Abstract
Nogo-B receptor (NgBR) was identified as a specific receptor for binding Nogo-B, and is essential for the stability of Niemann-Pick type C2 protein (NPC2) and NPC2-dependent cholesterol trafficking. Here, we report that NgBR expression levels decrease in the fatty liver and that NgBR plays previously unrecognized roles in regulating hepatic lipogenesis via NPC2-independent pathways. To further elucidate the pathophysiological role of NgBR in mammals, we generated NgBR liver-specific knockout mice and investigated the roles of NgBR in hepatic lipid homeostasis. Our results showed that NgBR knockout in mouse liver did not decrease either NPC2 levels or increase NPC2-dependent intracellular cholesterol levels. However, NgBR deficiency still resulted in remarkable cellular lipid accumulation that was associated with increased free fatty acids (FFA) and triglycerides (TG) in hepatocytes in vitro and in mouse livers in vivo. Mechanistically, NgBR deficiency specifically promotes the nuclear translocation of the liver X receptor alpha (LXRα) and increases the expression of LXRα-targeted lipogenic genes. LXRα knockout attenuates the accumulation of FFA and TG caused by NgBR deficiency. In addition, we elucidated the mechanisms by which NgBR bridges the AMP-activated protein kinase α (AMPKα) signaling pathway with LXRα nuclear translocation and LXRα-mediated lipogenesis. Our study demonstrates that NgBR is a specific negative regulator for LXRα–dependent hepatic lipogenesis and suggests that loss of NgBR is a potential trigger for inducing hepatic steatosis.
Introduction
Hepatic steatosis is caused by the abnormal accumulation of lipids within hepatocytes. It is the most common emerging liver disease, occurring in 21.4% of the US population (1). The liver X receptor α (LXRα) is a ligand-activated nuclear receptor involved in regulating the expression of lipogenic genes for biosynthesis of fatty acids (FAs) and triglycerides (TGs), both of which contribute to hepatic steatosis (2, 3). LXRα can be activated by endogenous agonists, such as the oxysterols 24-hydroxycholesterol and 24(S), 25-epoxycholesterol (4), or synthetic agonists, such as GW3965 and T0901317 (5-7). In addition to the promotion of reverse cholesterol transport (RCT) (8, 9), another major function of LXRα in the liver is the control of de novo lipogenesis, i.e., the biosynthesis of FAs (7). LXRα promotes lipogenesis through the induction of sterol-regulatory element-binding protein 1c (SREBP-1c), stearoyl CoA desaturase-1 (SCD-1), and fatty acid synthase (FASN) expression (5-7). Therefore, LXRα plays a critical role in regulating cholesterol homeostasis and hepatic lipogenesis.
Nogo-B is one isoform of the reticulon protein family and is involved in regulating blood vessel remodeling (10). NgBR has been identified as a receptor specific for Nogo-B and is essential for Nogo-B-stimulated endothelial cell migration and blood vessel formation (11, 12). NgBR interacts with Niemann-Pick type C2 protein (NPC2), a protein which transfers cholesterol between membranes (13). RNAi-mediated disruption of NgBR in vitro leads to decreased stability of NPC2, and increased intracellular free cholesterol accumulation (13). To further elucidate the physiological role of NgBR in regulating hepatic lipid homeostasis in vivo, we generated NgBR liver-specific knockout (NgBR LivKO) mice. Herein, we elucidate that NgBR has an NPC2-independent role for hepatic lipogenesis via AMPKα pathway-dependent translocation of LXRα into the nucleus.
Materials and Methods
NgBR gene targeting by homologous recombination
The NgBR gene is located at mouse chromosome 10:52.137.365-52.156.570 (ENSMUST00000023830), and consists of 5 exons. Exons 2-5 contain the cDNA sequence coding for the cytoplasmic domain of NgBR, which is the functional domain defined by previous publications (11, 13-15). Therefore, we specifically deleted the cytoplasmic domain of NgBR by inserting 2 LoxP sites outside exons 2-4 as shown in Figure S1A. A targeting vector in which NgBR exons 2 and 4 are flanked by a single upstream LoxP site, and a downstream FRT/neomycin resistance/FRT/LoxP cassette was constructed using a combination of ligation-mediated and recombineering techniques (16). NgBR gene fragments were retrieved from 129S strain bacterial artificial clones (Sanger Institute). A conditional NgBR knockout mouse was created by homologous recombination in 129S embryonic stem cells with subsequent implantation into C57BL/6 embryos to produce chimeric male founders. Crossbreeding with C57BL/6 females established germline transmission of the targeted NgBR allele. Correct gene targeting was confirmed by Southern blot analysis of genomic DNA isolated from established embryonic stem cells and offspring of chimeric mice (Fig. S1B). Upon further crossbreeding with B6.Cg-Tg(ACTFLPe)9205Dym/J mice (The Jackson Laboratory, Bar Harbor, ME) expressing Flp-recombinase under control of the human β-actin promoter, the FRT-flanked neomycin resistance cassette was excised from intron 5, resulting in a floxed allele in which exon 4 is flanked by a downstream LoxP site. Mice carrying the floxed allele were crossbred with C57BL/6 background mice for 10 generations
Animal studies
NgBR floxed mice were crossbred with albumin-specific Cre mice [Alb-Cre, B6.Cg-Tg(Alb-cre)21Mgn/J] (The Jackson Laboratory) on a C57BL/6J background. Alb-Cre efficiently induces hepatocyte-specific recombination of floxed genes to generate NgBR LivKO mice. To generate NgBR and LXRα double knockout (Double KO) mice, NgBR LivKO mice were crossbred with LXRα KO mice on a C57BL/6 background (The Jackson Laboratory). All animals were housed in a temperature-controlled environment with a 12/12 h light/dark cycles. All mice had free access to water and standard rodent chow or a Western diet (21.2% fat and 0.2% cholesterol; TD88137, Harlan Laboratories, Madison, WI). For rescue experiments, both littermate control (NgBR-floxed, no Cre) and NgBR LivKO mice were intraperitoneally (i.p.) injected with 100 μL metformin solution (250 mg/kg body weight/day) daily for one week. At the end of the experiment, mice were anesthetized and euthanized followed by collection of blood samples in EDTA-coated tubes and liver tissue samples. Plasma was isolated by centrifugation of blood samples for 20 min at 2,000 g at 4°C for determination of lipid composition. In addition, we crossbred NgBR floxed mice with globally inducible Cre mice (Rosa26R-ERT2Cre, The Jackson Laboratory) to generate NgBR inducible KO (NgBR iKO) mice. The NgBR global knockout was induced by i.p. injection of tamoxifen at 6-8 weeks of age. All of the animal experiments were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin.
Additional materials and methods are included in the Supplementary Materials.
Statistical analysis
The results of quantitative assays were analyzed using SPSS 16.0 for Windows. Data are presented as the mean ± standard error of the mean (SEM). Barlett’s test was performed prior to ANOVA. The statistical significance of differences was evaluated by ANOVA followed by Dunnett’s post-hoc analysis. Significance was defined as P<0.05.
Results
NgBR expression decreases in the fatty liver
To determine if hepatic NgBR expression is altered in patients with fatty liver disease, we used real-time PCR to quantify transcript levels of NgBR, SREBP-1c, and FASN in normal and diseased livers (human liver cDNA array was obtained from Origene). Figure 1A shows that NgBR expression is significantly decreased in the fatty liver samples compared to healthy controls. Further, this decrease in NgBR is associated with increased expression of SREBP-1c and FASN, two lipogenic genes causing hepatic steatosis (3). NgBR levels were also decreased in the liver of C57BL/6J mice fed a Western diet, which is evidenced by vacuole-containing lipid droplets in the H & E staining tissue sections (Fig. S2A) and increased expression of lipogenic genes (Fig. S2B).
