Abstract
Adolescence is a developmental period characterized by dramatic changes in cognition, risk-taking and social behavior. Although gonadal steroid hormones are well-known mediators of these behaviors in adulthood, the role gonadal steroid hormones play in shaping the adolescent brain and behavioral development has only come to light in recent years. Here we discuss the sex-specific impact of gonadal steroid hormones on the developing adolescent brain. Indeed, the effects of gonadal steroid hormones during adolescence on brain structure and behavioral outcomes differs markedly between the sexes. Research findings suggest that adolescence, like the perinatal period, is a sensitive period for the sex-specific effects of gonadal steroid hormones on brain and behavioral development. Furthermore, evidence from studies on male sexual behavior suggests that adolescence is part of a protracted postnatal sensitive period that begins perinatally and ends following adolescence. As such, the perinatal and peripubertal periods of brain and behavioral organization likely do not represent two discrete sensitive periods, but instead are the consequence of normative developmental timing of gonadal hormone secretions in males and females.
Keywords: adolescence, activational-organizational hypothesis, agonistic behavior, anxiety-like behavior, cortex, estrogen, ingestive behavior, sensitive periods, sexual behavior, synaptic pruning, testosterone
1.1 Introduction
Adolescence is a life transition characterized by striking changes in cognition, risk taking, and social behavior as individuals acquire the ability to function independently in adulthood (Spear, 2000; Steinberg, 2005). This gain of behavioral function requires significant development of the adolescent brain and often the reorganization of neural circuits, especially those regulating social behaviors. Recent work in both animals and humans reveals that reorganization of the adolescent brain involves many of the same developmental processes used during initial construction of the nervous system, including neurogenesis (Ahmed, et al., 2008; Eckenhoff & Rakic, 1988; He & Crews, 2007; Pinos, et al., 2001; Rankin, Partlow, McCurdy, Giles, & Fisher, 2003), programmed cell death (Nunez, Lauschke, & Juraska, 2001; Nunez, Sodhi, & Juraska, 2002), elaboration and pruning of dendritic arborizations and synapses (Andersen, Rutstein, Benzo, Hostetter, & Teicher, 1997; Huttenlocher & Dabholkar, 1997; Lenroot & Giedd, 2006; Sowell, Thompson, & Toga, 2004; Zehr, Todd, Schulz, McCarthy, & Sisk, 2006), and sexual differentiation (E. C. Davis, Shryne, & Gorski, 1996; Nunez, et al., 2001). Given the extent of neural plasticity during this time, the adolescent brain is particularly sensitive to experience and nervous system insult (Andersen, 2003; Spear, 2000), which likely contributes to the adolescent emergence of a number of psychiatric illnesses that disproportionately affect either females or males.
One of the hallmarks of adolescence is puberty (reproductive maturation), and there is a growing body of evidence that gonadal hormones, which become elevated during puberty, play a major role in shaping the brain and behavior during adolescence. Indeed, gonadal steroid hormones influence virtually all of the developmental processes listed above, yet our understanding of the intersection between adolescent brain development and gonadal hormones and the behavioral consequences of this interaction remains limited. This chapter will present the evidence from animal models that, by leaving their marks on the adolescent brain, both testicular and ovarian hormones program adult behaviors in a sex-dependent manner. Furthermore, adolescence may mark the end of a protracted postnatal sensitive period for the organizing actions of gonadal hormones on the developing brain.
1.2 Sensitive periods for the organizational effects of steroid hormones
Reproductive behavior is governed by sensitive periods of development during which testicular hormones serve as a type of “experience” that masculinizes and defeminizes the brain and peripheral tissues (Phoenix, Goy, Gerall, & Young, 1959; reviewed in Ward & Ward, 1985). For example, depriving males of testicular hormones during development via neonatal castration decreases masculine reproductive behavior in response to testosterone, and increases feminine responsiveness to estrogen and progesterone in adulthood. In the first paper to provide empirical evidence for behavioral masculinization by neonatal exposure to testosterone, Phoenix et al. (1959) hypothesized that the organizational effects of steroid exposure during early development program sex-specific activational responses to steroid hormones in adulthood. The criteria primarily used to distinguish between organizational and activational effects are defined as follows. First, organizational effects are permanent, and activational effects are transient. Second, organizational effects can only occur early in life (around the time of birth), and in particular, during a sensitive period. In contrast, activational effects usually occur in adulthood, and steroid hormones cannot activate behaviors until the underlying neural circuits have been organized. Since the time of this classic and important paper, other researchers have pointed out that the above criteria are too restrictive (Arnold & Breedlove, 1985), in light of evidence that steroid hormones can have enduring or permanent effects on the adult nervous system. For example, androgen treatment of adult female zebra finches, which do not normally sing, causes long-lasting increases in the volume of the brain nuclei underlying the production of song, and also induces singing behavior in these females (Gurney & Konishi, 1980). Thus, while sensitive periods always involve organizational change, organizational change does not always require a sensitive period.
For many years pubertal gonadal hormone secretions were thought only to activate adult behaviors. Specifically, pubertal secretions “activated” neural circuits that were “organized” by gonadal steroid hormones during the perinatal sensitive period. These scientific beliefs likely stemmed from the methods used to investigate the organizational effects of gonadal steroid hormones on behavior. For example, early studies employed neonatal castration followed by assessment of behavioral responses to steroid hormones in adulthood to determine the contribution of neonatal hormones to the process of behavioral masculinization and defeminization. However, because neonatal castration necessarily prevents exposure of the nervous system to hormone secretions during puberty, this approach confounded the contribution of neonatal hormones to the process of sexual differentiation of behavior with that of pubertal hormones. Furthermore, while some other early studies employed prepubertal castration as part of their experimental methods, the purpose of these investigations was not necessarily to assess the role of pubertal hormones in the masculinization and defeminization of reproductive behavior. Thus, while the results of some studies employing prepubertal castration suggested that the absence of testosterone during puberty altered adult reproductive behavior (Adkins-Regan, Orgeur, & Signoret, 1989; Ford, 1990; Gotz & Dorner, 1976; Larsson, 1967; Sodersten, 1973), the results of other studies did not (D’Occhio & Brooks, 1980; Dixon, 1993; Epple, Alveario, & Belcher, 1990; Larsson, Sodersten, Beyer, Morali, & Perez-Palacios, 1976; Shrenker, Maxson, & Ginsburg, 1985), and various methodological considerations made it difficult to directly compare the results of these studies or assess the effects of adolescent gonadal hormones on behavioral development. As such, it would be many years later before these questions were re-addressed in a systematic manner.
In the decades following these important early papers a few key methodological changes have allowed investigators to isolate the impact of pubertal gonadal hormone secretions on behavioral development from that of the perinatal period across several behavioral domains. These studies will be discussed in detail in the sections below. The preponderance of evidence that pubertal steroid hormones organize a wide range of adult behaviors prompted us to propose a two-stage model of behavioral development in which the perinatal period of steroid-dependent sexual differentiation is followed by a second wave of steroid-dependent neural organization during puberty and adolescence (Figure 1; Schulz, Molenda-Figueira, & Sisk, 2009; Schulz & Sisk, 2006; C.L. Sisk, Schulz, & Zehr, 2003; C. L. Sisk & Zehr, 2005). During the second wave, pubertal hormones first organize neural circuits in the developing adolescent brain, and then facilitate the expression of adult sex-typical behaviors in specific social contexts by activating those circuits. In this model, hormone-driven adolescent organization is viewed as a refinement of the sexual differentiation that occurred during perinatal neural development. That is, what occurs during perinatal brain organization determines the substrate upon which pubertal hormones act during adolescent organization. During the adolescent phase of organization, steroid-dependent refinement of neural circuits results in long-lasting structural changes that modify adult behavioral responses to hormones and socially-relevant sensory stimuli, outcomes again similar to those of the perinatal phase of organization.
Figure 1.
