Abstract
Calcitroic acid was isolated and characterized almost four decades ago but little is known about this important vitamin D metabolite. Four reported synthetic strategies to generate calcitroic acid are presented that highlight the scientific progress in the field of chemistry directed to vitamin D analog synthesis. The most recent synthesis described the generation of calcitroic acid with an overall yield of 12.8% in 13 steps. The endogenous formation of calcitroic acid has been demonstrated in perfused rat kidney using 24,25,26,27-tetranor-1,23(OH)2D3. Although, the majority of vitamin D metabolism is mediated by 24-hydoxylase (CYP24A1), it is not clear why the formation of calcitroic acid was not observed in the presence of recombinant CYP24A1 enzyme. Furthermore, it is not known if enzyme 1α-hydroxylase (CYP27B1) can convert calcioic acid into calcitroic acid. In addition to the lack of research investigating the endogenous formation of calcitroic acid the physiological role of calcitroic acid remains unknown. Only a few reports mentioned the biological activity of calcitroic acid in connection with the vitamin D receptor (VDR). When administered subcutaneous, calcitroic acid has anthracitic properties and elevates calcium blood levels when administered i.v.. In vitro, calcitroic acid at higher concentrations has been shown to bind VDR and induce gene transcription. However, these studies were not carried out in cells derived from target organs of calcitroic acid such as kidney, liver, and intestine. We can conclude that our current knowledge of calcitroic acid is limited and more studies are needed to identify its physiological role.
Graphical Abstract
Introduction
Calcitroic acid is a metabolite of vitamin D, which in turn is generated from cholesterol and sun exposure. Vitamin D research started in the beginning of the last century with the observation of Steenbock1 that irradiated food had antirachitic properties, a revolutionizing finding during the rickets-plagued industrial age. Although the molecule vitamin D was rapidly isolated,2 it took scientists forty years to understand that metabolism of vitamin D was essential to generate vitamin D analogs with biologic activity. The work was pioneered by Kodicek with in vivo dosing of 14C-labeled vitamin D2,3 however, the generation and administration of 3H-labeled vitamin analogs in the 60s led to the identification of vitamin D analog 25(OH)D3 with significant biological activity,4 followed by the most active analog 1,25(OH)2D3.5 These discoveries initiated many research programs in the fields of medicine, biochemistry, physiology, chemistry, and pharmacology; especially once the vitamin D receptor (VDR) was identified,6 and eventually cloned.7 VDR is part of superfamily of nuclear receptors and responsible for the regulations of genes involved in calcium homeostasis, cell proliferation, and cell differentiation. VDR also regulates the expression of p450 enzymes such as CYP24A18 and CYP27B19 as part of its ligand autoregulation and CYP3A4,10 CYP19A111 and CYP1A112 as a xenobiotic sensor similar to the pregnane X receptor (PXR) and constitutive androstane receptor (CAR).13 This article summarizes the discoveries around the “final” metabolite of vitamin D, calcitroic acid. It also highlights the biological activities of calcitroic acid, which in the presence of highly potent but short lived 1,25(OH)2D3 has been less studied. This work also demonstrates that after sixty years of vitamin D metabolism research, scientists are still identifying new metabolites and metabolic pathways of vitamin D.
Metabolic production of calcitroic acid
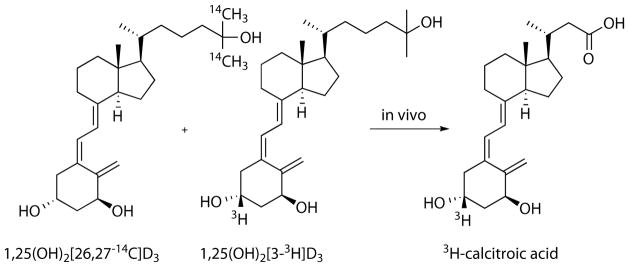
In 1965, it was observed that significant radioactivity was present in the aqueous extract of bile and intestine of 1α-3H-D3 dosed rats, however, none of these radioactive species were analyzed further.14 With improvement in rat bile duct cannulation, bile was collected for 24 h and separated chromatographically into five fractions including “faction D”, which after acid-hydrolysis separated into polar and less polar fractions.15 A glucuronide conjugate of a vitamin D analog was identified as a major component masking calcitroic acid, however, separation methods for acidic metabolites such an anion-exchange chromatography and derivatization with diazomethane were established. Although, researchers repeatedly reported large amounts of radioactivity for aqueous extract of liver and intestine from 3H-25(OH)D3 treated animals,16 it took another ten years until the first isolation and eventual naming of calcitroic acid.17 In their study, rats were treated with a combination of 14C and 3H labeled 1,25(OH)2D3 (Figure 1) and after four hours soluble contents of liver and intestine (with contents) were separated as a chloroform fraction and methanol/water fraction.
Figure 1.
In vivo experiment to identify metabolic products from 1,25(OH)2D3. The enrichment of 3H-labeled material in organ extracts indicated a modification of the side chain of 1,25(OH)2D3.
The aqueous layers of both tissues were enriched in 3H over 14C indicating that the side chain of 1,25(OH)2D3 was shortened during metabolism. Especially fractions of negatively charged compounds, after diethylaminoethyl cellulose separation (anion exchange), had significant amounts of radioactivity. 3H-calcitroic acid was isolated as methyl ester after esterification with diazomethane and characterized by mass spectrometry. Blood contained a small amount of 3H-calcitroic acid, however, large amounts were found in liver and especially in intestine plus content.