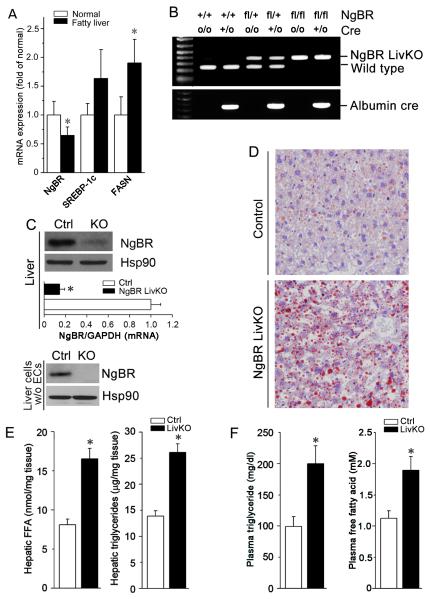
Figure 1. Loss of NgBR induces lipid accumulation in the mouse liver and hypertriglyceridemia.
(A) NgBR gene expression is decreased in human fatty liver, which is associated with increased expression of lipogenic genes SREBP-1c and FASN. A human liver cDNA panel including 8 healthy control samples and 4 fatty liver samples was purchased from Origene. Gene expression was determined by real-time PCR. * P<0.05; (B-F) NgBR liver-specific knockout (NgBR LivKO) mice were generated as described in the Methods. Both NgBR LivKO and littermate control (NgBR-floxed, no cre) mice were used to conduct the following experiments: (B) PCR analysis of the NgBR gene from mice that are wild-type (NgBR+/+:Creo/o), albumin-Cre (NgBR+/+:Cre+/o or NgBR+/+:Cre+/+), flox/+ (NgBRfl/+:Creo/o), flox/+:albumin-Cre (NgBRfl/+:Cre+/o or NgBRfl/+:Cre+/+), flox/flox (NgBRfl/fl:Creo/o), and flox/flox:albumin-cre (NgBRfl/fl:Cre+/o or NgBRfl/fl: Cre+/+). (C) Expression of NgBR protein and mRNA in the livers of littermate control and NgBR LivKO mice were determined by western blotting with whole liver protein extract (upper panel) or extract of hepatic cells after endothelial cells were removed (bottom panel) and real-time RT-PCR (upper panel) with total RNA isolated from whole liver tissues. *: P<0.05 vs. control (n=4). (D) Littermate control (NgBR-floxed, no cre) and NgBR LivKO female mice at 8 weeks old were fed a Western diet for 4 weeks. NgBR liver-specific knockout increased lipid accumulation determined by Oil Red O staining performed on the frozen liver sections. (E) Quantitative analysis showed that NgBR liver-specific knockout increased the levels of free fatty acid (FFA) and triglyceride (TG). Total lipid extract was prepared from liver tissues of littermate control and NgBR LivKO mice, *: P<0.05 vs. littermate control (n=5). (F) NgBR liver-specific knockout increased the levels of FFA and TG in plasma. Plasma samples were collected from littermate control (NgBR-floxed, no cre) and NgBR LivKO mice. FFA and TG levels were determined by assay kits. *: P<0.05 vs. littermate control (n=5).
NgBR deficiency results in lipid accumulation
To elucidate the effects of decreased NgBR expression on the pathogenesis of fatty liver disease, we generated NgBR LivKO mice as described in Methods. As shown in Figure 1B, single allele or double allele excision happened in heterozygous or homozygous floxed mice carrying Alb-Cre, respectively. NgBR protein and mRNA levels significantly decreased in the liver of NgBR LivKO mice, but some NgBR was still detected because of expression in liver endothelial cells (Fig. 1C, upper panel). When endothelial cells were removed from isolated hepatocytes by immunodepletion using CD31-conjugated beads, NgBR expression in the purified hepatocytes was undetectable (Fig. 1C, bottom panel).
Although NgBR global knockout is embryonic lethal in mice around E7.5 (17), NgBR LivKO mice are viable and do not have any defects in aspects of breeding, weight gain, food intake, and exterior appearance. However, Oil Red O staining demonstrates that NgBR LivKO induced severe lipid accumulation in the liver after consuming a Western diet for 4 weeks (Fig. 1D). Loss of hepatic NgBR expression substantially increased the amounts of free FA (FFA) and TG in hepatocytes and in plasma (Fig. 1E-F), but significantly decreased total and free cholesterol levels only in hepatocytes (Fig. S3). Lipid accumulation was also increased in the liver of NgBR LivKO mice fed normal chow (Fig. S4). Similarly, we observed increased total lipid content as well as increased hepatic FFA and TG levels in the liver of NgBR inducible KO (iKO) mice (Fig. S5) fed a Western diet. The above results suggest that loss of hepatic NgBR expression impairs hepatic lipid homeostasis and induces hypertriglyceridemia.
NgBR deficiency-induced accumulation of FFAs and TGs in the liver can be recapitulated in cultured HepG2 cells. We generated a stable NgBR knockdown HepG2 cell line (shNgBRi cells) by infecting HepG2 cells with lentivirus carrying small hairpin NgBR RNAi and puromycin selection (Fig. 2A). Compared to control cells infected with non-silencing small hairpin RNAi (shNSi cells), the results of Oil Red O staining show a severe lipid accumulation in shNgBRi cells (Fig. 2B). Quantitative lipid analysis showed that NgBR deficiency significantly increased cellular FFAs and TGs (Fig. 2C) and slightly decreased total and free CHO levels in HepG2 cells (Fig. S6D).
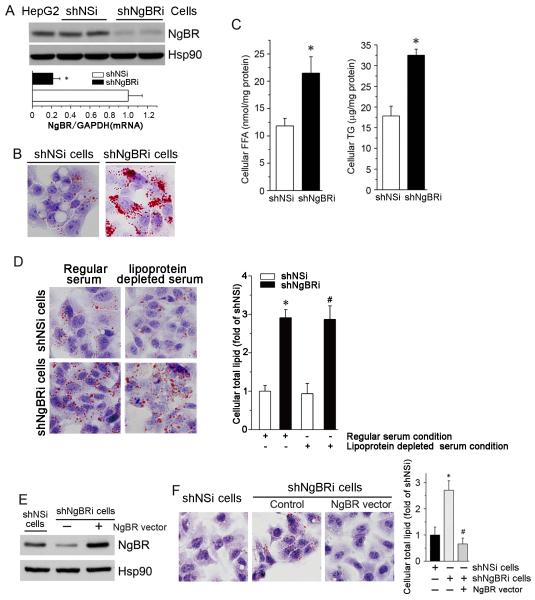
Figure 2. NgBR deficiency induces cellular lipid accumulation in vitro.
