demonstrated increased expression of these same three Wnts after DDC treatment (25). Moreover, cell specific analysis revealed that the EpCAM-enriched population is the primary source of these Wnts during biliary injury. Cholangiocytes have been shown to play an important role during cholestatic liver injury, secreting factors pivotal for bile duct repair (26). Similarly, in this study we have shown that Wnts secreted by proliferating biliary cells are necessary for the repair of cholestatic liver injury.
Interestingly, the function of Wnt7B and Wnt10A in our model is different from that of Wnt7A, although all three are secreted by EpCAM+ cells. Both Wnt7B and Wnt10A induce the proliferation of small cholangiocytes in an autocrine fashion. Previous studies have also identified a role for Wnt10A and Wnt7B in proliferation, regeneration, and self-renewal (27–29). Interestingly, Wnt7B was also found to be expressed in poorly-differentiated hepatocellular carcinoma (HCC) cell lines, which have higher proliferative indices than well-differentiated HCC (30, 31).
Wnt7A, on the other hand, acts in a paracrine fashion on neighboring hepatocytes to induce cellular reprogramming. Notably, Wnt7A has been shown to induce TCF-mediated transcriptional activation when added in combination with R-spondin, a modulator of Wnt signaling, which is in line with our findings that Wnt7A signals through the canonical Wnt pathway (32). Interestingly, a previous study demonstrated that depletion of macrophages, a cellular source of Wnt7A, resulted in loss of hepatocyte specification and formation of biliary structures during hepatocellular injury and regeneration (33). While these results differ from ours, it is important to note that the cellular source of Wnt7A and the type of liver injury differ, and thus activity of Wnt7A in liver may be context-specific.
We have previously shown that the number of A6-positive cells is decreased in β-catenin KO mice at early stages (2 weeks) of DDC compared to wild-type (WT) littermate controls, although there is a partial recovery at 4 weeks suggesting compensatory mechanisms (11). In the current study, Wls KO also showed a paucity of proliferating cholangiocytes after DDC, verifying an essential role for the Wnt/β-catenin axis in biliary repair. However, because in this model all epithelial-derived Wnts are inhibited regardless of function, we were unable to determine whether the primary role of Wnt signaling after cholestatic liver injury is to stimulate cholangiocyte proliferation or hepatocyte-to-cholangiocyte transdifferentiation. Furthermore, it was unclear whether β-catenin is required for Wnt-mediated cholangiocyte proliferation as well as hepatocyte transdifferentiation. Thus, the LRP5/6 KO mouse afforded us with an opportunity to address the extent to which these processes are β-catenin-dependent, as non-canonical Wnt signaling is intact in this model. We utilized BDL as a secondary model of cholestasis and ductular proliferation to further validate the findings in our DDC model. Because transdifferentiation was decreased but the number of ductular structures was unchanged in the LRP5/6 KO after BDL, we hypothesize that the Wnts involved in inducing cholangiocyte proliferation are independent of β-catenin and function through non-canonical signaling mechanisms. This was further supported by in vitro studies demonstrating that proliferation and cell viability were unimpaired in sm-cc cells after β-catenin suppression. Thus, the rebound in ductular response seen in β-catenin KO during later stages of DDC diet may be due to increased β-catenin independent cholangiocyte proliferation, which is compensating for absent β-catenin dependent hepatocyte transdifferentiation. Indeed, equivalent CK19 mRNA expression is seen in β-catenin KO and WT after 4 weeks of DDC diet (data not shown). Future experiments utilizing lineage tracing and Wnt-specific knockouts will help further eludicate the relative contribution of these two mechanisms to hepatobiliary repair. However it is important to note that while Wls KO showed significant mortality after 4 weeks of DDC diet, all β-catenin KO mice survived DDC diet. Thus, Wnt-driven cholangiocyte proliferation appears to be indispensable for hepatobiliary repair during cholestasis.
It is becoming increasingly apparent that hepatocytes are capable of transdifferentiating into cholangiocytes in models where biliary injury is the predominant insult to the liver. Early studies utilized in vitro organoid culture systems to demonstrate that isolated hepatocytes exposed to a defined culture medium organize into a defined histological architecture, with cholangiocytes covering the surface of the tissue exposed to media (34, 35). Additional studies employing strain-tagged cells demonstrated that the cholangiocytes were derived from hepatocytes that have undergone cellular reprogramming (4, 36). More recent work utilizing genetic mouse models and lineage tracing has confirmed that hepatocytes are capable of converting to a biliary lineage under conditions that induce chronic biliary injury (3, 5, 37). These results suggest that the number of hepatocytes expressing biliary markers increases over time during biliary injury, and that as cholestasis progresses, more and more hepatocytes are “recruited” to compensate for damage to or loss of the biliary epithelium.