Thus, the hypothesis that calcitroic acid is part of the enterohepatic circulation was subsequently confirmed with the identification of 3H-calcitroic acid in the bile duct.18 A more detailed analysis of tissue distribution of calcitroic acid using the techniques stated above using bolus administration of 1,25(OH)2-[3α-3H]D3 demonstrated a more complex metabolism.19 First, other acidic metabolites were found that were suggested to be polar calcitroic acid conjugates and second, calcitroic acid was found in the intestine of bile duct cannulated rats that suggests endogenous mucosal synthesis of calcitroic acid. In addition, only up to 50% of the radioactive material could be extracted from dried liver, another indication of complex metabolite formation.
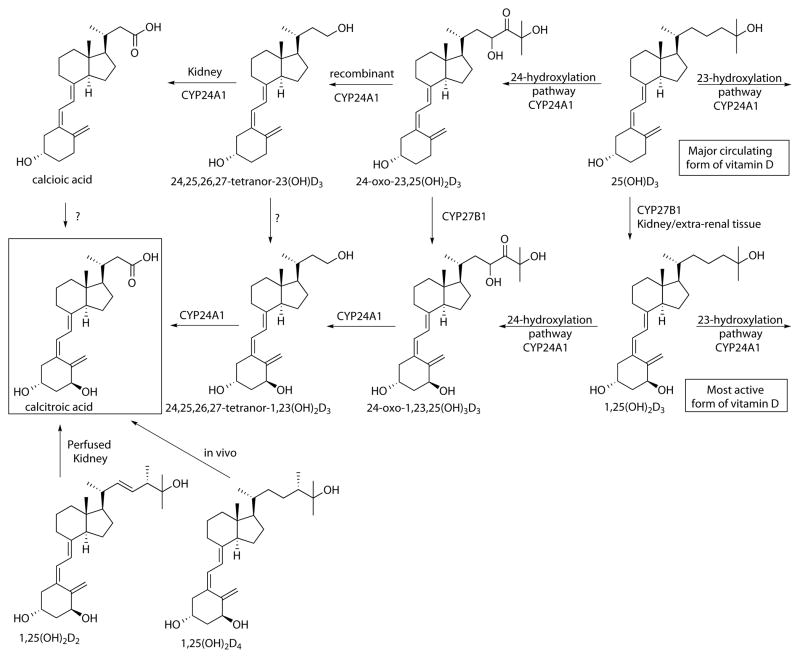
As pointed out above, the organ chloroform extracts received more attention and enabled researchers to identify one of the most pronounced pathways for vitamin D metabolism that eventually generates calcitroic acid. The first evidence of 24-hydroxylation of vitamin D was found in blood of [1,2-3H]D3-treated chicks, with the product initially believed to be 21,25(OH)2D3,20 but later identified as 24,25(OH)2D3 in chick kidney homogenates.21 It was further discovered that 1,25(OH)2D3 increases the production of 24,25(OH)2D3, suggesting for the first time an upregulation of 24-hydoxylase (CYP24A1) by 1,25(OH)2D3.22 CYP24A1 was first identified in chick kidney.23 Further characterization of the purified enzyme found in kidney mitochondria was accomplished by several groups24, 25 and eventual lead to cloning and expression of mitochondrial kidney CYP24A1.26 In the meantime, several new vitamin D metabolites were identified in vivo or perfused rat kidney including 24,25,26,27-tetranor-23(OH)D327 and 24,25,26,27-tetranor-1,23(OH)2D3, the most likely precursors for calcitroic acid (Scheme 1).28
Scheme 1.
Metabolism of vitamin D analogs to calcitroic acid.
The conversion of 24,25,26,27-tetranor-1,23(OH)2D3 into calcitroic acid was reported by two groups during the same year using perfused rat kidney.29, 30 In addition, it was shown that cultured bone cells mediated the same metabolism in addition to the earlier reported conversion of 25-(OH)D3 to 24,25-(OH)2D3.31 Thus, the expression of CYP24A1 is not limited to kidney. Analysis of recombinant human CYP24A1 confirmed that a single P450 enzyme catalyzed the six-step pathway from 1,25(OH)2D3 to calcitroic acid.32 In addition is was demonstrated that CYP24A1 also mediated the alternative C23 hydroxylation pathway, a four-step monooxygenation from 25-(OH)D3 to 25(OH)D3-26,23-lactone, which interestingly was not observed for rat CYP24A1.33 Human recombinant CYP24A1 was also able to catalyze the conversion of 25-(OH)D3 to 24,25,26,27-tetranor-23(OH)D3,34 however complete oxidation to calcioic acid was only observed in perfused rat kidney.35 In addition, calcitroic acid was found in perfused kidney when treated with supplement-derived 1,25(OH)2D236, although CYP24A1 seemed not to be the only enzyme involved in this process.37 Calcitroic acid was also identified in the bile of rats treated with 1,25(OH)2D4.38 Finally, a novel metabolism pathway of 1,25(OH)2D3 that includes the formation of epi-1,25(OH)2D3 has shown the formation of epi-calcitroic acid with the 3β-OH configuration.39
Vitamin D analogs bearing a α1 hydroxyl functionality like 1,25(OH)2D3 bind more strongly to VDR compared to their non 1α-hydroxylated counterpart (25-(OH)D3) and are therefore biologically more active at the same concentration. The identification, purification, and characterization of the responsible enzyme 25-hydroxyvitamin D3 1α-hydroxylase (CYP27B1) has been ongoing for five decades.40 Similar to CYP24A1, CYP27B1 expression is not limited to kidney mitochondria. Only recently it was shown that CYP27B1 is able to convert most vitamin D metabolites of the 24-hydroxylation pathway to their corresponding 1α hydroxyl products, however, 24,25,26,27-tetranor-23(OH)D3 and calcioic acid have not been investigated yet.41
Synthesis of calcitroic acid
The identification of physiological metabolites of vitamin D in the 20th century depended heavily on the use of radioactive compounds enabling identification of nano- to picomolar concentrations in combination with chromatography. Structural identification was demonstrated by co-elution with authentic samples that were isolated and characterized previously or generated by chemical synthesis. In addition, mass spectrometry enabled structural conformation of small amounts of material due to specific fragmentation patterns. The first synthesis of calcitroic acid was reported in 1981 (Scheme 2).42
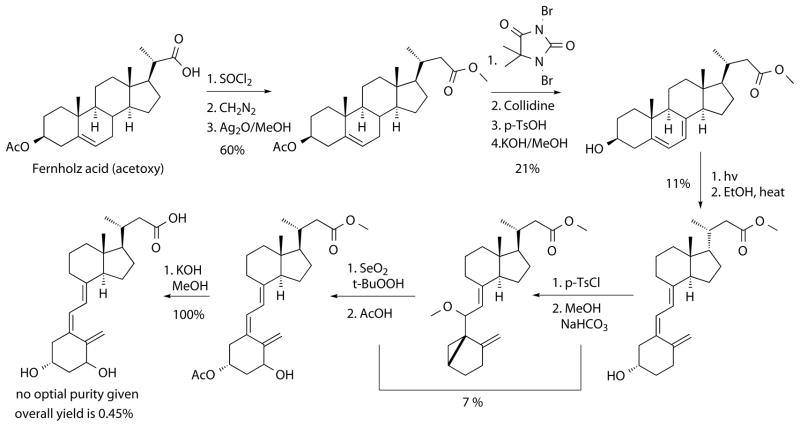
Scheme 2.
