Abstract
Adolescence is a time in development when significant changes occur in affective neurobiology. These changes provide a prolonged period of plasticity to prepare the individual for independence. However, they also render the system highly vulnerable to the effects of environmental stress exposures. Here, we review the human literature on the associations between stress-exposure and developmental changes in amygdala, prefrontal cortex, and ventral striatal dopaminergic systems during the adolescent period. Despite the vast differences in types of adverse exposures presented in his review, these neurobiological systems appear consistently vulnerable to stress experienced during development, providing putative mechanisms to explain why affective processes that emerge during adolescence are particularly sensitive to environmental influences.
Keywords: Human, Adolescence, Stress, Amygdala, Prefrontal Cortex, Ventral Striatum
Humans have one of the slowest rates of brain development of all species, taking years to reach maturity (Thompson & Nelson, 2011). On the one hand, this protracted development can be quite costly to the species, placing tremendous energy demands on parents and lengthening the period of time that offspring remain dependent on another member of the species. However, it has been argued that this long developmental period is in fact adaptive for the human species (Bjorklund, 1997; Tottenham, 2014). A developmental period that has been particularly elongated through evolution is adolescence (Thompson & Nelson, 2011). One of the adaptive values of a long adolescent period is a prolonged period of developmental plasticity. Thus, adolescence is a time of immense learning about the environment, in particular the social environment (Blakemore, 2008). However, this benefit of increased plasticity also renders developing neurobiology vulnerable to stressors that happen during development. Indeed, stressors incurred during development have different effects on brain function than if they are incurred in adulthood (Birn, Patriat, Phillips, Germain, & Herringa, 2014). Because of continued brain development extending into adolescence, this period is both a time when sensitivity to new environmental exposures (Casey et al., 2010; Somerville & Casey, 2010), particularly non-familial social stimuli, is high and when large individual differences in affective behaviors can be first observed (Casey, Pattwell, Glatt, & Lee, 2013; Gee & Casey, 2015). This review will discuss functional changes in human brain development and their intersections with psychosocial stressors.
A Foreword on Stress and Adolescence
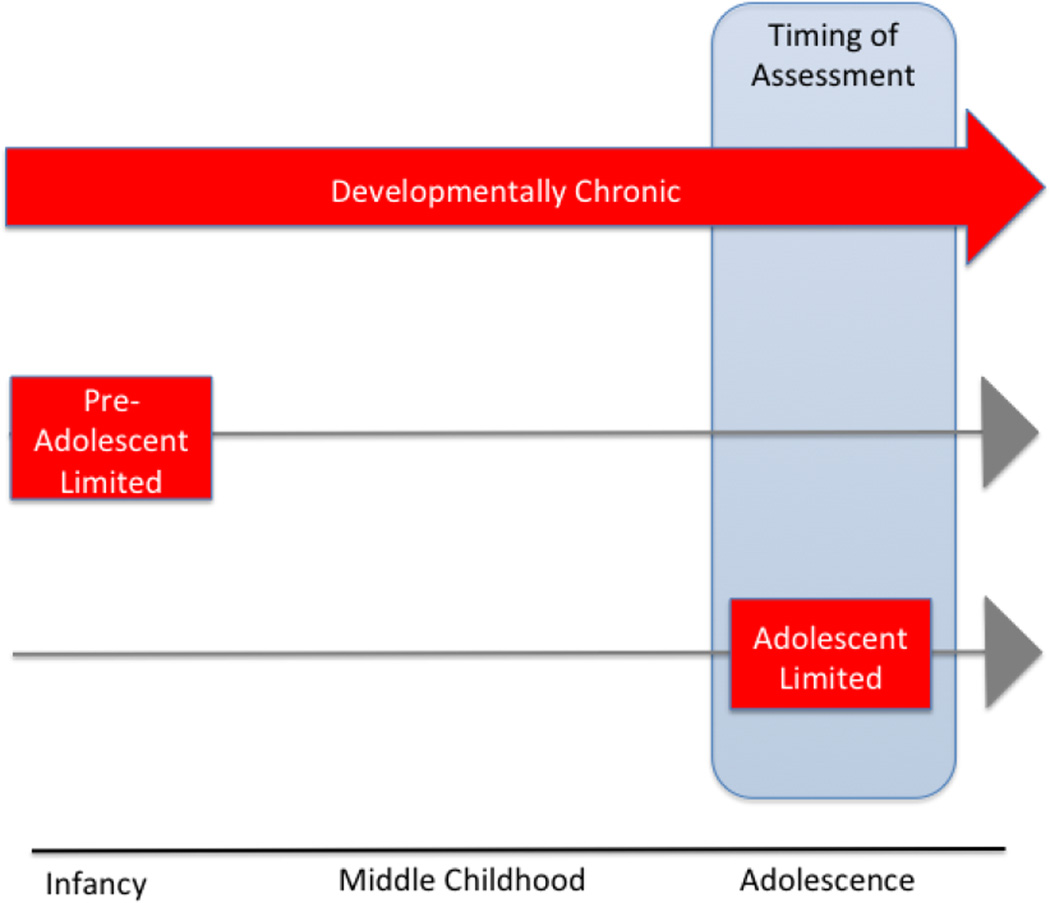
Stress is commonly defined as any environmental challenge that threatens the well-being of an organism (McEwen & Gianaros, 2011). The “environment” is a powerful agent of developmental change, but it is also very challenging to measure in humans. This problem becomes magnified when trying to assess and quantify exposure to stressors in the environment. Stressors in the environment can vary by type, degree, frequency, number, age of exposure, and duration since exposure (see Tottenham & Sheridan, 2010). Moreover, stressors during development can be challenging to ascertain because of reliance on retrospective reporting (Widom & Sherpard, 1996) as well as caregivers’ or adolescent’s reluctance or inability to report on stress exposure. More importantly for consideration of adolescent stress is defining the temporal parameters of the stress exposure in question. For illustration, imagine three different adolescents who are participating in stress research, all with three different patterns of stress exposures, as illustrated in Figure 1. There is first the case of developmentally chronic stress (top row), with stress-related behavioral and brain phenotypes measured in adolescence. The second would be a history of traumatic stress that was preadolescent-limited (e.g., to infancy or childhood), with stress-related behavioral and brain phenotypes measured in adolescence (middle row). The third would be current traumatic stress that is limited to adolescence and measured in adolescence (bottom row). The latter, adolescent limited stress, is very rare in the human literature and much more common in the animal literature, which unlike human research can control the timing of stress exposure. There are several possible reasons for this discrepancy; it could be that developmentally chronic stress is the most frequently occurring type of stress experience; alternatively, it is plausible that adolescent limited traumatic stress (in the absence of prior significant stress) is quite frequent, but perhaps more challenging to identify as research samples. As will be described, there are a handful of studies that have examined adolescent limited stressors, but these acute stressors tend to be milder daily stressors, rather than traumas. Moreover, it is typically challenging to distinguish with great precision which of these three types of developmental stress is being assessed in human studies.
Figure 1.
Different Chronicities of Adolescent Stress. (Top row) Developmentally chronic stress occurs chronically throughout development. (Middle row) Preadolescent-limited (to infancy or childhood) is comprised of a history of traumatic stress. (Bottom row) Adolescent limited describes current traumatic stress that is limited to adolescence.
Importantly, these three different types of stress histories can be associated with very different effects on brain development. For these reasons, it is critical to find convergence with animal models (e.g., see Romeo, Patel, Pham, & So, in press, this issue), which can much more precisely define and control the developmental timing of stressors. These cross species endeavors have identified significant concordance across rodent models of laboratory stress and findings in human adolescents who experience real-world stress (Callaghan, Sullivan, Howell, & Tottenham, 2014; Malter Cohen et al., 2013). These animal models complement human studies of adolescent stress, and therefore offer important translational value providing highly controlled information about developmental mechanisms.
The period of adolescence is especially important to consider when examining the effects of stress on neurobiology, whether it is living with a history of stress or experiencing concurrent stress. As reviewed elsewhere (Andersen & Teicher, 2008; Callaghan & Tottenham, 2016; Tottenham, 2014; Tottenham & Sheridan, 2010), stress exposures during development can have very different effects, and sometimes more potent effects, on the brain than when those exposures occur in adulthood. There are several reasons for differences from adults. First, there is the rapid brain development and increased plasticity of developing systems relative to the adult. Secondly, the developing brain is rich with stress hormone receptors (e.g., corticotropin releasing factor), even more so than in the adult (Avishai-Eliner, Yi, & Baram, 1996). Thirdly, the nature of brain developmental change is hierarchical, and therefore environmentally-induced alterations will not only impact stress-sensitive regions but also the development of the targets to which they will later project. However, it is less clear how individual periods within development (i.e., infancy versus childhood versus adolescence) result in differential outcomes on human brain development. There are studies suggesting that prolonged trauma during infancy can have the most profound effects on brain function (Cowell, Cicchetti, Rogosch, & Toth, 2015). On the other hand, retrospective reporting on recalled age of stress exposure has indicated later ages (e.g., early childhood or transition to adolescence) may be a sensitive period for volumetric development of subcortical neural regions (Anderson et al., 2008; Pechtel, Lyons-Ruth, Anderson, & Teicher, 2014). Most likely, the age of greatest impact will vary by domain of functioning. The human literature on developmental timing effects is much more limited than the animal literature for the reasons mentioned above, and probably the most conservative approach at this time is to appreciate that stressors during development have a profound, and usually different or greater, impact on brain function and to leverage translation across the animal literature (see Romeo et al., in press, this issue), which can much more easily control temporal factors associated with stress.
