Abstract
Frontotemporal lobar degeneration is a neurodegenerative disorder affecting over 50,000 people in the United States alone. The most common pathological subtype of FTLD is the presence of ubiquitinated TAR DNA binding protein 43 (TDP-43) accumulations in frontal and temporal brain regions at autopsy. While some cases of FTLD-TDP can be attributed to the inheritance of disease-causing mutations, the majority of cases arise with no known genetic cause. In 2010, the first genome-wide association study (GWAS) was conducted in patients with FTLD-TDP to determine potential genetic risk factors for this homogenous subgroup of dementia patients, leading to the identification of the TMEM106B locus on chromosome 7. In this manuscript, we review the initial discovery and replication studies describing TMEM106B variants as disease risk factors and modifiers in TDP-43 proteinopathies, such as FTLD-TDP caused by progranulin (GRN) or C9orf72 mutations, as well as Alzheimer’s disease and hippocampal sclerosis. We further summarize what is currently known about the previously uncharacterized TMEM106B protein and its role as a potential regulator of lysosomal function, and we discuss how modifying TMEM106B levels might uncover promising therapeutic strategies for individuals suffering from TDP-43 proteinopathy.
Keywords: TMEM106B, frontotemporal dementia, TDP-43, lysosomes, genetic association
Introduction
Frontotemporal lobar degeneration (FTLD) is the second leading cause of pre-senile neurodegeneration after Alzheimer’s disease (AD) and is characterized by the preferential atrophy of the frontal and temporal lobes of the brain. Since the development of this disease can occur across a large brain area, the symptoms at disease onset differ among individuals with this disorder. The term frontotemporal dementia (FTD) is most often used as an umbrella term for the spectrum of clinical manifestations. The most common of these subtypes is behavioral variant frontotemporal dementia (bvFTD) in which reported symptoms include abnormal behaviors such as poor hygiene, social disinhibition, or hoarding [7, 57]. Primary progressive aphasia (PPA) is another, less common, variety of FTD classified by language deterioration. PPA can be further sub-classified into three different presentations: semantic variant of PPA (svPPA) in which the patient finds it difficult to remember the meaning of words, non-fluent variant of PPA (nfvPPA) in which the patient is unable to adequately form words, or logopenic variant of PPA (lvPPA) in which the patient’s ability to retrieve words has deteriorated [25]. All FTD clinical subtypes can present with the motor neuron disease, amyotrophic lateral sclerosis (ALS; termed FTD-ALS), or with other movement disorders such as corticobasal syndrome, progressive supranuclear palsy, or parkinsonism [5].
Nearly 30–50% of FTD cases report a family history of dementia [24, 57, 58, 62, 85], suggesting a strong genetic component for this disease. The first gene discovered to cause FTLD was the microtubule-associated protein tau (MAPT) located on chromosome 17, in which patients exhibit intraneuronal aggregates of hyperphosphorylated tau in the affected brain regions at autopsy (FTLD-tau) [30]. However, MAPT mutations only account for a small percentage of familial FTLD cases. In contrast, the majority of FTLD patients display intranuclear and/or cytoplasmic accumulations of ubiquitinated proteins (FTLD-U) [32, 33, 40, 44]. The primary ubiquitinated protein was identified as the transcription factor TAR DNA binding protein 43 (TDP-43) [4, 51], now classified as FTLD-TDP. Mutations in the gene encoding progranulin (GRN) were found to be a frequent cause of FTLD-TDP [6, 16, 22, 72]. Biochemical and pathological assessments have determined that FTLD-causing GRN mutations invariably cause progranulin protein (PGRN) haploinsufficiency and subsequent TDP-43 proteinopathy [6, 16, 22, 41, 43]. Since this discovery in 2006, over 60 GRN mutations in approximately 230 families have been identified [13, 16, 22, 72]. However, GRN mutations do not account for all patients with TDP-43 inclusions. It was not until 2011 that two independent groups discovered the most common genetic cause of FTLD-TDP. This mutation was identified as a hexanucleotide repeat expansion, (GGGGCC)n, in the chromosome 9 open reading frame 72 (C9orf72) gene and mutation carriers can present with FTLD, FTLD-ALS, or ALS alone, yet they all have TDP-43 pathology [17, 48, 59]. The identification of a common genetic cause and subsequent pathological manifestation in both FTLD and ALS provided concrete evidence that these two diseases exist as a part of a common disease spectrum. C9orf72 and GRN mutations likely account for over half of all familial occurrences of FTLD-TDP; however, mutations in the genes encoding tank-binding kinase 1 (TBK1) and the valosin containing protein (VCP) have also been discovered as less common genetic causes in patients with FTLD-TDP [20, 23, 46, 54, 77, 84]. In rare cases of FTLD-U, the ubiquitinated protein is a TDP-43 protein family member known as fused in sarcoma (FUS) [50, 67], but there is no known genetic cause for this pathological subtype. Finally, FTLD-U is also observed in patients with causal mutations in the charged multivesicular body protein 2B (CHMP2B) gene [70]; however, the ubiquitinated protein in these individuals has not been identified. In summary, while genetic mutations can account for a large number of individuals suffering from FTLD-TDP, the cause of the majority of cases remains unknown.
In 2010, single nucleotide polymorphisms (SNPs) identified on chromosome 7 led to the discovery of the first genetic risk factor for FTLD-TDP. The SNPs were in a genomic region encoding the transmembrane protein 106B (TMEM106B) [76]. Research efforts have since focused on both the genetic correlations as well as the functional properties of this relatively uncharacterized transmembrane protein. In this review we focus on the key findings related to TMEM106B and its role in FTLD risk. Moreover, we discuss how TMEM106B levels are hypothesized to be the critical factor linking TMEM106B to disease pathogenesis, revealing TMEM106B as a potential drug target in FTLD and related TDP-43 proteinopathies.
Identification of TMEM106B as a risk factor for FTLD-TDP
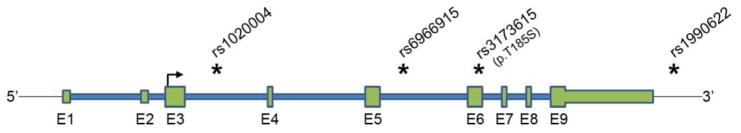
A subset of FTLD patients can be explained by inherited genetic mutations; however, a large portion of cases remain unexplained. In an attempt to find genetic risk factors for FTLD, Van Deerlin and colleagues focused on the homogenous population of cases with FTLD-TDP pathology and conducted a GWAS in 2509 control subjects and 515 subjects with pathologically-confirmed FTLD-TDP, some of which contained a GRN mutation [76]. This GWAS led to the discovery of 3 SNPs within a 68 kb region on chromosome 7p21.3 that associated with the occurrence of FTLD-TDP with genome-wide significance (rs6966915, rs102004, and top marker SNP rs1990622; p-value range = 5.00×10−11 to 1.08×10−11) (Fig. 1) [76]. The 3 significantly-associated SNPs were in the same linkage disequilibrium (LD) block as 9 other SNPs of nominal association, and all spanned the TMEM106B gene locus. In this case-control cohort, the minor allele of each significant SNP was greatly underrepresented in FTLD-TDP patients (32.1% versus 43.6% in controls for rs1990622 minor C-allele; p-value = 1.08×10−11), suggesting that individuals expressing the minor TMEM106B alleles are less likely to develop disease (odds ratio (OR) = 0.61 for the rs1990622 minor allele). For simplicity, we refer to the common TMEM106B variants as the risk alleles and the minor variants as the protective alleles throughout this manuscript. Van Deerlin and colleagues replicated their findings in a second pathologically-confirmed FTLD-TDP case-control cohort, but were unable to observe significant differences in a small third cohort containing living FTD patients of unspecified pathology. Of particular interest, Van Deerlin and colleagues stratified their initial GWAS cohort by GRN mutation status and found that the association of the top 3 SNPs remained significant in both groups, but was greatest in people with GRN-related FTLD-TDP [rs1990622 p-value = 1.34×10−9; OR = 0.34] in GRN carriers versus in non-GRN carriers (rs1990622 p-value = 6.90×10−7; OR = 0.68). This suggested that TMEM106B SNPs might have the greatest effect in people with GRN mutations, a rather unexpected finding given that GRN mutations had been considered near fully penetrant previous to this discovery [76]. In fact, since this initial report, while several groups tried to replicate the associations of TMEM106B in clinical and pathological cohorts of FTLD with variable success, all studies confirmed the highly significant effect of TMEM106B SNPs in modifying risk in GRN mutation carriers. Of note, the 12 TMEM106B SNPs included in the Van Deerlin et al. publication are in high linkage disequilibrium with only one coding SNP (rs3173615) located within the TMEM106B gene that results in a threonine to serine change at amino acid 185 (Figure 1). The potential functional implication of this TMEM106B variant and others on TMEM106B-related mechanisms is described starting on page 13.