First synthesis of calcitroic acid starting from a cholesterol precursor.
The acetoxy derivative of Fernholz acid, which is commercially available from Steraloids, was used as starting material and converted using an Arndt-Eistert reaction to the corresponding higher carboxy homologue. Allylic bromination and subsequent elimination installed the diene, which was converted in low yield under photochemical and thermal conditions to the methyl ester of calcioic acid. 1α-hydroxylation was achieved by synthesizing a cyclovitamin D derivative followed by allylic oxidation and cycloreversion with acidic acid. Hydrolysis gave calcitroic acid in an overall yield of 0.09%. The optical rotation was not given but the corresponding methyl ester co-eluded in HPLC with the derivatized material isolated from rat livers. Small improvements to this route increased the overall yield to 0.28%.43. Two years later a new route based on a previous synthesis of vitamin D analogs was reported with an overall yield of 0.36 % (Scheme 3).44
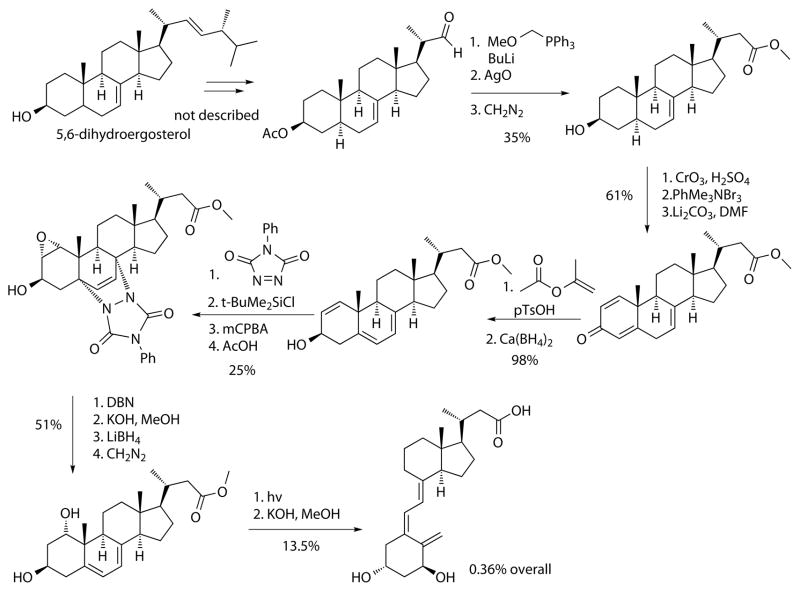
Scheme 3.
Synthesis of calcitroic acid using a provitamin D precursor.
The synthesis started with 5,6-dihydroergosterol, which was used to prepare the corresponding aldehyde. The carbon chain elongation was carried out by a Wittig reaction followed by demethylation, oxidation, and esterification. A quinone-like structure was generated by oxidation, α-carbon bromination, and elimination, which was subsequently isomerized and reduced to the allylic alcohol. The diene and the alcohol was protected, followed by oxidation and subsequent deprotection. The retro Diels-Alder reaction recreated the diene and conversion of the ester to the acid enabled a selective reduction of the epoxide followed by esterification. Finally, the seco-steroid scaffold was produced by light and subsequent saponification generating calcitroic acid in 0.36% overall yield. Importantly, the light-induced ring opening reaction reduced significantly the overall yield of both described methods. During the 80s and 90s many different synthetic strategies were explored to generate of vitamin D analogs, which led to the availability of many new synthons for their synthesis.45 The carbon chain elongation using a seco-steroid as starting material to produce calcitroic acid was introduced in 1990 (Scheme 4).46
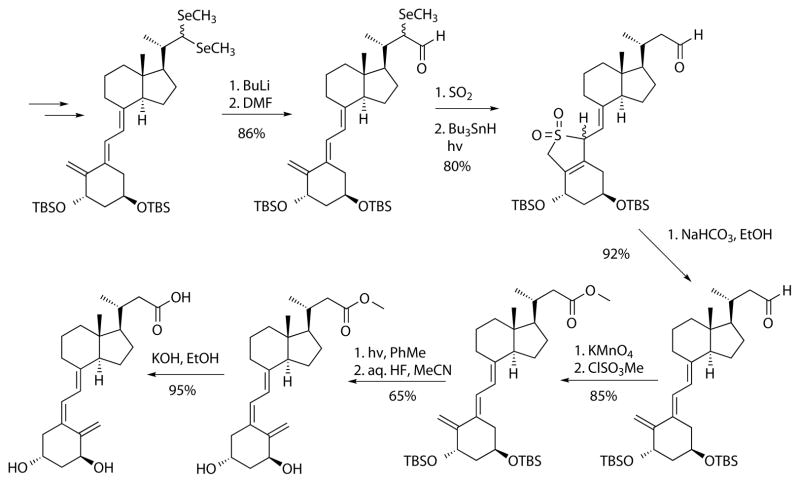
Scheme 4.