Stable NgBR knockdown HepG2 (shNgBRi) cells were generated as described in the supplemental information and used to conduct the following experiments. (A) NgBR knockdown in shNgBRi cells was characterized by western blotting and real time RT-PCR. *: P<0.05 vs. control (shNSi) cells (n=3). (B) Determination of cellular lipid accumulation by Oil Red O staining. (C) Quantitative analysis of cellular FFA and TG levels. *: P<0.05 vs. shNSi cells (n=3). (D) Control HepG2 cells (shNSi) and NgBR-deficient HepG2 cells (shNgBRi) were cultured in MEM medium containing 10% regular or lipoprotein-deficient serum (Kalen Biomedical, Montgomery village, MD) for 24 h followed by Oil Red O staining (left panel). Quantitative levels of total lipid content in shNSi and shNgBRi cells were determined as described in the Methods. *, #: P<0.05 vs. shNSi cells in MEM medium containing either regular or lipoprotein deficient serum, respectively (n=3). (E, F) Overexpression of NgBR attenuates NgBR deficiency-caused lipid accumulation. shNgBRi cells were transiently transfected with an NgBR expression vector for 24 h. NgBR expression was then determined by western blotting (E). Cellular lipid content was determined by Oil Red O staining and quantitative analysis, respectively (F). *: P<0.05 vs. shNSi cells (n=3); #: P<0.05 vs. shNgBRi cells without transfection of NgBR expression vector (n=3).
To determine if the accumulation of intracellular lipids is caused by lipid uptake, we cultured shNSi and shNgBRi HepG2 cells in culture medium containing 10% lipoprotein deficient serum (LPDS) or regular fetal bovine serum. The results of Oil Red O staining (Fig. 2D, left panel) show higher intracellular lipid levels in shNgBRi cells than shNSi cells, with similar accumulations in cells cultured in medium containing either regular serum or LPDS (Fig. 2D, right panel). These results suggest that the lipid accumulation in NgBR-deficient HepG2 cells is not attributable to increased lipid uptake.
We confirmed the specific effects of NgBR deficiency on lipid accumulation by overexpressing NgBR in shNgBRi HepG2 cells (Fig. 2E). As shown in Figure 2F, restored expression of NgBR in NgBR-deficient HepG2 cells reduced the total intracellular lipid content to normal levels. Taken together, these results suggest that NgBR deficiency results in hepatic lipid accumulation through increased cellular FFA and TG levels, which is casued by exacerbated hepatic lipogenesis.
NgBR deficiency does not affect hepatic NPC2 protein levels and intracellular free cholesterol in the physiological setting
A previous report showed that interaction between NgBR and NPC2 protein increases NPC2 stability and inhibits intracellular free cholesterol accumulation (13). To determine if NgBR deficiency influences NPC2-mediated intracellular cholesterol transport, we cultured shNgBRi cells in complete or serum-free medium. Compared to shNSi cells, NPC2 protein levels were substantially decreased in shNgBRi cells in the absence of serum (Fig. S6A, right two lanes), consistent with the previous report (13), but were unchanged when serum was present (Fig. S6A, left two lanes). Correspondingly, results of filipin staining show accumulated free cholesterol in shNgBRi cells cultured in serum-free medium (Fig. S6B, right bottom panel) but not in complete medium (Fig. S6B, left bottom panel).
We also determined the effects of NgBR deficiency on NPC2 expression and cholesterol levels in vivo. Compared to littermate controls, NgBR LivKO did not decrease NPC2 protein expression in mouse livers (Fig. S6C). Consistent with in vitro results (Fig. S6D), total and free cholesterol levels slightly decreased in the livers of NgBR LivKO mice (Fig. S3A). Taken together, these results suggest that NgBR deficiency does not impair either NPC2 expression or NPC2-mediated cholesterol transport under physiological conditions.
NgBR deficiency activates hepatic lipogenesis
To elucidate the underlying mechanisms by which NgBR deficiency induces hepatic FFA and TG accumulation, we examined the protein levels of lipogenic genes, such as FASN and SREBP-1c. As shown in Figure 3A, shNgBRi HepG2 cells had increased expression of FASN and the nuclear (mature) form of SREBP-1c (nSREBP-1c). In contrast, overexpression of NgBR in HepG2 cells reduced the protein levels of both FASN and nSREBP-1c (Fig. 3B). Consistently, increased expression of both precursor and mature forms of SREBP-1c (pSREBP-1c, nSREBP-1c), as well as FASN and SCD-1, was observed in the livers of NgBR LivKO mice (Fig. 3C). These in vitro and in vivo results confirm that the deficiency of hepatic NgBR expression activates hepatic lipogenesis.
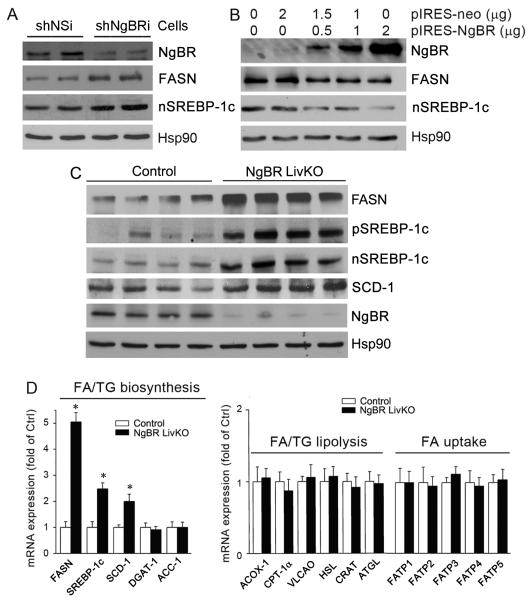
Figure 3. NgBR deficiency increases hepatic lipogenesis.
(A) Protein levels of NgBR, FASN, and mature SREBP-1c (nSREBP-1c) in shNSi and shNgBRi cells were determined by western blotting. (B) HepG2 cells were transfected with an NgBR overexpression vector at the indicated concentrations for 24 h followed by determination of NgBR, FASN, and nSREBP-1c protein expression by western blotting. (C) Total protein was extracted from the livers of littermate control (NgBR-floxed, no cre) and NgBR LivKO female mice (8 weeks old) that were fed a Western diet for 4 weeks, and protein levels of FASN, precursor of SREBP-1c (pSREBP-1c), nSREBP-1c, and SCD-1 were determined by western blotting. (D) Total RNA was extracted from liver samples of littermate control and NgBR LivKO mice, and expression of genes for FFA and TG biosynthesis, FFA/TG lipolysis and FFA uptake was determined by real-time RT-PCR. *: P<0.05 vs. control (n=4). The sequences of the primers are listed in Table S1.
To reveal the mechanism by which NgBR deficiency increases hepatic lipogenesis, we further examined the effects of NgBR LivKO on the transcription of genes regulating lipid biosynthesis and metabolism by real-time PCR array. The results show that NgBR deficiency increased the transcription of genes involved in FA biosynthesis (FASN, SREBP-1c, and SCD-1), but not the genes for TG biosynthesis (DGAT-1; Fig. 3D, left panel). In addition, the transcription of genes involved in FA or TG lipolysis or in FA uptake was not affected by NgBR deficiency (Fig. 3D, right panel). This same gene expression profile also was observed in the livers of NgBR iKO mice (Fig. S7). The above in vitro and in vivo results suggest that deficiency of NgBR expression activates hepatic lipogenesis, resulting in accumulation of FFA and TG in the liver.