Fig. 1.
TMEM106B genomic region. Schematic representation of the TMEM106B genomic region on chromosome 7p21. Introns are drawn in blue while exons are depicted in green, with coding regions drawn taller than non-coding regions. The transcription start site located in exon 3 (E3) is represented by an arrow showing transcriptional direction. Selected FTLD-TDP-associated SNPs are marked with an asterisk (*) to denote their relative location within the TMEM106B locus.
Validation of TMEM106B SNPs as risk factors across the genetic FTLD-TDP spectrum
A number of independent clinical and pathological cohorts of FTLD cases with and without GRN mutations have now been studied in an attempt to replicate the association between TMEM106B SNPs and FTLD risk (results described in Table 1; TMEM106B association studies are listed and summarized in Online Resource Tables 1 and 2). The first independent replication was published by Finch and colleagues in which all 3 initially identified TMEM106B SNPs (rs1990622, rs1020004, and rs6966915) were nominally associated with FTLD risk in a cohort containing 822 controls and 640 FTD cases, the majority of which were living and, thus, of unknown pathologic subtype. However, when only people with GRN mutations were considered, the frequency of the minor alleles for all 3 analyzed SNPs was significantly reduced as compared to healthy individuals (p-value range = 0.006 to 0.0009) [19]. Genotyping data revealed that only the frequency of people homozygous, not heterozygous, for the minor alleles of each of the 3 SNPs was significantly reduced in GRN mutation carriers versus controls (2.6% versus 19.1%, respectively; p-value = 0.009 for rs1990622) [19]. These findings suggested a recessive disease model where an individual must carry two copies of the minor allele in order for TMEM106B SNPs to confer protection against GRN-related FTLD-TDP. The extremely low frequency of homozygous TMEM106B minor allele carriers in GRN mutants further indicated that a significant number of individuals with GRN mutations and two minor TMEM106B alleles may remain without symptoms throughout life and simply do not show up in the clinic to participate in research studies. It is noteworthy to also consider that only one publication to date has identified a significant association in a largely clinical FTD cohort of unknown pathology [78], while 4 others have not [19, 29, 61, 76]. The inconsistent nature of these findings is likely a result of differences in the number of individuals who will present with TDP-43 pathology on autopsy. Thus, depending on the diagnostic inclusion criteria and relative contribution of patient ascertainment from Memory, Movement Disorder or ALS Clinics, a different percentage of patients will likely have an underlying TDP-43 proteinopathy.
Table 1.
TMEM106B risk association studies in GRN or C9orf72 mutation carriers
| Mutation group | Study | Group 1 (N) | Group 2 (N) | Model | SNP | Minor allele | p-valuea | Odds Ratiob |
|---|---|---|---|---|---|---|---|---|
| GRN | ||||||||
| Van Deerlin et al., 2010 | CON (2509) | GRN (89) | Allelic | rs1990622 | C | 1.34×10−9 | 0.34 | |
| Finch et al., 2011 | CON (822) | GRN (78) | Allelic | rs1990622 | C | 0.0003 | 0.51 | |
| Finch et al., 2011 | CON (822) | GRN (78) | Additive | rs1990622 | C | 0.003 | 0.57 | |
| Finch et al., 2011 | CON (822) | GRN (78) | Dominant | rs1990622 | C | 0.088 | 0.65 | |
| Finch et al., 2011 | CON (822) | GRN (78) | Recessive | rs1990622 | C | 0.003 | 0.12 | |
| Nicholson et al., 2013 | CON (822) | GRN (29) | Recessive | rs1990622 | C | 0.03 | 0.15 | |
| Gallagher et al., 2014 | CON (2509) | GRN (116) | Allelic | rs1990622 | C | <0.0001 | 0.37 | |
| Lattante et al., 2014 | CON (552) | GRN (76) | Allelic | rs1990622 | C | 0.0041 | 0.58 | |
| Lattante et al., 2014 | CON (552) | GRN (76) | Recessive | rs1990622 | C | 9.54×10−6 | 0 | |
| C9orf72 | ||||||||
| van Blitterswijk et al., 2014 | CON (376) | all C9 (260) | Recessive | rs3173615 | G | 0.014 | 0.57 | |
| van Blitterswijk et al., 2014 | CON (376) | FTD C9 (69) | Recessive | rs3173615 | G | 0.009 | 0.33 | |
| van Blitterswijk et al., 2014 | CON (376) | FTD/ALS C9 (71) | Recessive | rs3173615 | G | 0.017 | 0.38 | |
| van Blitterswijk et al., 2014 | CON (376) | ALS C9 (120) | Recessive | rs3173615 | G | 0.55 | 0.85 | |
| Gallagher et al., 2014 | CON (2509) | FTLD-TDP C9 (80) | Allelic | rs1990622 | C | 0.008 | 0.64 | |
| Lattante et al., 2014 | CON (552) | FTD C9 (95) | Allelic | rs1990622 | C | 0.47 | 1.12 | |
| Lattante et al., 2014 | CON (552) | FTD/ALS C9 (50) | Allelic | rs1990622 | C | 0.28 | 1.26 | |
| Lattante et al., 2014 | CON (552) | all C9 (145) | Recessive | rs1990622 | C | 0.085 | 1.49 | |
| Lattante et al., 2014 | CON (552) | FTD C9 (95) | Recessive | rs1990622 | C | 0.233 | 1.41 | |
| Lattante et al., 2014 | CON (552) | FTD/ALS C9 (50) | Recessive | rs1990622 | C | 0.163 | 1.67 |
Results from studies that assessed whether TMEM106B SNPs associate with disease risk in GRN or C9orf72 mutation carriers are summarized. Several publications include analyses of more than one SNP; thus, we summarize the findings of rs1990622 when available.
Reported p-values for the statistical models are not corrected for multiple testing.
Odds ratios are given with respect to the minor allele.
GRN: progranulin mutation carriers, C9orf72: chromosome 9 open reading frame 72 expansion carriers, N: number of individuals, FTD: frontotemporal dementia, FTLD-TDP: frontotemporal dementia lobar degeneration with TAR DNA binding protein 43 pathology, ALS: amyotrophic lateral sclerosis, SNP: single nucleotide polymorphism.