Synthesis of calcitroic acid from seco-steroid precursor.
The starting material was a seleno acetal, which was generated from the corresponding 1α-hydroxy vitamin D aldehyde derivative. Formylation gave a lithio-demethylseleno derivative as a carbon homologue, which was protected as a hetero Diels-Alder product with sulfur dioxide followed by radical deselenation induced by light. A retro Diels-Alder reaction was achieved under mild conditions followed by oxidation and esterification to the corresponding ester. Photoisomerization installed the natural Z-configuration of methyl ester of the protected calcitroic acid, which was subsequently deprotected and hydrolyzed to give calcitroic acid. The reactions described in Scheme 4 achieved high yields, however the route has many steps if the synthesis of the precursor would be included. Very recently, a synthesis using commercially available chemicals was reported, that for the first time enabled the synthesis of gram quantities of calcitroic acid (Scheme 5).47
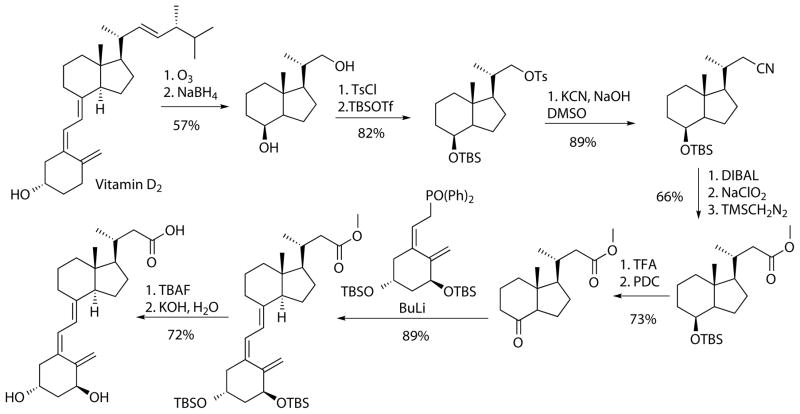
Scheme 5.
Synthesis of calcitroic acid starting from vitamin D2 and ring C synthon. Both compounds are commercially available. The overall yield is 12.8 % using 13 steps.
Originally developed to generated 13C calcitroic acid for metabolic research, this synthesis used the Inhoffen Lythgoe diol, which was readily synthesized from vitamin D2.48 The carbon elongation was accomplished by substitution of the tosylate with potassium cyanide, followed by reduction, oxidation, and esterification. Deprotection and oxidation installed the ketone to enable a Wittig Horner reaction with a commercially available organophosphine oxide (ring C synthon). Subsequent deprotection and hydrolysis produced calcitroic acid in 12.8% overall yield.
In vitro and in vivo characterization of calcitroic acid
The affinity of calcitroic acid to VDR was determined initially using 1,25(OH)2-[26,27-3H]D3 and VDR isolated from the chick intestines.19 The IC50 was 2.6 μM and 6.8 μM for calcitroic acid methyl ester and calcitroic acid, respectively. The IC50 measured with 25OH-[26,27-3H]D3 and vitamin binding protein, which was isolated from rat serum, was determined to be 10 μM for calcitroic acid. The antirachitic activity, determined by calcification of the epiphyseal plate of rats maintained for 2 weeks on a low phosphorus diet, was demonstrated following 7 day (12.5 ng/animal t.i.d.) subcutaneous treatment of calcitroic acid methyl ester or 7 day (50 ng/animal t.i.d.) treatment with calcitroic acid. In these studies, under the same treatment regime 5 ng/animal t.i.d. of calcitriol improved calcification 3 times greater than the corresponding 50 ng/animal dosing. Serum calcium levels were elevated in rats fed a low calcium diet for 3 week and administered a single i.v. dose of 2 μg/animal calcitroic acid and corresponding methyl ester. Calcium transport using intestinal permeability for calcium was not altered at this dose, although 50 ng i.v. of calcitriol increased the transport three-fold but also induced hypercalcemia.19 In vitro investigations were conducted a decade later using a luciferase assay under control of a mouse osteopontin vitamin D response element (VDRE) in G-361 melanoma epithelia cells. At 20 nM, calcitriol acid induced gene transcription significantly more than other 25-hydoxy vitamin D3 metabolites including the final product of 23-hydroxylation (23S,25R)-1α,25-dihydroxyvitamin D3-26,23-lactone.49 Similar results were observed with a luciferase transcription assay using a CYP24A1 promoter VDRE.50 However, at 20 nM calcitroic acid HaCaT cells (immortalized keratinocyte) did not show induction of CYP24A1 mRNA when incubated for 4h or 24h. Recently, calcitroic acid was shown to support binding of VDR and coregulator SRC1 (steroid receptor coactivator 1) in a two-hybrid assay with an EC50 of 870 nM.51 Interestingly, calcitroic acid did not inhibit the interaction between calcitriol and VDR at the concentrations used. Furthermore, calcitroic acid was able to induce upregulation of CYP24A1 in DU145 prostate cancer cells at 7.5 μM after 18 hours. At the same concentration, CYP24A1 mRNA production in the presence of calcitriol was not altered.