The nature of stressors can vary dramatically across adolescents. What is perhaps most striking across the human literature is that despite the vast differences between the nature of adverse experiences to which adolescents might be exposed, such as parental neglect/deprivation, abuse, community violence, and natural disasters, there are nonetheless common developmental outcomes observed across studies. Most commonly, adolescents with a history of stress exposure are at elevated risk for dysregulated affect, and this core feature gives rise to a wide range of psychosocial challenges, including anxiety, depression, personality disorders, externalizing problems, and eating disorders (Dvir, Ford, Hill, & Frazier, 2014). This review focuses on the current literature examining the underlying neural correlates of dysregulated affect in adolescence, which to date has largely focused on alterations in prefrontal cortex (PFC), amygdala, and ventral striatum. There are other regions that are certainly impacted by stress in adolescence (e.g., the hippocampus; cerebellum); however, for the scope of this paper, we focused on the amygdala, ventral striatum, and PFC since the function of these regions have received the most empirical attention in affective/motivational processes during adolescence.
In development, the increasing ability to regulate affect relies heavily on dynamic interactions between the amygdala, PFC, and striatum (Ernst et al., 2005; Hare, Tottenham, Davidson, Glover, & Casey, 2005; Somerville, Hare, & Casey, 2011; Somerville, Jones, & Casey, 2010). Broadly defined, both the amygdala and the ventral striatum are involved in affective associative learning. Research has shown that the amygdala and the ventral striatum exhibit responsivity to both positive (appetitive) and negative (aversive) stimuli (Paton, Belova, Morrison, & Salzman, 2006). However, there are also differences that have been noted in the literature. For example, the amygdala is typically implicated in identifying highly salient emotional stimuli and serving as an attentional gate for associative learning (e.g., Pearce & Hall, 1980). The ventral striatum is more likely to be implicated in reward-based learning, computing a prediction error based on anticipated outcomes (Li, Schiller, Schoenbaum, Phelps, & Daw, 2011; Schultz, Dayan, & Montague, 1997). Models of affective behavior posit that the organization between PFC, amygdala, and ventral striatum allows for the dynamic coordination of emotional learning and responding through Pavlovian and instrumental processes that link emotion to action.
The PFC is an association area that coordinates and regulates information throughout the brain. The amygdala has strong bidirectional projections with the PFC (Barbas, Saha, Rempel-Clower, & Ghashghaei, 2003), while the ventral striatum receives unidirectional projections from the amygdala and PFC, including excitatory projections that facilitate reward learning (Stuber et al., 2011), and sends indirect projections back to the PFC (Cardinal, Parkinson, Hall, & Everitt, 2002; Casey, 2015; Cho, Ernst, & Fudge, 2013). The strong connectivity from the amygdala to the ventral striatum (Cho et al., 2013) has been observed during human development starting as early as early childhood as measured by resting state functional connectivity (Fareri et al., 2015). However, what will be clear in this review is that to date, the functional significance of these amygdala-ventral striatum connections has not been thoroughly examined in human development, and much of the extant human developmental work has approached the function of these two regions in parallel rather than in coordination. We anticipate that understanding these connections will provide important information not only about typical adolescent affective behaviors, but also the impact of stress exposures in adolescents. However, current research on the development of these regions has already provided much insight into how environmental stress influences adolescent behavior and brain development, which will be reviewed below.
Although significant brain development occurs well before the start of adolescence, there are notable changes during adolescence, particularly in stress sensitive affective and cognitive systems. Moreover, adolescence is marked by increased stress and heightened stress reactivity, compared to children and adults (Dahl & Gunnar, 2009; Dorn, Dahl, Woodward, & Biro, 2006). For example, the hypothalamic-pituitary-adrenal (HPA) axis, which is one of the major stress axes in humans and produces cortisol, and undergoes tremendous change with the transition to adolescence (Adam, 2006; Netherton, Goodyer, Tamplin, & Herbert, 2004), both in basal levels as well as in response to laboratory stressors (e.g. public speaking), to a novel social stressor (e.g. peer rejection), and to academic stressors (Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001; Knutsson et al., 1997; Stroud, Papandonatos, Williamson, & Dahl, 2004). In fact, Gunnar and colleagues (2009) show nonlinear patterns of heightened basal HPA activity and greater stress reactivity to a stressor across development, with adolescents showing the most robust effects compared to children and young adults. There are many reasons for this change. First, there is an intimate association between the activity of the HPA axis and the hypothalamic-pituitary-gonadal axis, which is responsible for pubertal elevations in testosterone and estrogen (Dismukes, Shirtcliff, Hanson, & Pollak, 2015). Secondly, the HPA axis is particularly sensitive to social challenges, which may increase dramatically with adolescence (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009). Thirdly, there is mounting evidence from both rodent and human research to suggest that the transition to adolescence represents a time when HPA axis undergoes dramatic change. For example, the HPA axis exhibits a significant gain in reactivity following puberty. This change is especially important to understand for adolescents with a history of stress; pre-pubertally, previously-institutionalized adolescents exhibit flattened morning cortisol production, possibly reflecting a down-regulation of “cortisol as a counter-regulatory response to prolonged periods of elevated cortisol” (Quevedo, Johnson, Loman, Lafavor, & Gunnar, 2012). However, previously-institutionalized adolescents in mid- to late-puberty do not show this flattening (Gunnar et al., 2009; Hostinar, Johnson, & Gunnar, 2015; Quevedo et al., 2012; Romeo, 2010). These findings have led to the conclusion that “puberty probably allows the reprogramming of the HPA axis” (Gunnar et al., 2009). This transition occurs in parallel with large developments in subcortical and cortical neural regions, like the amygdala, PFC, and ventral striatum, whose activity is highly modulated by cortisol and related stress hormones, because circulating cortisol can easily pass through the blood-brain barrier and readily interacts with amygdala-PFC circuitry (Avishai-Eliner et al., 1996; Moriceau, Roth, Okotoghaide, & Sullivan, 2004) and also with the ventral striatum (Graf et al., 2013). This confluence of developmental changes may increase the susceptibility of adolescents to environmental stressors (developmentally chronic, pre-adolescent limited, or adolescent limited).
These aspects of adolescence provide a putative mechanism to explain the well-established findings that many mental illnesses associated with stress exposure emerge (or are first observed) during the adolescent period (Casey et al., 2013; Gee & Casey, 2015; Kessler et al., 2005). This paper will review the literature examining the associations between stressors and functional development of human subcortical-cortical circuitry during the adolescent period. Specifically, we will focus on amygdala-PFC circuitry and ventral striatal dopaminergic systems during adolescence. There were several motivations for this focus: the sensitivity of amygdala-PFC and ventral striatum to environmental stressors, particularly during adolescence; the significant developmental changes in amygdala-PFC and ventral striatum circuitry during the transitions into and out of the adolescent period; and the central role in amygdala-PFC and ventral striatum circuitry in individual differences in socio-affective behaviors that emerge during the transition into adolescence.
The goal of this review is to summarize what is currently known about stress during adolescence, how it impacts affective and motivational learning systems in adolescence, and how stress may exert neurobiological effects on circuitry that subserves these operations in the developing brain. There are, unfortunately, many types of stress exposures to which children and adolescents can be exposed, and each of these may be associated with stressor-unique effects on the brain (see Sheridan & McLaughlin, 2014). However, as will be discussed, there is nonetheless significant overlap across types of stress exposures on subcortical regions like amygdala and ventral striatum. Because of their intimate association with the social environment, the amygdala-PFC and ventral striatal dopaminergic systems are particularly sensitive targets of stress in adolescence. We will start with a brief review of the effects of stress on amygdala-PFC circuitry followed by the literature on ventral striatal dopaminergic systems. Although there is strong interconnectivity of these regions with each other, the organization of this review follows the human developmental stress literature, which to date, has typically studied these systems separately.
Amygdala-PFC Circuitry Development
Adolescence is a developmental period characterized by large changes in affect regulation and its underlying neural correlates. In the healthy adult, connections between the amygdala and PFC comprise the core circuitry involved in negative affect generation and regulation (Kim, Loucks, et al., 2011; Lee, Heller, van Reekum, Nelson, & Davidson, 2012; Phan, Wager, Taylor, & Liberzon, 2002; Toyoda et al., 2011). The neurobiology of negative affect generation has been extensively and best characterized at the level of the amygdala, which mediates threat learning and vigilance (Davis & Whalen, 2001). The PFC sends projections to the amygdala that modulate amygdala reactivity (Milad & Quirk, 2002). These projections, depending on their source, can function to attenuate or potentiate (Senn et al., 2014) affective responding, and thus these connections are fundamental to mature affect regulation (Banks, Eddy, Angstadt, Nathan, & Phan, 2007; Delgado, Nearing, Ledoux, & Phelps, 2008; Monk, 2008).
