Abstract
In the young of many mammalian species, including humans, a vigorous and highly rewarding social activity is abundantly expressed, known as social play behaviour. Social play is thought to be important for the development of social, cognitive and emotional processes and their neural underpinnings, and it is disrupted in pediatric psychiatric disorders. Here, we summarize recent progress in our understanding of the brain mechanisms of social play behaviour, with a focus on its rewarding properties. Opioid, endocannabinoid, dopamine and noradrenaline systems play a prominent role in the modulation of social play. Of these, dopamine is particularly important for the motivational properties of social play. The nucleus accumbens has been identified as a key site for opioid and dopamine modulation of social play. Endocannabinoid influences on social play rely on the basolateral amygdala, whereas noradrenaline modulates social play through the basolateral amygdala, habenula and prefrontal cortex. In sum, social play behaviour is the result of coordinated activity in a network of corticolimbic structures, and its monoamine, opioid and endocannabinoid innervation.
Keywords: Social play behaviour, Reward, Place conditioning, Operant conditioning, Opioids, Endocannabinoids, Dopamine, Noradrenaline, Prefrontal cortex, Nucleus accumbens, Amygdala
1. Introduction
During development, humans and animals acquire a wide variety of social behaviours that enable adaptive functioning directed at survival and reproduction in adulthood. Of these, one form of social behaviour that is particularly abundant during post-weaning development, is social play behaviour, also referred to as play fighting or rough-and-tumble play (Fagen,1981; Graham and Burghardt, 2010; Panksepp et al., 1984; Pellis and Pellis, 2009; Vanderschuren et al., 1997; Himmler et al., 2013; Vanderschuren and Trezza, 2014). One important function of social play behaviour is to facilitate the development of a rich and flexible social repertoire (Pellis and Pellis, 2009; Vanderschuren and Trezza, 2014), and as such, it can be considered a trigger for social development.
Social play behaviour is observed in the majority of mammalian species, including humans. It is most abundant from weaning until after puberty (Panksepp, 1981; Pellis and Pellis, 2009). In rodents, this covers the juvenile phase until mid-adolescence, equivalent to childhood through early/mid adolescence in humans (McCutcheon and Marinelli, 2009; Spear, 2000). Social play behaviour is known for its energetic, vigorous nature. It contains elements of aggressive, predatory and sexual behaviour, performed in a modified or exaggerated form (Panksepp et al., 1984; Pellis and Pellis, 2009; Vanderschuren et al., 1997). Furthermore, these behaviours are accompanied or preceded by explicit physical, facial or vocal signals that the intention of the behaviour is playful in nature. This makes social play behaviour typically easy to recognize and quantify. This is particularly true for several rodent species, including the rat. As a result, the vast majority of our knowledge on the neural underpinnings of social play behaviour stems from rat studies (Siviy and Panksepp, 2011; Trezza et al., 2010; Vanderschuren and Trezza, 2014), and the present overview therefore focuses on social play behaviour in rats.
Social play behaviour is a highly pleasurable, rewarding activity (Trezza et al., 2010; Trezza et al., 2011a; Vanderschuren, 2010), which was already recognized by Charles Darwin, who in The Descent of Man (1871) wrote that `Happiness is never better exhibited than by young animals, such as puppies, kittens and lambs, when playing together, like our own children'. On the one hand, this has spurred thinking about the importance and functions of social play, since most naturally rewarding activities such as feeding and sexual behaviour are clear promotors of survival, whereas this is less obvious for social play. On the other hand, this provides us with the opportunity to study social reward mechanisms in developing animals. The importance hereof should not be underestimated, because of the fact that social impairments, including aberrant social play, are core symptoms of pediatric mental disorders, such as autism, disruptive behaviour disorders, attention-deficit/hyperactivity disorder and early-onset schizophrenia (Alessandri, 1992; Helgeland and Torgersen, 2005; Jones et al., 1994; Jordan, 2003; Møller and Husby, 2000). In addition, studying the mechanisms of social play reward, in comparison with studies on other social (e.g. sexual behaviour), non-social (e.g. feeding) and artificial rewards (i.e. drugs of abuse) will paint a picture of how the brain processes pleasurable events and activities, and how these processes overlap or differ between different types of rewards (Berridge and Kringelbach, 2015).
Studying the neural mechanisms of social play behaviour therefore provides insight into how the brain processes positive social signals to generate meaningful social behaviour. The positive emotions that accompany social play contribute to emotional well-being, and as such are important for human health and animal welfare (Bateson, 2015; Ginsburg, 2007; Held and Špinka, 2011). In addition, the study of play will increase our knowledge on how adaptive social experiences shape proper brain development during childhood and adolescence (e.g. Crone and Dahl, 2012). Indeed, dysfunctional social interactions during childhood and adolescence are known to have a long-lasting negative impact on social abilities and cognitive function in humans (Braun and Bock, 2011; Cacioppo and Hawkley, 2009). Investigating the brain mechanisms that underlie social behaviour in the young will also enhance our understanding of child and adolescent mental disorders in which aberrant social behaviour is prominently manifest, such as autism, disruptive behaviour disorders, attention-deficit/hyperactivity disorder and early-onset schizophrenia (American Psychiatric Association, 2013). In the present review, we will provide an overview of studies on the neural underpinnings of social play behaviour in rats, with a focus on its rewarding properties.
1.1 Structure of social play behaviour in rats
In rats, an episode of social play behaviour usually starts off when a rat approaches a conspecific and attempts to touch its neck with the snout (Panksepp and Beatty, 1980; Pellis and Pellis, 1987; Poole and Fish, 1975; Vanderschuren et al., 1997). This behaviour is called pouncing or nape contact, and it is considered the most important parameter of play initiation, perhaps reflecting a motivational aspect of social play. Although pouncing most often occurs from the side, it can also occur from behind, in which case it superficially resembles sexual mounting. The most characteristic response to this play initiation is when the recipient rats rolls onto its dorsal surface, which is commonly known as `pinning', although pinning is not invariably preceded by pouncing. From this position, the animal on bottom will attempt to gain access to the initiating animals' neck area, so that pinning functions to prolong an ongoing play bout. Being pinned is an otherwise unusual posture for a rat and hence, it is easy to recognize. Note here that one animal on its back with the other standing over it also occurs during aggressive encounters. Thus, social play behaviour in rats clearly combines elements of sexual and aggressive behaviours. Importantly, there are clear distinctions in the microstructure of social play and aggressive behaviour (Blanchard and Blanchard, 1977; Pellis, 1988; Pellis and Pellis, 1987). Most important perhaps is the fact that the on-top and on-bottom positions alternate during social play, whereas this is obviously not the case during aggression. Furthermore, the targets of initiation/attack differ between social play and aggression: the nape of the neck for the former and rump, flanks, back for the latter.
Pinning and pouncing are considered to be the main indices for social play behaviour in rats, because they strongly co-vary with other playful social behaviours (such as following and wrestling) (Panksepp and Beatty, 1980), and are stimulated by brief periods of social isolation (hours to several days) (Niesink and Van Ree, 1989; Vanderschuren et al., 1995a; Vanderschuren et al., 2008). Indeed, responding to a pounce with a full rotation to supine is the most common response during the time when social play behaviour peaks in development (i.e. roughly between postnatal days 28–40). Before and after this period, male rats more often use a partial rotation strategy, whereby the hind legs stay on the ground, which can result in a brief period of upright wrestling. Interestingly, this shift in response to pouncing is much more pronounced in male rats, as compared to females. Thus, how a rat responds to play initiation is age- and sex-dependent (Pellis and Pellis, 1987; Pellis and Pellis, 1990). Another type of response to pouncing is evasion, whereby the recipient animal moves away. The initiator animal may then start to chase the recipient, so that evasion can also function to prolong the playful interaction. An alternative approach used to measure the effects of experimental manipulations on social play focuses on the measurement of the defensive tactics performed by the recipient of a playful attack (Himmler et al., 2013). The frequency of play fighting can be assessed by counting the number of playful nape attacks occurring per unit of time and playful defense can be measured as a percentage (number of attacks defended/total number of attacks × 100%; Himmler et al., 2013).
An important methodological issue that needs to be considered when performing environmental, genetic or pharmacological manipulations of social play is the strain of rats used. Thus, one should be aware that the magnitude of the experimentally-induced changes in social play parameters may differ between rat strains, and this needs to be considered in order to avoid ceiling or floor effects (Siviy et al., 2003; Reinhart et al., 2006; Siviy et al., 2011; Manduca et al., 2013, 2014).
Since social play behaviour involves behaviours that resemble social acts from different contexts, such as aggressive and sexual behaviour, it is important to provide signals to conspecifics that the intention of the behaviour is playful in nature (Palagi et al., 2016). In some animal species, there are specific signals that are used to communicate this intention, such as the `play-bow' in dogs, and particular facial and vocal expressions in primates. In rats, a jumpy type of gait is associated with social play (but not with sex or aggression). Furthermore, during social play, rats emit high-frequency, 50 kHz vocalizations (Knutson et al., 2002; Palagi et al., 2016; Wöhr and Schwarting, 2013), that are also associated with other rewarding activities. These vocalizations may signal positive mood or playful intent (Palagi et al., 2016; Wöhr and Schwarting, 2013). However, the relationship between social play behaviour and these high-frequency vocalizations is not as straightforward as initially assumed (Kisko et al., 2015; Manduca et al., 2014a), so that their exact initiating or facilitating contribution to social play remains to be determined.
1.2 Functions of social play behaviour
Play behaviour is widespread in the animal kingdom, yet does not appear to have an obvious direct function. This paradox has inspired a lively debate on the functions of play (Fagen, 1981; Graham and Burghardt, 2010; Groos, 1898; Huizinga, 1949; Martin and Caro, 1985; Panksepp et al., 1984; Pellis and Pellis, 2009; Small, 1899; Smith, 1982). With regard to the functions of social play behaviour in rats, laboratory experiments in the past decades have provided evidence that it facilitates the development of social, cognitive, emotional, and motor skills, in particular the ability to use these capacities flexibly in a changeable and unpredictable environment (Pellis and Pellis, 2009; Špinka et al., 2001; Vanderschuren and Trezza, 2014). We concede that these findings pertain to one form of play in one species, and extrapolations must therefore be made with caution.
By and large, the studies referred to above have investigated the long-term consequences of social isolation in young rats, in particular during the developmental period when social play is most abundant (i.e. temporary post-weaning social isolation, also referred to as `play deprivation'). It is beyond the scope of this review to provide an extensive overview of these studies, that we have recently summarized elsewhere (Vanderschuren and Trezza, 2014). In brief, these studies have found that play-deprived rats are particularly impaired under novel, changeable or challenging situations. For example, although play-deprived rats are well capable of displaying aggressive and defensive behaviour, they have trouble adjusting their behaviour to the context and circumstances. That is, when confronted with an aggressive resident rat, play-deprived rats evoke more aggression, incur more injuries, take more time to assume a submissive posture and show inappropriate exploration of the resident's territory after defeat (Van den Berg et al., 1999a; Von Frijtag et al., 2002). This kind of deficits stretches beyond the social domain. In tests of cognitive function, play-deprived rats show slower habituation to a novel environment, retarded response reversal learning, slower acquisition of a rat gambling task and increased premature responses in the 5-choice serial reaction time task when task contingencies unexpectedly changed (Baarendse et al., 2013; Einon et al., 1978; Einon and Morgan, 1977). Note that with sufficient training, or under baseline test conditions, there were no differences in task performance between play-deprived and control animals. Together, these findings resonate well with the hypothesis that play serves to equip animals for unexpected circumstances (Špinka et al., 2001), i.e. that by combining subsequences of behaviours out of their primary context, animals experiment with their own behaviour and in so doing, acquire a rich behavioural repertoire that they are able to use in a flexible way. Broadly speaking, play therefore serves to facilitate the development of functions such as flexibility and creativity (Bateson, 2015; Špinka et al., 2001). In addition to this, social play, most likely by virtue of its emotional dimensions, i.e. that play is accompanied by the sensation of excitement and pleasure, also has a function in emotional development. Studies addressing this have indeed shown that play-deprived animals show increased levels of anxiety (Leussis and Andersen, 2008; Lukkes et al., 2009; Wright et al., 1991) and augmented sensitivity to the positive effects of substances of abuse (Baarendse et al., 2014; Lesscher et al., 2015; Whitaker et al., 2013).
The studies summarized above all indicate that social play behaviour in rats has an important function in the development of brain and behaviour. However, these are all delayed benefits, that are highly unlikely to be the primary immediate drivers of social play. It is most likely its rewarding, pleasurable effects (Pellis and Pellis, 2009; Trezza et al., 2011a; Vanderschuren, 2010) that motivate animals to play in the short term. In other words, animals play because they enjoy doing so. In addition, its social aspects, i.e. seeking out pleasurable company, engaging in interactions with conspecifics, and establishing and maintaining social bonds, also contribute to the immediate motivation to play.
Interestingly, while early life adverse events have negative consequences on brain function and behavior, environmental enrichment in rats positively affects social play behaviour. Thus, it has been shown that maternal exposure to environmental enrichment before and during gestation increased social play behaviour in male (but not female) rats (Zuena et al. 2016). Similarly, post-weaning environmental enrichment was shown to counteract the deleterious effects of prenatal stress on play behaviour and corticosterone secretion in rats (Morley-Fletcher et al., 2003).
1.3 Rewarding properties of social play
Social play has a strong emotional component, its most characteristic feature being its high reward value (Panksepp et al, 1984; Vanderschuren et al., 1997; Trezza et al., 2011; Pellis and Pellis, 2009). The rewarding properties of social play are reflected by its ability to support T-maze learning and runway performance as well as place and operant conditioning. These behavioural paradigms have been used to disentangle several aspects of social play reward (Trezza et al., 2011a), including: 1. The subjective feeling of pleasure, i.e. hedonic impact or `liking'. 2. Approach behaviour towards or the willingness to work for social play, i.e. incentive motivation. 3. Associative learning and memory, i.e. cognitive aspects of social play.
1.3.1 Place conditioning
Place conditioning is among the most widely used tests to study the rewarding properties of natural and drug rewards in laboratory animals (Tzschentke, 2007, Bardo and Bevins, 2000). Place conditioning has been used to demonstrate the pleasurable aspects of (playful) social interactions (Calcagnetti and Schechter, 1992; Crowder and Hutto, 1992; Douglas et al., 2004; Lahvis et al., 2015; Panksepp and Lahvis, 2007; Van den Berg et al., 1999b), access to pups (Mattson et al., 2001), sexual behaviour (Camacho et al., 2004; Jenkins and Becker, 2003; Kippin and van der Kooy, 2003), as well as aggressive social interactions (Martinez et al., 1995; Tzschentke, 2007).
A typical place conditioning set-up consists of two or three linked chambers: two conditioning chambers with different visual, tactile and/or olfactory cues, sometimes separated by a third neutral middle compartment. The paradigm is based on the principle that through coupling of the pleasurable properties of social play with the distinct environmental cues of a particular chamber, these cues will come to elicit approach behaviour, so that a rat will spend more time in that environment, when allowed to choose. During conditioning, animals will have the opportunity to play with a conspecific in one chamber and they will be placed alone or with a non-playful partner (e.g., a partner treated with a drug that selectively suppresses social play) in the other chamber. Usually, one day after the last conditioning session, animals are placed in the middle compartment (or on the border of the two chambers, if a two-chamber setup is used) and the animal can freely move about the apparatus for a certain amount of time. The time spent in each of the chambers or the time spent in the play-paired chamber pre- vs post-conditioning is used as an indication of conditioned place preference (CPP).
Calcagnetti and Schechter (1992) were the first to demonstrate that CPP could be acquired with social play. Young rats (postnatal day (PND) 29–33) were conditioned with a partner that had been rendered non-playful by treatment with the muscarinic receptor antagonist scopolamine in one compartment, and with a playful partner in the other compartment. During testing, the rats significantly preferred the compartment previously paired with a playful social partner. Comparable findings were reported by Crowder and Hutto (1992), who used a hybrid of a place and an operant conditioning setup. Douglas et al. (2004) showed that isolated adolescent and adult rats of both sexes acquired social CPP, with adolescent males showing the strongest preference. No social CPP was found in group-housed adults whereas group-housed adolescents showed preference for the compartment previously paired with similarly housed partners. However, when socially housed adolescents were conditioned with isolated partners, no social CPP occurred. These results show that social play is most rewarding for isolated adolescent male rats and suggest that a comparable level of social motivation facilitates the rewarding experience of a social interaction.