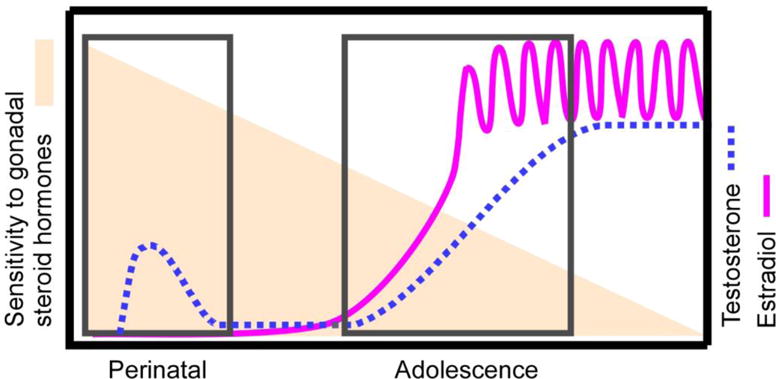
The Two-stage model of postnatal brain and behavioral development. The lines depict the time course for endogenous secretions of testosterone (dotted line) in males and estradiol in females (solid line) across postnatal development. The boxes highlight the times in which endogenous gonadal steroid hormones typically organize the developing brain. The shading indicates, based on current empirical evidence, sensitivity to the organizing actions of gonadal steroid hormones decreases across postnatal development. Note that while substantial evidence suggests that both male and female behaviors are organized by adolescent exposure to gonadal steroid hormones, the evidence thus far for decreasing sensitivity to the organizing actions of hormones across postnatal development is primarily in males in a limited number of species. Therefore, further testing of this empirical model may reveal a different pattern of sensitivity to gonadal steroid hormones in sexes of additional species.
1.3 Is adolescence a sensitive period distinct from the perinatal period?
Early studies showed that sexual behavior could not be activated by testosterone in prepubertal male hamsters, suggesting that a second window of sensitivity to the organizing effects of gonadal hormones may open at adolescence (Meek et al, 1997; Romeo et al, 2001; Romeo et al, 2002). Alternatively, organizational effects of steroid hormones may occur prior to puberty, but remain latent until requisite hormone-independent development occurs during adolescence. Therefore, we tested the hypothesis that adolescence marks the opening of a second sensitive period for the organizing actions of testosterone on adult male reproductive behavior. This hypothesis predicts that exposure to testosterone during adolescence, but not before or after adolescence, will result in full activational responses to testosterone in adulthood.
Male hamsters were GDX at 10d of age (after the perinatal period of sexual differentiation), and then exposed to 19 days of blank- or testosterone-filled silastic capsules either before puberty (10–29d of age), during the normal time of puberty (29–48d of age), or after puberty (64–83d of age). In adulthood, four weeks following capsule removal, all GDX’d males were implanted with testosterone-filled capsules and tested one week later with a receptive female. Both prepubertal and adolescent testosterone treatment, but not adult testosterone treatment, enhanced adult reproductive behavior, demonstrating that adolescence is not a discrete sensitive period for the organizing actions of testosterone on adult reproductive behavior (Figure 2). In addition, prepubertal testosterone treatment had the greatest impact on adult reproductive function, suggesting that the potential for testosterone to organize reproductive behavior decreases across postnatal development. Therefore, we propose the classical view of organizational and activational mechanisms of steroid action be revised to incorporate an extended window of decreasing postnatal sensitivity to the organization of adult social behavior by steroid hormones (Figure 1). If this is the case, then the two stages of hormone-dependent organization are driven by the two times that testicular hormones become elevated in males, and not by the opening/closing of two discrete sensitive periods.
Figure 2.
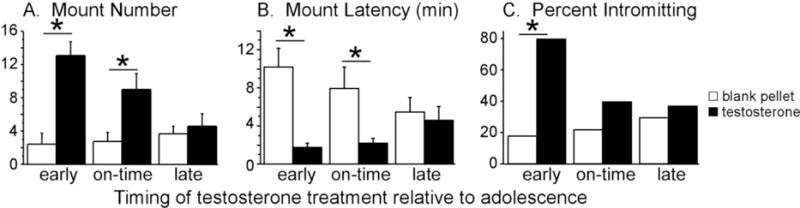
Effects of periadolescent testosterone exposure on adult reproductive behaviors. Testosterone treatments were designed to simulate early, on-time, and late pubertal development, and all behavior testing occurred in adulthood. Only pre- and mid-adolescent testosterone treatments facilitated mounting behavior in response to testosterone in adulthood. Adult intromissive behavior was only increased by pre-adolescent testosterone treatments. These data suggest that early testosterone treatments enhance behavioral responsiveness to testosterone in adulthood. Asterisk indicates a significant difference (p<0.05) between groups. Adapted from Schulz et al., (2009), Endocrinology 150 (9) 3690–3698.
A recent study in humans lends support to the possibility of an extended postnatal window of decreasing sensitivity to gonadal steroid hormones. Beltz and Berenbaum (2013) hypothesized that if sensitivity to organizational effects of gonadal steroid hormones decreases across adolescence, then the age at which adolescents undergo puberty should be inversely associated with the effectiveness of gonadal steroid hormones in organizing spatial (men) or verbal (women) ability. Participants reported whether they experienced specific pubertal events much earlier, somewhat earlier, the same, somewhat later, or much later than their peers to determine a pubertal timing score, and their verbal and spatial abilities were assessed. Among men, an effect of pubertal timing on three-dimensional mental rotations test scores was found, with early maturers performing better than late maturers. In contrast, no effects of pubertal timing on verbal or spatial ability were detected in women. The authors conclude that their findings are consistent with the hypothesis of declining sensitivity to the organizing actions of testosterone throughout adolescent development. Their data further highlight the sex-specific effects of gonadal steroid hormones during human adolescent development, as is observed in rodent species [for an earlier review of these questions in humans see Beltz and Berenbaum, (2011)].
The model presented in Figure 1 is based upon empirical findings presented in this review demonstrating that gonadal secretions organize the developing brain during both perinatal and adolescent development, and that experimental models have also demonstrated that sensitivity to the organizing actions of gonadal steroid hormones decreases across the postnatal period. Importantly, this model is intended to be theoretical and testable. Thus far, the studies that have directly tested the hypothesis of decreasing sensitivity to gonadal steroid hormones across adolescent development have been conducted primarily in males of only two species (hamsters and human), and for only two classes of behavior (reproductive and cognitive). In the sections that follow we discuss numerous studies that provide clear evidence for adolescent organizational effects of gonadal steroid hormones on a myriad of behaviors (in both sexes). We hope that future investigations will also test whether adolescence is a period of decreasing sensitivity for the organizing actions of gonadal steroid hormones. Such studies are needed to determine whether the model of decreasing adolescent sensitivity generalizes to multiple species, behaviors, and both sexes of a given species.
1.4 Hormone-dependent behavioral organization during puberty and adolescence
1.4.1 Male Reproductive Behavior
Much of the empirical evidence for hormonal organization of male reproductive behavior during puberty comes from studies in male Syrian hamsters. In this species, endocrine and behavioral puberty occurs between 4 and 7 weeks of age. Puberty begins with increases in testes weight and circulating testosterone (Miller, Whitsett, Vandenbergh, & Colby, 1977; C.L. Sisk & Turek, 1983; Vomachka & Greenwald, 1979), and culminates with attainment of adult-typical testosterone levels and maturation of reproductive behavior, which is activated by testosterone and its biologically active metabolites. The first tests of whether testicular hormones also organize reproductive behavior during adolescence were conducted by comparing the reproductive behaviors of adult males that underwent adolescent development in the presence or absence of testicular hormones. Specifically, males were gonadectomized (GDX) either before puberty at 21 days of age or after puberty at 63 days of age. Six weeks following GDX, males were testosterone-treated and behavior tested with a receptive female one week later. Males GDX prior to puberty displayed significantly fewer mounts, intromissions, and ejaculations than males GDX after puberty, suggesting that pubertal testicular secretions are necessary for complete masculinization of the developing adolescent brain. In a separate study, prepubertally GDX males treated with estradiol and progesterone in adulthood displayed increased lordosis behavior when paired with a male conspecific, demonstrating that testicular hormones during puberty and adolescence are also necessary for defeminization of the developing brain (Figure 3).
Figure 3.
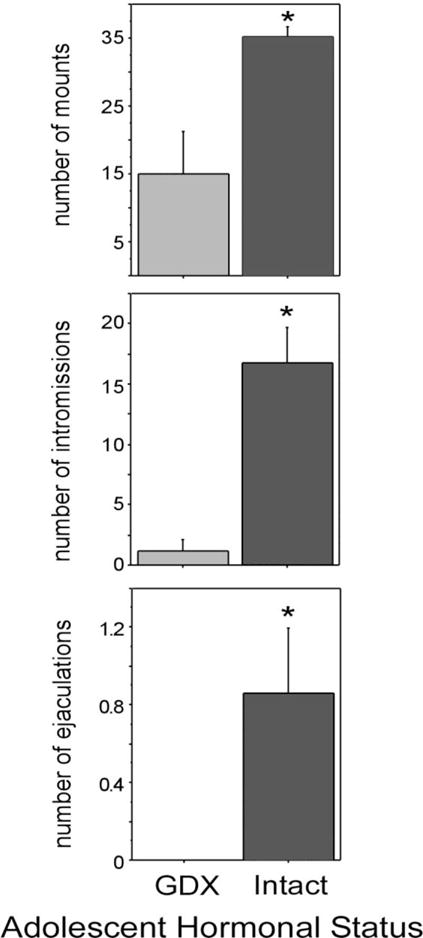
Mean number of mounts, intromissions and ejaculations displayed by sexually inexperienced males that were deprived of testicular hormones during adolescence (GDX during adolescence) and males exposed to testicular hormones during adolescence (intact during adolescence; GDX in adulthood) and tested for reproductive behavior 7 weeks later. All males were administered T for one week prior to behavior tests. All values are expressed as means ± SEM. Adapted from Schulz et al., (2004) Hormones and Behavior 45 (4) 242–249.