During the almost one hundred years of vitamin D research a tremendous quantity of knowledge has been accumulated, growing exponentially over the past 50 years. Much of this knowledge has been summarized in the “vitamin D” book in the editions of 1997, 2005, 2011, and 2016. However, it is obvious there is still much to learn especially regarding the physiological metabolites of vitamin D and their biological functions. Calcitroic acid is one of these molecules, which was identified 37 years ago yet we still know very little about it. One important factor impeding calcitroic acid research has been the unavailability of this compound. Hopefully, this will be overcome with the recently published new method from Meyer, et al.47 Even the older synthetic methods might inspire chemists to develop alternative new syntheses. A great deal of knowledge as gained on the metabolic formation of calcitroic acid using recombinant enzymes with further detail on their cofactors and enzyme kinetics. However, it is obvious that a physiologically relevant system such as a perfused kidney would reflect a more comprehensive metabolic system that gives a more complete picture, as demonstrated for the metabolism of 25(OH)D335 or 1,25(OH)2D2.36 Ultimately, in vivo studies will give the best and most relevant picture of the true metabolic conversion of vitamin D and interestingly these were the experiments conducted by the pioneers in this field. The in vivo experiments to identify vitamin D metabolites were carried out exclusively with radiolabeled vitamin D compounds, however, only a certain part of the radioactivity was recovered by extraction using chloroform and water/alcohol mixtures. In addition, the most pronounced and relatively easy to purify metabolites were identified with the technical and instrumentational limitation of that time. Recent developments in the area of tandem mass spectrometry has sparked new research into vitamin D metabolite identification with contributions by Slominski and Tuckey who unraveled a new metabolic pathway of vitamin D3 mediated by CYP11A1 and new metabolites formed from vitamin D2 in vitro.52, 53 In addition, matrix-assisted laser desorption/ionization imaging mass spectrometry now enables the analysis of metabolites from isolated tissue without extraction.54
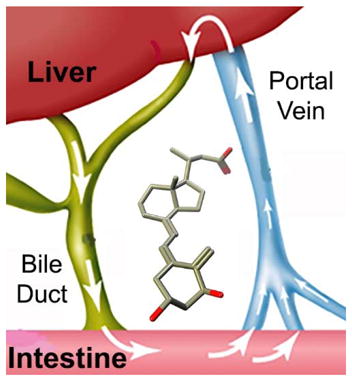
In vivo metabolism occurs in two phases, which include conjugation such as glucuronidation and amino acid and glutathione conjugation, as well as acetylation, sulfation, and methylation. Conjugation occurring in the kidney can lead to urinary excretion, however, conjugation in the liver can lead to intestinal secretion of conjugated vitamin D metabolites as bile, where bacteria and intestinal cells can reverse this process. Thus, non-conjugated vitamin D metabolites can be reabsorbed and further metabolized in the liver. The bile is very likely to carry the largest quantity of conjugates of vitamin D metabolites, which were isolated as polar methyl ester fraction and predicted 35 years ago.19 The intestine, liver, and kidney can be predicted to be exposed to the largest amount of vitamin D metabolites including calcitroic acid.
Evaluation of the biological function of calcitroic acid is important from a public health point view. Vitamin D supplements that include vitamin D3 and vitamin D2 are consumed by millions of people all-over the world. Some of the vitamin D is stored in fat tissue and activated gradually and some is activated directly and metabolized. Not all vitamin D is converted into 1,25(OH)2D3, but quickly converted into 24,25(OH)2D3 and other metabolites. Based on what we currently know, most of it will be converted into calcitroic acid. Thus, millions of people are exposed to significant amounts of calcitroic acid by taking vitamin D supplements and yet we still know very little about its biological function. In addition, calcitroic acid is a common metabolite of vitamin D based drugs. For instance, calcipotriol is converted into calcitroic acid in keratinocytes.55 Furthermore, the vitamin D analog paricalcitol is clinically approved for secondary hyperparathyroidism and alfacalcidol is used in Japan to treat osteoporosis. Tacalcitol and calcipotriol are approved to treat psoriasis. All these drugs are very likely to have a common metabolic product, calcitroic acid.
Calcitroic acid interacts weakly with VDR and is able to induce gene transcription mediated by VDR at higher concentrations. The 25(OH)D3 concentration in blood can be as high as 100 nM. When metabolized in the liver and accumulated in bile a concentration of calcitroic acid with biological activity can be reached. DeLuca demonstrated antirachitic activity of calcitroic acid when administrating a continuous low dose. Hypercalcemic effects of calcitroic acid were observed when a large dose was administered i.v. to rats. In addition, there might be biological effects of calcitroic acid, which has been attributed solely to 1,25(OH)2D3 when vitamin D was administered. One hypothesis proposed was that water-soluble metabolites of 25(OH)D3 that are secreted into the intestinal track may protect the colon from cancer-inducing accumulation of lithocholic acid by upregulation of CYP3A4.35 In addition, vitamin D has been shown to be beneficial for treating irritable bowel syndrome,56 which might be mediated in part by calcitroic acid. In summary, calcitroic acid is an important vitamin D metabolite with yet unknown physiological functions. However, current developments in mass spectrometry might enable more sophisticated investigations to unravel vitamin D metabolism further with an emphasis of in vivo processes and physiological effects of vitamin D metabolites in different parts of the human body.