However, amygdala-PFC connections are slow to develop. In rodents, long-range connections between the amygdala and medial PFC continue to develop well into adolescence (Cunningham, Bhattacharyya, & Benes, 2002; Johnson et al., 2016; Pattwell et al., 2016). In the human, this circuitry shows developmental changes across the first two decades of life. It has been posited that adolescence marks a new sensitive period for amygdala circuitry (Scherf, Smyth, & Delgado, 2013). Anatomically, there is considerable postnatal development in amygdala volume in infancy (Gilmore et al., 2012). Nonetheless, volumetric change continues to be observed into childhood and adolescence (Giedd et al., 1996; Uematsu et al., 2012). Some of these changes in volume correspond to pubertal change (Bramen et al., 2011; Goddings et al., 2014), suggesting that pubertal development is one important agent of developmental change in the amygdala during adolescence. Adolescence is a time of remarkable change in both functional and structural connections between the amygdala and PFC. Functional and structural connectivity between amygdala and PFC begins to exhibit adult-like patterns that serve regulatory function (Decety, Michalska, & Kinzler, 2012; Dougherty, Blankenship, Spechler, Padmala, & Pessoa, 2015; Gabard-Durnam et al., 2014; Gee, Humphreys, et al., 2013; Hare et al., 2008; Lebel et al., 2012; Perlman & Pelphrey, 2011; Silvers, Shu, Hubbard, Weber, & Ochsner, 2014; Swartz, Carrasco, Wiggins, Thomason, & Monk, 2014; Vink, Derks, Hoogendam, Hillegers, & Kahn, 2014). These strengthening connections are developing at the same time that high amygdala reactivity to emotional stimuli (faces, scenes) is observed (Decety et al., 2012; Gee, Humphreys, et al., 2013; Guyer et al., 2008; Hare et al., 2008; Swartz et al., 2014; Vink et al., 2014). Different models have been developed to account for these findings (e.g., imbalance model, (Casey, Galvan, & Somerville, 2016); dual systems accounts, (Steinberg, 2010); triadic model, (Ernst, 2014)). Each of these models emphasizes the large changes and the continued development of limbic-cortical circuitry during adolescence. Although these changes allow for continued plasticity throughout adolescence, the instability associated with large age-related change increases the vulnerability of neural systems to be influenced by environmental stress (Lupien, McEwen, Gunnar, & Heim, 2009) as we will discuss in the next section.
Amygdala-PFC Circuitry, Adolescence, and Developmentally Chronic/Pre-Adolescent Limited Stress
Across various types of traumatic stress exposures (e.g., parental neglect/deprivation, abuse, community violence, and natural disasters), alterations to amygdala function and amygdala functional connectivity to PFC during adolescence have been observed. These alterations correspond with the behavioral observations of increased fear reactivity (Fani et al., 2015), attentional biases towards threat (Fani et al., 2015; Troller-Renfree, McDermott, Nelson, Zeanah, & Fox, 2015) and difficulty with affect-related regulation (Tottenham et al., 2010) following significant stress exposure. What these exposures have in common is that they were experienced during a time of rapid brain development and are all threats to survival. The amygdala continues its development throughout adolescence, and its primary function is to gather information (both aversive and appetitive) that is important for survival. These aspects of the amygdala together with its abundance of HPA axis hormone receptors (Vazquez et al., 2006) position it well to be a target of these exposures.
Adverse caregiving is a significant early-life threat to the developing human, because of the absence of developmentally necessary regulation from the parent (see Tottenham, 2012 for review), and represents one of the earliest forms of psychological stress that a human can experience. One of the most highly replicable effects of caregiving adversity is amygdala hyperreactivity in response to emotional stimuli during adolescence (Garrett et al., 2012; Marusak, Martin, Etkin, & Thomason, 2015). Adolescents with a history of physical abuse and neglect (De Bellis & Hooper, 2012; McCrory et al., 2013) or exposure to family violence (McCrory et al., 2011) exhibit elevated amygdala reactivity in response to highly arousing emotional faces, even if they are presented preattentively (McCrory et al., 2013). This elevated amygdala reactivity to emotional faces has also been observed in adolescents who experience low parental warmth (Casement et al., 2014) or severe neglect and institutional care during infancy (Gee, Gabard-Durnam, et al., 2013; Maheu et al., 2010; Tottenham et al., 2011).
Significant stressors experienced later in development have also been associated with increased amygdala reactivity. In a group of healthy adolescents, the total number of stressors after the age of 4 years old was positively correlated with the magnitude of amygdala reactivity to emotional faces, suggesting the amygdala continues to be shaped by significantly stressful events beyond infancy and early childhood (Ganzel, Kim, Gilmore, Tottenham, & Temple, 2013). In a prospective study of adolescents, those who reported significant traumatic stress in the past year exhibited developmental increases in amygdala reactivity to emotional faces over a 2-year period, unlike those with low stress who exhibited age-related decreases. Moreover, those with significant traumatic stress were more likely to exhibit an amygdala trajectory similar to adolescents with a significant familial risk for depression (Swartz, Williamson, & Hariri, 2015). These data suggest that amygdala hyperactivity in adolescence might be an important biomarker for mental illness associated with affect dysregulation (Swartz, Knodt, Radtke, & Hariri, 2015). In support of this interpretation, McLaughlin and colleagues (2014) had the rare opportunity to have obtained brain scans using functional magnetic resonance imaging (fMRI) from adolescents prior to the terrorist attack on the Boston Marathon. Following the attack, these researchers could predict post-traumatic stress disorder (PTSD) symptomology based on the magnitude of amygdala response in the initial scan.
Stress exposure is also associated with alterations in the development of connections between the amygdala and PFC regions during adolescence. One means of measuring functional connectivity is to employ resting state measures, which assay spontaneous regional interactions that occur when a subject is not performing an explicit task and provides an index of the integrity of a functional connection between regions of interest. Adolescents with a history of child maltreatment (Herringa et al., 2013) or trauma (Pagliaccio et al., 2015; Thomason et al., 2015) exhibit weaker connectivity between amygdala and PFC regions (Nooner et al., 2013), including regions in the medial PFC (mPFC). The nature of amygdala-mPFC resting state connectivity has implications for future mental health; in adulthood, weaker connectivity is associated with increased trait anxiety (Kim, Gee, Loucks, Davis, & Whalen, 2011). In adolescence, weaker amygdala-mPFC connectivity has been shown to mediate the associations between stress and anxiety symptoms (Pagliaccio et al., 2015) and PTSD symptoms (Cisler, Scott Steele, Smitherman, Lenow, & Kilts, 2013) suggesting that stress-induced alterations to amygdala-mPFC connectivity during adolescence may underlie emotional difficulties following stress exposures in development. In a more direct test of this hypothesis using a prospective design, Burghy and colleagues (2012) followed subjects from the age of 4 years old to 18 years old. In this study, stress experienced at 4 years old was associated with increased cortisol levels in childhood, which predicted altered functional connectivity between the amygdala and mPFC in adolescence. Amygdala-mPFC connectivity in adolescence was associated with anxiety and depression, although in different ways – amygdala-mPFC connectivity was negatively correlated with anxiety symptoms, but it was positively correlated with depression symptoms. These findings are important because they provide compelling evidence that developmental stress increases the risk for internalizing problems, and this association is mediated by alterations to amygdala-mPFC function during adolescence. This effect was only observed in females, suggesting a possible neurobiological basis for commonly observed sex differences in risk for internalizing problems (Nolen-Hoeksema, 1987).
Amygdala-PFC connectivity has also been examined in adolescence while participants are engaged in behavioral tasks. Connectivity measured during these manipulations offer an important complement to resting state measures because they index the coactivation of two regions that is elicited by a particular stimulus or behavioral demand. Across different types of stress exposures, task-based measures have demonstrated altered functional connectivity between the amygdala and PFC. For example, in response to viewing negative facial expressions, it has been shown that both trauma exposure (Wolf & Herringa, 2016) and a history of early-institutional rearing (Gee, Gabard-Durnam, et al., 2013) is followed by greater inverse correlations between amygdala and mPFC. This pattern is more typical of healthy adults (e.g., Lee et al., 2012), suggesting an acceleration or temporary enhancement of function during development. The magnitude of these effects has been shown to be associated with avoidance behaviors (Wolf & Herringa, 2016). Whether these patterns of task-elicited connectivity reflect risk or resilience remains unclear – although further research is needed, one possible interpretation of altered connectivity is that it is an adaptation that helps the individual meet immediate emotion regulation needs, even if they are not optimal emotion regulation strategies in the long term (Callaghan & Tottenham, 2016); in support of this interpretation, the more adultlike patterns in amygdala-PFC regulatory connectivity in response to viewing fearful faces have been associated with lower current anxiety symptoms in previously-institutionalized youth (Gee, Gabard-Durnam, et al., 2013). Similarly, during an aversive learning paradigm, adolescents with a history of institutional care are more likely to exhibit adult-like patterns of amygdala functional connections with both ventral and dorsal medial PFC (Silvers et al., 2016). The dorsal region of medial PFC has been associated with more amplification of negative affect (Maier et al., 2012). This difference in both a regulatory region as well as an amplification region raises important questions about the relative balance between excitatory and inhibitory influences to and from the amygdala following stress exposure.