NgBR deficiency activates LXRα pathway
To determine the role of LXRα in NgBR deficiency-induced lipogenesis, we initially examined LXRα expression and nuclear translocation in vitro. Figure 4A demonstrates that NgBR deficiency did not change the protein levels of LXRα in total cellular lysates, but dramatically increased LXRα levels in the nucleus, with proportionally reduced LXRα levels in the cytosol. These results suggest that NgBR deficiency promotes LXRα translocation from the cytosol into the nucleus. Immunofluorescent staining also demonstrated that the majority of LXRα was localized in the cytosol of shNSi cells, but in the nuclei of shNgBRi cells (Fig. 4B). Overexpression of NgBR in shNgBRi-cells caused most of the LXRα to relocate into the cytosol, suggesting that LXRα nuclear translocation is reversible (Fig. 4B). Similarly, LXRα protein levels in total liver lysates did not change in NgBR LivKO mice, but nuclear levels increased more than 2-fold, while cytosolic levels decreased (Fig. 4C). The similar distribution pattern of LXRα was observed in the liver of NgBR iKO mouse (Fig. S8). Furthermore, the results of both immunofluorescent and immunohistochemical staining of LXRα in mouse liver sections also demonstrated that NgBR LivKO induced LXRα nuclear translocation (Fig. 4D-E). The specificity of LXRα antibody for Western blot and immunostaining has been validated by using LXRα knockout mouse tissues (Fig. S9). In addition, NgBR deficiency had no effect on the nuclear translocation of LXRβ and retinoid X receptor (RXR) (Fig. S10), demonstrating that NgBR deficiency specifically induces the nuclear translocation of LXRα. Consistent with previous reports (5-7), treatment of HepG2 cells with GW3965, a synthetic LXRα ligand, resulted in intracellular lipid accumulation (Fig. 4F), a similar phenotype to shNgBRi cells, which implies that LXRα may play a critical role in NgBR deficiency induced lipogenesis.
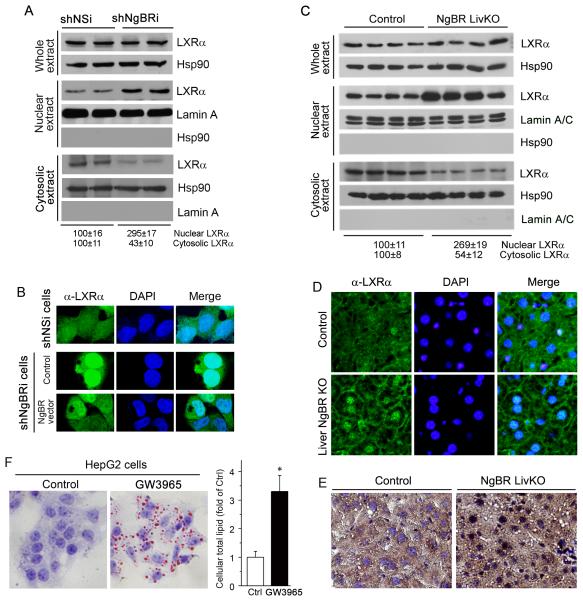
Figure 4. NgBR deficiency promotes LXRα nuclear translocation and activates LXRα pathway.
(A) Protein levels of LXRα in total cellular, cytosolic and nuclear extracts of shNSi and shNgBRi cells were determined by western blotting. (B) NgBR deficiency promotes the nuclear translocation of LXRα. Localization of LXRα in shNSi cells, shNgBRi cells, and shNgBRi cells transfected with the plasmid DNA of NgBR-HA were determined by immunofluorescent staining. (C) Total, cytosolic and nuclear proteins were extracted from liver tissue samples of littermate control and NgBR LivKO female mice (n=4). Expression of LXRα was determined by western blotring. (D, E) Localization of LXRα in the frozen liver sections of littermate control and NgBR LivKO female mice was determined by immunofluorescent (D) and immunohistochemical (E) staining. (F) LXR activation increases lipid accumulation. HepG2 cells were treated with LXR ligand (GW3965, 10 μM) for 24 h followed by determination of cellular lipid contents with Oil Red O staining and quantitative analysis. *: P<0.05 vs. control (n=3).
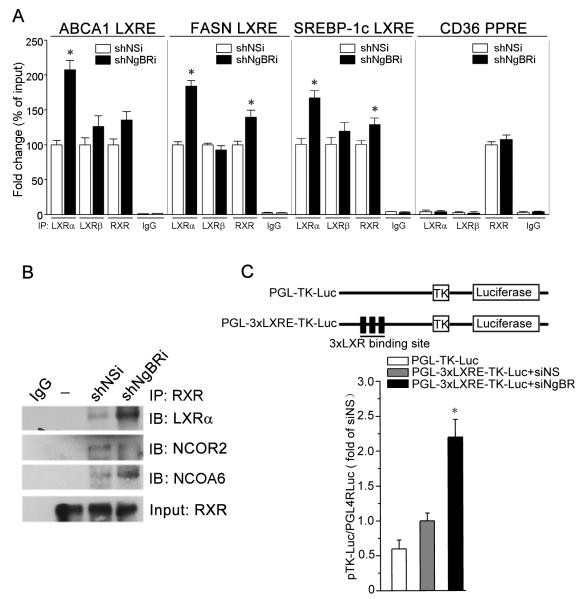
We further confirmed the effects of NgBR deficiency on LXRα–mediated gene transcription. The binding of the LXR response element (LXRE) to LXRα, LXRβ, or RXR protein was determined by chromatin immunoprecipitation/quantitative PCR (ChIP-qPCR) assays. ABCA1, FASN, and SREBP-1c genes contain the LXRE (18) while the CD36 gene contains a peroxisome proliferator-activated receptor γ response element (PPRE) (19). Figure 5A shows that NgBR deficiency significantly increased the binding of LXRα to the LXREs of ABCA1, FASN, and SREBP-1c and moderately increased the binding of RXR, presumably because of the increased formation of the LXRα/RXR heterodimer in the nucleus (18). However, the binding of LXRβ to the LXREs was not affected. Little binding of LXRα or LXRβ to the PPRE of CD36 was observed, which indirectly demonstrates the specificity of the LXRα and LXRβ antibodies. The binding of RXR to the PPRE of CD36 was not influenced by NgBR deficiency, which suggests that NgBR does not affect RXR cellular distribution (Fig. S10B). Increased amounts of LXRα and nuclear receptor co-activator 6 (NCOA6) and decreased amounts of nuclear receptor co-repressor 2 (NCOR2) were detected in the complex co-immunoprecipitated by anti-RXR antibody from shNgBRi cells (Fig. 5B). An LXRE reporter assay also demonstrated that NgBR knockdown increased the LXRE-dependent luciferase activity as compared to controls (Fig. 5C). These results indicate that NgBR deficiency-induced nuclear translocation of LXRα increases the transcription of LXRα target genes.
Figure 5. NgBR deficiency increases LXRα transcriptional activity.
(A) Chromatin was isolated from confluent shNSi or shNgBRi cells. After determination of input, the immunoprecipitation was conducted with normal IgG, anti-LXRα, LXRβ or RXR antibody followed by real-time PCR. *P<0.05 vs. the corresponding control (n=4). (B) The LXRα/RXR heterodimer was immunoprecipitated by anti-RXR antibody followed by determination of LXRα, NCOR2, and NCOA6 in the complex by western blotting. (C) NgBR deficiency increased LXRE promoter activity. Activities of firefly and Renilla luciferases in the isolated cell lysate were determined using the Dual-Luciferase Reporter Assay System (Promega). *: P<0.05 vs. control (n=3).
In addition, NgBR deficiency in vitro and in vivo increases expression of other LXRα target genes that are involved in reverse cholesterol transport (RCT, e.g., ABCA1, ABCG1/5/8) (Fig. S11A and S11B). As shown in Figure S11C and S11D, the induction levels of LXRα target genes in NgBR knockdown HepG2 cells is equal to the levels in control HepG2 cells treated with 1 μM GW3965. In addition, NgBR deficiency increases the response of HepG2 cells to GW3965 stimulation (Fig. S11C and S11D).
