Abstract
Adolescence is a time of extensive neuroanatomical, functional and chemical reorganization of the brain, which parallels substantial maturational changes in behavior and cognition. Environmental factors that impinge on the timing of these developmental factors, including stress and drug exposure, increase the risk for psychiatric disorders. Indeed, antecedents to affective and psychotic disorders, which have clinical and pathophysiological overlap, are commonly associated with risk factors during adolescence that predispose to these disorders. In the context of schizophrenia, psychosis typically begins in late adolescence/early adulthood, which has been replicated by animal models. Rats exposed during gestational day (GD) 17 to the mitotoxin methylazoxymethanol acetate (MAM) exhibit behavioral, pharmacological, and anatomical characteristics consistent with an animal model of schizophrenia. Here we provide an overview of adolescent changes within the dopamine system and the PFC and review recent findings regarding the effects of stress and cannabis exposure during the peripubertal period as risk factors for the emergence of schizophrenia-like deficits. Finally, we discuss peripubertal interventions appearing to circumvent the emergence of adult schizophrenia-like deficits.
Keywords: animal models, development, adolescence, dopamine, stress, cannabinoids, schizophrenia
1. Introduction
Adolescence is a slowly developing transitional period between infancy and adulthood characterized by extensive behavioral, hormonal and neurochemical changes (Spear, 2000). These processes are mediated by substantial maturational changes in brain structure, neurochemistry and function, which are the outcome of dynamic interactions between genes and environmental factors (i.e. early life insults, stress and drug exposure) (Keshavan et al., 2014). Importantly, the impact of several interacting variables during this period increases the risk for onset of major psychiatric disorders such as schizophrenia, substance abuse and affective disorders (Paus et al., 2008), all of which involve dysregulation of the dopamine (DA) system (Goto and Grace, 2007). This may be due, in part, to changes in dopaminergic function during this period (Wahlstrom et al., 2010). For example, shifts in the balance between mesocortical and mesolimbic systems might account for adolescent behaviors of increased risk, reward seeking and incentive salience and may underlie the risk for mood, addictive and psychotic disorders (Keshavan et al., 2014). Indeed, as nicely discussed by Lichenstein and co-authors in this issue (Lichenstein et al., 2016), developmental changes in the brain, including those within the prefrontal cortex (PFC), contribute to age-specific behavioral characteristics of adolescence, including an increase in the propensity to use drugs and exaggerated sensitivity to stress (Romeo and McEwen, 2006; Spear, 2000).
Schizophrenia is regarded as a neurodevelopmental disorder in which alterations start early in development and culminate in the emergence of psychosis usually during late adolescence or adulthood. Individuals suffering from schizophrenia manifest a range of behavioral changes, including positive symptoms (symptoms added on to a normal personality, such as hallucinations and delusions), negative symptoms (characteristics missing, such as social interactions and motivation), as well as cognitive impairment. Cognitive deficits and negative symptoms are present even before the onset of psychosis and are frequently associated with poor functional outcome (Lesh et al., 2011; Kahn et al., 2015). Although the current management goals with antipsychotics are restricted to improving positive symptoms, the amelioration of negative and cognitive symptoms remain a largely unmet medical need (Kahn et al., 2015).
While schizophrenia is a uniquely human disorder which its etiology and pathophysiology are not completely known, it is amenable to study in animal models with appropriate limitations. Therefore, one can glean from human imaging and pharmacological studies which systems seem to have a primary involvement in the disorder (e.g., hippocampus, prefrontal cortex, dopamine), and examine in animals how these systems function in the normal state (Modinos et al., 2015). We can also incorporate information regarding genetic and environmental risk factors into rodent models to examine how these can negatively impact the normal balance present in these systems. Therefore, a give-and-take between clinical imaging and pharmacological studies and normal and developmental rodent models can be an effective strategy for advancing the field.
Several developmental disruption models have been advanced, including early life immune activation, neonatal ventral hippocampal lesions, and the MAM model; nonetheless, despite the multifaceted mechanisms used by such interventions, the adult animal pathophysiology is remarkably similar in nature (Meyer and Feldon, 2009; Modinos et al., 2015; Tseng et al., 2009). Importantly, this is consistent with the concept that schizophrenia is a disorder with multiple etiologies.
It is commonly accepted that both genetic and environmental factors contribute to the development of schizophrenia. Given that, currently, no universal genetic biomarker of the risk of transition into psychosis is available (Millan et al., 2016) and the fact that the opportunity for intervention on environmental factors is clearly easier than on genes, the focus of this review will be on environmental risk factors. Among them, stress sensitivity and exposure to drugs of abuse (such as cannabis) are linked to the later transition into psychosis (Kahn et al., 2015). We will also discuss some neurodevelopmental underpinnings of risk for psychopathology during adolescence since an improved understanding of them is crucial for the development of better therapeutic approaches and interventions to prevent the transition to psychosis.
To examine the pathophysiology of schizophrenia, we employ a neurodevelopmental disruption model that uses the mitotoxin methylazoxymethanol acetate (MAM), which is administered to pregnant rats at gestational day (GD) 17 and leads to aberrant methylation of DNA interfering with neurogenesis (Hoareau et al., 2006), which produces many characteristics consistent with schizophrenia-like deficits, including neuroanatomical changes (thinning of limbic cortices, parvalbumin (PV) loss), behavioral deficits (prepulse inhibition of startle, reversal learning, latent inhibition, social interaction) and pharmacologic responses (increased locomotion to amphetamine) in addition to an increase in VTA DA neuron population activity (i.e. number of DA neurons firing) that is driven by hyperactivity in the ventral hippocampus (Flagstad et al., 2004; Grace, 2015; Lodge and Grace, 2007; Moore et al., 2006). In addition, consistent with the onset of psychosis in schizophrenia patients, most of the alterations related to the positive symptoms become evident only after puberty, while social withdrawal and cognitive deficits are present both before and after puberty (summarized in Table 1). Thus, this model is ideal for exploring the link between altered embryonic neurogenesis and the transition into a schizophrenia-like phenotype in the adult. Using this model, we review recent findings regarding modulation of DA dysregulation in the context of schizophrenia. We highlight studies suggesting that the peripubertal period, corresponding to mid- to late- adolescence in humans, constitutes a sensitive period for the etiology of schizophrenia and discuss how environmental factors influencing the DA system may confer risk towards onset of schizophrenia-like deficits. Furthermore, we review novel peripubertal interventions that have been shown to prevent and/or attenuate the emergence of neurobehavioral phenotypes that parallel deficits in schizophrenia.
Table 1.
Abnormalities observed in adolescent and adult MAM animals
| Test | Adolescence | Adulthood | Reference |
|---|---|---|---|
| Volume of the mPFC | - | ↓ | (Mackowiak et al., 2014) |
| Volume of lateral and third ventricle | ↑ | ↑ | (Chin et al., 2011) |
| Social Interaction | ↓ | ↓ | (Le Pen et al., 2006) |
| Spatial recognition memory | - | ↓ | (Le Pen et al., 2006) |
| PPI test | - | ↓ | (Le Pen et al., 2006; Mackowiak et al., 2014) |
| Locomotor response to MK-801 | - | ↑ | (Le Pen et al., 2006) |
| Locomotor response to amphetamine | - | ↑ | (Chen et al., 2014; Moore et al., 2006) |
| VTA DA neuron activity | ↑ | ↑ | (Chen et al., 2014) |
| PV loss in the hippocampus | ↓ | ↓ | (Chen et al., 2014; Gill and Grace, 2014) |
| GAD67 mRNA levels in the mPFC | ↓ | (Mackowiak et al., 2014) | |
| Anxiety | ↑ | ↑ | (Du and Grace, 2013) |
| BLA activity | ↑ | ↑ | (Du and Grace, 2016a) |
| Stress responsivity | ↑ | ↑ | (Zimmerman et al., 2013) |
↓: impairment; ↑: increase; -: no change; BLA: basolateral amygdala; DA: dopamine; mPFC: medial prefrontal cortex; PPI: prepulse inhibition; PV: parvalbumin; VTA: ventral tegmental area
2. Adolescent Changes in the Dopamine System: Focus on Dopamine Control of PFC Activity
In addition to early neurodevelopmental processes, such as cell migration and differentiation, the development of brain circuits during adolescence is of clear importance for schizophrenia and other psychiatric disorders.