The initial studies assessing TMEM106B SNPs as disease risk factors in FTLD-TDP were conducted prior to the discovery of C9orf72 repeat expansion in 2012. Since this discovery, two independent groups found TMEM106B SNPs to also associate with one’s risk of developing FTLD and/or ALS caused by C9orf72 mutations [21, 75] (results described in Table 1). In these C9orf72 mutant cohorts, the frequency of individuals carrying the minor allele of TMEM106B SNPs [rs1990622 and/or rs3173615 in LD with rs1990622 (Fig. 1)] was significantly reduced, although not as markedly as in cohorts of GRN mutation carriers [21, 75]. Interestingly, when van Blitterswijk et al. analyzed C9orf72 repeat expansion carriers in groups based on their predominant disease presentation as either FTLD, FTLD-ALS, or ALS, it was shown that TMEM106B SNPs specifically protect against the development of FTLD but not ALS, with less TDP-43 burden in the brains of C9orf72 expansion carriers homozygote for the protective TMEM106B alleles as compared to risk allele carriers [75]. These findings are in agreement with an earlier examination of TMEM106B SNPs in a clinical cohort of ALS patients in which TMEM106B SNPs rs1990622 and rs1020004 were not associated with disease risk, but did significantly associate with cognitive function in ALS patients, with individuals homozygous for the rs1990622 minor allele having better cognitive performance than individuals heterozygous or homozygous for the major risk alleles [79]. The second study of TMEM106B in C9orf72 expansion carriers (of all clinical presentations) surprisingly reported an earlier age at onset and death in C9orf72 expansion carriers with the protective TMEM106B allele, which may seem counterintuitive at first glance. However, this finding may be in line with the van Blitterswijk et al. study (thoroughly summarized by Deming and Cruchaga [18]), and a consequence of the different effect of TMEM106B on ALS versus FTLD presentations of the disease. For example, TMEM106B may protect against the development of FTLD but not ALS, leading to an overall earlier age at onset and death associated with the ALS phenotype in the group of protective allele carriers. A third examination of rs1990622 in a French cohort of C9orf72 mutation carriers with FTD and FTD-ALS did not report a significant association in their C9orf72 mutant cohort, even though the association in GRN mutation carriers was found to be significant [38] (Table 1). This suggests that the association of TMEM106B risk variants might be different in individual C9orf72 populations. Importantly, despite the variable composition of genetically defined FTLD-TDP patient cohorts, TMEM106B SNP frequencies remained significantly underrepresented in a pathologically-confirmed cohort of FTLD-TDP patients even after excluding all carriers of GRN mutations and C9orf72 expansions [75], signifying an effect of TMEM106B on all FTLD-TDP patients irrespective of the underlying genetic defect. The selective impact of TMEM106B SNPs on TDP-43 pathology in cortical brain areas (as seen in FTLD-TDP) as compared to the spinal cord (as seen in ALS) remains unexplained at present, but may potentially provide insight into TMEM106B expression and function in future studies. To better understand this discrepancy, future studies could potentially focus on the differences in TMEM106B levels and/or distribution in the brain versus the spinal cord. Additionally, lysosomal function has been suggested to play a role in TDP-43 aggregation [8, 81, 82]; thus, it is also possible that TMEM106B-related lysosomal phenotypes are different between cortical and motor neurons.
TMEM106B variants associate with disease risk in other TDP-43 proteinopathies
Research related to TMEM106B SNPs in disease has repeatedly concluded that TMEM106B variants only associate with disease risk in FTLD cohorts with confirmed or mutation-predicted TDP-43 pathology. However, a number of other neurodegenerative diseases also present with TDP-43 pathology, including AD, Lewy body dementia (LBD), and hippocampal sclerosis (HpScl) [2, 87]. Thus, research has been conducted to determine whether TMEM106B variants might be associated with risk of general TDP-43 proteinopathy not specific to an FTLD presentation (study groups and outcome measures are summarized in Online Reference Tables 1 and 2). A study performed by Rutherford et al. in 2012 was the first to report that TMEM106B SNPs might be important for the pathological presentation of AD [64]. More specifically, this research group examined the association of rs1990622 in a pathologically-confirmed AD cohort of over 900 subjects, comparing rs1990622 genotypic frequencies in AD patients with and without TDP-43 pathology. The percentage of individuals homozygous for the rare allele in AD patients without TDP-43 pathology was similar to previously published non-demented cohorts (approximately 21%) indicating no significant association of rs1990622 in this subgroup. However, in AD patients with TDP-43 pathology, this percentage was significantly less, with only 13% of individuals homozygous for the minor TMEM106B allele, suggesting TMEM106B rare variants protected against the development of TDP-43 even in the setting of AD [64]. HpScl is also common in elderly patients with dementia, including FTLD-TDP and AD, and TDP-43 pathology accompanies over 70% of those individuals [2]. The Rutherford et al. study indicated that TMEM106B SNPs strongly associate with one’s risk of developing HpScl in AD, with 6% of individuals with AD-HpScl homozygous for the rs190622 minor allele [64]. This strong association was also found by Murray and colleagues who reported no individuals homozygous for the minor allele of rs1990622 in neither an AD-HpScl cohort of 132 subjects, nor in a 30 person cohort of pure HpScl, revealing TMEM106B as the strongest genetic indicator of AD-HpScl and HpScl known to date [47]. Later studies conducted by Nelson et al. and Yu et al. also reported a significant underrepresentation of TMEM106B protective alleles in patients with HpScl [49, 86], with further assessments indicating lower TDP-43 pathological burden in patients homozygous for the protective TMEM106B alleles [86]. Genetic assessments were also conducted in a cohort of LBD in which the minor TMEM106B allele of rs1990622 was 17% less represented in people with accompanying HpScl than those without HpScl, although this did not reach statistical significance possibly due to a smaller sample size (n=35 versus 109, respectively) [3]. Finally, in a GWAS study conducted this year, SNPs in TMEM106B showed a near genome-wide significant interaction with APOE genotype, providing further evidence that TMEM106B alleles might modify AD risk [34]. These studies collectively suggest that TMEM106B SNPs are risk factors for the development and severity of TDP-43 proteinopathy in non-FTLD disorders such as AD and HpScl.
TMEM106B variants associate with brain volume and connectivity
TMEM106B variants have been studied in the context of non-demented individuals either as part of the general population or in asymptomatic individuals carrying GRN mutations. Adams and colleagues addressed whether TMEM106B variants might be associated with subclinical, structural brain changes in non-demented elderly individuals, possibly leaving a subgroup of subjects more susceptible to the pathophysiology of FTLD or related disorders. This group discovered that the risk allele of rs1990622 accompanied significantly reduced volume of the superior temporal gyrus, most markedly in the left hemisphere [1]. Interestingly, this area of the brain includes structures important for language processing, often affected in FTLD patients [36, 42, 60]. In line with this study, Premi et al. discovered asymptomatic GRN mutation carriers portray significantly decreased brain connectivity of the left frontoparietal network as compared to non-demented individuals, and that this phenotype was worsened in people expressing two copies of the risk rs1990622 allele [56]. Taken together, these analyses might suggest that TMEM106B SNPs confer risk for TDP-43 proteinopathies by predisposing affected brain regions to FTLD-related pathogenesis.