Tasks involving effortful emotion regulation have further revealed atypical amygdala-PFC functional connectivity in adolescence following stress exposure. Marusak and colleagues (2015) have shown that while performing an emotional conflict task that involves categorizing facial affect while ignoring an overlying emotion word, group differences related to trauma exposure emerged in amygdala and PFC regions (Marusak et al., 2015). Specifically, trauma-exposed youths exhibited amygdala-PFC connectivity patterns consistent with poor affect regulatory function. Correspondingly, the trauma exposed group also exhibited a failure to dampen amygdala reactivity and poorer regulatory skill. Cognitive reappraisal tasks (Ochsner et al., 2004) are another means of measuring emotion regulation skills, and they assess effortful attempts to dampen negative emotions elicited by negative images. During attempts to decrease responses to negative stimuli, maltreated adolescents were more likely to recruit prefrontal regulatory regions than controls to reach the same level of emotion regulation (McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015). Taken together, these task-based measures suggest that not only is amygdala reactivity altered by stress during adolescence, but also the nature of the communication between amygdala and prefrontal circuits is altered. These alterations may include adaptations for achieving the best possible affect regulation. It is possible that given the hierarchical nature of brain development and the early development of the amygdala, development of connectivity with prefrontal regions in adolescence depends very much on the earlier emerging function of the amygdala (as suggested by Gee, Gabard-Durnam, et al., 2013; Gee, Humphreys, et al., 2013). That is, stress-induced hyperreactivity of the amygdala may increase the risk for atypical connections with the PFC that can differ in nature, timing, or both.
Development of Ventral Striatal Dopaminergic Motivational Systems
In addition to large changes in amygdala-PFC circuitry, adolescence is a also developmental period characterized by significant changes in reward sensitivity, cognitive regulation and their neural correlates (Steinberg, 2005). The ventral striatum supports reward-related processes, such as reward based learning (Fiorillo, Tobler, & Schultz, 2003), through receipt of significant dopaminergic inputs from the ventral tegmental area and substantia nigra (Haber, 2011). The dopamine system, which undergoes significant maturation during adolescence (Andersen & Teicher, 2008, 2009; Rosenberg & Lewis, 1995; Spear, 2009), is also sensitive to the effects of stress. In adult rodents, acute stress induces increased extracellular levels of dopamine in the nucleus accumbens (Abercrombie, Keefe, Di Frischia, & Zigmond, 1989; Kalivas & Duffy, 1995; Ungless, Argilli, & Bonci, 2010), which is mediated through stress hormones (Kreek & Koob, 1998; Rouge-Pont, Deroche, Le Moal, & Piazza, 1998). Stress also increases firing rates (Anstrom & Woodward, 2005) and synaptic adaptation in dopamine neurons (Saal, Dong, Bonci, & Malenka, 2003) which is thought to amplify reward salience (Mather & Lighthall, 2012; Starcke & Brand, 2012). This section will review evidence suggesting that stress triggers motivational and reward seeking behavior.
Much insight on this topic has been gleaned from animal studies. Rodents in the juvenile period evince robust dopamine reactivity in stressful contexts. Postnatal stress in young rat pups leads to elevated levels of dopamine (Huppertz-Kessler, Poeschl, Hertel, Unsicker, & Schenkel, 2012). Juvenile versus adult rats show an enhanced stress response when exposed to cues of predation threat (Wright, Muir, & Perrot, 2012). Moreover, prenatal (Silvagni, Barros, Mura, Antonelli, & Carboni, 2008) and early life (Jezierski, Zehle, Bock, Braun, & Gruss, 2007) stress exposure leads to enhanced dopamine release and functional alterations of dopaminergic pathways in adolescence (Jezierski, Zehle, Bock, Braun, Gruss, 2007).
Stress-related alterations of dopamine release have also been demonstrated in adult humans (Starcke and Brand, 2012). Using positron emission tomography (PET), researchers have shown that a laboratory stressor increases dopamine release in the adult human brain (Scott, Heitzeg, Koeppe, Stohler, & Zubieta, 2006), which is correlated with higher cortisol levels (Pruessner, Champagne, Meaney, & Dagher, 2004), and that stress-induced cortisol levels were positively associated with amphetamine-induced dopamine release in the ventral striatum (Wand et al., 2007).
In humans as well, there are significant normative developmental changes ventral striatum during adolescence. Across many fMRI studies to date, researchers have shown significant changes in ventral striatal reactivity during adolescence. Most of these studies report that adolescents evince stronger activation of the ventral striatum in response to primary (Galván & McGlennen, 2013), secondary (Cohen et al., 2010; Galván et al., 2006; van Leijenhorst et al., 2010), and social rewards (Guyer et al., 2008; Telzer, Fuligni, Lieberman, & Galván, 2013) (Bjork et al., 2004). This increase in ventral striatum reactivity parallels the adolescent maturation of the dopamine system observed in animal models (Andersen & Teicher, 2008, 2009; Rosenberg & Lewis, 1995; Spear, 2009). These neural findings are paralleled by heightened reward sensitivity, risk taking and motivational behavior in adolescents as compared to other age groups (see Galván, 2013 for review). Interestingly, environmental conditions have been found to exacerbate (Phuong & Galvan, in press) or diminish (Do & Galván, 2015) these effects, underscoring the malleability of the mesolimbic system during this crucial period of development.
Ventral Striatum, Adolescence, and Developmentally Chronic/Pre-Adolescent Limited Stress
Like the amygdala and the PFC, the ventral striatum seems highly influenced by stress in adolescents. Young adults with a history of childhood stress exhibit blunted ventral striatal response when processing a monetary reward (Boecker et al., 2014; Hanson et al., 2016). Research in younger samples shows that this effect of stress on reward-related circuitry can emerge during adolescence. Adolescents with a history of institutional caregiving exhibit blunted ventral striatal response (Mehta et al., 2010). Specifically, at the group level, adolescents with a history of institutional care do not differentiate reward predicting cues of varying value (low, medium, high) in the ventral striatum, whereas control individuals showed greater responses to medium and high reward cues compared to low. Goff and colleagues (2012) showed that group differences in ventral striatal response to positive facial emotion between individuals with a history of institutional care and a typically-raised comparison group emerge first in adolescence. In this study, adolescence was also the time when depression symptoms increased significantly, and ventral striatum hypoactivity correlated with depressive symptomology in the adversity-exposed group (Goff et al., 2012). Research performed independently both Hanson et al. (2015) and Schneider et al. (2012) has shown that even less extreme forms of caregiving adversity (i.e., low maternal affiliation or emotional neglect) are associated with alterations in ventral striatal reactivity. Importantly, Hanson et al., (2015) showed that a history of emotional neglect was associated with decreased functional connectivity (perhaps even negative) between amygdala and ventral striatum in response to reward, providing a possible mechanism linking the elevated amygdala reactivity to blunted ventral striatum functioning in adolescents with a history of stress.
These findings of blunted ventral striatum responsivity during adolescence following early life stress might explain behavioral effects in reward-related paradigms often observed following stress exposures. The balloon analogue risk taking task (BART)(Lejuez et al., 2002) is a task that involves subjects’ pumping up a virtual balloon that can grow larger with corresponding increasing reward but can potentially explode (if pumped too much) with a complete loss of rewards. It provides a measure of risk-taking under conditions of potential reward and has been associated with ventral striatal function in adults (Rao, Korczykowski, Pluta, Hoang, & Detre, 2008). Consistent with blunted ventral striatal reactivity described above, adolescents with a history of institutional caregiving exhibit less risk taking (i.e., they pump the balloon less) compared to same aged peers without a history of early adversity (Humphreys et al., 2015; Loman, Johnson, Quevedo, Lafavor, & Gunnar, 2014). Similarly, adolescents with a history of maltreatment and a diagnosis of depression are less like to select a risky choice (with high reward) during a two-choice decision-making task involving probabilistic monetary gains (Guyer et al., 2006). These findings are consistent with working models (e.g., Auerbach, Admon, & Pizzagalli, 2014; Goff & Tottenham, 2015), which posit that early life stress may render the adolescent vulnerable to depression because of its effects on ventral striatal development during this sensitive time.
