Abstract
Adolescence is defined as a transitional period between childhood and adulthood characterized by changes in social interaction and acquisition of mature cognitive abilities. These changes have been associated with the maturation of brain regions involved in the control of motivation, emotion, and cognition. Among these regions, the protracted development of the human prefrontal cortex during adolescence has been proposed to underlie the maturation of cognitive functions and the regulation of affective responses. Studies in animal models allow us to test the causal contribution of specific neural processes in the development of the prefrontal cortex and the acquisition of adult behavior. This review summarizes the cellular and synaptic mechanisms occurring in the rodent prefrontal cortex during adolescence as a model for understanding the changes underlying human prefrontal development.
Keywords: adolescence, prefrontal cortex, interneurons, GABA, dopamine, glutamate, cannabinoid
1. Adolescence in animal models
Adolescence is typically defined as a transitional period between childhood and adulthood. However, it is important to highlight that the exact span of adolescence varies across the different species likely due to genetic, environmental, and social factors. Behaviorally, adolescence is characterized by increased experimentation, changes in social interaction, and cognitive development with the ultimate goal of achieving independence and skills required for survival as an adult (Spear, 2000). Such changes in behavior have been rightly associated with the development and maturation of brain regions and neuronal circuitry involved in the control of motivation, emotion, and cognition. Nonetheless, the precise neurobiological mechanisms underlying the maturation of human behavior during adolescence can only be inferred from psychological and imaging studies in combination with behavioral pharmacology and data collected from post-mortem brain samples. Studies in animal models allow us to test hypotheses based on these clinical observations, and determine the causal contribution of specific neural processes in the regulation of each maturational event in acquisition of adult behavior.
Similar to their human counterparts, non-human primates display risk-taking, novelty seeking, and increased vigilance during adolescence (Spear, 2000). In laboratory rodents, adolescence is accompanied by a peak in play behavior, increased exploratory activity and impulsivity, and can be conservatively defined within postnatal days (P) 30 to 50 (Spear, 2000). Despite the behavioral idiosyncrasies of each species, a common developmental theme during adolescence is the acquisition of mature cognitive abilities in the domains of decision-making, behavioral inhibition, and working memory, all of which have been ascribed to the maturation of specific functional domains within the prefrontal cortex (PFC). The PFC integrates contextual and emotional information required for goal-directed behaviors and affect regulation. The importance of understanding the developmental trajectory of the PFC is underscored by multiple findings showing its impaired function in addiction (Chambers et al., 2003) and psychiatric diseases whose onset occurs during the periadolescent period, such as schizophrenia and affective disorders (Hoftman and Lewis, 2011).
We and others have been able to identify clear neurobiological changes occurring during adolescence in rodent models that carry deep significance for PFC maturation. Among these are pre- and postsynaptic differences in neurotransmission and the gain of neuromodulatory capacity that ultimately affect PFC processing of afferent information and output.
2. Anatomical and neurochemical changes in the PFC during adolescence
This review focuses on the developmental changes affecting the PFC because of its well-described role in acquisition of mature cognitive abilities across several species (Fuster, 2001). Indeed, the PFC integrates information from many cortical and subcortical structures including the ventral hippocampus, the amygdala and the mediodorsal thalamus, and also receives neuromodulatory inputs from catecholaminergic and serotonergic nuclei in the brainstem.
Early imaging analyses of developmental trajectories in humans established that the dorsal-ventrolateral cortex and the medial temporal lobe, which includes the hippocampus and the amygdala, undergo significant changes in volume from late childhood to adulthood (Sowell and Jernigan, 1998). During the first two decades of life, the gray matter in the frontal cortex experiences a significant decrease in volume at the same time that temporal structures increase (Sowell and Jernigan, 1998; Suzuki et al., 2005). The consistent thinning of neocortical structures observed in humans in cross-sectional and longitudinal studies (Giedd et al., 1999; Gogtay et al., 2004; Mills et al., 2014; Sowell et al., 2003) occurs at a time of synaptic pruning (Huttenlocher and Dabholkar, 1997; Huttenlocher et al., 1982; Petanjek et al., 2011) of presumably glutamatergic synapses, whereas the increase in temporal lobe’s volume is thought to result from the elevated myelination occurring in the hippocampal formation starting in adolescence (Benes, 1989; Benes et al., 1994). Importantly, the reported anatomical and connectivity changes experienced by frontal structures (Paus et al., 1999) are associated with the protracted maturation of working memory (Satterthwaite et al., 2013) and increased emotional regulation (Gee et al., 2013; Swartz et al., 2014) during adolescence.
To understand whether inputs to the PFC affect its functional maturation, we have focused on the developmental trajectories of specific afferents to the rat PFC, especially the ones where anatomical evidence of a peri-adolescent change has been reported.
2.1. Dopamine innervation in the PFC
The role of dopamine in the modulation of PFC transmission was reported shortly after the unequivocal identification of dopamine in the cortex (Thierry et al., 1973). Dopamine innervation in the rodent PFC can be detected early after birth, starting in the deep layers of the cortex (Kalsbeek et al., 1988). This innervation changes qualititatively and quantitatively in fiber caliber and density at different rates within the PFC, reaching a stable state between P20 and P35 for the supragenual region of the PFC. Dopamine innervation continues to develop in prelimbic PFC areas until P60, after which there are no visible changes. Of note, dopamine fibers are found in abundance in layer I and layers V–VI, with fewer fibers in layer III. Similar distribution patterns of dopamine innervation can be detected in the primate PFC with subtle variations depending on the subregion and primate species used in the study. However, dopamine innervation in the primate PFC is typically more extensive at the regional and laminar level than in the rodent PFC (Goldman-Rakic et al., 1989; Lewis et al., 1987; Lewis et al., 1998; Raghanti et al., 2008). A preliminary analysis of the developmental trajectory of dopamine fibers in rhesus monkey suggests that cortical layers experience a peak in dopamine innervation during adolescence, albeit this change was only significant in layer III where the lowest abundance of dopamine fibers is found in this species (Rosenberg and Lewis, 1994). Importantly, the only comparative analysis made among primates suggests that the primary difference in dopamine innervation between apes and other old world monkeys is the more even distribution displayed across cortical layers for the latter (Raghanti et al., 2008). Overall, the anatomical distribution of dopamine innervation would predict that PFC output to subcortical areas through layers V–VI would be highly sensitive to dopamine modulation in all species, whereas the effects of dopamine in layers II–III remain best studied in great apes.