TMEM106B is a lysosomal protein
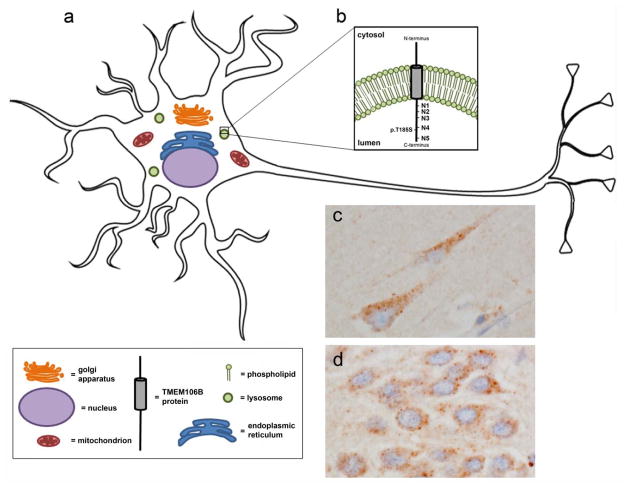
TMEM106B belongs to the TMEM106 family of proteins that are between 200–300 amino acids (aa) in length with relatively unknown function, consisting of TMEM106A, TMEM106B, and TMEM106C. Since the identification of TMEM106B as an FTLD risk factor, research has been underway to further understand the role of this protein in normal brain and cellular function. Immunohistochemical assessments of TMEM106B expression in human brain tissue have been conducted with newly developed antibodies targeting this protein. These assessments indicate that TMEM106B is expressed in neurons (Fig. 2) [11, 14, 52], with expression also observed in glial and endothelial cells [11, 52]; however, the specificity of these antibodies has not been validated in TMEM106B knockout systems. Within the cell, in vitro assays uncovered that TMEM106B is a single-pass, type 2 integral membrane glycoprotein predominantly located in the membranes of endosomes and lysosomes (Fig. 2) [9, 12, 14, 37, 52, 73]. The transmembrane domain is predicted to range from aa 96–118 (http://smart.embl-heidelberg.de/smart/show_motifs.pl?ID=Q9NUM4&), and five sequence motifs of post-translational N-gylcosylation (N-X-T/S) span its luminal domain. Simple glycans are added to three of the asparagine (N) residues (N145, N151, and N164), and complex glycans are added to the most C-terminal motifs at N183 and N256 [37]. Mutagenesis experiments have indicated that the first three glycosylation sites are not critical for TMEM106B localization; however, loss of complex glycans on residue N183 impairs TMEM106B forward transport to endosomes/lysosomes and results in ER retention of the mutant protein. Additionally, N256 complex glycosylation is necessary for TMEM106B sorting, since N256 mutants were trafficked from the endoplasmic reticulum (ER) to the plasma membrane instead of endosomal/lysosomal compartments [37]. Once in the lysosomal membrane, TMEM106B undergoes evolutionarily-conserved regulated intramembrane proteolysis (RIP) [10]. In the case of TMEM106B, this mechanism is a two-step process involving the initial release of the luminal domain by an unknown enzyme, leaving an N-terminal fragment (NTF) still anchored in the membrane [10]. The NTF is further cleaved by the aspartyl protease, signal peptide peptidase-like 2a (SLPP2a), to create an intracellular domain (ICD) that is rapidly degraded. followed by intramebrane processing of the transmembrane stump [39]. It is not yet known whether this processing is misregulated in FTLD, how RIP affects full-length TMEM106B function, or whether TMEM106B fragments created by RIP have a function of their own.
Fig. 2.
TMEM106B is found in the lysosomal membrane of neurons. (a) Representation of a neuron and various intracellular compartments, including the lysosome. (b) Magnification of the lysosomal membrane in which TMEM106B resides as a type II transmembrane protein, with the relative location of the p.T185S variant and 5 glycosylation sites (N1–N5) depicted in the luminal region. (c–d) Immunohistochemical labeling of TMEM106B in human brain shows a punctate TMEM106B-immunoreactive pattern in pyramidal neurons of the hippocampus (c) and in granular neurons of the dentate gyrus (d).
TMEM106B levels affect lysosomal function
Expression studies in cell culture models have led to several independent reports indicating that TMEM106B levels are critical for lysosome size, acidification, function, and transport in various cell types. The first of these studies was conducted in 2012 by Chen-Plotkin and colleagues who showed that TMEM106B overexpression significantly enhances the size of LAMP-1/TMEM106B-positive structures [14]. This study and others have reported that these large structures derived by TMEM106B overexpression do not acidify properly to create mature and/or functional lysosomes, causing a delay in endolysosomal-dependent degradation and concomitant cytotoxicity [9, 12, 14, 73]. Additional data by Brady et al. showed that in addition to larger lysosomal size, TMEM106B overexpression in cultured N2a cells causes a concomitant reduction in the total number of lysosomes per cell [9]. Later publications showed that TMEM106B overexpression in primary neurons did not affect cell viability despite the fact that the previously reported effects of TMEM106B overexpression on lysosomal size and/or acidification were replicated [12, 73]. Moreover, overexpression experiments in primary neurons were unable to show that the overall number of lysosomes was reduced [73], which might signify distinct TMEM106B-mediated phenotypes in various cell types. Nonetheless, increased TMEM106B levels in neurons drastically reduced the number of mobile LAMP-1-positive structures upon TMEM106B overexpression, with the majority of LAMP-1-positive structures accumulating in the cell soma [73]. Enhanced TMEM106B expression has also been shown to cause the translocation of transcription factor EB (TFEB) to the nucleus and to upregulate gene expression from the Coordinated Lysosomal Expression and Regulation (CLEAR) gene network [73]. A shift in the location of TFEB from the lysosome to the nucleus has previously been shown to cause increased CLEAR network expression and this phenomenon is commonly used as a marker of lysosomal stress [65, 69]. Taken together, these findings indicate that TMEM106B upregulation results in lysosomal defects, including improper lysosomal formation, poor lysosomal acidification, reduced lysosomal transport, and increased lysosomal stress.
A study by Schwenk et al. knocked down the expression of TMEM106B in HeLa cells and found that reduced TMEM106B expression leads to a clustering of lysosomes near the nucleus that can be rescued upon reintroducing TMEM106B [66]. In primary rat hippocampal neurons, the clustering of lysosomes in response to Tmem106b reduction was not as pronounced, nor did it affect cell viability; however, loss of neuronal Tmem106b expression significantly enhanced retrograde lysosomal motility in dendrites and reduced dendritic branching [66, 73], leaving the remaining, principle dendrite longer in length with reduced expression of synaptic proteins [66]. Interestingly, restoring the balance between retrograde and anterograde lysosomal trafficking rescued the dendritic branching phenotype observed in neurons [66], suggesting that the reduced dendritic branching observed with Tmem106b loss was due to the Tmem106b-mediated changes in lysosomal trafficking. Importantly, TMEM106B knockdown in primary neurons did not trigger TFEB translocation [73], nor did it affect the pH-dependent proteolytic processing of cathepsin B [66] signifying that TMEM106B loss does not induce lysosomal stress or chages in lysosomal pH. However, Stagi and colleagues did report significantly reduced lysosomal size in neurons upon TMEM106B depletion [73]. These findings could indicate that proper TMEM106B levels are critical to maintain proper lysosomal function.
It should be noted that many of the studies of TMEM106B function and/or levels involve biochemical analyses of TMEM106B by Western blotting. The molecular weight of the TMEM106B protein is predicted at approximately 31 kilodaltons (kDa); however, reports have rarely observed TMEM106B at this molecular weight. In fact, the majority of functional studies on the TMEM106B protein report a molecular weight at approximately 40 or 80 kDa depending on the denaturing conditions, the antibody used, and the cellular system utilized for analysis [8, 9, 14, 37, 52]. Since most initial reports on TMEM106B function have required the generation of previously uncharacterized TMEM106B antibodies for protein detection, this may have contributed to the inconsistent TMEM106B detection patterns. It is likely, however, that the protein detected at 40 kDa is the glycosylated monomeric form of TMEM106B, whereas the band observed at 80 kDa is likely dimeric TMEM106B protein. Some groups report a loss of dimeric and only slightly increased monomeric TMEM106B signal when protein denaturation is conducted at temperatures higher than 4 °C [9, 14], likely because the antibody used in these studies has higher affinity for the dimeric form as compared to monomeric TMEM106B [37, 52]. Thus, it is important to consider these discrepancies when comparing functional studies of this relatively uncharacterized protein, especially when the nature of the biologically relevant form (or forms) of TMEM106B are unknown.