Adolescent-Specific Daily Stressors
There are many types of daily stressors that emerge during adolescence (Persike & Seiffge-Krenke, 2012) including family, academic (Arnett, 2002; Bynner, 2000), and peer (Eccles et al., 1993; Smetana, Campione-Barr, & Metzger, 2006) pressures. For example, demanding coursework and preparation for college placement often lead to increases in school related stress (McAndrew, Akande, Turner, & Sharma, 1998). Socially, adolescents frequently experience stressors in the domain of romantic relationships and peer networks (Hand & Furman, 2009; Kuttler & LaGreca, 2004). Also common is elevated stress associated with developing more intimate friendships while maintaining family relationships and establishing autonomy (Laursen & Collins, 1994; Nieder & Seiffge-Krenke, 2001). Ethnographic data demonstrates that these effects are observed globally, as adolescents from over 140 cultures report high feelings of stress in these domains (Schlegel, 2001). However, as noted in the foreword, there is limited research on acute effects of stress on adolescent neurobiological development in humans. We summarize here what has been found in research on the human adolescent amygdala, ventral striatum, and PFC in response to daily stressors.
Amygdala-PFC Circuitry
Based on adult findings, amygdala-PFC circuitry responds strongly and immediately to acute stressors (Oei et al., 2012; van Marle, Hermans, Qin, & Fernandez, 2010). These changes have been associated with acute administration of the stress hormone, glucocorticoids (Henckens, van Wingen, Joels, & Fernandez, 2010). Although there are no studies that have examined the effects of acute stress on amygdala-PFC circuit functioning during adolescence, the implications from the adult work provide strong motivation for the hypothesis that amygdala-PFC circuitry are highly reactive to environmental stressors. Moreover, significant and prolonged stressors during development might have even more pronounced effects on the long-term programming of these circuits.
Ventral Striatum
In adults, acute stress yields alterations in neural activation of mesolimbic-prefrontal circuitry, including the striatum, insula, thalamus, and prefrontal regions (Kogler et al., 2015; Pruessner et al., 2008), which may be due to the stress-related increases in dopamine release in ventral striatum (VS) and orbital frontal cortex (OFC) observed in animal models (Ungless, Argilli, & Bonci, 2010) and human positron emission tomography (PET) studies (Pruessner, Champagne, Meaney, & Dagher, 2004). Research shows that this enhanced dopamine release is magnified in individuals who also evince increased cortisol levels in response to psychosocial stressors (Pruessner et al., 2004). The behavioral consequences of these neurochemical and neural activation changes in response to stress include enhanced reward salience and greater reward-biased decisions (Porcelli & Delgado, 2009; Putman, Antypa, Crysovergi, & van der Does, 2010; Starcke & Brand, 2012).
Although there has been much less research on the effects of concurrent adversity in adolescence (see Romeo et al., in press, this issue, for rodent studies), Casement and colleagues (2014) examined the prospective associations between challenging social situations for girls at 11–12 years old and neural activation during anticipation of reward when they were 15 years old. There was a negative association between ventral striatum response and maternal warmth, suggesting at a minimum that the ventral striatum may respond quite differently to stressors that are experienced during the adolescent period. Galván and McGlennen (2012) used a daily diary approach to monitor and compare 14–17 year-old adolescents’ daily stress to that of 18–21 year-old emerging adults. Results indicated that adolescent participants made more risky decisions during high stress relative to low stress, suggesting that stress impacts behavior in a similar, albeit more exaggerated, manner in adolescents relative to adults (Galván & McGlennen, 2012).
Similarly, Phuong and Galván (in press) used an ecological momentary assessment (EMA) approach to monitor adolescent participants' naturalistic stress for two weeks. Each participant visited the laboratory twice, once on a day when they endorsed a high level of daily stress and once on a day when they endorsed a low level of daily stress. This novel approach allowed for the examination of within-person, as well as developmental effects, thereby precluding potential confounds related to individual differences in laboratory-stress reactivity. At the lab, participants underwent fMRI scanning during a risky decision making task. The findings showed that adolescents (aged 14–17 years) made more risky choices than adults (ages 25–30 years) following a stressful day. This effect could not be explained by adolescents simply “being riskier” in general as there were no developmental differences following a self-reported minimally stressful day. The behavioral differences between adolescents and adults that emerged on stressful days were associated with developmental differences in neural recruitment as well. Adolescents showed less engagement of regions that have previously been associated with risk monitoring, including the orbital frontal cortex and insula, than adults (Phuong and Galván, in press). Adolescent girls also showed enhanced activation of the caudate, a region implicated in reward. In a follow-up study, the authors found that the relationship between insula response and risky behavior was exacerbated in individuals who reported regularly sleeping less than the 7 hours per night that is recommended by the National Sleep Foundation (Phuong and Galván, under review).
Prefrontal Cortex
The prefrontal cortex and its connections with subcortical regions is one of the latest to develop in the human brain, and therefore it is vulnerable to the effects of stressors during adolescence. Certainly, there is a rich literature in adults showing that acute stress negatively affects cognition (Janis, 1993; Keinan, 1987; Mather & Lighthall, 2012; Porcelli & Delgado, 2009; Preston, Buchanan, Stansfield, & Bechara, 2007), in learning, memory, decision and inhibition domains (Mather and Lighthall, 2012; Roozendaal, 2002; Sandi, 2013; Wolf, 2006). For instance, response inhibition performance in adult males is significantly impaired following acute stress (Scholz et al., 2009). Animal research has also found that rodents (Hennessy, Cohen, & Rosen, 1973; Micco, McEwen, & Shein, 1979) and monkeys exposed to stress-level cortisol treatments have impaired response inhibition (Lyons, Lopez, Yang, & Schatzberg, 2000), which is mediated via stress-induced atrophy of prefrontal neurons (Liston et al., 2006; Radley et al., 2004). These findings have been instrumental in establishing the mechanism by which acute stress can dysregulate cognition.
More recent research has focused on determining whether these stress effects on cognition and PFC extend to adolescent populations. Using the EMA approach described above, Rahdar and Galvan (2014) monitored adolescent participants' naturalistic stress for two weeks. Each participant visited the laboratory twice, once on a day when they endorsed a high level of daily stress and once on a day when they endorsed a low level of daily stress. At the lab, participants performed a Go/No-go task while undergoing fMRI to assess cognitive control and as a probe for prefrontal function. Behaviorally, all participants exhibited worse response inhibition under high, versus low, stress states, an effect that was significantly stronger in adolescents. At the neural level, there was a significant age by stress interaction, such that adolescents exhibited less recruitment of the dorsolateral prefrontal cortex (DLPFC) during inhibition under high-stress versus low-stress; adults evinced the opposite activation pattern in DLPFC. These data provide further support that the developing brain may be a more vulnerable target to the cognitive and neurobiological effects of stress (Rahdar & Galván, 2014).
Future Directions
Across several laboratories, samples, and paradigms, the human literature has demonstrated a strong association between stress-exposure and altered development of neuro-affective/motivational systems in adolescence. The effects of stress on affective, motivational, and cognitive systems during adolescence have largely been studied in parallel rather than in conjunction. However, it is becoming increasingly clear that the amygdala, PFC, and ventral striatum operate together during affective processes as they emerge during adolescence (see Casey et al., 2016; Ernst, 2014; Somerville, van den Bulk, & Skwara, 2014). Indeed, it is possible that direct stress-induced alterations in one region can produce cascading and indirect stress-alterations in the others. For example, stress-induced alterations to the early developing amygdala could have downstream effects as a result of unidirectional amygdala-ventral striatum projections (e.g., Cho et al., 2013) and the late development of the prefrontal cortex (e.g., Gee et al., 2013). This hypothesis requires longitudinal testing. Moreover, the nature of information represented by each region is less modular than distributed in the human. For example, the ventral striatum represents negatively valenced cues as well as positively valenced cues (Levita et al., 2009), and the amygdala is highly responsive to reward in addition to being sensitive to aversive cues (Murray, 2007). Computational modeling approaches with adult fMRI data have shown that the unique computations of the amygdala and ventral striatum work together to encode different parameters of environmental contingencies (Li et al., 2011). This approach, although not yet applied to data from developmental populations, might be a powerful one for understanding how stress influences adolescent brain function.
Implications for mental health: Research Domain Criteria Framework
There are significant mental health implications for delineating the adolescent processes that are affected by developmental stress. Identifying the mechanisms targeted by exposure to stress and how they are affected during adolescence is central to understanding the developmental emergence of mental illnesses (e.g., anxiety and depression) within the Research Domain Criteria (RDoC) framework (see Kozak & Cuthbert, 2016, for discussion). For example, the affective dimensions of negative affect and positive affect and the cognitive constructs of cognitive control and working memory are all at risk for alteration by stress and all contribute to diagnoses of anxiety and depression. By identifying which dimensions are more or less affected by particular types of stress exposures at different levels of analysis, including brain circuitry, behavior physiology, environment, and development, we may have more power to predict mental health course with greater specificity for an individual. However, development is highly dynamic, which presents additional challenges, as well as opportunities, to understanding the emergence of mental health problems within an RDoC framework (Casey, Oliveri, & Insel, 2014). Knowledge of normative developmental changes in the brain and how they interact with timing of stress exposures and the timing of phenotypic manifestation is necessary for a more complete understanding of the pathophysiology and etiology of mental illness. These domains of functioning will also each have its own developmental timeline, and they will interact with each other during development; thus interactions between dimensions should be considered across infancy, childhood, and adolescence. The significant changes that occur during the transitions into and during adolescence, both at the level of neurobiology and environment, render adolescence a vulnerable period for stress-related alterations to the mechanisms that underlie affective processes. Moreover, future work that continues to characterize how adolescent brain development is influenced by environmental stressors can also provide insight into the mechanisms for recovery and adaptation.
