Abstract
We propose that structural and functional connectivity of the anterior cingulate cortex (ACC) represents a critical component of adolescent developmental psychopathology. We hypothesize that connectivity of the ACC, a hub for integrating cognitive, affective, and social information to guide self-regulation across domains, supports adaptive development of self-regulation during adolescence and that, conversely, disrupted maturation of ACC connectivity contributes to the development of depression. To integrate findings on typical development, we report results of a meta-analysis of diffusion imaging findings of typical adolescent development of the cingulum and anterior thalamic radiation, the tracts most relevant to ACC connectivity, and provide a critical review of the literature on ACC functional connectivity. Finally, we review the evidence for altered structural and functional connectivity in adolescents with depression. Although the evidence for our claim is persuasive, a more comprehensive understanding of the ACC’s role depends upon future investigations with sophisticated modeling of networks, prospective and longitudinal designs, and examination of structure-function associations.
Keywords: adolescence, anterior cingulate cortex, connectivity, development, affect regulation, depression
Adolescence, spanning from around the time of pubertal onset, roughly age 9–12 (Crone and Dahl, 2012), until the early- to mid-20s (Crews et al., 2007; Spear, 2000), is a period of dramatic neuroplasticity when widespread developmental changes in neural structure and function are thought to underlie improvements in cognitive, affective, and social domains (Andersen, 2003; Casey, 2015; Luna et al., 2015). In particular, maturation of macroscopic brain networks, through a combination of developing of neurotransmitter systems (Gomes et al., 2016), improved white matter microstructural integrity and changing patterns of task-related and intrinsic neural dynamics, promotes the computational specialization of the neural circuitry necessary to meet the demands of an individual’s environment (Luna et al., 2015). Importantly, this period of plasticity coincides with a dramatic rise in the incidence of several forms of serious psychiatric illness, including depression, that are thought to arise when the typical course of adolescent brain development is disrupted (Paus et al., 2008). Despite extensive investigation of human development and psychopathology, the precise neurobiological pathways underlying the emergence of depression, a common, recurrent, and serious form of psychopathology that typically begins during adolescence (Davey et al., 2008), still remain poorly understood. Elucidating the neurodevelopmental processes that lay the foundation for adaptive adolescent functioning will aid in the identification of neural markers that predict risk for adolescent psychopathology and guide the design of novel prevention and intervention approaches.
Goals of the Current Review
Here, we posit that connectivity of the ACC as a key neural substrate of adolescent neurodevelopment that supports the development of adult self-regulatory capabilities and that can contribute to psychopathology during adolescence when its development is aberrant. Because of the ACC’s location, role as a hub for global neural network dynamics, and critical function in integrating multimodal cognitive, affective, and social information to guide adaptive affect and behavioral regulation (Shenhav et al., 2013), we hypothesize that healthy development of ACC is essential for mental health as young people proceed through adolescence and into adulthood. Additionally, because altered affect regulation is a fundamental characteristic of depression, we propose that both structural and functional connectivity of this essential region are altered in adolescents with depression. This focus stands in contrast to prior work on the developmental neuroscience of adolescent psychopathology, which has primarily focused on either global developmental changes (Giedd et al., 2015; Power et al., 2010; Uda et al., 2015), such as decreasing volume of gray matter over adolescence, or the protracted development of the prefrontal cortex and its potential functional consequences for disorders involving self-regulation (Casey, 2015; Geier, 2013). Similarly, conceptual papers on the function of the ACC have neglected its relevance to adolescent development and developmental psychopathology (Drevets et al., 2008; Shenhav et al., 2013; Weston, 2012). In all, the extant body of literature has failed to appreciate the unique and critical role of the anterior cingulate cortex (ACC) in adolescent brain and behavioral development.
To support our hypothesis, we present a meta-analysis of white matter development during adolescence in two tracts that are central to structural connectivity of the ACC: the cingulum and the anterior thalamic radiations (ATR). Thus, we integrate findings across the large number of developmental studies using diffusion tensor imaging (DTI) to assess white matter structure. Although there is a rich cross-sectional literature examining age effects on white matter microstructure, there is a much smaller body of research using longitudinal data to address developmental changes in white matter across adolescence. Extant longitudinal work is limited by a small number of follow-up assessments, short inter-scan intervals, and inconsistency in the white matter tracts included. Furthermore, although many studies have examined patterns of white matter development across the lifespan, very few have focused specifically on adolescence in order to characterize the trajectory of microstructural changes during this critical developmental period. Therefore, the present meta-analysis aims to more clearly delineate the pattern of cingulum and ATR development in adolescence to establish whether microstructure of these pathways continues to mature throughout this period.
Next, we provide a critical, integrative review of the development of functional connectivity of the ACC. As this literature does not readily lend itself to meta-analytic techniques because of the variety of data analytic strategies applied, we limit our review to a discussion and interpretation of findings across studies. We then extend the consideration of ACC connectivity during adolescence to depression. Depression emerges during adolescence and is most consistently related to ACC structure and function, and its core feature—altered affect regulation is—conceptually linked to a primary function of the ACC (Pizzagalli, 2011; Rive et al., 2013). Finally, we identify gaps in the literature and weaknesses in studies to date, providing recommendations for future research. Throughout, we use a framework of developmental psychopathology, which approaches adolescence as a vulnerable period during which typical development must be understood as a foundation for the deviations that result in atypical development.
The Anterior Cingulate Cortex
The ACC, or the anterior portion of the medial cerebral cortex that lies below the frontal cortex and superior to the corpus callosum (Nolte, 2008), is hypothesized to play a central role in processing information from a broad range of domains in order to guide adaptive behavior (Gasquoine, 2013; Holroyd and Yeung, 2012; Lavin et al., 2013; Shenhav et al., 2013; Weston, 2012). Overall, the ACC is active during a wide range of contexts and has been implicated in an array of different functions, including error and conflict monitoring (Botvinick et al., 2004), affective processing (Etkin et al., 2011), social processing (Beckmann et al., 2009), and reward learning and decision-making (Haber and Behrens, 2014). Several recent reviews have sought to integrate these seemingly diverse functions of the ACC into an overarching function, suggesting that the ACC compiles multimodal information (Lavin et al., 2013; Luna et al., 2015; Shackman et al., 2011; Shenhav et al., 2013) in order to estimate the cognitive and behavioral requirements of different responses (Shenhav et al., 2013; Weston, 2012) and adaptively select and maintain behavioral options (Gasquoine, 2013; Holroyd and Yeung, 2012). A common feature of these accounts is their designation of the ACC as a hub for information processing that integrates inputs from multiple domains and systems: affective, social, cognitive, and visceral (Gasquoine, 2013; Holroyd and Yeung, 2012; Lavin et al., 2013; Luna et al., 2015; Shackman et al., 2011; Shenhav et al., 2013; Weston, 2012). Based on this integration, the ACC is postulated to drive activation in premotor and motor cortical regions as well as basal ganglia, cerebellum and PFC to guide cognitive and behavioral output (Shenhav et al., 2013; Weston, 2012). Given its putative role as the hub connecting multimodal inputs and sophisticated outputs, connectivity between the ACC and other cortical and subcortical brain regions is of particular interest for adolescent development.
The ACC can be further subdivided into subgenual, rostral, and dorsal regions, which are thought to have distinct functional specializations, with a transition along a dorsal to rostral continuum from cognitive to affective processes (Mohanty et al., 2007). The subgenual ACC (sgACC), located ventral to the genu of the corpus callosum (also referred to as the ventral ACC), has been strongly implicated in the automatic modulation of affect (Drevets et al., 2008; Phillips et al., 2008). This region is thought to correspond to the infralimbic cortex in the rodent brain (Öngür and Price, 2000). The dorsal ACC (dACC) is comprised of the cingulate gyrus and sulcus from the genu of the corpus callosum to the plane of the anterior commissure (also known as the midcingulate cortex) and interacts with motor regions to select actions based on integrated information about long term goals and reward contingencies (Rive et al., 2013; Shackman et al., 2011; Shenhav et al., 2013). This region of the ACC is thought to be unique to humans and has no homologous regions in the primate or rodent brain (Vogt et al., 2013). The rostral ACC (rACC; also referred to as pregenual ACC), situated anterior to the genu of the corpus callosum, shows strong connectivity with both dorsal and subgenual divisions and is hypothesized to coordinate autonomic, visceromotor, and endocrine components of affective responses (Sturm et al., 2013). The rACC is considered homologous to rodent ACC (i.e., area d32), although some cytoarchitectural distinctions have been noted between species (Vogt et al., 2013).
ACC and the Development of Adaptive Self-Regulation
Adolescence is an important period for the development of adaptive self-regulation - the ability to monitor and modify one’s reactions to meet one’s goals (Thompson, 1994) - which facilitates improved interpersonal functioning and the establishment of long-term objectives as individuals make the transition to adult status and roles (Farley and Kim-Spoon, 2014). Adolescence is widely conceptualized as a time of social-affective reorientation (Blakemore and Mills, 2014; Crone and Dahl, 2012; Somerville et al., 2010). Across this period, youth develop increased independence, form more intimate friendships, begin romantic relationships, become strongly aware of their status among peers, and are subject to increasing educational and occupational demands that necessitate increasingly complex self-regulatory capabilities. Therefore, the development of mature self-regulation is a critical task of adolescence, as it is required to achieve many of the central goals of adulthood. These include obtaining an education, contributing to the workforce, initiating and maintaining romantic relationships, forming a supportive social network, and regulating behavior in order to thrive in multiple domains (Brown et al., 2008; Pfeifer et al., 2011; Schulenberg et al., 2004).
In particular, adolescents have been shown to make more maladaptive or “risky” decisions in the presence of peers (Chein et al., 2011) and in affectively arousing contexts (Figner et al., 2009) relative to adults. Age-related improvements in decision-making in social and affective contexts are hypothesized to derive from neurodevelopmental changes in regions related to estimating reward value of environmental information and developing interactions between subcortical and cortical systems that support aspects of decision making that rely on complex cost-benefit calculations (Hartley and Somerville, 2015). Given the putative role of the ACC in integrating affective, social and cognitive information to guide action selection, maturation of ACC connectivity is a likely mechanism supporting more adaptive self-regulation and decision-making under such complex cost-benefit scenarios.
In addition to supporting typical adolescent development, developing ACC connectivity has important implications for risk for psychopathology during this period. In particular, ACC dysfunction has been strongly implicated in depression in both adult and adolescent samples. Depression is characterized by difficulty modulating negative affect, as well as a decrease in goal-directed behaviors, functions that are closely tied to ACC function. Congruently, a recent meta-analysis including over 500 participants demonstrated that depressed youth display aberrant activation in both dorsal and subgenual ACC (Miller et al., 2015). Furthermore, direct modulation of subgenual ACC function has been identified as a promising treatment for depression (Berlim et al., 2014), suggesting that disrupted ACC function is a fundamental feature of the disorder that may play a causal role in the onset and maintenance of depression.
