Abstract
Background & Aims
Brain-gut axis signaling modifies gastrointestinal symptomatology. Altered neural processing of intestinal pain signals involves interoceptive brain regions in adults with functional and inflammatory gastrointestinal disorders. Although these disorders frequently present in childhood, there are no published studies in youth. We determined if neural processing of somatic pain stimuli differs in adolescents and young adults (AYA) with irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD), as compared to healthy controls (HC).
Methods
IBS and IBD AYA (16–20 y) underwent anticipated and thermal pain stimuli of low and high intensity on their forearm and simultaneous blood oxygen level-dependent functional magnetic resonance imaging. Data from adult HC were used for comparison. Subjects answered surveys evaluating alexithymia, anxiety, depression, and pain catastrophizing. Group data were compared using Linear Mixed Effects and ANOVA.
Results
Study groups were similar by sex but not age. Significant group by pain condition interactions were observed in interoceptive brain regions during pain anticipation, and within perceptual brain regions during perceived pain. Higher activation within interoceptive brain regions during anticipated pain was observed in IBS compared to IBD and HC subjects. IBD patients demonstrated increased activation in perceptual brain regions during experienced pain as compared to IBS and HC.
Conclusions
IBS and IBD AYA demonstrate altered neural processing of somatic pain compared with each other and with HC. Our results suggest that neuromodulatory interventions targeting interoceptive brain circuits in IBS and perceptual brain regions in IBD may be effective.
Keywords: functional magnetic resonance imaging, irritable bowel syndrome, inflammatory bowel disease, interoception
INTRODUCTION
Perception of gastrointestinal (GI) pain varies individually and is modified by signaling between the GI tract and the central nervous system, otherwise known as the brain-gut axis. Studies to date have evaluated pain perception and thresholds in adults with GI disorders and have found that pain perception in these conditions can be functionally altered. Two particular disease entities of interest are inflammatory bowel disease (IBD), typically characterized by periods of GI inflammation accompanied by abdominal pain and diarrhea frequently with blood, and irritable bowel syndrome (IBS), also characterized by abdominal pain and change in bowel movement frequency but in the absence of demonstrated GI inflammation. Chang et al. demonstrated differences in perceptual responses to rectosigmoid balloon distension in adults with IBS and IBD, as compared with healthy controls1. Specifically, adults with IBS demonstrated a lower balloon inflation threshold for perceived discomfort while adults with IBD demonstrated a higher threshold for perceived discomfort as compared across the three groups. Zhou et al. also demonstrated differences in psychophysical responses to experimental somatic pain stimuli in IBS subjects2.
Functional neuroimaging research suggests that IBS pathophysiology may relate to a core impairment in interoception, the neural mechanism by which humans sense the physiologic well-being state of the body and through which homeostasis and adaptive emotion processing is achieved3. Larsson et al. demonstrated a correlation between GI tract perceptual responses (to both true and anticipated stimuli) and greater blood oxygen level dependent (BOLD) signals at brain regions associated with interoception (the cingulate gyrus, insula and thalamus)4. Similarly, functional neuroimaging research in adults has demonstrated the importance of the insula in modulating the intrinsic functional connectivity of major networks in the resting brain that is related to IBS symptomatology, with greater connectivity predicting greater intensity of symptoms, particularly chronic visceral pain5. Visceral placebo analgesia (verbal suggestion of analgesia accompanied by placebo infusion intravenously) has been shown to modulate pain perception in IBS adults (as compared to healthy controls6 and IBD7 adults) via enhanced brain activity at interoceptive brain regions6. Anticipation of visceral pain has also been shown to result in brain activation patterns involving interoceptive brain areas in IBS adult females8. Similar studies have yet to be performed in youth with IBS despite the fact that IBS symptoms are quite prevalent in adolescents (17% of high school students and 8% of middle school students in a community-based sample)9.
Functional neuroimaging research has also been performed in adult IBD patients. In general, the IBS population has shown more distinct differences in fMRI findings by brain regions (see above) as compared to the IBD population and to healthy controls. However, some differences have nevertheless been noted in IBD patients as compared with healthy controls. Specifically, attenuation of activation in somatosensory areas during pain and stool sensations with visceral balloon inflation has been seen in the IBD versus healthy control populations10. Similar to the IBS population, fMRI studies have yet to be performed in youth with IBD despite the fact that IBD commonly presents during the adolescent years11.
Persons with IBS12 and IBD13 frequently have symptoms affecting non-visceral body systems as well, which suggests that the abnormal sensitivity in these populations extends beyond the gastrointestinal system. However, neural processing of extra-visceral or somatic pain stimuli has not been evaluated using fMRI in adult or pediatric patients with functional (IBS) or organic (IBD) gastrointestinal disease. Nevertheless, fMRI neural processing has been evaluated in healthy controls and in other clinical populations demonstrating pain dysregulation. For example, anorexia nervosa subjects demonstrate an interoceptive brain activation mismatch between anticipation of and objective responses to somatic pain stimuli suggesting altered integration or perception of body signals14. The biobehavioral view of pain suggests that pain is an experience with both physiological and psychological factors, and that understanding both these factors is required to effectively understand pain14. Consistent with this view, the cognitive behavioral theory posits that the degree to which sensory aspects are distressing and/or disabling depends on the way it is interpreted by the patient (e.g., pain-related cognitions) and affects the way they cope with or react to it (e.g., pain-induced behaviors)15. Anticipation shapes pain experiences, which are altered in individuals with anxiety16, depression17, and chronic pain conditions18.