TMEM106B binding partners
To identify proteins that might interact with TMEM106B, Stagi and colleagues conducted a yeast two-hybrid screen using either the TMEM106B cytoplasmic N-terminus (aa 1-96) or luminal C-terminus (aa 118-274) for bait and a prey library consisting of human adult brain proteins. Using this technique in combination with immunoprecipitation experiments, Stagi and colleagues determined that the N-terminus of TMEM106B is involved in interactions with itself and its family member, TMEM106C [73]. The data regarding whether TMEM106C resides in lysosomal structures has been discrepant, with one group showing at least partial localization in LAMP-1-positive structures and another showing no TMEM106C and LAMP-1 overlap [10, 73]. Therefore, additional analyses would be required to determine whether TMEM106 family members B and C might have similar function. The N-terminus of TMEM106B was also found to interact with endocytic adaptor proteins such as clathrin heavy chain (CLTC) and the μ1 subunit of adipocyte protein 2 (AP2M1) [73]. These protein interactions together with TMEM106B’s endolysosomal localization would imply that the TMEM106B cytoplasmic domain possibly participates in the delivery of endocytic cargos to the lysosome. In agreement with this hypothesis, Jun and colleagues reported that TMEM106B also directly binds to CHMP2B [35], known to be a member of the endosomal sorting complexes required for transport III (ESCRT-III) complex that regulates endolysosomal protein trafficking and autophagic structure formation [27, 28]. One additional report identified the C-terminus of microtubule-associated protein 6 (Map6) to also directly bind the N-terminus of Tmem106b by utilizing mass spectrometry analysis of rat brain Tmem106b immunoprecipitation samples [66]. Interestingly, Map6 was found to have a function reciprocal to Tmem106b since Map6 knockdown was shown to rescue the dendritic branching phenotype caused by reduced Tmem106b expression, and overexpression of Map6 mimics Tmem106b loss [66]. Neither study identified proteins that interact with the luminal C-terminus of TMEM106B. This could be explained by the fact that all procedures were performed at cytoplasmic pH, which is significantly higher than the pH of the lysosomal lumen. Thus additional analyses are necessary to determine TMEM106B C-terminal interactions.
What is the TMEM106B functional variant?
Further insight regarding how this relatively uncharacterized protein leads to risk or protection from developing FTLD-TDP can be obtained by understanding which SNP or SNPs are responsible for the association, and to determine how these SNPs affect TMEM106B regulation and/or function. The top SNPs originally identified to associate with FTLD-TDP were part of a GWAS study that included a panel of 550,000 common SNPs [76]. The use of these SNP panels is effective for GWAS to identify risk loci; however, the SNP most significantly associated with the development of FTLD-TDP is unlikely to be the actual SNP within the LD block responsible for the genetic association, and any one of the dozens of SNPs in this LD block, or a combination of these SNPs, could be functionally relevant. SNP rs3173615 is the only coding variant in the rs1990622-related LD block, located within exon 6 (of 9 total exons) of TMEM106B at codon 185 (Fig. 1). This missense variant leads to a coding change from the more common, highly conserved threonine (T185; risk allele) to a serine (S185; protective allele); however, in silico analyses predict only a mild functional effect of this single aa change on TMEM106B function. Alternatively, rs1990622 or other non-coding variants in the LD region might be regulatory variants affecting gene expression or splicing. In fact, in silico prediction analyses suggest that rs1990622 affects the binding of the transcription factor known as CCCTC-binding factor (CTCF) (http://regulomedb.org/snp/chr7/12283786).
TMEM106B associated variants and TMEM106B expression levels
In the original manuscript by Van Deerlin et al., the authors described higher TMEM106B mRNA levels in the frontal cortex of individuals expressing the risk TMEM106B variant for rs1990622 as compared to individuals homozygous for the protective allele [76]. The authors also reported that people with FTLD-TDP have significantly higher amounts of TMEM106B mRNA as compared to non-demented individuals, although this effect was driven by the GRN mutation carriers and might have been a result of this disease group having less individuals homozygous for the minor allele in this small cohort (n=25) [76]. Moreover, they queried a publicly-available mRNA-by-SNP database to determine genetic regulators of TMEM106B mRNA expression in lymphoblasts cells, and found that rs1990622 was significantly correlated with TMEM106B mRNA expression [76]. While an increase in TMEM106B mRNA expression provides a plausible pathogenic mechanism supported by functional studies showing increased TMEM106B expression can cause detriment to lysosomal-related structures, the effect of the associated SNPs on TMEM106B mRNA levels was not confirmed in other brain mRNA expression studies [15, 78, 86]. However, these studies were all performed using postmortem brain tissue with possibly variable levels of neuronal loss and cell type composition which may have masked possible SNP-expression associations. Future studies on TMEM106B levels in human brain should consider that variability in TMEM106B levels might be a SNP-dependent effect, or could be a consequence of disease-related lysosomal dysfunction. In fact, in vitro and in vivo models of lysosomal dysfunction showed that Tmem106b protein levels are significantly increased in these model systems [26, 37]; thus, SNP-related changes in post-mortem tissue TMEM106B protein levels might be best addressed using non-diseased tissues.
Evidence also exists to suggest that coding SNP rs3173615 might directly affect TMEM106B protein levels, independent of mRNA expression levels. As previously described, this missense variant is located in codon 185 of the TMEM106B protein (Fig. 2), which is only two aa downstream from a critical site of N-glycosylation at residue 183 (N813) that is part of the N-X-T/S glycosylation consensus sequence [37]. Upon transient overexpression, Nicholson and colleagues found that the T185 isoform of TMEM106B led to a nearly two-fold increase in TMEM106B protein expression as compared to the S185 isoform, despite equal mRNA expression levels. They further showed that the higher levels of TMEM106B T185 protein were due to more rapid lysosomal degradation of the TMEM106B S185 protein, and that this difference in expression was abrogated when a mutation in the N183 glycosylation site was introduced [52]. Potential p.T185S-mediated changes in N183 glycosylation would likely be modest since both variants of TMEM106B are found to adequately localize to endo/lysosomal compartments [9, 52]; however, rs3173615-dependent effects on TMEM106B protein levels could contribute to the protective effects observed in FTLD pathogenesis.
Finally, independent of TMEM106B SNPs, it has been suggested that TMEM106B expression in the brain is regulated by the microRNA-132 cluster of microRNAs previously implicated in neurodevelopment [45, 80]. MicroRNAs regulate gene expression in a sequence-specific manner, and disruption of microRNA production or processing can cause transcriptional imbalances leading to cellular dysfunction. Chen-Plotkin et al. reported that the levels of three microRNAs found in the microRNA-132 cluster are significantly reduced in FTLD brains as compared to healthy controls, with the strongest effects observed in GRN mutation carriers [14]. Furthermore, TMEM106B protein levels were also observed as markedly increased in GRN mutation carriers [14]. Yu and colleagues later showed that levels of microRNA-132 significantly correlate with TDP-43 burden in elderly individuals with TDP-43 pathology [86]. These reports provide yet another mechanism by which TMEM106B levels might be critical for the onset of TDP-43-related disorders.