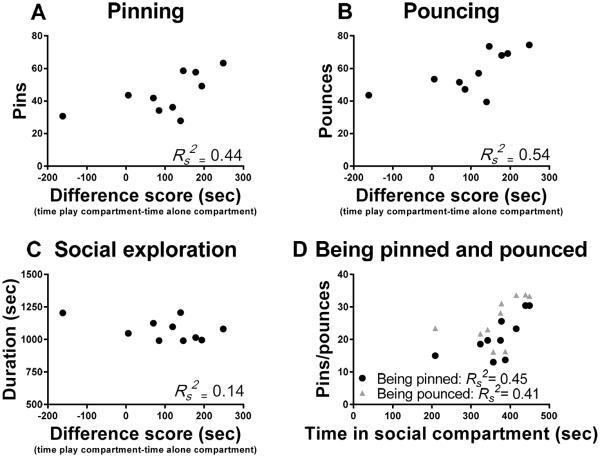
Trezza et al. (2009b) demonstrated social play-induced CPP in animals that were socially isolated during conditioning, while animals isolated for a shorter period of time (i.e. 3.5 hours) before conditioning showed a trend towards significant CPP. Importantly, this isolation period has been shown to induce a half-maximal increase in the amount of social play behaviour (Niesink and Van Ree 1989; Vanderschuren et al., 1995c, -2008). No CPP developed in animals that were group-housed or housed with an adult rat. Importantly, rats conditioned with a partner treated with methylphenidate, a drug that reduces play-related behaviours without affecting general social interest (Achterberg et al., 2015; Vanderschuren et al., 2008), did not develop CPP, which underscores the importance of social play for the development of CPP. In support of this notion, we have found that the total amounts of pins and pounces during the conditioning sessions positively correlate with the magnitude of CPP. Thus, the more the animals played, the larger the CPP. This correlation was not observed for social exploration. When the play data were analyzed taking initiator and recipient of the play interaction into account (i.e. pinning/pouncing vs being pounced or pinned), both being pinned and being pounced were found to correlate positively with CPP. Importantly, since being pinned requires an active response of the animal (i.e. rotating to its dorsal surface), this suggests that it is the active engagement in social play, rather than being the initiator of the interaction, that determines its rewarding properties (Figure 1).
Figure 1. Correlations of social play parameters with conditioned place preference.
A significant positive relationship was found between the mean amount of pins (A) and pounces (B) during the eight conditioning sessions of social play-induced conditioned place preference with the difference between time spent in the social compartment and the time spent in the non-social compartment during the preference test (pins: rs = 0.66, p= 0.04; pounces: rs = 0.73, p= 0.02). No significant correlation was found between the mean amount of social exploration during conditioning sessions and difference between the time spent in the social compartment and the time spent in the non-social compartment during the preference test (C: rs = −0.37, p= 0.29). In addition, there was a significant positive relationship between the mean amount of being pinned and being pounced and the time spent in the social compartment (D: being pinned: rs = 0.67, p= 0.03; being pounced: rs = 0.64, p= 0.05, n=10). Data were analyzed using Spearman's correlation coefficient.
In apparent contrast to the above findings, Kummer et al. (2011) and Peartree et al. (2012) found that social interaction without physically engaging in play can also support the development of CPP, albeit to a lesser extent than active social play itself. Thus, whereas two pairings with a playful partner were sufficient to develop CPP, eight pairings were required to establish CPP when the social partner was confined behind a barrier or when a ball was used as a stimulus (to evoke object play) (Peartree et al., 2012). When tactile stimulation was prevented during the social interaction, so that only visual and olfactory information could be exchanged, place aversion was found (Kummer et al., 2011). Combined, these results show whereas social play may not be strictly required for CPP to emerge, being able to actively engage in this activity marked facilitates the development of CPP, indicating the social play is the most rewarding component of social behaviour in young rats.
The interaction between social and drug reward in rats has also been studied using place conditioning experiments. Studies by Thiel et al. (2008, -2009) have demonstrated that social play can also be used to enhance the rewarding properties of drugs of abuse such as cocaine and nicotine and vice versa. Using a sub-effective conditioning paradigm, in which each condition alone (i.e. either drug or social play) was not sufficient to produce CPP, the two rewards together interacted to produce CPP, although both nicotine and cocaine reduced play itself. Grotewold et al. (2014) reported comparable findings with social interaction and cocaine. These studies are important for our understanding of the effects of social context on drug reward (El Rawas and Saria, 2016; Trezza et al., 2014; Zernig and Pinheiro, 2015). All in all, there is ample evidence to show that social play can induce CPP, which provides an excellent opportunity to study the pleasurable aspects of social play behaviour.
1.3.2 Operant conditioning
Social play, like palatable food, drugs of abuse and several social behaviours can be used to support operant conditioning, the procedure by which an animal can obtain a reward by performing an arbitrary action, such as pressing a lever, poking its nose in a hole or touching a screen. An operant conditioning chamber (often called `skinner box', after B.F. Skinner, one of the main instigators of operant conditioning research) typically consists of a computer controlled chamber with levers, nose-poke holes or a touchscreen, and cue lights to steer the animal's behaviour. When an animal makes a required response, it receives a reward, so that the animal learns the contingency between its response (e.g. lever-pressing) and the delivery of the reward. This increases the likelihood that the animal will repeat the action, a phenomenon known as reinforcement. Different reinforcement schedules can be used to gauge distinct aspects of the animals' behaviour. Of these, the progressive-ratio (PR) schedule of reinforcement was developed to specifically study motivation for rewards (Hodos, 1961; Richardson and Roberts, 1996). Under a PR schedule, the number of responses to obtain the next reward is increased after every obtained reward, until the animal stops responding. The maximal number of responses performed to obtain one single reward, i.e. the breakpoint, is generally used as a measure for incentive motivation. Operant conditioning has been employed using different social rewards (Trezza et al., 2011a), such as access to a receptive female (Everitt and Stacey, 1987), access to pups (Lee at al., 1999) or (playful) social handling by an experimenter (Davis and Perusse, 1988). The first ever operant conditioning experiments with social play were performed in primates. Mason et al. (1962) tested in 2 young chimpanzees whether they were willing to press a lever to interact with an experimenter. Importantly, of the social behaviours on offer, they found that social play most powerfully supported responding. In a follow-up study (Mason et al., 1963), the reinforcing properties of food and social interaction were compared, in which the incentive value of the food was manipulated by testing the animals when hungry of satiated and by changing the palatability of the presented food. Social interaction consisted of being petted by the experimenter or social play with the experimenter. Food was preferred when animals were hungry or highly palatable food was present and the animals preferred play over petting. The most intriguing finding of the study was that even when the animals were hungry or when highly palatable food was available, the chimpanzees still chose play half of the time.
Recently, we developed an operant conditioning paradigm for social play reward in rats (Achterberg et al., 2016a; Achterberg et al., 2016b). In this setup, rats are trained to lever press for brief episodes of social play behaviour. In order to assess the motivational properties of social play, we used a PR schedule of reinforcement. Consistent with the notion that social isolation increases social play (Niesink and Van Ree, 1989; Panksepp and Beatty, 1980; Vanderschuren et al., 1995a; Vanderschuren et al., 2008) by enhancing the motivation to play, we found that responding for social play, as well as its performance during reinforced periods, was higher in rats isolated for 24 hr before testing than in animals isolated for 2 hr. Moreover, as will be discussed in detail below, we found that the performance of social play and responding for social play could be pharmacologically dissociated. This indicates that the motivation for play and its performance are modulated through distinct, although likely overlapping, neural systems. Together, these results show that operant conditioning can be used to assess motivational aspects of social play behaviour.
Motivational properties of social play have previously also been studied in a T-maze set-up (Humphreys and Einon, 1981; Ikemoto and Panksepp, 1992; Normansell and Panksepp, 1990; Werner and Anderson, 1976). In this paradigm, animals are placed in a `startbox' at the bottom of a T-shaped maze and after a short delay are allowed to move towards one of the arms of the maze. This can be used to determine preference for certain stimuli as a measure for reward and motivation, whereby movement speed or choice latency are often used as readout parameters. In addition, mnemonic aspects of reward processes can be assessed. Compared to group-raised animals, it has been shown that social isolation-reared adolescent rats chose the opportunity for social interaction more often compared to a palatable food reward (Ikemoto and Panksepp, 1992). Futhermore, young rats preferred a playing partner in a `goal box' compared to a social but non-playful partner (Humphreys and Einon, 1981; Normansell and Panksepp, 1990; Werner and Anderson, 1976). In addition, dominant adolescent males in a play couple needed less time to traverse a T-maze for the opportunity to play compared to their subordinate play partners and vice versa (Panksepp et al., 1984).
2. Neuropharmacology of social play
Much of our knowledge on the neural underpinnings of social play behaviour derives from pharmacological intervention studies (Siviy and Panksepp, 2011; Trezza et al., 2010; Vanderschuren et al., 1997). In this section, we will provide an overview of the studies that have used pharmacological manipulations. For the most part, these have been systemic treatments, but these have more recently been followed up by intracranial administration studies. Because the main topic of the present review is the positive, rewarding properties of social play, we will focus on neural systems implicated in reward signaling, i.e. opioids, cannabinoids, dopamine and noradrenaline.
2.1 Opioids
The endogenous opioid system has been widely implicated in reward mechanisms, i.e. the positive emotional properties of food, sex and drugs of abuse (Berridge and Kringelbach, 2015; Le Merrer et al., 2009; Van Ree et al., 1999). Indeed, the opioid system, consisting of three prototypes of endogenous ligands (endorphins, enkephalins and dynorphins) and three receptor types (mu, delta and kappa) (Akil et al., 1984; Le Merrer et al., 2009), is perhaps the most widely studied neuromodulator system in the context of social play behaviour.
The finding that stimulation of opioid neurotransmission by treating rats with the opioid receptor agonist morphine increases social play behaviour was first reported in the 1980s (Panksepp et al., 1980; Panksepp et al., 1985), and this finding has been replicated many times since (Manduca et al., 2014a; Niesink and Van Ree, 1989; Normansell and Panksepp, 1990; Trezza and Vanderschuren, 2008a; Trezza and Vanderschuren, 2008b; Vanderschuren et al., 1995a; Vanderschuren et al., 1995c). Conversely, treatment with opioid receptor antagonists, such as naloxone and naltrexone, reduces social play behaviour (Beatty and Costello, 1982; Jalowiec et al., 1989; Niesink and Van Ree, 1989; Normansell and Panksepp, 1990; Panksepp et al., 1980; Panksepp et al., 1985; Siegel et al., 1985; Siegel and Jensen, 1986; Trezza and Vanderschuren, 2009). Analysis of the effect of the opioid receptor agonist morphine on social play behaviour showed that it does not primarily change the structure of this behaviour. Rather, the internal coherence of social play may be enhanced, so that playful interactions are prolonged (Vanderschuren et al., 1995c). The facilitating effect of morphine on social play was not profoundly affected by testing the animals in an unfamiliar context, suggesting that this drug does not alter play by enhancing feelings of safety or reducing anxiety in an novel environment (Trezza and Vanderschuren, 2008b; Vanderschuren et al., 1995a). Interestingly, a low dose of morphine, that had no net stimulating effect on social play, did attenuate the initial reduction in social play in an unfamiliar environment, suggesting that the increase in social play induced by higher doses of this drug is at least in part the result of a facilitation of the initiation of this behaviour (Vanderschuren et al., 1995a). This is consistent with the observation that pouncing, a measure of play initiation, is also enhanced when only one animal in a test pair is treated. When behaviour was analyzed in each animal separately, it appeared that both pinning and pouncing were increased in the morphine-treated rat, but these behaviours were unchanged in control animals. Thus, the increase in pouncing is the result of the enhancement of this behaviour in the morphine-treated animal (Trezza and Vanderschuren, 2008b). More recently, it was found that the effect of morphine on social play is more pronounced in Wistar rats compared to Sprague Dawley rats, suggesting that genetic background influences the effect of this drug on social play. However, since Sprague Dawley rats showed higher baseline levels of play, it can as yet not be excluded that a ceiling effect confounded the effect of morphine in this strain (Manduca et al., 2014a). Moreover, the stimulating effects of morphine on social play have also been observed in Long Evans rats (Normansell and Panksepp, 1990; Panksepp et al., 1985), indicating that although genetic background may be of influence, this effect is not specific to one single strain of rats.
The play-stimulating effects of morphine can be attributed to the endorphin system and to stimulation of mu-opioid receptors (Niesink and Van Ree, 1989; Trezza et al., 2011b; Vanderschuren et al., 1995b). Systemic treatment of rats with beta-endorphin or with a muopioid receptor agonist stimulated social play behaviour and treatment with a mu-opioid receptor antagonist reduced it, whereas treatment with delta opioid receptor ligands did not affect social play behaviour. In contrast, kappa-opioid receptor agonist treatment suppressed social play behaviour (Niesink and Van Ree, 1989; Vanderschuren et al., 1995b). The nucleus accumbens has been identified as an important site of action of opioids to modulate social play behaviour (Trezza et al., 2011b; Vanderschuren et al., 1995d). Thus, social play behaviour reduced in vivo opioid receptor binding in the rostral nucleus accumbens, suggesting that social play evokes release of opioid peptides (Vanderschuren et al., 1995d). Moreover, intra-nucleus accumbens treatment with morphine, beta-endorphin or the mu-opioid receptor agonist DAMGO stimulated social play behaviour, whereas the delta-opioid receptor agonist DPDPE, met-enkaphalin or the enkephalinase inhibitor thiorphan did not affect social play, and the mu-opioid receptor antagonist CTAP and the kappa opioid receptor agonist U69593 inhibited social play behaviour. Importantly, the play-enhancing effects of systemic morphine treatment could be blocked by intra-nucleus accumbens infusions of naloxone, demonstrating that stimulation of nucleus accumbens opioid receptors is necessary and sufficient to augment the expression of social play behaviour (Trezza et al., 2011b).
Subsequent studies have investigated the involvement of opioids in the rewarding properties of social play behaviour more directly. The first of these (Normansell and Panksepp, 1990) investigated the effects of systemic treatment with the opioid receptor agonist morphine and the opioid receptor antagonist naloxone on behaviour in a social play-rewarded T-maze setup. Although opioid drug treatment did not affect choice behaviour or latency in the task, morphine stimulated, whereas naloxone reduced the expression of social play behaviour in the goal box. Furthermore, naloxone accelerated and morphine retarded extinction. These findings are consistent with the well-documented role of opioids in the expression of social play behaviour, and hint at an involvement of opioids in the incentive motivation for social play, apparent as altered extinction (Normansell and Panksepp, 1990). The lack of effect on choice behaviour and latency could either mean that these aspects of play motivation are not under the influence of opioid neurotransmission, or that they are less sensitive parameters of play motivation. Consistent with the effect of systemic naloxone treatment, intra-nucleus accumbens treatment with CTAP blocked the development of social play-induced conditioned place preference (Trezza et al., 2011b).
Opioid neurotransmission has also been implicated in the positive effects of non-opioid drugs on social play. Thus, blockade of opioid receptors with naloxone attenuated the play-facilitating effects of indirect cannabinoid agonists (i.e. the anandamide hydrolysis inhibitor URB597 and the anandamide transport inhibitor VDM-11) (Trezza and Vanderschuren, 2008a; Trezza and Vanderschuren, 2009), nicotine (Trezza et al., 2009a) and alcohol (Varlinskaya and Spear, 2009; but see Trezza et al., 2009a).
In sum, the endogenous opioid system plays a critical role in the modulation of social play behaviour in rats. The available evidence strongly points at the mu-opioid receptor system, and the nucleus accumbens as an important site of action for opioid influences on social play. Opioids modulate the expression of social play, perhaps by interfering with the internal coherence of this behaviour- i.e. by prolonging playful social activity (Vanderschuren et al., 1995c), and also the pleasurable and motivational properties of social play are under the influence of opioids as well.
2.2 Cannabinoids
Endocannabinoids are lipid signaling messengers that activate the same receptors as delta-9-tetrahydrocannabinol (THC), the active component of Cannabis sativa. The most studied endocannabinoids, anandamide and 2-arachidonoylglycerol (2-AG), are synthesized following neuronal depolarization (Mechoulam et al., 2014; Piomelli, 2003). Once released from postsynaptic neurons, they bind to presynaptic G-protein coupled cannabinoid receptors (CB1 and CB2). Finally, their actions are terminated by uptake via one or more endocannabinoid membrane transporters, followed by degradation by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), hydrolytic enzymes that provide the primary clearance routes for anandamide and 2-AG, respectively (Mechoulam et al., 2014; Piomelli, 2003).