Acknowledgments
We thank D. Stafford (Director of the MIDD) for the excellent comments for this manuscript.
Funding Sources. This work was supported by the University of Wisconsin–Milwaukee, the Milwaukee Institute for Drug Discovery, the UWM Research Growth Initiative, NIH R03DA031090, the UWM Research Foundation, the Lynde and Harry Bradley Foundation, and the Richard and Ethel Herzfeld Foundation.
Keywords
- Vitamin D
An important pro-vitamin that can be obtained from diet or generated in the body from 7-dehydrocholersterol. This vitamin is important for bone homeostasis and cell proliferation and differentiation.
- Calcitroic acid
A major metabolite of vitamin D that is primarily formed in the liver and secreted as bile into the intestine.
- CYP24A1
A metabolizing enzyme that is responsible for the formation of many vitamin D analogs including calcitroic acid.
- Vitamin D receptor
A receptor for vitamin D analogs that mediates, among others, the transcription of genes responsible for calcium homeostasis cell proliferation and differentiation.
- CYP27B1
A P450 enzyme that activates vitamin D analogs by hydroxylation primarily in the kidney.
- Antirachitic
The ability of a compound to cure rickets by balancing calcium homeostasis.
- Metabolisms
Degradation of compounds in our body mediated by two phases. Firstly, the oxidation, reduction and hydrolysis mediated by enzymes and secondly the conjugation to specific compounds to facilitate transport and reuptake.
- Conjugation
The enzyme-mediated reactions of phase I metabolites with hydrophilic compounds such as glucuronic acid, sulfate, glutathione, or glycine.
References
- 1.Steenbock H, Black A. Fat-soluble vitamins. XVII The induction of growthpromoting and calcifying properties in a ration by exposure to ultraviolet light. J Biol Chem. 1924;61:405–422. [Google Scholar]
- 2.Askew FA, Bourdillon RB, Bruce HM, Jenkins RGC, Webster TA. The Distillation of Vitamin D. Proc R Soc. 1930;B107:76–90. [Google Scholar]
- 3.Chalk KJ, Kodicek E. The association of 14C-labelled vitamin D2 with rat serum proteins. Biochem J. 1961;79:1–7. doi: 10.1042/bj0790001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blunt JW, DeLuca HF, Schnoes HK. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1968;7:3317–3322. doi: 10.1021/bi00850a001. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF, Schnoes HK, DeLuca HF, Suda T, Cousins RJ. Isolation and identification of 1,25-dihydroxycholecalciferol. A metabolite of vitamin D active in intestine. Biochemistry. 1971;10:2799–2804. doi: 10.1021/bi00790a023. [DOI] [PubMed] [Google Scholar]
- 6.Brumbaugh PF, Haussler MR. 1Alpha,25-dihydroxyvitamin D3 receptor: competitive binding of vitamin D analogs. Life Sci. 1973;13:1737–1746. doi: 10.1016/0024-3205(73)90120-3. [DOI] [PubMed] [Google Scholar]
- 7.McDonnell DP, Mangelsdorf DJ, Pike JW, Haussler MR, O’Malley BW. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987;235:1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- 8.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Jones G, Prosser DE. Chapter 3: The activating enzymes of vitamin D metabolism (25- and 1alpha-hydroxylase) In: Feldman D, Pike JW, Adams JS, editors. Vitamin D. 3. Academic Press; San Diego, CA, USA: 2011. [Google Scholar]
- 10.Wang Z, Schuetz EG, Xu Y, Thummel KE. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J Steroid Biochem Mol Biol. 2013;136:54–58. doi: 10.1016/j.jsbmb.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundqvist J, Hansen SK, Lykkesfeldt AE. Vitamin D analog EB1089 inhibits aromatase expression by dissociation of comodulator WSTF from the CYP19A1 promoter-a new regulatory pathway for aromatase. Biochim Biophys Acta. 2013;1833:40–47. doi: 10.1016/j.bbamcr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Matsunawa M, Akagi D, Uno S, Endo-Umeda K, Yamada S, Ikeda K, Makishima M. Vitamin D receptor activation enhances benzo[a]pyrene metabolism via CYP1A1 expression in macrophages. Drug Metab Dispos. 2012;40:2059–2066. doi: 10.1124/dmd.112.046839. [DOI] [PubMed] [Google Scholar]
- 13.Reschly EJ, Krasowski MD. Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds. Curr Drug Metab. 2006;7:349–365. doi: 10.2174/138920006776873526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callow RK, Kodicek E, Thompson GA. Metabolism of tritiated vitamin D. Proc R Soc Lond B Biol Sci. 1966;164:1–20. doi: 10.1098/rspb.1966.0010. [DOI] [PubMed] [Google Scholar]
- 15.Bell PA, Kodicek E. Investigations on metabolites of vitamin D in rat bile. Separation and partial identification of a major metabolite. Biochem J. 1969;115:663–669. doi: 10.1042/bj1150663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai HC, Wong RG, Norman AW. Studies on calciferol metabolism. IV Subcellular localization of 1,25-dihydroxy-vitamin D 3 in intestinal mucosa and correlation with increased calcium transport. J Biol Chem. 1972;247:5511–5519. [PubMed] [Google Scholar]