In order to evaluate whether pain processing in youth with GI disorders involves interoceptive pathways in the brain, we evaluated neural responses to somatic pain and its anticipation in adolescents and young adults (AYA) with pain from IBS and IBD in comparison to healthy controls (HC). Since symptomatology in AYA with gastrointestinal disease is frequently not limited to the gastrointestinal tract, we evaluated a somatic pain stimulus. We hypothesized that pain processing of a somatic stimulus in AYA with IBS, as compared to HC and AYA with IBD, would result in greater brain activity in interoception-related brain regions, We also hypothesized that pain processing of a somatic stimulus in AYA with IBD, as compared to HC and AYA with IBS, would demonstrate attenuation in pain detection-related brain regions.
METHODS
We performed an evaluation of the neurobiology of pain anticipation and pain processing among adolescents and young adults with IBS and IBD, and HC. All study subjects completed surveys and a functional magnetic resonance imaging (fMRI) scan and protocol. This study received local IRB approval and informed consent and/or assent was obtained from all subjects.
Subjects
Inclusion criteria for participation included: being right-handed, being an adolescent or young adult (AYA) 16–20 years old with IBS or IBD and having abdominal pain during disease exacerbations. IBS participants met Rome III criteria for IBS19 as confirmed by a pediatric gastroenterologist, while IBD participants had been diagnosed by a pediatric gastroenterologist. Subjects with diagnosed psychiatric disorder and/or psychotropic medication exposure within the past 6 months, irremovable ferromagnetic implant, inability to follow protocol, and/or requirement for sedation for MRI were excluded from participation. Data from ten healthy right-handed young adult HC (age 18–22 years) with no psychiatric history or past or current gastrointestinal symptoms previously studied as part of a prior neuroimaging study and using the identical study protocol20 were used for comparison.
Surveys
A battery of surveys and behavior measures assessed baseline emotional and stress states postulated to affect interoception awareness, including alexithymia, anxiety, and depression, in all study subjects. GI symptomatology was measured in IBS and IBD patients; HC had previously reported no GI symptomatology.
Alexithymia
The Toronto Alexithymia Scale-2021 measures difficulty identifying feelings, difficulty describing feelings, and externally orientated thinking and was used to determine patients’ levels of alexithymia which has been associated with pain sensitivity.
Anxiety
The Spielberger State-Trait Anxiety Inventory assessed level/severity of baseline anxiety22. State anxiety refers to anxiety related to temporary stressors, while trait anxiety refers to daily anxiety.
Depression
The Beck Depression Inventory-II, a 21-question multiple-choice self-report inventory that measures depressive symptoms23, was used to screen for and characterize depressive symptoms; higher scores indicate increasingly severe depressive symptoms.
Pain catastrophizing
Temperament specifically related to pain was measured using an adaptation of Sullivan’s Pain Catastrophizing Scale for children24. Pain catastrophizing has previously been related to perceived pain intensity25, and pain expression26.
GI Symptomatology
Disease symptomatology in IBS and IBD is similar. We used the Gastrointestinal Symptoms Rating Scale27 to assess abdominal pain, dyspepsia, indigestion, and bowel dysfunction (symptoms are rated on a Likert scale of 0 (no symptoms) to 3 (most symptomatic/worst)) and characterize GI symptomatology of IBS and IBD youth in the study.
Experimental Pain Paradigm
A validated pain anticipation paradigm was used20. Briefly, the paradigm had two temporal conditions (anticipation, stimulus) with three stimulus conditions for anticipation (cued high pain, low pain, or uninformed pain) and two stimulus conditions for stimulus (high pain or low pain). This paradigm was selected to allow evaluation of not only actual stimuli but also the adaptive emotional responses (i.e. anxiety) related to anticipation of the stimuli in studied youth. Thermal stimuli, experienced as moderately (6 sec; 47.5°C) and mildly (6 sec; 45.5°C) painful to the subject, were delivered in a pseudo-random and counterbalanced order through a 9 cm2 thermode (Medoc TSA-II, Ramat-Yishai, Israel) securely fastened to the subject’s left volar forearm. Prior to scanning, subjects were pre-tested with several non-painful and painful temperature stimuli to ensure that temperatures were well tolerated.
Post-Scanner Anxiety and Pain Ratings
To measure the subjective experience of the task, subjects rated anticipatory anxiety, as well as the intensity and unpleasantness of the perceived pain (0=“no anxiety/pain sensation/unpleasantness” to 10=“extreme anxiety/pain sensation/unpleasantness”) after the scan. Subjects were instructed to provide separate ratings for the low and high pain stimuli.
fMRI Protocol
Two fMRI runs (412 brain volumes/run) sensitive to blood oxygenation level-dependent (BOLD) contrast were collected for each subject using a 3.0 Tesla GE Signa EXCITE scanner (GE Healthcare, Milwaukee) (T2*-weighted echo planar imaging with partial k-space sampling, TR=1500ms, TE=30ms, flip angle=80, FOV=23cm, 64×64 matrix, 30 2.8mm 1.2mm gap axial slices) while they performed the paradigm described above. FMRI acquisitions were time-locked to the onset of the task. During the same experimental session, a high-resolution T1-weighted image (FSPGR, TR=8ms, TE=3ms, TI=450ms, flip angle=12, FOV=25cm, 172 1mm sagittal slices, 256×256 matrix) was obtained for anatomical reference.