Highlights.
Adolescent changes in affective neurobiology increases vulnerability to stressors
Amygdala, prefrontal cortex, and ventral striatum are consistent targets of stress
Potential mechanisms for affect dysregulation that emerges in adolescence
Acknowledgments
This work was supported by the National Institute of Mental Health (R01MH091864 to N.T.), the Dana Foundation (to N.T.), the National Science Foundation (BCS0963750 to A.G.) and the Jacobs Foundation (to A.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie E, Keefe K, Di Frischia D, Zigmond M. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens and medial frontal cortex. Journal of Neurochemistry. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Adam E. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31:664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher M. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neuroscience. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher M. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neuroscience Biobehavioral Reviews. 2009;33(4):516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary Evidence for Sensitive Periods in the Effect of Childhood Sexual Abuse on Regional Brain Development. Journal of Neuropsychiatry and Clinical Neurosciences. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom K, Woodward D. Restraint increases dopaminergic burst firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. doi: 10.1038/sj.npp.1300730. [DOI] [PubMed] [Google Scholar]
- Arnett J. The psychology of globalization. American Psychologist. 2002;57:774–783. doi: 10.1037/0003-066X.57.10.774. [DOI] [PubMed] [Google Scholar]
- Auerbach RP, Admon R, Pizzagalli DA. Adolescent depression: stress and reward dysfunction. Harv Rev Psychiatry. 2014;22(3):139–148. doi: 10.1097/HRP.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, Baram TZ. Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Developmental Brain Research. 1996;91(2):159–163. doi: 10.1016/0165-3806(95)00158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ. Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress Anxiety. 2014;31(10):880–892. doi: 10.1002/da.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J, Knutson B, Fong G, Caggiano D, Bennett S, Hommer D. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neuroscience. 2004;24(8):1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund DF. The role of immaturity in human development. Psychol Bull. 1997;122(2):153–169. doi: 10.1037/0033-2909.122.2.153. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9(4):267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Boecker R, Holz NE, Buchmann AF, Blomeyer D, Plichta MM, Wolf I, Laucht M. Impact of early life adversity on reward processing in young adults: EEG-fMRI results from a prospective study over 25 years. PLoS One. 2014;9(8):e104185. doi: 10.1371/journal.pone.0104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Forbes EE, Chen J, Toga AW, Sowell ER. Puberty influences medial temporal lobe and cortical gray matter maturation differently in boys than girls matched for sexual maturity. Cereb Cortex. 2011;21(3):636–646. doi: 10.1093/cercor/bhq137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynner J. Social change and the sequencing of developmental transitions. In: Crockett L, Silbereisen R, editors. Negotiating adolescence in times of social change. Cambridge, MA: Cambridge University Press; 2000. pp. 89–103. [Google Scholar]
- Callaghan BL, Sullivan RM, Howell B, Tottenham N. The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits--a cross-species analysis. Dev Psychobiol. 2014;56(8):1635–1650. doi: 10.1002/dev.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences. 2016 doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26(3):321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, Forbes EE. Girls' challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Dev Cogn Neurosci. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Somerville LH. Beyond simple models of adolescence to an integrated circuit-based account: A commentary. Dev Cogn Neurosci. 2016;17:128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Levita L, Libby V, Pattwell SS, Ruberry EJ, Somerville LH. The storm and stress of adolescence: insights from human imaging and mouse genetics. Dev Psychobiol. 2010;52(3):225–235. doi: 10.1002/dev.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Pattwell SS, Glatt CE, Lee FS. Treating the developing brain: implications from human imaging and mouse genetics. Annu Rev Med. 2013;64:427–439. doi: 10.1146/annurev-med-052611-130408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YT, Ernst M, Fudge JL. Cortico-amygdala-striatal circuits are organized as hierarchical subsystems through the primate amygdala. J Neurosci. 2013;33(35):14017–14030. doi: 10.1523/JNEUROSCI.0170-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Scott Steele J, Smitherman S, Lenow JK, Kilts CD. Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: a network-level analysis among adolescent girls. Psychiatry Res. 2013;214(3):238–246. doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;13(6):669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell RA, Cicchetti D, Rogosch FA, Toth SL. Childhood maltreatment and its effect on neurocognitive functioning: Timing and chronicity matter. Dev Psychopathol. 2015;27(2):521–533. doi: 10.1017/S0954579415000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Gunnar M. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Development and Psychopathology. 2009;21(1):1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hooper SR. Neural substrates for processing task-irrelevant emotional distracters in maltreated adolescents with depressive disorders: a pilot study. J Trauma Stress. 2012;25(2):198–202. doi: 10.1002/jts.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cereb Cortex. 2012;22(1):209–220. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dismukes AR, Shirtcliff EA, Hanson JL, Pollak SD. Context influences the interplay of endocrine axes across the day. Dev Psychobiol. 2015;57(6):731–741. doi: 10.1002/dev.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do K, Galván A. FDA cigarette warning labels lower craving and elicit frontoinsular activation in adolescent smokers. Social Cognitive and Affective Neuroscience. 2015;10(11):1484–1496. doi: 10.1093/scan/nsv038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn L, Dahl RE, Woodward H, Biro F. Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Dougherty LR, Blankenship SL, Spechler PA, Padmala S, Pessoa L. An fMRI Pilot Study of Cognitive Reappraisal in Children: Divergent Effects on Brain and Behavior. J Psychopathol Behav Assess. 2015;37(4):634–644. doi: 10.1007/s10862-015-9492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir Y, Ford JD, Hill M, Frazier JA. Childhood maltreatment, emotional dysregulation, and psychiatric comorbidities. Harv Rev Psychiatry. 2014;22(3):149–161. doi: 10.1097/HRP.0000000000000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JS, Midgley C, Wigfield C, Buchanan C, Reuman D, Flanagan C, DM I. Development during adolescence: The impact of stage-environment fit on young adolescents' experiences in schools and families. American Psychologist. 1993;48:90–101. doi: 10.1037//0003-066x.48.2.90. [DOI] [PubMed] [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25(4):1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM, Jovanovic T. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex. 2015;64:249–259. doi: 10.1016/j.cortex.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Gabard-Durnam L, Goff B, Flannery J, Gee DG, Lumian DS, Tottenham N. Normative development of ventral striatal resting state connectivity in humans. Neuroimage. 2015;118:422–437. doi: 10.1016/j.neuroimage.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo C, Tobler P, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. Neuroimage. 2014;95C:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A. The Teenage Brain: Sensitivity to Rewards. Current Directions in Psychological Science. 2013;22:88–93. doi: 10.1177/0963721413476512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, Hare T, Parra C, Penn J, Voss H, Glover G, Casey B. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván A, McGlennen K. Daily stress increases risky decision-making in adolescents: a preliminary study. Developmental Psychobiology. 2012;54:433–440. doi: 10.1002/dev.20602. [DOI] [PubMed] [Google Scholar]
- Galván A, McGlennen K. Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. Journal of Cognitive Neuroscience. 2013;25:284–296. doi: 10.1162/jocn_a_00326. [DOI] [PubMed] [Google Scholar]
- Ganzel BL, Kim P, Gilmore H, Tottenham N, Temple E. Stress and the healthy adolescent brain: evidence for the neural embedding of life events. Dev Psychopathol. 2013;25(4 Pt 1):879–889. doi: 10.1017/S0954579413000242. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety. 2012;29(5):449–459. doi: 10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Casey BJ. The Impact of Developmental Timing for Stress and Recovery. Neurobiol Stress. 2015;1:184–194. doi: 10.1016/j.ynstr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. doi: 33/10/4584 [pii] 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4-18 years. Journal of Comparative Neurology. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Shen D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cereb Cortex. 2012;22(11):2478–2485. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings AL, Mills KL, Clasen LS, Giedd JN, Viner RM, Blakemore SJ. The influence of puberty on subcortical brain development. Neuroimage. 2014;88:242–251. doi: 10.1016/j.neuroimage.2013.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.12.010. doi: S0306-4522(12)01191-8 [pii] 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Tottenham N. Early-life adversity and adolescent depression: mechanisms involving the ventral striatum. CNS Spectr. 2015;20(4):337–345. doi: 10.1017/S1092852914000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Gasser PJ. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci. 2013;33(29):11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev Psychopathol. 2009;21(1):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, Masten CL, Jazbec S, Pine DS, Ernst M. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1059–1067. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Ernst M. A developmental examination of amygdala response to facial expressions. J Cogn Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S. Neuroanatomy of reward: A view from the ventral striatum. In: Gottfried J, editor. Neurobiology of Sensation and Reward. Boca Raton FL: CRC Press; 2011. [PubMed] [Google Scholar]