Dopamine terminals in both rodent and primates are remarkably similar at the ultrastructural level forming contacts onto somas, spines, and dendritic shafts, particularly in the distal region of the dendritic tree (Goldman-Rakic et al., 1989; Seguela et al., 1988). Dopamine terminals usually co-exist with an asymmetric (likely excitatory) synapse (Goldman-Rakic et al., 1989; Verney et al., 1990) and have been found closely apposed to dendritic processes and somas of GABA-positive cells (Verney et al., 1990). The highest frequency of such DA-GABA apposition was found within layers V–VI in the rat medial PFC (Benes et al., 1993). Some GABA-positive soma or dendrites in close contiguity with dopamine terminals also receive GABA-positive axonal inputs from local interneurons (Sesack et al., 1995; Verney et al., 1990). Given the number of potential contacts, the exact ontogeny and outcome of each interaction during development has not been fully described, with the exception of a significant increase in dopamine contacts onto layers V–VI GABAergic cells observed in rats during the transition to young adulthood (P60) (Benes et al., 1996). It was later demonstrated that dopamine terminals preferentially contact a subclass of GABAergic cells that express parvalbumin (Sesack et al., 1998).
The distribution of cortical dopamine receptors in primates and rodents follows closely the anatomical pattern of dopamine innervation (Gaspar et al., 1995; Muly et al., 1998). Overall, almost all pyramidal neurons and GABAergic interneurons in the PFC express both classes of D1 and D2 receptors to some extent (Bouthenet et al., 1987; Gaspar et al., 1995; Muly et al., 1998; Santana et al., 2009). In rodents, approximately 90–80% of D1 and D2 receptors co-localize in pyramidal neurons, with layers V–VI displaying the highest expression level (Santana et al., 2009; Vincent et al., 1993). The few studies measuring dopamine receptor binding have shown that both D1 and D2 receptors increase globally in the frontal cortex until young adulthood (P60) with no reported receptor pruning (reviewed in (Tarazi and Baldessarini, 2000)).
2.2. Hippocampal innervation in the PFC
Afferents originating from the hippocampal formation make monosynaptic contacts onto PFC neurons (Carr and Sesack, 1996; Ferino et al., 1987; Hoover and Vertes, 2007; Swanson, 1981). Of particular interest is the ventral hippocampal-to-PFC glutamatergic pathway, which innervates largely pyramidal output neurons through asymmetric excitatory axospinous contacts (Carr and Sesack, 1996) and a subclass of GABAergic interneurons expressing parvalbumin (Gabbott et al., 2002). More importantly, the integrity of this pathway is needed for sustaining proper working memory processes (Floresco et al., 1997; Friedman and Goldman-Rakic, 1988; Wang and Cai, 2006), a cognitive function that becomes mature during young adulthood (Luna et al., 2004).
Imaging studies in humans have revealed that increases in total hippocampal volume do occur during postnatal development, some of which extend to the late adolescence/young adulthood period depending on the sub-region analyzed (Goddings et al., 2014; Gogtay et al., 2006; Suzuki et al., 2005). Nonetheless, there is a paucity of anatomical information on the timing by which ventral hippocampal innervation to the PFC reaches maturity during adolescence, with the exception of the studies by Benes et al described above (Benes, 1989; Benes et al., 1994). Newer technologies, in particular diffusion tensor imaging tractography, promise to fill this gap in knowledge and address when anatomical hippocampal-PFC connectivity arises during development and whether its integrity shows specific periods of vulnerability (Fani et al., 2015; Robinson et al., 2015).
2.3. Amygdalar innervation in the PFC
The PFC also receives monosynaptic glutamatergic inputs from the amygdala (Bacon et al., 1996; Krettek and Price, 1977; McDonald, 1996; Verwer et al., 1996), a pathway that is involved in the consolidation of learning and memory, and emotional regulation (Davis and Whalen, 2001). Proper strengthening of the amygdalar-PFC connectivity is thought to be required for PFC modulation of amygdala-dependent behaviors (Garcia et al., 1999; Hariri et al., 2003). In fact, early life disruption of such connectivity has been correlated to some extent with the severity of affective disorders (Burghy et al., 2012).
Amygdalocortical fibers can be observed shortly after birth with a bilaminar pattern arising in the medial PFC between P12 and P16 in rats (Cunningham et al., 2002; Verwer et al., 1996). This pattern reflects preferential innervation of pyramidal neurons of layers II and V of the PFC, as the majority of these contacts form asymmetric excitatory axospinous synapses (Cunningham et al., 2002), and innervation of GABAergic interneurons through axosomatic and axodendritic contacts (Cunningham et al., 2008; Gabbott et al., 2006). The density of amygdalar innervation continues to increase in both layers II and V up to P65, with layer V of the infralimbic region of the PFC experiencing the greatest increase (Cunningham et al., 2002). These results indicate that the amygdala projection to the PFC increases progressively until early adulthood and is likely to regulate prefrontal output through its action on pyramidal cells and interneurons.
3. Functional remodeling of PFC neurocircuitry during adolescence
The structural changes occurring during adolescence are modest compared to the ones experienced after birth and during childhood, yet magnification of this developmental window reveals that these subtle changes in “hardware” carry a profound significance in the refinement of neuronal signaling. The follow sections will review major findings from our laboratory and others highlighting adolescence as a critical period for the maturation of both glutamatergic and GABAergic transmission in the PFC, particularly due to a differential weight of neuromodulators such as monoamines and cannabinoids. The discovery of dopamine as a bona fide neurotransmitter in the PFC opened the question of whether it played a role in motivated behavior similar to what had been described in striatal circuits. More recently, the cannabinoid system has emerged as a master regulator of PFC plasticity. Both of these systems also display unique age-dependent changes that directly impact PFC activity.
3.1. Modulation of PFC activity by dopamine
Dopamine modulation of PFC activity has been implicated in the regulation of key cognitive processes, many of which mature during adolescence such as working memory, inhibitory control and attention (Goldman-Rakic et al., 2000; Horvitz, 2000; Jay, 2003; O'Donnell, 2003). At the cellular level, dopamine’s action in the PFC is dictated by the functional state of local excitatory and inhibitory activity during cortical maturation (O'Donnell, 2010; Tseng et al., 2009). It has been proposed that dopamine may facilitate the maturation of PFC-dependent cognitive functions by virtue of a D1 receptor-dependent enhancement of prefrontal NMDA transmission (O'Donnell, 2010; Tseng et al., 2009) given the fact that activation of D1 receptors in the PFC improves memory retrieval and working memory (Floresco and Phillips, 2001; Seamans et al., 1998), and co-activation of D1 and NMDA receptors in the PFC is required for appetitive instrumental learning in adult rats (Baldwin et al., 2002).