Statistical Analysis
Demographic and survey data were analyzed according to group assignment (IBS, IBD, or HC) using standard statistical comparison methods. For continuous measures, ANOVA analyses were used for group comparisons; for non-continuous measures, Wilcoxon rank test was used for group comparisons. For survey data group comparisons involving control subjects, because demographic analyses demonstrated notable differences in groups by age, age was added as a covariate in ANCOVA comparison analyses. Analyses were performed using JMP software version 11 (Cary, NC).
fMRI Statistical Analysis
All imaging data were analyzed with the Analysis of Functional NeuroImages (AFNI) software package28 as in prior studies20. Briefly, preprocessed (corrected for: slice-dependent time shifts, interleaved acquisition, rigid body head motion, and despiked29) time series data for each individual were analyzed using a multiple regression model corrected for autocorrelation consisting of three anticipation-related and two stimulus-related regressors. Anticipation-related regressors consisted of: 1) Anticipation of moderately painful heat stimulation, i.e., high pain anticipation; and 2) Anticipation of mildly painful heat stimulation, i.e., low pain anticipation. Since the uninformed cue did not contribute to our understanding of the specific mechanism of interest, this condition was modeled as regressor of no interest. Stimulus-related regressors consisted of: 1) Application of moderately painful heat, i.e., high pain stimulation; and 2) Application of mildly painful heat, i.e., low pain stimulation. Six additional regressors were included in the model as nuisance regressors: one outlier regressor to account for physiological and scanner noise (i.e., the ratio of brain voxels outside of 2SD of the mean at each acquisition), three movement regressors to account for residual motion (in the roll, pitch, and yaw directions), and regressors for baseline and linear trends to account for signal drifts. To reduce the false positives induced by cross correlations of the time series data were fit using the AFNI program 3dREMLfit. A Gaussian filter with a full width-half maximum of 4mm was applied to the voxel-wise percent signal change data to account for individual variation in the anatomical landmarks. Data from each subject were normalized to Talairach coordinates.
Voxel-wise percent signal change for high and low pain anticipation and high and low pain were entered into a linear mixed effects model with Group (IBS/IBD/Control) and Condition (low pain/high pain) entered as fixed factors, subjects entered as a random factor, and age as a covariate. Analyses were done with the AFNI function 3dLME.R, which uses statistical program R (www.cran.org) and the nlme library. Results are displayed that showed significant Group by Condition interaction effects for pain anticipation and pain experience. A Monte Carlo simulation (iterations =10,000) using AlphaSim was used to determine that for a search volume within the whole brain a cluster size of 768mm3 was required to control for multiple comparisons maintaining an alpha of 0.05. The cluster F-values were calculated by averaging the voxel based F-values in each cluster. The average percent signal change was extracted from regions of activation for post-hoc correlational analysis. The between group t-tests were performed in the regions that showed significant Group by Condition interactions (the resultant t-values are displayed). Spearman’s correlation analyses were also performed between extracted percent signal change for Group by Condition interactions during anticipation and stimulation and the Spielberger State-Trait Anxiety Inventory within each patient group. Analyses were performed using JMP software version 11 (Cary, NC).
RESULTS
Demographic and Clinical Data
Twenty right-handed AYA 16–20 years old with IBS (n=10) and IBD (n=10) and abdominal pain during disease exacerbations were recruited for participation in the study protocol from a tertiary-care, academic, pediatric gastroenterology center. Ten right-handed adults who were otherwise healthy without gastrointestinal symptoms underwent the same study protocols. Patient and HC groups did not differ by race (80% v. 50% v. 30% White, IBS v. IBD v. HC, p=0.11), ethnicity (40% v. 20% v. 20% Hispanic, p=0.51), or sex (80% v. 50% v. 60% female, p=0.37). Age differed among study groups, with IBS (median (IQR): 17 (16, 17) years) and IBD (18 (17, 19) years) groups being on average 3 and 2 years younger than HC (20 (20, 21) years), respectively (p<0.0001).
Anxiety, Depression, Pain, and Gastrointestinal symptom Data
Survey scores are reported in Table 1. Both IBS and IBD patients demonstrated greater alexithymia symptoms compared to HC (F=11.55, p<0.001). In addition, both IBS and IBD patients reported greater state (F=5.18, p=0.01) and trait anxiety (F=8.79, p=0.001) as compared to HC. Furthermore, measures of depression were higher in IBS patients compared to HC F=5.47, p=0.01) but not between IBD and HC. In contrast, neither pain catastrophizing nor pain ratings during the fMRI protocol differed across groups (F=0.06–1.84; all p>0.05). Anxiety ratings were greater in IBS patients than IBD or HC at rest (F=3.48, p<0.05) but not during other times during the fMRI protocol. IBS patients reported greater gastrointestinal symptomatology as compared to IBD patients at the time of study (p=0.03). HC did not report any gastrointestinal symptoms.
TABLE 1.
Survey responses by Group (Mean (SD))
| Survey | IBS | IBD | Healthy Control | p-value* |
|---|---|---|---|---|
| Alexithymia | 52 (10) | 48 (11) | 31 (7) | 0.0001 |
| Anxiety (State) | 39 (11) | 35 (12) | 24 (4) | 0.007 |
| Anxiety (Trait) | 38 (10) | 40 (11) | 27 (5) | 0.006 |
| Depression | 11 (10) | 10 (9) | 1 (2) | 0.01 |
| Pain catastrophizing | 26 (12) | 24 (11) | 9 (6) | 0.001 |
| Gastrointestinal symptoms | 0.5 (0.3) | 0.2 (0.2) | N/A | 0.03 |
| Pain Scores during Low Heat | 2 (2) | 2.5 (2.5) | 1.5 (1.5) | 0.90 |
| Pain Scores during High Heat | 6 (2) | 6.5 (3) | 6 (2) | 0.92 |
| Anxiety Scores at Rest | 4.5 (3.7) | 1.3 (2.2) | 0.1 (0.3) | .046 |
| Anxiety Scores prior to Low Heat | 2 (2) | 2.5 (2.5) | 1.5 (1.5) | 0.85 |
| Anxiety Scores prior to High Heat | 6 (3) | 6.5 (2.5) | 6.5 (2) | 0.81 |
Results expressed as mean (standard deviation).
p-value of ANCOVA analyses comparing responses by group, controlling for age.