β-catenin and its downstream targets have been shown to be upregulated in hepatic organoid cultures under conditions that promote hepatocyte-to-cholangiocyte transdifferentiation (38), implicating a role for Wnt signaling in this process. Underscoring the functional significance of these observations, we found that S45D mice, which express excess β-catenin in liver, had an increased number of A6-positive hepatocytes compared to WT when subjected to long-term cholestatic injury (20). Importantly, this finding was coincident with a decrease in markers of biliary injury, as assessed by serum alkaline phosphatase levels. Further analysis of the S45D mice at earlier time points after DDC has shown an upregulation of biliary markers EpCAM, CK19, and Sox9. Interestingly, while loss of LRP5/6 from liver led to fewer A6-positive hepatocytes after BDL, we do not see a corresponding decrease in markers of cholestasis such as serum alkaline phosphatase, which may be due to the relatively short duration of the injury.
Previous studies and we have demonstrated that transdifferentiation of hepatocytes expressing Sox9 occurs primarily in the periportal area (3, 39). In light of our findings, it is likely that the concentration of Wnt7A, and thus transdifferentiation of hepatocytes, is highest in the periportal region due to the continuous injury to the bile ducts. The injury provides a stimulus for compensatory proliferation of cholangiocytes, which are also simultaneously secreting Wnt7A to recruit surrounding hepatocytes to participate in biliary repair.
In conclusion, our results have demonstrated that β-catenin signaling regulates hepatobiliary repair in a Wnt-dependent manner. These findings mandate a comprehensive analysis of the role of Wnt/β-catenin signaling in transdifferentiation and amelioration of cholestasis, and may provide novel therapeutics for treating cholestatic liver disease.
Supplementary Material
Acknowledgments
We thank Sucha Singh for invaluable technical assistance. We also thank Dr. Valentina Factor and Dr. Gianfranco Alpini for generously providing essential reagents for this study. This study was funded by NIH grants 1R01DK62277 and 1R01DK100287 and Endowed Chair for Experimental Pathology to SPSM, and NIH grant 1R01DK103775 to KNB.
Abbreviations
- Wls
Wntless
- KO
knockout
- Wls-LKO
liver-specific Wls KO
- WT
wild-type
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- ALT
serum alanine aminotransferases
- AST
aspartate aminotransferase
- PC
pericentral area
- PP
periportal area
- PT
portal triad area
- PC
parenchymal cells
- Kp
Kupffer cells
- EC
endothelial cells
- BEC
biliary epithelial cells
- HC
hepatocytes
- HSC
hepatic stellate cells
- Alb
albumin
- HCC
hepatocellular carcinoma
- TCF
T cell factor
- GS
glutamine sythetase
- IHC
immunohistochemistry
- WB
Western blot
- PCR
polymerase chain reaction
References
- 1.Poupon R, Chazouilleres O, Poupon RE. Chronic cholestatic diseases. J Hepatol. 2000;32:129–140. doi: 10.1016/s0168-8278(00)80421-3. [DOI] [PubMed] [Google Scholar]
- 2.Fickert P, Stoger U, Fuchsbichler A, Moustafa T, Marschall HU, Weiglein AH, Tsybrovskyy O, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanger K, Zong Y, Maggs LR, Shapira SN, Maddipati R, Aiello NM, Thung SN, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michalopoulos GK, Barua L, Bowen WC. Transdifferentiation of rat hepatocytes into biliary cells after bile duct ligation and toxic biliary injury. Hepatology. 2005;41:535–544. doi: 10.1002/hep.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarlow BD, Pelz C, Naugler WE, Wakefield L, Wilson EM, Finegold MJ, Grompe M. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu WY, Bird TG, Boulter L, Tsuchiya A, Cole AM, Hay T, Guest RV, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monga SP. beta-Catenin Signaling and Roles in Liver Homeostasis, Injury, and Tumorigenesis. Gastroenterology. 2015;148:1294–1310. doi: 10.1053/j.gastro.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010;48:554–558. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Mowry LE, Nejak-Bowen KN, Okabe H, Diegel CR, Lang RA, Williams BO, et al. beta-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 2014;60:964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apte U, Thompson MD, Cui S, Liu B, Cieply B, Monga SP. Wnt/beta-catenin signaling mediates oval cell response in rodents. Hepatology. 2008;47:288–295. doi: 10.1002/hep.21973. [DOI] [PubMed] [Google Scholar]