Structural and Functional Connectivity of the ACC
Given the ACC’s role as a hub for processing and integrating many types of information (van den Heuvel and Sporns, 2013), the development of its structural and functional connectivity of this region may be a neural mechanism of adaptive self-regulation in adolescence.
Structural ACC Connectivity
The ACC sits at the intersection of the brain’s cognitive, affective, and action networks (Haber and Behrens, 2014) and is directly connected to limbic and subcortical structures as well as prefrontal and motor cortical regions (Beckmann et al., 2009). The current review focuses on the cingulum bundle and anterior thalamic radiations, as these represent the primary white matter pathways linking the ACC with its distributed afferent and efferent targets.
Cingulum
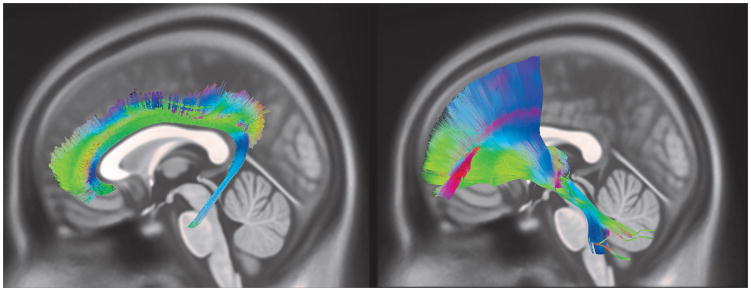
The cingulum bundle is a major white matter pathway that runs along the anterior-posterior axis of each hemisphere and facilitates communication between frontal, parietal, and temporal cortices as well as subcortical structures, including the striatum and hippocampus (See Figure 1). Based on the topography of cingulum fibers, this pathway can be divided into subgenual, dorsal, and parahippocampal/temporal sections that differ with regard to their spatial location, microstructural characteristics, and connectivity profiles (Jones et al., 2013). Based on these subdivisions, several studies of adolescent development divide this pathway into its anterior cingulate and posterior hippocampal components (e.g., Rollins et al., 2010; Simmonds et al., 2014). In these instances, the current meta-analysis will focus on age-related changes in the anterior cingulate division of the cingulum, containing fibers that connect subgenual and dorsal regions of the ACC to other anterior and posterior cingulate regions, prefrontal, motor, temporal, and parietal cortical areas, as well as subcortical structures including the amygdala, hippocampus, striatum, and hypothalamus (Beckmann et al., 2009; Heilbronner and Haber, 2014; Jones et al., 2013).
Figure 1.
Illustration of the (a) cingulum and (b) anterior thalamic radiations (ATR). Original figure generated to illustrate the pathways of interest in the current review. Deterministic tractography was used to track the cingulum and ATR using diffusion spectrum imaging data from an atlas generated from a sample of 60 neurologically healthy participants (the sample and atlas generation process are described in (Jarbo and Verstynen, 2015; Yeh et al., 2016). Color-coded images show the orientation of the principal direction of diffusion of each streamline, with red, blue, and green representing diffusion along x-, y-, and z-axes, respectively.
Anterior thalamic radiations (ATR)
The ATR is the primary conduit for communication between the ACC and subcortical limbic structures. This pathway links the anterior thalamic nuclei and the ACC and medial frontal regions via the anterior limb of the internal capsule and anterior corona radiata (Catani and Thiebaut de Schotten, 2012; Lobel et al., 2009) (See Figure 1). The anterior thalamic nuclei are considered to be part of the limbic system, as they receive input from the hippocampus and hypothalamic mammillary bodies (Catani and Thiebaut de Schotten, 2012). Therefore, the ATR facilitates the integration of affective and visceral information with other cognitive functions of the ACC.
Different studies of structural connectivity across development examine distinct portions of this pathway, including the anterior limb of the internal capsule (i.e. Lebel et al., 2008), anterior corona radiata (i.e. Verhoeven et al., 2010), and/or applying tractography to define the ATR (i.e. Peters et al., 2012), each of which will be included in the current meta-analysis. However, it is important to note that the anterior limb of the internal capsule and anterior corona radiata contain frontostriatal and frontopontine fibers in addition to anterior thalamic projections (Catani and Thiebaut de Schotten, 2012). Therefore, developmental changes in the anterior limb of the internal capsule and anterior corona radiata may not be specific to the ATR, and must be interpreted with caution.
Measuring structural ACC connectivity
Diffusion MRI is a neuroimaging method that quantifies structural connectivity by measuring the direction and magnitude of water diffusion in neural tissue (Dell'Acqua and Catani, 2012; Hagmann et al., 2006). Fractional anisotropy (FA) is the most common diffusion MRI-based measure of white matter microstructure, with higher values indicative of greater structural integrity and improved organization, cohesion, and compactness of white matter fiber tracts (Beaulieu, 2002; Bonekamp et al., 2007; Paus, 2010; Walhovd et al., 2014). Several other metrics can also be calculated, including mean diffusivity (MD), which estimates of the overall magnitude of diffusion, axial diffusivity (AD), which measures diffusion along the primary direction of diffusion, and radial diffusivity (RD), which reflects the amount of diffusion perpendicular to the primary diffusion direction. Although the precise cellular mechanisms of change in each diffusion metric remain unclear, higher FA and AD coupled with lower RD and MD are generally considered to reflect better structural integrity, increased myelination, and greater cohesion of white matter pathways.
Functional ACC Connectivity
In addition to its direct structural connections, the ACC plays a central role in two of the basic functional neural networks: the default mode network (DMN) and the salience network (SN). The DMN is a canonical resting-state network that includes the medial prefrontal cortex, posterior cingulate cortex, lateral parietal cortex and rACC (Greicius et al., 2003; Raichle, 2015), and that has been implicated in an array of functions related to self-referential mental activity, including autobiographical memory, self-monitoring, self-regulation, and source monitoring (Fair et al., 2008; Northoff, 2014), as well as related social cognitive functions (Spreng et al., 2009). Perhaps not surprisingly, serious psychopathology is associated with hyperactive, hyperconnected DMN (Broyd et al., 2009; Whitfield-Gabrieli and Ford, 2012). The salience network (SN) is a neural network primarily comprised of the dACC and anterior insula, which has been implicated in the identification of salient internal and external stimuli to guide behavior (Menon and Uddin, 2010).
Further supporting the central role of the ACC in integrating across multiple domains, seed-based approaches have demonstrated that resting-state functional connectivity of the ACC is distributed widely throughout the brain. Dorsal activation is correlated with activation of sensorimotor circuits, rostral activity is linked to PFC activation, and transitional zones of connectivity appear to be functionally connected to both prefrontal and sensorimotor brain regions and promote coordination among these networks (Margulies et al., 2007). Therefore, based on its structural and functional connectivity, the ACC is uniquely well equipped to process multimodal information and to integrate this information to guide adaptive behavior.
Measuring functional ACC connectivity
Functional connectivity MRI (fcMRI) is a method of inferring functional connectivity between different brain regions based on temporal correlations in neural signal during the performance of a task or at rest (Power et al., 2010). Temporal coherence in spontaneous low-frequency signal fluctuations at rest is hypothesized to reflect a history of co-activation suggesting a functional relationship between regions (Ernst et al., 2015). Several statistical methods have been applied to fcMRI data, including seed-based approaches, (e.g., psychophysiologic interaction), graph analysis techniques on region-to-region connectivity, and independent/principal component analysis (Power et al., 2010), which vary in the degree to which they are hypothesis- or data-driven (Ernst et al., 2015). Seed-based approaches select a small number of regions a priori and assess correlations between fluctuations the signal in these regions and fluctuations in voxels throughout the rest of the brain. Graph theory methods involve parcellating the brain into a large number of different regions, or “nodes”, and simultaneously analyzing pairwise correlations in coordination, or “edges”, between each node and other nodes. Finally, studies using independent/principal component analysis use decomposition methods to group regions based on their temporal coherence in neural signal. In the current review, we will include studies using all types of fcMRI analysis.
ACC Connectivity and Developmental Psychopathology
Based on its critical role in promoting self regulation, the development of ACC connectivity is likely to play an important role in the development of depression during adolescence. The most compelling developmental psychopathology model of depression proposes that depression emerges during adolescence because of the occurrence of the simultaneous development in multiple, interacting domains (Davey et al., 2008). Specifically, dramatic changes in peer social orientation, neural reward circuitry, executive function, reward motivation, and self-relevant goals during adolescence are postulated to combine, in vulnerable youth, to create a foundation for depression. Congruently, a recent meta-analytic review of neural response in adolescent depression reported that ACC is consistently disrupted across a variety of task contexts (Miller et al., 2015). At a network level, studies have consistently found that depression is associated with enhanced connectivity within the anterior DMN (including the rACC) (Kaiser et al., 2015; Mulders et al., 2015) and reduced connectivity between the DMN and the frontoparietal control network (Mulders et al., 2015).
Typical Development of Structural ACC Connectivity: Meta-Analysis
Methods
Study Selection
For the purposes of the current review, we chose to include studies with human participants from age 10–20 (at minimum) and tested the effect of age on FA of the cingulum and/or ATR. Adolescence is broadly defined as the transitional period between childhood and adulthood (Crone and Dahl, 2012), but the precise onset and offset of adolescence are difficult to delineate (Spear, 2000). Traditionally, this period is thought to begin around the time of pubertal onset, roughly age 9–12 (Crone and Dahl, 2012) and end with relative independence from the parents (Casey, 2015) during the early- to mid-20s (Crews et al., 2007; Spear, 2000). Because studies of adolescent development have varied considerably in the age groups they include or have examined development in a wider range of the lifespan, many of the studies included an age range broader than 10–20 years.
We conducted literature searches with PubMed, Google Scholar, and Ovid Medline using search terms adolescen*, (development* OR maturation OR age-related), and (diffusion AND (weighted OR tensor)) OR DTI OR TBSS). Reference lists were also examined to identify additional studies meeting these inclusion criteria. Using this search strategy, 37 studies were identified that met our inclusion criteria. From the total body of studies identified, those reporting regression coefficients corresponding to the linear, quadratic, or logarithmic effect of age on FA of the cingulum (N=10 studies) and/or ATR (N=7 studies) were selected for inclusion in the meta-analysis.