Linking TMEM106B to other FTLD disease proteins
It has been known that the lysosome is a critical player in the pathogenesis of neurodegenerative disorders such as AD and Parkinson’s diseases [53, 63]. However, the role of lysosomal function in FTLD-TDP and other TDP-43 proteinopathies was not as evident until the recent discovery that individuals homozygous for the loss-of-function mutations in GRN develop a lysosomal storage disorder [71]. Since the effect of TMEM106B SNPs is most significant in GRN mutation carriers, a key area of interest has been to link TMEM106B and its associated variants to PGRN biology. The first report linking TMEM106B SNPs to PGRN levels was published by Finch et al. who showed that non-demented individuals expressing the protective, minor allele of TMEM106B SNPs had significantly higher levels of PGRN in plasma [19]. However, plasma PGRN levels were only found increased by approximately 3% and this data was not replicated by other groups [19, 68]. A link between TMEM106B and GRN was later obtained using RNA sequencing (RNAseq) data that showed a negative correlation between TMEM106B mRNA and GRN mRNA levels, likely due to SNP-dependent changes in GRN gene methylation [86].
In vitro assessments have also been conducted to determine whether overexpression of TMEM106B (independent of the associated p.T185S variant) affects PGRN levels or localization. Such endeavors determined that both the TMEM106B S185 and T185 isoforms similarly co-localize with PGRN in LAMP-1-positive compartments and significantly induce PGRN upregulation in response to TMEM106B overexpression [9, 52, 66]. However, given the detrimental effect of TMEM106B overexpression on lysosomal function [9, 12, 52, 66, 73], the increase in PGRN expression observed in these experiments is likely a consequence of global lysosomal dysfunction and not specific to PGRN. While important, studies focused on the effect of TMEM106B levels on the cellular function of PGRN unfortunately have not been performed.
Similar to PGRN, Busch et al. recently assessed whether TMEM106B interacts either directly or indirectly with C9orf72. Data obtained in this study suggested that TMEM106B overexpression was unable to exert toxic lysosomal phenotypes when C9orf72 was not present; thus, siRNA-mediated reduction of C9orf72 expression in HEK293 and HeLa cells rescued TMEM106B-dependent increases in lysosomal size [12]. Additionally, C9orf72 loss rescued TMEM106B-induced changes in lysosomal acidification and subsequent cytotoxicity [12]. While this study suggests a mechanistic link between TMEM106B and C9orf72, it is more difficult to determine how this might translate in patients with C9orf72 repeat expansions. Based on these findings, one would expect that loss of C9orf72, as observed in mutation carriers, would be protective against TMEM106B-related cellular changes. Further study into TMEM106B and C9orf72-related mechanisms is required to fully understand how these proteins might be functionally related and whether relative expression patterns of these two proteins could explain selective protection in C9orf72 expansion carriers against FTLD but not ALS in homozygous TMEM106B minor allele carriers.
A study conducted by Jun et al. aimed to establish a link between TMEM106B and the FTLD-related protein, CHMP2B. Patients harboring FTLD-causing CHMP2B mutations are much rarer than those with GRN mutations or C9orf72 expansions [55]. Nonetheless, one feature found in patients with disease-causing CHMP2B mutations is the presence of large vacuolar structures in the brain that label positive for endosomal proteins, suggesting an endosomal-related disease mechanism [31]. Interestingly, Jun and colleagues found endogenous Tmem106b to partially co-localize to both wild-type CHMP2B- and mutant CHMP2B-positive structures in mouse neurons [35], further highlighting a potential role for TMEM106B in the endolysosomal pathway. Moreover, this study showed that T185 TMEM106B more readily binds to CHMP2B than S185 TMEM106B, and that T185 TMEM106B-CHMP2B binding is enhanced with the mutant form of CHMP2B [35]. Also, overexpression of T185 TMEM106B showed a greater reduction in autophagic flux, commonly used to measure autophagic degradation activity, versus the S185 variant of TMEM106B, and this difference was independent of total TMEM106B levels [35]. Whether these CHMP2B-related differences in TMEM106B variants contribute to the observed associations in all forms of FTLD-TDP remains to be determined.
Conclusions and future directions
An overwhelming body of evidence has revealed TMEM106B variants as significant contributors to one’s risk of developing various TDP-43 proteinopathies, both in patients harboring disease-causing mutations, and in subjects with TDP-43 pathology of unknown cause. The major theme connecting all of these studies is the link between TMEM106B and TDP-43, and more than one study has suggested that TMEM106B SNPs can modify TDP-43 pathological burden [75, 86]. Therefore, it is of particular interest that no study to date has functionally linked TMEM106B and TDP-43. In fact, mislocalized TDP-43 can be cleared through the process of lysosomal degradation and autophagy, and improper TDP-43 clearance has been attributed by several groups to be, in part, due to impairments in these cellular processes [8, 74, 81, 83]. Thus, future studies should be executed to elucidate how TMEM106B might be involved in TDP-43 clearance.
Studies performed in various cellular systems has provided evidence suggesting that TMEM106B levels, at least in part, are likely involved in TMEM106B-related risk for TDP-43 proteinopathies. Furthermore, multiple genetic association studies have suggested that the mechanism by which TMEM106B SNPs confer disease risk is by mediating TMEM106B levels, either through transcriptional and/or post translational regulation. Therefore, one possible therapeutic avenue that might be administered for patients with TDP-43-related diseases would be to reduce the levels of TMEM106B in the brain. While several studies describe a relationship between TMEM106B and endolysosomal function, it remains unknown as to what TMEM106B actually does in these cellular compartments. Furthermore, little information exists regarding the potential consequences of brain or systemic TMEM106B loss. Tmem106b knockout mice have been generated and are currently being used by several groups to better study this gene in the context of aging-related disorders. In fact, these mice have not yet been shown to have gross pathological deficits or a reduced lifespan, suggesting that Tmem106b is not critical for survival (unpublished observations, personal communication). However, based on the few publications that have studied TMEM106B reduction, it is possible that complete loss of TMEM106B might affect lysosomal trafficking and/or neuronal development [66, 73] without affecting survival, indicating that partial reduction of TMEM106B levels might have the greatest clinical promise; yet, these analyses have yet to be conducted in a model organism.
In summary, TMEM106B is the most significant genetic risk factor for individuals with FTLD-TDP and related proteinopathies. Since the original discovery six years ago, the field of FTLD has gained considerable ground regarding the role of the lysosome in disease pathogenesis. However, research efforts obtained to date have only skimmed the surface of how TMEM106B is related to these disease mechanisms, and more work is urgently needed to evaluate this protein as a promising therapeutic target for patients suffering from these devastating disorders.