Although analogous experiments to examine the effects of prepubertal GDX on adult male sexual behavior have not been performed in rats, there is nevertheless evidence that testosterone, acting during the prepubertal and early pubertal period, masculinizes and defeminizes neural circuits underlying sexual behavior. Bloch and Mills (1995) examined adult behavioral responses to either testosterone or to estradiol and progesterone in male rats that had been GDX as neonates and then treated with testosterone or vehicle for a two week period of time during the juvenile/early puberty period (15–30 days of age). After testosterone priming in adulthood, rats receiving testosterone from 15–30 days of age displayed more mounts and intromissions compared with rats that received vehicle. In addition, after estradiol/progesterone priming in adulthood, rats receiving testosterone from 15–30 days of age displayed reduced lordosis and proceptive behaviors compared with rats that received vehicle. Thus, testosterone exposure during the juvenile/early pubertal period is capable of masculinizing and defeminizing sexual behavior. Although these studies in rats did not specifically investigate the role of pubertal testosterone in the expression of adult sexual behavior, they do confirm that testosterone can exert organizational influences on the prepubertal/early pubertal brain well beyond the maximally sensitive neonatal period during which initial sexual differentiation normally occurs.
1.4.2 Male agonistic behavior
Given that many social behaviors change dramatically across the adolescent period, adolescent exposure to gonadal hormones may induce organizational change in a host of male social behaviors. Indeed, organizational effects of gonadal hormones during adolescence have also been found for scent marking and territorial aggression in species as diverse as tree shrews, mice and gerbils. In tree shrews, prepubertal castration prevents testosterone from activating scent marking in adulthood (Eichmann & Holst, 1999). Similarly, mice and gerbils both display testosterone-dependent aggressive behavior in adulthood, and the ability of testosterone to activate adult aggression is substantially reduced in prepubertally castrated males (Lumia, Raskin, & Eckhert, 1977; Shrenker, et al., 1985).
As with reproductive behavior, much of what is known about pubertal organization of male agonistic behaviors comes from study of the Syrian hamster. Adult male hamsters exhibit testosterone-modulated scent marking behavior in adulthood by rubbing specialized sebaceous glands located on their dorsolateral flanks onto objects in their environment. In adults, this flank marking behavior is essential for the maintenance of dominance relationships between males, and dominant males flank mark at higher levels than submissive males (Ferris, Axelson, Shinto, & Albers, 1987; Johnston, 1970). Testosterone’s ability to regulate flank marking behavior changes across adolescence, as prepubertal testosterone-treatment fails to elicit flank marking behavior during social interactions with age- and weight-matched males (Schulz, Menard, Smith, Albers, & Sisk, 2006). Furthermore, males GDX before puberty, but not after puberty, display reduced flank marking in response to testosterone treatment in adulthood (Figure 4A), suggesting that adolescent exposure to testosterone programs flank marking responses to testosterone during adult male social interactions. Flank marking behavior is regulated in part by vasopressin V1a receptors in the lateral septum, and pubertal testosterone may organize the expression of V1a, as V1a receptor binding is significantly greater in prepubertally GDX males compared with males GDX in adulthood (Figure 4B; 4C).
Figure 4.
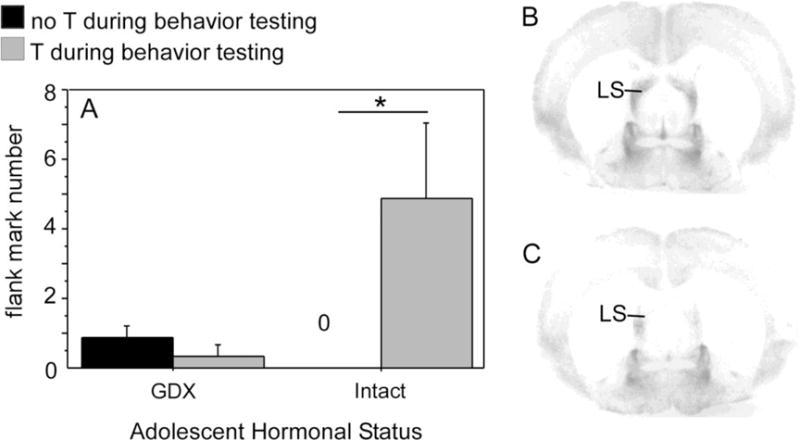
(A) Mean number of flank marks exhibited by adult males deprived of testicular hormones during adolescence (GDX during adolescence) and males exposed to testicular hormones during adolescence (intact during adolescence; GDX in adulthood). Adult testosterone treatment significantly increased flank marking behavior during a resident/intruder test in males who were gonad-intact during adolescence (GDX in adulthood) but not males where were GDX prior to adolescence. (B&C) Photomicrographs of V1a receptor binding in the lateral septum (LS) of two testosterone-treated adult males that were either deprived of gonadal hormones during adolescence (B) or exposed to gonadal hormones during adolescence (C). Males deprived of gonadal hormones during adolescence (B) displayed significantly greater V1a receptor binding than males exposed to gonadal hormones during adolescence (C). Adapted from Schulz et al., (2006), Hormones and Behavior 50 (3) 477–483.
During the first social encounter between two unfamiliar male hamsters in a neutral environment, an aggressive interaction initially occurs, and a dominant-subordinate relationship is typically established within a few minutes. In subsequent encounters, there is little aggression per se, but the dominant-subordinate relationship is maintained through flank marking by both males, with the dominant male flank-marking more frequently than the subordinate male. This pattern of behavior, in which overt aggression is replaced by non-life threatening flank marking, is an example of social proficiency or competence, defined as the ability of an animal to make adaptive changes in behavior as a result of social experience. Castration before puberty, but not after, disrupts this experience-dependent pattern of behavior (Figure 5; De Lorme & Sisk, 2013). Specifically, males GDX before puberty and T-replaced in adulthood display lower levels of flank marking overall, even if they are the dominant male. Furthermore, when prepubertally GDX males are re-introduced after the dominant/subordinate relationship was established in a prior encounter, they once again engage in overt aggression to re-establish the relationship, instead of maintaining the relationship via flank-marking. Thus, pubertal testosterone programs social proficiency, in addition to programming activation of flank-marking (Figure 5).
Figure 5.
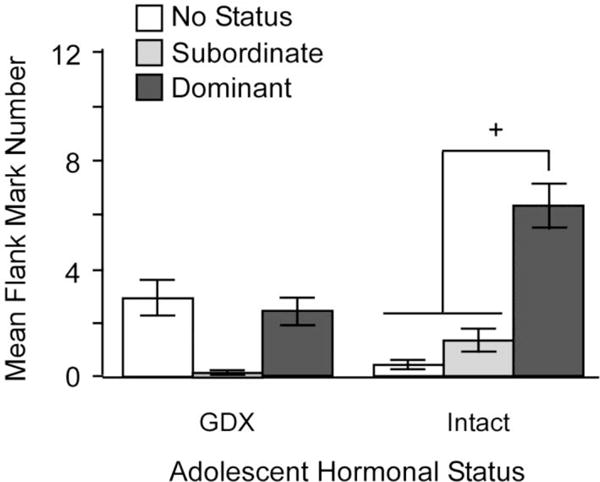
Mean number of flank marks across 6 trials is dependent on an interaction between pubertal testosterone, status and trial number. Status only affected the number of flank marks in males that were exposed to testicular hormones during adolescence (intact; GDX and T-replaced in adulthood), with dominant males flank marking significantly more than no-status and subordinate intact males (+ = p < 0.05). There were no differences between no-status, subordinate or dominant males that were deprived of testicular hormones during adolescence (GDX during adolescence) and T-replaced in adulthood prior to behavioral testing. Adapted from Delorme and Sisk (2013), Physiology & Behavior (112–113) 1–7.