Data Analysis
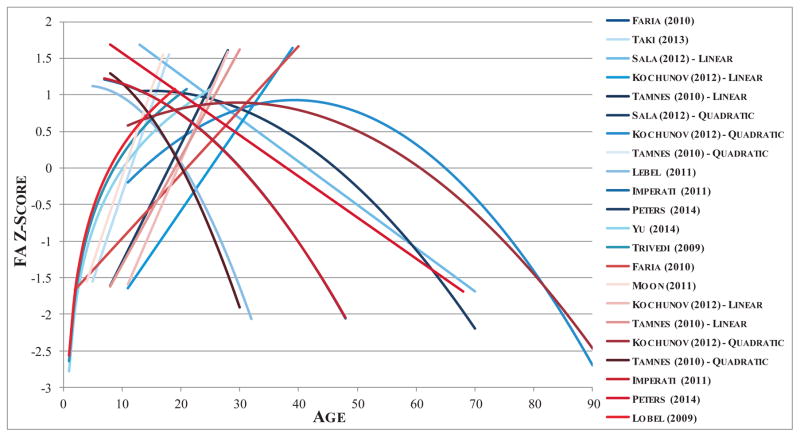
From all relevant studies, we extracted the reported regression coefficient for the influence of age on multiple DTI-based diffusivity measures (e.g., FA) and used these parameters to simulate a predicted trajectory of different white matter metrics across the range of ages included in each study. For studies reporting linear effects of age, a linear model was used to simulate metrics of white matter microstructure at each age, whereas for studies reporting quadratic effects of age, a quadratic model was used. In instances when studies parcellated the cingulum into anterior and posterior (hippocampal) divisions, only diffusivity metrics corresponding to the anterior region were selected for inclusion. For the ATR, diffusivity measures were included that correspond to the anterior limb of the internal capsule, anterior corona radiata, and the ATR as a whole. For studies reporting results from multiple regression models (i.e. linear and quadratic models), separate datasets were generated for each model. FA values were converted to z-scores in order to standardize across studies, producing an estimate of the predicted direction and relative magnitude of change in FA at each age for each pathway (See Figure 2). Simulated data from each regression model from each study were aggregated to form a composite dataset of simulated white matter integrity across development and linear and quadratic effects of age on FA were tested on this composite set. Each study was dummy coded and included as a covariate to control for variation across study samples and methods, as well as to account for the fact that some studies contributed multiple data points to the analysis. The likelihood ratio test was used to determine whether the quadratic model was a significantly better fit to the data relative to the linear model for each study. Finally, linear and quadratic regression models were also applied within the 10–20 age range, in order to specifically assess the direction, trajectory, and significance of change in white matter microstructure during adolescence. The same method was used to combine across studies that reported regression coefficients for mean, radial, and axial diffusivity of the cingulum and/or ATR.
Figure 2.
Illustration of meta-analysis methods. Regression models reported for each pathway were used to simulate fractional anisotropy (FA) data for each age within the range included in each study. FA values were converted to z-scores in order to standardize across studies, producing an estimate of the predicted direction and magnitude of change in FA at each age for each pathway. Blue lines represent regression models of developmental change in FA of the cingulum. Red lines represent regression models for age effects on FA in the anterior thalamic radiations.
Results
Cingulum
We identified 10 studies of age associations with white matter microstructure of the cingulum bundle, which also reported regression coefficients corresponding to general linear regression models. Three studies included multiple regression models, resulting in a total of 13 models included in the current meta-analysis (See bolded studies in Table 2 and results in Table 3).
Table 2.
Diffusion Tensor Imaging Studies of Typical Development of FA within the Cingulum and Anterior Thalamic Radiations
| N | Age | FA Changea | T | b-value | Directions | Cingulum ROI | ATR ROI | |
|---|---|---|---|---|---|---|---|---|
| Longitudinal Region-of-Interest Studies | ||||||||
| Brouwer 2012 | 126 | 9–12 | ↑ | 1.5 | 1000 | 32 | Yes | - |
| Lebel 2011 | 103 | 5–32 | ↑ | 1.5 | 1000 | 6 | Yes | - |
| Simmonds 2014 | 128 | 8–29 | ↑ | 3 | 800 | 6 | Yes | Yes |
|
| ||||||||
| Cross-sectional Region-of-Interest Studies | ||||||||
| Bonekamp 2007 | 40 | 5–19 | ↑ | 1.5 | 1000 | 15 | Yes | Yes |
| Cancelliere 2013 | 202 | 0–18 | - | 1.5 | 1000 | 15 | - | Yes |
| Clayden 2012 | 53 | 8–16 | ↑ | 1.5 | 1000 | 20 | Yes | Yes |
| Faria 2010 | 35 | 2–40 | ↑ | 1.5 | 700 | 32 | Yes | Yes |
| Grieve 2011 | 213 | 7–86 | ↑ | 1.5 | 1250 | 12 | Yes | - |
| Imperati 2011 | 144 | 7–48 | ↑ | 3 | 1000 | 64 | Yes | Yes |
| Kochunov 2012 | 831 | 11–90 | ↑ | 3 | 700 | 55 | Yes | Yes |
| Lebel 2008 | 202 | 5–30 | ↑ | 1.5 | 1000 | 6 | Yes | Yes |
| Lebel 2012 | 403 | 5–83 | ↑ | 1.5 | 1000 | 6 | Yes | Yes |
| Lobel 2009 | 72 | 0–19 | ↑ | 1.5 | 1000 | 6 | - | Yes |
| Moon 2011 | 87 | 4–17 | ↑ | 3 | 1000 | 6 | - | Yes |
| Peters 2014 | 296 | 8–68 | ↑↓b | 3 | 1000 | 31 | Yes | Yes |
| Qiu 2008 | 75 | 6–26 | ↑ | 3 | 1000 | 6 | - | Yes |
| Rollins 2010 | 32 | 6–18 | ↑ | 3 | 700 | 30 | Yes | Yes |
| Sala 2012 | 84 | 13–70 | ↓ | 1.5 | 900 | 12 | Yes | - |
| Snook 2005 | 60 | 8–27 | ↑ | 1.5 | 1000 | 6 | - | Yes |
| Stadlbauer 2008 | 38 | 18–88 | - | 3 | 1000 | 6 | Yes | - |
| Supekar 2010 | 33 | 7–22 | ↑ | 3 | 850 | 23 | Yes | - |
| Taki 2013 | 290 | 5–18 | ↑ | 3 | 1000 | 32 | Yes | - |
| Tamnes 2010 | 168 | 8–30 | ↑ | 1.5 | 700 | 30 | Yes | - |
| Trivedi 2009 | 51 | 0–21 | ↑ | 1.5 | 700 & 1000 | 10 | Yes | - |
| Verhoeven 2010 | 78 | 0–24 | ↑ | 3 | 800 | 45 | Yes | Yes |
| Westlye 2010 | 430 | 8–85 | ↑ | 1.5 | 700 | 30 | Yes | Yes |
| Yu 2014 | 65 | 0–25 | ↑ | 3 | 1000 | 30 | Yes | - |
|
| ||||||||
| Longitudinal Voxelwise Studies | Cingulum Sig | ATR Sig | ||||||
| Bava 2010 | 22 | 16–21 | ↑ | 3 | 2000 | 15 | - | Yes |
| Giorgio 2010 | 24 | 13–18 | ↑ | 1.5 | 1000 | 60 | Yes | Yes |
| Wang 2012 | 16 | 13–17 | ↑ | 1.5 | 1000 | 32 | Yes | Yes |
|
| ||||||||
| Cross-sectional Voxelwise Studies | ||||||||
| Ashtari 2007 | 24 | 10–20 | ↑ | 1.5 | 1000 | 25 | Yes | - |
| Barnea-Goralay 2005 | 34 | 6–19 | - | 1.5 | 900 | 6 | - | Yes |
| Brauer 2011 | 20 | 5–32 | ↑ | 1.5 | 1000 | 60 | Yes | Yes |
| Faria 2010 | Listed above | - | - | |||||
| Giorgio 2008 | 42 | 13.5–42 | - | 1.5 | 1000 | 60 | - | - |
| Peters 2012 | 78 | 8–21 | ↑ | 1.5 | 1000 | 31 | Yes | Yes |
| Qiu 2008 | Listed above | Yes | Yes | |||||
| Qiu 2010 | 96 | 11–25 | ↑ | 3 | 1000 | 12 | Yes | Yes |
| Schmithorst 2002 | 33 | 5 – 18 | - | 3 | 814/994 | 6 | - | Yes |
| Stevens 2009 | 50 | 11–37 | ↑ | 3 | 1000 | 12 | - | - |
| Taki 2013 | Listed above | - | - | |||||
Note. Studies included in the meta-analysis are bolded. Characteristics of studies reporting both ROI and voxelwise analyses are listed only once.
The direction of FA change refers to the adolescent period, not lifespan change.
Peters et al. (2014) found increased FA of the cingulum, and decreased FA for the ATR.
Table 3.
Meta-Analytic Results for Linear and Quadratic Effects of Age on Cingulum Microstructure.
| Model | # Studies | # Models | Age Range | Beta (Age) | p | Beta (Age2) | p | R2 | Adjusted R2 | SSE |
|---|---|---|---|---|---|---|---|---|---|---|
| FA | ||||||||||
| Linear | 10 | 13 | 1–90 | −0.474 | .001 | - | - | 0.119 | 0.094 | 427.95 |
| Quadratic | 10 | 13 | 1–90 | 1.597 | .001 | -2.096 | .001 | 0.406 | 0.388 | 288.621* |
| Linear | 10 | 13 | 10–20 | 0.218 | .001 | - | - | 0.859 | 0.844 | 12.173 |
| Quadratic | 10 | 13 | 10–20 | 0.834 | .021 | 0.619 | .084 | 0.862 | 0.846 | 11.891 |
|
| ||||||||||
| MD | ||||||||||
| Linear | 4 | 6 | 1–70 | 0.512 | .001 | - | - | 0.137 | 0.11 | 168.277 |
| Quadratic | 4 | 6 | 1–70 | −2.485 | .001 | 2.841 | .001 | 0.472 | 0.453 | 102.886* |
| Linear | 4 | 6 | 10–20 | −0.23 | .001 | - | - | 0.836 | 0.817 | 7.313 |
| Quadratic | 4 | 6 | 10–20 | −0.95 | .122 | 0.722 | .236 | 0.841 | 0.818 | 7.109 |
|
| ||||||||||
| AD | ||||||||||
| Linear | 4 | 6 | 1–70 | −0.983 | .001 | - | - | 0.547 | 0.534 | 99.68 |
| Quadratic | 4 | 6 | 1–70 | −2.105 | .001 | 1.112 | .001 | 0.615 | 0.602 | 84.788* |
| Linear | 4 | 6 | 10–20 | −0.151 | .002 | - | - | 0.897 | 0.885 | 5.795 |
| Quadratic | 4 | 6 | 10–20 | 0.263 | .551 | −0.415 | .389 | 0.898 | 0.885 | 5.712 |
|
| ||||||||||
| RD | ||||||||||
| Linear | 4 | 6 | 1–70 | 0.509 | .001 | - | - | 0.146 | 0.123 | 187.807 |
| Quadratic | 4 | 6 | 1–70 | −2.421 | .001 | 2.904 | .001 | 0.608 | 0.595 | 86.245* |
| Linear | 4 | 6 | 10–20 | −0.173 | .002 | - | - | 0.862 | 0.846 | 4.78 |
| Quadratic | 4 | 6 | 10–20 | −0.618 | .270 | 0.445 | .424 | 0.864 | 0.845 | 4.721 |
Note. Boldface text indicates model with better fit according to likelihood ratio test.