fMRI Data
Heat Pain Anticipation
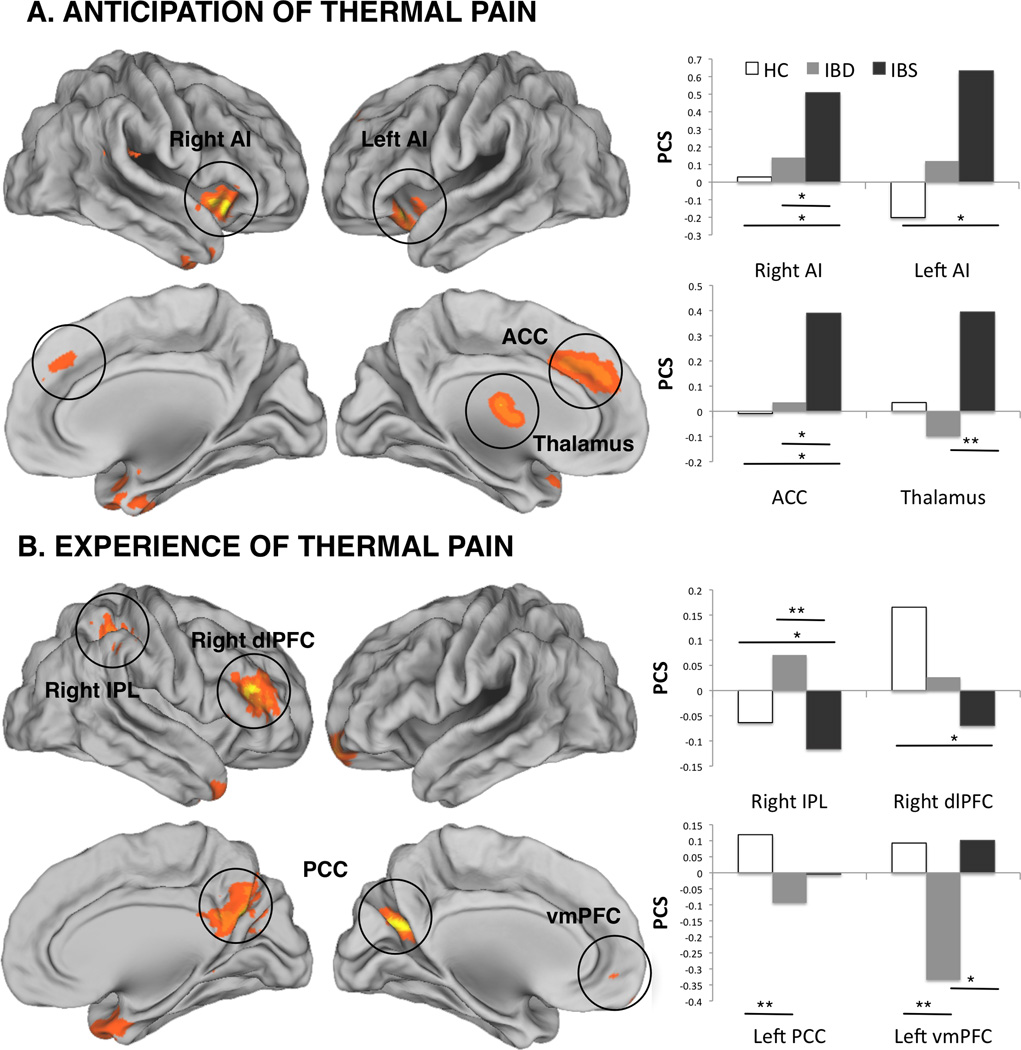
Figure 1A and Table 3 show significant whole-brain group (IBS/IBD/HC) by condition (high/low pain cue) interaction effects within left anterior cingulate cortex (ACC), left thalamus, left anterior insula (AI), right uncus, and right AI. Further examination of these differences with the between-group t-tests (Table 2) indicated that patients with IBS demonstrated significantly increased activation within the ACC and bilateral AI as compared to the HC group and increased activation within the ACC, thalamus and right AI but lower activation at the right uncus when compared to the IBD group (T’s>2.04, p’s<0.05). No differences were noted during the anticipation of pain between the IBD and HC groups (p>0.05, all comparisons).
FIGURE 1.
Whole brain group (HC, IBD, IBS) by condition (low, high) interaction during anticipation (A) and experience (B) of thermal pain. Bar graphs indicate percent signal changes (PSC) in the areas that showed significant interaction effects. AI – anterior insula; ACC – anterior cingulate cortex; IPL – inferior parietal lobule; dlPFC – dorsolateral prefrontal cortex; PCC – posterior cingulate cortex; vmPFC – ventromedial prefrontal cortex. * p<0.05; ** p<0.01. See Text and Tables 2, 3 for details.
TABLE 3.