Adolescence is accompanied by maturational processes in the cortical and limbic circuits (Spear, 2000), which are characterized by neuronal maturation and rearrangement processes, such as myelination, synaptic pruning, and dendritic plasticity (De Bellis et al., 2001). Also, maturation of neurotransmitter systems such as the glutamatergic, the dopaminergic, and the GABAergic systems within the PFC occur during this period (Spear, 2000). These maturational processes are essential for normal adult behavioral performance and can also be disrupted by environmental factors (Andersen, 2003).
Normal PFC function is dependent on the interplay of several neuronal types and their modulation by afferents. Glutamate pyramidal neurons are the primary PFC output and their activity is regulated by local inhibitory GABAergic interneurons as well as by excitatory projections from other pyramidal neurons, enabling the synchronization of cortical neuron activity. Thus, PFC function depends on a proper excitation-inhibition balance. Many aspects of this circuitry change during adolescence (O'Donnell, 2010).
In non-human primates, the density of GABA interneuron processes increases during adolescence and then markedly declines (Erickson and Lewis, 2002; Lewis, 1997). Human studies have documented the maturation of GABA interneuron circuits during adolescence as well (Uhlhaas et al., 2009). The DA system also undergoes significant alterations during adolescence. In non-human primates, concentrations of DA shift gradually toward anterior regions until adolescence, when DA concentrations are highest in the prefrontal cortex (Irwin et al., 1994). The density of DA innervation of the medial PFC (mPFC) in macaques, assessed with tyrosine hydroxylase staining, rises during the preadolescent period until adolescence and then declines (Rosenberg and Lewis, 1995). DA receptors also change postnatally. The density of D1, D2, and D4 receptors increases progressively until adolescence in rats (Tarazi and Baldessarini, 2000), and then at least D1 receptors are pruned (Andersen et al., 2000).
The efficacy of the excitation–inhibition balance in the PFC is adjusted by DA during adolescence (O'Donnell, 2010). Previous studies by O’Donnell and colleagues have demonstrated dramatic differences in the response to dopamine ligands in preadolescent rats when compared to adult rats (O'Donnell, 2010, 2011; Tseng and O'Donnell, 2007a, b). Whereas D1 agonists were able to excite, at both prepubertal and post adolescent ages, the fast-spiking interneurons which are identified by their content of the calcium-binding protein parvalbumin (PV), D2 activation switches from being mildly inhibitory prepubertally to strongly excitatory in young adult rats (Tseng and O'Donnell, 2007a, b). Changes in the DA modulation of pyramidal neuron ability to sustain persistent activity during adolescence have also been observed. D1 modulation of responses mediated by N-methyl-aspartate (NMDA) receptors in PFC pyramidal neurons revealed that although D1 agonists enhance NMDA responses in all age groups (Tseng and O'Donnell, 2004; Wang and O'Donnell, 2001), the ability to induce persistent depolarizations resembling up states by D1-NMDA co-activation emerges only during early adolescence (Tseng and O'Donnell, 2005). Altogether, these data have been interpreted as evidence that circuitries that underlie excitation–inhibition balance within PFC seems to be refined in their connectivity in the transition to young adult stages (O'Donnell, 2012). This change would produce a more effective filtering out of irrelevant information in the adult PFC.
Given that DA–glutamate-GABA interactions within the PFC continue to develop after puberty (O'Donnell, 2010), a disruption on the development of these interactions during this period could result in dramatic changes responsible for functional and behavioral deficits relevant to schizophrenia as suggested by rodent models (O'Donnell, 2011). Such changes are likely implicated in the clinical onset of symptoms that takes place typically in late adolescence or early adulthood. However, the mechanisms underlying the development of pathological changes that cause the syndrome to develop during this time are still not fully understood. Additionally, this significant age-dependent dynamic range of alterations in the cortical networks may confer the adolescent brain with enhanced vulnerability to environmental stimuli, such as stress and drugs of abuse (Lewis et al., 2012; Uhlhaas, 2013). For example it could be that genetic predisposition leads to deficits in cortical regulation of stress, or it could be that environmental stressors lead to system disruption, or a combination of both factors, with the latter being the most accepted model.
3. Regulation of Dopamine System Function
The DA system is unique among the brain’s modulatory systems in that it has discrete projections to brain regions implicated in a variety of functions ranging from motor behaviors to affect and cognition (Grace, 2016). This is because the DA system consists of a number of subcomponents that are topographically distinct with respect to their location, projection site, and function. In the rat, for example, the most medial portion of the midbrain DA system is the ventral tegmental area (VTA), which projects to the reward-related nucleus accumbens/ventral striatum. However, at the transition between the lateral VTA and the substantia nigra (SN) are DA neurons in lateral VTA that project to associative striatum, and lateral SN projecting primarily to the motor- and habit-formation related dorsolateral and dorsomedial striatum, respectively (Ikemoto, 2007). Thus, DA neurons that have distinct projection sites and afferent drive are capable of independently regulating activity states and modulating information flow discretely in separate circuits with distinct functions (Grace et al., 2007).
VTA DA neurons exhibit several activity states that are regulated by multiple afferent systems and have important functional implications (Figure 1). In vivo studies in awake and anesthetized rats have shown that approximately half of VTA DA neurons are not firing spontaneously (Freeman and Bunney, 1987; Freeman et al., 1985; Grace and Bunney, 1984b). Of those DA neurons that are firing, the firing can take the form of single-spike discharge or burst firing. Single-spike firing in midbrain DA neurons is characterized by a slow irregular tonic firing pattern (i.e. tonic activity) (Grace and Bunney, 1984b, 1985). Behaviorally salient stimuli, such as presentation of an unexpected reward or a stimulus associated with a rewarding (Schultz, 1998) or aversive (Lammel et al., 2011) event, trigger a transition into a rapid burst-firing mode in these neurons (i.e. phasic firing) (Grace and Bunney, 1984a). Thus, burst firing is the behaviorally relevant phasic response to stimuli and its amplitude is dependent on the number of DA neurons that are firing spontaneously.
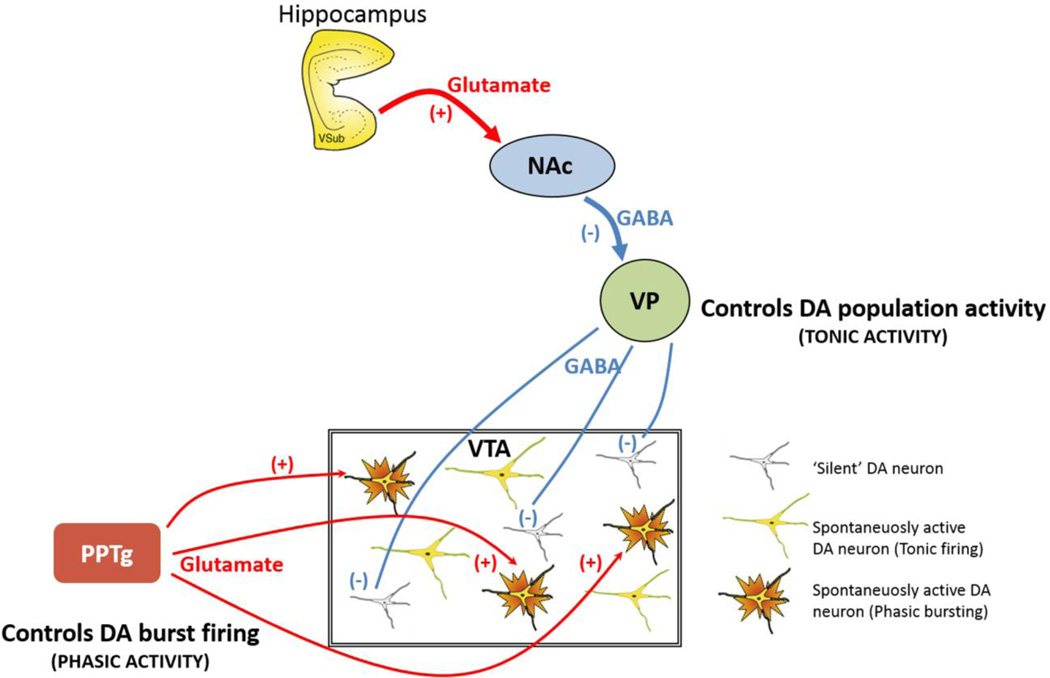
Figure 1.