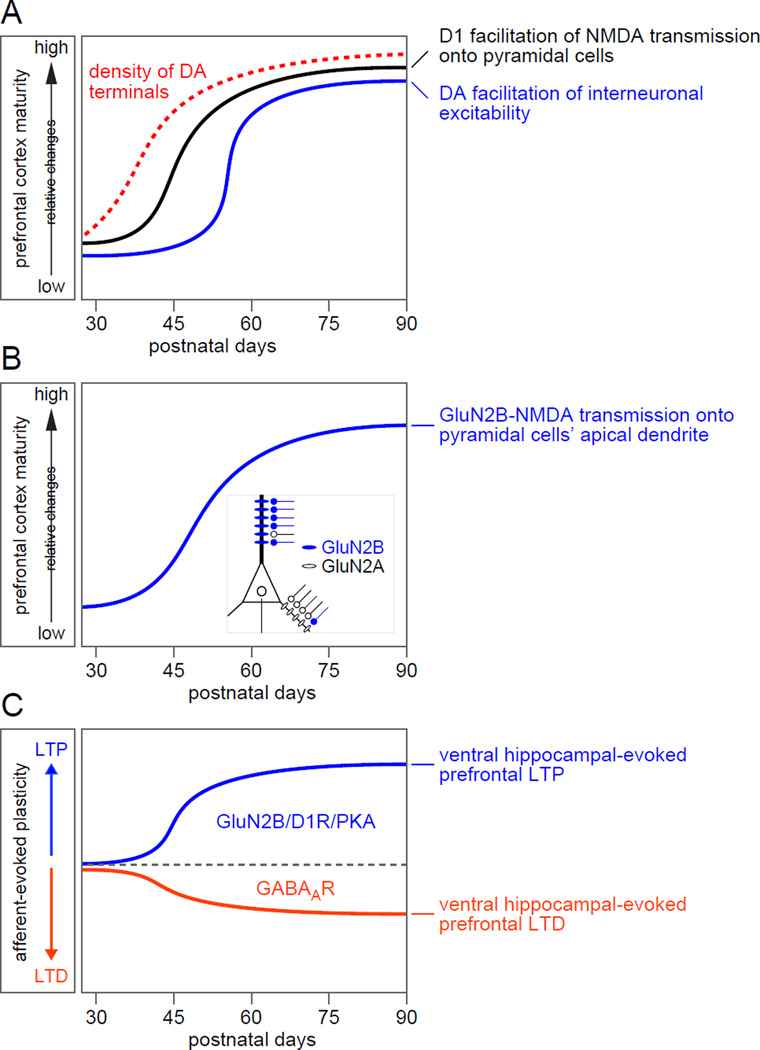
Dopamine D1 receptor stimulation in the adult PFC can elicit long-lasting plateau depolarizations in pyramidal neurons, an effect that requires NMDA receptor transmission and postsynaptic calcium-dependent signaling (Tseng and O'Donnell, 2005). Such D1 receptor-mediated facilitation of plateau depolarizations in the PFC emerges only after P45 (Tseng and O'Donnell, 2005) (Fig 1A), when dendritic sodium and calcium regenerative transients become effective in coupling distal apical dendritic activity with somata to sustain plateau potentials in pyramidal neurons (Heng et al., 2011b; Zhu, 2000). Certainly, D1-NMDA interactions could differentially regulate PFC synaptic function depending on the stage of cortical maturation and the level of receptor expression. Thus, an important feature to enable the development of a functionally mature PFC network includes the acquisition of adult levels of D1 (Flores-Barrera et al., 2014; Leslie et al., 1991; Monyer et al., 1994; Tarazi and Baldessarini, 2000; Tarazi et al., 1999; Williams, 1993; Williams et al., 1993) and NMDA receptors combined with intrinsic physiological changes during adolescence (Heng et al., 2011b; Tseng and O'Donnell, 2005; Zhu, 2000). The late adolescent emergence of D1 receptor-mediated facilitation of NMDA receptor transmission (Flores-Barrera et al., 2014; Tseng and O'Donnell, 2005) would therefore have a strong impact in regulating PFC plasticity during the transition to adulthood and the subsequent acquisition of cognitive abilities associated with adult behaviors.
Figure 1. Postnatal development of dopamine and glutamatergic transmission in the PFC.
(A) D1 receptor modulation of NMDA transmission in deep-layer pyramidal neurons increases after P45 to reach steady state, adult level by around P60 (Tseng and O’Donnell 2005; Flores-Barrera et al., 2014). A similar pattern of dopamine (DA) innervation was observed in the PFC. Dopaminergic fibers can be found in deep layers soon after birth, yet the density of DA innervation in the prelimbic cortex continues to increase until P60 (Kalsbeek et al, 1988). Similarly, DA modulation of GABAergic activity in the PFC undergoes developmental regulation such that D1 and D2 receptor-mediated facilitation of interneuronal excitability becomes markedly enhanced after P50 (Tseng and O’Donnell 2007). (B) GluN2B-NMDA receptor transmission begins to emerge in the apical dendrite of layer V pyramidal neurons in the PFC around P45 (Flores-Barrera et al., 2014). This developmental change is required to strengthening PFC processing of ventral hippocampal inputs (Flores-Barrera et al., 2014) and to enable the increased D1 receptor modulation of NMDA-mediated responses described in A. (C) PFC processing of ventral hippocampal drive is also developmentally regulated as revealed by the mechanisms contributing to the induction of prefrontal LTP and LTD following high-frequency stimulation of the ventral hippocampus (Caballero et al., 2014; Flores-Barrera et al., 2014). While prefrontal LTP is dependent on local GluN2B-NMDA transmission, D1 receptor (D1R) activation and protein-kinase A (PKA) signaling, prefrontal LTD relies on the recruitment of local GABAergic interneurons and GABA-A receptor (GABAAR)-mediated transmission.
