Abstract
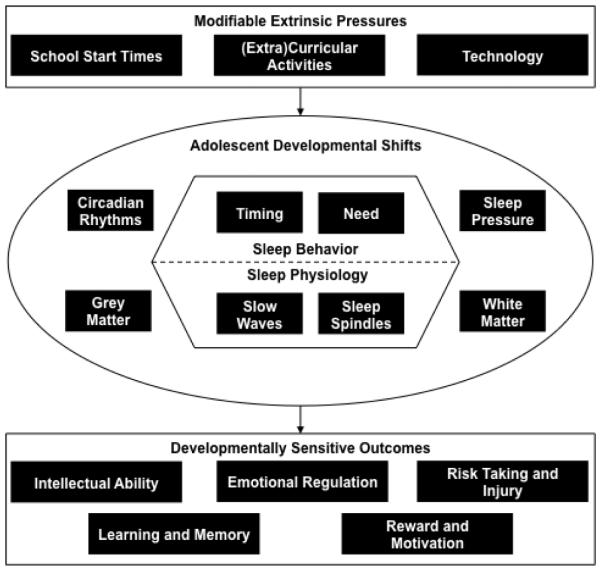
Sleep is a core behavior of adolescents, consuming up to a third or more of each day. As part of this special issue on the adolescent brain, we review changes to sleep behaviors and sleep physiology during adolescence with a particular focus on the sleeping brain. We posit that brain activity during sleep may provide a unique window onto adolescent cortical maturation and compliment waking measures. In addition, we review how sleep actively supports waking cognitive functioning in adolescence. Though this review is focused on sleep in healthy adolescents, the striking comorbidity of sleep disruption with nearly all psychiatric and developmental disorders (for reviews see 1,2) further highlights the importance of understanding the determinants and consequences of adolescent sleep for the developing brain. Figure 1 illustrates the overarching themes of our review, linking brain development, sleep development, and behavioral outcomes.
Adolescent Sleep Behavior
Adolescence is a time of increased independence and emergence of new social roles, all of which affect behavior: sleep is no exception. Driven in part by this newly acquired autonomy, combined with delays in the circadian timing system (reviewed in T. Lee chapter) and changes to the homeostatic sleep regulating system that provide greater tolerance for sleep pressure 3, bedtimes become later with each passing year during adolescence. Rise times, by contrast, are more often determined by school start times and thus remain unchanged or move earlier 4. Whether using self-report 4 or objectively recorded sleep 5, studies show that US teens lose about 90 minutes of sleep each school night from grade 6 (about 11-12 years old) to grade 12 (about 17-18 years old). With both approaches, the average school-night total sleep time for the youngest adolescents was about 8.4 h and about 6.9 h in the high school seniors. A more recent report from the Center for Disease Control using data from the Youth Behavior Risk Surveillance Data from 2007, 2009, 2011, and 2014 (N = 50,370 US students) found that two thirds of students in grades 9 to 12 reported 7 h or less sleep on school nights 6. Trends are similar in other countries and circumstances appear worst for adolescents living in Southeast Asia. Yang et al. in 2005 7, for example, showed that teens’ reported school-night bedtimes progressively later than in the US from grades 5/6 (10:42 pm ± 78 m) to grades 11/12 (12:54 am ± 84 m) and that nightly total sleep time for school nights was nearly 3 h less in the older versus younger adolescents: 8 h 18 m vs. 5 h 24 min, much shorter in year 12 than in the US.
Despite the dwindling time spent asleep, studies suggest that sleep “need”,per se does not undergo dramatic changes during adolescence. An early longitudinal study, following adolescents yearly from 10-12 till 15-18 years of age found that when given ten hours of sleep-opportunity, adolescents slept an average of approximately 9.25 hours irrespective of age or maturational stage. Further evidence for the stability of sleep need comes from Ohayon and colleagues 8, indicating a decline in sleep duration on school days, but no change on non-school days, leading the authors to conclude that the school day decline is driven by environmental rather than biological factors. Given the pervasive discrepancy between sleep need and sleep obtained for most teens, understanding the consequences of chronic insufficient sleep is paramount. For example, a laboratory study in which 10th graders slept on a self-selected school night schedule, found that during a morning nap opportunity (08:30, roughly equivalent to the first or second class-period of American high schools), participants fell asleep in approximately 5 minutes, nearly half the time the same participants took to fall asleep later in the day. About 50% of the sample fell asleep in less than 2 minutes and directly into REM sleep. This study indicates that these 10th graders may in fact suffer from pathological sleepiness during the start of the school day, perhaps a result of a simultaneous delay in their circadian rhythms and the abridgement of their sleep opportunity by earlier school-start times 9. Thus, starting the school day sleepy and unprepared for the cognitive and social challenges of adolescence is quotidian for many teens.