- 17.Esvelt RP, Schnoes HK, DeLuca HF. Isolation and characterization of 1 alpha-hydroxy-23-carboxytetranorvitamin D: a major metabolite of 1,25-dihydroxyvitamin D3. Biochemistry. 1979;18:3977–3983. doi: 10.1021/bi00585a021. [DOI] [PubMed] [Google Scholar]
- 18.Onisko BL, Esvelt RP, Schnoes HK, DeLuca HF. Metabolites of 1 alpha, 25-dihydroxyvitamin D3 in rat bile. Biochemistry. 1980;19:4124–4130. doi: 10.1021/bi00558a034. [DOI] [PubMed] [Google Scholar]
- 19.Esvelt RP, De Luca HF. Calcitroic acid: biological activity and tissue distribution studies. Arch Biochem Biophys. 1981;206:403–413. doi: 10.1016/0003-9861(81)90107-7. [DOI] [PubMed] [Google Scholar]
- 20.Suda T, DeLuca HF, Schnoes HK, Ponchon G, Tanaka Y, Holick MF. 21,25-dihydroxycholecalciferol. A metabolite of vitamin D3 preferentially active on bone. Biochemistry. 1970;9:2917–2922. doi: 10.1021/bi00816a025. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF, Schnoes HK, DeLuca HF, Gray RW, Boyle IT, Suda T. Isolation and identification of 24,25-dihydroxycholecalciferol, a metabolite of vitamin D made in the kidney. Biochemistry. 1972;11:4251–4255. doi: 10.1021/bi00773a009. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y, DeLuca HF. Stimulation of 24,25-dihydroxyvitamin D3 production by 1,25-dihydroxyvitamin D3. Science. 1974;183:1198–1200. doi: 10.1126/science.183.4130.1198. [DOI] [PubMed] [Google Scholar]
- 23.Knutson JC, DeLuca HF. 25-Hydroxyvitamin D3-24-hydroxylase. Subcellular location and properties. Biochemistry. 1974;13:1543–1548. doi: 10.1021/bi00704a034. [DOI] [PubMed] [Google Scholar]
- 24.Burgos-Trinidad M, Brown AJ, DeLuca HF. Solubilization and reconstitution of chick renal mitochondrial 25-hydroxyvitamin D3 24-hydroxylase. Biochemistry. 1986;25:2692–2696. doi: 10.1021/bi00357a061. [DOI] [PubMed] [Google Scholar]
- 25.Ohyama Y, Okuda K. Isolation and characterization of a cytochrome P-450 from rat kidney mitochondria that catalyzes the 24-hydroxylation of 25-hydroxyvitamin D3. J Biol Chem. 1991;266:8690–8695. [PubMed] [Google Scholar]
- 26.Ohyama Y, Noshiro M, Okuda K. Cloning and expression of cDNA encoding 25-hydroxyvitamin D3 24-hydroxylase. FEBS Lett. 1991;278:195–198. doi: 10.1016/0014-5793(91)80115-j. [DOI] [PubMed] [Google Scholar]
- 27.Jones G, Kung M, Kano K. The isolation and identification of two new metabolites of 25-hydroxyvitamin D3 produced in the kidney. J Biol Chem. 1983;258:12920–12928. [PubMed] [Google Scholar]
- 28.Reddy GS, Tserng KY, Thomas BR, Dayal R, Norman AW. Isolation and identification of 1,23-dihydroxy-24,25,26,27-tetranorvitamin D3, a new metabolite of 1,25-dihydroxyvitamin D3 produced in rat kidney. Biochemistry. 1987;26:324–331. doi: 10.1021/bi00375a045. [DOI] [PubMed] [Google Scholar]
- 29.Reddy GS, Tserng KY. Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry. 1989;28:1763–1769. doi: 10.1021/bi00430a051. [DOI] [PubMed] [Google Scholar]
- 30.Makin G, Lohnes D, Byford V, Ray R, Jones G. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem J. 1989;262:173–180. doi: 10.1042/bj2620173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner RT, Puzas JE, Forte MD, Lester GE, Gray TK, Howard GA, Baylink DJ. In vitro synthesis of 1 alpha,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proc Natl Acad Sci U S A. 1980;77:5720–5724. doi: 10.1073/pnas.77.10.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaki T, Sawada N, Komai K, Shiozawa S, Yamada S, Yamamoto K, Ohyama Y, Inouye K. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur J Biochem. 2000;267:6158–6165. doi: 10.1046/j.1432-1327.2000.01680.x. [DOI] [PubMed] [Google Scholar]
- 33.Sakaki T, Sawada N, Nonaka Y, Ohyama Y, Inouye K. Metabolic studies using recombinant escherichia coli cells producing rat mitochondrial CYP24 CYP24 can convert 1alpha,25-dihydroxyvitamin D3 to calcitroic acid. Eur J Biochem. 1999;262:43–48. doi: 10.1046/j.1432-1327.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- 34.Inouye K, Sakaki T. Enzymatic studies on the key enzymes of vitamin D metabolism; 1 alpha-hydroxylase (CYP27B1) and 24-hydroxylase (CYP24) Biotechnol Annu Rev. 2001;7:179–194. doi: 10.1016/s1387-2656(01)07037-5. [DOI] [PubMed] [Google Scholar]
- 35.Reddy GS, Omdahl JL, Robinson M, Wang G, Palmore GT, Vicchio D, Yergey AL, Tserng KY, Uskokovic MR. 23-carboxy-24,25,26,27-tetranorvitamin D3 (calcioic acid) and 24-carboxy-25,26,27-trinorvitamin D3 (cholacalcioic acid): end products of 25-hydroxyvitamin D3 metabolism in rat kidney through C-24 oxidation pathway. Arch Biochem Biophys. 2006;455:18–30. doi: 10.1016/j.abb.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerman DR, Reinhardt TA, Kremer R, Beitz DC, Reddy GS, Horst RL. Calcitroic acid is a major catabolic metabolite in the metabolism of 1 alpha-dihydroxyvitamin D-2. Arch Biochem Biophys. 2001;392:14–22. doi: 10.1006/abbi.2001.2419. [DOI] [PubMed] [Google Scholar]