Dopamine regulation of PFC inhibition is also developmentally regulated. Despite the initial in vivo studies revealing that dopamine exerts an overall inhibitory effect on PFC activity (Bunney and Aghajanian, 1976; Ferron et al., 1984; Lewis and O'Donnell, 2000), the precise underlying mechanism of such inhibitory action remained unclear and controversial for many decades. In addition to the inhibition mediated by D2 receptor stimulation (Tseng and O'Donnell, 2004), GABAergic interneurons in the PFC also express D1 and D2 receptors (Gaspar et al., 1995; Mrzljak et al., 1996; Muly et al., 1998; Smiley et al., 1994; Vincent et al., 1993), and evidence suggest that part of the inhibitory action of dopamine in the PFC is due to local facilitation of GABAergic activity (Gorelova et al., 2002; Gulledge and Jaffe, 1998; Pirot et al., 1992; Tseng et al., 2006; Tseng and O'Donnell, 2004). Recordings of GABAergic interneurons in the juvenile PFC (P15–35) revealed that only D1, not D2 receptors, increase excitability of fast-spiking interneurons (Gorelova et al., 2002; Tseng et al., 2006). It is during late adolescence (after P50; Fig 1A) that a powerful excitatory action by D2 receptor signaling onto GABAergic activity emerges in the PFC (Tseng et al., 2006; Tseng and O'Donnell, 2007). This is perhaps one of the most interesting and unexpected finding ever described in the field of dopamine. While dopamine control of GABAergic activity in the juvenile/immature PFC is strictly D1 receptor-mediated, the net effect of mesocortical dopamine in the adult PFC is to drive GABAergic firing (fast-spiking and non-fast spiking interneurons) by both D1- and D2 receptor-dependent mechanisms (Tseng et al., 2006; Tseng and O'Donnell, 2007). Thus, the involvement of both dopamine receptors in facilitating prefrontal GABAergic activity presumably causes a powerful inhibitory control in the PFC and influences the timing and spatial selectivity of local ensembles and their computational capacity (Lew and Tseng, 2014).
In summary, the late adolescent acquisition of dopamine-dependent control of excitatory and inhibitory transmission could provide a critical neurobiological step to fine-tuning PFC output activity responsible for the maturation of cognitive processes. A more efficient recruitment of prefrontal GABAergic activity by dopamine will limit PFC neuronal firing in response to asynchronous inputs, whereas the arrival of strong coincident excitatory inputs (e.g., from the hippocampus) is expected to favor a D1 receptor-mediated facilitation of NMDA receptor transmission (Flores-Barrera et al., 2014) such that the representation encoded in the PFC would be reinforced and synchronized ensembles of neuronal activity would be enabled.
3.2. Glutamatergic control of PFC activity
As summarized above, dopamine-dependent regulation of neuronal excitability in the PFC increases during adolescence in a manner that correlates to some extent with the augmented L-type Ca2+ function and postsynaptic PKA-dependent signaling observed after P40 (Heng et al., 2011b). In addition to these postsynaptic changes, it has long been recognized that proper coordination of input-specific presynaptic activity also contributes to PFC maturation during adolescence (Maroun and Richter-Levin, 2003; Tseng et al., 2009). Of particular interest is the developmental regulation of glutamatergic inputs carrying contextual and emotional information from the ventral hippocampus (Floresco et al., 1997; Seamans et al., 1998) and amygdala (Garcia et al., 1999; Gilmartin and Helmstetter, 2010; Milad and Quirk, 2012), as disruptions of these pathways impact the acquisition of cognitive abilities associated with adult behavior (Best and Miller, 2010; Casey et al., 2000; Tseng et al., 2009). Certainly, ongoing remodeling of several anatomical features within the hippocampal-PFC and amygdalar-PFC connectivity continues throughout adolescence (Benes, 1989; Cressman et al., 2010; Cunningham et al., 2002).
At the functional level, changes in PFC response to ventral hippocampal stimulation have been studied for some time in adult animals (Jay, 2003). We have learned from these studies that the monosynaptic hippocampal-PFC pathway is modulated by NMDA receptor transmission and D1 receptor-mediated signaling (Gurden et al., 2000), yet little is known of how its functional connectivity changes during adolescence. Our recent studies in rats revealed that glutamatergic plasticity within the hippocampal-PFC pathway undergoes distinct developmental trajectory from that elicited from the basolateral amygdala. While stimulation of the amygdala induces a form of LTP in the PFC that is already enabled by P30, prefrontal LTP elicited following hippocampal stimulation does not emerge until P50 (Caballero et al., 2014; Flores-Barrera et al., 2014). Notably, both forms of LTP require activation of NMDA receptors in the PFC. Interestingly, an intact GluN2B transmission was required only for sustaining prefrontal LTP elicited from the ventral hippocampus (Flores-Barrera et al., 2014). Together, these results indicate that the delayed strengthening of the ventral hippocampal-PFC pathway is dictated by the late-adolescent expression of GluN2B function in the PFC (Fig 1B).
A change in the subunit composition of NMDA receptors has been shown to directly impact plasticity in cortical circuits (Zhao et al., 2005) with a decreasing contribution of GluN2B to that of GluN2A transmission over the course of postnatal development (Dumas, 2005; Wang et al., 2008). However, recent studies have challenged this traditional view by highlighting that GluN2B-containing NMDA receptors are key for a variety of PFC-dependent functions in adult animals including working memory processes (Wang et al., 2008; Wang et al., 2013) and trace fear conditioning (Gilmartin et al., 2013). Accordingly, we found that GluN2B transmission in the PFC does emerges late in adolescence (Fig 1B) to enable the expression of hippocampal-to-PFC LTP in an input-specific manner (Fig 1C) (Flores-Barrera et al., 2014). Interestingly, the functional acquisition of GluN2B transmission in the PFC was observed only at glutamatergic synapses driving the apical dendrite of layer V pyramidal neurons after P50 (Fig 1B) (Flores-Barrera et al., 2014). Note that this compartment-specific developmental event takes place ~20 days after major structural remodeling of apical dendritic length and complexity has been completed (i.e., P30; (Heng et al., 2011b; Zhu, 2000)), and ~1week after the apical dendrite becomes functionally coupled to the soma (i.e., P42; (Zhu, 2000)). The slow kinetic of GluN2B-containing NMDA receptors (Vicini et al., 1998) certainly provides a functional advantage for computing input integration (Wang, 1999) and selectively amplify contextually salient information originating from ventral hippocampus. Thus, the late adolescent incorporation of GluN2B transmission into a functionally mature apical dendrite could be seen as a key neurobiological step towards PFC maturation by virtue of enhancing the input-specific capacity of afferent integration and processing.