Given the well-established role of endocannabinoids in the modulation of emotional, motivational and cognitive states (Fattore et al., 2010; Lutz, 2009; Zanettini et al., 2011), it is not surprising that they are involved in social play behaviour. Studies with systemic drug administration have shown that pharmacological interference with endocannabinoid neurotransmission has different effects on social play behaviour in rats, depending on the pharmacological tool used. Thus, when rats were treated with drugs that directly stimulate CB1 cannabinoid receptors, such as the synthetic cannabinoid receptor agonist WIN55,212-2 (Trezza and Vanderschuren, 2008a, b), methanandamide, a metabolically stable analogue of anandamide (Trezza and Vanderschuren, 2009), or with THC, the active component of Cannabis sativa (Trezza et al., 2014), social play was markedly reduced. These effects were mediated by activation of CB1 cannabinoid receptors, since they were prevented by pretreatment with the CB1 cannabinoid receptor antagonist/inverse agonist SR141716A. Exposure to a moderate dose of THC during pregnancy and lactation also decreased social play behaviour in the offspring (Trezza et al., 2008). Collectively, these results are consistent with findings showing that both acute (Genn et al., 2004; van Ree et al., 1984) and chronic (O'Shea et al., 2006; Schneider and Koch, 2005; Schneider et al., 2008) treatment with cannabinoid receptor agonists reduced social interaction in rats. The reduction in social play induced by direct cannabinoid receptor agonists may be the consequence of the widespread activation of CB1 cannabinoid receptors in multiple brain areas, including in regions where increased cannabinoid activity causes a cognitive and emotional state that is incompatible with the adequate execution of complex social acts.
An alternative pharmacological approach to interfere with endocannabinoid neurotransmission is to use drugs that target the enzymes involved in endocannabinoid uptake or hydrolysis. Since endocannabinoids are synthesized and released on-demand following neuronal depolarization, this pharmacological approach has the advantage of increasing local endocannabinoid neurotransmission, allowing to clarify the physiological role of endocannabinoids in neurobehavioural functions (Bari et al., 2006; Petrosino and Di Marzo, 2010). Selective inhibition of anandamide metabolism using the FAAH inhibitor URB597 increased social play behaviour in rats (Manduca et al., 2014b; Trezza et al., 2012; Trezza and Vanderschuren, 2008a, b). Similar effects were induced by the anandamide transporter inhibitor VDM11 (Trezza and Vanderschuren, 2009). These results suggest that, during social play, anandamide is released in brain areas mediating this behaviour, and that the increased anandamide tone induced by drugs that interfere with either anandamide uptake or hydrolysis makes rats more playful. To support this possibility, it has been shown that, during social play, anandamide but not 2-AG levels increased in the rat nucleus accumbens (NAc), amygdala (Trezza et al., 2012) and dorsal striatum (Marco et al., 2011). Systemic administration of the FAAH inhibitor URB597 magnified the social play-induced increase in anandamide levels in the amygdala, but not the NAc (Trezza et al., 2012), pointing to a crucial role of the amygdala in anandamide modulation of social play. Indeed, while infusion of URB597 into both the basolateral amygdala (BLA) and NAc increased social play, blockade of CB1 cannabinoid receptors with the antagonist/inverse agonist SR141716A in the BLA, but not NAc, prevented the play-enhancing effects of systemic administration of URB597 (Trezza et al., 2012). Collectively, these data identify the BLA as an important site of action through which anandamide signaling positively modulates social play behaviour.
Close interactions between the endocannabinoid, dopaminergic and endogenous opioid systems have been implicated in the positive subjective properties of natural and drug rewards (Gardner, 2005; van Ree et al., 1999). Indeed, a functional interplay between these neurotransmitter systems in affective responses and social behaviours has also been described (Fattore et al., 2010; Loureiro et al., 2015). In line with this kind of functional interaction, it has been shown that the effects of indirect cannabinoid agonists on social play behaviour were blocked by pretreatment with the cannabinoid receptor antagonist/inverse agonist SR141716A, the opioid receptor antagonist naloxone or the dopamine receptor antagonist α-flupenthixol (Trezza and Vanderschuren, 2008a; -2009). More recently, we have shown that endocannabinoid-dopamine interactions in social play occur via the NAc, since intra NAc administration of α-flupenthixol counteracted the increase in social play behaviour induced by systemic administration of the anandamide hydrolysis inhibitor URB597 and the opioid receptor agonist morphine (Manduca et al., 2016). The mostly likely mechanism underlying such interaction is the modulation of the activity of ventral tegmental dopamine neurons by endocannabinoids, that alter the functional activity of the GABA and glutamate inputs received by dopaminergic cells (Gardner, 2005). Moreover, endocannabinoids have been implicated in the play-enhancing effects of nicotine and ethanol in rats (Trezza et al., 2009a), a finding in line with the role of the endocannabinoid system in the reinforcing effects of these drugs (Economidou et al., 2006; Gamaleddin et al., 2015; Pava and Woodward, 2012).
It is known that the behavioural effects of cannabinoid drugs depend on both genetic and environmental factors (Haller et al., 2009; Naidu et al., 2007). In line with this notion, the effects of URB597 on social play behaviour in rats were found to be strain-dependent, as URB597 increased social play in Wistar but not Sprague–Dawley rats (Manduca et al., 2014b). The lack of effects of URB597 in Sprague–Dawley animals may be due to a ceiling effect, since rats from the Sprague-Dawley strain show higher baseline levels of social play behaviour than Wistar rats (Manduca et al., 2014a; Manduca et al., 2014b). As for the role of environmental factors, URB597 increased social play when rats were tested either in a familiar or unfamiliar test cage (Manduca et al., 2014b; Trezza and Vanderschuren, 2008b), and either under high or low light conditions (Manduca et al., 2014b). The effects of URB597 on social play, however, were influenced by the level of social activity of the test partner: thus, URB597 enhanced the responsiveness to play solicitation, but only when reciprocated by an equally socially motivated test partner (Trezza and Vanderschuren, 2008c).
More recently, we investigated whether endocannabinoids differentially modulate the motivational and pleasurable properties of social play behaviour (Achterberg et al., 2016b). We found that URB597, administered at a dose known to increase the expression of social play behaviour, did not affect social play-induced operant responding or social play-induced conditioned place preference (CPP). The CB1 cannabinoid receptor antagonist/inverse agonist SR141716A reduced operant responding when administered at a dose known to decrease the expression of social play behaviour, although this effect may be secondary to concurrent drug-induced stereotypic behaviours (i.e., grooming and scratching) (Tallett et al., 2007). Collectively, these data indicate that endocannabinoids likely drive the expression of social play behaviour as a whole, without differentially affecting its motivational or pleasurable properties.
The role of 2-AG in social play behaviour has recently been investigated using the dual FAAH/MAGL inhibitor JZL195 (Long et al., 2009). At a dose that enhanced brain levels of 2-AG, but not anandamide, JZL195 increased social play behaviour. This effect was antagonized by pretreatment with the cannabinoid receptor antagonist/inverse agonist SR141716A (Manduca et al., 2015). The effects of JZL195 were behaviourally specific, since at the dose that increased social play, JZL195 did not alter social exploration, anxiety or locomotor activity, nor did it induce other cannabimimetic effects, such as catalepsy or hypothermia (Manduca et al., 2015). These findings provide the first evidence for a role of 2-AG in social play behaviour in rats, which resonates well with recent studies showing an involvement of 2-AG signaling in social reward (Wei et al., 2016) and social defeat stress (Tomas-Roig et al., 2016) in adult mice.
To summarize, endocannabinoids modulate the performance of social play behaviour in rats. In the case of anandamide, this modulation is strain- and context-dependent, occurs in concert with the endogenous opioid and dopaminergic systems and involves limbic brain areas, such as the NAc and amygdala. The brain areas involved in the modulation of social play behaviour by 2-AG, and whether such modulation involves interactions with other neurotransmitter systems, remain to be elucidated.
2.3 Dopamine
Given the rewarding properties of social play, and the well-documented role of dopamine neurotransmission in motivational processes and incentive salience (Berridge, 2007; Floresco, 2015; Kelley, 2004; Robbins and Everitt, 2007; Salamone and Correa, 2012), it is very likely that dopamine also modulates social play behaviour. Indeed, treatment with non-selective dopamine receptor antagonists, such as haloperidol, chlorpromazine, alpha-flupenthixol, the dopamine D1 receptor antagonist SCH-23390 and the D2 receptor antagonist eticlopride has been reported to inhibit social play (Beatty et al., 1984; Holloway and Thor, 1985; Humphreys and Einon, 1981; Niesink and Van Ree, 1989; Siviy et al., 1996; Trezza and Vanderschuren, 2009). Moreover, the play-enhancing effects of indirect cannabinoid agonists such as the anandamide hydrolysis inhibitor URB597 and the anandamide transport inhibitor VDM-11, as well as those of nicotine and ethanol (but not morphine), were blocked by systemic pretreatment with an ineffective dose of alpha-flupenthixol (Trezza et al., 2009a; Trezza and Vanderschuren, 2008a; Trezza and Vanderschuren, 2009).
In contrast to the relatively straightforward effects of dopamine receptor antagonists on social play behaviour, the effects of dopamine receptor agonist treatment on social play have been reported to be quite variable. Treatment with the non-selective dopamine receptor agonist apomorphine was found to increase (Beatty et al., 1984; Vanderschuren et al., 2008) as well as decrease social play (Niesink and Van Ree, 1989), and treatment with selective dopamine D1 (SKF-38393) and D2 (quinpirole) receptor agonists suppressed social play behaviour (Siviy et al., 1996). There is also a sizeable body of evidence to show that psychostimulant drugs, such as amphetamine, methylphenidate and cocaine, that increase dopamine neurotransmission by inhibiting dopamine reuptake or promoting its release, are powerful suppressors of social play (Achterberg et al., 2014a; Beatty et al., 1982; Beatty et al., 1984; Ferguson et al., 2000; Humphreys and Einon, 1981; Sutton and Raskin, 1986; Thor and Holloway, Jr., 1983; Vanderschuren et al., 2008). However, the effects of amphetamine and methylphenidate were found to rely on noradrenergic neurotransmission, whereas the effect of cocaine only partially depends on dopamine (Achterberg et al., 2014a; Vanderschuren et al., 2008) (see below). Indeed, selectively increasing extracellular dopamine levels using the reuptake blocker GBR-12909 did not alter social play (Vanderschuren et al., 2008). It appears therefore that an optimal level of dopamine is required for the expression of social play behaviour, whereby both stimulating and reducing dopaminergic neurotransmission can disrupt social play, reminiscent of the `inverted U/shaped' dose-dependent effects of prefrontal dopamine and noradrenaline on working memory (Robbins and Arnsten, 2009). In this regard, one also needs to bear in mind that with systemic drug treatment, dopaminergic neurotransmission is altered in a variety of brain regions, which may lead to a dysbalance in dopamine signaling between different circuits (Cools and Robbins, 2004). Interestingly, in Fischer 344 rats, that are known to display profoundly less social play in comparison to other widely used strains of laboratory rats, dopamine function has been shown to be disrupted. Thus, electrically evoked vesicular dopamine release, as measured using fast scan cyclic voltammetry in brain slices, was reduced in ventral and dorsal striatum compared to Sprague-Dawley rats. Moreover, dopamine turnover was reduced in the striatum and prefrontal cortex (Siviy et al., 2011). Remarkably, dopamine release in the striatum evoked by high potassium concentrations or amphetamine, which is thought to mobilize cytoplasmic pools of neurotransmitter, was higher in slices from Fischer 344 rats (Siviy et al., 2015). Together, these data indicate that there is a dysbalance between dopamine availability from vesicular (i.e., sensitive to electrical stimulation) versus cytoplasmic stores (i.e., sensitive to amphetamine) in this rat strain. This aberration in dopamine signaling may result in impaired dopaminergic modulation of social play and thus profoundly reduced levels of social play in Fischer 344 rats.
Given that dopamine in the nucleus accumbens has been particularly implicated in motivation and incentive salience (Berridge, 2007; Floresco, 2015; Kelley, 2004; Robbins and Everitt, 2007; Salamone and Correa, 2012), a recent study investigated the involvement of nucleus accumbens dopamine in social play behaviour (Manduca et al., 2016). This study found that intra-accumbens infusion of amphetamine and the dopamine receptor agonist apomorphine (but not the dopamine reuptake blocker GBR-12909) enhanced social play, in a dopamine D1- and D2 receptor-dependent manner. Moreover, intra-accumbens treatment with the non-selective dopamine receptor antagonist alpha-flupenthixol inhibited the play-stimulating effects induced by systemic treatment with the opioid receptor agonist morphine and the anandamide hydrolysis inhibitor URB597. Last, alpha-flupenthixol at a higher dose reduced social play, but only in animals that were maximally motivated to play as a result of longer social isolation (Manduca et al., 2016). This study sheds new light on the role of dopamine in social play, by pinpointing the nucleus accumbens as an important site of action of dopamine. Thus, the confusing findings of dopamine agonist treatments summarized above may be the result of changes in dopamine signaling in different brain regions affecting social play behaviour in counteracting ways.
In view of the role of dopamine in incentive motivation (Berridge, 2007; Floresco, 2015; Kelley, 2004; Robbins and Everitt, 2007; Salamone and Correa, 2012), we recently studied how dopamine modulates the motivation for social play behaviour. Thus, treatment with cocaine, methylphenidate and the dopamine reuptake blocker GBR-12909 increased responding for social play under a PR schedule of reinforcement. Consistent with a role of dopamine in this increased motivation for social play, the effects of cocaine and methylphenidate on responding were blocked by pretreatment with an inactive dose of the non-selective dopamine receptor antagonist alpha-flupenthixol. Treatment with a higher dose of alpha-flupenthixol reduced responding for social play. Intriguingly, cocaine and methylphenidate reduced the expression of social play during reinforced play periods, and these effects were not altered by alpha-flupenthixol (see below) (Achterberg et al., 2016a), supporting the notion that motivation for play and its expression are mediated through distinct neural mechanisms.
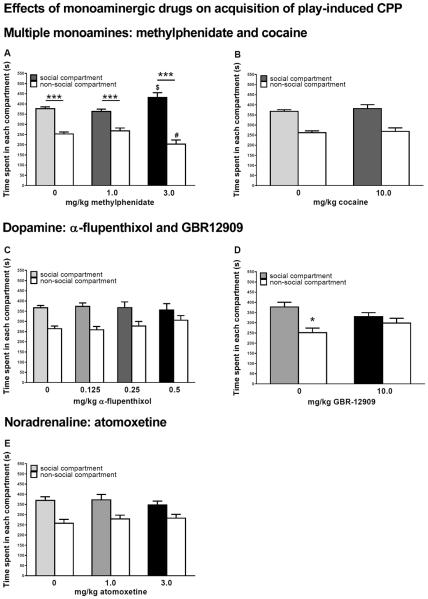
In parallel, we have also investigated the role of dopamine in social play-induced CPP. As mentioned above, Trezza et al. (2009b) showed when animals were conditioned with a methylphenidate-treated partner, this blocked the development of CPP. Remarkably, in a follow-up study, we found that treating both animals in couple with methylphenidate results in a significant preference for the social compartment (Figure 2A). This is probably the result of the positive affective properties of methylphenidate itself, since the rats also showed a significant drug-induced CPP after conditioning with methylphenidate (Table 1). Treatment with cocaine (10 mg/kg) neither affected social play-induced place conditioning (Figure 2B) nor produced CPP by itself (Table 1). It may perhaps be surprising that methylphenidate, but not cocaine induced CPP, as both drugs have been widely reported to support CPP (Tzschentke, 1998; Tzschentke, 2007). This is most likely the result of the fact that these drugs were administered 30 min before conditioning, consistent with the pretreatment interval we used when studying the effects of cocaine and methylphenidate on social play itself (Achterberg et al., 2014a; Vanderschuren et al., 2008), and on social play-induced CPP. It is known that cocaine-induced CPP is diminished when a delay is introduced between cocaine treatment and conditioning, whereby even a conditioned place aversion can result (Ettenberg et al., 1999). That treatment with methylphenidate did induce CPP may be the result of the slower kinetics of methylphenidate compared to cocaine (Gerasimov et al., 2000; Volkow and Swanson, 2003), so that the positive affective properties of methylphenidate but not cocaine survive a 30 min post-treatment delay. Consistent with the notion that dopamine is not primarily involved in the pleasurable properties of rewards (Berridge, 2007; Salamone and Correa, 2012), we found that systemic treatment with alpha-flupenthixol had minimal, if any, effects on social play-induced CPP (Figure 2C), even at doses that did reduce the expression of this behaviour (Trezza and Vanderschuren, 2009). Perhaps the most remarkable finding in this series of experiments is that treatment with the dopamine reuptake blocker GBR12909 disrupted the development of social play-induced CPP (Figure 2D), at a dose that neither affected place conditioning by itself (Table 1), nor influenced the expression of social play behaviour (Achterberg et al., 2016a; Vanderschuren et al., 2008). The underlying mechanisms of this effect are unclear. Apparently, an increased level of dopaminergic neurotransmission in multiple brain areas is incompatible with the development of social play-induced CPP, perhaps as a result of a dysbalance in dopamine transmission between different brain regions (Cools and Robbins, 2004), whereby functions that underlie the positive subjective properties of social play and/or learning a place-reward association become disrupted.