- 12.Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Jr, Dar MJ, Khillan J, Dai C, et al. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–1613. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong Z, Baker JJ, Zylstra-Diegel CR, Williams BO. Lrp5 and Lrp6 play compensatory roles in mouse intestinal development. J Cell Biochem. 2012;113:31–38. doi: 10.1002/jcb.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan X, Apte U, Micsenyi A, Kotsagrelos E, Luo JH, Ranganathan S, Monga DK, et al. Epidermal growth factor receptor: a novel target of the Wnt/beta-catenin pathway in liver. Gastroenterology. 2005;129:285–302. doi: 10.1053/j.gastro.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 16.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 17.Okabe M, Tsukahara Y, Tanaka M, Suzuki K, Saito S, Kamiya Y, Tsujimura T, et al. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–1960. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 18.Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Ueno Y, Alpini G, Yahagi K, Kanno N, Moritoki Y, Fukushima K, Glaser S, et al. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 20.Thompson MD, Awuah P, Singh S, Monga SP. Disparate cellular basis of improved liver repair in beta-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Am J Pathol. 2010;177:1812–1822. doi: 10.2353/ajpath.2010.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger BZ, Jacquemin P, et al. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yovchev MI, Locker J, Oertel M. Biliary fibrosis drives liver repopulation and phenotype transition of transplanted hepatocytes. J Hepatol. 2016;64:1348–1357. doi: 10.1016/j.jhep.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lachmann A, Xu H, Krishnan J, Berger SI, Mazloom AR, Ma’ayan A. ChEA: transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics. 2010;26:2438–2444. doi: 10.1093/bioinformatics/btq466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, Penfold ME, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh T, Kamiya Y, Okabe M, Tanaka M, Miyajima A. Inducible expression of Wnt genes during adult hepatic stem/progenitor cell response. FEBS Lett. 2009;583:777–781. doi: 10.1016/j.febslet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Alvaro D, Mancino MG, Glaser S, Gaudio E, Marzioni M, Francis H, Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Han D, Wang L, Feng H. Down-regulation of Wnt10a affects odontogenesis and proliferation in mesenchymal cells. Biochem Biophys Res Commun. 2013;434:717–721. doi: 10.1016/j.bbrc.2013.03.088. [DOI] [PubMed] [Google Scholar]
- 28.Long A, Giroux V, Whelan KA, Hamilton KE, Tetreault MP, Tanaka K, Lee JS, et al. WNT10A promotes an invasive and self-renewing phenotype in esophageal squamous cell carcinoma. Carcinogenesis. 2015;36:598–606. doi: 10.1093/carcin/bgv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuzugullu H, Benhaj K, Ozturk N, Senturk S, Celik E, Toylu A, Tasdemir N, et al. Canonical Wnt signaling is antagonized by noncanonical Wnt5a in hepatocellular carcinoma cells. Mol Cancer. 2009;8:90. doi: 10.1186/1476-4598-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hino N, Higashi T, Nouso K, Nakatsukasa H, Tsuji T. Apoptosis and proliferation of human hepatocellular carcinoma. Liver. 1996;16:123–129. doi: 10.1111/j.1600-0676.1996.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim KA, Wagle M, Tran K, Zhan X, Dixon MA, Liu S, Gros D, et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol Biol Cell. 2008;19:2588–2596. doi: 10.1091/mbc.E08-02-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulter L, Govaere O, Bird TG, Radulescu S, Ramachandran P, Pellicoro A, Ridgway RA, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michalopoulos GK, Bowen WC, Mule K, Stolz DB. Histological organization in hepatocyte organoid cultures. Am J Pathol. 2001;159:1877–1887. doi: 10.1016/S0002-9440(10)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limaye PB, Bowen WC, Orr AV, Luo J, Tseng GC, Michalopoulos GK. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2008;47:1702–1713. doi: 10.1002/hep.22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalopoulos GK, Bowen WC, Mule K, Lopez-Talavera JC, Mars W. Hepatocytes undergo phenotypic transformation to biliary epithelium in organoid cultures. Hepatology. 2002;36:278–283. doi: 10.1053/jhep.2002.34858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekiya S, Suzuki A. Hepatocytes, rather than cholangiocytes, can be the major source of primitive ductules in the chronically injured mouse liver. Am J Pathol. 2014;184:1468–1478. doi: 10.1016/j.ajpath.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Limaye PB, Alarcon G, Walls AL, Nalesnik MA, Michalopoulos GK, Demetris AJ, Ochoa ER. Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development. Lab Invest. 2008;88:865–872. doi: 10.1038/labinvest.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.