1.4.3 Male Anxiety-Related Behavior
In comparison to male hamsters, male rats exhibit low-levels of aggression when they meet in a neutral environment. However, the degree of friendliness that male rats display is different in familiar and unfamiliar environments: male rats spend less time in social interactions in a novel environment than they do in a familiar environment. The reduction in social interaction is a masculine response to a novel environment, as female rats are unfazed by it and spend similar amounts of time interacting in familiar and unfamiliar environments (Primus & Kellogg, 1990). Novel environments are considered anxiogenic to males because pretreatment with anxiolytic drugs prevents the reduction in social interaction normally induced by them (File, 1985; File & Hyde, 1978). The anxiogenic effect of a novel environment in male rats is not present until adulthood. Whereas adult males display reduced social interactions in novel environments, prepubertal males do not (Primus & Kellogg, 1989). Although this response to novel environments is not regulated by testosterone in adulthood, depriving male rats of testosterone during adolescence prevents its development altogether, i.e., social interactions are not reduced in a novel environment in prepubertally GDX rats (Primus & Kellogg, 1990). Testosterone replacement during the time of puberty in prepubertally GDX rats permits the development of the masculine response (reduced social interaction) to a novel environment (Primus and Kellogg, 1990). This organizational effect of testosterone is mediated by its aromatized metabolite estradiol, as treatment with the aromatase inhibitor fadrozole during the time of puberty prevents development of the response to a novel environment (Kellogg & Lundin, 1999). More recent work has examined how the presence or absence of testosterone during puberty influences the behavior of male rats in other tests of anxiety. These studies show that compared with male rats GDX in adulthood, prepubertally GDX male rats spend more time in the open arms of an elevated plus maze and more time in the light section of a light-dark box, indicating in both cases that prepubertal GDX results in a less anxious phenotype in adulthood (Brown, Kulbarsh, Spencer, & Duval, 2015). Together, these studies support the idea that the presence of testicular hormones during adolescence organizes anxiety-like behaviors in male rats, specifically making them more anxious after adolescence than before.
1.4.4 Female Behavior
1.4.4.1 Feminizing and demasculinizing effects of ovarian hormones
Ovarian hormones also organize female social behaviors during adolescence, and the effects of adolescent ovarian hormones vary depending on the specific social behavior in question. Studies demonstrate that ovarian hormones during adolescence are capable of either feminizing (enhancing female-typical attributes), masculinizing (enhancing male-typical attributes), or defeminizing (suppressing female-typical attributes) adult behavior. Recently, an estrogen-deficient aromatase knock-out mouse model has provided new opportunities to study the effects of estradiol during development (Bakker, Honda, Harada, & Balthazart, 2002). Although the aromatase gene knock-out prevents biosynthesis of estrogen, estrogen receptors are fully-functional in this model, thereby providing a unique opportunity to study the effects of exogenous estradiol administration on female behavioral development (Bakker & Baum, 2008). Female knock-out mice display significantly less lordosis behavior compared to wildtype or heterozygous mice following adult ovariectomy and hormone treatment, suggesting that exposure to endogenous estrogen during adolescence feminizes reproductive responses to estradiol and progesterone (Bakker, et al., 2002). In a second study, estradiol was systematically administered during development either prior to the onset of normative ovarian secretions of gonadal steroid hormones (postnatal days 5–15), or the earliest timeframe for ovarian secretions gonadal steroid hormones (postnatal days 15–25). Interestingly, whereas administration of estradiol between days 5–15 had no effect on lordosis behavior in wildtype or knockout animals, administration between days 15–25 significantly increased lordosis behavior in the aromatase knockout animals (Brock, Baum, & Bakker, 2011). These data provide compelling evidence for the feminization of female reproductive behavior by estradiol during early adolescent development in female mice.
Rough and tumble play in female rats is also actively feminized by ovarian steroid hormones. Males and females display striking differences in play behavior. During adolescence, males transition from a playful defense strategy of a full supine position when contacted by a male conspecific to a partial supine position (the adult posture). In contrast, females do not show this change in play behavior across adolescence (Field & Pellis, 2008; Pellis, 2002). However, females ovariectomized (OVX) neonatally or prior to puberty display the male-typical adolescent transition in play defense posture, suggesting that ovarian hormones during adolescence actively feminize and demasculinize play responses in females. Indeed, neonatal testosterone administration does not produce the male-typical play pattern (Smith, Forgie, & Pellis, 1998). Thus, play behavior in rats is a very interesting example of active feminization and demasculinization by adolescent ovarian steroid hormones.
Food guarding and ingestive behaviors are feminized by ovarian steroid hormones during adolescence. Rats display food guarding behaviors in which sexually-dimorphic postural strategies are employed to defend a food source (Field, Whishaw, Forgie, & Pellis, 2004). Both neonatal and pubertal OVX shifts female defense strategies toward a more male-like pattern, whereas adult OVX has no effect. Thus, these data suggest that ovarian hormones actively feminize food defense strategies during the neonatal and/or adolescent periods. Pubertal estradiol also feminizes ingestive responses to metabolic signals in rats (Swithers, McCurley, Hamilton, & Doerflinger, 2008). Treatment with mercaptoacetate, a drug that interferes with fatty acid oxidation, increases food intake in male but not female rats. Prepubertally OVX females display a male-like response to mercaptoacetate and increase their food intake in adulthood, whereas adult OVX females do not increase food intake in response to mercaptoacetate. Furthermore, this effect of prepubertal OVX is prevented by estradiol replacement during puberty, indicating a role for estradiol (not progesterone) in organizing (feminizing) the response to metabolic challenge (Swithers et al., 2008).
Recent evidence also suggests that maternal behavior is feminized by ovarian hormones during adolescence. Female mice OVX prior to puberty spend less time with pups, take longer to retrieve them, and retrieve fewer of them, as compared to either females that are OVX after puberty in adulthood. However, these maternal behaviors are preserved in prepubertally OVX females that receive estradiol during the time of puberty (Kercmar, Snoj, Tobet, & Majdic, 2014), again providing evidence that estradiol, in some behavioral contexts, actively feminizes the adolescent brain.
1.4.4.2 Defeminizing and masculinizing effects of ovarian hormones
In contrast to the feminizing effects of ovarian hormones during adolescence on food guarding, ingestive, and maternal behaviors, ovarian hormones cause species-specific defeminization and masculinization of mating behavior. In female Syrian hamsters, prepubertal, but not postpubertal, OVX decreases lordosis latency and increases overall lordosis duration in response to adult estradiol and progesterone treatment (Figure 6; Schulz & Sisk, 2006), suggesting that ovarian hormone exposure during adolescence defeminizes adult lordosis behavior. In addition, estradiol treatment following prepubertal OVX also defeminizes adult behavior, indicating that estradiol is the ovarian hormone driving behavioral defeminization during adolescence (Schulz & Sisk, 2006). While it may be surprising that ovarian hormones defeminize female lordosis behavior during adolescence, estrogen-receptor mediated behavioral defeminization also occurs during the perinatal period of development (Clemens & Gladue, 1978; Coniglio, Paup, & Clemens, 1973; Paup, Coniglio, & Clemens, 1972; for review see Wallen & Baum, 2002). Whether ovarian hormone-induced defeminization of lordosis behavior during adolescent development negatively impacts female reproductive success is not known. One possibility is that behavioral defeminization is a trade-off for estradiol-dependent organization of behaviors that facilitate reproductive success. For example, female hamsters are notoriously aggressive (e.g. Payne & Swanson, 1970), and socially dominant females give birth to larger litters than socially subordinate females (Huck, Lisk, & McKay, 1988). Perhaps adolescent ovarian hormones facilitate social dominance/aggression in female hamsters, as has been demonstrated for adolescent testicular hormones in male hamsters (Schulz, et al., 2006; Schulz & Sisk, 2006). Thus, although defeminization of lordosis behavior by estradiol during adolescence reduces the duration of mating interactions with males, organization of other behavioral systems may ensure overall reproductive female success.
Figure 6.