Group differences in fMRI signaling in the Group (IBS/IBD/HC) by condition (high/low pain cue) interaction [anticipation] and group (IBS/IBD/HC) by condition (high/low pain stimulus) interaction [pain experience]
| Brain Region | HC | IBD | IBS | IBS > IBD | IBS> HC | IBD > HC |
|---|---|---|---|---|---|---|
| Pain Anticipation | ||||||
|
Anterior Cingulate Cortex (ACC) |
−0.01 | 0.04 | 0.39 | 2.3* | 2.6* | NS |
| Thalamus | 0.03 | −0.1 | 0.39 | 3.0** | NS | NS |
| L. Anterior Insula (LAI) | −0.2 | 0.12 | 0.63 | NS | 2.6* | NS |
| R. Anterior Insula (RAI) | −0.01 | 0.21 | −0.33 | 2.5* | 2.7* | NS |
| R. Uncus | 0.03 | 0.14 | 0.51 | −2.7* | NS | NS |
| Pain Experience | ||||||
|
R. Superior Temporal Gyrus (STG) |
0.13 | −0.08 | −0.25 | NS | −2.7* | NS |
| L. Cerebellum | 0.06 | 0.07 | −0.14 | −2.5* | −2.3* | NS |
| L. Lentiform Nucleus (LN) | 0.11 | 0.01 | −0.12 | NS | −2.8** | NS |
|
R. Inferior Parietal Lobule (IPL) |
−0.06 | 0.07 | −0.12 | −3.2** | NS | 2.2* |
| R. Posterior Cingulate (PCC) | 0.11 | −0.1 | −0.11 | NS | −2.6* | −2.2* |
|
R. dorsolateral Prefrontal(RdlPFC) |
0.17 | 0.03 | −0.07 | NS | −2.7* | NS |
| Cerebellum | −0.03 | 0.12 | −0.12 | −2.7* | NS | NS |
| L. Posterior Cingulate (PCC) | 0.12 | −0.09 | −0.01 | NS | NS | −3.1** |
|
L. ventromedial prefrontal (vmPFC) |
0.09 | −0.33 | 0.1 | 2.4* | NS | −2.8** |
IBS = functional gastrointestinal disorder; IBD = inflammatory bowel disease; HC = healthy control;
< 0.01;
< 0.05
TABLE 2.
Group (IBS/IBD/HC) by condition (high/low pain cue) interaction [anticipation] and group (IBS/IBD/HC) by condition (high/low pain stimulus) interaction [pain experience] within the whole brain analysis.
| Brain Region | Volume (mm3) |
Talairach Coordinates | F statistic | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Pain Anticipation | |||||
| L. Anterior Cingulate Cortex (ACC) | 4288 | −7 | 31 | 36 | 5.0 |
| L. Thalamus | 1408 | −4 | −13 | 12 | 4.6 |
| L. Anterior Insula (LAI) | 1152 | −35 | 19 | −12 | 5.2 |
| R. Anterior Insula (RAI) | 896 | 33 | 18 | −5 | 5.4 |
| R. Uncus | 1024 | 35 | 1 | −29 | 5.3 |
| Pain Experience | |||||
| R. Superior Temporal Gyrus (STG) | 1984 | 32 | 10 | −32 | 4.6 |
| L.Cerebellum | 1984 | −13 | −59 | −25 | 4.8 |
| L. Lentiform Nucleus (LN) | 1792 | −29 | −9 | 2 | 4.9 |
| R. Inferior Parietal Lobule (IPL) | 1408 | 39 | −40 | 45 | 5.2 |
| R. Posterior Cingulate Cortex (RPCC) | 1344 | 8 | −61 | 26 | 4.7 |
| R. dorsolateral Prefrontal Cortex (RdlPFC) | 1216 | 38 | 31 | 19 | 4.9 |
| Cerebellum | 1152 | 1 | −42 | −14 | 4.5 |
| L.Posterior Cingulate Cortex (PCC) | 1024 | −23 | −63 | 20 | 5.2 |
| L. ventromedial prefrontal cortex (vmPFC) | 768 | −18 | 51 | −4 | 4.7 |
Heat Pain Experience
Figure 1B and Table 3 shows significant whole-brain group (IBS/IBD/HC) by condition (high/low pain stimulus) interaction effects within right superior temporal gyrus (STG), bilateral cerebellum, left lentiform nucleus (LN), right inferior parietal lobule (IPL), bilateral posterior cingulate cortex (PCC), right dorsolateral prefrontal cortex (dlPFC), and left medial frontal gyrus (MFG). Further examination of these differences with the between-group t-tests (Table 2) indicated that the patients with IBS demonstrated lower activation within bilateral cerebella and right IPL, and higher activation at the left MFG when compared to IBD patients; and significantly lower activation within right STG, left cerebellum, left LN, right PCC, and right dlPFC when compared to HC. Furthermore, when compared to the HC group, IBD participants demonstrated higher activation within the right IPL and lower activation within bilateral PCC and left MFG.
fMRI Data and Anxiety Data
Since anxiety plays an important role in both functional and organic GI disorders, we examined whether trait anxiety (daily anxiety) measures related to brain activation in our sample. We found that in IBS, trait anxiety correlated positively with brain activation in the ACC during pain anticipation (ρ =0.71, p=0.02) and with brain activation within ventromedial prefrontal during pain stimuli (ρ =0.63, p<0.05). In other words, those IBS subjects with highest trait anxiety showed highest ACC activation during anticipation of pain and highest ventromedial prefrontal activation during pain experience. In contrast, trait anxiety scores did not correlate with brain activity in IBD patients. The differences in correlation coefficients between IBS and IBD groups did not meet significance but showed tendency (ACC: z=1.1, p=0.14; VMPFC: z=1.4, p=0.07).
DISCUSSION
This is the first study to demonstrate significant differences in the neural processing of anticipated and actual thermal somatic pain stimuli in AYA with IBS and IBD as compared to each other and with healthy volunteers. In particular, our findings demonstrate a high emotional response to pain anticipation in the anterior cingulate and right anterior insula, brain areas associated with interoception and emotion, among AYA with IBS as compared to IBD AYA and HC. In contrast, AYA with IBD showed anticipatory brain responses similar to that in HC. However, during pain, AYA with IBD showed more intense neural signaling as compared to AYA with IBS and HC within the right inferior parietal lobule, an area involved in attention to environmental stimuli30, and more deactivation within the ventromedial prefrontal cortex, an area within the default mode network, which is active when the brain is at rest or not attending to a given task31. Taken together, our results are consistent with the idea that AYA with IBS show increased emotional reactivity to the upcoming pain stimuli driven by increased anxiety, while those with IBD do not and may rather show increased cognitive control when pain is actually present. Although prior studies have evaluated gastrointestinal pain stimuli in adult patients with IBS and IBD as compared to HC1,7,32, this is the first report to our knowledge to evaluate anticipation and processing of somatic pain stimuli in the setting of IBS and IBD and the first to evaluate pain processing in AYA with IBS and IBD.