Tonic and phasic dopamine (DA) neuron regulation. DA neurons are known to generate their own activity through a pacemaker conductance. However, a substantial population of DA neurons is not firing spontaneously, being held in a hyperpolarized state by GABA-mediated inhibitory inputs from the ventral pallidum (VP). The VP, in turn, is controlled by a pathway originating in the ventral subiculum (vSub) of the hippocampus. The vSub projects to the nucleus accumbens (NAc), which in turn inhibits the VP. Therefore, when the vSub is excited, it drives NAc inhibition of the VP, releasing the dopamine neurons from inhibition. By contrast, phasic burst firing is dependent on glutamatergic inputs arising from several areas, primary among these being the pedunculopontine tegmentum (PPTg). This afferent system works to regulate activity states within the population of DA neurons, because only neurons that are firing spontaneously can burst fire; hyperpolarized neurons have a magnesium block of the NMDA channel and won’t change state. Therefore the PPTg provides the phasic signal, whereas the VP, by controlling the number of DA neurons firing, determines the tonic gain.
These two activity states are regulated by different systems (Figure 1). Tonic firing is modulated by a powerful GABAergic input from the ventral pallidum (VP), which is capable of hyperpolarizing the neuron below threshold for firing. In this manner, the VP controls the proportion of DA neurons that are firing spontaneously (Floresco et al., 2003), which is referred to as tonic firing or population activity. An important regulatory pathway arises from the ventral subiculum (vSub) of the hippocampus; thus, activation of the vSub drives neuronal firing in the nucleus accumbens (NAc), which, in turn, inhibits the VP and releases dopamine neurons from inhibition. This pathway controls the tonic baseline state of the dopamine system (Figure 1) (Grace, 2012; Grace et al., 2007).
Burst firing is driven by a glutamatergic input arising primarily from the brainstem pedunculopontine tegmentum (PPTg) acting on NMDA receptors (Floresco et al., 2003; Grace, 2012). Given that burst firing is potently driven by glutamate actions on NMDA receptors (Chergui et al., 1993) and that NMDA receptors are only activated on neurons that are depolarized, only spontaneously active DA neurons are capable of transitioning to burst (i.e. phasic) firing. This is because if the neuron is hyperpolarized, there is a magnesium block of the NMDA channel that prevents activation (Mayer et al., 1984). Thus, in order for glutamate to activate NMDA receptors and drive burst firing, the neurons must be in a depolarized, spontaneously active state (i.e. tonic activity). Collectively, these data indicate that tonic activity is an important parameter with respect to the functional output of the DA system, as it sets the level of responsivity of the system to more rapid phasic stimuli (Lodge and Grace, 2006). For this reason, we suggest that the proportion of DA neurons that are spontaneously active sets the baseline tone of responsivity of the DA system, thereby serving as the “gain” for the phasic response (Grace, 2012).
In sum, in their basal state, spontaneously active DA neurons discharge in a slow, irregular tonic firing pattern. The behaviorally salient, rapid phasic response of DA neurons is represented by burst firing, although burst firing only occurs in neurons that exhibit tonic activity. The number of DA neurons firing (i.e. tonic or population activity) is controlled by a potent GABAergic input from the VP that holds a subset of neurons in a non-firing state. Therefore, the PPTg drives the phasic burst firing, whereas the VP controls the number of DA neurons that can be driven to burst firing in such a way that the amplitude of the response depends on the level of tonic activity of the DA system, which represents the gain (Figure 1) (Grace et al., 2007).
4. Dopamine System Dysregulation in a Neurodevelopmental Model of Schizophrenia
Increased activity in the DA system (i.e. DA hyperresponsivity) is thought to underlie the psychotic symptoms of schizophrenia. This has been best exemplified by the use of antipsychotic drugs, all of which are D2 antagonists, to treat schizophrenia (Kapur and Remington, 2001). Furthermore, repeated and/or high doses of drugs that release DA or increase DA neurotransmission (i.e. DA agonists) induce psychotic symptoms in healthy individuals and exacerbate psychosis in schizophrenia patients (Angrist et al., 1974; Janowsky et al., 1978). Neuroimaging studies have demonstrated enhanced fluorodopa uptake in patients with schizophrenia as well as increased amphetamine-induced DA release, as measured by raclopride displacement, in the striatum of schizophrenia patients compared with controls; the amplitude of these increases is correlated with severity of symptoms and the worsening of psychosis (Howes et al., 2009; Laruelle and Abi-Dargham, 1999). Relevant to prevention and early intervention in schizophrenia is the fact that striatal dopamine synthesis capacity seems to be elevated in ultrahigh- risk patients who went on to develop psychosis, suggesting that the onset of psychosis is preceded by presynaptic dopaminergic dysfunction (Howes et. al, 2011). Furthermore, in the psychotic transition group, there was a direct relationship between the magnitude of dopaminergic dysfunction and the severity of prodromal symptoms. Collectively, these findings establish a strong link between DA transmission and the positive psychotic symptoms of schizophrenia (Guillin et al., 2007). However, there is little evidence for a dysfunction within the DA system itself; instead, focus has been turned into a dysregulation of the DA system by afferent structures.
One of these structures is the hippocampus, which is also pivotal in the pathophysiology of schizophrenia (Harrison, 2004; Herold et al., 2013). Neuroimaging studies have revealed that the anterior hippocampus, which is functionally equivalent to the ventral hippocampus in rodents (Grace, 2012), is hyperactive in schizophrenia (Heckers and Konradi, 2015), and the hyperactivity corresponds to psychosis (Silbersweig et al., 1995). Moreover, postmortem studies have shown that schizophrenia patients exhibit a substantial reduction of PV in GABAergic interneurons of the hippocampus (Benes et al., 2007; Konradi et al., 2011), but also of the PFC (Beasley et al., 2002; Kalus et al., 2002; Lewis et al., 2001). This PV loss is thought to disrupt inhibitory networks and contribute to the hyperactivity and loss of evoked gamma rhythms within the PFC and the hippocampus (Koenig et al., 2012; Lisman et al., 2008; Spencer et al., 2003; Volk and Lewis, 2010). Deficits in prefrontal cortical function likely contribute to cognitive impairments and working memory deficits (Gonzalez-Burgos et al., 2015), whereas aberrant hippocampal function is associated with positive symptom severity (Schobel et al., 2009).
Similar observations have been seen in a variety of animal models of schizophrenia, suggesting that hippocampal overdrive leads to a hyper-responsive DA state. The developmental disruption model based on the administration of MAM on GD 17 produces a number of features consistent with the pathophysiology of schizophrenia, including DA and hippocampal hyperactivity as well as a loss of PV neuron function. In these rats, the hippocampus is hyperactive based on cell firing and rhythmic activity, and this pathologically enhanced drive from the vSub results in a doubling in the number of spontaneously firing DA neurons in the VTA (i.e. higher population activity) (Lodge and Grace, 2007). vSub inactivation completely abolished elevated DA population activity in MAM rats, thereby establishing a causal link between excessive hippocampal activity and DA system hyper-responsivity in MAM rats. Like schizophrenia patients, MAM animals also exhibit a reduction in PV-expressing interneurons within the vSub and PFC and display deficits in gamma oscillatory activity (Gill and Grace, 2014; Lodge et al., 2009). En masse, these data indicate that a loss of PV interneuron function in the vSub results in alterations in vSub output that drives a hyper-responsive DA system that underlies the positive symptoms of schizophrenia (Grace, submitted).
5. Environmental factors contributing to adolescent onset of schizophrenia-like deficits
Schizophrenia is a developmental disorder that typically manifests in adolescence or early adulthood (Harrop and Trower, 2001). Indeed, most subjects diagnosed with schizophrenia experience their first psychotic break in late adolescence or early adulthood (Lieberman, 2006), suggesting that adolescence is a critical period for schizophrenia (Selemon and Zecevic, 2015). Converging evidence from clinical and preclinical studies have highlighted an important role for environmental factors, such as exposure to stress and drugs of abuse, during developmentally sensitive periods as risk factors for the emergence of psychiatric disorders, including schizophrenia (Thompson et al., 2004; van Os et al., 2010). Notably, both of these factors can have long-term effects on DA responsivity (Belujon and Grace, 2015; Burke and Miczek, 2014; Thompson et al., 2004). Adolescence is a time when stress reactivity is high and the stress response systems of hypothalamic-pituitary adrenal (HPA) axis are still undergoing maturation (Romeo and McEwen, 2006) and it is also a time of increased experimentation with drugs of abuse (Spear, 2000); therefore, we focus on these two factors within the context of the MAM model of schizophrenia.