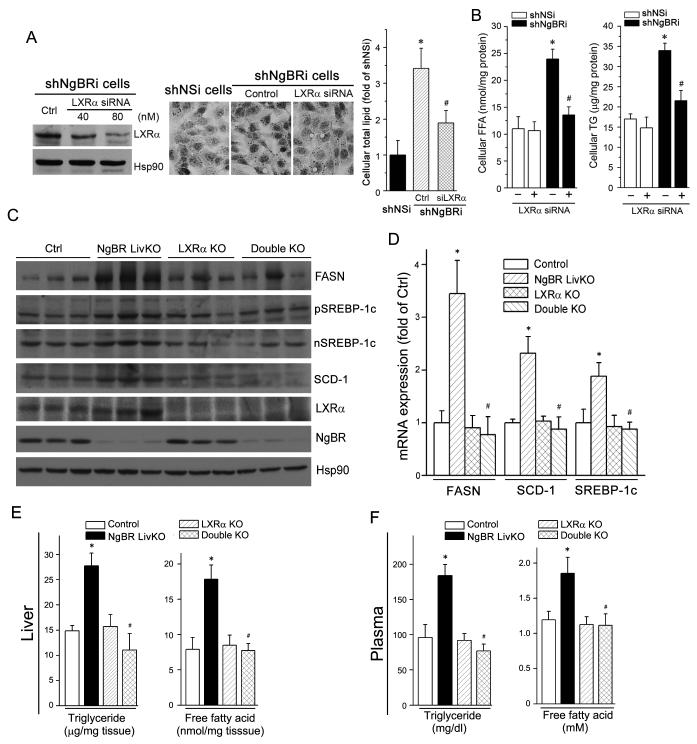
Indeed, knockdown of LXRα expression ameliorated lipid accumulation in shNgBRi cells, which was determined by Oil Red O staining (Fig. 6A). Furthermore, lipid profile analysis results demonstrate that LXRα knockdown specifically attenuated the increase of cellular FFA and TG levels induced by NgBR deficiency (Fig. 6B). To determine if the hepatic lipogenesis induced by NgBR deficiency is mainly mediated through activation of LXRα in vivo, we crossbred NgBR LivKO mice with LXRα KO mice to generate LXRα and NgBR double knockout (dKO) mice. Compared to NgBR LivKO mice, lack of LXRα expression blocked NgBR deficiency-induced expression of FASN, SREBP-1c, and SCD-1 protein and mRNA in the liver (Fig. 6C-D). Consequently, hepatic/plasma TG and FFA levels in the dKO mice (Fig. 6E-F) were reduced to the levels observed in littermate control or LXRα KO mice. However, hepatic cholesterol levels in dKO mice were at the same levels as in LXRα KO mice (Fig. S3A) because deficiency of LXRα expression impaired the hepatic RCT pathway that can result in hepatic cholesterol accumulation (9, 20). No significant difference of plasma cholesterol levels was determined among control, single KO and dKO mice (Fig. S3B). Taken together, both in vitro and in vivo results demonstrate that NgBR deficiency induces hepatic lipogenesis by activating LXRα, which results in accumulation of FFA and TG in both the liver and plasma.
Figure 6. LXRα knockout reduces hepatic lipogenesis in the liver of NgBR LivKO mice.
(A) LXRα knockdown prevents NgBR deficiency-induced lipid accumulation. ShNgBRi cells were transfected with non-silencing siRNA (Ctrl) or LXRα siRNA followed by determination of LXRα expression by western blotting (left panel). Cellular lipid contents were determined by Oil Red O staining (middle panel) and quantitative analysis (right panel). *: P<0.05 vs. shNSi cells; #: P<0.05 vs. shNgBRi cells alone. (B) LXRα knockdown reduces NgBR deficiency-induced accumulation of FFA and TG. shNSi and shNgBRi cells were transfected with LXRα siRNA (80 nM) followed by quantitative analysis of cellular FFA and TG. *: P<0.05 vs. shNSi cells; #: P<0.05 vs. shNgBRi cells alone. (C-F) Lack of LXRα expression attenuates NgBR deficiency-induced lipogenesis in vivo. NgBR and LXRα double knockout (dKO) mice were generated by crossbreeding LXRα KO mice with NgBR LivKO mice. NgBR-floxed (Ctrl), NgBR LivKO, LXRα KO, and dKO female mice at 8 weeks old were fed a Western diet for 4 weeks. Liver and plasma samples were individually collected and used for the following assays. (C) Expression of FASN, (p/n)SREBP-1c, SCD-1, LXRα, and NgBR protein was determined by western blotting; (D) expression of FASN, SCD-1, and SREBP-1c mRNA was determined by real-time RT-PCR and normalized to GAPDH; *: P<0.05 vs. control mice; #: P<0.05 vs. NgBR LivKO mice; (E, F) FFA and TG levels in the liver (E) and plasma (F) were determined with assay kits; *: P<0.05 vs. control mice; #: P<0.05 vs. NgBR LivKO mice (n=6).
NgBR deficiency impairs AMPKα pathway
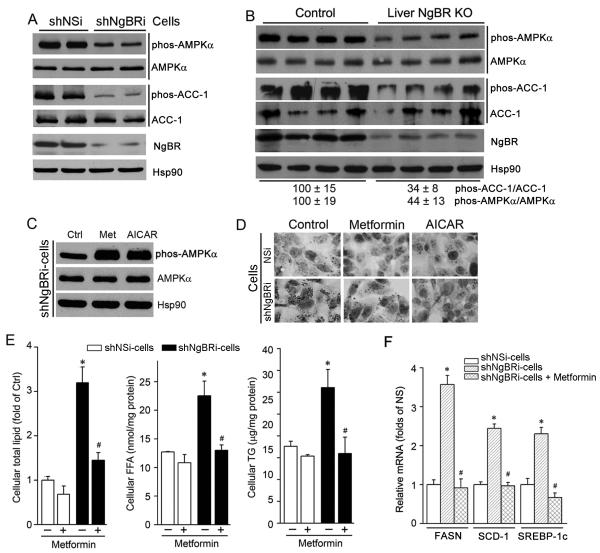
AMPKα is a critical kinase regulating glucose and lipid metabolism (21-23). Figure 7A shows that NgBR deficiency did not affect AMPKα expression, but reduced the phosphorylation of AMPKα (phos-AMPKα) (upper panel), impairing its activation. NgBR deficiency also decreased the phosphorylation of acetyl-CoA carboxylase-1 (phos-ACC-1), a target gene of activated AMPKα (22, 23). Consistently, decreased phos-AMPKα and phos-ACC-1 were also observed in the livers of NgBR LivKO mice (Fig. 7B) and NgBR iKO mice (Fig. S12), although expression of AMPKα and ACC-1 was not affected. These in vitro and in vivo results reveal that NgBR deficiency impairs the AMPKα pathway.
Figure 7. NgBR deficiency impairs the AMPKα pathway.
(A, B) NgBR deficiency inhibits phosphorylation of hepatic AMPKα and ACC-1 in vitro and in vivo. Total cellular protein was extracted from shNSi and shNgBRi cells (A), or from the livers of littermate control and NgBR LivKO female mice that were fed a Western diet for 4 weeks from 8-week old (B). Expression of total AMPKα, phos-AMPKα, total ACC-1 and phos-ACC-1 was determined by western blotting. (C) shNgBRi cells were treated with metformin (Met, 10 μM) or AICAR (1 mM) overnight followed by determination of phos-AMPKα and AMPKα by western blotting. (D-F) AMPKα activation prevents NgBR deficiency-induced lipid accumulation in HepG2 cells. Both shNSi and shNgBRi cells were treated with metformin (10 μM) or AICAR (1 mM) overnight. Cells were used to determine lipid content by Oil Red O staining (D) and lipid quantitative analysis (E), and expression of FASN, SCD-1, and SREBP-1c mRNA were determined by real-time RT-PCR (F). *: P<0.05 vs. shNSi cells; #: P<0.05 vs. shNgBRi cells without treatment (n=3).