In addition to LTP, ventral hippocampal stimulation can potentiate GABAergic transmission in the PFC (see below section 3.3) and subsequently induce a form of LTD that also emerges around P50 (Fig 1C) (Caballero et al., 2014; Thomases et al., 2014). Interestingly, plasticity elicited from the basolateral amygdala typically results in prefrontal LTP instead of LTD (Caballero et al., 2014; Thomases et al., 2014) despite the fact that amygdalar inputs do drive feedforward inhibition in the PFC via activation of local interneurons (Dilgen et al., 2013). It is therefore conceivable that ventral hippocampal inputs exert a much powerful control of prefrontal GABAergic plasticity than those from the basolateral amygdala. Thus, the late-adolescent onset of hippocampal-dependent LTP and LTD could contribute to the functional maturation of PFC processing of context-dependent information through an input-specific remodeling of pre/postsynaptic mechanisms. More research is warranted to identify the precise role of LTP and LTD at later stages of brain maturation.
3.3. Local GABAergic regulation of PFC activity
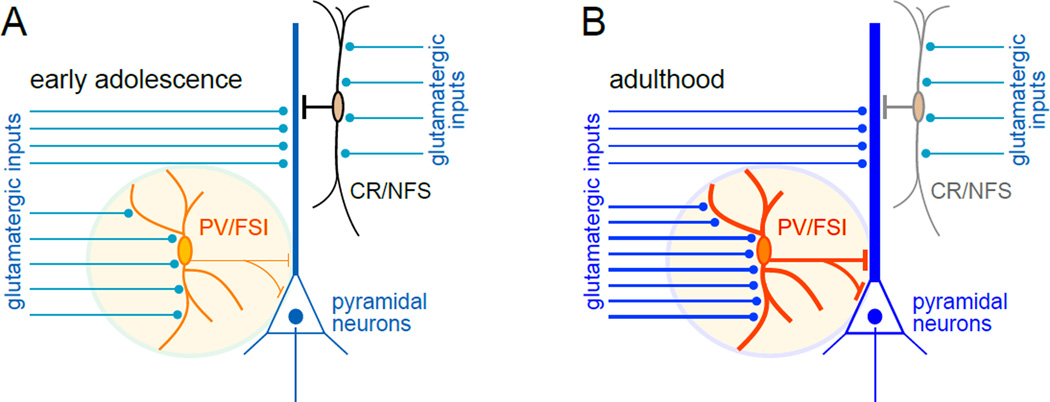
In addition to the different mechanisms contributing to the refinement of glutamatergic activity in the PFC, local GABAergic transmission also undergoes major functional remodeling during adolescence. The most consistent changes in prefrontal GABAergic function are at the level of local GABAergic interneurons, specifically those expressing the calcium binding proteins parvalbumin (PV) and calretinin (CR) (Caballero et al., 2013; Erickson and Lewis, 2002; Fung et al., 2010). These two populations show opposite developmental patterns during adolescence (Fig 2), such that PV interneurons upregulate their expression whereas CR decreases sharply during this period (Caballero et al., 2013). Given that PV expression strongly depends on glutamatergic signaling (Behrens et al., 2007), our results indicate this switch in dominance may be partially due to the observed increase in excitatory inputs selectively impinging upon PV-positive, fast-spiking interneurons during adolescence (Caballero et al., 2013). Moreover, these events coincide with a period of increased GABAergic activity onto layers V–VI pyramidal neurons detected as a 30% increase in the frequency of spontaneous postsynaptic inhibitory potentials (Cass et al., 2014). Together, these findings indicate that maturation of PV-positive/fast-spiking interneurons is likely responsible for the periadolescent increase in GABAergic transmission observed in the PFC (Fig 2). The contribution of other populations of GABAergic interneurons in this process remains to be determined.
Figure 2. Periadolescent changes in GABAergic function are associated with shifts in dominance of GABAergic populations in the PFC.
(A) Early adolescent rats exhibit lower levels of PV expression in the PFC when compared to adults, a developmental hallmark that is associated with diminished glutamatergic transmission onto PV-positive, fast-spiking interneurons (PV/FSI). On the other hand, the expression of CR in the PFC is higher during early adolescence and lower in adulthood. However, the levels of glutamatergic transmission onto CR-positive, non-fast spiking interneurons (CR/NFS) remain unchanged throughout adolescence. (B) In the adult PFC, the frequency of glutamatergic synaptic activity onto PV/FSI increases significantly in tandem with a marked upregulation of PV expression. No developmental changes are observed in glutamatergic transmission onto CR/NFS, yet there is a robust decrease of CR expression in adults. The identity of such glutamatergic inputs remains to be defined.
Among the postsynaptic factors that could account for the observed periadolescent increase in GABAergic transmission is the subunit switch in GABA-A receptors from α2 to α1. Such compositional changes have been shown to result in fast inhibitory transmission in other cortical regions due to the properties of α1-containing GABA-A receptors (Vicini et al., 2001). Recently, these subunit changes have been observed in the monkey dorsolateral PFC (Datta et al., 2014; Hashimoto et al., 2009), and are also likely to be driven by the advent of specific excitatory inputs to the PFC.
Our lab has studied the identity of the excitatory inputs that prompt maturational changes in GABAergic transmission in the PFC during adolescence. These studies have revealed the excitatory afferents from the ventral hippocampus to the PFC undergoes functional changes during adolescence (Thomases et al., 2013). Rats younger than P40 were not able to induce the typical hippocampal-induced inhibition observed in animals older than P45 or P60. Importantly, the adult pattern of inhibition was fully dependent on GABA-A receptor transmission as it was blocked with local application of picrotoxin in the PFC (Thomases et al., 2013). Whether specific PFC inputs (e.g., ventral hippocampus) trigger the maturation of GABAergic interneurons or prior GABAergic maturation is needed to enable PFC processing of hippocampal inputs remains unknown (Caballero and Tseng, 2012).
In any case, we do know that disturbances in dopamine and cannabinoid systems as well as NMDAR-mediated transmission during adolescence are sufficient to elicit a state of PFC disinhibition that can be traced to a disruption in prefrontal GABAergic signaling (Cass et al., 2014; Cass et al., 2013; Thomases et al., 2014; Thomases et al., 2013). In addition, the age-dependent maturation of PFC GABAergic function could explain the adolescent acquisition of cognitive functions and emotional regulation subserved by different PFC domains. One such functional implication is the hippocampal gating of amygdalar inputs in the PFC, which can only be detected after P40 (Thomases et al., 2014) and might be critical for the developmental acquisition of PFC control over amygdala-dependent processes.