Figure 2. Effect of dopaminergic and noradrenergic drugs on social play-induced conditioned place preference (CPP).
Place conditioning was performed as previously described (Trezza et al., 2009; Achterberg et al., 2012, 2014b).
(A) Methylphenidate (1–3 mg/kg) altered the acquisition of social play-induced CPP (Fcompartment(1,114)=163.79, p<0.001; Ftreatment(2,114)=0.01, p=0.99; Fcompartment × treatment(2,114)=8.73, p<0.001; 0 mg/kg: n=34, 1.0 mg/kg: n=20, 3.0 mg/kg: n=10). At the lowest dose, it did not affect social play-induced CPP (0 mg/kg: t(33)=7.49, p<0.001; 1.0 mg/kg: t(19)=4.18, p=0.001), but treatment with 3 mg/kg methylphenidate increased the preference for the social compartment (3.0 mg/kg: t(9)=5.87, p=0.001; t(39)soc0-soc3=−2.64, p=0.01; t(39)nsoc0-nsoc3=2.33, p=0.03).
(B) Cocaine (10 mg/kg) did not affect the acquisition of social play-induced CPP. The animals showed a preference for the social compartment (Fcompartment(1,86)=61,77 p<0.001; Ftreatment(1,86)=0.50, p=0.48; Fcompartment × treatment(1,86)=0.08, p=0.78; 0 mg/kg: n=24, 10 mg/kg: n=10).
(C) The acquisition of social play-induced CPP was not affected by treatment with the dopamine receptor antagonist α-flupenthixol (Fcompartment(1,104)=42.89, p<0.001; Ftreatment(3,104)=0.24, p=0.85; Fcompartment × treatment(3,104)=0.97, p=0.41; 0 mg/kg: n=24, 0.125 mg/kg: n=12, 0.25 mg/kg: n=10, 0.5 mg/kg: n=10)
(D) The acquisition of social play-induced CPP was blocked by treatment with the dopamine reuptake inhibitor GBR-12909 (Fcompartment (1,40)=12.65, p=0.001; Ftreatment(1,40)<0.001, p=0.99; Fcompartment × treatment(1,40)=4.630, p=0.04; t(11)social compartment -non-social compartment 0 mg/kg=2.88, p=0.01; t(9)social compartment -non-social compartment 10 mg/kg=0.77, p=0.46; 0 mg/kg: n=12, 10 mg/kg: n=10).
(E) Treatment with the noradrenaline reuptake inhibitor atomoxetine did not affect the acquisition of social play-induced CPP (Fcompartment (1,70)=28.05, p<0.001; Ftreatment(2,70)=0.20, p=0.82; Fcompartment × treatment(2,70)=0.73, p=0.49; 0 mg/kg: n=18, 1.0 mg/kg: n=10, 3.0 mg/kg: n=10).
Data are shown as mean+SEM, social compartment vs non-social compartment: *p<0.05, ***p<0.001. Time spent in social compartment, 0 mg/kg vs 3.0 mg/kg methylphendiate $p<0.05; time spent in non-social compartment, 0 mg/kg vs 3.0 mg/kg methylphenidate #p<0.05. Data were analysed using a two-way analysis of variance (ANOVA) with compartment and treatment as between subject effects. When appropriate, ANOVA was followed up by Student's paired t-tests.
Table 1.
Effect of treatment with monoaminergic drugs on the acquisition of conditioned place preference
| Drug | Mean±SEM * | Statistics | Conclusion |
|---|---|---|---|
|
| |||
| Cocaine | 0 mg/kg: | Fcompartment(1,36)=0.05, p=0.83 | No effect |
| 0 mg/kg n=10 | Veh: 321.84±18.21 | Ftreatment(2,36)=0.38, p=0.54 | |
| 10 mg/kg n=10 | Veh: 311.07±17.19 | Fcompartment x treatment(2,36)=0.14, | |
| 10 mg/kg: | p=0.72 | ||
| Drug: 326±20.73 | |||
| Veh: 329±12.94 | |||
|
| |||
| Methylphenidate | 0 mg/kg: | Fcompartment(1,84)=13.75, p<0.001 | 3 mg/kg methylphenidate induced conditioned place preference |
| 0 mg/kg n=20 | Veh: 309.86±23.76 | Ftreatment(2,84)=0.31, p=0.74 | |
| 1.0 mg/kg n=11 | Veh: 290.81±15.41 | Fcompartment x treatment(2,84)=6.82, | |
| 3.0 mg/kg n=11 | 1.0 mg/kg: | p=0.002 | |
| Drug: 320.73±17.71 | |||
| Veh: 304.55±16.49 | Post hoc Drug vs Veh: | ||
| 3.0 mg/kg: | 0 mg/kg: t(9)=−1.56, p=0.15 | ||
| Drug: 393.84±15.62 | 1.0 mg/kg: t(10)=0.40, p=0.70 | ||
| Veh: 234.82±18.73 | 3.0 mg/kg: t(10)=4.84, p=0.001 | ||
|
| |||
| GBR12909 | 0 mg/kg: | Fcompartment(1,82)=0.57, p=0.45 | No effect |
| 0 mg/kg n=20 | Veh: 309.89±21.78 | Ftreatment(2,82)=0.85, p=0.43 | |
| 3.0 mg/kg n=10 | Veh: 290.81±23.45 | Fcompartment x treatment(2,82)=0.09, | |
| 10.0 mg/kg n=11 | 3.0 mg/kg: | p=0.92 | |
| Drug: 323.39±21.89 | |||
| Veh: 320.77±22.63 | |||
| 10.0 mg/kg: | |||
| Drug: 300.71 ±21.92 | |||
| Veh: 287.87±23.42 | |||
Drug: time spent in drug-paired compartment, Veh: time spent in vehicle-paired compartment.
In sum, an important modulatory role for dopamine in social play behaviour has been identified. It plays an important role in the motivation for social play (Achterberg et al., 2016a), and it underlies some of the stimulating effects on social play of non-dopaminergic drugs (Manduca et al., 2016; Trezza et al., 2009a; Trezza and Vanderschuren, 2008a), whereby the nucleus accumbens is a critical site of action (Manduca et al., 2016). The role of dopamine in the rewarding properties of social play that support the development of CPP remains to be investigated in more detail.
2.4 Noradrenaline
Noradrenergic neurotransmission has been implicated in a variety of cognitive processes, including learning, attention and flexibility (Aston-Jones and Cohen, 2005; Berridge and Waterhouse, 2003; Robbins and Arnsten, 2009; Roozendaal and McGaugh, 2011). In addition, it plays a role in the generation and perception of emotions, whereby there is an emerging body of work to indicate that noradrenaline is involved in reward processes (Bouret and Richmond, 2015; Ventura et al., 2005). Given that the expression of social play likely depends on a complex interplay between these cognitive and emotional processes, it is well conceivable that noradrenaline is important for proper performance of social play behaviour. Treatment with the noradrenaline reuptake inhibitor atomoxetine has been demonstrated to inhibit social play, which was prevented by pretreatment with the α2-noradrenaline receptor antagonist RX821002 (Vanderschuren et al., 2008). Interestingly, treatment with α2-noradrenaline receptor antagonists produced differential effects on social play. RX821002 enhanced, whereas yohimbine decreased social play behaviour (Vanderschuren et al., 2008; Normansell and Panksepp, 1985; Siviy and Baliko, 2000). Treatment with the α2-noradrenaline receptor agonist clonidine was also found to reduce social play behaviour, and this effect was attenuated by pretreatment with yohimbine (Beatty et al., 1984a; Siviy et al., 1994). Siviy and colleagues (1994) also reported a role of α1-noradrenaline receptors in the modulation of social play behaviour. In their study, the α1-adrenoreceptor agonist St-587 did not affect, while the α1-adrenoreceptor antagonist prazosin reduced social play behaviour. Furthermore, pretreatment with St-587 prevented the play-reducing effect of prazosin (Siviy et al., 1994). Propranolol, the antagonist of β-noradrenaline receptors, has been found to decrease social play behaviour as well (Beatty et al., 1984).
Treatment with psychostimulant drugs, such as methylphenidate, amphetamine and cocaine has been consistently reported to suppress social play behaviour (Achterberg et al., 2014a; Beatty et al., 1982; Beatty et al., 1984; Ferguson et al., 2000; Humphreys and Einon, 1981; Sutton and Raskin, 1986; Thor and Holloway, 1983; Vanderschuren et al., 2008). These drugs elevate levels of endogenous noradrenaline, dopamine and serotonin by acting as neurotransmitter reuptake inhibitors, whereas amphetamine also induces the release of these neurotransmitters. Pharmacological analysis showed that the play-reducing effect of methylphenidate was mediated by noradrenergic neurotransmission: social play was suppressed by the noradrenaline reuptake inhibitor atomoxetine, but not by the selective dopamine reuptake inhibitor GBR12909. In addition, the play suppressing effect of methylphenidate could be blocked by pretreatment with an α2- (RX821002), but not an α1-(prazosin) or β- (propranolol) noradrenergic antagonist, or dopamine receptor antagonists (Vanderschuren et al, 2008). In a follow up study (Achterberg et al., 2014b), the play-suppressive effects of amphetamine and cocaine were studied. Like methylphenidate, the play-suppressant effect of amphetamine was antagonized by the α2-noradrenaline receptor antagonist RX821002 but not by the dopamine receptor antagonist α-flupenthixol. Remarkably, the effects of cocaine on social play were not antagonized by α2 noradrenergic, dopaminergic, or serotonergic receptor antagonists, administered either alone or in combination. However, the effects of a subeffective dose of cocaine could be enhanced by a combination of subeffective doses of the serotonin reuptake inhibitor fluoxetine, the dopamine reuptake inhibitor GBR12909, and the noradrenaline reuptake inhibitor atomoxetine. These data demonstrate that methylphenidate and amphetamine suppresses social play through stimulation of α2-noradrenaline receptors. On the other hand, cocaine reduces social play by simultaneous increases in noradrenaline, dopamine, and serotonin neurotransmission.
The brain regions contributing to the play-suppressant effect of methylphenidate have also been investigated. Infusion of methylphenidate and atomoxetine into the anterior cingulate cortex, infralimbic cortex, BLA, and habenula inhibited social play, but not social exploratory behaviour or locomotor activity. Methylphenidate administration into the prelimbic, medial/ventral orbitofrontal, and ventrolateral orbitofrontal cortex, mediodorsal thalamus, or nucleus accumbens shell was ineffective in affecting social play behaviour. These results show that the social play-suppressant effects of methylphenidate and atomoxetine are mediated through a distributed network of prefrontal and limbic subcortical regions implicated in cognitive control and emotional processes (Achterberg et al., 2015).
Using our setup in which rats respond for social play under a PR schedule of reinforcement (Achterberg et al., 2016a; Achterberg et al., 2016b), we showed that treatment with the psychostimulant drugs methylphenidate and cocaine increased responding for social play, but suppressed its expression during reinforced play periods (see above). These effects were further investigated by treating rats with the dopamine reuptake inhibitor GBR12909 and the noradrenaline reuptake inhibitor atomoxetine. GBR12909 increased responding for social play but did not affect its expression, whereas atomoxetine-treated rats displayed decreased responding for social play as well as reduced expression. The effects of methylphenidate and cocaine on responding for social play, but not their play-suppressant effects, were blocked by pretreatment with the dopamine receptor antagonist alpha-flupenthixol. Conversely, pretreatment with the α2-noradrenaline receptor antagonist RX821002 prevented the play-suppressant effect of methylphenidate, but left its enhancing effect on responding for social play unaltered. Together, these data indicate that the play-suppressant effects of methylphenidate (as well as atomoxetine) depend on noradrenergic neurotransmission, whereas it is dopaminergic neurotransmission that mediates psychostimulant-induced increase in the motivation for social play. With regard to a role for noradrenaline in social play reward, we found that treatment with atomoxetine did not affect social play-induced CPP (Figure 2E).
Place conditioning can also be used to investigate the cognitive aspects of reward processes. For example, Trezza et al. (2009) showed that social play-induced CPP could be reinstated after extinction by a single play session in the play-associated compartment. In order to understand the role of noradrenaline in the dynamics of positive social memories, the effect of propranolol, a β-adrenoceptor antagonist known to influence a variety of memory processes (Nader and Hardt, 2009; Sara, 2000), on acquisition, consolidation, retrieval and reconsolidation of social play-induced CPP in adolescent rats was evaluated (Achterberg et al., 2012). Systemic treatment with propranolol, immediately before or after a CPP test (which is essentially a memory retrieval session), attenuated the expression of CPP 24 h later. Following extinction, CPP could be reinstated in saline- but not in propranolol-treated rats, indicating that propranolol treatment had persistently disrupted the CPP memory trace. Furthermore, propranolol did not affect acquisition, consolidation or retrieval of social play-induced CPP, suggesting that β-noradrenergic receptors are specifically involved in the re-storage of long-term social reward memory after retrieval, i.e. the reconsolidation process (Achterberg et al., 2012). In a follow-up study, we found that the reconsolidation of social play-induced CPP depended on stimulation of glucocortocoid receptors and to a lesser extent NMDA receptors, whereas mineralocorticoid and CB1-cannabinoid receptors were not involved (Achterberg et al., 2014b).
2.5 Other systems
Aside from opioids, cannabinoids, dopamine and noradrenaline, pharmacological studies on the neural mechanisms of social play behaviour have investigated a range of other neurotransmitter systems as well (Siviy and Panksepp, 2011; Trezza et al., 2010; Vanderschuren et al., 1997). With regard to influences on the positive, rewarding aspects of social play behaviour, we here restrict ourselves to the effects of drugs that may alter social play reward.
The stimulating effects of alcohol on social play have been well-documented. Thus, treatment with low doses of alcohol increased social play behaviour (Trezza et al., 2009a; Varlinskaya et al., 2001; Varlinskaya and Spear, 2002; Varlinskaya and Spear, 2006; Varlinskaya and Spear, 2009). These effects were not secondary to changes in locomotion (Trezza et al., 2009a; Varlinskaya et al., 2001; Varlinskaya and Spear, 2006). In addition, they were not likely to be a result of the anxiolytic properties of alcohol. That is, the effects of alcohol on social play were largely comparable in familiar and unfamiliar test environments and not associated with an anxiolytic effect on the elevated plus maze (Trezza et al., 2009a). Moreover, the facilitating effects of alcohol on social play were particularly apparent in early adolescent rats, suggesting that it is the playful aspects of social interaction, that are most abundant at this age, that are facilitated by alcohol. Older animals appeared to be more sensitive to the social disinhibition (i.e. relief of the reduction in social interaction in an unfamiliar environment) by alcohol (Varlinskaya and Spear, 2002; Varlinskaya and Spear, 2006). In terms of mechanism of action, dopamine and nicotine receptor stimulation is involved in the alcohol-induced increase in social play (Trezza et al., 2009a), whereas mixed findings have been reported regarding the role of opioid neurotransmission (Trezza et al., 2009a; Varlinskaya and Spear, 2009).