- Hand L, Furman W. Rewards and costs in adolescent other-sex friendships: Comparison to same-sex friendships and romantic relationships. Social Development. 2009;18:270–287. [Google Scholar]
- Hanson JL, Albert D, Iselin AM, Carre JM, Dodge KA, Hariri AR. Cumulative stress in childhood is associated with blunted reward-related brain activity in adulthood. Soc Cogn Affect Neurosci. 2016;11(3):405–412. doi: 10.1093/scan/nsv124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, Williamson DE. Blunted Ventral Striatum Development in Adolescence Reflects Emotional Neglect and Predicts Depressive Symptoms. Biol Psychiatry. 2015;78(9):598–605. doi: 10.1016/j.biopsych.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57(6):624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. doi: S0006-3223(08)00359-4 [pii] 10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joels M, Fernandez G. Time-dependent effects of corticosteroids on human amygdala processing. J Neurosci. 2010;30(38):12725–12732. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy J, Cohen M, Rosen A. Adrenocortical influences upon the extinction of an appetitive runway response. Physiology and Behavior. 1973;11:767–770. doi: 10.1016/0031-9384(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ, Essex MJ. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A. 2013;110(47):19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev Sci. 2015;18(2):281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Lee SS, Telzer EH, Gabard-Durnam LJ, Goff B, Flannery J, Tottenham N. Exploration-exploitation strategy is dependent on early experience. Dev Psychobiol. 2015;57(3):313–321. doi: 10.1002/dev.21293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz-Kessler C, Poeschl J, Hertel R, Unsicker K, Schenkel J. Effects of a new postnatal stress model on monoaminergic neurotransmitters in rat brains. Brain and Development. 2012;34:274–279. doi: 10.1016/j.braindev.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Janis I. Decision-making under stress. In: Goldberger L, Breznitz S, editors. Handbook of stress: Theoretical and clnical aspects. 2nd. New York: Free Press; 1993. pp. 56–74. [Google Scholar]
- Jezierski G, Zehle S, Bock J, Braun K, Gruss M. Early stress and chronic methylphenidate cross-sensitize dopaminergic responses in the adolescent medial prefrontal cortex and nucleus accumbens. Journal of Neurochemistry. 2007;103:2234–2244. doi: 10.1111/j.1471-4159.2007.04927.x. [DOI] [PubMed] [Google Scholar]
- Johnson CM, Loucks A, Peckler H, Thomas A, Janak P, Wilbrecht L. Long-range orbitofrontal and amygdala axons show divergent patterns of maturation in the frontal cortex across adolescence. Developmental Cognitive Neuroscience. 2016;18:113–120. doi: 10.1016/j.dcn.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Research. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- Keinan G. Decision making under stress: scanning of alternative under controllable and uncontrollable threats. Journal of Personality and Social Psychology. 1987;52:639–644. doi: 10.1037//0022-3514.52.3.639. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. doi: 62/6/593 [pii] 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21(7):1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223(2):403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings P, Granger D, Usher B, Zahn-Waxler C. Adrenocrotical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Developmental psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Knutsson U, Dahlgren J, Marcus C, Rosberg S, Bronnegard M, Stierna P, et al. Circadian cortisol rhythms in healthy boys and girls: Relationship with age, growth, body composition, and pubertal development. Journal of Clinical Endocrinology and Metabolism. 1997;82:536–540. doi: 10.1210/jcem.82.2.3769. [DOI] [PubMed] [Google Scholar]
- Kogler I, Muller VI, Chang A, Eickoff SB, Fox PT, Gur RC, Derntl B. Psychosocial vs physiological stress--Meta-analyses on deactivations and activations of the neural correlates of the stress response. Neuroimage. 2015;119:235–251. doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak MJ, Cuthbert BN. The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics. Psychophysiology. 2016;53(3):286–297. doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- Kreek M, Koob G. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Kuttler A, LaGreca A. Linkages among adolescent girls' romantic relationships, best friendships, and peer networks. Journal of Adolescence. 2004;27:395–414. doi: 10.1016/j.adolescence.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Laursen B, Collins W. Interpersonal conflict during adolescence. Psychological Bulletin. 1994;115:197–209. doi: 10.1037/0033-2909.115.2.197. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ. Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage. 2012;62(3):1575–1581. doi: 10.1016/j.neuroimage.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J Exp Psychol Appl. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44(3):1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nat Neurosci. 2011;14(10):1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson M, Niogi S, Ulug A, Casey B. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Loman MM, Johnson AE, Quevedo K, Lafavor TL, Gunnar MR. Risk-taking and sensation-seeking propensity in postinstitutionalized early adolescents. J Child Psychol Psychiatry. 2014;55(10):1145–1152. doi: 10.1111/jcpp.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. doi: nrn2639 [pii] 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lyons D, Lopez J, Yang C, Schatzberg A. Stress-level cortisol treatment impairs inhibitory control of behavior in monkeys. Journal of Neuroscience. 2000;20:7816–7821. doi: 10.1523/JNEUROSCI.20-20-07816.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn Affect Behav Neurosci. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S, Szalkowski A, Kamphausen S, Perlov E, Feige B, Blechert J, Tuscher O. Clarifying the role of the rostral dmPFC/dACC in fear/anxiety: learning, appraisal or expression? PLoS One. 2012;7(11):e50120. doi: 10.1371/journal.pone.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci U S A. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40(5):1250–1258. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Lighthall N. Both risk and reward are processed differently in decisions made under stress. Current Directions in Psychological Science. 2012;21:36–41. doi: 10.1177/0963721411429452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAndrew F, Akande A, Turner S, Sharma Y. A cross-cultural ranking of stressful life events in Germany, India, South Africa, and the United States. Journal of Cross-Cultural Psychology. 1998;29:717–727. [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, Viding E. Amygdala activation in maltreated children during pre-attentive emotional processing. Br J Psychiatry. 2013;202(4):269–276. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, Viding E. Heightened neural reactivity to threat in child victims of family violence. Curr Biol. 2011;21(23):R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan MA. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress Anxiety. 2014;31(10):834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child Maltreatment and Neural Systems Underlying Emotion Regulation. J Am Acad Child Adolesc Psychiatry. 2015;54(9):753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SC, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci. 2010;22(10):2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Micco D, McEwen B, Shein W. Modulation of behavioral inhibition in appetitive extinction following manipulation of adrenal steroids in rats: implications for the involvement of the hippocampus. Journal of Comparitive Physiology and Psychology. 1979;93:323–329. doi: 10.1037/h0077560. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. doi: 10.1038/nature01138 nature01138 [pii] [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Dev Psychopathol. 2008;20(4):1231–1250. doi: 10.1017/S095457940800059X. doi: S095457940800059X [pii] 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int J Dev Neurosci. 2004;22(5–6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. doi: 10.1016/j.ijdevneu.2004.05.011 S0736574804000668 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11(11):489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehyroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;31:664–679. doi: 10.1016/s0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Nieder T, Seiffge-Krenke I. Coping with stress in different phases of romantic development. Journal of Adolescence. 2001;24:297–311. doi: 10.1006/jado.2001.0407. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Sex differences in unipolar depression: evidence and theory. Psychol Bull. 1987;101(2):259–282. [PubMed] [Google Scholar]
- Nooner KB, Mennes M, Brown S, Castellanos FX, Leventhal B, Milham MP, Colcombe SJ. Relationship of trauma symptoms to amygdala-based functional brain changes in adolescents. J Trauma Stress. 2013;26(6):784–787. doi: 10.1002/jts.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Oei NY, Veer IM, Wolf OT, Spinhoven P, Rombouts SA, Elzinga BM. Stress shifts brain activation towards ventral 'affective' areas during emotional distraction. Soc Cogn Affect Neurosci. 2012;7(4):403–412. doi: 10.1093/scan/nsr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, Belden AC, Barch DM. Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J Abnorm Psychol. 2015;124(4):817–833. doi: 10.1037/abn0000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439(7078):865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Lee FS. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun. 2016;7:11475. doi: 10.1038/ncomms11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87(6):532–552. [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SB, Pelphrey KA. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J Exp Child Psychol. 2011;108(3):607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persike M, Seiffge-Krenke I. Competence in coping with stress in adolescents from three regions of the world. Journal of Youth and Adolescence. 2012;41:863–879. doi: 10.1007/s10964-011-9719-6. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phuong J, Galván A. Acute Stress Increases Risky Decisions and Dampens Prefrontal Activation Among Adolescent Boys. Neuroimage. doi: 10.1016/j.neuroimage.2016.08.067. (in press) [DOI] [PubMed] [Google Scholar]
- Phuong J, Galván A. Sleep Loss Amplifies the Relationship Between Stress and Neural Correlates of Risky Decision Making. (under review) [Google Scholar]