3.4. PFC modulation by cannabinoids
The CB1 receptor undergoes a clear developmental regulation during postnatal development, which is accompanied by relative changes in the concentrations of endogenous cannabinoids (Berrendero et al., 1998). More specifically, CB1 receptor mRNA expression decreases from juveniles to adults in the cerebral cortex of rats (Berrendero et al., 1998). Similarly, we have found the levels of CB1 receptor mRNA decrease sharply during adolescence particularly in the limbic/associative regions of the PFC (Heng et al., 2011a). Although the functional impact of this reduction has not been fully examined, a developmental loss of CB1 receptors would predict an important change in the known endocannabinoid-mediated modulation of both glutamatergic and GABAergic transmission in the PFC (Auclair et al., 2000; Bacci et al., 2004; Bodor et al., 2005; Fortin and Levine, 2007; Harkany et al., 2004). Accordingly, we have found that glutamatergic synaptic transmission onto layer V pyramidal neurons in the adult PFC is subjected to less CB1 receptor-dependent presynaptic inhibition than the PFC of their juvenile/early adolescent counterparts (Heng et al., 2011a). Future studies are needed to assess whether a similar downregulation of CB1 receptor-dependent modulation of GABAergic function occurs in the PFC during adolescence. CB1 receptor expression can be found in PFC GABAergic interneurons (Tsou et al., 1998), in particular among the cholecystokinin-positive and calbindin-positive subpopulations (Eggan et al., 2010; Marsicano and Lutz, 1999; Wedzony and Chocyk, 2009).
The neurobiological significance of a developmental regulation of CB1 receptor expression and function during adolescence is not entirely understood, in part due to our limited knowledge of the specific neuronal circuits developing during adolescence. However, insights can be gained from epidemiological studies showing an association between cannabis abuse during adolescence and increased risk of developing psychosis and cognitive impairments later in life (Caspi et al., 2005; Henquet et al., 2005; Meier et al., 2012), although such a link is not cannabis specific (Chambers et al., 2003). Despite the complex mixture of natural cannabinoids present in cannabis (Elsohly and Slade, 2005), a common finding from these studies is the negative impact observed on cognitive functions associated with working memory and decision making processes (Kanayama et al., 2004; Meier et al., 2012; Schweinsburg et al., 2008; Solowij et al., 2002), many of which are refined during adolescence and depend on PFC maturation. Similar PFC-dependent deficits have been observed in rodent models following chronic exposure to CB1 receptor agonists during adolescence (O'Shea et al., 2004; Raver et al., 2013; Renard et al., 2013; Schneider and Koch, 2003; Schneider et al., 2008). Based on these studies, it has been proposed that a sustained elevation of CB1 receptor signaling in the PFC by exogenous cannabinoids could contribute to the cognitive deficits seen in chronic cannabis abusers (Caballero and Tseng, 2012; Realini et al., 2009). Our recent studies in rats indicate that the neuronal deficits induced by repeated cannabinoid exposure (i.e., CB1 receptor agonist administration) during adolescence are at the level of PFC GABAergic circuitry, an impairment that is strictly age-dependent such that GABAergic functionality is unaffected when cannabinoid treatment occurs in adulthood (Cass et al., 2014). Such specific windows of susceptibility to cannabinoids suggest that untimely CB1 receptor stimulation during adolescence hinders the proper development of the PFC. The exact mechanisms by which CB1 receptor signaling enable PFC maturation remain to be determined.
4. Summary and Conclusions
The progressive structural and neurochemical adjustments combined with distinctive physiological changes occurring in the PFC during adolescence are crucial for establishing appropriate information processing mechanisms that can support more complex behavioral outcomes. As reviewed here, susceptibility to developmental insults is certainly high during the periadolescent transition period, an impact that is likely to disrupt PFC maturation and functioning through adulthood (Cass et al., 2014; Cass et al., 2013; Thomases et al., 2014; Thomases et al., 2013). We propose that any early developmental event capable of altering the mechanisms underlying the protracted trajectory of PFC maturation and functional remodeling could result in abnormal assembly of PFC circuits, which would not become apparent until adolescence when proper fine tuning of PFC GABAergic activity is integrated to enable input selectivity and increase PFC output capacity (Lew and Tseng, 2014).
Highlights.
Adolescence occurs in mammals as a period for acquisition of prefrontal maturity
Dramatic functional changes occur in the prefrontal cortex during adolescence
Prefrontal maturation is required to support complex behavioral outcomes
Preventing such maturation leads to an inadequate control of prefrontal output
Acknowledgments
Grant sponsor: National Institute of Mental Health grants R01-MH086507 and R01-MH105488
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 1996;720:211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Kelley AE. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci. 2002;22:1063–1071. doi: 10.1523/JNEUROSCI.22-03-01063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Molloy R. Dopamine-immunoreactive axon varicosities form nonrandom contacts with GABA-immunoreactive neurons of rat medial prefrontal cortex. Synapse. 1993;15:285–295. doi: 10.1002/syn.890150405. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Molloy R, Khan Y. Increased interaction of dopamine-immunoreactive varicosities with GABA neurons of rat medial prefrontal cortex occurs during the postweanling period. Synapse. 1996;23:237–245. doi: 10.1002/(SICI)1098-2396(199608)23:4<237::AID-SYN1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Garcia-Gil L, Hernandez ML, Romero J, Cebeira M, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. Localization of mRNA expression and activation of signal transduction mechanisms for cannabinoid receptor in rat brain during fetal development. Development. 1998;125:3179–3188. doi: 10.1242/dev.125.16.3179. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81:1641–1660. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouthenet ML, Martres MP, Sales N, Schwartz JC. A detailed mapping of dopamine D-2 receptors in rat central nervous system by autoradiography with [125I]iodosulpride. Neuroscience. 1987;20:117–155. doi: 10.1016/0306-4522(87)90008-x. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Aghajanian GK. Dopamine and norepinephrine innervated cells in the rat prefrontal cortex: pharmacological differentiation using microiontophoretic techniques. Life Sci. 1976;19:1783–1789. doi: 10.1016/0024-3205(76)90087-4. [DOI] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct. 2013 Feb 12; doi: 10.1007/s00429-013-0508-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Thomases DR, Flores-Barrera E, Cass DK, Tseng KY. Emergence of GABAergic-dependent regulation of input-specific plasticity in the adult rat prefrontal cortex during adolescence. Psychopharmacology (Berl) 2014;231:1789–1796. doi: 10.1007/s00213-013-3216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Tseng KY. Association of Cannabis Use during Adolescence, Prefrontal CB1 Receptor Signaling, and Schizophrenia. Front Pharmacol. 