- 37.Horst RL, Omdahl JA, Reddy S. Rat cytochrome P450C24 (CYP24) does not metabolize 1,25-dihydroxyvitamin D2 to calcitroic acid. J Cell Biochem. 2003;88:282–285. doi: 10.1002/jcb.10359. [DOI] [PubMed] [Google Scholar]
- 38.Tachibana Y, Tsuji M. Study on the metabolites of 1 alpha,25-dihydroxyvitamin D-4. Steroids. 2001;66:93–97. doi: 10.1016/s0039-128x(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 39.Rhieu SY, Annalora AJ, Wang G, Flarakos CC, Gathungu RM, Vouros P, Sigueiro R, Mourino A, Schuster I, Palmore GT, Reddy GS. Metabolic stability of 3-epi-1alpha,25-dihydroxyvitamin D3 over 1 alpha 25-dihydroxyvitamin D3: metabolism and molecular docking studies using rat CYP24A1. J Cell Biochem. 2013;114:2293–2305. doi: 10.1002/jcb.24576. [DOI] [PubMed] [Google Scholar]
- 40.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–134. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 41.Tang EK, Tieu EW, Tuckey RC. Expression of human CYP27B1 in Escherichia coli and characterization in phospholipid vesicles. FEBS J. 2012;279:3749–3761. doi: 10.1111/j.1742-4658.2012.08736.x. [DOI] [PubMed] [Google Scholar]
- 42.DeLuca HF, Schnoes HK, Esvelt RP. Progress ofor preparing calcitroic acid and its esters. 4260804 A US. 1981
- 43.Esvelt RP, Fivizzani MA, Paaren HE, Schnoes HK, DeLuca HF. Synthesis of Calcitroic Acid, a Metabolite of 1a,25-Dihydroxycholecalciferol. J Org Chem. 1981;46:465–458. [Google Scholar]
- 44.de Costa BR, Makk N, Midgley JM, Modi NT, Watt RA, Whalley WB. Unsaturated Steroids. Part 12.’ Synthesis of 1a,3b-Dihydroxy-24-nor-9,10-secochola-5,7,10(19)trien-23-oic (Calcitroic) Acid and of the Cholic-and 25-Homocholic Acid Analogues. J Chem Soc, Perkin Trans I. 1985;7:1331–1336. [Google Scholar]
- 45.Zhu GD, Okamura WH. Synthesis of Vitamin-D (Calciferol) Chemical Reviews. 1995;95:1877–1952. [Google Scholar]
- 46.Calverley MJ. The Seleno-Acetal Route to Side-Chain-Modified 1-Alpha-Hydroxy-Vitamin-D Analogs - Stereoselective Synthesis of the New 22z Isomer of Mc903 (Calcipotriol) Synlett. 1990:157–159. [Google Scholar]
- 47.Meyer D, Rentsch L, Marti R. Efficient and scalable total synthesis of calcitroic acid and its C-13-labeled derivative. RSC Advances. 2014;4:32327–32334. [Google Scholar]
- 48.Posner GH, Lee JK, White MC, Hutchings RH, Dai HY, Kachinski JL, Dolan P, Kensler TW. Antiproliferative hybrid analogs of the hormone 1 alpha,25-dihydroxyvitamin D-3: Design, synthesis, and preliminary biological evaluation. J Org Chem. 1997;62:3299–3314. doi: 10.1021/jo970049w. [DOI] [PubMed] [Google Scholar]
- 49.Harant H, Andrew PJ, Reddy GS, Foglar E, Lindley IJ. 1alpha,25-dihydroxyvitamin D3 and a variety of its natural metabolites transcriptionally repress nuclear-factor-kappaB-mediated interleukin-8 gene expression. Eur J Biochem. 1997;250:63–71. doi: 10.1111/j.1432-1033.1997.00063.x. [DOI] [PubMed] [Google Scholar]
- 50.Harant H, Spinner D, Reddy GS, Lindley IJ. Natural metabolites of 1alpha,25-dihydroxyvitamin D(3) retain biologic activity mediated through the vitamin D receptor. J Cell Biochem. 2000;78:112–120. [PubMed] [Google Scholar]
- 51.Teske KA, Bogart JW, Sanchez LM, Yu OB, Preston JV, Cook JM, Silvaggi NR, Bikle DD, Arnold LA. Synthesis and evaluation of vitamin D receptor-mediated activities of cholesterol and vitamin D metabolites. Eur J Med Chem. 2016;109:238–246. doi: 10.1016/j.ejmech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014;144(Pt A):28–39. doi: 10.1016/j.jsbmb.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slominski AT, Kim TK, Shehabi HZ, Tang EK, Benson HA, Semak I, Lin Z, Yates CR, Wang J, Li W, Tuckey RC. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014;383:181–192. doi: 10.1016/j.mce.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lietz CB, Gemperline E, Li L. Qualitative and quantitative mass spectrometry imaging of drugs and metabolites. Adv Drug Deliv Rev. 2013;65:1074–1085. doi: 10.1016/j.addr.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masuda S, Strugnell S, Calverley MJ, Makin HL, Kremer R, Jones G. In vitro metabolism of the anti-psoriatic vitamin D analog, calcipotriol, in two cultured human keratinocyte models. J Biol Chem. 1994;269:4794–4803. [PubMed] [Google Scholar]
- 56.Sprake EF, Grant VA, Corfe BM. Vitamin D3 as a novel treatment for irritable bowel syndrome: single case leads to critical analysis of patient-centred data. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-007223. [DOI] [PMC free article] [PubMed] [Google Scholar]