- Porcelli A, Delgado MR. Acute stress modulates risk taking in financial decision-making. Psychological Science. 2009;20:278–283. doi: 10.1111/j.1467-9280.2009.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston S, Buchanan T, Stansfield R, Bechara A. Effects of anticipatory stress on decision making in a gambling task. Behavioral Neuroscience. 2007;121:257–262. doi: 10.1037/0735-7044.121.2.257. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Champagne F, Meaney M, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [C-11] raclopride. Journal of Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and magnetic resonance imaging studies. Biol. Psychiatry. 2008;63(2):234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Putman P, Antypa N, Crysovergi P, van der Does W. Expogenous cortisol acutely influence motivated decision making in healthy young men. Psychopharmacology (Berl) 2010;208:257–263. doi: 10.1007/s00213-009-1725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Johnson A, Loman M, Lafavor T, Gunnar M. The Confluence of Adverse Early Experience and Puberty on the Cortisol Awakening Response. Int J Behav Dev. 2012;36(1):19–28. doi: 10.1177/0165025411406860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley J, Sisti H, Hao J, Rocher A, McCall T, Hof P, McEwen BS. Chronic behavioral stress induces apical dendritic reorganization. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rahdar A, Galván A. The cognitive and neurobiological effects of daily stress in adolescents. Neuroimage. 2014;15:267–273. doi: 10.1016/j.neuroimage.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART) Neuroimage. 2008;42(2):902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31(2):232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Patel R, Pham L, So VM. Adolescence and the ontogeny of the hormonal stress response in male and female rats and mice. Neurosci Biobehav Reviews. doi: 10.1016/j.neubiorev.2016.05.020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B. Stress and memory: Opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiology of Learning and Memory. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- Rosenberg D, Lewis D. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. Journal of Comparitive Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Deroche V, Le Moal M, Piazza P. Individual differences in stress-induced dopamine release in the nucleus accumbens are influencedy by corticosterone. European Journal of Neuroscience. 1998;10:3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka R. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sandi C. Stress and Cognition. Cognitive Science. 2013;4:245–261. doi: 10.1002/wcs.1222. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Smyth JM, Delgado MR. The amygdala: an agent of change in adolescent neural networks. Horm Behav. 2013;64(2):298–313. doi: 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel A. The global spread of adolescent culture. In: Crocket L, Silbereisen R, editors. Negotiating adolescence in times of social change. New York, NY: Cambridge University Press; 2001. pp. 63–86. [Google Scholar]
- Schneider S, Brassen S, Bromberg U, Banaschewski T, Conrod P, Flor H, Consortium I. Maternal interpersonal affiliation is associated with adolescents' brain structure and reward processing. Transl Psychiatry. 2012;2:e182. doi: 10.1038/tp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz U, LaMarca R, UM N, Aberle I, Ehlert U, Hornung R, Kliegel M. Go no-go performance under psychological stress: Beneficial effects of implementation intention. Neurobiology of Learning and Memory. 2009;91:89–92. doi: 10.1016/j.nlm.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Scott D, Heitzeg M, Koeppe R, Stohler C, Zubieta J. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. Journal of Neuroscience. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, Luthi A. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 2014;81(2):428–437. doi: 10.1016/j.neuron.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, McLaughlin KA. Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci. 2014;18(11):580–585. doi: 10.1016/j.tics.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvagni A, Barros V, Mura C, Antonelli M, Carboni E. Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: an 'in vivo' microdialysis study in adolescent and young adult rats. European Journal of Neuroscience. 2008;28:744–758. doi: 10.1111/j.1460-9568.2008.06364.x. [DOI] [PubMed] [Google Scholar]
- Silvers JA, Lumian DS, Gabard-Durnam L, Gee DG, Goff B, Fareri DS, Tottenham N. Previous Institutionalization Is Followed by Broader Amygdala-Hippocampal-PFC Network Connectivity during Aversive Learning in Human Development. J Neurosci. 2016;36(24):6420–6430. doi: 10.1523/JNEUROSCI.0038-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Shu J, Hubbard AD, Weber J, Ochsner KN. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev Sci. 2014 doi: 10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smetana J, Campione-Barr N, Metzger A. Adolescent development in interpersonal and societal contexts. Annual Review of Psychology. 2006;57:255–284. doi: 10.1146/annurev.psych.57.102904.190124. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Current Opinion in Neurobiology. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain Cogn. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, van den Bulk BG, Skwara AC. Response to: "the triadic model perspective for the study of adolescent motivated behavior". Brain Cogn. 2014;89:112–113. doi: 10.1016/j.bandc.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Spear L. Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology. 2009;21(1):87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K, Brand M. Decision making under stress: a selective review. Neuroscience and Biobehavioral Reviews. 2012;36:1228–1248. doi: 10.1016/j.neubiorev.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9(2):69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Stroud L, Papandonatos G, Williamson D, Dahl R. Sex differences in the effects of pubertal development on responses to a corticotropin-releasing hormone challenge: The Pittsburgh psychobiologic studies. Annals of the New York Academy of Sciences. 2004;1021:348–351. doi: 10.1196/annals.1308.043. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475(7356):377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage. 2014;86:212–220. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85(3):505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, Hariri AR. Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. Am J Psychiatry. 2015;172(3):276–283. doi: 10.1176/appi.ajp.2014.14020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E, Fuligni A, Lieberman M, Galván A. Meaningful family relationships: neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience. 2013;25:374–387. doi: 10.1162/jocn_a_00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Marusak HA, Tocco MA, Vila AM, McGarragle O, Rosenberg DR. Altered amygdala connectivity in urban youth exposed to trauma. Soc Cogn Affect Neurosci. 2015;10(11):1460–1468. doi: 10.1093/scan/nsv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JL, Nelson AJ. Middle childhood and modern human origins. Human Nature. 2011;22(3):249–280. doi: 10.1007/s12110-011-9119-3. [DOI] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species-expected caregiving. Dev Psychobiol. 2012;54(6):598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. The importance of early experiences for neuro-affective development. Curr Top Behav Neurosci. 2014;16:109–129. doi: 10.1007/7854_2013_254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated Amygdala Response to Faces Following Early Deprivation. Developmental Science. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry T, Nurse M, Gilhooly T, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and emotion regulation difficulties. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan M. A Review of adversity, the amygdala and the hippocampus: A Consideration of developmental timing. Frontiers in Human Neuroscience. 2010;3:1–18. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Li XY, Wu LJ, Zhao MG, Descalzi G, Chen T, Zhuo M. Interplay of amygdala and cingulate plasticity in emotional fear. Neural Plast. 2011;2011:813749. doi: 10.1155/2011/813749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S, McDermott JM, Nelson CA, Zeanah CH, Fox NA. The effects of early foster care intervention on attention biases in previously institutionalized children in Romania. Dev Sci. 2015;18(5):713–722. doi: 10.1111/desc.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7(10):e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless M, Argilli E, Bonci A. Effects of stress and aversion on dopamine neurons: Implications for addiction. Neuroscience and Biobehavioral Reviews. 2010;35:151–156. doi: 10.1016/j.neubiorev.2010.04.006. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L, Zanolie K, van Meel C, Westenberg P, Rombouts S, Crone E. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescents. Cerebral Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. Neuroimage. 2010;53(1):348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Bailey C, Dent GW, Okimoto DK, Steffek A, Lopez JF, Levine S. Brain corticotropin-releasing hormone (CRH) circuits in the developing rat: effect of maternal deprivation. Brain Res. 2006;1121(1):83–94. doi: 10.1016/j.brainres.2006.08.104. doi: S0006-8993(06)02638-2 [pii] 10.1016/j.brainres.2006.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Wand G, Oswald L, McCaul M, Wong D, Johnson E, Zhou Y, Kumar A. Association of amphetamine-induced striatal dopamine release and cortisol responses to psychological stress. Neuropsychopharmacology. 2007;32:2310–2320. doi: 10.1038/sj.npp.1301373. [DOI] [PubMed] [Google Scholar]
- Widom CS, Sherpard RL. Accuracy of Adult Recollections of Childhood Victimization: Part 1. Childhood Physical Abuse. Psychol Assess. 1996;8(4):412–421. [Google Scholar]
- Wolf O. Effects of stress hormones on the structure and function of the human brain. Expert Review of Endocrinology Metabolism. 2006;1:623–632. doi: 10.1586/17446651.1.5.623. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Herringa RJ. Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology. 2016;41(3):822–831. doi: 10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright L, Muir K, Perrot T. Enhanced stress responses in adolescent versus adult rats exposed to cues of predation threat, and peer interaction as a predictor of adult defensiveness. Developmental Psychobiology. 2012;54:47–69. doi: 10.1002/dev.20575. [DOI] [PubMed] [Google Scholar]