FA
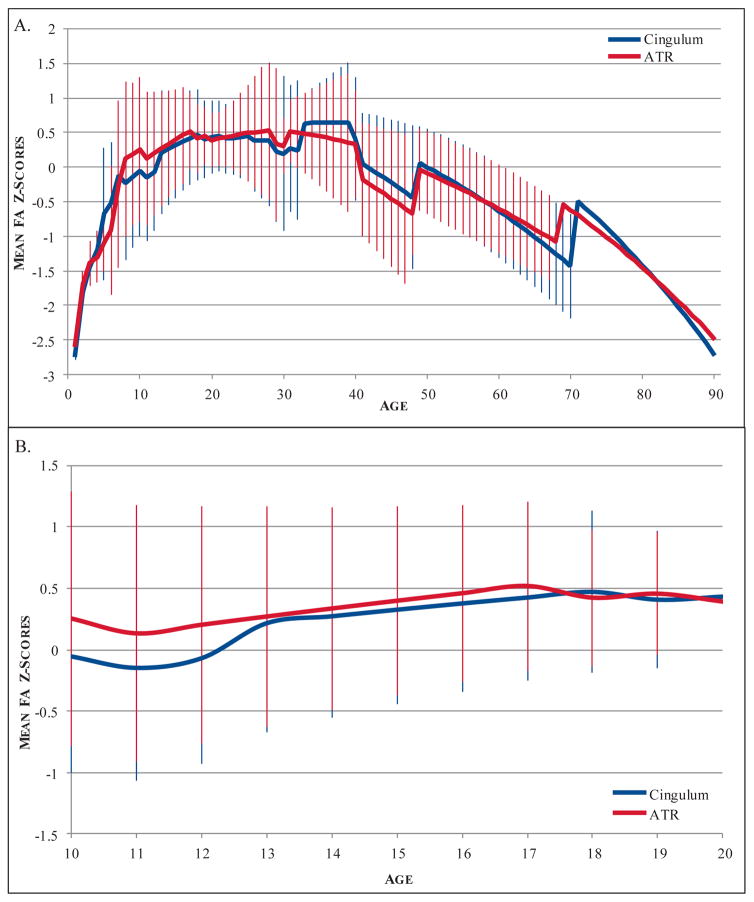
Across all 13 models, significant effects of age were found for the linear (beta = −.474, p < .001) and quadratic (age beta = 1.597, p < .001; age2 beta = −2.096, p < .001) regression models. The likelihood ratio test demonstrated that the quadratic model was a better fit to the data including the entire range of ages (deviance = 139.33, p < .05). Looking specifically at the adolescent period (age 10–20), a significant linear (beta = .218, p < .001) and trend-level quadratic (age beta = .835, p = .021; age2 beta = −.619, p = .084) effect of age on FA was also observed. In this case, the quadratic model was not a significantly better fit to the data (deviance = .282, NS), and the linear model was retained (See Figure 3).
Figure 3.
Developmental trajectories of cingulum and ATR fractional anisotropy. A) Lifetime developmental trajectory of FA from age 0–90. B) Trajectory of FA across adolescence, age 10–20. Lines represent mean FA z-scores from all models included. Because the composite model predictions combine both linear and quadratic effects, both linear and quadratic models are displayed. Error bars depict standard deviations.
MD, RD, and AD
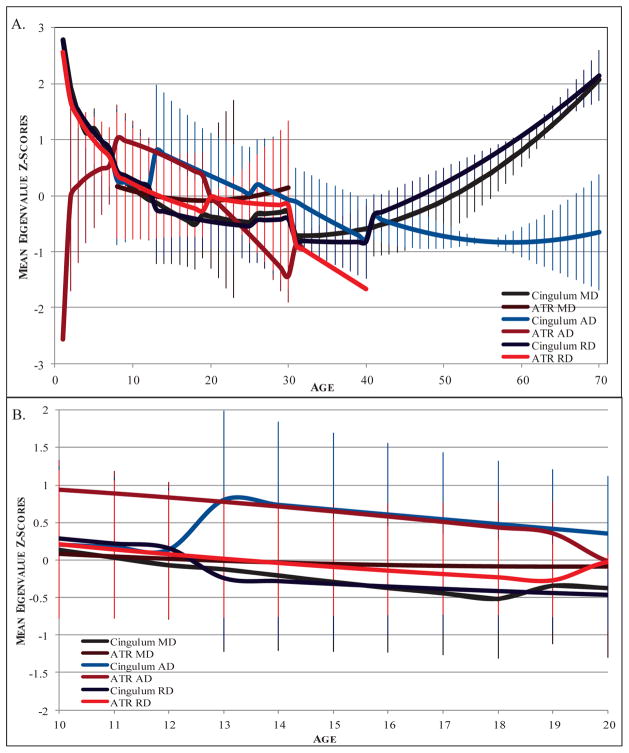
Four studies reported regression coefficients for the effect of age on non-FA diffusivity metrics MD, RD, and AD. Two studies contributed 2 models, resulting in a total of 6 models for inclusion. Across the age range included, significant linear and quadratic effects of age were found for each measure (all p < .001), and the quadratic models were found to fit the data better (all p < .05; see Table 3 for full results). Additionally, within the adolescent period (age 10–20), significant linear effects of age were observed for MD, RD, and AD (all p < .01), whereas quadratic age effects were not significant (See Figure 4).
Figure 4.
Developmental trajectories of cingulum and ATR MD, RD, and AD. A) Lifetime developmental trajectory of MD, RD and AD from age 0–70. B) Trajectory of MD, RD, and AD across adolescence, age 10–20. Lines represent mean z-scores for each measure across all models included. Both linear and quadratic effects are displayed. Error bars depict standard deviations. MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity.
ATR
Seven studies reported regression coefficients for age effects on white matter microstructure of the ATR, contributing a total of 9 regression models (See bolded studies in Table 2 and results in Table 4).
Table 4.
Meta-Analytic Results for Linear and Quadratic Effect of Age on White Matter Microstructure of Anterior Thalamic Radiation
| Model | # Studies | # Models | Age Range | Beta (Age) | p | Beta (Age2) | p | R2 | Adjusted R2 | SSE |
|---|---|---|---|---|---|---|---|---|---|---|
| FA | ||||||||||
| Linear | 7 | 9 | 1–90 | −0.7 | .001 | - | - | 0.272 | 0.251 | 225.691 |
| Quadratic | 7 | 9 | 1–90 | 0.903 | .001 | −1.616 | .001 | 0.431 | .412 | 176.459* |
| Linear | 7 | 9 | 10–20 | 0.168 | .001 | - | - | 0.842 | 0.824 | 9.55 |
| Quadratic | 7 | 9 | 10–20 | 0.588 | .218 | −0.422 | .377 | 0.843 | .824 | 9.459 |
|
| ||||||||||
| MD - Only 1 study reported regression coefficients corresponding to age effect on MD | ||||||||||
|
| ||||||||||
| AD | ||||||||||
| Linear | 3 | 4 | 1–40 | −0.807 | .001 | - | - | 0.536 | 0.517 | 46.401 |
| Quadratic | 3 | 4 | 1–40 | 0.508 | .052 | −1.351 | .001 | 0.638 | 0.62 | 36.162* |
| Linear | 3 | 4 | 10–20 | −0.655 | .001 | - | - | 0.467 | 0.411 | 2.969 |
| Quadratic | 3 | 4 | 10–20 | 0.836 | .516 | −1.499 | .248 | 0.486 | 0.417 | 2.862 |
|
| ||||||||||
| RD | ||||||||||
| Linear | 3 | 4 | 1–40 | −0.672 | .001 | - | - | .372 | 0.347 | 62.764 |
| Quadratic | 3 | 4 | 1–40 | −1.092 | .002 | 0.432 | .201 | 0.383 | 0.351 | 61.72 |
| Linear | 3 | 4 | 10–20 | −0.216 | .005 | - | - | 0.805 | 0.785 | 4.285 |
| Quadratic | 3 | 4 | 10–20 | −0.549 | .488 | 0.334 | .672 | 0.806 | 0.78 | 4.264 |
Note. Only 1 study reported regression coefficients for MD so this measure was excluded from the meta-analysis for the anterior thalamic radiations. Boldface text indicates model with better fit according to likelihood ratio test.
FA
Across the entire age range (age 0–90), significant linear (beta = −.7, p < .001) and quadratic (age beta = .903, p < .001; age2 beta = −1.616, p < .001) effects of age were found. Again, the quadratic model was found to be a better fit to the data (deviance = 49.232, p < .05). Within adolescence (age 10–20), a significant linear (beta = .168, p < .001) effect of age was present, whereas quadratic terms were not significant (See Figure 3).
MD, RD, and AD
Only 1 study included in the literature review reported regression coefficients for MD, so this measure was excluded from the meta-analysis. Three studies provided a total of 4 models testing age effects on ATR RD and AD. Linear effects of age were found for both (both p < .001), whereas quadratic effects were only found for AD (See Table 4 for full results). Within adolescence, linear decreases in both RD and AD were found (both p < .01).
Summary of Typical Development of Structural ACC Connectivity
Collectively, results of the literature review and meta-analysis converge in reporting a curvilinear course of cingulum and ATR development, characterized by increasing FA across adolescence that peaks in the third decade of life (See Figure 3). An inverse pattern of developmental trajectories was seen for MD and RD measures, suggesting that the age-related effects on white matter pathways relates, in part, to changes in underlying myelination integrity (Budde et al., 2009; Song et al., 2005). Additionally, these findings suggest that microstructural development may progress at different rates at different points in adolescence, and future research should explore how variation in the trajectory of white matter development may relate to the development of cognitive, affective, and social skill across adolescence.
Typical Development of Functional ACC Connectivity: Review
Throughout the brain—but particularly for the ACC—patterns of functional connectivity undergo ongoing remodeling between childhood and adulthood. Adolescent brain development features the increasing prominence of the ACC in the brain’s functional networks. Overall, a transition from local to distributed connectivity takes place, whereby children show greater resting-state connectivity between local regions and adults display a more distributed pattern of long-range connectivity throughout the brain (Power et al., 2010). Furthermore, several distinct neural networks strengthen their connectivity while others weaken across development (Ernst et al., 2015).