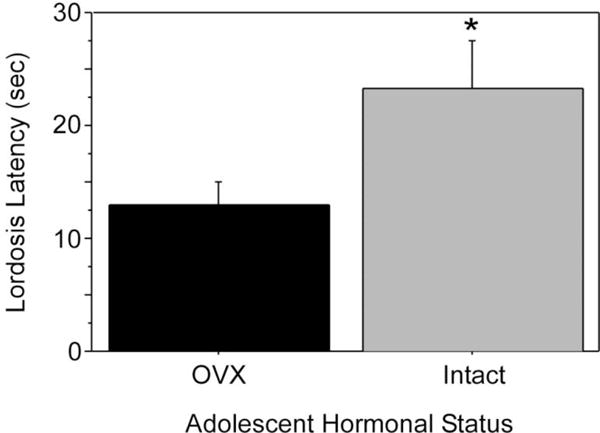
Pubertal ovarian hormones defeminize lordosis behavior. Females were exposed to (intact during adolescence; OVX in adulthood) or deprived of ovarian hormones during adolescence (OVX during adolescence). All females were estradiol and progesterone primed in adulthood prior to behavioral testing with a stud male. Females exposed to ovarian hormones during adolescence (intact) displayed significantly longer lordosis latencies than females deprived of adolescent ovarian hormones (OVX) when both groups were estradiol and progesterone primed and paired with a male in adulthood. Asterisk indicates P < 0.05. Adapted from Schulz and Sisk (2006), Molecular and Cellular Endocrinology (254–255) 120–126.
Defeminizing and masculinizing effects of gonadal steroid hormones during adolescence have also been found in female rats. Exogenous testosterone administration in early adolescence defeminizes lordosis as well as proceptive solicitation behaviors (Bloch, Mills, & Gale, 1995). In addition, in comparison to females OVX after adolescence, females OVX before adolescence display less testosterone-induced mounting and spend significantly less time with females than males during partner preference tests, suggesting that adolescent ovarian hormones masculinize mating behavior (de Jonge, Muntjewerff, Louwerse, & van de Poll, 1988). Interestingly, the neonatal hormonal environment may determine the extent to which adolescent ovarian hormones masculinize behavior. Adolescent ovarian hormone exposure has little effect on the reproductive behavior of neonatally androgenized female rats (de Jonge, et al., 1988). Thus, developmental processes occurring neonatally alter the neural substrate on which gonadal hormones act during the adolescent period, which fits with a model of decreasing sensitivity of neural circuits to the organizing actions of steroid hormones across postnatal development as discussed above.
1.5 Neurobiological mechanisms underlying hormone-dependent organization of the adolescent brain
Thus far we have focused on the behaviors that are organized by testicular and ovarian hormones during adolescence. With just the few exceptions noted above, the upshot of these adolescent organizational influences is to further masculinize and defeminize behaviors in males and to feminize and demasculinize behaviors in females. For the most part, the neurobiological mechanisms of behavioral organization are not yet known. However, it is clear that pubertal gonadal hormones exert organizational influences on the structure of brain regions involved in the behaviors that are organized during adolescence. In many instances, adolescent organization of brain structure results in further sexual differentiation of these brain regions, which is the focus of this section. The brain regions discussed below are structurally sexually dimorphic, i.e., there are sex differences in overall size, neuron or glial cell number, dendritic complexity, or connectivity. Like behavior, sex differences in these structural features are first programmed (sexually differentiated) during perinatal development through differential exposure of males and females to testicular hormones, and then they emerge or become exaggerated during adolescence by the pubertal elevation in gonadal hormones. Hormone-dependent organization of brain structure during adolescence involves many of the same developmental processes that are in play during the perinatal organizational period, e.g., cell death and survival and synapse proliferation and elimination. It is presumed that adolescent organization of structure has something to do with adolescent organization of behavior, but at this time, we can only point to correlational relationships between hormone-dependent organization of structure and behavior during adolescence in animal models. In humans, strides are being made toward a better understanding of the relationships among structural and functional changes in the adolescent brain, adolescent maturation of executive function and social cognition, and pubertal hormones in males and females, and this literature is reviewed elsewhere in this special issue (Gur and Gur, this issue).
1.5.1 Adolescent development of hypothalamic cell groups: anteroventral periventricular nucleus (AVPV) and sexually dimorphic nucleus of the hypothalamus (SDN)
The rat AVPV is one of the few examples of a female-biased sexual dimorphism, i.e., it is larger and contains more neurons in females compared with males. The AVPV integrates a hormonal signal from the ovaries (elevated estradiol levels) with a circadian signal from the suprachiasmatic nucleus to provide the neural trigger for generation of the preovulatory surge of luteinizing hormone (LH; reviewed in Simerly, 2002). This neuroendocrine positive feedback response develops during puberty in female rats (Andrews, Mizejewski, & Ojeda, 1981), and is sexually differentiated—male rats are incapable of generating an LH surge at any age (Corbier, 1985; Gogan, Beattie, Hery, Laplante, & Kordon, 1980). Although sex differences in AVPV structure are programmed during perinatal development, the sex difference in AVPV volume emerges during pubertal development (E. C. Davis, et al., 1996), along with the capacity for females to generate an LH surge. The SDN is a prominent feature of the male hypothalamus in a variety of mammalian species, and was the first identified male-biased sexual dimorphism, with the SDN of males being larger in volume and having more neurons than the SDN of females due to the masculinizing effects of testosterone during perinatal development (Gorski, 1985). The precise behavioral role for the male SDN has remained elusive, as discrete lesions of the SDN have surprisingly subtle, if any, effects on male sexual behavior. There is some indication that the SDN may be involved in sexual motivation and partner selection, at least in male ferrets (Baum, Carroll, Cherrv, & Tobet, 1990).
Gonadal hormone modulation of cell number and cell group volume is a potential mechanism for the active maintenance of sexual dimorphisms in the AVPV and SDN during adolescent development. Ahmed and colleagues used the cell birthdate marker bromodeoxyuridine (BrdU) to identify cells born during early puberty in the adult AVPV and SDN (Figure 7; Ahmed, et al., 2008). BrdU-immunoreactive cells were more numerous in the AVPV of females as compared to males, whereas they were more numerous in the SDN of males compared to females. Furthermore, these sex differences in BrdU cells paralleled sex differences in cell group volume. Prepubertal GDX eliminated sex differences in the number of BrdU-immunoreactive cells in adulthood, and resulted in corresponding changes in cell group volume. Specifically, in females, prepubertal OVX reduced the number of AVPV BrdU cells and volume but did not affect the number of BrdU cells in the neighboring SDN. In males, prepubertal castration reduced BrdU cells and volume of the SDN but not AVPV. Some BrdU cells in both sexes also expressed either NeuN or GFAP, indicating that cells added to sexually dimorphic regions during puberty differentiate into functional neurons and glial cells (Ahmed, et al., 2008). Thus, pubertal hormones modulate either cell proliferation or survival in sexually dimorphic cell groups in a sex- and brain region-dependent manner, and thereby contribute to the maintenance of structural and functional sex differences during adolescent brain development.
Figure 7.
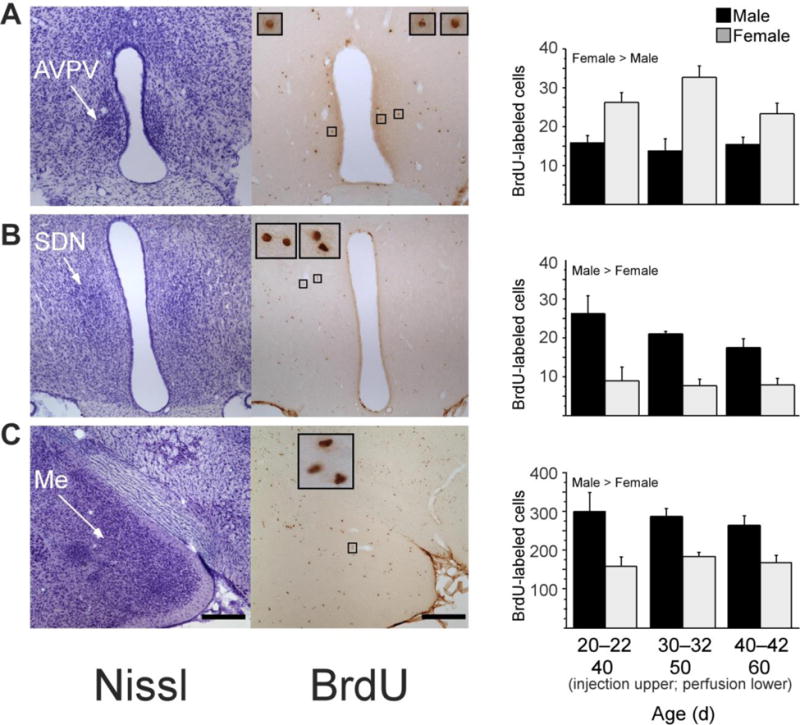
New cells are added during puberty to the AVPV, SDN and medial amygdala in male and female rats. Left photomicrographs, thionin-stained sections; right photomicrographs, BrdU labeled cells in nearby sections from the same rat; insets, BrdU-labeled cells framed in small boxes at x10 higher magnification. Rats received a daily injection of 300 mg per kg body weight of BrdU on three consecutive days at either 20–22, 30–32 or 40–42 d of age (n ¼ 6–8 per age and sex). BrdU is incorporated into DNA during the S phase of the cell cycle and can be later visualized to identify cells replicating at the time of BrdU administration. Brain tissue was collected 20 d after the first BrdU injection, on 40, 50 or 60 d of age, respectively. Quantitative analyses of BrdU-labeled cells revealed that during puberty, significantly more cells were added to AVPV (A) in females than in males, whereas significantly more cells were added to SDN (B) and medial amygdala (Me; C) in males than in females. Data are means ± s.e.m. Scale bars, 250 mm in lower-magnification images. Adapted from Ahmed et al., (2008), Nature Neuroscience 11 (9) 995–997.