Knockdown of AMPKα expression in HepG2 cells with validated siRNAs also resulted in decreased phos-AMPKα and phos-ACC-1 (Fig. S13A, left panel) while increasing LXRα nuclear translocation (Fig. S13A, right panel) as well as increasing cellular total lipid, FFA and TG levels (Fig. S13B-C). Knockdown of AMPKα, but not its target ACC-1, induced expression of lipogenic genes, such as FASN, SREBP-1c, and SCD-1 (Fig. S14B) and increased cellular FFA and TG levels (Fig. S14C). In addition, inhibition of ACC-1 expression had no effect either on expression of lipogenic genes or on cellular lipid levels in siNgBR cells (Fig. S14B-C, siACC-1+siNgBR vs. siNgBR).
In contrast, treatment of shNgBRi cells with the AMPKα activators metformin and AICAR increased phos-AMPKα (Fig. 7C). Consequently, accumulation of total lipid, FFA, and TG, as well as induction of FASN, SCD-1, and SREBP-1c gene expression in shNgBRi cells were reduced to similar levels as in shNSi cells (Fig. 7D-F). Therefore, both in vitro and in vivo studies suggest that activation of lipogenesis by NgBR deficiency is also through inactivation of AMPKα pathway.
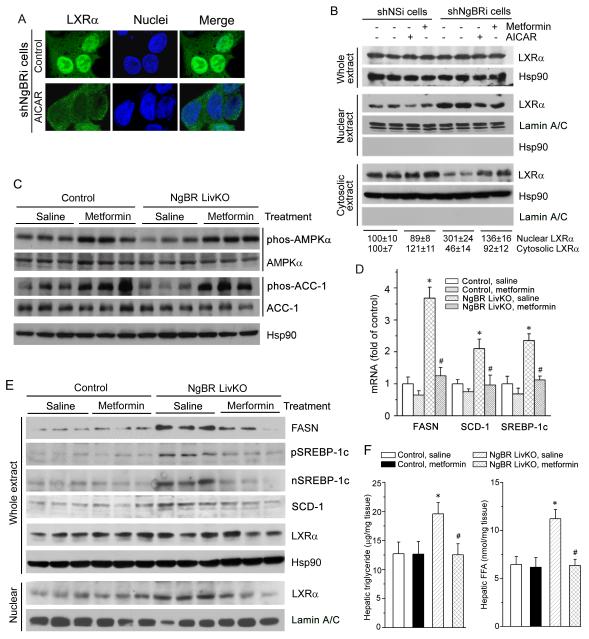
Given the concurrence of inactivation of AMPKα and activation of LXRα pathways in NgBR-deficient hepatic cells in vitro and in vivo, we further wished to determine the connection between AMPKα and LXRα in the context of NgBR deficiency. We initially treated shNgBRi cells with AICAR, a specific AMPKα activator, and examined the nuclear translocation of LXRα by immunofluorescent staining and western blotting. As shown in Figure 8A, the majority of LXRα was present in the nuclei of shNgBRi cells. However, treatment with AICAR resulted in the re-localization of LXRα from the nucleus to cytosolic compartments. Consistently, the western blots showed that although activation of AMPKα by metformin or AICAR had little effect on LXRα expression in both shNSi and shNgBRi cells (Fig. 8B, upper panel) and only slightly affected LXRα cellular distribution in shNSi cells (Fig. 8B, left half of middle and bottom panel), either metformin or AICAR treatment substantially decreased LXRα levels in the nuclear fraction (Fig. 8B, right half of middle panel) and increased LXRα levels in the cytosolic fraction (Fig. 8B, right half of bottom panel) in shNgBRi cells.
Figure 8. Activation of AMPKα blocks NgBR deficiency-induced LXRα activation and hepatic lipid accumulation.
(A, B) Activation of AMPKα blocks NgBR deficiency-induced nuclear translocation of LXRα in vitro. NgBR-deficient cells (shNgBRi) were treated with AICAR (1 mM) overnight followed by determination of LXRα localization by immunofluorescent staining (A). Both shNSi and shNgBRi cells were treated with metformin (10 μM) and AICAR (1 mM) overnight followed by determination of LXRα protein levels in cytosolic, nuclear, and whole cellular extracts by western blotting (B). (C-F) Littermate control and NgBR LivKO female mice at 8 weeks old were i.p. injected daily with saline (control) or metformin solution (250 mg/kg body weight/day) for one week. Total protein, RNA, lipid, and nuclear protein were prepared from mouse liver samples, for the following assays. Expression of phos-AMPKα, AMPKα, phos-ACC-1, and ACC-1 protein in total protein extract (C), expression of FASN, pSREBP-1c, nSREBP-1c, SCD-1, and LXRα protein in total protein extract, and LXRα protein in nuclear extract (E), expression of FASN, SCD-1, and SREBP-1c mRNA in total RNA (D), and TG and FFA levels in lipid extract (F) were determined by western blotting, real time RT-PCR, and assay kits. *: P<0.05 vs. control mice; #: P<0.05 vs. NgBR LivKO mice receiving saline injection (n=3).
In vivo, administration of metformin increased phos-AMPKα and phos-ACC-1 in the livers of both littermate controls and NgBR LivKO mice (Fig. 8C). Consequently, metformin treatment abolished the induction of FASN, SCD-1, and SREBP-1c mRNA (Fig. 8D) and protein expression (Fig. 8E, right half) in the livers of NgBR LivKO mice, but did not affect expression of these lipogenic genes in control mice (Fig. 8E, left half). Meanwhile, compared to control mice, the increased FFA and TG levels in the livers of NgBR LivKO mice on regular rodent chow were reduced to normal levels as in littermate control mice after metformin treatment (Fig. 8F). These results suggest that AMPKα activation can effectively attenuate activation of LXRα and rescue the hepatic lipogenesis defects caused by NgBR deficiency.
Discussion
In this study, we elucidated previously unrecognized roles of NgBR in regulating hepatic lipogenesis in vitro and in vivo. Our study: (a) revealed that NgBR deficiency did not change NPC2 expression or NPC2-mediated cholesterol trafficking under physiological conditions; (b) demonstrated that NgBR deficiency resulted in increased hepatic FFA and TG levels by activating lipogenesis; (c) demonstrated that NgBR deficiency-induced lipogenesis is mediated by simultaneous inactivation of AMPKα and activation of LXRα pathways, thereby increasing expression of lipogenic genes; and (d) elucidated the connection between AMPKα and LXRα in which activation of AMPKα can prevent LXRα nuclear translocation and attenuate LXRα-dependent lipogenesis. Together, these findings suggest that loss of NgBR expression in human fatty liver is a potential risk factor for inducing hepatic steatosis via enhancing LXRα-mediated lipogenesis.