White matter pathways connect a majority of common neural networks (van den Heuvel et al., 2009), but regions can also display strong functional connectivity in the absence of known structural connections (Honey et al., 2009). Therefore, within a given network, some regions may be directly connected via major white matter tracts, whereas others may take several steps to communicate with one another. Importantly, when known structural connections are present, white matter microstructure has been shown to predict functional connectivity (Damoiseaux and Greicius, 2009; Honey et al., 2009). Thus, improved white matter integrity of the cingulum and ATR may directly strengthen functional connectivity of the ACC and contribute to improved integration between cognitive, affective, and social domains. Functional connections associated with higher-level social-cognitive and affective functions show a more protracted developmental course than connections related to motor and attentional control, supporting the notion that age-related changes in functional ACC connectivity may underlie the maturation of key higher-level capabilities across adolescence.
Development of Default Mode and Salience Network Connectivity
The DMN and SN both show a strong developmental pattern of increasing functional network connectivity across adolescence. Consistent with the dorsal to rostral continuum of cognitive and affective functions of the ACC (Mohanty et al., 2007), the sgACC and rACC become more strongly connected to the DMN, whereas dACC shows increasing connectivity with the SN during this period. Within the DMN, regions display only limited connectivity in childhood and become strongly functional connected by adulthood (Fair et al., 2008). In particular, anterior DMN connectivity of the rACC was shown to increase significantly between age 7 and 18 (Sole-Padulles et al., 2016). Similarly, looking at connectivity of the SN across development, significantly stronger functional connectivity between the dACC and anterior insula has been observed among adults relative to children (Uddin et al., 2011), with dACC connectivity increasing significantly between age 7–18 (Sole-Padulles et al., 2016). Furthermore, a direct comparison of structural and functional connectivity of the DMN across development identified the cingulum to be the most immature link in this network in childhood, and demonstrated that cingulum FA was positively correlated with DMN functional connectivity in adulthood (Supekar et al., 2010). Therefore, ongoing development of cingulum microstructure in adolescence may contribute to increased functional connectivity of the DMN, and facilitate the self- and social-cognitive functions this network supports.
ACC Connectivity and Adolescent Depression
Building on the meta-analytic and literature review findings that the ACC’s structural and functional connectivity develop substantially during adolescence, we turn to the role of ACC connectivity in the development of depression in adolescence. We review the findings from DTI and fcMRI studies (both resting-state and task-based approaches), with attention to young people with clinical-level psychopathology or at risk for psychopathology, primarily because of family history. We searched for studies of functional connectivity during task or resting contexts in adolescents (age 10–20) with depression (Table 5). We did not exclude studies based on technique for computing functional connectivity or for focus on ACC as a seed region. We did not include treatment studies or studies focusing on remitted depression, as they address questions different from those we are posing.
Table 5.
Structural and Functional ACC Connectivity in Adolescent Depression
| Structural Connectivity | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Adolescents at High Risk for Depression | ||||||||
|
| ||||||||
| HR n | LR n | Mean Age | FA Difference | Tract | T | b-value | Directions | |
|
|
||||||||
| Huang 2011 | 18 | 13 | 15.6 | ↓ | Cingulum | 3 | 1000 | 30 |
| Keedwell 2012 | 18 | 15 | 22.2 | ↓ | Cingulum | 3 | 1000 | 30 |
|
| ||||||||
| Adolescents with Depression | ||||||||
|
| ||||||||
| MD n | TD n | Mean Age | FA Difference | Tract | T | b-value | Directions | |
|
|
||||||||
| Cullen 2010 | 14 | 14 | 16.8 | ↓ | Cingulum | 3 | 1000 | 30 |
| Henderson 2013 | 17 | 16 | 16.6 | ↓ | Cingulum, ATR | 3 | 1000 | 12 |
| LeWinn 2014 | 52 | 42 | 16.1 | ↓ | ATR | 3 | 1500 | 30 |
|
| ||||||||
| Functional Connectivity | ||||||||
|
| ||||||||
| Adolescents at High Risk for Depression | ||||||||
|
| ||||||||
| HR n | LR n | Mean Age | ACC Connectivity | Regions with Connectivity to ACC | Subregion | Context | Method | |
|
|
||||||||
| Chai 2015 | 27 | 16 | 11.2 | ↓ | dlPFC | sgACC | Rest | mPFC/PCC, dlPFF amygdala seeds |
|
| ||||||||
| ↑ | mPFC/PCC | |||||||
|
| ||||||||
| Clasen 2014 | 11 | 13 | 13.6 | ↓ | rIFG | dACC | Rest | rIFG seed |
|
| ||||||||
| Kerestes 2016 | 29 | 43 | 14 | - | - | - | Viewing happy faces | Putamen, dlPFC seeds |
|
| ||||||||
| Adolescents with Depressive Symptoms | ||||||||
|
| ||||||||
| n | Mean Age | ACC Connectivity | Regions with Connectivity to ACC | Subregion | Context | Method | ||
| Strikwerda-Brown 2014 | 72 | 16.47 | ↓ | PCC, angular gyrus, dlPFC, dmPFC, vmPFC | sgACC | Rest | sgACC seed | |
|
| ||||||||
| Davey 2015 | 56 | 16.5 | ↑ | Amygdala | sgACC | Rest | Amygdala seed | |
|
| ||||||||
| Adolescents with Current Depression | ||||||||
|
| ||||||||
| MD N | TD N | Mean Age | ACC Connectivity | Regions with Connectivity to ACC | Subregion | Context | Method | |
|
|
||||||||
| Connolly 2013 | 23 | 36 | 16 | ↑ | Insula, amygdala | sgACC | Rest | 4 sgACC seeds |
|
| ||||||||
| Cullen 2009 | 12 | 14 | 16.6 | ↓ | mPFC, STG, Insula | sgACC | Rest | sgACC, dACC, amygdala seeds |
|
| ||||||||
| Gabbay 2013 | 21 | 21 | 16.7 | ↑ | Caudate | sgACC, pgACC | Rest | 6 striatal seeds |
|
| ||||||||
| Geng 2016 | 26 | 31 | 15.6 | - | - | - | Rest | Hippocampus seed |
|
| ||||||||
| Kim 2015 | 22 | 20 | 14 | - | - | - | Rest | Amygdala, PCC seeds |
|
| ||||||||
| Pannekoek 2014 | 26 | 26 | 15 | ↑ | Amygala | pgACC dACC |
Rest | Amygdala, seeds dACC, PCC |
|
| ||||||||
| ↓ | mPFC | |||||||
|
| ||||||||
| Blom 2012 | 31 | 36 | 16 | ↑ | Insula | dACC | Viewing affective faces | Insula seed |
|
| ||||||||
| Ho 2014 | 19 | 19 | 16 | ↓ | mPFC, insula, fusiform, precuneus | sgACC | Viewing fearful faces | sgACC seed |
|
| ||||||||
| ↑ | Amygdala | |||||||
|
| ||||||||
| Ho 2015 | 26 | 37 | 16 | ↑ | mPFC, PCC | dACC | Viewing affective faces | mPFC, PCC seeds |
|
| ||||||||
| Kerestes 2016 | 29 | 43 | 15.3 | - | - | - | Viewing Happy Faces | Putamen, dlPFC seeds |
|
| ||||||||
| Perlman 2012 | 14 | 14 | 15.4 | ↑ | Amygdala | sgACC | Emotion regulation task | Amygdala seed |
Note: Papers are listed by name of first author; et al. is implied. Upward arrow indicates greater value in clinical or high-risk group than in typically developing (TD) group. HR: high-risk; LR: low-risk; FA: fractional anisotropy; MD: major depression; ATR: anterior thalamic radiations; ACC: anterior cingulate cortex; PCC: posterior cingulate cortex; dlPFC: dorsolateral prefrontal cortex; dmPFC: dorsomedial PFC; vmPFC: ventromedial PFC; sgACC: subgenual ACC; mPFC: medial PFC; STG: superior temporal gyrus; dACC: dorsal ACC; pgACC: pregenual ACC.
Structural ACC connectivity
Adolescents at high risk for depression
Among adolescents at high familial risk for depression, two previous studies have identified decreased FA of the cingulum relative to adolescents with no family history of mood disorder (Huang et al., 2011; Keedwell et al., 2012). These results support the hypothesis that aberrant cingulum microstructure may confer greater risk for MDD. However, given the preliminary nature of the existing literature, future studies are necessary to support these hypotheses.
Adolescents with depression
Altered white matter integrity of the cingulum (Cullen et al., 2010; Henderson et al., 2013) and ATR (Henderson et al., 2013; LeWinn et al., 2014) has been reported in adolescents with depression relative to typically developing adolescents. Furthermore, among depressed adolescents, lower cingulum FA has been linked to greater depression severity whereas poorer integrity of the ATR is associated with higher anhedonia, a symptom involving difficulty with motivation for or enjoyment of rewarding experiences and reflecting disruption of reward systems (Henderson et al., 2013). These results support the hypothesis that disrupted development of structural ACC connectivity is implicated in the emergence of affective problems in adolescence and suggests that white matter integrity of the cingulum and ATR has a significant impact on symptom presentation and illness severity.
Functional ACC connectivity
Given the available evidence that white matter integrity is distributed in tracts connecting the ACC to various cortical and subcortical regions throughout the brain, functional connectivity of the ACC among depressed adolescents is also likely to deviate from that in typically developing youth. In particular, adolescents with depression are postulated to exhibit disruptions in both affective and cognitive domains—with over-engagement of negative affect systems (i.e. sgACC, Phillips et al., 2008), under-engagement of positive affect systems (e.g. Forbes and Dahl, 2012), and ineffective engagement of cognitive or self-regulatory systems (Rayner et al., 2016). Additionally, depressed adults have been reported to show increased activation of the DMN in association with higher levels of rumination compared to healthy adults and hyperactivation of the SN in response to negative stimuli (Hamilton et al., 2013).
Adolescents at high risk for affective disorders
A small number of studies have examined premorbid differences in resting-state connectivity among individuals at high risk for depression based on family history prior to the onset of clinically significant depressive symptoms. These studies provide evidence for premorbid abnormalities in ACC connectivity among high-risk youth, including greater connectivity between the the sgACC and the default mode network, reduced connectivity between the sgACC and the dlPFC (Chai et al., 2015), as well as decreased resting-state connectivity between the dACC and the rostral inferior frontal gyrus relative to those with no family history of depression (Clasen et al., 2014).
Adolescents with depression
Several studies have examined resting-state connectivity among adolescents with current depression and provide preliminary evidence to suggest that these individuals are characterized by aberrant connectivity of the sgACC. Specifically, Cullen et al. (2009) demonstrated decreased resting-state connectivity between the sgACC and distributed frontal cortical regions. Lending additional support to these findings, Strikwerda-Brown et al. (2014) found that weaker resting-state connectivity between the sgACC and dPFC was associated with higher depressive symptoms in a psychiatrically healthy sample of adolescents, and that decreased connectivity over 2 years predicted increased depressive symptoms over the same follow-up interval. Additionally, there is also evidence to suggest that depressed adolescents display increased resting-state sgACC connectivity with the amygdala (Connolly et al., 2013) and striatum (Gabbay et al., 2013). These data were corroborated by findings that greater sgACC-amygdala connectivity at rest was associated with greater magnitude of negative affect in a sample of healthy adolescents (Davey et al., 2015).