1.5.2 Adolescent development of the posterodorsal medial amygdala (MePD)
The MePD integrates internal signals (e.g. gonadal hormones) and external cues from the environment (e.g. pheromones) to coordinate the display of social behaviors in rodents. The MePD shows a male-biased sexual dimorphism in volume in adulthood, particularly in the right hemisphere (Cooke, Stokas, & Woolley, 2007; Morris, Jordan, King, Northcutt, & Breedlove, 2008). Although the MePD is sexually dimorphic prior to puberty, the dimorphism becomes significantly greater across adolescent development. One factor contributing to this adolescent change is a sexually-dimorphic addition of new cells. For example, when male and female rats are injected with the cell birth-datemarker bromodeoxyuridine (BrdU) during adolescence, the number of BrdU-labeled cells in the MePD is higher in males than in females (Figure 7). Furthermore, the sex differences in BrdU-labeled cell number corresponds with sex differences in regional volume determined from analysis of Nissl-stained sections (Ahmed, et al., 2008).
These adolescent changes in MePD volume and cell number are due, at least in part, to increases in astrocyte number in males. For example, the majority of BrdU-labeled cells also express the astrocytic glial marker GFAP, indicating that astrocytes are driving the male-biased sexual dimorphism in MePD cell number and volume during adolescence (Ahmed, et al., 2008). Gonadal hormones influence this dimorphism, as prepubertal GDX reduces the pubertal addition of new astrocytes to the MePD in males but not in females (Ahmed, et al., 2008). Importantly, a separate study suggests that pubertally born MePD astrocytes are functionally integrated into MePD circuitry, as some BrDU-labeled cells (specific cell phenotype not yet known) are activated (express fos) after a sexual encounter with a receptive female (Mohr & Sisk, 2013). Furthermore, males carrying the testicular feminization mutation (tfm) of the androgen receptor and females do not display MePD increases in astrocyte number and branching during adolescence. In contrast, wildtype males display normative increases in MePD astrocyte number and branching, indicating that normative androgen receptor function is necessary for the adolescent development of this sexual dimorphism (Johnson, Breedlove, & Jordan, 2013; Johnson, Schneider, DonCarlos, Breedlove, & Jordan, 2012). Thus, strong evidence suggests that adolescent changes in astrocyte number and branching in males contributes to the sexual dimorphism in MePD cell volume.
The MePD projects to the bed nucleus of the stria terminalis (BNST), and the BNST is part of the extended amygdala, considered to be a neural circuit regulating a variety of social behaviors in both males and females (Newman, 1999). A microscopic analysis of the human BNST from postmortem samples showed that sex differences in BNST volume were not present in samples obtained during either fetal development or childhood and puberty (<16 yrs of age; (Chung, De Vries, & Swaab, 2002). However, there was a clear sex difference in BNST volume in samples obtained in adulthood (22–49 yrs of age), with the male BNST about 40% larger than the female BNST. Mean volume of the male BNST was larger in the adult samples compared with the childhood/pubertal samples, but volume of the female BNST was similar in the two age samples. Thus, it appears that the human BNST enlarges over the course of adolescent development in males, but not in females, and this results in the emergence of a male-biased sexual dimorphism in the BNST during adolescence. Whether testicular or ovarian hormones are involved in this sex difference is not known.
1.5.3 Adolescent development of the medial prefrontal cortex (mPFC)
The rodent mPFC is involved in regulation of male sexual behavior, play, and social dominance (Bell, McCaffrey, Forgie, Kolb, & Pellis, 2009; J. F. Davis, et al., 2010; Wang, et al., 2011), and is sexually dimorphic in adult rats, with mPFC volume being greater in males than in females. This sexual dimorphism emerges during puberty and is due in part to sex differences in cell death and synaptic pruning. The number of ventral mPFC neurons is similar in male and female rats in early adolescence (P35), but by P90, males have significantly more neurons than females because of a decrease in neuron number in females during adolescence (Figure 8; Markham, Morris, & Juraska, 2007). Furthermore, prepubertal GDX prevents the decline in neuron (and glia) number in females but not in males, suggesting that ovarian hormones drive the emergent sexual dimorphism during adolescence (Koss, Lloyd, Sadowski, Wise, & Juraska, 2015). In addition to decreases in neuron number observed in females, dendritic spines significantly decrease in both sexes between days 35 and 90, but only females show a loss of mPFC basilar dendrites (Koss, Belden, Hristov, & Juraska, 2014). Frontal cortex white matter volume increases across adolescent development in rodents, more so in males than in females, resulting in a male-biased sexual dimorphism (Willing & Juraska, 2015). This emergent sexual dimorphism also appears to be driven by ovarian hormones because prepubertal GDX significantly increases white matter volume in females but does not affect white matter volume in males (Koss, et al., 2015). Thus, under normative developmental conditions, the emergence of sex differences in mPFC gray and white matter during adolescence in rats are due to the actions of ovarian, not testicular, hormones on cell death, synaptic pruning, and myelination (Juraska & Willing, 2016). The situation appears to be different for adolescent development of white matter in humans, as the steeper increase in white matter volume in adolescent boys compared with girls is related to higher levels of testosterone (Perrin, et al., 2008; see also Gur and Gur in this volume).
Figure 8.
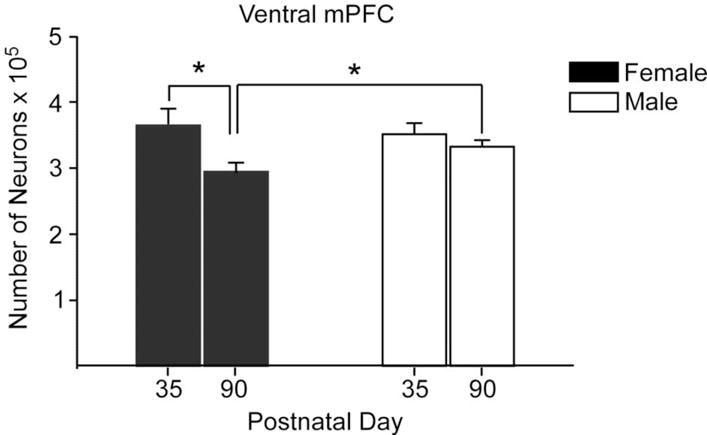
The number of neurons in the ventral portion of the male and female mPFC at P35 and in adulthood at P90. There was a loss of neurons in females between these ages, resulting in a sex difference in adulthood. n = 9–11 per group, * indicates p ≤ 0.02. Redrawn with permission from Markham et al., (2007), Neuroscience (144) 961–968.
1.6 Conclusions
Research over the past 25 years clearly identifies adolescence as a developmental period during which gonadal steroid hormones organize the brain and behavior, often resulting in further sexual differentiation. Organization of the adolescent brain builds on the sexual differentiation of neural circuits that was initiated primarily by testicular hormones acting on the developing male brain during the perinatal period of hormone-dependent organization. During the adolescent period of organization, both testicular and ovarian hormones play active roles in shaping adolescent brain development in males and females. The perinatal and peripubertal periods of organization do not appear to represent two distinct sensitive periods, but instead are a function of times during development during which gonadal hormones are elevated in the two sexes. Many types of behaviors are organized during adolescence, ranging from social behavior to ingestive behavior to cognitive function, although not all of these behaviors are necessarily organized in both sexes. Hormones organize the adolescent brain via many of the same mechanisms in play during hormonal organization of the perinatal brain, including cell proliferation and survival and synapse formation and elimination. As such, postnatal development appears to be a protracted period of sensitivity to the organizing actions of gonadal steroid hormones on the developing brain. Empirical evidence suggests that sensitivity to the organizing actions of gonadal steroid hormones may decrease across postnatal development, at least in males. Much remains to be learned about the specific neural mechanisms underlying hormone-dependent adolescent organization of behavior, and research providing causal links between structural changes and behavioral changes is sorely needed. Understanding the mechanisms of ovarian hormone organization of brain and behavior in females is a particularly ripe area for research because ovarian hormones do not play an active role in perinatal organization, and organizational influences of ovarian hormones during adolescence have only recently been described. Finally, it will be important to learn the extent to which gonadal hormones organize adolescent behaviors and neural circuits that aren’t necessarily sexually differentiated, and how much of what we have learned from animal models generalizes to humans.