5.1 Developmental Stress
Stress is a known risk factor for the onset of a variety of psychiatric illnesses (de Kloet et al., 2005; Lupien et al., 2009). Indeed, the onset of schizophrenia during adolescence or early adulthood is often associated with stressful life events (Holtzman et al., 2013), which can precipitate or exacerbate the psychotic symptoms of schizophrenia (Corcoran et al., 2003; Phillips et al., 2007; Sinclair et al., 2014; Walker et al., 2008). This may be due to deleterious effects of stress in the hippocampus (Sapolsky, 1996) and PV neurons (Czeh et al., 2005), both of which are critical for DA system pathology characteristic of schizophrenia.
In humans, young individuals that are at high risk for schizophrenia experience abnormally high reactivity to stress (Pruessner et al., 2011) and are more likely to develop psychosis if they have decreased tolerance to stress (Yung et al., 2005). This increased responsivity to stressors is also observed in rodent models of schizophrenia. For example, adolescent stress unmasked latent schizophrenia-like signs in mice exposed to prenatal immune activation (Giovanoli et al., 2013). Also, we observed that juvenile MAM rats emitted significantly more 22 kHz vocalizations (i.e. alarm calls), spent more time vocalizing, emitted calls at a higher rate and showed greater freezing in response to acute footshock stress compared to controls and older (i.e. adult) animals (Zimmerman et al., 2013). Moreover, adolescent MAM rats showed a disruption in HPA axis function, as indicated by blunted corticosterone secretion in response to acute footshock that persisted after 10 days of stress exposure. Furthermore, adolescent MAM rats displayed higher levels of anxiety-like behavior in the elevated plus-maze (Du and Grace, 2013). Collectively, these findings suggest that juvenile and adolescent MAM rats are more sensitive to the effects of stress. Importantly, these findings are consistent with epidemiological studies of adolescents at risk for schizophrenia indicating that those who show high stress sensitivity and increased anxiety tended to be those that eventually converted to schizophrenia later in life (Devylder et al., 2013; Owens et al., 2005).
Increased anxiety and responsivity to stressors was found to correlate with amygdala hyperactivity in adolescent MAM animals (Du and Grace, 2016a), suggesting that amygdala stress-induced hyperactivity during adolescence may lead to loss of vSub PV neuron function. Since amygdala responsivity is potently regulated by the mPFC (Rosenkranz et al., 2003), yet another structure implicated in the etiology of schizophrenia (Tse et al., 2015; Volk and Lewis, 2010), it seems plausible that failure of the mPFC to mitigate the effects of stress during this period may trigger PV loss in the vSub, thereby leading to hippocampal hyperactivity and DA system overdrive, along with disruption of other hippocampal targets underlying the negative symptoms of schizophrenia (Thompson et al., 2004). Additionally, it is well-known that stressful events lead to oxidative stress which is characterized by a disturbance in the balance between the production of reactive oxygen species and antioxidant defenses. Interestingly, increased oxidative stress at the end of adolescence/early adulthood has been implicated in PV loss in the vSub (Steullet et al., 2010). Given that pubertal/adolescent stress is a contributing factor in the transition to psychosis, controlling stress during this vulnerable period may circumvent these pathological changes and prevent the emergence of psychosis later in life (Figure 2).
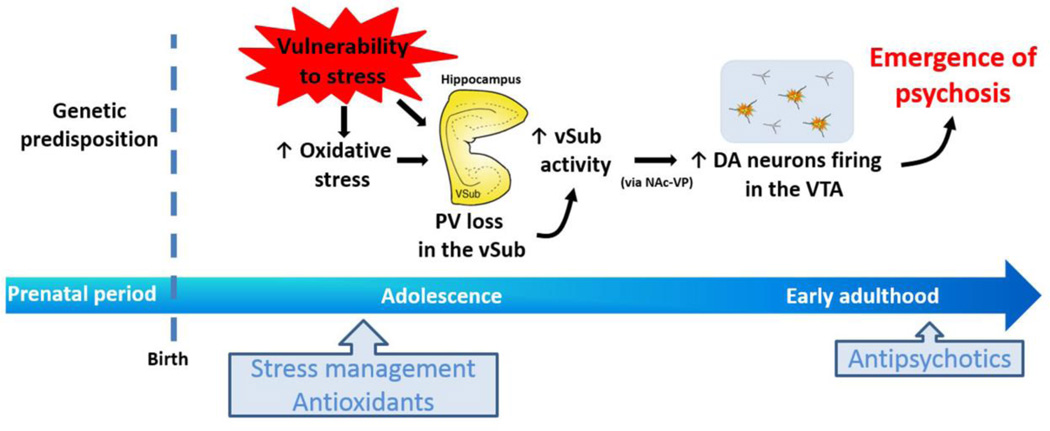
Figure 2.
Genetic predisposition would heighten stress responsivity during adolescence. Extreme stress exposure leads to parvalbumin (PV) loss in the ventral subiculum (vSub) of hippocampus, which can be a consequence of enhanced oxidative stress. This PV loss contributes to the vSub hyperactivity and, consequently, alterations in vSub output (via nucleus accumbens and ventral pallidum; NAc-VP) that drives a hyper-responsive dopamine (DA) system in the ventral tegmental area (VTA) that underlies the emergence of psychosis at late adolescence/early adulthood. Thus, early interventions using stress management and antioxidants would be useful to prevent the transition into schizophrenia, while antipsychotics are effective at onset of psychosis.
5.2 Adolescent cannabis exposure
Adolescence seems to be a vulnerable developmental period for the consequences of cannabis exposure (Schneider, 2008), and evidence suggests that early cannabis use is associated with increased risk of adverse developmental outcomes (Silins et al., 2014). Additionally, it has been shown that those with an established vulnerability to psychotic disorders are especially sensitive to the effects of cannabis (van Os et al., 2002).
In fact, animal studies showed that combining a neonatal PFC lesion with repeated pubertal cannabinoid administration resulted in higher impairment in social behavior (Schneider and Koch, 2005) and object recognition memory (Schneider and Koch, 2007). Furthermore, adolescent exposure to Δ9-tetrahydrocannabinol, the major psychotomimetic compound present in cannabis, worsened disruption of prepulse inhibition induced by isolation rearing (Malone and Taylor, 2006), suggesting that adolescent cannabinoid administration in vulnerable individuals might induce enhanced behavioral disturbances. Recently, we evaluated if cannabinoid exposure during adolescence would exacerbate schizophrenia-like signs in control versus MAM rats (Gomes et al., 2015). As expected, MAM-treated rats showed deficits in behavioral flexibility, augmented locomotor response to amphetamine and increased number of spontaneously active DA neurons in the VTA. Interestingly, pubertal treatment with the CB1/2 receptor agonist WIN55,212-2, from PD40 through PD65, in normal animals induced similar changes at adulthood as those observed in MAM rats, supporting the notion that adolescence exposure to cannabinoids may represent a risk factor for developing schizophrenia-like signs. The factors responsible for these changes have not been delineated. But, similar to MAM rats, there is evidence indicating that cannabinoid exposure during adolescence may induce PV loss (Zamberletti et al., 2014).
Contrary to our expectations, WIN55,212-2 treatment did not exacerbate the changes observed in MAM-treated rats. Intriguingly, although WIN55,212-2-treated MAM rats showed a higher number of spontaneously active DA neurons in the VTA that was similar to vehicle-treated MAM rats, adolescent WIN55,212-2 exposure in MAM rats tended to normalize the amphetamine-induced hyperlocomotion (Gomes et al., 2015). The reason for this is unclear, particularly given that it has been consistently shown that there are parallel changes between the number of DA neurons firing spontaneously in the VTA and locomotor response to amphetamine. This suggests that the pubertal exposure to WIN55,212-2 in MAM rats may have induced compensatory changes that are downstream from DA neuron activity. However, this needs to be further investigated.
While epidemiological studies indicate an association between cannabis use and susceptibility to schizophrenia (Casadio et al., 2011; Silins et al., 2014), it would be a challenge to evaluate if subgroups of patients that may develop schizophrenia are protected by cannabis use since it would require a large prospective study employing a still tightly controlled substance. Given data suggesting that cannabis may have anxiolytic actions, it may be that in a state of increased anxiety cannabis exposure can mitigate the effects of stress; alternately, the increased DA drive produced by the cannabinoid agonist during adolescence may desensitize the postsynaptic response to amphetamine in MAM rats despite the presence of maximal DA neuron activity. Therefore, it is clear that the relationship between cannabinoid exposure and susceptibility to disease states in rodent models is more complex than may be predicted, and it is not readily evident whether one can predict if cannabis will have protective or deleterious effects in a given individual.