Findings have been relatively more mixed regarding sgACC connectivity to the insula, with both increased (Connolly et al., 2013) and decreased (Cullen et al., 2009) connectivity reported across studies. However, this discrepancy may be due to the fact that one study included adolescents who were listening to music during the resting-state scan (Cullen et al., 2009). Alternatively, resting-state connectivity of the sgACC in depressed adolescents may differ for different subregions of the insula, which have distinct profiles of connectivity and function (Uddin, 2015). Future studies are necessary to clarify the nature and significance of sgACC-insula connectivity in adolescents with depression.
Very few studies have reported aberrant functional connectivity of the dACC and rACC in depressed adolescents. Pannekoek et al. (2014) found decreased connectivity between the dACC and several frontal cortical regions, as well as reduced negative connectivity between the rACC and the bilateral amygdala. Gabbay et al. (2013) reported that greater resting state connectivity between the rACC and the caudate predicted greater anhedonia among depressed adolescents. These studies suggest that aberrant ACC connectivity among depressed adolescents may extend beyond the sgACC, but future studies are necessary to conclusively establish whether these individuals display abnormal patterns of dACC or rACC connectivity at rest.
Studies of task-based functional connectivity are consistent with resting-state findings of higher sgACC-amygdala connectivity among depressed adolescents. During passive viewing of negative stimuli (strongly fearful faces) (Ho et al., 2014), as well as during active affect regulation (cognitive reappraisal of negative images) (Perlman et al., 2012), depressed adolescents display stronger functional connectivity between the sgACC and the amygdala compared to typically developing adolescents. Also in line with the resting-state literature, depressed adolescents show decreased functional connectivity between the sgACC and the middle frontal gyrus, as well as the insula/putamen, fusiform gyrus, and precuneus/posterior cingulate (Ho et al., 2014).
Task-based functional connectivity studies have also shed light on altered dACC connectivity in adolescent depression. Specifically, when adolescents view affectively valenced faces, greater functional connectivity has been reported between the dACC and the mPFC (Ho et al., 2015), as well as the anterior and middle insular cortex (Henje Blom et al., 2015). The latter finding may be indicative of aberrant functioning of the salience network in adolescent depression (Henje Blom et al., 2015), and future research is necessary to assess how salience network functioning may differ between depressed and typically developing adolescents.
Summary of ACC functional connectivity in adolescent affective disorders
Across studies, adolescents with depression exhibit greater functional connectivity between the sgACC and amygdala (Connolly et al., 2013; Davey et al., 2015; Ho et al., 2014; Perlman et al., 2012) coupled with reduced connectivity between sgACC and frontal cortical regions (Cullen et al., 2009; Ho et al., 2014; Strikwerda-Brown et al., 2015) both at rest and during tasks, which could reflect an overengagement of negative affect systems in tandem with inefficient top-down regulation, resulting in ineffective regulation of affective responding. Furthermore, increased functional connectivity between the dACC and insula during affective processing may contribute to difficulty switching from ruminative and self-referential processing (Henje Blom et al., 2015) and supports the idea that depressed adolescents are characterized by overengagement of the SN to negative stimuli.
Integration of Structural and Functional ACC Connectivity in Adolescent Depression
In all, both structural and functional connectivity of the ACC appear to be disrupted in adolescent depression. Depressed adolescents display reduced white matter integrity of the cingulum and ATR, as well as stronger sgACC-amygdala connectivity, weaker functional connectivity between the sgACC and frontal cortical regions, and stronger dACC-insula functional connectivity when viewing affective faces. While still preliminary, this literature supports the widespread disruption of ACC connectivity in adolescent depression that is consistent with amplified negative affective reactivity, a bias towards negative stimuli in the environment, and disrupted top-down affect regulation, which may collectively impair goal-directed self-regulatory processes driven by the ACC. Given that alterations in ACC connectivity have been reported in adolescents at high risk for depression prior to illness onset, we hypothesize that aberrant ACC connectivity is a premorbid characteristic that contributes to the onset of MDD. Altered ACC connectivity could be attributable to genetic variation (Braskie et al., 2012), environmental impoverishment (Wang and Young, 2014), or exposure to stress (Howell et al., 2013; Sanjuan et al., 2013) or maltreatment (Choi et al., 2009; Huang et al., 2012) during development. Subsequently, social withdrawal and academic underachievement in the context of active depressive symptoms could exacerbate aberrant patterns of ACC connectivity and contribute to a negative long-term trajectory of mental health and socioeconomic status.
Discussion
Limitations of studies to date
The current review and meta-analysis finds that both structural and functional ACC connectivity develop across adolescence, and proposes that age-related changes in the ACC’s structural and functional networks facilitate improved affect regulation and the establishment of adult-level skills necessary to navigate the transition to adulthood. Accordingly, we assert that aberrant ACC connectivity is an important neural mechanism of risk for depression. The literature linking altered structural and functional ACC connectivity to depression in adolescence supports this claim.
Although there has been a recent surge in the number of studies looking at the development of neural network characteristics and their implications for psychopathology, there are several limitations of this literature that must be improved upon in future research. Many studies of white matter development included in the current meta-analysis include small samples, limiting the strength of the conclusions that can be drawn from this data. Additionally, the vast majority of studies use cross-sectional designs, and those reporting longitudinal data include very short follow-up intervals, limiting the strength of the conclusions that can be drawn from this work. Additionally, studies vary in their definitions of adolescence, so the ages of participants included is not consistent. Although adolescence is broadly considered to be the period when individuals transition from dependence on parents to relative independence (Spear, 2000), the precise onset and offset of adolescence are difficult to delineate. As a result, there are discrepancies between the age ranges included in different studies. Furthermore, the predominance of cross-sectional studies is a significant barrier to understanding whether aberrant ACC connectivity is a stable premorbid risk factor, a cause, a concurrent correlate, or a consequence of adolescent psychopathology. Studies that follow larger samples throughout adolescence are necessary to more conclusively delineate typical and maladaptive patterns of ACC development during this period. Additionally, this literature is characterized by substantial methodological heterogeneity, including the magnet strength and imaging parameters applied, analytical methods, and statistical approaches employed across different studies, which significantly limits opportunities for replication between studies.
For functional connectivity findings, interpretation is complicated by the use of rest in some studies and task in others and the use of different types of tasks in task fMRI studies (e.g., cognitive control vs. affective processing). Methodologically, studies used different strategies for computing functional connectivity and, for seed-based functional connectivity, did not consistently select the ACC or a subregion of the ACC as seed. Thus, we interpret the pattern of findings cautiously, given the small number of reports and the differences across them. In terms of both investigations of neural circuitry and methodology for conducting such investigations, the field is changing rapidly. As more developmental and clinical neuroscience studies report their findings, we will have a more comprehensive picture of the connectivity of the ACC as it is disrupted in adolescent depression.
Additionally, there is only a small, heterogeneous literature available on ACC connectivity and adolescent depression. Furthermore, studies that focus on single disorders may be limited in their potential to uncover the neural mechanisms of psychopathology, as psychiatric disorders have heterogeneous symptom presentations and rarely occur in isolation, often co-occurring with other conditions. Indeed, the functions attributed to the ACC and its neural network impact behavior across several domains, and are not specific to one type of psychopathology. Research geared towards elucidating behavioral correlates of ACC function and dysfunction may be more useful for improving our understanding of the role of ACC connectivity relative to studies of discrete clinical populations.
Furthermore, a large proportion of earlier work examining adaptive neural development and risk for psychopathology in adolescence has focused on identifying specific abnormalities in a single brain region or small group of regions. More recently, researchers have come to appreciate that this approach may not be sufficiently complex to further our understanding of brain function in health and disease and there has been a paradigm shift towards studying how brain regions interact across time (Menon, 2011). Nonetheless, this remains a new area of research and substantial future work is necessary to improve our understanding of developing neural network dynamics and their role in adaptive functioning as well as adolescent psychopathology.
Next Steps
Although the current review and meta-analysis provide strong support for the hypothesis that the development of structural ACC connectivity is ongoing throughout adolescence and into young adulthood, substantial future work is necessary to establish the course of microstructural development during the adolescent period and elucidate the cellular basis of these changes. To this end, longitudinal studies with multiple imaging assessments across adolescence are necessary, as well as translational animal studies to relate developmental white matter changes to underlying cellular processes during adolescence. However, consistent with challenges to studying human adolescents, translational research on adolescent ACC development is complicated by controversy over operationalizing adolescence (Spear, 2015), as well as delineating brain regions that are homologous to the human ACC (Vogt et al., 2013).
Additionally, future research is needed to firmly establish links between structural connectivity, functional connectivity, patterns of neural activation and behavior across typical development and among depressed youth. Powerful recent findings have begun to reveal how anatomical connectivity impacts functional connectivity and patterns of coactivation also modulate the structural integrity of white matter pathways (Luna et al., 2015). However, the mechanisms of these associations remain poorly understood. Future research should address how structural and functional connectivity influence each other and elucidate the time course of these effects as well as potential moderators. Additionally, future work should directly address how improvements in different higher-order functions relate to cingulum and ATR microstructural development and whether functional connectivity mediates these associations.
Furthermore, future research is necessary to elucidate how the maturation of neurotransmitter systems moderates the development of structural and functional ACC connectivity in adolescence. In particular, the dopamine system undergoes substantial alterations during this period, characterized by a shift towards greater cortical dopaminergic innervation in adolescence relative to childhood, when striatal dopaminergic projections are more dominant (see Gomes et al., this issue for review). Changes in dopaminergic functioning in adolescence are hypothesized to have an important influence on prefrontal cortical synaptic plasticity and myelination (Davey et al., 2008), suggesting that dopamine system changes may meaningfully impact the development of cingulum and ATR microstructure as well as functional ACC connectivity.
Another crucial step is to examine network-to-network functional connectivity. Because the brain comprises a set of interdependent networks that require coordination across and within networks to guide adaptive behavior (Dosenbach et al., 2008; Luna et al., 2015), it is important to move beyond a single-network emphasis. With the field of developmental connectomics gaining momentum (Hagmann et al., 2012), more studies are taking this approach. Recent relevant investigations have addressed alterations in the balance of network function in, for example, adolescent depression (Jacobs et al., 2014) and other psychopathology (e.g. Wang et al., 2016).