Treatment with low doses of nicotine has been reported to increase social play behaviour. This effect was independent of changes in locomotion and anxiety, and blocked by pretreatment with a nicotine (mecamylamine), dopamine (alpha-flupenthixol), or opioid (naloxone) receptor antagonist (Trezza et al., 2009a). Other substances with positive subjective properties that have been investigated in the context of play include the non-competitive NMDA-receptor antagonist MK-801, that was reported to have biphasic effects on social play (i.e. facilitation at low doses and reduction at higher doses) (Siviy et al., 1995). The play-enhancing effect of MK-801 is likely mediated by blockade of the NR2B subunit of the NMDA receptor. Thus, treatment with the NR2B antagonist ifenprodil, but not the NR2A antagonist PEAQX was found to increase social play (Morales et al., 2013). Treatment with the benzodiazepine drug diazepam reduced social play and increased non-playful social exploratory behaviour at a dose that had anxiolytic effects in the elevated plus-maze (Trezza et al., 2009a), providing additional evidence for a dissociation between social play behaviour and anxiety. The lateral septum is a candidate region for the modulation of social play behaviour by GABA and glutamate, since the concentration of these neurotransmitters was found to increase in this structure during social play (Bredewold et al., 2015). Last, recent studies have started to investigate the role of vasopressin and oxytocin, neuropeptides implicated in a range of social behaviours (Donaldson and Young, 2008; Meyer-Lindenberg et al., 2011; Stoesz et al., 2013) in social play in rats and hamsters. Intracerebroventricular treatment with the vasopressin receptor antagonist (CH2)5Tyr(Me2)AVP was found to reduce social play behaviour in male rats, yet enhanced it in females (Veenema et al., 2013). Intriguingly, when this antagonist was infused into the lateral septum, opposite effects were found: an increase in social play in males, and a reduction in female rats (Veenema et al., 2013). These latter effects were subsequently found to depend on context, as the play-enhancing effect of intra-septal treatment with the vasopressin receptor antagonist in male rats were observed when the animals were tested in their home cages, but not in a novel, unfamiliar environment (Bredewold et al., 2014). In females, intra-septum infusion of the vasopressin receptor antagonist decreased social play in the home cage, but not in a novel cage. In addition, treatment with vasopressin into the lateral septum suppressed social play when tested in the home (but not novel) cage, and intra-septum oxytocin treatment reduced social play in the novel (but not the home) cage (Bredewold et al., 2014). Remarkably, intracerebroventricular or intra-septal infusions of an oxytocin receptor antagonist did not affect social play behaviour in either sex or environment in these studies (Veenema et al., 2013; Bredewold et al., 2014). An earlier study in male hamsters has also identified the anterior hypothalamus as a site of action for vasopressin to modulate social play, since infusion of the vasopressin receptor antagonist d(CH2)5Tyr(Me)AVP into this region reduced social play behaviour (Cheng and Delville, 2009). Together, these studies point at an intricate modulation of social play behaviour by vasopressin and oxytocin, that depends on sex, familiarity of the test environment, and brain region (Bredewold et al., 2014; Cheng and Delville, 2009; Veenema et al., 2013).
3. Neural modulation of social play
The neural mechanisms of social play behaviour in rats have been investigated using lesion, site-specific injection of selective agonists and antagonists and immediate early gene expression studies. Although our understanding of the brain mechanisms of social play is far from complete, these studies have so far provided a most interesting glimpse into how limbic and corticostriatal circuits modulate social play.
3.1 Frontal cortex
The prefrontal cortex plays an integral role in higher cognitive, so-called executive functions, such as attention, planning, working memory and decision making (e.g. Floresco, 2013; Miller and Cohen, 2001; Robbins and Arnsten, 2009). Since social behaviours, in which one has to perceive, interpret and respond to the emotions, intentions and actions of others, are inherently unpredictable, involvement in social play of the frontal cortical brain regions that facilitate flexible navigation in a changeable environment is to be expected.
The earliest studies that have investigated the involvement of (frontal) cortical regions in social play have used neonatal ablation of cortical tissue. The earliest of these studies (Panksepp et al., 1994) found that neonatal removal of the frontal cortex modified the structure of social play, in that lesioned animals showed shorter pin duration, suggesting that intact frontal cortex function facilitates the prolongation of a playful interaction. Somewhat consistent, later studies found that neonatal lesion of the medial prefrontal cortex reduced pinning, as a result of the fact that the lesioned animals used a different strategy to respond to play initiation, i.e. more evasions and partial rotations and less complete rotations (Bell et al., 2009; Schneider and Koch, 2005). By contrast, neonatal lesions of the orbital frontal cortex caused animals to be unable to adjust their response to play initiation to the dominance status of their partners (Pellis et al., 2006). Together, these studies suggest that the different frontal cortical regions subserve the fine-tuning of social play behaviour, consistent with the involvement of the frontal cortex in executive functions.
Since the aforementioned studies used neonatal lesions, it is not clear whether the effects on later play are of organizational or activational origin, i.e. whether the prefrontal cortex modulates the development or expression of social play- or both. This issue was circumvented in a more recent intracranial pharmacology study, in which different regions of the prefrontal cortex were temporally inactivated using infusions of the GABA receptor agonists baclofen and muscimol (Van Kerkhof et al., 2013a). This study showed that inactivation of the prelimbic, infralimbic and medial/ventral orbitofrontal cortex reduced social play. In this study, the structure of social play did not seem to be markedly altered as much as its quantity, supporting the notion that the prefrontal cortex, perhaps by fine-tuning the response to a conspecific's behaviour, facilitates the prolongation of a playful interaction. However, given that the frontal cortex is functionally heterogeneous (Dalley et al., 2004; Heidbreder and Groenewegen, 2003), it is not likely that these three regions play a unitary role in social play. Indeed, a recent study, aimed at elucidating the neural sites of action through which methylphenidate and atomoxetine suppress social play, found that infusion of these drugs into the anterior cingulate and infralimbic cortex reduced social play (Achterberg et al., 2015). In contrast, methylphenidate infusions into the prelimbic, medial/ventral orbitofrontal or ventrolateral orbital cortex were ineffective (Achterberg et al., 2015).
Involvement of the frontal cortex in social play has also been investigated using immediate early gene studies, in which expression of genes like c-fos are used as a measure for cellular activity. In one of these studies, no change in c-fos in anterior cingulate and medial/ventral orbital cortex was found after social play (Gordon et al., 2002), although a subsequent study (Gordon et al., 2003) did find an increase in expression of brain-derived neurotrophic factor (BDNF) in a more lateral, motor part of frontal cortex. In a recent, extensive investigation of social play-induced cellular activity, increases in c-fos expression after social play were found in a range of frontal areas, including anterior cingulate, prelimbic, medial orbital and ventrolateral orbital cortex, whereas social play decreased c-fos expression in dorsolateral orbital cortex (Van Kerkhof et al., 2014). In addition, c-fos expression in these prefrontal regions correlated with the striatal regions to which they project, suggesting involvement of prefronto-striatal projections in the expression of social play behaviour. Interestingly, even though social play did not increase the expression of c-fos in the BLA, c-fos activity in the BLA after social play correlated with play-induced c-fos expression in ventral striatum, medial prefrontal cortex and orbitofrontal cortex. Thus, activity in amygdalo-striatal pathways, as well as in the reciprocal projections between the BLA and the frontal cortex, likely underlies the expression of social play behaviour (Van Kerkhof et al., 2014).
3.2 Striatum
In general terms, the striatum, as an integral part of the basal ganglia, subserves the generation of voluntary motor output on the basis of emotional and cognitive information (Cardinal et al., 2002; Mogenson et al., 1980; Voorn et al., 2004; Yin et al., 2008). It is therefore reasonable to assume an important role for striatal function in social play behaviour. Indeed, it has been shown in nonhuman primates that striatum size correlated with the rate of social play behaviour, but not non-playful social behaviour (Graham, 2011). Even though it is a relatively homogeneous structure in terms of cytoarchitecture, the striatum is anatomically and functionally heterogeneous (Alexander et al., 1986; Voorn et al., 2004; Yin et al., 2008; Zahm, 2000). In this regard, the striatum can be subdivided in several ways; the most widely used division is between a dorsal and a ventral part, often referred to as caudate-putamen and nucleus accumbens, respectively. The dorsal part is often subdivided into a medial and a lateral portion, and the nucleus accumbens into core and shell areas. That said, a gradual division from ventromedial (including nucleus accumbens shell and olfactory tubercle) to dorsolateral (nucleus accumbens core/dorsomedial striatum to dorsolateral striatum) is probably more valid on the basis of anatomical inputs and function (Voorn et al., 2004).
Consistent with the idea that the striatum, in particular its midbrain dopamine innervation, mediates the generation of appropriate motor output in social play, it was shown that neonatal lesioning of striatal dopamine resulted in animals showing shorter play bouts and truncated play sequences (Pellis et al., 1993). More recently, the involvement of different striatal subregions in social play was investigated using temporary pharmacological inactivation using either a combination of baclofen and muscimol or the AMPA receptor antagonist DNQX (Van Kerkhof et al., 2013a). These experiments demonstrated that inactivation of the nucleus accumbens core with baclofen/muscimol increased social play duration, whereas inactivation of the dorsomedial striatum with DNQX enhanced social play. These findings resonate well with the notion that inhibition of nucleus accumbens neuronal activity facilitates reward-related behaviour (Carlezon and Thomas, 2009; Taha and Fields, 2006), perhaps by disinhibition of downstream basal ganglia circuits. In addition, the dorsomedial striatum has been implicated in behavioural inhibition in laboratory tasks of impulsive behaviour (Eagle and Baunez, 2010), suggesting that pharmacologically attenuating dorsomedial striatal function may disinhibit social play.
Within the ventral striatum, dopamine, opioids and endocannabinoids act to modulate several aspects of reward-related behaviour (Berridge, 2007; Berridge and Kringelbach, 2015; Floresco, 2015; Kelley, 2004; Parsons and Hurd, 2015; Robbins and Everitt, 2007; Salamone and Correa, 2012). In general terms, it is thought that dopamine plays an important role in incentive salience and motivation, whereas opioids and endocannabinoids rather mediate the pleasurable aspects of rewards. Indeed, these neurotransmitter systems in the nucleus accumbens have also been implicated in social play. As summarized above, stimulating nucleus accumbens dopamine neurotransmission enhances social play, whereas blocking nucleus accumbens dopamine receptors reduces it, and attenuates the play-enhancing effects of systemic morphine (opioid) and URB597 (cannabinoid) treatment (Manduca et al., 2016). In addition, stimulation of nucleus accumbens mu-opioid receptors increases, and blocking these reduces social play behaviour, whereby the nucleus accumbens core and shell are comparably sensitive (Trezza et al., 2011b). Last, infusions of the anandamide hydrolysis inhibitor URB597 into the nucleus accumbens increase social play as well, and social play increases anandamide levels in the nucleus accumbens. However, blockade of nucleus accumbens CB1 receptors does not reduce social play, or alter the play-enhancing effect of systemic treatment with URB597 (Trezza et al., 2012). In contrast, the play-suppressant effects of the dopamine/noradrenaline reuptake inhibitor methylphenidate do not rely on the nucleus accumbens (Achterberg et al., 2015). Thus, the nucleus accumbens is an important site of action for opioids and dopamine to mediate the pleasurable and motivational properties of social play, whereas the effects of endocannabinoids on social play likely rely for a substantial part on brain regions outside the striatum (see below).
Consistent with a functional role for the striatum in social play as described above, social play-induced increases in c-fos expression were found in both dorsal and ventral striatum (Gordon et al., 2002; Van Kerkhof et al., 2014), and as mentioned earlier, the increases in striatum activity correlated with its prefrontal and amygdaloid inputs.
3.3 Amygdala
In situ hybridization experiments in rats showed that social play increased the expression of BDNF (Gordon et al., 2003), but not the immediate early gene c-fos (Gordon et al., 2002) in the entire amygdala. A more recent study analyzed social play-induced c-fos activity within the four subregions of the amygdala (van Kerkhof et al., 2014): after social play, increased c-fos expression was found in the lateral amygdala, but not in the central or medial amygdala or in the BLA (van Kerkhof et al., 2014). Interestingly, even though cellular activity within the BLA was not significantly increased after social play, it correlated with activity in its prefrontal and striatal afferents and efferents. Activity within the medial amygdala was also found to be correlated with activity within the medial prefrontal, orbital and agranular insular cortex (van Kerkhof et al., 2014).
Lesion studies confirmed the involvement of the amygdala in social play. Meaney and coworkers showed that juvenile (PND 21) electrolytic lesions of the amygdala reduced social play behaviour in male but not female rats (Meaney et al., 1981), suggesting that sex differences in the anatomy and physiology of the amygdala during development have a differential influence on the social play of male and female rats. Later studies extended this finding by showing that not only juvenile (PND 21) but also neonatal (PND 7) excitotoxic lesions of the amygdala reduced social play (Daenen et al., 2002; Wolterink et al., 2001). In the latter studies, only male rats were used, which leaves the question open as to whether these lesion effects are sex-dependent.
Studies that investigated the role of epigenetic proteins in sexually dimorphic behaviors support the notion that the amygdala is involved in sex differences in social play. For instance, neonatal (PND 0 – 2) suppression of the expression of the gene expression regulator methyl-CpG-binding protein 2 within the amygdala reduced social play behaviour on PND 25 – 29, but only in male rats (Kurian et al., 2008). Corepressors in the amygdala, that influence gene transcription through their association with histone deacetylase complexes, also have a role in the sexual differentiation of social play behavior. Indeed, reducing the expression of the nuclear receptor corepressor in the amygdala within the first 36 h of life allowed for further masculinization of social play behaviour, since it increased social play in male but not female rats (Jessen et al., 2010). These findings suggest that the nuclear receptor corepressor in the amygdala blunts sex differences in social play behaviour. The amygdala may also underlie the sexually dimorphic effects of prenatal inflammation in rats, since prenatal immune challenge with the bacterial endotoxin lipopolysaccharide reduced social play behaviour and vasopressin mRNA expression in the medial amygdala in male but not female offspring (Taylor et al., 2012).
The amygdala is an important brain site underlying cannabinoid and noradrenergic modulation of social play behaviour. Thus, social play increased the levels of the endocannabinoid anandamide in the amygdala, as well as the phosphorylation of CB1 cannabinoid receptors, which is thought to be a consequence of receptor activation. Furthermore, infusion of the anandamide hydrolysis inhibitor URB597 into the BLA increased social play, whereas the stimulating effect on social play induced by systemic treatment with URB597 was blocked by intra-BLA infusion of the CB1 cannabinoid receptor antagonist/inverse agonist SR141716A (Trezza et al., 2012). Conversely, infusion of the dopamine/noradrenaline reuptake inhibitor methylphenidate and the selective noradrenaline reuptake inhibitor atomoxetine into the BLA suppressed social play (Achterberg et al., 2015). In the BLA, noradrenaline reduces neuronal activity via α2-adrenoceptors (Buffalari and Grace, 2007; Ferry et al., 1997; Johnson et al., 2011). Since the play-suppressant effect of systemic administration of methylphenidate and atomoxetine are exerted through stimulation of α2-adrenoceptors (Vanderschuren et al., 2008), the inhibition of social play induced by intra-BLA administration of these drugs may be the result of reduced BLA output. As for the role of the amygdala in opioid modulation of social play, analysis of opioid receptor density showed that mu- (but not delta- or kappa-)opioid receptor binding was increased in the BLA after social isolation between PND 22 – 35 followed by five weeks of social housing (Van den Berg et al., 1999c). However, autoradiographic experiments reported no changes in opioid receptor binding in the amygdala upon social play (Vanderschuren et al., 1995d).
Together, these studies in rats emphasize the role of the amygdala in social play behaviour. In particular, the amygdala appears to be involved in sex differences in social play, and in cannabinoid and noradrenergic modulation of this behaviour. Moreover, the size of the amygdala in nonhuman primates has been correlated with the presence of social play behaviour both in non-sexual (Lewis and Barton, 2006) and sexual (Pellis and Iwaniuk, 2002) contexts, with a larger size being associated with more time spent on play behaviour. On the basis of these studies, it has been proposed that social play helps to develop amygdala-mediated behaviours, such as social assessment, social recognition and response to facial expression (Lewis and Barton, 2006).
3.4 Habenula, thalamus and hypothalamus
In recent years, the role of the habenula in social play has received a great deal of attention, since this brain structure regulates monoaminergic neurotransmission (Lecourtier and Kelly, 2007) and it is involved in reward and cognitive processes (Lecourtier and Kelly, 2007; Proulx et al., 2014). In situ hybridization experiments in rats showed that short-term social isolation (up to 24 hr) increased c-fos expression within the habenula, and that a subsequent episode of social play reduced this activity within the medial sector of the lateral habenula (van Kerkhof et al., 2013b). These results suggest that the habenula mediates the negative emotional aspects of social isolation, which is mitigated by the opportunity for subsequent social play. Furthermore, pharmacological inactivation of the habenula with a mixture of the GABA receptor agonists baclofen and muscimol resulted in a reduction in social play behaviour. Interestingly, different effects were observed on play solicitation (i.e. pouncing) and on the response to solicitation (i.e. pinning), with the latter being more sensitive to habenula inactivation than the former (van Kerkhof et al., 2013b). Therefore, the habenula appears to be particularly involved in the responsiveness to play solicitation.
We have recently shown that the habenula is also involved in the play suppressant effects of methylphenidate and atomoxetine. Indeed, both drugs reduced social play when infused into the habenula (Achterberg et al., 2015). Two possible mechanisms may explain these effects: functional inhibition of the habenula by methylphenidate and atomoxetine may have reduced the positive emotional properties of social play, or may have influenced cognitive aspects of social play, perhaps in concert with prefrontal regions (Achterberg et al., 2015).