LXRα is a master gene regulating lipid metabolism in the liver (24, 25), and its translocation to the nucleus is an essential step for its activation (2, 18, 26, 27). When LXRα translocates into the nucleus, it forms an obligate heterodimer with RXR. The current model for LXRα activation postulates that the LXRα-RXR heterodimer binds to LXREs in LXRα target genes which are pre–associated with co-repressors such as silencing mediator for retinoic acid and thyroid hormone receptors as well as nuclear receptor co-repressor (18, 28). Following the binding, the co-repressors are released and co-activators are recruited, thereby resulting in activation of expression of target genes involved in RCT and cholesterol metabolism, as well as FA and TG biosynthesis (18). The results of the dKO mice in our study (Fig. 6) clearly elucidate that NgBR deficiency-induced hepatic lipogenesis is dependent on LXRα because LXRα KO abolishes the induction of lipogenic genes and reduces the increased TG and FFA levels in the livers and plasma of NgBR LivKO mice to normal levels. Mechanistically, our results demonstrate that there are certain amounts of LXRα localized in the cytosol of control cells/tissue (Fig. 4A, 4C); however, NgBR deficiency selectively promotes the nuclear translocation of LXRα (Fig. 4A-4E) and increases LXRα target gene expression (Fig. 3A, 3C, 3D, S7A). Our results suggest that the localization of LXRα is regulated by AMPKα signaling and that nuclear translocation of LXRα enhances LXRα-dependent hepatic lipogenesis.
NgBR was initially identified as the receptor for Nogo-B (11). High affinity of Nogo-B binding to NgBR is sufficient for Nogo-B-mediated chemotaxis and tube formation of endothelial cells (11). Recent reports also demonstrated that NgBR is essential for developmental angiogenesis in zebrafish (12) and is involved in the epithelial-mesenchymal transition of breast tumor cells (29). However, the primary physiological functions of NgBR are still unclear. Analysis of the NgBR sequence reveals its C-terminal domain has high homology with cis-isoprenyltransferase (cis-IPTase) (11). Using the C-terminal domain of NgBR as bait, several interacting proteins were identified by the yeast 2-hybrid approach, one of which was NPC2 (13). NPC2 is a soluble glycoprotein that binds cholesterol and transfers cholesterol between membranes. A previous publication demonstrated that RNAi-mediated disruption of NgBR leads to decreased stability of NPC2 due to lack of glycosylation and results in increased intracellular cholesterol accumulation under serum-free conditions (13). Further studies in Dr. Sessa’s laboratory revealed that NgBR is a critical subunit of cis-IPTase, which is involved in the synthesis of dolichol that is an intermediate product required for protein glycosylation (14, 15). However, as shown in Figure S6, NPC2 protein levels do not decrease either in the livers of NgBR LivKO mice or in NgBR-deficient HepG2 cells cultured in serum, presumably because dolichol and glycosylation defects in NgBR-deficient cells can be compensated by exogenous dolichol present in serum. Interestingly, lipid accumulation still increases in NgBR-deficient mouse livers and HepG2 cells via a NPC2-independnet pathway. Our results demonstrate that, under physiological conditions, NgBR deficiency increases the biosynthesis of FFA and TG by the AMPKα-LXRα pathway, which is different from cholesterol accumulation caused by decreased NPC2 protein levels as reported previously (13). In Figure S15, we demonstrated that Western diet increases total XBP-1 and sXBP-1, which is a transcription factor that regulates proper functioning of cellular stress response (30). The result of sXBP-1 overexpression decreasing the expression of NgBR in HepG2 cells (Fig. S15B) suggests the ER stress induced by Western diet is the key causing the decreased expression of NgBR. The precise molecular mechanisms by which sXBP-1 regulates NgBR transcription needs further investigation.
Synthetic LXR agonists, such as GW3965 and T0901317, have been used for improving RCT and preventing atherosclerosis (2). However, hepatic lipogenesis induced by LXR activation limits the clinical application of LXR agonists for atherosclerosis treatment (6, 7, 31). Our results show that NgBR-deficient mice have a similar lipogenic phenotype as shown in the livers of mice receiving LXR agonist treatment, increasing FFA and TG due to increased expression of FASN and SCD-1. As shown in Figure 6, depletion of LXRα expression prevents FFA and TG accumulation while decreasing FASN, SREBP-1c, and SCD-1 expression in the context of NgBR deficiency both in vitro and in vivo. Interestingly, overexpression of NgBR substantially ameliorates the LXRα activation-induced lipid accumulation in HepG2 cells (Fig. S16). Meanwhile, activation of AMPKα can efficaciously block NgBR-mediated LXRα nuclear translocation and lipogenesis both in vitro and in vivo (Fig. 8). Our results clearly suggest that NgBR deficiency-induced hepatic lipogenesis is mediated by LXRα activation, and that NgBR plays an important role in controlling LXRα activity. Although NgBR deficiency increases ABCA1 and ABCG1/5/8 (Fig. S11A), NgBR deficiency only slightly reduced intracellular cholesterol (Fig. S3A, S6D). This is consistent with a previous publication (20) showing that LXR activation with T0901317 only reduces hepatic cholesterol by about 15%, although hepatic ABCA1 transcript levels increase 2-fold. A previous report demonstrated that plasma cholesterol is regulated by induced ABCA1 in the intestine (8), suggesting that LXRα activation in hepatic cells has limited contribution to RCT. The tissue-specific contribution of NgBR-mediated LXRα activation requires further investigation.
A previous study showed that AMPK attenuated hepatic steatosis by phosphorylating and inhibiting SREBP activity (32). Our results (Fig. 8E) further elucidate that metformin does not change total or nuclear SREBP-1c levels in the liver of control mice, but AMPKα activation reduces expression of SREBP-1c in the liver of NgBR LivKO mice by a LXRα-dependent manner because LXRα knockout abolishes the induction of SREBP-1c expression/maturation in the livers of NgBR LivKO mice (Fig. 6C). These findings suggest that AMPKα might be an upstream regulator for controlling LXRα-dependent lipogenesis. As shown in Figure S13, knockdown of AMPKα in HepG2 cells increases lipogenesis and the nuclear translocation of LXRα. Conversely, activation of AMPKα prevents the nuclear translocation of LXRα and subsequent hepatic lipogenesis in shNgBRi cells (Fig. 7C-7E, 8A) and the livers of NgBR LivKO mice (Fig. 8E, 8F). In association with activation of AMPKα, expression of lipogenic genes in shNgBRi cells and the livers of NgBR LivKO mice was restored to normal levels (Fig. 7F, 8E). These results imply that AMPKα agonists may ameliorate the undesirable effects of LXRα agonists for atherosclerosis treatment. Although AMPK-regulated ACC-1 phosphorylation is also involved in lipogenesis and FFA oxidation (33), we determined that ACC-1 knockdown has little effect on lipogenesis in shNgBRi cells (Fig. S14). Our results support a mechanism by which NgBR bridges the AMPKα signaling pathway with LXRα nuclear translocation as well as LXRα-mediated lipogenesis.
Although simple hepatic steatosis is not a life-threatening disease, it causes liver malfunction and insulin resistance. Day et al. (34) initially proposed a “two-hit” model to explain the progression from simple TG accumulation in hepatocytes (hepatic steatosis, first hit) to hepatic steatosis with inflammation and fibrosis (steatohepatitis, second hit), which results in the end stages of liver diseases, such as cirrhosis and hepatocellular carcinoma. Hepatic steatosis arises from an imbalance between TG acquisition and removal. TGs are assembled by coupling three FAs to a glycerol backbone via esterification. The FAs used for hepatic TG formation are derived from 3 sources: 1) diet, 2) de novo synthesis, and 3) adipose tissue secretion (35, 36). FA is a central lipid component resulting in TG accumulation. Our results demonstrate that NgBR deficiency promotes de novo lipogenesis in the liver, and decreased NgBR expression is associated with the development of fatty liver in humans (Fig. 1A) and in mice (Fig. S2). Therefore, our study is the first to elucidate the physiological role of NgBR in regulating hepatic lipid homeostasis. Our study suggests that the NgBR LivKO mouse might be a suitable model for non-alcoholic fatty liver disease, since it recapitulates the features of hepatic steatosis, and that loss of NgBR expression in the liver is a potential risk factor for inducing hepatic steatosis by promoting LXRα nuclear translocation and increasing LXRα-dependent lipogenesis.