Acknowledgments
Rosa Rademakers is funded by National Institutes of Health grants P50 AG016574, P50 NS072187, R01 NS080882 and R01 NS076471, the ALS Therapy Alliance, and the Consortium for Frontotemporal Dementia. Alexandra Nicholson is funded the Mayo Clinic Edward C. Kendall Research Fellowship. Images of TMEM106B neuronal staining are courtesy of Dr. Dennis Dickson.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Adams HH, Verhaaren BF, Vrooman HA, Uitterlinden AG, Hofman A, van Duijn CM, et al. TMEM106B influences volume of left-sided temporal lobe and interhemispheric structures in the general population. Biol Psychiatry. 2014;76:503–508. doi: 10.1016/j.biopsych.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki N, Murray ME, Ogaki K, Fujioka S, Rutherford NJ, Rademakers R, et al. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathol. 2015;129:53–64. doi: 10.1007/s00401-014-1358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 5.Baizabal-Carvallo JF, Jankovic J. Parkinsonism, movement disorders and genetics in frontotemporal dementia. Nat Rev Neurol. 2016;12:175–185. doi: 10.1038/nrneurol.2016.14. [DOI] [PubMed] [Google Scholar]
- 6.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 7.Boxer AL, Miller BL. Clinical features of frontotemporal dementia. Alzheimer Dis Assoc Disord. 2005;19(Suppl 1):S3–6. doi: 10.1097/01.wad.0000183086.99691.91. [DOI] [PubMed] [Google Scholar]
- 8.Brady OA, Meng P, Zheng Y, Mao Y, Hu F. Regulation of TDP-43 aggregation by phosphorylation and p62/SQSTM1. J Neurochem. 2011;116:248–259. doi: 10.1111/j.1471-4159.2010.07098.x. [DOI] [PubMed] [Google Scholar]
- 9.Brady OA, Zheng Y, Murphy K, Huang M, Hu F. The frontotemporal lobar degeneration risk factor, TMEM106B, regulates lysosomal morphology and function. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady OA, Zhou X, Hu F. Regulated intramembrane proteolysis of the frontotemporal lobar degeneration risk factor, TMEM106B, by signal peptide peptidase-like 2a (SPPL2a) J Biol Chem. 2014;289:19670–19680. doi: 10.1074/jbc.M113.515700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busch JI, Martinez-Lage M, Ashbridge E, Grossman M, Van Deerlin VM, Hu F, et al. Expression of TMEM106B, the frontotemporal lobar degeneration-associated protein, in normal and diseased human brain. Acta Neuropathol Commun. 2013;1:36. doi: 10.1186/2051-5960-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch JI, Unger TL, Jain N, Skrinak RT, Charan RA, Chen-Plotkin AS. Increased expression of the frontotemporal dementia risk factor TMEM106B causes C9orf72-dependent alterations in lysosomes. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerami C, Scarpini E, Cappa SF, Galimberti D. Frontotemporal lobar degeneration: current knowledge and future challenges. J Neurol. 2012;259:2278–2286. doi: 10.1007/s00415-012-6507-5. [DOI] [PubMed] [Google Scholar]
- 14.Chen-Plotkin AS, Unger TL, Gallagher MD, Bill E, Kwong LK, Volpicelli-Daley L, et al. TMEM106B, the risk gene for frontotemporal dementia, is regulated by the microRNA-132/212 cluster and affects progranulin pathways. J Neurosci. 2012;32:11213–11227. doi: 10.1523/JNEUROSCI.0521-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruchaga C, Graff C, Chiang HH, Wang J, Hinrichs AL, Spiegel N, et al. Association of TMEM106B Gene Polymorphism With Age at Onset in Granulin Mutation Carriers and Plasma Granulin Protein Levels. Arch Neurol. 2011 doi: 10.1001/archneurol.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 17.DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deming Y, Cruchaga C. TMEM106B: a strong FTLD disease modifier. Acta Neuropathol. 2014;127:419–422. doi: 10.1007/s00401-014-1249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finch N, Carrasquillo MM, Baker M, Rutherford NJ, Coppola G, Dejesus-Hernandez M, et al. TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology. 2011;76:467–474. doi: 10.1212/WNL.0b013e31820a0e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freischmidt A, Wieland T, Richter B, Ruf W, Schaeffer V, Muller K, et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci. 2015;18:631–636. doi: 10.1038/nn.4000. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher MD, Suh E, Grossman M, Elman L, McCluskey L, Van Swieten JC, et al. TMEM106B is a genetic modifier of frontotemporal lobar degeneration with C9orf72 hexanucleotide repeat expansions. Acta Neuropathol. 2014;127:407–418. doi: 10.1007/s00401-013-1239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- 23.Gijselinck I, Van Mossevelde S, van der Zee J, Sieben A, Philtjens S, Heeman B, et al. Loss of TBK1 is a frequent cause of frontotemporal dementia in a Belgian cohort. Neurology. 2015;85:2116–2125. doi: 10.1212/WNL.0000000000002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman JS, Farmer JM, Wood EM, Johnson JK, Boxer A, Neuhaus J, et al. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology. 2005;65:1817–1819. doi: 10.1212/01.wnl.0000187068.92184.63. [DOI] [PubMed] [Google Scholar]
- 25.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotzl JK, Mori K, Damme M, Fellerer K, Tahirovic S, Kleinberger G, et al. Common pathobiochemical hallmarks of progranulin-associated frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis. Acta Neuropathol. 2014;127:845–860. doi: 10.1007/s00401-014-1262-6. [DOI] [PubMed] [Google Scholar]
- 27.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 2011;21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Henne WM, Stenmark H, Emr SD. Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez I, Rosende-Roca M, Alegret M, Mauleon A, Espinosa A, Vargas L, et al. Association of TMEM106B rs1990622 marker and frontotemporal dementia: evidence for a recessive effect and meta-analysis. J Alzheimers Dis. 2015;43:325–334. doi: 10.3233/JAD-132432. [DOI] [PubMed] [Google Scholar]
- 30.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 31.Isaacs AM, Johannsen P, Holm I, Nielsen JE. Frontotemporal dementia caused by CHMP2B mutations. Curr Alzheimer Res. 2011;8:246–251. doi: 10.2174/156720511795563764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, et al. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- 33.Josephs KA, Holton JL, Rossor MN, Godbolt AK, Ozawa T, Strand K, et al. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol. 2004;30:369–373. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 34.Jun G, Ibrahim-Verbaas CA, Vronskaya M, Lambert JC, Chung J, Naj AC, et al. A novel Alzheimer disease locus located near the gene encoding tau protein. Mol Psychiatry. 2016;21:108–117. doi: 10.1038/mp.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jun MH, Han JH, Lee YK, Jang DJ, Kaang BK, Lee JA. TMEM106B, a frontotemporal lobar dementia (FTLD) modifier, associates with FTD-3-linked CHMP2B, a complex of ESCRT-III. Mol Brain. 2015;8:85. doi: 10.1186/s13041-015-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- 37.Lang CM, Fellerer K, Schwenk BM, Kuhn PH, Kremmer E, Edbauer D, et al. Membrane orientation and subcellular localization of transmembrane protein 106B (TMEM106B), a major risk factor for frontotemporal lobar degeneration. J Biol Chem. 2012;287:19355–19365. doi: 10.1074/jbc.M112.365098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lattante S, Le Ber I, Galimberti D, Serpente M, Rivaud-Pechoux S, Camuzat A, et al. Defining the association of TMEM106B variants among frontotemporal lobar degeneration patients with GRN mutations and C9orf72 repeat expansions. Neurobiol Aging. 2014;35:2658 e2651–2655. doi: 10.1016/j.neurobiolaging.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 39.Lemberg MK. Intramembrane proteolysis in regulated protein trafficking. Traffic. 2011;12:1109–1118. doi: 10.1111/j.1600-0854.2011.01219.x. [DOI] [PubMed] [Google Scholar]
- 40.Lipton AM, White CL, 3rd, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol. 2004;108:379–385. doi: 10.1007/s00401-004-0900-9. [DOI] [PubMed] [Google Scholar]
- 41.Mackenzie IR, Baker M, Pickering-Brown S, Hsiung GY, Lindholm C, Dwosh E, et al. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain. 2006;129:3081–3090. doi: 10.1093/brain/awl271. [DOI] [PubMed] [Google Scholar]
- 42.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackenzie IR, Rademakers R. The molecular genetics and neuropathology of frontotemporal lobar degeneration: recent developments. Neurogenetics. 2007;8:237–248. doi: 10.1007/s10048-007-0102-4. [DOI] [PubMed] [Google Scholar]