Regarding the role of diencephalic brain structures in social play, lesions of dorsomedial thalamus (DMT) or parafascicular area of the thalamus (PFA) reduced the frequency of pinning, with the magnitude of the decrease being larger following PFA damage (Siviy and Panksepp, 1985). Lesions of the PFA also reduced play solicitation behaviours and made rats insensitive to the play-modulating effects of naloxone and morphine (Siviy and Panksepp, 1985). Conversely, animals with lesions of the DMT did not show changes in play solicitation and were sensitive to the play-enhancing effects of morphine, but not to the play-suppressive effects of naloxone (Siviy and Panksepp, 1985). Additional evidence that the thalamus is involved in opioid modulation of social play comes from autoradiographic experiments in rats, that showed social play-induced changes in opioid receptor binding in the dorsolateral and paratenial thalamic nuclei (Vanderschuren et al., 1995d).
Lewis and Barton found a correlation between social play and the volume of the hypothalamus in nonhuman primates (Lewis and Barton, 2006). The hypothalamus becomes sexually differentiated by gonadal hormones during the perinatal period, giving rise to sexually differentiated behaviours (Negri-Cesi et al., 2008; Swaab et al., 2002). Thus, the hypothalamus may be involved in sex differences in social play. Studies in rodents support this possibility. For instance, Paul and coworkers found a positive correlation between social play and vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus (PVN) of male but not female rats (Paul et al., 2014). Furthermore, Madden and Zup (2014) reported that the reduction in social play behaviour induced by perinatal hyperserotonemia was associated with alterations in the number of oxytocinergic cells in the PVN of the female offspring only. Together, these studies indicate that neuropeptides in the PVN regulate the development of social play in a sexually dimorphic manner. Similarly to the thalamus, opioid neurotransmission in the hypothalamus is involved in social play behaviour. Social play-induced changes in opioid receptor binding in the paraventricular and arcuate nuclei of the hypothalamus have been reported (Vanderschuren et al., 1995d). Collectively, these studies indicate that both thalamic and hypothalamic brain areas are involved in social play behaviour.
4. Discussion
Social play is being increasingly recognized as an important social activity for humans and animals alike. It is intrinsically rewarding, supports social bonding and in the long run facilitates social, cognitive and emotional development (e.g. Bateson, 2015; Burghardt, 2010; Pellis and Pellis, 2009; Vanderschuren and Trezza, 2014). It is therefore of great importance to understand the brain mechanisms underlying social play behaviour, not least because social deficits in child and adolescent psychiatric disorders remain as yet very difficult to treat. In the present review, we have summarized the neural underpinnings of social play behaviour in rats, with a particular focus on that aspect that spurs the immediate expression of this behaviour: its rewarding, pleasurable properties (Vanderschuren, 2010).
The studies into the neural mechanisms of social play have identified the nucleus accumbens as an important region through which dopamine may mediate the motivation for play, and opioids and perhaps endocannabinoids modulate its pleasurable effects (Manduca et al., 2016; Trezza et al., 2011b; Trezza et al., 2012). Importantly, pharmacological studies have demonstrated functional interactions between opioid, endocannabinoid and dopamine neurotransmission in the modulation of social play behaviour (Manduca et al., 2016; Trezza and Vanderschuren, 2008a), providing evidence to support the notion that pleasurable and motivational properties are entwined during the proper expression of this behaviour. In addition, the BLA is involved in the endocannabinoid modulation of social play (Trezza et al., 2012), suggesting that functional interactions between the BLA and the nucleus accumbens (Cardinal et al., 2002; Janak and Tye, 2015; Voorn et al., 2004) are likely involved in the generation of social play (Van Kerkhof et al., 2014). Noradrenergic neurotransmission as well as prefrontal cortex activity have been implicated in cognitive aspects of social play (Achterberg et al., 2015; Bell et al., 2009; Pellis et al., 2006; Van Kerkhof et al., 2013a), although the exact cognitive functions that are supported by these mechanisms in the context of social play remain to be identified. Together, the studies discussed in the present review show that social play behaviour is mediated by a distributed network of corticolimbic structures and their opioid, dopaminergic, endocannabinoid and noradrenergic innervation, that integrates the cognitive and emotional aspects of play into coherent behavioural output.
These data summarized here show that the last two or three decades have witnessed a profound increase in our understanding of the neural mechanisms of social play behaviour (Siviy and Panksepp, 2011; Trezza et al., 2010; Vanderschuren and Trezza, 2014). Even so, compared to food or drug reward, the knowledge of the playful brain is still quite limited, and much remains to be gained in this field. Arguably, most progress has been made using neural manipulation techniques, such as intracranial drug infusions, that directly tax brain processes involved in this behaviour. Indeed, the recent application of online measurement techniques of brain chemistry, using for example in vivo microdialysis (Bredewold et al., 2015; Robinson et al., 2011), holds considerable promise to increase knowledge in this area. In addition, the application and refinement of place and operant conditioning methods (Achterberg et al., 2016a; Achterberg et al., 2016b; Calcagnetti and Schechter, 1992; Thiel et al., 2008; Trezza et al., 2011a), to investigate the pleasurable and motivational properties of social play, as well as certain cognitive processes involved in this behaviour (Achterberg et al., 2012; Achterberg et al., 2014b), have allowed for the explicit dissection of neural mechanisms underlying sub-components of social play. Although these models have helped us study social reward mechanisms (e.g. Trezza et al., 2011b), they have also pointed us to potential drawbacks. In our own experience, this has been most prominent in pharmacological studies on social play-induced CPP, in particular when drugs are used that possess rewarding or aversive properties themselves. In this case, proper care should be taken to make sure that positive or negative affective effects of the drugs themselves do not occlude the interpretation of the experimental findings. This can, for example, be done by comparing CPP scores between drug-plus-play versus drug-only treatment groups. Moreover, in the case that drugs are used that by themselves influence the expression of social play behaviour, one must be aware that a reduction in CPP can be the result of reduced rewarding effects of social play, but also of a mere decrease in the amount of social play displayed, which may have reduced the pleasurable aspects of the social episode. Ideally, social play should therefore be quantified during conditioning (e.g. Thiel et al., 2008; Fig. 1), albeit that this is very labour intensive. A particularly challenging thought is whether we will be able to model cognitive aspects of social play in more detail. Clearly, place and operant conditioning models can be used to study processes related to learning and memory in the context of social play (Achterberg et al., 2012; Achterberg et al., 2014b), whereby it will be of interest to investigate whether and how play-associated cues influence behaviour, using for example second-order conditioning (Everitt and Robbins, 2000), as has in the past been done for sexual behaviour (Everitt, 1990). In addition, pertinent study of the processes related to, for example, social perception, behavioural flexibility, social cooperation and intentionality (Adolphs, 2010; Frith and Frith, 2012) will help us to understand further how social play behaviour comes about.
Several other important questions remain. First, the neural mechanisms of social play summarized here are reminiscent of those that are thought mediate other natural rewards, such as feeding and sex, and that are hijacked by artificial rewards such as drugs of abuse (Everitt and Robbins, 2016; Kelley et al., 2005; Kelley and Berridge, 2002; Nesse and Berridge, 1997; Volkow and Morales, 2015). It remains to be demonstrated therefore, which mechanisms in the brain support those aspects of social play that are specific to this behaviour, and that set it apart from ingesting (palatable) food, copulation or using psychoactive drugs. As alluded to above, these may include functions related to social perception and cooperation, but also cognitive functions such as impulse control, decision making and flexibility (Adolphs, 2010; Frith and Frith, 2012). Interestingly, whereas it is reasonable to assume that the latter three cognitive functions need to be optimally aligned for the proper performance of social play, it is their breakdown that is thought to play a prominent role in the pathophysiology of drug addiction (De Wit, 2009; Goldstein and Volkow, 2011; Jentsch and Taylor, 1999; Perry and Carroll, 2008; Verdejo-García et al., 2008). This provides a theoretical, as well as a neural basis to dissociate adaptive social from maladaptive addictive behaviour. A second issue that is relatively unexplored is the sex differences in social play behaviour. Thus, differences in the structure and intensity of social play in male and female rats have been well documented (Pellis et al., 1997). Moreover, when one accepts that social play facilitates social, emotional and cognitive development, and that male and female animals require distinct (but likely overlapping) social, emotional and cognitive skills in adulthood, then it follows that the neural mechanisms of social play should diverge between male and female animals. Yet, apart perhaps from amygdala function in social play (Kurian et al., 2008; Meaney et al., 1981), effects of alcohol (Varlinskaya et al., 2015) and the role of vasopressin and oxytocin (Bredewold et al., 2014; Veenema et al., 2013), neural underpinnings of sex differences (whereby commonalities between males and females are equally important) have not been studied in detail. One last important question, that partially relates to the previous one, is whether the neural mechanisms of social play behaviour are distinct between species. Indeed, the social, emotional and cognitive needs during adult life profoundly differ between animal species, and the structure and function of social play does so too (Biben, 2002; Graham and Burghardt, 2010; Palagi et al., 2016; Pellis and Pellis, 1998). Hence, the neural underpinnings of social play are also likely to diverge between species, although it is, of course, likely that certain aspects of play related to perception, cognition and reward may rely on common brain mechanisms. As most of our knowledge on the neural underpinnings of social play comes from rat studies (see e.g. Cheng et al., 2008; Colonnello et al., 2011; Guard et al., 2002, for exceptions), it is important to investigate whether social play in other species is supported by comparable or distinct brain mechanisms.
In conclusion, our understanding of the brain processes underlying social play behaviour has markedly increased in recent years. Even though much remains to be investigated, the corticolimbic, opioid, dopamine, endocannabinoid and noradrenergic mechanisms that have been implicated in social play start to paint a picture of how this developmentally important, highly rewarding behaviour is mediated. We are confident that further study into this topic will increase our understanding of how the brain processes positive social signals to generate appropriate social behaviour, how social play contributes to the development of brain and behaviour, and how stimulation of social play can increase health and welfare of humans and animals.
Highlights.
-
-
Social play behaviour is a highly rewarding social activity abundant in young animals
-
-
Social play behaviour facilitates the development of brain and behaviour
-
-
The rewarding properties of social play can be studied using pertinent paradigms
-
-
Social play behaviour is mediated by a distributed network of corticolimbic structures
-
-
Opioids, dopamine and endocannabinoids interact in the modulation of social play
Acknowledgements
We would like to dedicate this manuscript to the memory of Professor Frauke Ohl, chair of the Department of Animals in Science and Society, who passed away on 28 January 2016. We remember her as an inspiring colleague, mentor, and friend. Our work on social play behaviour during the last decade has been generously supported by the National Institute on Drug Abuse (R01 DA022628 to L.J.M.J.V.), the Netherlands Organization for Scientific Research (NWO; Veni grant 91611052 to V.T.), the European Union (Marie Curie Career Reintegration Grant PCIG09-GA-2011-293589 to V.T.) and the Dutch Top Institute Pharma (project T5-107). We thank Mandy Aalderink for assistance with the experiments shown in Figure 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Achterberg EJM, Trezza V, Siviy SM, Schrama L, Schoffelmeer ANM, Vanderschuren LJMJ. Amphetamine and cocaine suppress social play behavior in rats through distinct mechanisms. Psychopharmacology. 2014a;231:1503–1515. doi: 10.1007/s00213-013-3272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterberg EJM, Trezza V, Vanderschuren LJMJ. Beta-adrenoreceptor stimulation mediates reconsolidation of social reward-related memories. PLoS One. 2012;7:e39639. doi: 10.1371/journal.pone.0039639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterberg EJM, Trezza V, Vanderschuren LJMJ. Glucocorticoid receptor antagonism disrupts reconsolidation of social reward-related memories in rats. Behav. Pharmacol. 2014b;25:216–225. doi: 10.1097/FBP.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterberg EJM, van Swieten MMH, Driel NV, Trezza V, Vanderschuren LJMJ. Dissociating the role of endocannabinoids in the pleasurable and motivational properties of social play behaviour in rats. Pharmacol. Res. 2016b;110:151–158. doi: 10.1016/j.phrs.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterberg EJM, Van Kerkhof LWM, Damsteegt R, Trezza V, Vanderschuren LJMJ. Methylphenidate and atomoxetine inhibit social play behavior through prefrontal and subcortical limbic mechanisms in rats. J. Neurosci. 2015;35:161–169. doi: 10.1523/JNEUROSCI.2945-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achterberg EJM, Van Kerkhof LWM, Servadio M, Van Swieten MMH, Houwing DJ, Aalderink M, Driel NV, Trezza V, Vanderschuren LJMJ. Contrasting roles of dopamine and noradrenaline in the motivational properties of social play behavior in rats. Neuropsychopharmacology. 2016a;41:858–868. doi: 10.1038/npp.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–767. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: biology and function. Ann. Rev. Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Alessandri SM. Attention, play, and social behavior in ADHD preschoolers. J. Abnorm. Child Psychol. 1992;20:289–302. doi: 10.1007/BF00916693. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baarendse PJJ, Counotte DS, O'Donnell P, Vanderschuren LJMJ. Early social experience is critical for the development of cognitive control and dopamine modulation of prefrontal cortex function. Neuropsychopharmacology. 2013;38:1485–1494. doi: 10.1038/npp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baarendse PJJ, Limpens JHW, Vanderschuren LJMJ. Disrupted social development enhances the motivation for cocaine. Psychopharmacology. 2014;231:1695–1704. doi: 10.1007/s00213-013-3362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology. 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bari M, Battista N, Fezza F, Gasperi V, Maccarrone M. New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev. Med. Chem. 2006;6:257–268. doi: 10.2174/138955706776073466. [DOI] [PubMed] [Google Scholar]
- Bateson P. Playfulness and creativity. Curr. Biol. 2015;25:R12–R16. doi: 10.1016/j.cub.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Costello KB. Naloxone and play fighting in juvenile rats. Pharmacol. Biochem. Behav. 1982;17:905–907. doi: 10.1016/0091-3057(82)90470-1. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Costello KB, Berry SL. Suppression of play fighting by amphetamine: effects of catecholamine antagonists, agonists and synthesis inhibitors. Pharmacol. Biochem. Behav. 1984;20:747–755. doi: 10.1016/0091-3057(84)90194-1. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Dodge AM, Dodge LJ, Panksepp J. Psychomotor stimulants, social deprivation and play in juvenile rats. Pharmacol. Biochem. Behav. 1982;16:417–422. doi: 10.1016/0091-3057(82)90445-2. [DOI] [PubMed] [Google Scholar]
- Bell HC, McCaffrey DR, Forgie ML, Kolb B, Pellis SM. The role of the medial prefrontal cortex in the play fighting of rats. Behav. Neurosci. 2009;123:1158–1168. doi: 10.1037/a0017617. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biben M. Squirrel monkey play fighting: making the case for a cognitive training function for play. In: Bekoff M, Byers JA, editors. Animal play. Cambridge University Press; Cambridge: 2002. pp. 161–180. [Google Scholar]
- Blanchard RJ, Blanchard DC. Aggressive behavior in the rat. Behav. Biol. 1977;21:197–224. doi: 10.1016/s0091-6773(77)90308-x. [DOI] [PubMed] [Google Scholar]
- Bouret S, Richmond BJ. Sensitivity of locus ceruleus neurons to reward value for goal-directed actions. J. Neurosci. 2015;35:4005–4014. doi: 10.1523/JNEUROSCI.4553-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Bock J. The experience-dependent maturation of prefronto-limbic circuits and the origin of developmental psychopathology: implications for the pathogenesis and therapy of behavioural disorders. Dev. Med. Child. Neurol. 2011;53(Suppl. 4):14–18. doi: 10.1111/j.1469-8749.2011.04056.x. [DOI] [PubMed] [Google Scholar]
- Bredewold R, Schiavo JK, van der Hart M, Verreij M, Veenema AH. Dynamic changes in extracellular release of GABA and glutamate in the lateral septum during social play behavior in juvenile rats: Implications for sex-specific regulation of social play behavior. Neuroscience. 2015;307:117–127. doi: 10.1016/j.neuroscience.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredewold R, Smith CJW, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front. Behav. Neurosci. 2014;8:216. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J. Neurosci. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt GM. The comparative reach of play and brain: perspective, evidence, and implications. Am. J. Play. 2010;2:338–356. [Google Scholar]
- Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends Cogn. Sci. 2009;13:447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social interaction in juvenile rats. Physiol. Behav. 1992;51:667–672. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Camacho F, Sandoval C, Paredes R. Sexual experience and conditioned place preference in male rats. Pharmacol. Biochem. Behav. 2004;78:419–425. doi: 10.1016/j.pbb.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl. 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S-Y, Delville Y. Vasopressin facilitates play fighting in juvenile golden hamsters. Physiol Behav. 2009;98:242–246. doi: 10.1016/j.physbeh.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Cheng S-Y, Taravosh-Lahn K, Delville Y. Neural circuitry of play fighting in golden hamsters. Neuroscience. 2008;156:247–256. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Colonnello V, Iacobucci P, Fuchs T, Newberry RC, Panksepp J. Octodon degus. A useful animal model for social-affective neuroscience research: basic description of separation distress, social attachments and play. Neurosci. Biobehav. Rev. 2011;35:1854–1863. doi: 10.1016/j.neubiorev.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Philos. Transact. Ser. A Math. Phys. Eng Sci. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav. Brain Res. 2002;136:571–582. doi: 10.1016/s0166-4328(02)00223-1. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Davis H, Perusse R. Human-based social interaction can reward a rat's behavior. Animal Learn. Behav. 1988;16:89–92. [Google Scholar]
- De Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci. Biobehav. Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716 A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology. 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev. Psychobiol. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ, Kibbler CC. Brief periods of socialization and later behavior in the rat. Dev. Psychobiol. 1978;11:213–225. doi: 10.1002/dev.420110305. [DOI] [PubMed] [Google Scholar]
- El Rawas R, Saria A. The two faces of social interaction reward in animal models of drug dependence. Neurochem. Res. 2016;41:492–499. doi: 10.1007/s11064-015-1637-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol. Biochem. Behav. 1999;64:507–512. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci. Biobehav. Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology. 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 2016;67:1–28. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): II. Effects of preoptic area lesions, castration, and testosterone. J. Comp. Psychol. 1987;101:407–419. [PubMed] [Google Scholar]
- Fattore L, Melis M, Fadda P, Pistis M, Fratta W. The endocannabinoid system and nondrug rewarding behaviours. Exp. Neurol. 2010;224:23–36. doi: 10.1016/j.expneurol.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Fagen R. Animal play behavior. Oxford University Press; Oxford: 1981. [Google Scholar]
- Ferguson SA, Frisby NB, Ali SF. Acute effects of cocaine on play behaviour of rats. Behav. Pharmacol. 2000;11:175–179. doi: 10.1097/00008877-200004000-00010. [DOI] [PubMed] [Google Scholar]
- Ferry B, Magistretti PJ, Pralong E. Noradrenaline modulates glutamate-mediated neurotransmission in the rat basolateral amygdala in vitro. Eur. J. Neurosci. 1997;9:1356–1364. doi: 10.1111/j.1460-9568.1997.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB. Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions. Front. Neurosci. 2013;7:62. doi: 10.3389/fnins.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu. Rev. Psychol. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Mechanisms of social cognition. Annu. Rev. Psychol. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- Gamaleddin IH, Trigo JM, Gueye AB, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. Role of the endogenous cannabinoid system in nicotine addiction: novel insights. Front. Psychiatry. 2015;6:41. doi: 10.3389/fpsyt.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol. Biochem. Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Genn RF, Tucci S, Marco EM, Viveros MP, File SE. Unconditioned and conditioned anxiogenic effects of the cannabinoid receptor agonist CP 55,940 in the social interaction test. Pharmacol. Biochem. Behav. 2004;77:567–573. doi: 10.1016/j.pbb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methylphenidate administration: a microdialysis and locomotor activity study. J. Pharmacol. Exp. Ther. 2000;295:51–57. [PubMed] [Google Scholar]
- Ginsburg KR, Committee on Communications and the Committee on Psychosocial Aspects of Child and Family Health The importance of play in promoting healthy child development and maintaining strong parent-child bonds. Pediatrics. 2007;119:182–191. doi: 10.1542/peds.2006-2697. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon NS, Burke S, Akil H, Watson SJ, Panksepp J. Socially-induced brain `fertilization': play promotes brain derived neurotrophic factor transcription in the amygdala and dorsolateral frontal cortex in juvenile rats. Neurosci. Lett. 2003;341:17–20. doi: 10.1016/s0304-3940(03)00158-7. [DOI] [PubMed] [Google Scholar]
- Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res. Bull. 2002;57:651–659. doi: 10.1016/s0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- Graham KL. Coevolutionary relationship between striatum size and social play in nonhuman primates. Am. J. Primatol. 2011;73:314–322. doi: 10.1002/ajp.20898. [DOI] [PubMed] [Google Scholar]
- Graham KL, Burghardt GM. Current perspectives on the biological study of play: signs of progress. Q. Rev. Biol. 2010;85:393–418. doi: 10.1086/656903. [DOI] [PubMed] [Google Scholar]
- Groos K. The play of animals. D. Appleton; New York: 1898. [Google Scholar]
- Grotewold SK, Wall VL, Goodell DJ, Hayter C, Bland ST. Effects of cocaine combined with a social cue on conditioned place preference and nucleus accumbens monoamines after isolation rearing in rats. Psychopharmacology. 2014;231:3041–3053. doi: 10.1007/s00213-014-3470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard HJ, Newman JD, Roberts RL. Morphine administration selectively facilitates social play in common marmosets. Dev. Psychobiol. 2002;41:37–49. doi: 10.1002/dev.10043. [DOI] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology. 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso- ventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Held SDE, Špinka M. Animal play and animal welfare. Anim. Behav. 2011;81:891–899. [Google Scholar]
- Helgeland MI, Torgersen S. Stability and prediction of schizophrenia from adolescence to adulthood. Eur. Child Adolesc. Psychiatry. 2005;14:83–94. doi: 10.1007/s00787-005-0436-0. [DOI] [PubMed] [Google Scholar]
- Himmler BT, Pellis VC, Pellis SM. Peering into the dynamics of social interactions: measuring play fighting in rats. J. Vis. Exp. 2013;71:e4288. doi: 10.3791/4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:934–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Holloway WR, Jr., Thor DH. Interactive effects of caffeine, 2-chloroadenosine and haloperidol on activity, social investigation and play fighting of juvenile rats. Pharmacol. Biochem. Behav. 1985;22:421–426. doi: 10.1016/0091-3057(85)90043-7. [DOI] [PubMed] [Google Scholar]
- Huizinga J. Homo ludens: A study of the play-element in culture. Routledge & Kegan; London: 1949. [Google Scholar]
- Humphreys AP, Einon DF. Play as a reinforcer for maze-learning in juvenile rats. Anim. Behav. 1981;29:259–270. [Google Scholar]
- Ikemoto S, Panksepp J. The effects of early social isolation on the motivation for social play in juvenile rats. Dev. Psychobiol. 1992;25:261–274. doi: 10.1002/dev.420250404. [DOI] [PubMed] [Google Scholar]
- Jalowiec JE, Calcagnetti DJ, Fanselow MS. Suppression of juvenile social behavior requires antagonism of central opioid systems. Pharmacol. Biochem. Behav. 1989;33:697–700. doi: 10.1016/0091-3057(89)90409-7. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm. Behav. 2003;43:503–507. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Jessen HM, Kolodkin MH, Bychowski ME, Auger CJ, Auger AP. The nuclear receptor corepressor has organizational effects within the developing amygdala on juvenile social play and anxiety-like behavior. Endocrinology. 2010;151:1212–1220. doi: 10.1210/en.2009-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LR, Hou M, Prager EM, Ledoux JE. Regulation of the Fear Network by Mediators of Stress: Norepinephrine Alters the Balance between Cortical and Subcortical Afferent Excitation of the Lateral Amygdala. Front. Behav. Neurosci. 2011;5:23. doi: 10.3389/fnbeh.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. The Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Jordan R. Social play and autistic spectrum disorders. Autism. 2003;7:347–360. doi: 10.1177/1362361303007004002. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci. Biobehav. Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J. Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisko TM, Euston DR, Pellis SM. Are 50-khz calls used as play signals in the playful interactions of rats? III. The effects of devocalization on play with unfamiliar partners as juveniles and as adults. Behav. Processes. 2015;113:113–121. doi: 10.1016/j.beproc.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol. Bull. 2002;128:961–977. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Kummer K, Klement S, Eggart V, Mayr MJ, Saria A, Zernig G. Conditioned place preference for social interaction in rats: contribution of sensory components. Front. Behav. Neurosci. 2011;5:80. doi: 10.3389/fnbeh.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J. Neurosci. 2008;28:7137–7142. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis GP, Panksepp JB, Kennedy BC, Wilson CR, Merriman DK. Social conditioned place preference in the captive ground squirrel (Ictidomys Tridecemlineatus): Social reward as a natural phenotype. J. Comp. Psychol. 2015;129:291–303. doi: 10.1037/a0039435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci. Biobehav. Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav. Brain Res. 1999;100:15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JAJ, Befort K, Kieffer B. Reward processing by the opioid system in the brain. Physiol. Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesscher HMB, Spoelder M, Rotte MD, Janssen MJ, Hesseling P, Lozeman-van 't Klooster JG, Baars AM, Vanderschuren LJMJ. Early social isolation augments alcohol consumption in rats. Behav. Pharmacol. 2015;26:673–680. doi: 10.1097/FBP.0000000000000165. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Lewis KP, Barton RA. Amygdala size and hypothalamus size predict social play frequency in nonhuman primates: a comparative analysis using independent contrasts. J. Comp. Psychol. 2006;120:31–37. doi: 10.1037/0735-7036.120.1.31. [DOI] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X, Burston JJ, Sim-Selley LJ, Lichtman AH, Wiley JL, Cravatt BF. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc. Natl. Acad. Sci. USA. 2009;106:20270–20275. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loureiro M, Renard J, Zunder J, Laviolette SR. Hippocampal cannabinoid transmission modulates dopamine neuron activity: impact on rewarding memory formation and social interaction. Neuropsychopharmacology. 2015;40:1436–1447. doi: 10.1038/npp.2014.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes JL, Mokin MV, Scholl JL, Forster GL. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm. Behav. 2009;55:248–256. doi: 10.1016/j.yhbeh.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Lutz B. Endocannabinoid signals in the control of emotion. Curr. Opin. Pharmacol. 2009;9:46–52. doi: 10.1016/j.coph.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Madden AM, Zup SL. Effects of developmental hyperserotonemia on juvenile play behavior, oxytocin and serotonin receptor expression in the hypothalamus are age and sex dependent. Physiol. Behav. 2014;128:260–269. doi: 10.1016/j.physbeh.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Manduca A, Campolongo P, Palmery M, Vanderschuren LJMJ, Cuomo V, Trezza V. Social play behavior, ultrasonic vocalizations and their modulation by morphine and amphetamine in Wistar and Sprague-Dawley rats. Psychopharmacology. 2014a;231:1661–1673. doi: 10.1007/s00213-013-3337-9. [DOI] [PubMed] [Google Scholar]
- Manduca A, Morena M, Campolongo P, Servadio M, Palmery M, Trabace L, Hill MN, Vanderschuren LJMJ, Cuomo V, Trezza V. Distinct roles of the endocannabinoids anandamide and 2-arachidonoylglycerol in social behavior and emotionality at different developmental ages in rats. Eur. Neuropsychopharmacol. 2015;25:1362–1374. doi: 10.1016/j.euroneuro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Manduca A, Servadio M, Campolongo P, Palmery M, Trabace L, Vanderschuren LJMJ, Cuomo V, Trezza V. Strain- and context-dependent effects of the anandamide hydrolysis inhibitor URB597 on social behavior in rats. Eur. Neuropsychopharmacol. 2014b;24:1337–1348. doi: 10.1016/j.euroneuro.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Manduca A, Servadio M, Damsteegt R, Campolongo P, Vanderschuren LJMJ, Trezza V. Dopaminergic neurotransmission in the nucleus accumbens modulates social play behavior in rats. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.22. doi: 10.1038/npp.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Rapino C, Caprioli A, Borsini F, Maccarrone M, Laviola G. Social encounter with a novel partner in adolescent rats: activation of the central endocannabinoid system. Behav. Brain Res. 2011;220:140–145. doi: 10.1016/j.bbr.2011.01.044. [DOI] [PubMed] [Google Scholar]
- Martin P, Caro TM. On the functions of play and its role in behavioral development. Adv. Study Behav. 1985;15:59–103. [Google Scholar]
- Martinez M, Guillen-Salazar F, Salvador A, Simon VM. Successful intermale aggression and conditioned place preference in mice. Physiol. Behav. 1995;58:323–328. doi: 10.1016/0031-9384(95)00061-m. [DOI] [PubMed] [Google Scholar]
- Mason WA, Hollis JH, Sharpe LG. Differential responses of chimpanzees to social stimulation. J. Comp. Physiol. Psychol. 1962;55:1105–1110. [Google Scholar]
- Mason WA, Saxon SV, Sharpe LG. Preferential responses of young chimpanzees to food and social rewards. Psychol. Rec. 1963;13:341–345. [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav. Neurosci. 2001;115:683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur. J. Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Dodge AM, Beatty WW. Sex-dependent effects of amygdaloid lesions on the social play of prepubertal rats. Physiol. Behav. 1981;26:467–472. doi: 10.1016/0031-9384(81)90175-x. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Hanus LO, Pertwee R, Howlett AC. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 2014;15:757–764. doi: 10.1038/nrn3811. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Møller P, Husby R. The initial prodrome in schizophrenia: searching for naturalistic core dimensions of experience and behavior. Schizophrenia Bull. 2000;26:217–232. doi: 10.1093/oxfordjournals.schbul.a033442. [DOI] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, Spear LP. Low doses of the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, induces social facilitation in adolescent male rats. Behav Brain Res. 2013;250:18–22. doi: 10.1016/j.bbr.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. Eur J Neurosci. 2003;18:3367–74. doi: 10.1111/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat. Rev. Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology. 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, Colciago A, Pravettoni A, Casati L, Conti L, Celotti F. Sexual differentiation of the rodent hypothalamus: hormonal and environmental influences. J. Steroid. Biochem. Mol. Biol. 2008;109:294–299. doi: 10.1016/j.jsbmb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278:63–66. doi: 10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- Niesink RJM, Van Ree JM. Involvement of opioid and dopaminergic systems in isolation- induced pinning and social grooming of young rats. Neuropharmacology. 1989;28:411–418. doi: 10.1016/0028-3908(89)90038-5. [DOI] [PubMed] [Google Scholar]
- Normansell L, Panksepp J. Effects of clonidine and yohimbine on the social play of juvenile rats. Pharmacol. Biochem. Behav. 1985;22:881–883. doi: 10.1016/0091-3057(85)90540-4. [DOI] [PubMed] [Google Scholar]
- Normansell L, Panksepp J. Effects of morphine and naloxone on play-rewarded spatial discrimination in juvenile rats. Dev. Psychobiol. 1990;23:75–83. doi: 10.1002/dev.420230108. [DOI] [PubMed] [Google Scholar]
- O'Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J. Psychopharmacol. 2006;20:611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- Palagi E, Burghardt GM, Smuts B, Cordoni G, Dall'Olio S, Fouts HN, Rehakova-Petru M, Siviy SM, Pellis SM. Rough-and-tumble play as a window on animal communication. Biol. Rev. 2016;91:311–327. doi: 10.1111/brv.12172. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The ontogeny of play in rats. Dev. Psychobiol. 1981;14:327–332. doi: 10.1002/dev.420140405. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Beatty WW. Social deprivation and play in rats. Behav. Neural Biol. 1980;30:197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskenazi FG. Endogenous opioids and social behavior. Neurosci. Biobehav. Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Jalowiec JE, DeEskenazi FG, Bishop P. Opiates and play dominance in juvenile rats. Behav. Neurosci. 1985;99:441–453. doi: 10.1037//0735-7044.99.3.441. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Normansell L, Cox JF, Siviy SM. Effects of neonatal decortication on the social play of juvenile rats. Physiol. Behav. 1994;56:429–443. doi: 10.1016/0031-9384(94)90285-2. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Siviy SM, Normansell L. The psychobiology of play: theoretical and methodological perspectives. Neurosci. Biobehav. Rev. 1984;8:465–492. doi: 10.1016/0149-7634(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015;16:579–594. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul MJ, Terranova JI, Probst CK, Murray EK, Ismail NI, de Vries GJ. Sexually dimorphic role for vasopressin in the development of social play. Front. Behav. Neurosci. 2014;8:58. doi: 10.3389/fnbeh.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pava MJ, Woodward JJ. A review of the interactions between alcohol and the endocannabinoid system: implications for alcohol dependence and future directions for research. Alcohol. 2012;46:185–204. doi: 10.1016/j.alcohol.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peartree NA, Hood LE, Thiel KJ, Sanabria F, Pentkowski NS, Chandler KN, Neisewander JL. Limited physical contact through a mesh barrier is sufficient for social reward-conditioned place preference in adolescent male rats. Physiol. Behav. 2012;105:749–756. doi: 10.1016/j.physbeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis SM. Agonistic versus amicable targets of attack and defense: consequences for the origin, function and descriptive classification of play-fighting. Aggr. Behav. 1988;14:85–104. [Google Scholar]