Pain is a multidimensional experience that affects overall wellbeing of an individual. Interoception is the integrated neural representation of all aspects of the condition of the body in a system responsible for maintaining homeostasis33. In the case of gut homeostasis, the intestine provides information to the central nervous system (CNS) via the enteric nervous system (ENS). The CNS in response then communicates to the ENS to affect gut homeostasis. Data to date demonstrate bidirectional effects of brain-gut communications. Recent evidence suggests that interoceptive inputs from the gut, including those generated by intestinal microbes, may influence emotional arousal and affective behaviors34. Similarly, researchers have demonstrated that psychological distress can produce clinical symptoms and increase disease activity among patients with IBS and IBD35. Furthermore, activation of the CNS corticotropin-releasing factor system (activated during stress) in the mouse model can alter GI motility and produce clinical symptoms such as diarrhea and also exacerbate existing IBD symptoms and/or induce the flare-up of IBD symptoms36.
Interoception appears to be processed in the brain primarily within the insular cortex and the ACC33. Both the insular cortex and the ACC are implicated in perceiving the unpleasantness of pain, generating an emotional response to pain, and controlling our motivational behaviors37. In the current study, we demonstrate a high component of neural signaling in interoceptive areas of the brain (ACC and insular cortices) highly associated with emotion in patients with IBS as compared to both patients with IBD and HC. These areas are also part of the interconnected salience network that shows abnormally tight coupling with the default mode and executive control networks in adolescents with IBS38. Interestingly, the increase in brain activity in patients with IBS occurred primarily during pain anticipation and not during actual pain stimuli. The increased activation in the MFG in IBS, compared to IBD, patients has been associated with pain anticipation in prior studies and most probably has a cognitive or modulatory, rather than sensory, role39. The current findings may indicate that actual pain is not required for such altered neural processing but rather anticipation of pain alone may trigger dysregulation of brain signals in areas of the brain involved in interoception.
Other patient populations with classically defined psychiatric illness have demonstrated altered neural signaling and processing in brain regions vital to interoceptive processing in response to pain anticipation or stress induction, including patients with depression20 and those with eating disorders40. High activity in interoceptive regions to situational cues, in particular, may be associated with increased disease symptoms40 and/or increased susceptibility/risk for disease relapse41. According to Letham’s fear-avoidance model, increased activity in these interoceptive brain regions may be a learned response that ultimately leads to an invalid state and exaggerated pain perception42. Similarly, Paulus and Stein proposed a role for interoceptive brain regions in triggering anxiety and avoidance behaviors with potential pain amplification43. This may be the mechanism for pain amplification in our studied IBS AYA subjects, in whom we found a significant relationship between trait anxiety and increased brain activity in interoceptive regions (i.e. anterior cingulate cortex). Errors in the processing of interoceptive information may be key in understanding the neural mechanism of IBS and may indicate an exaggerated response to the concept of imminent pain.
Functional MRI findings in patients with IBS implicate the CNS as having a role in the pathogenesis of IBS44,45. Similarly, our findings support that patients with IBS have a CNS-induced component to their gastrointestinal disease presentation. Antidepressants and psychological therapies have also been demonstrated as effective treatments for IBS46. Our findings suggest that neuromodulatory interventions may be helpful to reduce dysfunctional pain signal processing in adolescent patients with IBS. Real-time fMRI feedback studies in adults have already demonstrated that neuromodulation of brain signaling at the ACC can effectively reduce pain severity perception47, and neuromodulatory interventions including mindfulness training have been shown to modulate ACC and insula signaling37.
In regards to pain processing in IBD, the only region that showed significantly higher pain activation in IBD compared to IBS and HC groups was IPL, an area important in cognitive control of emotion48 and pain49. The IPL is part of the frontoparietal control network that plays an important role in cognitive pain modulation7. Activation at the IPL has been associated with reduced pain sensations when subjects are prompted incorrectly to anticipate a particular thermal pain stimulus48. Interestingly, IBD also showed more deactivation within medial prefrontal cortex (mPFC), compared to both groups and more deactivation within the PCC compared to the controls. Both the mPFC and PCC areas lie within the Default Mode Network (DMN), thought to be implicated in self- referential cognitive processing, self-awareness and self-monitoring31. The mPFC is involved in pain perception during spontaneous pain changes in patients with chronic back pain patients, with high-frequency brain activity oscillations correlating positively with pain ratings50. Taken together, our findings suggest that cognitive diversion techniques may be the mechanism used in IBD to down regulate or modulate the pain experience. Our data demonstrate similar activation patterns of brain regions in patients with IBS and IBD as others have demonstrated in adult patients with IBS and IBD in response to aversive visceral stimuli32. These findings suggest that altered processing of pain signals is not isolated to the inputs or sensations from the intestine in patients with IBS and IBD, but affects global sensory inputs as well.