- 44.Mackenzie IR, Shi J, Shaw CL, Duplessis D, Neary D, Snowden JS, et al. Dementia lacking distinctive histology (DLDH) revisited. Acta Neuropathol. 2006;112:551–559. doi: 10.1007/s00401-006-0123-3. [DOI] [PubMed] [Google Scholar]
- 45.Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, et al. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer H, Weihl CC. The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J Cell Sci. 2014;127:3877–3883. doi: 10.1242/jcs.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murray ME, Cannon A, Graff-Radford NR, Liesinger AM, Rutherford NJ, Ross OA, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014;128:411–421. doi: 10.1007/s00401-014-1302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, et al. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–690. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson PT, Wang WX, Partch AB, Monsell SE, Valladares O, Ellingson SR, et al. Reassessment of risk genotypes (GRN, TMEM106B, and ABCC9 variants) associated with hippocampal sclerosis of aging pathology. J Neuropathol Exp Neurol. 2015;74:75–84. doi: 10.1097/NEN.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 52.Nicholson AM, Finch NA, Wojtas A, Baker MC, Perkerson RB, 3rd, Castanedes-Casey M, et al. TMEM106B p.T185S regulates TMEM106B protein levels: implications for frontotemporal dementia. J Neurochem. 2013;126:781–791. doi: 10.1111/jnc.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- 54.Pottier C, Bieniek KF, Finch N, van de Vorst M, Baker M, Perkersen R, et al. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol. 2015;130:77–92. doi: 10.1007/s00401-015-1436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pottier C, Ravenscroft TA, Sanchez-Contreras M, Rademakers R. Genetics of FTLD: Overview and what else we can expect from genetic studies. J Neurochem. 2016 doi: 10.1111/jnc.13622. [DOI] [PubMed] [Google Scholar]
- 56.Premi E, Formenti A, Gazzina S, Archetti S, Gasparotti R, Padovani A, et al. Effect of TMEM106B polymorphism on functional network connectivity in asymptomatic GRN mutation carriers. JAMA Neurol. 2014;71:216–221. doi: 10.1001/jamaneurol.2013.4835. [DOI] [PubMed] [Google Scholar]
- 57.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- 59.Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rohrer JD, Geser F, Zhou J, Gennatas ED, Sidhu M, Trojanowski JQ, et al. TDP-43 subtypes are associated with distinct atrophy patterns in frontotemporal dementia. Neurology. 2010;75:2204–2211. doi: 10.1212/WNL.0b013e318202038c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rollinson S, Mead S, Snowden J, Richardson A, Rohrer J, Halliwell N, et al. Frontotemporal lobar degeneration genome wide association study replication confirms a risk locus shared with amyotrophic lateral sclerosis. Neurobiol Aging. 2011;32:758 e751–757. doi: 10.1016/j.neurobiolaging.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Rosso SM, Donker Kaat L, Baks T, Joosse M, de Koning I, Pijnenburg Y, et al. Frontotemporal dementia in The Netherlands: patient characteristics and prevalence estimates from a population-based study. Brain. 2003;126:2016–2022. doi: 10.1093/brain/awg204. [DOI] [PubMed] [Google Scholar]
- 63.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 64.Rutherford NJ, Carrasquillo MM, Li M, Bisceglio G, Menke J, Josephs KA, et al. TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology. 2012;79:717–718. doi: 10.1212/WNL.0b013e318264e3ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sardiello M, Palmieri M, di Ronza A, Medina DL, Valenza M, Gennarino VA, et al. A gene network regulating lysosomal biogenesis and function. Science. 2009;325:473–477. doi: 10.1126/science.1174447. [DOI] [PubMed] [Google Scholar]
- 66.Schwenk BM, Lang CM, Hogl S, Tahirovic S, Orozco D, Rentzsch K, et al. The FTLD risk factor TMEM106B and MAP6 control dendritic trafficking of lysosomes. Embo J. 2014 doi: 10.1002/embj.201385857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seelaar H, Klijnsma KY, de Koning I, van der Lugt A, Chiu WZ, Azmani A, et al. Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol. 2010;257:747–753. doi: 10.1007/s00415-009-5404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serpente M, Fenoglio C, Clerici F, Bonsi R, Arosio B, Cioffi SM, et al. Transmembrane protein 106B gene (TMEM106B) variability and influence on progranulin plasma levels in patients with Alzheimer’s disease. J Alzheimers Dis. 2015;43:757–761. doi: 10.3233/JAD-141167. [DOI] [PubMed] [Google Scholar]
- 69.Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15:647–658. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Skibinski G, Parkinson NJ, Brown JM, Chakrabarti L, Lloyd SL, Hummerich H, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37:806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 71.Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, et al. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am J Hum Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snowden JS, Pickering-Brown SM, Mackenzie IR, Richardson AM, Varma A, Neary D, et al. Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain. 2006;129:3091–3102. doi: 10.1093/brain/awl267. [DOI] [PubMed] [Google Scholar]
- 73.Stagi M, Klein ZA, Gould TJ, Bewersdorf J, Strittmatter SM. Lysosome size, motility and stress response regulated by fronto-temporal dementia modifier TMEM106B. Mol Cell Neurosci. 2014;61:226–240. doi: 10.1016/j.mcn.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urushitani M, Sato T, Bamba H, Hisa Y, Tooyama I. Synergistic effect between proteasome and autophagosome in the clearance of polyubiquitinated TDP-43. J Neurosci Res. 2010;88:784–797. doi: 10.1002/jnr.22243. [DOI] [PubMed] [Google Scholar]
- 75.van Blitterswijk M, Mullen B, Nicholson AM, Bieniek KF, Heckman MG, Baker MC, et al. TMEM106B protects C9ORF72 expansion carriers against frontotemporal dementia. Acta Neuropathol. 2014 doi: 10.1007/s00401-013-1240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet. 2010;42:234–239. doi: 10.1038/ng.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Zee J, Gijselinck I, Pirici D, Kumar-Singh S, Cruts M, Van Broeckhoven C. Frontotemporal lobar degeneration with ubiquitin-positive inclusions: a molecular genetic update. Neurodegener Dis. 2007;4:227–235. doi: 10.1159/000101847. [DOI] [PubMed] [Google Scholar]
- 78.van der Zee J, Van Langenhove T, Kleinberger G, Sleegers K, Engelborghs S, Vandenberghe R, et al. TMEM106B is associated with frontotemporal lobar degeneration in a clinically diagnosed patient cohort. Brain. 2011;134:808–815. doi: 10.1093/brain/awr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vass R, Ashbridge E, Geser F, Hu WT, Grossman M, Clay-Falcone D, et al. Risk genotypes at TMEM106B are associated with cognitive impairment in amyotrophic lateral sclerosis. Acta Neuropathol. 2011;121:373–380. doi: 10.1007/s00401-010-0782-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang IF, Guo BS, Liu YC, Wu CC, Yang CH, Tsai KJ, et al. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc Natl Acad Sci U S A. 2012;109:15024–15029. doi: 10.1073/pnas.1206362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang IF, Tsai KJ, Shen CK. Autophagy activation ameliorates neuronal pathogenesis of FTLD-U mice: a new light for treatment of TARDBP/TDP-43 proteinopathies. Autophagy. 2013;9:239–240. doi: 10.4161/auto.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X, Fan H, Ying Z, Li B, Wang H, Wang G. Degradation of TDP-43 and its pathogenic form by autophagy and the ubiquitin-proteasome system. Neurosci Lett. 2010;469:112–116. doi: 10.1016/j.neulet.2009.11.055. [DOI] [PubMed] [Google Scholar]
- 84.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 85.Wood EM, Falcone D, Suh E, Irwin DJ, Chen-Plotkin AS, Lee EB, et al. Development and validation of pedigree classification criteria for frontotemporal lobar degeneration. JAMA Neurol. 2013;70:1411–1417. doi: 10.1001/jamaneurol.2013.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA. The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology. 2015;84:927–934. doi: 10.1212/WNL.0000000000001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–370. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]