6. Prevention of schizophrenia-like deficits in animal models by peripubertal/adolescent intervention
The use of antipsychotic drugs as a treatment for schizophrenia has several limitations. For example, antipsychotic administration may produce unwanted side effects, and this mode of treatment suffers from low efficacy (Lieberman et al., 2005). Furthermore, there is substantial evidence suggesting that the biological changes underlying the development of schizophrenia may already be active in the prepsychotic (i.e. prodromal) phase (Phillips et al., 2002). Moreover, there is evidence showing that the duration of untreated psychosis is associated with a worsened prognosis in schizophrenia (Wyatt et al., 1997). This period involves increasing symptoms and gradual functional decline that begin several months to years before clinical onset and constitutes a critical period for preventive efforts (Addington and Heinssen, 2012; Goulding et al., 2013). Thus, a better approach may be to develop preventive interventions that target the prepsychotic phase (i.e. prodromal period), which may prevent the transition to psychosis in susceptible individuals at risk for schizophrenia (Birchwood and Macmillan, 1993; Phillips et al., 2002). In addition to MAM rats, this idea has been applied in other two animal models of schizophrenia based on neurodevelopmental disruption, the neonatal ventral hippocampus lesion (NVHL) and maternal immune activation (MIA). While the NVHL model involves experimental ibotenic acid lesions of the ventral hippocampus in neonatal rats (PD7-9) (O'Donnell, 2012), the MIA is based on the premise that maternal exposure to infection in pregnancy would increase risk for developing schizophrenia in the offspring (Meyer and Feldon, 2010). The most commonly used and well-established MIA model involves the administration of the viral mimic polyriboinosinic-polyribocytidilic acid (Poly I:C) to pregnant rodents (Meyer and Feldon, 2010). Both models produce a variety of behavioral and neurochemical abnormalities resembling schizophrenia that emerge after puberty (for review, (Meyer and Feldon, 2010; O'Donnell, 2012)). Similar to MAM rats, a loss of PV, enhanced DA system responsivity and excessive reactivity to stress have also been observed in these two models (Cabungcal et al., 2014; Meyer et al., 2008; Tseng et al., 2009). Below, we review recent studies regarding the effects of prepubertal and adolescent interventions on these rodent models (Table 2).
Table 2.
Preventive interventions during juvenile/adolescence period in animal models based on neurodevelopmental disruption
| Intervention | Period of intervention |
Animal model | Preventive effects on | Test age | Reference |
|---|---|---|---|---|---|
| Diazepam (5 mg/kg/day) |
PD31-40 | MAM | Heightened anxiety, AIH, PV loss, increased VTA DA neuron activity, BLA hyperactivity and CS-induced increase in BLA theta power |
>PD62 | (Du and Grace, 2013; 2016a and 2016b) |
| Risperidone (0.045 mg/kg/day) |
PD35-56 | NVHL | AIH | PD57 | (Richtand et al., 2006) |
| Cognitive training | PD35-37 | NVHL | Cognitive impairment and enhanced cognition-associated synchrony of neural oscillations between the hippocampi |
>PD60 | (Lee et al., 2012) |
| N-acetyl-cysteine (drinking water, 900 mg/L) |
PD5-50 | NVHL | PPI and MMN disruption, PV loss, electrophysiological deficits in the PFC |
>PD60 | (Cabungcal et al., 2014) |
| Ebselen (10 mg/kg/day) |
PD35-50 | NVHL | PPI impairment | PD60 | (Cabungcal et al., 2014) |
| Apocynin (drinking water, 750 mg/L) |
PD5-50 | NVHL | PPI impairment | PD60 | (Cabungcal et al., 2014) |
| Clozapine (7.5 mg/kg/day) |
PD34-47 | MIA (PolyI:C) | Brain structural changes (ventricular enlargement, hippocampal reduction), LI impairment, and AIH |
>PD90 | (Piontkewitz et al., 2009) |
| Risperidone (0.045 or 1.2 mg/kg/day) |
PD34-47 or PD48-61 |
MIA (PolyI:C) | Brain structural changes (ventricular enlargement, hippocampal reduction, PV loss), LI impairment, and AIH |
>PD90 | (Piontkewitz et al., 2011, 2012a; Piontkewitz et al., 2012b) |
| Clozapine (15 mg/kg/day) |
PD35-65 | MIA (PolyI:C) | PPI and LI impairment | >PD90 | (Meyer et al., 2010) |
| Fluoxetine (20 mg/kg/day) |
PD35-65 | MIA (PolyI:C) | PPI impairment, and AIH | >PD90 | (Meyer et al., 2010) |
| Haloperidol (3 mg/kg/day) |
PD35-65 | MIA (PolyI:C) | LI impairment, and AIH | >PD90 | (Meyer et al., 2010) |
| Celecoxib (2.5–10 mg/kg/day) |
PD35-46 | MIA (PolyI:C) | Locomotor response to MK-801 | >PD90 | (Zavitsanou et al., 2014) |
AIH: amphetamine-induced hyperlocomotion; BLA: basolateral amygdala; CS: conditioned stimulus; DA: dopamine; LI: latent inhibition; MAM: methylazoxymethanol acetate model; MIA: maternal immune activation; MMN: mismatch negativity; NVHL: neonatal ventral hippocampus lesion; PD: postnatal day; PFC: prefrontal cortex; PPI: prepulse inhibition; PV: parvalbumin; VTA: ventral tegmental area.
6.1 Diazepam
As discussed in the previous section, MAM rats exhibit disrupted stress responsivity during early development (Zimmerman et al., 2013). Given the role of stress in the etiology of schizophrenia (Corcoran et al., 2003; Holtzman et al., 2013), it seems plausible that one may be able to attenuate schizophrenia-like brain and behavioral abnormalities by targeting aberrant stress responsivity during the peripubertal period. This idea was tested by peripubertally treating MAM animals with the anxiolytic drug diazepam. In MAM rats, peripubertal (PND31-40) diazepam administration reversed adolescent anxiety-like behavior in the EPM, and prevented the increase in VTA DA population activity and blunted the behavioral hyperresponsivity to amphetamine during adulthood (Du and Grace, 2013). In addition, peripubertal diazepam treatment reduced freezing behaviors induced by conditioned stimuli (CS), and normalized atypical (i.e. higher) neuronal firing rates within the basolateral amygdala (BLA) of MAM rats during both the peripubertal and adult period (Du and Grace, 2016a). Peripubertal diazepam administration attenuated the CS-induced increase in BLA theta power in adult animals, suggesting a persistent normalization of this structure by this treatment. Furthermore, although MAM rats exhibit a reduction of PV content but not PV interneurons in the hippocampus evident by PND27 (Gill and Grace, 2014; Lodge et al., 2009), rats treated with diazepam peripubertally had more PV interneurons in the vSub of the hippocampus compared to MAM rats without this treatment (Du and Grace, 2016b). A similar pattern was observed for the density of PV interneurons, in which MAM rats with peripubertal administration of diazepam had higher density, albeit significantly lower than control rats. Therefore, it appears that peripubertal diazepam administration effectively protects some of the PV loss known to occur in the hippocampus of MAM animals (Gill and Grace, 2014; Lodge et al., 2009). In sum, this work suggests that MAM treatment makes animals more susceptible to the deleterious effects of stress and that controlling (i.e. attenuating) stress responses during the peripubertal period may circumvent the process leading to the emergence of behavioral abnormalities and hyperdopaminergic state observed in adult MAM rats (Du and Grace, 2013; 2016b). Although an absence of effects induced by diazepam treatment to adult MAM animals would underscore that adolescence is as period of particular opportunity for intervention, these studies looked only at adolescent diazepam treatment.