Highlights.
Adolescence is a sensitive period for the effects of hormones on brain and behavior
Testicular hormones masculinize and defeminize social and reproductive behaviors
Ovarian hormones have both feminizing and defeminizing effects on female behavior
Gonadal steroid hormones drive many brain structural changes during adolescence
Adolescence may be part of a protracted postnatal steroid-sensitive period
Acknowledgments
This work was supported by NIH R01 MH090091 and R01 MH068764 to Cheryl L. Sisk and IK2 BX001562 to Kalynn M. Schulz.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kalynn M. Schulz, Email: kschulz3@utk.edu.
Cheryl L. Sisk, Email: sisk@msu.edu.
References
- Adkins-Regan E, Orgeur P, Signoret JP. Sexual differentiation of reproductive behavior in pigs: defeminizing effects of prepubertal estradiol. Horm Behav. 1989;23:290–303. doi: 10.1016/0018-506x(89)90068-8. [DOI] [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Mizejewski GJ, Ojeda SR. Development of estradiol-positive feedback on luteinizing hormone release in the female rat: a quantitative study. Endocrinology. 1981;109:1404–1413. doi: 10.1210/endo-109-5-1404. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Frontiers in Neuroendocrinology. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Honda SI, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. Journal of Neuroscience. 2002;22:9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Carroll RS, Cherrv JA, Tobet SA. Steroidal control of behavioural, neuroendocrine and brain sexual differentiation: studies in a carnivore, the ferret. J Neuroendocrinol. 1990;2:401–418. doi: 10.1111/j.1365-2826.1990.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Bell HC, McCaffrey DR, Forgie ML, Kolb B, Pellis SM. The Role of the Medial Prefrontal Cortex in the Play Fighting of Rats. Behavioral Neuroscience. 2009;123:1158–1168. doi: 10.1037/a0017617. [DOI] [PubMed] [Google Scholar]
- Beltz AM, Berenbaum SA. Cognitive effects of variations in pubertal timing: is puberty a period of brain organization for human sex-typed cognition? Horm Behav. 2013;63:823–828. doi: 10.1016/j.yhbeh.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: Effects of prenatal and pubertal organizational hormones. Frontiers in Neuroendocrinology. 2011;32:183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Mills R. Prepubertal testosterone treatment of neonatally gonadectomized male rats: defeminization and masculinization of behavioral and endocrine function in adulthood. Neurosci Biobehav Rev. 1995;19:187–200. doi: 10.1016/0149-7634(94)00064-8. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Mills R, Gale S. Prepubertal testosterone treatment of female rats: defeminization of behavioral and endocrine function in adulthood. Neurosci Biobehav Rev. 1995;19:177–186. doi: 10.1016/0149-7634(95)00065-m. [DOI] [PubMed] [Google Scholar]
- Brock O, Baum MJ, Bakker J. The Development of Female Sexual Behavior Requires Prepubertal Estradiol. Journal of Neuroscience. 2011;31:5574–5578. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, Kulbarsh KD, Spencer KA, Duval C. Peri-pubertal exposure to testicular hormones organizes response to novel environments and social behaviour in adult male rats. Horm Behav. 2015;73:135–141. doi: 10.1016/j.yhbeh.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WC, De Vries GJ, Swaab DF. Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. J Neurosci. 2002;22:1027–1033. doi: 10.1523/JNEUROSCI.22-03-01027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens LG, Gladue BA. Feminine sexual behavior in rats enhanced by prenatal inhibition of androgen aromatization. Horm Behav. 1978;11:190–201. doi: 10.1016/0018-506x(78)90048-x. [DOI] [PubMed] [Google Scholar]
- Coniglio LP, Paup DC, Clemens LG. Hormonal specificity in the suppression of sexual receptivity of the female golden hamster. J Endocrinol. 1973;57:55–61. doi: 10.1677/joe.0.0570055. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Stokas MR, Woolley CS. Morphological sex differences and laterality in the prepubertal medial amygdala. Journal of Comparative Neurology. 2007;501:904–915. doi: 10.1002/cne.21281. [DOI] [PubMed] [Google Scholar]
- Corbier P. Sexual differentiation of positive feedback: effect of hour of castration at birth on estradiol-induced luteinizing hormone secretion in immature male rats. Endocrinology. 1985;116:142–147. doi: 10.1210/endo-116-1-142. [DOI] [PubMed] [Google Scholar]
- D’Occhio MJ, Brooks DE. Effects of androgenic and oestrogenic hormones on mating behaviour in rams castrated before and after puberty. J Endocrinol. 1980;86:403–411. doi: 10.1677/joe.0.0860403. [DOI] [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- Davis JF, Loos M, Di Sebastiano AR, Brown JL, Lehman MN, Coolen LM. Lesions of the Medial Prefrontal Cortex Cause Maladaptive Sexual Behavior in Male Rats. Biological Psychiatry. 2010;67:1199–1204. doi: 10.1016/j.biopsych.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge FH, Muntjewerff JW, Louwerse AL, van de Poll NE. Sexual behavior and sexual orientation of the female rat after hormonal treatment during various stages of development. Horm Behav. 1988;22:100–115. doi: 10.1016/0018-506x(88)90034-7. [DOI] [PubMed] [Google Scholar]
- De Lorme KC, Sisk CL. Pubertal testosterone programs context-appropriate agonistic behavior and associated neural activation patterns in male Syrian hamsters. Physiol Behav. 2013:112–113. 1–7. doi: 10.1016/j.physbeh.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon AF. Sexual and aggressive behavior of adult male marmosets (Callithrix jacchus) castrated neonatally, prepubertally, or in adulthood. Physiol Behav. 1993;54:301–307. doi: 10.1016/0031-9384(93)90115-v. [DOI] [PubMed] [Google Scholar]
- Eckenhoff MF, Rakic P. Nature and fate of proliferative cells in the hippocampal dentate gyrus during the life-span of the rhesus monkey. Journal of Neuroscience. 1988;8:2729–2747. doi: 10.1523/JNEUROSCI.08-08-02729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichmann F, Holst DV. Organization of territorial marking behavior by testosterone during puberty in male tree shrews. Physiol Behav. 1999;65:785–791. doi: 10.1016/s0031-9384(98)00230-3. [DOI] [PubMed] [Google Scholar]
- Epple G, Alveario MC, Belcher AM. Copulatory behavior of Adult Tamarins (Saguinus fuscicollis) castrated as neonates or juveniles: effect of testosterone treatment. Horm Behav. 1990;24:470–483. doi: 10.1016/0018-506x(90)90036-w. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Axelson JF, Shinto LH, Albers HE. Scent marking and the maintenance of dominant/subordinate status in male golden hamsters. Physiol Behav. 1987;40:661–664. doi: 10.1016/0031-9384(87)90114-4. [DOI] [PubMed] [Google Scholar]
- Field EF, Pellis SM. The brain as the engine of sex differences in the organization of movement in rats. Arch Sex Behav. 2008;37:30–42. doi: 10.1007/s10508-007-9270-4. [DOI] [PubMed] [Google Scholar]
- Field EF, Whishaw IQ, Forgie ML, Pellis SM. Neonatal and pubertal, but not adult, ovarian steroids are necessary for the development of female-typical patterns of dodging to protect a food item. Behavioral Neuroscience. 2004;118:1293–1304. doi: 10.1037/0735-7044.118.6.1293. [DOI] [PubMed] [Google Scholar]
- File SE. Animal models for predicting clinical efficacy of anxiolytic drugs: social behaviour. Neuropsychobiology. 1985;13:55–62. doi: 10.1159/000118163. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JJ. Differentiation of sexual behaviour in pigs. J Reprod Fertil Suppl. 1990;40:311–321. [PubMed] [Google Scholar]
- Gogan F, Beattie IA, Hery M, Laplante E, Kordon D. Effect of neonatal administration of steroids or gonadectomy upon oestradiol-induced luteinizing hormone release in rats of both sexes. J Endocrinol. 1980;85:69–74. doi: 10.1677/joe.0.0850069. [DOI] [PubMed] [Google Scholar]
- Gorski RA. The 13th J. A. F. Stevenson memorial lecture. Sexual differentiation of the brain: possible mechanisms and implications. Can J Physiol Pharmacol. 1985;63:577–594. doi: 10.1139/y85-098. [DOI] [PubMed] [Google Scholar]
- Gotz F, Dorner G. Sex hormone-dependent brain maturation and sexual behaviour in rats. Endokrinologie. 1976;68:275–282. [PubMed] [Google Scholar]