- Pellis SM, Castañeda E, McKenna MM, Tran-Nguyen LTL, Whishaw IQ. The role of the striatum in organizing sequences of play fighting in neonatally dopamine-depleted rats. Neurosci. Lett. 1993;158:13–15. doi: 10.1016/0304-3940(93)90600-p. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Field EF, Smith LK, Pellis VC. Multiple differences in the play fighting of male and female rats: implications for the causes and functions of play. Neurosci. Biobehav. Rev. 1997;21:105–120. doi: 10.1016/0149-7634(95)00060-7. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Hastings E, Shimizu T, Kamitakahara H, Komorowska J, Forgie ML, Kolb B. The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and nonplayful social contexts. Behav. Neurosci. 2006;120:72–84. doi: 10.1037/0735-7044.120.1.72. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Iwaniuk AN. Brain system size and adult-adult play in primates: a comparative analysis of the roles of the non-visual neocortex and the amygdala. Behav. Brain Res. 2002;134:31–39. doi: 10.1016/s0166-4328(01)00455-7. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggr. Behav. 1987;13:227–242. [Google Scholar]
- Pellis SM, Pellis VC. Differential rates of attack, defense, and counterattack during the developmental decrease in play fighting by male and female rats. Dev. Psychobiol. 1990;23:215–231. doi: 10.1002/dev.420230303. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. Play fighting of rats in comparative perspective: a schema for neurobehavioral analyses. Neurosci. Biobehav. Rev. 1998;23:87–101. doi: 10.1016/s0149-7634(97)00071-7. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC. The playful brain: venturing to the limits of neuroscience. Oneworld Publications; Oxford: 2009. [Google Scholar]
- Perrin G, Ferreira G, Meurisse M, Mouly A, Levy F. Social recognition memory requires protein synthesis after retrieval. Behav. Neurosci. 2007;121:148–155. doi: 10.1037/0735-7044.121.1.148. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr. Opin. Investig. Drugs. 2010;11:51–62. [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Poole TB, Fish J. An investigation of playful behavior in Rattus norvegicus and Mus musculus (Mammalia) J. Zool. Lond. 1975;175:61–71. [Google Scholar]
- Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat. Neurosci. 2014;17:1146–1152. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart CJ, McIntyre DC, Metz GA, Pellis SM. Play fighting between kindling-prone (FAST) and kindling-resistant (SLOW) rats. J Comp Psychol. 2006;120:19–30. doi: 10.1037/0735-7036.120.1.19. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J. Neurosci. Meth. 1996;1:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology. 2007;191:433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Zitzman DL, Smith KJ, Spear LP. Fast dopamine release events in the nucleus accumbens of early adolescent rats. Neuroscience. 2011;176:296–307. doi: 10.1016/j.neuroscience.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Memory modulation. Behav. Neurosci. 2011;125:797–824. doi: 10.1037/a0026187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: Toward a neurobiology of remembering. Learn. Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology. 2005;30:944–957. doi: 10.1038/sj.npp.1300634. [DOI] [PubMed] [Google Scholar]
- Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict. Biol. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- Siegel MA, Jensen RA. The effects of naloxone and cage size on social play and activity in isolated young rats. Behav. Neural Biol. 1986;45:155–168. doi: 10.1016/s0163-1047(86)90739-9. [DOI] [PubMed] [Google Scholar]
- Siegel MA, Jensen RA, Panksepp J. The prolonged effects of naloxone on play behavior and feeding in the rat. Behav. Neural Biol. 1985;44:509–514. doi: 10.1016/s0163-1047(85)91024-6. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Baliko CN. A further characterization of alpha-2 adrenoceptor involvement in the rough-and-tumble play of juvenile rats. Dev. Psychobiol. 2000;37:25–34. doi: 10.1002/1098-2302(200007)37:1<25::aid-dev4>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Crawford CA, Akopian G, Walsh JP. Dysfunctional play and dopamine physiology in the Fischer 344 rat. Behav. Brain Res. 2011;220:294–304. doi: 10.1016/j.bbr.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siviy SM, Fleischhauer AE, Kuhlman SJ, Atrens DM. Effects of alpha-2 adrenoceptor antagonists on rough-and-tumble play in juvenile rats: evidence for a site of action independent of non-adrenoceptor imidazoline binding sites. Psychopharmacology. 1994;113:493–499. doi: 10.1007/BF02245229. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Fleischhauer AE, Kerrigan LA, Kuhlman SJ. D2 dopamine receptor involvement in the rough-and-tumble play behavior of juvenile rats. Behav. Neurosci. 1996;110:1168–1176. doi: 10.1037//0735-7044.110.5.1168. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Line BS, Darcy EA. Effects of MK-801 on rough-and-tumble play in juvenile rats. Physiol. Behav. 1995;57:843–847. doi: 10.1016/0031-9384(94)00361-8. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Love NJ, DeCicco BM, Giordano SB, Seifert TL. The relative playfulness of juvenile Lewis and Fischer-344 rats. Physiol Behav. 2003;80:385–94. doi: 10.1016/j.physbeh.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Siviy SM, McDowell S, Eck SR, Turano A, Akopian G, Walsh JP. Effects of amphetamine on striatal dopamine release, open-field activity, and play in Fischer 344 and Sprague-Dawley rats. Behav. Pharmacol. 2015;26:720–732. doi: 10.1097/FBP.0000000000000191. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Panksepp J. Dorsomedial diencephalic involvement in the juvenile play of rats. Behav. Neurosci. 1985;99:1103–1113. doi: 10.1037//0735-7044.99.6.1103. [DOI] [PubMed] [Google Scholar]
- Siviy SM, Panksepp J. In search of the neurobiological substrates for social playfulness in mammalian brains. Neurosci. Biobehav. Rev. 2011;35:1821–1830. doi: 10.1016/j.neubiorev.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Small WS. Notes on the psychic development of the young white rat. Am. J. Psychol. 1899;11:80–100. [Google Scholar]
- Smith PK. Does play matter? Functional and evolutionary aspects of animal and human play. Behav. Brain Sci. 1982;5:139–184. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Špinka M, Newberry RC, Bekoff M. Mammalian play: training for the unexpected. Q. Rev. Biol. 2001;76:141–168. doi: 10.1086/393866. [DOI] [PubMed] [Google Scholar]
- Stoesz BM, Hare JF, Snow WM. Neurophysiological mechanisms underlying affiliative social behavior: Insights from comparative research. Neurosci. Biobehav. Rev. 2013;37:123–132. doi: 10.1016/j.neubiorev.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Sutton ME, Raskin LA. A behavioral analysis of the effects of amphetamine on play and locomotor activity in the post-weaning rat. Pharmacol. Biochem. Behav. 1986;24:455–461. doi: 10.1016/0091-3057(86)90541-1. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Chung WC, Kruijver FP, Hofman MA, Ishunina TA. Sexual differentiation of the human hypothalamus. Adv. Exp. Med. Biol. 2002;511:75–100. doi: 10.1007/978-1-4615-0621-8_6. discussion 100–105. [DOI] [PubMed] [Google Scholar]
- Taha SA, Fields HL. Inhibitions of nucleus accumbens neurons encode a gating signal for reward-directed behavior. J. Neurosci. 2006;26:217–222. doi: 10.1523/JNEUROSCI.3227-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallett AJ, Blundell JE, Rodgers RJ. Grooming, scratching and feeding: role of response competition in acute anorectic response to rimonabant in male rats. Psychopharmacology. 2007;195:27–39. doi: 10.1007/s00213-007-0880-2. [DOI] [PubMed] [Google Scholar]
- Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol. Sex Differ. 2012;3:15. doi: 10.1186/2042-6410-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Dep. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor DH, Holloway WR., Jr. Play soliciting in juvenile male rats: effects of caffeine, amphetamine and methylphenidate. Pharmacol. Biochem. Behav. 1983;19:725–727. doi: 10.1016/0091-3057(83)90352-0. [DOI] [PubMed] [Google Scholar]
- Tomas-Roig J, Piscitelli F, Gil V, Del Rio JA, Moore TP, Agbemenyah H, Salinas-Riester G, Pommerenke C, Lorenzen S, Beissbarth T, Hoyer-Fender S, Di Marzo V, Havemann-Reinecke U. Social defeat leads to changes in the endocannabinoid system: An overexpression of calreticulin and motor impairment in mice. Behav. Brain Res. 2016;303:34–43. doi: 10.1016/j.bbr.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJJ, Vanderschuren LJMJ. Prosocial effects of nicotine and ethanol in adolescent rats through partially dissociable neurobehavioral mechanisms. Neuropsychopharmacology. 2009a;34:2560–2573. doi: 10.1038/npp.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse JJ, Vanderschuren LJMJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol. Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJMJ. On the interaction between drugs of abuse and adolescent social behavior. Psychopharmacology. 2014;231:1715–1729. doi: 10.1007/s00213-014-3471-z. [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Cassano T, Macheda T, Dipasquale P, Carratu MR, Gaetani S, Cuomo V. Effects of perinatal exposure to delta-9-tetrahydrocannabinol on the emotional reactivity of the offspring: a longitudinal behavioral study in Wistar rats. Psychopharmacology. 2008;198:529–537. doi: 10.1007/s00213-008-1162-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Vanderschuren LJMJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev. Cogn. Neurosci. 2011a;1:444–457. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Vanderschuren LJMJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009b;19:659–669. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJM, Vanderschuren LJMJ. Nucleus accumbens m-opioid receptors mediate social reward. J. Neurosci. 2011b;31:6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Manduca A, Petrosino S, Van Kerkhof LWM, Pasterkamp RJ, Zhou YP, Campolongo P, Cuomo V, Di Marzo V, Vanderschuren LJMJ. Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. J. Neurosci. 2012;32:14899–14908. doi: 10.1523/JNEUROSCI.0114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology. 2008a;197:217–227. doi: 10.1007/s00213-007-1025-3. [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Cannabinoid and opioid modulation of social play behavior in adolescent rats: Differential behavioral mechanisms. Eur. Neuropsychopharmacol. 2008b;18:519–530. doi: 10.1016/j.euroneuro.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJMJ. Divergent effects of anandamide transporter inhibitors with different target selectivity on social play behavior in adolescent rats. J. Pharmacol. Exp. Ther. 2009;328:343–350. doi: 10.1124/jpet.108.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog. Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict. Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 1999a;34:129–138. [PubMed] [Google Scholar]
- Van den Berg CL, Pijlman FT, Koning HA, Diergaarde L, Van Ree JM, Spruijt BM. Isolation changes the incentive value of sucrose and social behaviour in juvenile and adult rats. Behav. Brain Res. 1999b;106:133–142. doi: 10.1016/s0166-4328(99)00099-6. [DOI] [PubMed] [Google Scholar]
- Van den Berg CL, Van Ree JM, Spruijt BM, Kitchen I. Effects of juvenile isolation and morphine treatment on social interactions and opioid receptors in adult rats: behavioural and autoradiographic studies. Eur. J. Neurosci. 1999c;11:3023–3032. doi: 10.1046/j.1460-9568.1999.00717.x. [DOI] [PubMed] [Google Scholar]
- Van Kerkhof LWM, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJMJ. Social play behavior in rats is mediated by functional activity in medial prefrontal cortex and striatum. Neuropsychopharmacology. 2013a;38:1899–1909. doi: 10.1038/npp.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhof LW, Damsteegt R, Trezza V, Voorn P, Vanderschuren LJMJ. Functional integrity of the habenula is necessary for social play behaviour in rats. Eur. J. Neurosci. 2013b;38:3465–3475. doi: 10.1111/ejn.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kerkhof LWM, Trezza V, Mulder T, Gao P, Voorn P, Vanderschuren LJMJ. Cellular activation in limbic brain systems during social play behaviour in rats. Brain Struct. Funct. 2014;219:1181–1211. doi: 10.1007/s00429-013-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ree JM, Gerrits MAFM, Vanderschuren LJMJ. Opioids, reward and addiction: an encounter of biology, psychology and medicine. Pharmacol. Rev. 1999;51:341–396. [PubMed] [Google Scholar]
- Van Ree JM, Niesink RJ, Nir I. delta 1-Tetrahydrocannabinol but not cannabidiol reduces contact and aggressive behavior of rats tested in dyadic encounters. Psychopharmacology. 1984;84:561–565. doi: 10.1007/BF00431467. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ. How the brain makes play fun. Am. J. Play. 2010;2:315–337. [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology. 1995a;117:225–231. doi: 10.1007/BF02245191. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Spruijt BM, Van Ree JM. m- and k-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur. J. Pharmacol. 1995b;276:257–266. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Niesink RJM, Van Ree JM. The neurobiology of social play behavior in rats. Neurosci. Biobehav. Rev. 1997;21:309–326. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Spruijt BM, Hol T, Niesink RJM, Van Ree JM. Sequential analysis of social play behavior in juvenile rats: effects of morphine. Behav. Brain Res. 1995c;72:89–95. doi: 10.1016/0166-4328(96)00060-5. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Stein EA, Wiegant VM, Van Ree JM. Social play alters regional brain opioid receptor binding in juvenile rats. Brain Res. 1995d;680:148–156. doi: 10.1016/0006-8993(95)00256-p. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Trezza V. What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms. In: Andersen SL, Pine D, editors. The neurobiology of childhood; Curr. Top. Behav. Neurosci. Springer; New York: 2014. pp. 189–212. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Trezza V, Griffioen-Roose S, Schiepers OJG, Van Leeuwen N, De Vries TJ, Schoffelmeer ANM. Methylphenidate disrupts social play behavior in adolescent rats. Neuropsychopharmacology. 2008;33:2946–2956. doi: 10.1038/npp.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol. Clin. Exp. Res. 2002;26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev. Psychobiol. 2006;48:146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol. Clin. Exp. Res. 2009;33:991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol. Clin. Exp. Res. 2001;25:377–385. [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell EM, Spear LP. Sex differences in sensitivity to the social consequences of acute ethanol and social drinking during adolescence. Behav. Brain Res. 2015;282:6–13. doi: 10.1016/j.bbr.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, de Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology. 2013;38:2554–2561. doi: 10.1016/j.psyneuen.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Alcaro A, Puglisi-Allegra S. Prefrontal cortical norepinephrine release is critical for morphine-induced reward, reinstatement and dopamine release in the nucleus accumbens. Cereb. Cortex. 2005;15:1877–1886. doi: 10.1093/cercor/bhi066. [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008;32:777–810. doi: 10.1016/j.neubiorev.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am. J. Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, Schot M, van den Bos R, Spruijt BM. Individual housing during the play period results in changed responses to and consequences of a psychosocial stress situation in rats. Dev. Psychobiol. 2002;41:58–69. doi: 10.1002/dev.10057. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJMJ, Groenewegen HJ, Robbins TW, Pennartz CMA. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wei D, Lee D, Li D, Daglian J, Jung KM, Piomelli D. A role for the endocannabinoid 2-arachidonoyl-sn-glycerol for social and high-fat food reward in male mice. Psychopharmacology. 2016;233:1911–1919. doi: 10.1007/s00213-016-4222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner CM, Anderson DF. Opportunity for interaction as reinforcement in a T-maze. Pers. Soc. Psychol. Bull. 1976;2:166–169. [Google Scholar]
- Whitaker LR, Degoulet M, Morikawa H. Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron. 2013;77:335–345. doi: 10.1016/j.neuron.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wöhr M, Schwarting RKW. Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell Tissue Res. 2013;354:81–97. doi: 10.1007/s00441-013-1607-9. [DOI] [PubMed] [Google Scholar]
- Wolterink G, Daenen LE, Dubbeldam S, Gerrits MA, van Rijn R, Kruse CG, Van Der Heijden JA, Van Ree JM. Early amygdala damage in the rat as a model for neurodevelopmental psychopathological disorders. Eur. Neuropsychopharmacol. 2001;11:51–59. doi: 10.1016/s0924-977x(00)00138-3. [DOI] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol. Behav. 1991;50:129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur. J. Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front. Behav. Neurosci. 2011;5:57. doi: 10.3389/fnbeh.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Pinheiro BS. Dyadic social interaction inhibits cocaine-conditioned place preference and the associated activation of the accumbens corridor. Behav. Pharmacol. 2015;26:580–594. doi: 10.1097/FBP.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuena AR, Zinni M, Giuli C, Cinque C, Alemà GS, Giuliani A, Catalani A, Casolini P, Cozzolino R. Maternal exposure to environmental enrichment before and during gestation influences behaviour of rat offspring in a sex-specific manner. Physiol Behav. 2016;163:274–287. doi: 10.1016/j.physbeh.2016.05.010. [DOI] [PubMed] [Google Scholar]