6.2 Antipsychotics
In addition to several ethical concerns, studies in individuals in the early clinical stages of the schizophrenia yet before the development of the full clinical phenotype do not support a prophylactic effect of the treatment with atypical antipsychotic drugs reduce the risk of progression to first-episode psychosis beyond the period of active treatment (McGlashan et al., 2006; McGorry et al., 2002). In animals, clozapine and risperidone (0.045 and 1.2 mg/kg) treatment during adolescence/early adulthood in animals exposed to prenatal immune activation prevented structural and behavioral abnormalities associated with schizophrenia (Piontkewitz et al., 2011; Piontkewitz et al., 2009; Piontkewitz et al., 2012b). The treatment with the same low dose of risperidone on PD35-56 in NVHL rats prevented amphetamine-induced hyperlocomotion (Richtand et al., 2006). Additionally, using the MIA model, it was observed that clozapine and risperidone (low dose) treatment were devoid of any negative effects in control offspring. On the other hand, haloperidol and risperidone at a high dose, while being effective in Poly I:C offspring, were shown to induce deleterious effects in controls (Meyer et al., 2010; Piontkewitz et al., 2011). However, how these drugs produced this impact is unclear, given that there is no evidence for a hyperdopaminergic state at this period (Moore et al., 2006). Instead, this may be related to the HPA axis suppression associated with these drugs (Walker et al., 2008). These results underscore the need to screen a variety of compounds and doses in prevention studies in animal models.
6.3 Antidepressants
Antidepressants have been reported to be beneficial to treatment of prodromal schizophrenia in adolescents (Cornblatt et al., 2007). In subjects at high clinical risk for psychosis, the combination of cognitive behavioral therapy and antidepressants was associated with a reduced risk of transition to psychosis (Fusar-Poli et al., 2015). In animals, fluoxetine treatment to adolescent rats in the MIA model of schizophrenia normalized prepulse inhibition disruption and enhanced locomotor response to amphetamine at adulthood (Meyer et al., 2010). However, at the same time, similar to that observed with the antipsychotics haloperidol and risperidone, repeated fluoxetine induced negative effects on normal behavioral in control subjects, such as a disrupted latent inhibition and increased locomotor response to the NMDA receptor antagonist MK-801 (Meyer et al., 2010). Effects induced by antidepressant treatment to adult animals in the MIA model were not investigated.
6.4 Cognitive training
Cognitive impairment is a core feature of schizophrenia and is frequently associated with poor long-term functional outcome (Elvevag and Goldberg, 2000; Lesh et al., 2011). Interestingly, it was observed that cognitive training during adolescence prevented the cognitive impairment in adult NVHL rats and the benefits generalized beyond the training task. This early training also normalized brain function assessed by interhippocampal synchrony of cognition-related neural oscillations (Lee et al., 2012). These findings indicate that prophylactic cognitive therapy at an early age can offer promise for improving functional outcomes of people at risk for schizophrenia. Indeed, the benefits of cognitive remediation therapy are greater in younger patients (Wykes et al., 2009). These data indicate that intervention strategies may not be limited to medications.
6.5 Antioxidants
Several environmental risk factors for schizophrenia lead to increased oxidative stress, alteration of antioxidant systems, and often permanently decreased PV expression (Do et al., 2015). As discussed above, PV cells are sensitive to severe stress (Czeh et al., 2005; Milner et al., 2013). The PV impairment after environmental trauma could be mediated by oxidative stress. Indeed, it was shown that superoxide overproduction causes the decrease in PV expression in an NMDA receptor hypofunction model of schizophrenia and social isolated animals (Behrens et al., 2007; Harte et al., 2007; Schiavone et al., 2009), suggesting that redox dysregulation could affect PV interneurons.
A large postnatal treatment with the antioxidant N-acetyl cysteine that started prior to the ventral hippocampus lesion and continued through early adulthood (PD5-PD50) prevented the loss of PV in the PFC, as well as circumvented electrophysiological and behavioral deficits (prepulse inhibition and mismatch negativity disruption) relevant to schizophrenia in NVHL rats at adulthood (Cabungcal et al., 2014). A reversal of PPI deficits was also observed after juvenile and adolescent treatment with two other antioxidants, ebselen and apocynin. Interestingly, while most of the schizophrenia-like changes in NVHL rats become evident only after puberty, an enhanced oxidative stress was observed at PD21 (Cabungcal et al., 2014), indicating that a redox dysregulation during a critical developmental period can disrupt normal PV interneuron maturation resulting in the electrophysiological and behavioral deficits. Therefore, the authors suggest that presymptomatic oxidative stress yields abnormal adult brain function in a developmentally compromised brain, indicating the redox modulation as a potential target for early intervention. Moreover, evidence showing that immature PV interneurons may have a less robust antioxidant defense system could explain why early intervention is required. Indeed, the vulnerability of PFC immature PV interneurons is associated with the absence of fully mature perineuronal nets, which protects these cells against oxidative stress (Cabungcal et al., 2013; Do et al., 2015).
6.6 Anti-inflammatory drugs
The involvement of inflammatory changes in the pathophysiology of schizophrenia has been recently highlighted (Kirkpatrick and Miller, 2013). It has also been suggested that anti-inflammatory drugs may be used as adjunct treatment in schizophrenia (Sommer et al., 2014). Also, data from rodent models show that the early attenuation of inflammation prevents schizophrenia-like signs at adulthood. For example, in rats exposed to prenatal immune activation celecoxib treatment during adolescence prevented the increased locomotor response to MK-801 at adulthood (Zavitsanou et al., 2014). In humans, celecoxib was effective when used as an add-on treatment to antipsychotic in early onset schizophrenia (Muller et al., 2010) but not in chronic schizophrenia (Rapaport et al., 2005), suggesting that the earlier the intervention the better the outcome.
7. Conclusion
Adolescence is a period of marked change in brain circuits that enable the transition from adolescent impulsivity and reckless behavior into more effective adult coping strategies. As such, this represents a time during which an organism can test the limits of the environment and decide on the best actions to ensure survival and success. However, this is also a time of enhanced plasticity in brain circuits, in which deleterious environmental impacts can lead to disruption of system stability and the emergence of psychiatric disorders. Schizophrenia, as with many other disorders, is believed to arise from a combination of a genetic predisposition and an environmental insult. Therefore, unlike Huntington’s disease, in which the presence of a genetic deficit is always associated with later emergence of pathology, in the case of schizophrenia what seems to be inherited is a predisposition to the disorder, with environmental factors flipping the switch between normal and pathological states. Our studies in the MAM rat show that MAM does not “cause” schizophrenia. Instead, the presence of heightened response to stressors peripubertally and the ability to circumvent the transition to a schizophrenia-like state by stress attenuation during this period suggests that the MAM instead is causing the rat to show heightened stress responsivity, with the stress leading to PV loss and emergence of psychosis (Grace, submitted) (Figure 2). There have been a number of studies that have attempted to find the risk genes for schizophrenia, with limited success. Our results suggest that a more effective approach may be to examine which genes predispose the individual to heightened stress responses as an antecedent to schizophrenia. This, combined with behavioral screens for stress responsivity in at-risk individuals may point to a more effective intervention of stress alleviation during the critical adolescent period that may prevent PV neuron loss and the transition to schizophrenia in adulthood.
Highlights.
Adolescence is a critical period for risk factors that predispose to schizophrenia.
The DA system and the PFC undergo dramatic changes during this period.
We review effects of stress and drug exposure in the MAM model of schizophrenia.
Adolescence is a sensitive period for exacerbation and prevention of schizophrenia.
Acknowledgments
This work was supported by USPHS MH57440, MH191180, MH104320, and T32-MH016804.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
AAG has received funds from Johnson & Johnson, Lundbeck, Pfizer, GSK, Merck, Takeda, Dainippon Sumitomo, Otsuka, Lilly, Roche, Asubio, Abbott, Autofony, and Janssen. FVG and MRC declare no conflict of interest.