The guiding conceptual model of current research in psychopathology is that this construct is captured perhaps more appropriately in a set of dimensions related to affect, cognition, and behavior rather than in a set of diagnostic categories based on phenomenology and self-report. As approaches such as NIMH’s Research Domain Criteria (aka RDoC; https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml) initiative increasingly influence the operationalization of psychopathology and developmental psychopathology approaches gain prominence, we will see more research addressing a range of important but underappreciated issues. From an RDoC-style or dimensional perspective, these issues include common, transdiagnostic factors (e.g., P or general psychopathology, Caspi et al., 2014); dimensions related to multiple forms of psychopathology (e.g., reward or “positive valence system” disruption); and multi-method approaches to operationalizing these dimensions. From a developmental psychopathology perspective, these issues include longitudinal designs, examining atypical development in the context of typical development, and modeling within-person trajectories to understand the unfolding of psychopathology over time. An invaluable tool in this progress will be the application of sophisticated data analytic methods combined with large data sets, possibly promoted through data sharing. We expect to see more studies using functional and structural connectivity of the ACC or other central regions as predictors in large multi-method models of adolescent psychopathology, similar to what has been done in recent papers on alcohol use in the large, multi-site IMAGEN study of adolescent development (Nees et al., 2012; Whelan et al., 2014).
We are limited to describing the literature using samples from studies using discrete, DSM-defined groups and employing case-control designs because that approach has been the norm for developmental psychopathology and clinical neuroscience research. But soon we hope that we will be able to write a similar conceptual paper by citing a literature that is more diverse, generalizable, and developmentally informed in order to more appropriately capture the mechanisms of developmental psychopathology.
Finally, while not directly applicable to treatment or prevention efforts, we argue that research on the neuroscience of developmental psychopathology should have clinical relevance. Whether applied to evaluating the influence of standard treatments on changes in functional connectivity in depression, as in the case of pharmacologic treatment for depression (Gudayol-Ferre et al., 2015), using brain function to predict the outcome or mechanisms of effective treatments such as electroconvulsive therapy (Perrin et al., 2012; van Waarde et al., 2015), or examining the mechanistic targets for novel brain stimulation techniques such as repetitive transcranial magnetic stimulation (rTMS, Baeken et al., 2014), understanding connectivity in neural networks can generate progress in therapeutics. Not surprisingly, given its importance to developmental psychopathology, connectivity of the ACC has been implicated in each of these research directions for depression (e.g. Barrett et al., 2004; Leaver et al., 2016; Spati et al., 2015). Research in this field could also, ultimately, identify new brain targets for intervention. While some have expressed despair about the future of treatment for psychopathology (Hyman, 2014), a path toward alleviating suffering should surely include greater attention to the development of serious psychopathology through a more comprehensive understanding of brain development.
Conclusions
In all, connectivity of the ACC is important to typical adolescent development and risk for adolescent depression. This is consistent with our guiding hypothesis: development of the ACC and associated self regulatory abilities are a key process of adolescent developmental psychopathology. Structural and functional connectivity of the ACC develop during adolescence, as indicated by our meta-analytic findings on structure and review of the literature on function. Furthermore, altered patterns of both structural and functional connectivity have been observed among adolescents at high risk for mood disorders and those with current depression. The field still has considerable opportunity for studies of structure-function association, prospective developmental studies of psychopathology, and studies examining brain structure and function as part of a complex set of mechanisms and risk factors. Furthermore, future research is needed to examine the influence of ACC development on risk for other forms of adolescent psychopathology, such as anxiety, bipolar disorder and substance use disorder. The current review provides a valuable step toward understanding the developmental psychopathology and developmental neuroscience of adolescence.
Table 1.
Common Abbreviations
| Abbreviation | Full Text |
|---|---|
| ACC | Anterior cingulate cortex |
| AD | Axial diffusivity |
| ATR | Anterior thalamic radiations (also known as the anterior thalamic pathway, anterior thalamic projections, anterior thalamocortical pathway, anterior corticothalamic projections) |
| DTI | Diffusion tensor imaging |
| FA | Fractional anisotropy |
| MD | Mean diffusivity |
| RD | Radial diffusivity |
| ROI | Region of interest |
| rsfMRI | Resting-state functional magnetic resonance imaging |
| RSN | Resting state network |
| SUD | Substance use disorder |
Highlights.
Anterior cingulate cortex (ACC) is key to adolescent developmental psychopathology.
A meta-analysis shows linear adolescent development of ACC-relevant tracts.
ACC functional connectivity increases to default mode and salience networks.
ACC connectivity is disrupted in adolescent depression.
Acknowledgments
The authors thank Rachel LePage for assistance with references and Marissa Cross for contributions to table preparation. This work was supported by National Science Foundation Graduate Research Fellowship to Sarah D. Lichenstein (DGE), NIH grants to Erika E. Forbes (R01 MH104418, PI: EE Forbes; R01 MH093605, MPIs: KE Keenan, AE Guyer & EE Forbes; and R01 MH103230, MPIs: J Wildes & EE Forbes;), and a NARSAD Independent Investigator Award to Erika E. Forbes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Baeken C, Marinazzo D, Wu GR, Van Schuerbeek P, De Mey J, Marchetti I, Vanderhasselt MA, Remue J, Luypaert R, De Raedt R. Accelerated HF-rTMS in treatment-resistant unipolar depression: Insights from subgenual anterior cingulate functional connectivity. World J Biol Psychiatry. 2014;15:286–297. doi: 10.3109/15622975.2013.872295. [DOI] [PubMed] [Google Scholar]
- Barrett J, Della-Maggiore V, Chouinard PA, Paus T. Mechanisms of action underlying the effect of repetitive transcranial magnetic stimulation on mood: behavioral and brain imaging studies. Neuropsychopharmacology. 2004;29:1172–1189. doi: 10.1038/sj.npp.1300411. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlim MT, McGirr A, Van den Eynde F, Fleck MP, Giacobbe P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31–38. doi: 10.1016/j.jad.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horska A. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34:733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braskie MN, Jahanshad N, Toga AW, McMahon KL, de Zubicaray GI, Martin NG, Wright MJ, Thompson PM. How a common variant in the growth factor receptor gene, NTRK1, affects white matter. Bioarchitecture. 2012;2:181–184. doi: 10.4161/bioa.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Suppl 4):S290–310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Meier MH, Ramrakha S, Shalev I, Poulton R, Moffitt TE. The p Factor: One General Psychopathology Factor in the Structure of Psychiatric Disorders? Clinical psychological science : a journal of the Association for Psychological Science. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. Atlas of Human Brain Connections. Oxford University Press; New York: 2012. [Google Scholar]
- Chai XJ, Hirshfeld-Becker D, Biederman J, Uchida M, Doehrmann O, Leonard JA, Salvatore J, Kenworthy T, Brown A, Kagan E, de Los Angeles C, Gabrieli JD, Whitfield-Gabrieli S. Altered Intrinsic Functional Brain Architecture in Children at Familial Risk of Major Depression. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Developmental Science. 2011;14:F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesworth BM, Hamilton CB, Walton DM, Benoit M, Blake TA, Bredy H, Burns C, Chan L, Frey E, Gillies G, Gravelle T, Ho R, Holmes R, Lavallee RL, MacKinnon M, Merchant AJ, Sherman T, Spears K, Yardley D. Reliability and validity of two versions of the upper extremity functional index. Physiotherapy Canada Physiotherapie Canada. 2014;66:243–253. doi: 10.3138/ptc.2013-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Jeong B, Rohan ML, Polcari AM, Teicher MH. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen PC, Beevers CG, Mumford JA, Schnyer DM. Cognitive control network connectivity in adolescent women with and without a parental history of depression. Dev Cogn Neurosci. 2014;7:13–22. doi: 10.1016/j.dcn.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, Frank G, Hendren R, Max JE, Paulus MP, Tapert SF, Banerjee D, Simmons AN, Yang TT. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol Psychiatry. 2013;74:898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat Rev Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Camchong J, Bell CJ, Houri A, Kumra S, Lim KO, Castellanos FX, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, Kurma S, Lim KO. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49:173–183. e171. doi: 10.1097/00004583-201002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain structure & function. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Davey CG, Whittle S, Harrison BJ, Simmons JG, Byrne ML, Schwartz OS, Allen NB. Functional brain-imaging correlates of negative affectivity and the onset of first-episode depression. Psychol Med. 2015;45:1001–1009. doi: 10.1017/S0033291714002001. [DOI] [PubMed] [Google Scholar]
- Davey CG, Yucel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neurosci Biobehav Rev. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua F, Catani M. Structural human brain networks: hot topics in diffusion tractography. Curr Opin Neurol. 2012;25:375–383. doi: 10.1097/WCO.0b013e328355d544. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Torrisi S, Balderston N, Grillon C, Hale EA. FMRI functional connectivity applied to adolescent neurodevelopment. Annual Review of Clinical Psychology. 2015;11:361–377. doi: 10.1146/annurev-clinpsy-032814-112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley JP, Kim-Spoon J. The development of adolescent self-regulation: reviewing the role of parent, peer, friend, and romantic relationships. J Adolesc. 2014;37:433–440. doi: 10.1016/j.adolescence.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Mackinlay RJ, Wilkening F, Weber EU. Affective and deliberative processes in risky choice: age differences in risk taking in the Columbia Card Task. J Exp Psychol Learn Mem Cogn. 2009;35:709–730. doi: 10.1037/a0014983. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Research Review: Altered reward function in adolescent depression: what, when and how? J Child Psychol Psychiatry. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Li Q, Bangaru SD, Panzer AM, Alonso CM, Castellanos FX, Milham MP. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52:628–641. e613. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci Biobehav Rev. 2013;37:340–348. doi: 10.1016/j.neubiorev.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Geier CF. Adolescent cognitive control and reward processing: implications for risk taking and substance use. Horm Behav. 2013;64:333–342. doi: 10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology. 2015;40:43–49. doi: 10.1038/npp.2014.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Rincon-Cortes M, Grace AA. Adolescence as a period of vulnerability and intervention in schizophrenia: Insights from the MAM model. Neurosci Biobehav Rev. 2016 doi: 10.1016/j.neubiorev.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudayol-Ferre E, Pero-Cebollero M, Gonzalez-Garrido AA, Guardia-Olmos J. Changes in brain connectivity related to the treatment of depression measured through fMRI: a systematic review. Front Hum Neurosci. 2015;9:582. doi: 10.3389/fnhum.2015.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Behrens TE. The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders. Neuron. 2014;83:1019–1039. doi: 10.1016/j.neuron.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Grant PE, Fair DA. MR connectomics: a conceptual framework for studying the developing brain. Frontiers in systems neuroscience. 