This study has several limitations. First, as our study is a cross-sectional evaluation, we cannot assign causation to our findings. We acknowledge the small sample size, which may thus not adequately represent fully the studied populations and limit our ability to fully control for multiple confounders. In addition, we acknowledge the slight discrepancy in age distribution between the control and study groups in the paper. We controlled for these differences in all of the performed analyses and demonstrate notable differences in the neural processing and anticipation of somatic pain signals between AYA with IBS and IBD, as well as in contrast with HC. Although the age contribution cannot be completely ruled out, the insula cortex, an epicenter of interoceptive processing, does not mature until the mid third decade of life (20s)8,10. Therefore, we are confident that the observed differences were not influenced by slight age differences between the groups in our study. Further study is needed to determine whether these differential responses ultimately translate into gastrointestinal symptomatology as implied. In addition, whether these demonstrated responses in adolescence and young adulthood ultimately contribute to changes in adult neuroanatomy and function remains to be fully evaluated. Finally, in our prior work, adults were able to undergo the fMRI protocol without increased anxiety or other adverse reactions. Similarly, IBS and IBD AYA did not express any differences in anxiety scores during the fMRI protocol as compared with controls. Also, standardized somatic pain stimuli were determined through preliminary pain tolerance screening rather than based on subject-determined thresholds. Nevertheless, every subject was pre-tested before the MRI scan to ensure similar subjective pain ratings to chosen temperature stimulations. The groups did not differ in pain experienced (Table 1) during either low or high pain stimulation conditions.
CONCLUSION
We demonstrate altered neural processing of somatic pain signals in AYA with both IBS and IBD in comparison to each other and to HC. Specifically, the IBS group showed significantly greater activation in brain regions associated with interoception and decreased activation in brain regions associated with pain signal processing as compared with IBD and HC, while the IBD group demonstrated altered activation in brain pain processing regions in comparison with HC. Disorder-specific neuromodulatory interventions specifically targeting interoceptive brain areas may thus be effective for pain control in AYA with IBS while interventions targeting pain processing regions may be helpful in AYA with IBD.
What is Known/What is New.
What is known about this subject?
Functional and inflammatory gastrointestinal disorder patients frequently report pain affecting extra-intestinal systems, suggesting abnormal sensitivity beyond the gastrointestinal system.
Neural processing of extra-visceral pain has not been evaluated in patients with gastrointestinal disorders.
What are the new findings and/or what is the impact on clinical practice?
AYA with IBS and IBD exhibit significantly different neural processing of anticipated and actual somatic pain stimuli compared to each other and with controls.
IBS AYA show increased emotional reactivity and anxiety to anticipated pain, while IBD AYA show increased cognitive control when pain is actually present.
Acknowledgments
Source of Funding: Funding support received from the Clinical and Translational Research Institute, UCSD, the Price Foundation, and the Peterson Foundation. The project described was partially supported by the National Institutes of Health, Grant UL RR031980 for years 1 & 2 of CTSA funding and/or UL1TR000100 during year 3 and beyond of CTSA funding and in part by I01-CX-000816 from the United States (U.S.) Department of Veterans Affairs CS R&D Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or VA.
Abbreviations
- ACC
anterior cingulate cortex
- AI
anterior insula
- ANOVA
analysis of variance
- ANCOVA
analysis of covariance
- dlPFC
dorsolateral prefrontal cortex
- fMRI
functional magnetic resonance imaging
- IPL
inferior parietal lobule
- IBD
inflammatory bowel disease
- IBS
irritable bowel syndrome
- LN
lentiform nucleus
- MFG
medial frontal gyrus
- PCC
posterior cingulate cortex
- STG
superior temporal gyrus
- UCSD
University of California San Diego
Footnotes
Conflicts of Interests/Disclosures: All authors do not have any potential conflicts that are relevant to the manuscript.
Author Contributions: JH was involved in the study concept and design, obtained funding, provided study supervision, and participated in statistical analysis, interpretation of data, and drafting of the original manuscript. IS was involved in the study concept and design, provided technical support for the study, and participated in acquisition of data, statistical analysis, interpretation of data, and critical revision of the manuscript. LT was involved in acquisition of the data and critical revision of the manuscript. AS was involved in interpretation of the data and critical revision of the manuscript. WK was involved in study concept and design, provided material support for the study, and aided in obtaining funding and in critical revision of the manuscript.
REFERENCES
- 1.Chang L, Munakata J, Mayer EA, et al. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2000;47:497–505. doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Q, Fillingim RB, Riley JL, 3rd, Malarkey WB, Verne GN. Central and peripheral hypersensitivity in the irritable bowel syndrome. Pain. 2010;148:454–461. doi: 10.1016/j.pain.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary pharmacology & therapeutics. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 4.Larsson MB, Tillisch K, Craig AD, et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142:463 e3–472 e3. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong JY, Kilpatrick LA, Labus JS, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:14252–14259. doi: 10.1523/JNEUROSCI.1683-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HF, Hsieh JC, Lu CL, et al. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain. 2012;153:1301–1310. doi: 10.1016/j.pain.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Schmid J, Langhorst J, Gass F, et al. Placebo analgesia in patients with functional and organic abdominal pain: a fMRI study in IBS, UC and healthy volunteers. Gut. 2014 doi: 10.1136/gutjnl-2013-306648. [DOI] [PubMed] [Google Scholar]
- 8.Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126:79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. The Journal of pediatrics. 1996;129:220–226. doi: 10.1016/s0022-3476(96)70246-9. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein CN, Frankenstein UN, Rawsthorne P, Pitz M, Summers R, McIntyre MC. Cortical mapping of visceral pain in patients with GI disorders using functional magnetic resonance imaging. The American journal of gastroenterology. 2002;97:319–327. doi: 10.1111/j.1572-0241.2002.05464.x. [DOI] [PubMed] [Google Scholar]