References
- Addington J, Heinssen R. Prediction and prevention of psychosis in youth at clinical high risk. Annu Rev Clin Psychol. 2012;8:269–289. doi: 10.1146/annurev-clinpsy-032511-143146. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Angrist B, Sathananthan G, Wilk S, Gershon S. Amphetamine psychosis: behavioral and biochemical aspects. J Psychiatr Res. 1974;11:13–23. doi: 10.1016/0022-3956(74)90064-8. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Regulation of dopamine system responsivity and its adaptive and pathological response to stress. Proc Biol Sci. 2015;282 doi: 10.1098/rspb.2014.2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchwood M, Macmillan F. Early intervention in schizophrenia. Aust N Z J Psychiatry. 1993;27:374–378. doi: 10.3109/00048679309075792. [DOI] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl) 2014;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, Counotte DS, Lewis EM, Tejeda HA, Piantadosi P, Pollock C, Calhoon GG, Sullivan EM, Presgraves E, Kil J, Hong LE, Cuenod M, Do KQ, O'Donnell P. Juvenile antioxidant treatment prevents adult deficits in a developmental model of schizophrenia. Neuron. 2014;83:1073–1084. doi: 10.1016/j.neuron.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, Do KQ. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc Natl Acad Sci U S A. 2013;110:9130–9135. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio P, Fernandes C, Murray RM, Di Forti M. Cannabis use in young people: the risk for schizophrenia. Neurosci Biobehav Rev. 2011;35:1779–1787. doi: 10.1016/j.neubiorev.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Chen L, Perez SM, Lodge DJ. An augmented dopamine system function is present prior to puberty in the methylazoxymethanol acetate rodent model of schizophrenia. Dev Neurobiol. 2014;74:907–917. doi: 10.1002/dneu.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci. 1993;5:137–144. doi: 10.1111/j.1460-9568.1993.tb00479.x. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, Malaspina D. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lencz T, Smith CW, Olsen R, Auther AM, Nakayama E, Lesser ML, Tai JY, Shah MR, Foley CA, Kane JM, Correll CU. Can antidepressants be used to treat the schizophrenia prodrome? Results of a prospective, naturalistic treatment study of adolescents. J Clin Psychiatry. 2007;68:546–557. doi: 10.4088/jcp.v68n0410. [DOI] [PubMed] [Google Scholar]
- Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, Fuchs E. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology. 2005;30:67–79. doi: 10.1038/sj.npp.1300581. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Beers SR, Hall J, Frustaci K, Masalehdan A, Noll J, Boring AM. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature reviews. Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Devylder JE, Ben-David S, Schobel SA, Kimhy D, Malaspina D, Corcoran CM. Temporal association of stress sensitivity and symptoms in individuals at clinical high risk for psychosis. Psychol Med. 2013;43:259–268. doi: 10.1017/S0033291712001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Cuenod M, Hensch TK. Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophr Bull. 2015;41:835–846. doi: 10.1093/schbul/sbv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38:1881–1888. doi: 10.1038/npp.2013.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA. Amygdala hyperactivity in MAM model of schizophrenia is normalized by peripubertal diazepam administration. Neuropsychopharmacology. 2016a doi: 10.1038/npp.2016.42. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Grace AA. Aberrant anxiety in schizophrenia: a potential target for early intervention. 2016b In preparation. [Google Scholar]
- Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14:1–21. [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology. 2004;29:2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Bunney BS. Activity of A9 and A10 dopaminergic neurons in unrestrained rats: further characterization and effects of apomorphine and cholecystokinin. Brain Res. 1987;405:46–55. doi: 10.1016/0006-8993(87)90988-7. [DOI] [PubMed] [Google Scholar]
- Freeman AS, Meltzer LT, Bunney BS. Firing properties of substantia nigra dopaminergic neurons in freely moving rats. Life Sci. 1985;36:1983–1994. doi: 10.1016/0024-3205(85)90448-5. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Frascarelli M, Valmaggia L, Byrne M, Stahl D, Rocchetti M, Codjoe L, Weinberg L, Tognin S, Xenaki L, McGuire P. Antidepressant, antipsychotic and psychological interventions in subjects at high clinical risk for psychosis: OASIS 6-year naturalistic study. Psychol Med. 2015;45:1327–1339. doi: 10.1017/S003329171400244X. [DOI] [PubMed] [Google Scholar]
- Gill KM, Grace AA. Corresponding decrease in neuronal markers signals progressive parvalbumin neuron loss in MAM schizophrenia model. Int J Neuropsychopharmacol. 2014;17:1609–1619. doi: 10.1017/S146114571400056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, Schedlowski M, Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Guimaraes FS, Grace AA. Effects of pubertal cannabinoid administration on attentional set-shifting and dopaminergic hyper-responsivity in a developmental disruption model of schizophrenia. Int J Neuropsychopharmacol. 2015;18:1–10. doi: 10.1093/ijnp/pyu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. The dopamine system and the pathophysiology of schizophrenia: a basic science perspective. Int Rev Neurobiol. 2007;78:41–68. doi: 10.1016/S0074-7742(06)78002-3. [DOI] [PubMed] [Google Scholar]
- Goulding SM, Holtzman CW, Trotman HD, Ryan AT, Macdonald AN, Shapiro DI, Brasfield JL, Walker EF. The prodrome and clinical risk for psychotic disorders. Child Adolesc Psychiatr Clin N Am. 2013;22:557–567. doi: 10.1016/j.chc.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dopamine system dysregulation by the hippocampus: implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology. 2012;62:1342–1348. doi: 10.1016/j.neuropharm.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dopamine system dysregulation and the pathophysiology of schizophrenia: insights from the methylazoxymethanol acetate model. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.11.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Regulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016 doi: 10.1038/nrn.2016.57. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984a;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984b;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333:271–284. doi: 10.1016/0006-8993(85)91581-1. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- Harrop C, Trower P. Why does schizophrenia develop at late adolescence? Clin Psychol Rev. 2001;21:241–265. doi: 10.1016/s0272-7358(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm (Vienna) 2007;114:893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2015;167:4–11. doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold CJ, Lasser MM, Schmid LA, Seidl U, Kong L, Fellhauer I, Thomann PA, Essig M, Schroder J. Hippocampal volume reduction and autobiographical memory deficits in chronic schizophrenia. Psychiatry Res. 2013;211:189–194. doi: 10.1016/j.pscychresns.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Hoareau C, Hazane F, Le Pen G, Krebs MO. Postnatal effect of embryonic neurogenesis disturbance on reelin level in organotypic cultures of rat hippocampus. Brain Res. 2006;1097:43–51. doi: 10.1016/j.brainres.2006.04.075. [DOI] [PubMed] [Google Scholar]
- Holtzman CW, Trotman HD, Goulding SM, Ryan AT, Macdonald AN, Shapiro DI, Brasfield JL, Walker EF. Stress and neurodevelopmental processes in the emergence of psychosis. Neuroscience. 2013;249:172–191. doi: 10.1016/j.neuroscience.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, Murray RM, McGuire P. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am. J. Psychiatry. 2011;168:1311–1317. doi: 10.1176/appi.ajp.2011.11010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeill T, Chan P, Forno LS, Murphy GM, Jr, Di Monte DA, Sandy MS, Langston JW. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3:251–265. [PubMed] [Google Scholar]
- Janowsky DS, Leichner P, Parker D, Judd L, Huey L, Clopton P. Methylphenidate and serum prolactin in man. Psychopharmacology (Berl) 1978;58:43–47. doi: 10.1007/BF00426788. [DOI] [PubMed] [Google Scholar]
- Kalus P, Bondzio J, Federspiel A, Muller TJ, Zuschratter W. Cell-type specific alterations of cortical interneurons in schizophrenic patients. Neuroreport. 2002;13:713–717. doi: 10.1097/00001756-200204160-00035. [DOI] [PubMed] [Google Scholar]
- Kapur S, Remington G. Dopamine D(2) receptors and their role in atypical antipsychotic action: still necessary and may even be sufficient. Biol Psychiatry. 2001;50:873–883. doi: 10.1016/s0006-3223(01)01251-3. [DOI] [PubMed] [Google Scholar]
- Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, O’Donovan M, Correll CU, Kane JM, van Os J, Insel TR. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Giedd J, Lau JY, Lewis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ. Inflammation and schizophrenia. Schizophr Bull. 2013;39:1174–1179. doi: 10.1093/schbul/sbt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig T, van Swam C, Dierks T, Hubl D. Is gamma band EEG synchronization reduced during auditory driving in schizophrenia patients with auditory verbal hallucinations? Schizophr Res. 2012;141:266–270. doi: 10.1016/j.schres.2012.07.016. [DOI] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131:165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Gourevitch R, Hazane F, Hoareau C, Jay TM, Krebs MO. Peri-pubertal maturation after developmental disturbance: a model for psychosis onset in the rat. Neuroscience. 2006;143:395–405. doi: 10.1016/j.neuroscience.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Lee H, Dvorak D, Kao HY, Duffy AM, Scharfman HE, Fenton AA. Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron. 2012;75:714–724. doi: 10.1016/j.neuron.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichenstein SD, Verstynen T, Forbes EE. Adolescent brain development and psychopathology: a case for connectivity of the anterior cingulate cortex in affective and substance use. Biol Psychiatry. 2016 doi: 10.1016/j.neubiorev.2016.07.024. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA. Neurobiology and the natural history of schizophrenia. J Clin Psychiatry. 2006;67:e14. [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK Clinical Antipsychotic Trials of Intervention Effectiveness, I. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mackowiak M, Bator E, Latusz J, Mordalska P, Wedzony K. Prenatal MAM administration affects histone H3 methylation in postnatal life in the rat medial prefrontal cortex. Eur Neuropsychopharmacol. 2014;24:271–289. doi: 10.1016/j.euroneuro.2013.05.013. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. The effect of Delta9-tetrahydrocannabinol on sensorimotor gating in socially isolated rats. Behav Brain Res. 2006;166:101–109. doi: 10.1016/j.bbr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Zipursky RB, Perkins D, Addington J, Miller T, Woods SW, Hawkins KA, Hoffman RE, Preda A, Epstein I, Addington D, Lindborg S, Trzaskoma Q, Tohen M, Breier A. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163:790–799. doi: 10.1176/ajp.2006.163.5.790. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Yung AR, Phillips LJ, Yuen HP, Francey S, Cosgrave EM, Germano D, Bravin J, McDonald T, Blair A, Adlard S, Jackson H. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59:921–928. doi: 10.1001/archpsyc.59.10.921. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res. 2009;204:322–334. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2010;36:607–623. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, Gallinat J, Giedd J, Grayson DR, Heinrichs M, Kahn R, Krebs MO, Leboyer M, Lewis D, Marin O, Marin P, Meyer-Lindenberg A, McGorry P, McGuire P, Owen MJ, Patterson P, Sawa A, Spedding M, Uhlhaas P, Vaccarino F, Wahlestedt C, Weinberger D. Altering the course of schizophrenia: progress and perspectives. Nat Rev Drug Discov. 2016 doi: 10.1038/nrd.2016.28. In press. [DOI] [PubMed] [Google Scholar]
- Milner TA, Burstein SR, Marrone GF, Khalid S, Gonzalez AD, Williams TJ, Schierberl KC, Torres-Reveron A, Gonzales KL, McEwen BS, Waters EM. Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse. 2013;67:757–772. doi: 10.1002/syn.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G, Allen P, Grace AA, McGuire P. Translating the MAM model of psychosis to humans. Trends Neurosci. 2015;38:129–138. doi: 10.1016/j.tins.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60:253–264. doi: 10.1016/j.biopsych.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Krause D, Dehning S, Musil R, Schennach-Wolff R, Obermeier M, Moller HJ, Klauss V, Schwarz MJ, Riedel M. Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res. 2010;121:118–124. doi: 10.1016/j.schres.2010.04.015. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18:306–312. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–492. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. Cortical disinhibition in the neonatal ventral hippocampal lesion model of schizophrenia: new vistas on possible therapeutic approaches. Pharmacol Ther. 2012;133:19–25. doi: 10.1016/j.pharmthera.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Owens DG, Miller P, Lawrie SM, Johnstone EC. Pathogenesis of schizophrenia: a psychopathological perspective. Br J Psychiatry. 2005;186:386–393. doi: 10.1192/bjp.186.5.386. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips LJ, Francey SM, Edwards J, McMurray N. Stress and psychosis: towards the development of new models of investigation. Clin Psychol Rev. 2007;27:307–317. doi: 10.1016/j.cpr.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Yung AR, Yuen HP, Pantelis C, McGorry PD. Prediction and prevention of transition to psychosis in young people at incipient risk for schizophrenia. Am J Med Genet. 2002;114:929–937. doi: 10.1002/ajmg.b.10790. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophr Bull. 2011;37:1257–1269. doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology. 2012a;62:1273–1289. doi: 10.1016/j.neuropharm.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biol Psychiatry. 2009;66:1038–1046. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Piontkewitz Y, Bernstein HG, Dobrowolny H, Bogerts B, Weiner I, Keilhoff G. Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain Behav Immun. 2012b;26:353–363. doi: 10.1016/j.bbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Iyer SN, Faridi K, Joober R, Malla AK. Stress and protective factors in individuals at ultra-high risk for psychosis, first episode psychosis and healthy controls. Schizophr Res. 2011;129:29–35. doi: 10.1016/j.schres.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Delrahim KK, Bresee CJ, Maddux RE, Ahmadpour O, Dolnak D. Celecoxib augmentation of continuously ill patients with schizophrenia. Biol Psychiatry. 2005;57:1594–1596. doi: 10.1016/j.biopsych.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Taylor B, Welge JA, Ahlbrand R, Ostrander MM, Burr J, Hayes S, Coolen LM, Pritchard LM, Logue A, Herman JP, McNamara RK. Risperidone pretreatment prevents elevated locomotor activity following neonatal hippocampal lesions. Neuropsychopharmacology. 2006;31:77–89. doi: 10.1038/sj.npp.1300791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann N Y Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, Krause KH. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology. 2005;30:944–957. doi: 10.1038/sj.npp.1300634. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. The effect of chronic peripubertal cannabinoid treatment on deficient object recognition memory in rats after neonatal mPFC lesion. Eur Neuropsychopharmacol. 2007;17:180–186. doi: 10.1016/j.euroneuro.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623. doi: 10.1038/tp.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, et al. A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378:176–179. doi: 10.1038/378176a0. [DOI] [PubMed] [Google Scholar]
- Silins E, Horwood LJ, Patton GC, Fergusson DM, Olsson CA, Hutchinson DM, Spry E, Toumbourou JW, Degenhardt L, Swift W, Coffey C, Tait RJ, Letcher P, Copeland J, Mattick RP Cannabis Cohorts Research, C. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1:286–293. doi: 10.1016/S2215-0366(14)70307-4. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Purves-Tyson TD, Allen KM, Weickert CS. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology (Berl) 2014;231:1581–1599. doi: 10.1007/s00213-013-3415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40:181–191. doi: 10.1093/schbul/sbt139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Canbugcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, Cuenod M, Do KQ. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;17:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Thompson JL, Pogue-Geile MF, Grace AA. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophr Bull. 2004;30:875–900. doi: 10.1093/oxfordjournals.schbul.a007139. [DOI] [PubMed] [Google Scholar]
- Tse MT, Piantadosi PT, Floresco SB. Prefrontal cortical gamma-aminobutyric acid transmission and cognitive function: drawing links to schizophrenia from preclinical research. Biol Psychiatry. 2015;77:929–939. doi: 10.1016/j.biopsych.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007a;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007b;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ. Dysconnectivity, large-scale networks and neuronal dynamics in schizophrenia. Curr Opin Neurobiol. 2013;23:283–290. doi: 10.1016/j.conb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol. 2002;156:319–327. doi: 10.1093/aje/kwf043. [DOI] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prefrontal cortical circuits in schizophrenia. Curr Top Behav Neurosci. 2010;4:485–508. doi: 10.1007/7854_2010_44. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Wang J, O'Donnell P. D(1) dopamine receptors potentiate nmda-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex. 2001;11:452–462. doi: 10.1093/cercor/11.5.452. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Green MF, Tuma AH. Long-term morbidity associated with delayed treatment of first admission schizophrenic patients: a re-analysis of the Camarillo State Hospital data. Psychol Med. 1997;27:261–268. doi: 10.1017/s0033291796004345. [DOI] [PubMed] [Google Scholar]
- Wykes T, Reeder C, Landau S, Matthiasson P, Haworth E, Hutchinson C. Does age matter? Effects of cognitive rehabilitation across the age span. Schizophr Res. 2009;113:252–258. doi: 10.1016/j.schres.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell'Olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- Zamberletti E, Beggiato S, Steardo L, Jr, Prini P, Antonelli T, Ferraro L, Rubino T, Parolaro D. Alterations of prefrontal cortex GABAergic transmission in the complex psychotic-like phenotype induced by adolescent delta-9-tetrahydrocannabinol exposure in rats. Neurobiol Dis. 2014;63:35–47. doi: 10.1016/j.nbd.2013.10.028. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Lim CK, Purves-Tyson T, Karl T, Kassiou M, Banister SD, Guillemin GJ, Weickert CS. Effect of maternal immune activation on the kynurenine pathway in preadolescent rat offspring and on MK801-induced hyperlocomotion in adulthood: amelioration by COX-2 inhibition. Brain Behav Immun. 2014;41:173–181. doi: 10.1016/j.bbi.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Zimmerman EC, Bellaire M, Ewing SG, Grace AA. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38:2131–2139. doi: 10.1038/npp.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]