- Gurney ME, Konishi M. Hormone induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208:1380–1382. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacology Biochemistry and Behavior. 2007;86:327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Huck UW, Lisk RD, McKay MV. Social-Dominance and Reproductive Success in Pregnant and Lactating Golden-Hamsters (Mesocricetus-Auratus) under Seminatural Conditions. Physiology & Behavior. 1988;44:313–319. doi: 10.1016/0031-9384(88)90031-5. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johnson RT, Breedlove SM, Jordan CL. Androgen receptors mediate masculinization of astrocytes in the rat posterodorsal medial amygdala during puberty. J Comp Neurol. 2013;521:2298–2309. doi: 10.1002/cne.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RT, Schneider A, DonCarlos LL, Breedlove SM, Jordan CL. Astrocytes in the rat medial amygdala are responsive to adult androgens. J Comp Neurol. 2012;520:2531–2544. doi: 10.1002/cne.23061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE. Scent marking, olfactory communication and social behavior in the golden hamster, Mesocricetus auratus. Ph. D. Dissertation. Rockefeller; New York: 1970. [Google Scholar]
- Juraska JM, Willing J. Pubertal onset as a critical transition for neural development and cognition. Brain Res. 2016 doi: 10.1016/j.brainres.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg CK, Lundin A. Brain androgen-inducible aromatase is critical for adolescent organization of environment-specific social interaction in male rats. Horm Behav. 1999;35:155–162. doi: 10.1006/hbeh.1998.1508. [DOI] [PubMed] [Google Scholar]
- Kercmar J, Snoj T, Tobet SA, Majdic G. Gonadectomy prior to puberty decreases normal parental behavior in adult mice. Hormones and Behavior. 2014;66:667–673. doi: 10.1016/j.yhbeh.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss WA, Belden CE, Hristov AD, Juraska JM. Dendritic Remodeling in the Adolescent Medial Prefrontal Cortex and the Basolateral Amygdala of Male and Female Rats. Synapse. 2014;68:61–72. doi: 10.1002/syn.21716. [DOI] [PubMed] [Google Scholar]
- Koss WA, Lloyd MM, Sadowski RN, Wise LM, Juraska JM. Gonadectomy Before Puberty Increases the Number of Neurons and Glia in the Medial Prefrontal Cortex of Female, but Not Male, Rats. Developmental Psychobiology. 2015;57:305–312. doi: 10.1002/dev.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K. Testicular hormone and Developmental changes in mating behavior of the male rat. J Comp Physiol Psychol. 1967;63:223–230. doi: 10.1037/h0024358. [DOI] [PubMed] [Google Scholar]
- Larsson K, Sodersten P, Beyer C, Morali G, Perez-Palacios G. Effects of estrone, estradiol and estriol combined with dihydrotestosterone on mounting and lordosis behavior in castrated male rats. Horm Behav. 1976;7:379–390. doi: 10.1016/0018-506x(76)90009-x. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lumia AR, Raskin LA, Eckhert S. Effects of androgen on marking and aggressive behavior of neonatally and prepubertally bulbectomized and castrated male gerbils. Journal of Comparative and Physiological Psychology. 1977;91:1377–1389. [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Miller LL, Whitsett JM, Vandenbergh JG, Colby DR. Physical and behavioral aspects of sexual maturation in male golden hamsters. J Comp Physiol Psychol. 1977;91:245–259. doi: 10.1037/h0077315. [DOI] [PubMed] [Google Scholar]
- Mohr MA, Sisk CL. Pubertally born neurons and glia are functionally integrated into limbic and hypothalamic circuits of the male Syrian hamster. Proc Natl Acad Sci U S A. 2013;110:4792–4797. doi: 10.1073/pnas.1219443110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, King ZA, Northcutt KV, Breedlove SM. Sexual dimorphism and steroid responsiveness of the posterodorsal medial amygdala in adult mice. Brain Research. 2008;1190:115–121. doi: 10.1016/j.brainres.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Lauschke DM, Juraska JM. Cell death in the development of the posterior cortex in male and female rats. J Comp Neurol. 2001;436:32–41. [PubMed] [Google Scholar]
- Nunez JL, Sodhi J, Juraska JM. Ovarian hormones after postnatal day 20 reduce neuron number in the rat primary visual cortex. J Neurobiol. 2002;52:312–321. doi: 10.1002/neu.10092. [DOI] [PubMed] [Google Scholar]
- Paup DC, Coniglio LP, Clemens LG. Masculinization of the female golden hamster by neonatal treatment with androgen or estrogen. Horm Behav. 1972;3:123–131. doi: 10.1016/0018-506x(72)90014-1. [DOI] [PubMed] [Google Scholar]
- Payne AP, Swanson HH. AGONISTIC BEHAVIOUR BETWEEN PAIRS OF HAMSTERS OF SAME AND OPPOSITE SEX IN A NEUTRAL OBSERVATION AREA. Behaviour. 1970;36:259–&. [PubMed] [Google Scholar]
- Pellis SM. Sex differences in play fighting revisited: traditional and nontraditional mechanisms of sexual differentiation in rats. Arch Sex Behav. 2002;31:17–26. doi: 10.1023/a:1014070916047. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Herve PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: Role of testosterone and androgen receptor. Journal of Neuroscience. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Pinos H, Collado P, Rodriguez-Zafra M, Rodriguez C, Segovia S, Guillamon A. The development of sex differences in the locus coeruleus of the rat. Brain Research Bulletin. 2001;56:73–78. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–643. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav. 1990;24:311–323. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- Rankin SL, Partlow GD, McCurdy RD, Giles ED, Fisher KRS. Postnatal neurogenesis in the vasopressin and oxytocin-containing nucleus of the pig hypothalamus. Brain Research. 2003;971:189–196. doi: 10.1016/s0006-8993(03)02350-3. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Menard TA, Smith DA, Albers HE, Sisk CL. Testicular hormone exposure during adolescence organizes flank-marking behavior and vasopressin receptor binding in the lateral septum. Horm Behav. 2006;50:477–483. doi: 10.1016/j.yhbeh.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006:254–255. 120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Shrenker P, Maxson SC, Ginsburg BE. The role of postnatal testosterone in the development of sexually dimorphic behaviors in DBA/1Bg mice. Physiol Behav. 1985;35:757–762. doi: 10.1016/0031-9384(85)90408-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Schulz KM, Zehr JL. Puberty: A finishing school for male social behavior. Annals of the New York Academy of Sciences. 2003;1007:189–198. doi: 10.1196/annals.1286.019. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Turek FW. Developmental time course of pubertal and photoperiodic changes in testosterone negative feedback on gonadotropin secretion in the golden hamster. Endocrinology. 1983;112:1208–1216. doi: 10.1210/endo-112-4-1208. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Smith LK, Forgie ML, Pellis SM. Mechanisms underlying the absence of the pubertal shift in the playful defense of female rats. Dev Psychobiol. 1998;33:147–156. [PubMed] [Google Scholar]
- Sodersten P. Estrogen-activated sexual behavior in male rats. Horm Behav. 1973;4:247–256. doi: 10.1016/0018-506x(73)90009-3. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Swithers SE, McCurley M, Hamilton E, Doerflinger A. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav. 2008;54:471–477. doi: 10.1016/j.yhbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomachka AJ, Greenwald GS. The development of gonadotropin and steroid hormone patterns in male and female hamsters from birth to puberty. Endocrinology. 1979;105:960–966. doi: 10.1210/endo-105-4-960. [DOI] [PubMed] [Google Scholar]
- Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 4. Elsevier; 2002. pp. 385–423. [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. Bidirectional Control of Social Hierarchy by Synaptic Efficacy in Medial Prefrontal Cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- Ward IL, Ward OB. Sexual behavior differentiation: Effects of prenatal manipulations in rats. In: Adler N, Pfaff D, Goy RW, editors. Handbook of behavioral neurobiology. Vol. 7. New York: Plenum Press; 1985. pp. 77–97. [Google Scholar]
- Willing J, Juraska JM. THE TIMING OF NEURONAL LOSS ACROSS ADOLESCENCE IN THE MEDIAL PREFRONTAL CORTEX OF MALE AND FEMALE RATS. Neuroscience. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, Sisk CL. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. Journal of Neurobiology. 2006;66:578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]