Supplementary Material
Acknowledgement
This work was supported in part by start-up funds from the Divisions of Pediatric Surgery and Pediatric Pathology, Medical College of Wisconsin (MCW); the Advancing a Healthier Wisconsin endowment to MCW; AHA SDG 0730079N and NIH R01HL108938 to QRM; AHA postdoctoral fellowship 13POST13940002 to BZ; 2013 CTSI Pilot Awards (UL1TR000055, Advancing a Healthier Wisconsin) and NICHD RO3HD073274 to RJT; and National Science Foundation of China grants 81272460 and 81473204 to JH. Mass spectrometric analyses were performed in the mass spectrometry facility of the Department of Pharmacology and Toxicology, MCW. We thank Meghann Sytsma and Melissa Stauffer for editing the manuscript.
Footnotes
Author Contributions: W.H., W.Z., Y.C., U.R., R.T., Y.D., Z.L., and B.Z. conducted the experiments; M.J.T. and R.E.K. were responsible for the cholesterol measurements; Q.R.M., J.H., and W.H. designed the experiments and wrote the paper; J.F. and H.W. generated NgBR floxed mice; K.Z. and M.J.T. provided reagents and edited the paper; Q.R.M. and J.H. were responsible for overall integration and execution of the scientific approaches.
References
- 1.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, Koteish A, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calkin AC, Tontonoz P. Liver x receptor signaling pathways and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1513–1518. doi: 10.1161/ATVBAHA.109.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ducheix S, Montagner A, Theodorou V, Ferrier L, Guillou H. The liver X receptor: a master regulator of the gut-liver axis and a target for non alcoholic fatty liver disease. Biochem Pharmacol. 2013;86:96–105. doi: 10.1016/j.bcp.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 5.Grefhorst A, Elzinga BM, Voshol PJ, Plosch T, Kok T, Bloks VW, van der Sluijs FH, et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 6.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–7609. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo Sasso G, Murzilli S, Salvatore L, D'Errico I, Petruzzelli M, Conca P, Jiang ZY, et al. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 10.Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, Tempst P, et al. A new role for Nogo as a regulator of vascular remodeling. Nature Medicine. 2004;10:382–388. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 11.Miao RQ, Gao Y, Harrison KD, Prendergast J, Acevedo LM, Yu J, Hu F, et al. Identification of a receptor necessary for Nogo-B stimulated chemotaxis and morphogenesis of endothelial cells. Proc Natl Acad Sci U S A. 2006;103:10997–11002. doi: 10.1073/pnas.0602427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao BF, Chun CZ, Liu Z, Horswill MA, Pramanik K, Wilkinson GA, Ramchandran R, et al. Nogo-B receptor is essential for angiogenesis in zebrafish via Akt pathway. Blood. 2010;116:5423–5433. doi: 10.1182/blood-2010-02-271577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison KD, Miao RQ, Fernandez-Hernando C, Suarez Y, Davalos A, Sessa WC. Nogo-B receptor stabilizes Niemann-Pick type C2 protein and regulates intracellular cholesterol trafficking. Cell Metab. 2009;10:208–218. doi: 10.1016/j.cmet.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison KD, Park EJ, Gao NG, Kuo A, Rush JS, Waechter CJ, Lehrman MA, et al. Nogo-B receptor is necessary for cellular dolichol biosynthesis and protein N-glycosylation. Embo Journal. 2011;30:2490–2500. doi: 10.1038/emboj.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park EJ, Grabinska KA, Guan Z, Stranecky V, Hartmannova H, Hodanova K, Baresova V, et al. Mutation of Nogo-B receptor, a subunit of cis-prenyltransferase, causes a congenital disorder of glycosylation. Cell Metab. 2014;20:448–457. doi: 10.1016/j.cmet.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahs SA, Hille MT, Shi QZ, Weiler H, Montgomery RR. A conditional knockout mouse model reveals endothelial cells as the principal and possibly exclusive source of plasma factor VIII. Blood. 2014;123:3706–3713. doi: 10.1182/blood-2014-02-555151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rana U, Liu Z, Kumar SN, Zhao B, Hu W, Bordas M, Cossette S, et al. Nogo-B receptor deficiency causes cerebral vasculature defects during embryonic development in mice. Dev Biol. 2016;410:190–201. doi: 10.1016/j.ydbio.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jedidi I, Couturier M, Therond P, Gardes-Albert M, Legrand A, Barouki R, Bonnefont-Rousselot D, et al. Cholesteryl ester hydroperoxides increase macrophage CD36 gene expression via PPARalpha. Biochem Biophys Res Commun. 2006;351:733–738. doi: 10.1016/j.bbrc.2006.10.122. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Breevoort SR, Angdisen J, Fu MG, Schmidt DR, Holmstrom SR, Kliewer SA, et al. Liver LXR alpha expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. Journal of Clinical Investigation. 2012;122:1688–1699. doi: 10.1172/JCI59817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7. doi: 10.1016/j.ceb.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology (Bethesda) 2014;29:99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higuchi N, Kato M, Shundo Y, Tajiri H, Tanaka M, Yamashita N, Kohjima M, et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. 2008;38:1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- 25.Korach-Andre M, Parini P, Larsson L, Arner A, Steffensen KR, Gustafsson JA. Separate and overlapping metabolic functions of LXRalpha and LXRbeta in C57Bl/6 female mice. Am J Physiol Endocrinol Metab. 2010;298:E167–178. doi: 10.1152/ajpendo.00184.2009. [DOI] [PubMed] [Google Scholar]
- 26.Cha JY, Repa JJ. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem. 2007;282:743–751. doi: 10.1074/jbc.M605023200. [DOI] [PubMed] [Google Scholar]
- 27.Faulds MH, Zhao C, Dahlman-Wright K. Molecular biology and functional genomics of liver X receptors (LXR) in relationship to metabolic diseases. Curr Opin Pharmacol. 2010;10:692–697. doi: 10.1016/j.coph.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 29.Zhao B, Xu B, Hu W, Song C, Wang F, Liu Z, Ye M, et al. Comprehensive proteome quantification reveals NgBR as a new regulator for epithelial-mesenchymal transition of breast tumor cells. J Proteomics. 2015;112:38–52. doi: 10.1016/j.jprot.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Schadewijk A, van't Wout EF, Stolk J, Hiemstra PS. A quantitative method for detection of spliced X-box binding protein-1 (XBP1) mRNA as a measure of endoplasmic reticulum (ER) stress. Cell Stress Chaperones. 2012;17:275–279. doi: 10.1007/s12192-011-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodwin B, Watson MA, Kim H, Miao J, Kemper JK, Kliewer SA. Differential regulation of rat and human CYP7A1 by the nuclear oxysterol receptor liver X receptor-alpha. Mol Endocrinol. 2003;17:386–394. doi: 10.1210/me.2002-0246. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen ZP, O'Neill HM, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 35.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon HJ, Cha BS. Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World J Hepatol. 2014;6:800–811. doi: 10.4254/wjh.v6.i11.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.