2012;6:43. doi: 10.3389/fnsys.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl 1):S205–223. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: an intrinsic functional organization perspective. Neurobiol Dis. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Somerville LH. The neuroscience of adolescent decision-making. Current opinion in behavioral sciences. 2015;5:108–115. doi: 10.1016/j.cobeha.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Haber SN. Frontal cortical and subcortical projections provide a basis for segmenting the cingulum bundle: implications for neuroimaging and psychiatric disorders. J Neurosci. 2014;34:10041–10054. doi: 10.1523/JNEUROSCI.5459-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SE, Johnson AR, Vallejo AI, Katz L, Wong E, Gabbay V. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psychiatry. 2013;4:152. doi: 10.3389/fpsyt.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henje Blom E, Connolly CG, Ho TC, LeWinn KZ, Mobayed N, Han L, Paulus MP, Wu J, Simmons AN, Yang TT. Altered insular activation and increased insular functional connectivity during sad and happy face processing in adolescent major depressive disorder. J Affect Disord. 2015;178:215–223. doi: 10.1016/j.jad.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, Frank G, Max JE, Wu J, Chan M, Tapert SF, Simmons AN, Yang TT. Emotion-Dependent Functional Connectivity of the Default Mode Network in Adolescent Depression. Biol Psychiatry. 2015;78:635–646. doi: 10.1016/j.biopsych.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Yang G, Wu J, Cassey P, Brown SD, Hoang N, Chan M, Connolly CG, Henje-Blom E, Duncan LG, Chesney MA, Paulus MP, Max JE, Patel R, Simmons AN, Yang TT. Functional connectivity of negative emotional processing in adolescent depression. J Affect Disord. 2014;155:65–74. doi: 10.1016/j.jad.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Yeung N. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn Sci. 2012;16:122–128. doi: 10.1016/j.tics.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, McCormack KM, Grand AP, Sawyer NT, Zhang X, Maestripieri D, Hu X, Sanchez MM. Brain white matter microstructure alterations in adolescent rhesus monkeys exposed to early life stress: associations with high cortisol during infancy. Biology of mood & anxiety disorders. 2013;3:21. doi: 10.1186/2045-5380-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Fan X, Williamson DE, Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36:684–691. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Gundapuneedi T, Rao U. White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:2693–2701. doi: 10.1038/npp.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE. Revitalizing psychiatric therapeutics. Neuropsychopharmacology. 2014;39:220–229. doi: 10.1038/npp.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RH, Jenkins LM, Gabriel LB, Barba A, Ryan KA, Weisenbach SL, Verges A, Baker AM, Peters AT, Crane NA, Gotlib IH, Zubieta JK, Phan KL, Langenecker SA, Welsh RC. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PLoS One. 2014;9:e104366. doi: 10.1371/journal.pone.0104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarbo K, Verstynen TD. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J Neurosci. 2015;35:3865–3878. doi: 10.1523/JNEUROSCI.2636-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Christiansen KF, Chapman RJ, Aggleton JP. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia. 2013;51:67–78. doi: 10.1016/j.neuropsychologia.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Chapman R, Christiansen K, Richardson H, Evans J, Jones DK. Cingulum white matter in young women at risk of depression: the effect of family history and anhedonia. Biol Psychiatry. 2012;72:296–302. doi: 10.1016/j.biopsych.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Lavin C, Melis C, Mikulan E, Gelormini C, Huepe D, Ibanez A. The anterior cingulate cortex: an integrative hub for human socially-driven interactions. Frontiers in neuroscience. 2013;7:64. doi: 10.3389/fnins.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver AM, Espinoza R, Pirnia T, Joshi SH, Woods RP, Narr KL. Modulation of intrinsic brain activity by electroconvulsive therapy in major depression. Biological psychiatry : cognitive neuroscience and neuroimaging. 2016;1:77–86. doi: 10.1016/j.bpsc.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- LeWinn KZ, Connolly CG, Wu J, Drahos M, Hoeft F, Ho TC, Simmons AN, Yang TT. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J Am Acad Child Adolesc Psychiatry. 2014;53:899–909. 909 e891–897. doi: 10.1016/j.jaac.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobel U, Sedlacik J, Gullmar D, Kaiser WA, Reichenbach JR, Mentzel HJ. Diffusion tensor imaging: the normal evolution of ADC, RA, FA, and eigenvalues studied in multiple anatomical regions of the brain. Neuroradiology. 2009;51:253–263. doi: 10.1007/s00234-008-0488-1. [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annu Rev Neurosci. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CH, Hamilton JP, Sacchet MD, Gotlib IH. Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry. 2015;72:1045–1053. doi: 10.1001/jamapsychiatry.2015.1376. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, Heller W, Ho MH, Banich MT, Webb AG, Warren SL, Miller GA. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstadt-Klein S, Steiner S, Poustka L, Banaschewski T, Barker GJ, Buchel C, Conrod PJ, Garavan H, Heinz A, Gallinat J, Lathrop M, Mann K, Artiges E, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka MN, Spanagel R, Struve M, Loth E, Schumann G, Flor H. Determinants of early alcohol use in healthy adolescents: the differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–995. doi: 10.1038/npp.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte J. The Human Brain: An Introduction to its Functional Anatomy. Mosby Elsevier; Philadelphia: 2008. [Google Scholar]
- Northoff G. How is our self altered in psychiatric disorders? A neurophenomenal approach to psychopathological symptoms. Psychopathology. 2014;47:365–376. doi: 10.1159/000363351. [DOI] [PubMed] [Google Scholar]
- Öngür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys, and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain Cogn. 2010;72:26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Simmons AN, Wu J, Hahn KS, Tapert SF, Max JE, Paulus MP, Brown GG, Frank GK, Campbell-Sills L, Yang TT. Amygdala response and functional connectivity during emotion regulation: a study of 14 depressed adolescents. J Affect Disord. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, Schwarzbauer C. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A. 2012;109:5464–5468. doi: 10.1073/pnas.1117206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, Malhotra AK. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr Bull. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, 3rd, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The restless brain: how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci. 2015:370. doi: 10.1098/rstb.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner G, Jackson G, Wilson S. Cognition-related brain networks underpin the symptoms of unipolar depression: Evidence from a systematic review. Neurosci Biobehav Rev. 2016;61:53–65. doi: 10.1016/j.neubiorev.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhe HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013;37:2529–2553. doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Rollins NK, Glasier P, Seo Y, Morriss MC, Chia J, Wang Z. Age-related variations in white matter anisotropy in school-age children. Pediatr Radiol. 2010;40:1918–1930. doi: 10.1007/s00247-010-1744-1. [DOI] [PubMed] [Google Scholar]
- Sanjuan PM, Thoma R, Claus ED, Mays N, Caprihan A. Reduced white matter integrity in the cingulum and anterior corona radiata in posttraumatic stress disorder in male combat veterans: a diffusion tensor imaging study. Psychiatry research. 2013;214:260–268. doi: 10.1016/j.pscychresns.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg JE, Bryant AL, O'Malley PM. Taking hold of some kind of life: how developmental tasks relate to trajectories of well-being during the transition to adulthood. Dev Psychopathol. 2004;16:1119–1140. doi: 10.1017/s0954579404040167. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. Neuroimage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sole-Padulles C, Castro-Fornieles J, de la Serna E, Romero S, Calvo A, Sanchez-Gistau V, Padros-Fornieles M, Baeza I, Bargallo N, Frangou S, Sugranyes G. Altered Cortico-Striatal Connectivity in Offspring of Schizophrenia Patients Relative to Offspring of Bipolar Patients and Controls. PLoS One. 2016;11:e0148045. doi: 10.1371/journal.pone.0148045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Spati J, Hanggi J, Doerig N, Ernst J, Sambataro F, Brakowski J, Jancke L, grosse Holtforth M, Seifritz E, Spinelli S. Prefrontal thinning affects functional connectivity and regional homogeneity of the anterior cingulate cortex in depression. Neuropsychopharmacology. 2015;40:1640–1648. doi: 10.1038/npp.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiology & behavior. 2015 doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Strikwerda-Brown C, Davey CG, Whittle S, Allen NB, Byrne ML, Schwartz OS, Simmons JG, Dwyer D, Harrison BJ. Mapping the relationship between subgenual cingulate cortex functional connectivity and depressive symptoms across adolescence. Soc Cogn Affect Neurosci. 2015;10:961–968. doi: 10.1093/scan/nsu143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, Levenson RW. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc Cogn Affect Neurosci. 2013;8:468–474. doi: 10.1093/scan/nss023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of definition. In: Fox NA, editor. The development of emotion regulation: Biological and behavioral considerations. 1994. pp. 25–52. Monographs of the Society for Research in Child Development. [PubMed] [Google Scholar]
- Uda S, Matsui M, Tanaka C, Uematsu A, Miura K, Kawana I, Noguchi K. Normal development of human brain white matter from infancy to early adulthood: a diffusion tensor imaging study. Dev Neurosci. 2015;37:182–194. doi: 10.1159/000373885. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- van Waarde JA, Scholte HS, van Oudheusden LJ, Verwey B, Denys D, van Wingen GA. A functional MRI marker may predict the outcome of electroconvulsive therapy in severe and treatment-resistant depression. Mol Psychiatry. 2015;20:609–614. doi: 10.1038/mp.2014.78. [DOI] [PubMed] [Google Scholar]
- Verhoeven JS, Sage CA, Leemans A, Van Hecke W, Callaert D, Peeters R, De Cock P, Lagae L, Sunaert S. Construction of a stereotaxic DTI atlas with full diffusion tensor information for studying white matter maturation from childhood to adolescence using tractography-based segmentations. Hum Brain Mapp. 2010;31:470–486. doi: 10.1002/hbm.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Hof PR, Zilles K, Vogt LJ, Herold C, Palomero-Gallagher N. Cingulate area 32 homologies in mouse, rat, macaque and human: cytoarchitecture and receptor architecture. J Comp Neurol. 2013;521:4189–4204. doi: 10.1002/cne.23409. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Johansen-Berg H, Karadottir RT. Unraveling the secrets of white matter--bridging the gap between cellular, animal and human imaging studies. Neuroscience. 2014;276:2–13. doi: 10.1016/j.neuroscience.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Young KM. White matter plasticity in adulthood. Neuroscience. 2014;276:148–160. doi: 10.1016/j.neuroscience.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Wang YL, Yang SZ, Sun WL, Shi YZ, Duan HF. Altered functional interaction hub between affective network and cognitive control network in patients with major depressive disorder. Behav Brain Res. 2016;298:301–309. doi: 10.1016/j.bbr.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Weston CS. Another major function of the anterior cingulate cortex: the representation of requirements. Neurosci Biobehav Rev. 2012;36:90–110. doi: 10.1016/j.neubiorev.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Carvalho FM, Conrod PJ, Flor H, Fauth-Buhler M, Frouin V, Gallinat J, Gan G, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Martinot JL, Nees F, Ortiz N, Paillere-Martinot ML, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Strohle A, Schumann G, Garavan H. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Badre D, Verstynen T. Connectometry: A statistical approach harnessing the analytical potential of the local connectome. Neuroimage. 2016;125:162–171. doi: 10.1016/j.neuroimage.2015.10.053. [DOI] [PubMed] [Google Scholar]