2012;3:101. doi: 10.3389/fphar.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry. 2014;19:536–543. doi: 10.1038/mp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass DK, Thomases DR, Caballero A, Tseng KY. Developmental disruption of gamma-aminobutyric acid function in the medial prefrontal cortex by noncontingent cocaine exposure during early adolescence. Biol Psychiatry. 2013;74:490–501. doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman VL, Balaban J, Steinfeld S, Shemyakin A, Graham P, Parisot N, Moore H. Prefrontal cortical inputs to the basal amygdala undergo pruning during late adolescence in the rat. J Comp Neurol. 2010;518:2693–2709. doi: 10.1002/cne.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Increasing Interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb Cortex. 2008;18:1529–1535. doi: 10.1093/cercor/bhm183. [DOI] [PubMed] [Google Scholar]
- Datta D, Arion D, Lewis DA. Developmental Expression Patterns of GABAA Receptor Subunits in Layer 3 and 5 Pyramidal Cells of Monkey Prefrontal Cortex. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dilgen J, Tejeda HA, O'Donnell P. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. J Neurophysiol. 2013;110:221–229. doi: 10.1152/jn.00531.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC. Late postnatal maturation of excitatory synaptic transmission permits adult-like expression of hippocampal-dependent behaviors. Hippocampus. 2005;15:562–578. doi: 10.1002/hipo.20077. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169:1651–1661. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, Norrholm SD, Bradley B, Ressler KJ, Jovanovic T. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex. 2015;64:249–259. doi: 10.1016/j.cortex.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferino F, Thierry AM, Glowinski J. Anatomical and electrophysiological evidence for a direct projection from Ammon's horn to the medial prefrontal cortex in the rat. Exp Brain Res. 1987;65:421–426. doi: 10.1007/BF00236315. [DOI] [PubMed] [Google Scholar]
- Ferron A, Thierry AM, Le Douarin C, Glowinski J. Inhibitory influence of the mesocortical dopaminergic system on spontaneous activity or excitatory response induced from the thalamic mediodorsal nucleus in the rat medial prefrontal cortex. Brain Res. 1984;302:257–265. doi: 10.1016/0006-8993(84)90238-5. [DOI] [PubMed] [Google Scholar]
- Flores-Barrera E, Thomases DR, Heng LJ, Cass DK, Caballero A, Tseng KY. Late adolescent expression of GluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling. Biol Psychiatry. 2014;75:508–516. doi: 10.1016/j.biopsych.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DA, Levine ES. Differential effects of endocannabinoids on glutamatergic and GABAergic inputs to layer 5 pyramidal neurons. Cereb Cortex. 2007;17:163–174. doi: 10.1093/cercor/bhj133. [DOI] [PubMed] [Google Scholar]
- Friedman HR, Goldman-Rakic PS. Activation of the hippocampus and dentate gyrus by working-memory: a 2-deoxyglucose study of behaving rhesus monkeys. J Neurosci. 1988;8:4693–4706. doi: 10.1523/JNEUROSCI.08-12-04693.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gabbott P, Headlam A, Busby S. Morphological evidence that CA1 hippocampal afferents monosynaptically innervate PV-containing neurons and NADPH-diaphorase reactive cells in the medial prefrontal cortex (Areas 25/32) of the rat. Brain Res. 2002;946:314–322. doi: 10.1016/s0006-8993(02)02487-3. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139:1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learning & memory (Cold Spring Harbor, N.Y. 2010;17:289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learning & Memory. 2013 doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci U S A. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB. Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci. 1998;18:9139–9151. doi: 10.1523/JNEUROSCI.18-21-09139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Harkany T, Holmgren C, Hartig W, Qureshi T, Chaudhry FA, Storm-Mathisen J, Dobszay MB, Berghuis P, Schulte G, Sousa KM, Fremeau RT, Jr, Edwards RH, Mackie K, Ernfors P, Zilberter Y. Endocannabinoid-independent retrograde signaling at inhibitory synapses in layer 2/3 of neocortex: involvement of vesicular glutamate transporter 3. J Neurosci. 2004;24:4978–4988. doi: 10.1523/JNEUROSCI.4884-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011a;65:278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng LJ, Markham JA, Hu XT, Tseng KY. Concurrent upregulation of postsynaptic L-type Ca(2+) channel function and protein kinase A signaling is required for the periadolescent facilitation of Ca(2+) plateau potentials and dopamine D1 receptor modulation in the prefrontal cortex. Neuropharmacology. 2011b;60:953–962. doi: 10.1016/j.neuropharm.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquet C, Murray R, Linszen D, van Os J. The environment and schizophrenia: the role of cannabis use. Schizophr Bull. 2005;31:608–612. doi: 10.1093/schbul/sbi027. [DOI] [PubMed] [Google Scholar]
- Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull. 2011;37:493–503. doi: 10.1093/schbul/sbr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, De Courten C, Garey LJ, Van der Loos H. Synaptic development in human cerebral cortex. Int J Neurol. 1982;16–17:144–154. [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol. 2003;69:375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology (Berl) 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Robertson MW, Cutler AJ, Bennett JP., Jr Postnatal development of D1 dopamine receptors in the medial prefrontal cortex, striatum and nucleus accumbens of normal and neonatal 6-hydroxydopamine treated rats: a quantitative autoradiographic analysis. Brain Res Dev Brain Res. 1991;62:109–114. doi: 10.1016/0165-3806(91)90195-o. [DOI] [PubMed] [Google Scholar]
- Lew SE, Tseng KY. Dopamine modulation of GABAergic function enables network stability and input selectivity for sustaining working memory in a computational model of the prefrontal cortex. Neuropsychopharmacology. 2014;39:3067–3076. doi: 10.1038/npp.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BL, O'Donnell P. Ventral tegmental area afferents to the prefrontal cortex maintain membrane potential 'up' states in pyramidal neurons via D(1) dopamine receptors. Cereb Cortex. 2000;10:1168–1175. doi: 10.1093/cercor/10.12.1168. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Sesack SR, Levey AI, Rosenberg DR. Dopamine axons in primate prefrontal cortex: specificity of distribution, synaptic targets, and development. Adv Pharmacol. 1998;42:703–706. doi: 10.1016/s1054-3589(08)60845-5. [DOI] [PubMed] [Google Scholar]
- Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: colocalization of excitatory amino acids and projections to the limbic circuit. J Comp Neurol. 1996;365:367–379. doi: 10.1002/(SICI)1096-9861(19960212)365:3<367::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, McDonald K, Ward A, Poulton R, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Muly EC, 3rd, Szigeti K, Goldman-Rakic PS. D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci. 1998;18:10553–10565. doi: 10.1523/JNEUROSCI.18-24-10553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P. Dopamine gating of forebrain neural ensembles. Eur J Neurosci. 2003;17:429–435. doi: 10.1046/j.1460-9568.2003.02463.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell P. Adolescent maturation of cortical dopamine. Neurotox Res. 2010;18:306–312. doi: 10.1007/s12640-010-9157-3. [DOI] [PubMed] [Google Scholar]
- O'Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirot S, Godbout R, Mantz J, Tassin JP, Glowinski J, Thierry AM. Inhibitory effects of ventral tegmental area stimulation on the activity of prefrontal cortical neurons: evidence for the involvement of both dopaminergic and GABAergic components. Neuroscience. 1992;49:857–865. doi: 10.1016/0306-4522(92)90362-6. [DOI] [PubMed] [Google Scholar]
- Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Neuroscience. 2008;155:203–220. doi: 10.1016/j.neuroscience.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver SM, Haughwout SP, Keller A. Adolescent cannabinoid exposure permanently suppresses cortical oscillations in adult mice. Neuropsychopharmacology. 2013;38:2338–2347. doi: 10.1038/npp.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res. 2009;60:132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Renard J, Krebs MO, Jay TM, Le Pen G. Long-term cognitive impairments induced by chronic cannabinoid exposure during adolescence in rats: a strain comparison. Psychopharmacology (Berl) 2013;225:781–790. doi: 10.1007/s00213-012-2865-z. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Barron DS, Kirby LA, Bottenhorn KL, Hill AC, Murphy JE, Katz JS, Salibi N, Eickhoff SB, Fox PT. Neurofunctional topography of the human hippocampus. Hum Brain Mapp. 2015;36:5018–5037. doi: 10.1002/hbm.22987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, Gennatas ED, Hopson R, Jackson C, Prabhakaran K, Bilker WB, Calkins ME, Loughead J, Smith A, Roalf DR, Hakonarson H, Verma R, Davatzikos C, Gur RC, Gur RE. Functional maturation of the executive system during adolescence. J Neurosci. 2013;33:16249–16261. doi: 10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology. 2003;28:1760–1769. doi: 10.1038/sj.npp.1300225. [DOI] [PubMed] [Google Scholar]
- Schneider M, Schomig E, Leweke FM. Acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol. 2008;13:345–357. doi: 10.1111/j.1369-1600.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguela P, Watkins KC, Descarries L. Ultrastructural features of dopamine axon terminals in the anteromedial and the suprarhinal cortex of adult rat. Brain Res. 1988;442:11–22. doi: 10.1016/0006-8993(88)91427-8. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Melchitzky DS, Lewis DA. Dopamine innervation of a subclass of local circuit neurons in monkey prefrontal cortex: ultrastructural analysis of tyrosine hydroxylase and parvalbumin immunoreactive structures. Cereb Cortex. 1998;8:614–622. doi: 10.1093/cercor/8.7.614. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS. D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci U S A. 1994;91:5720–5724. doi: 10.1073/pnas.91.12.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J. Cognitive functioning of long-term heavy cannabis users seeking treatment. Jama. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Jernigan TL. Further MRI evidence of late brain maturation: Limbic volume increases and changing asymmetries during childhood and adolescence. Developmental Neuropsychology. 1998;14:599–617. [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hagino H, Nohara S, Zhou SY, Kawasaki Y, Takahashi T, Matsui M, Seto H, Ono T, Kurachi M. Male-specific volume expansion of the human hippocampus during adolescence. Cereb Cortex. 2005;15:187–193. doi: 10.1093/cercor/bhh121. [DOI] [PubMed] [Google Scholar]
- Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–154. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage. 2014;86:212–220. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci. 1999;21:43–49. doi: 10.1159/000017365. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Blanc G, Sobel A, Stinus L, Glowinski J. Dopaminergic terminals in the rat cortex. Science. 1973;182:499–501. doi: 10.1126/science.182.4111.499. [DOI] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Meyer JD, Caballero A, Tseng KY. Early adolescent MK-801 exposure impairs the maturation of ventral hippocampal control of basolateral amygdala drive in the adult prefrontal cortex. J Neurosci. 2014;34:9059–9066. doi: 10.1523/JNEUROSCI.1395-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomases DR, Cass DK, Tseng KY. Periadolescent exposure to the NMDA receptor antagonist MK-801 impairs the functional maturation of local GABAergic circuits in the adult prefrontal cortex. J Neurosci. 2013;33:26–34. doi: 10.1523/JNEUROSCI.4147-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Mallet N, Toreson KL, Le Moine C, Gonon F, O'Donnell P. Excitatory response of prefrontal cortical fast-spiking interneurons to ventral tegmental area stimulation in vivo. Synapse. 2006;59:412–417. doi: 10.1002/syn.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine-glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Verney C, Alvarez C, Geffard M, Berger B. Ultrastructural Double-Labelling Study of Dopamine Terminals and GABA-Containing Neurons in Rat Anteromedial Cerebral Cortex. Eur J Neurosci. 1990;2:960–972. doi: 10.1111/j.1460-9568.1990.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Verwer RW, Van Vulpen EH, Van Uum JF. Postnatal development of amygdaloid projections to the prefrontal cortex in the rat studied with retrograde and anterograde tracers. J Comp Neurol. 1996;376:75–96. doi: 10.1002/(SICI)1096-9861(19961202)376:1<75::AID-CNE5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci. 1993;13:2551–2564. doi: 10.1523/JNEUROSCI.13-06-02551.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res. 2006;175:329–336. doi: 10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Wang H, Stradtman GG, 3rd, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Natl Acad Sci U S A. 2008;105:16791–16796. doi: 10.1073/pnas.0804318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, Morrison JH, Wang XJ, Arnsten AF. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. J Neurosci. 1999;19:9587–9603. doi: 10.1523/JNEUROSCI.19-21-09587.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K, Chocyk A. Cannabinoid CB1 receptors in rat medial prefrontal cortex are colocalized with calbindin- but not parvalbumin- and calretinin-positive GABA-ergic neurons. Pharmacol Rep. 2009;61:1000–1007. doi: 10.1016/s1734-1140(09)70161-6. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Williams K, Russell SL, Shen YM, Molinoff PB. Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron. 1993;10:267–278. doi: 10.1016/0896-6273(93)90317-k. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Zhu JJ. Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. J Physiol. 2000;526(Pt 3):571–587. doi: 10.1111/j.1469-7793.2000.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]