- 11.Gasparetto M, Guariso G. Highlights in IBD Epidemiology and Its Natural History in the Paediatric Age. Gastroenterology research and practice. 2013;2013:829040. doi: 10.1155/2013/829040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lackner JM, Ma CX, Keefer L, et al. Type, rather than number, of mental and physical comorbidities increases the severity of symptoms in patients with irritable bowel syndrome. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2013;11:1147–1157. doi: 10.1016/j.cgh.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Have M, Brakenhoff LK, van Erp SJ, et al. Back/joint Pain, Illness Perceptions and Coping are Important Predictors of Quality of Life and Work Productivity in Patients with Inflammatory Bowel Disease: a 12-month Longitudinal Study. Journal of Crohn's & colitis. 2015;9:276–283. doi: 10.1093/ecco-jcc/jju025. [DOI] [PubMed] [Google Scholar]
- 14.Strigo IA, Matthews SC, Simmons AN, et al. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: evidence of interoceptive dysregulation. The International journal of eating disorders. 2013;46:23–33. doi: 10.1002/eat.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keefe FJ, Brown GK, Wallston KA, Caldwell DS. Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain. 1989;37:51–56. doi: 10.1016/0304-3959(89)90152-8. [DOI] [PubMed] [Google Scholar]
- 16.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Association of major depressive disorder with altered functional brain response during anticipation and processing of heat pain. Archives of general psychiatry. 2008;65:1275–1284. doi: 10.1001/archpsyc.65.11.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martikainen IK, Pecina M, Love TM, et al. Alterations in endogenous opioid functional measures in chronic back pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:14729–14737. doi: 10.1523/JNEUROSCI.1400-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. Journal of gastrointestinal and liver diseases : JGLD. 2006;15:237–241. [PubMed] [Google Scholar]
- 20.Strigo IA, Matthews SC, Simmons AN. Decreased frontal regulation during pain anticipation in unmedicated subjects with major depressive disorder. Translational psychiatry. 2013;3:e239. doi: 10.1038/tp.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagby RM, Parker JD, Taylor GJ. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. Journal of psychosomatic research. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 22.Speilberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) 1983 [Google Scholar]
- 23.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 24.Crombez G, Bijttebier P, Eccleston C, et al. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104:639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 25.Goubert L, Crombez G, Van Damme S. The role of neuroticism, pain catastrophizing and pain-related fear in vigilance to pain: a structural equations approach. Pain. 2004;107:234–241. doi: 10.1016/j.pain.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Vervoort T, Caes L, Trost Z, Sullivan M, Vangronsveld K, Goubert L. Social modulation of facial pain display in high-catastrophizing children: an observational study in schoolchildren and their parents. Pain. 2011;152:1591–1599. doi: 10.1016/j.pain.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 27.Kulich KR, Madisch A, Pacini F, et al. Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: a six-country study. Health Qual Life Outcomes. 2008;6:12. doi: 10.1186/1477-7525-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. ComputBiomedRes. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 29.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434–1448. doi: 10.1016/j.neuropsychologia.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 32.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 34.Neufeld KA, Kang N, Bienenstock J, Foster JA. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol. 2011;4:492–494. doi: 10.4161/cib.4.4.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacs Z, Kovacs F. Depressive and anxiety symptoms, dysfunctional attitudes and social aspects in irritable bowel syndrome and inflammatory bowel disease. International journal of psychiatry in medicine. 2007;37:245–255. doi: 10.2190/PM.37.3.a. [DOI] [PubMed] [Google Scholar]
- 36.Stengel A, Tache Y. Corticotropin-releasing factor signaling and visceral response to stress. Experimental biology and medicine. 2010;235:1168–1178. doi: 10.1258/ebm.2010.009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haase L, Thom NJ, Shukla A, et al. Mindfulness-based training attenuates insula response to an aversive interoceptive challenge. Social cognitive and affective neuroscience. 2014 doi: 10.1093/scan/nsu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Silverman A, Kern M, et al. Excessive coupling of the salience network with intrinsic neurocognitive brain networks during rectal distension in adolescents with irritable bowel syndrome: a preliminary report. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2015 doi: 10.1111/nmo.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaylord SA, Palsson OS, Garland EL, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. 2011;106:1678–1688. doi: 10.1038/ajg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans S, Lung KC, Seidman LC, Sternlieb B, Zeltzer LK, Tsao JC. Iyengar yoga for adolescents and young adults with irritable bowel syndrome. Journal of pediatric gastroenterology and nutrition. 2014;59:244–253. doi: 10.1097/MPG.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen CT, Lu PL. Thalamus and pain. Acta anaesthesiologica Taiwanica : official journal of the Taiwan Society of Anesthesiologists. 2013;51:73–80. doi: 10.1016/j.aat.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Lethem J, Slade PD, Troup JD, Bentley G. Outline of a Fear-Avoidance Model of exaggerated pain perception--I. Behaviour research and therapy. 1983;21:401–408. doi: 10.1016/0005-7967(83)90009-8. [DOI] [PubMed] [Google Scholar]
- 43.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain structure & function. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 45.Chang L. Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder? Gastroenterology clinics of North America. 2005;34:271–279. doi: 10.1016/j.gtc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Ford AC, Quigley EM, Lacy BE, et al. Effect of Antidepressants and Psychological Therapies, Including Hypnotherapy, in Irritable Bowel Syndrome: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2014 doi: 10.1038/ajg.2014.148. [DOI] [PubMed] [Google Scholar]
- 47.Mantini D, Caulo M, Ferretti A, Romani GL, Tartaro A. Noxious somatosensory stimulation affects the default mode of brain function: evidence from functional MR imaging. Radiology. 2009;253:797–804. doi: 10.1148/radiol.2533090602. [DOI] [PubMed] [Google Scholar]
- 48.Seo D, Olman CA, Haut KM, Sinha R, MacDonald AW, 3rd, Patrick CJ. Neural correlates of preparatory and regulatory control over positive and negative emotion. Social cognitive and affective neuroscience. 2014;9:494–504. doi: 10.1093/scan/nst115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunckley P, Wise RG, Aziz Q, et al. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience. 2005;133:533–542. doi: 10.1016/j.neuroscience.2005.02.041. [DOI] [PubMed] [Google Scholar]
- 50.Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]