Measurement and Key Principles of Adolescent Sleep
Sleep can be measured in a variety of ways, including self-reports, actigraphy, and polysomnography (PSG). Self-reports are useful for assessing perceived sleep difficulties and daytime functioning and are, for example, part of the diagnostic criteria for certain psychiatric illnesses 10. Several specific self-report sleep scales have been developed, including one focused on assessing the chronic sleep reduction of adolescence 11. Actigraphy usually involves wearing a small wrist-worn watch-like device that can delineate sleep and waking based on motion; such devices can provide measures of sleep in broad strokes (e.g., sleep duration, nocturnal arousals) and can be used to assess sleep over long periods of time (e.g., several weeks or months). Research-grade activity monitors have validated and open-sourced algorithms to estimate sleep, whereas newer commercially available activity monitors often provide summary measures of sleep and daytime physical activity using proprietary software inaccessible to the user. Validation studies of such devices are limited and have provided mixed results for specificity and sensitivity (e.g., 12,13). Although actigraphy and self-reports are important tools, the gold standard for quantifying sleep, is polysomnography (PSG), which requires continuous measurement of electroencephalogram (EEG), electrooculogram (EOG), and electromyogram (EMG). The combination of these physiological signals is used to divide sleep into two states: non rapid eye movement (NREM) sleep (which is further subdivided into 3 or 4 stages) and REM sleep.
The EEG signal also provides access to several cortical oscillations observed only in the sleeping brain. Two such oscillations that occur in NREM sleep are the subject of much study: slow waves and sleep spindles. Slow waves are low frequency (0.4 to 4.6 Hz), high amplitude oscillations generated primarily in the cortex, although the thalamus has been suggested to play a role in modulating these oscillations 14. These slow waves are plentiful at the beginning of the night and show an exponential decline over the course of sleep (reviewed in 15). Furthermore, slow waves show an increase in incidence and amplitude following sleep deprivation 15. The preferential occurrence of slow waves at the beginning of sleep and the increase when sleep deprived highlight the proposed role of these oscillations as markers of the sleep homeostatic system 16. Unlike slow waves, which dominate sleep EEG activity for several hours in the healthy adolescent brain, sleep spindles are transient (1-2 seconds) oscillations with a frequency between 11 and 16 Hz. Sleep spindles are generated through thalamocortical loops (see 17) and functional roles in sleep consolidation and declarative memory systems have been attributed to this activity (reviewed in 18).
The magnitude of these and other EEG oscillations can be calculated from EEG signals using the Fourier transform, which computes the strength of the EEG’s constituent frequencies. Therefore, EEG slow wave activity (SWA) is often defined as total power (μV2) in the 0.4 - 4.6 Hz frequency range. Spindles are similarly summarized by total EEG power in the 11 to 16 Hz range. Because the spectral frequency of spindles varies from person to person and across development, the frequency of the peak power in this band is sometimes used as a measure of spindle activity sensitivity to inter-individual variation.
Sleep Physiology in Adolescence
In addition to the developmental shifts that occur in circadian rhythms and sleep homeostasis during development, clear maturational changes are observed in the oscillatory physiology outlined above. These trajectories are likely driven by maturational modifications to brain anatomy during this time. The most striking change to the sleep EEG is a marked reduction in the EEG amplitude and power of the sleep EEG signal, which occurs earlier for girls than boys and is in part tied to pubertal maturation 19. This reduction in EEG power of up to 40% from pre- to post-puberty is seen across EEG frequencies, within both waking and sleep states19-22. This reduction is likely driven by significant declines in cortical grey matter which take place during adolescence. Direct support for this association comes from one study that measured both grey matter (i.e., structural MRI) and sleep EEG power in participants ages 8 to 19 years and found correlations between these measures over a wide range of cortical regions 23. Furthermore, both measures manifested an age-dependent decline, further supporting the hypothesis that the decline in sleep EEG power is driven by reductions in grey matter volume. A separate study that used high-density EEG to measure cortical activity at a large number of cortical regions found a developmental progression of maximal sleep slow wave activity (spectral sleep EEG power between 0.6 to 4.6 Hz; SWA) from posterior to anterior cortical regions 24. This progression is similar to observations from longitudinal MRI studies regarding regional maximal cortical grey matter volume 25.
Another developmental change manifest in the adolescent brain is an increase in white matter volume. Although direct evidence for an association between white matter volume and measures of the sleep EEG is lacking, one EEG measure of connectivity—sleeping EEG coherence— showed a linear increase in a study of adolescents similar to adolescent changes in white matter volume 26. As with the decline in sleep EEG power, this increase in coherence is found across frequencies and sleep states, indicating an anatomical substrate. Furthermore, the peak spectral frequency of sleep spindles also shows a linear increase across adolescence 20,21,27,28. Again, although direct evidence is lacking, we have hypothesized that this sleep spindle frequency increase reflects a measure of cortical myelination 29. Interestingly, the rate of change in sleep EEG power and coherence are not correlated, suggesting separate processes 30.
Sleep: An Active Role in Brain Development?
Sleep is not only an opportunity to measure otherwise unperturbed brain activity, but recent studies also suggest that sleep itself may play an active role in sculpting the adolescent brain. Using two-photon microscopy in adolescent mice, for example, Maret and colleagues found that synaptic spine elimination was higher during sleep than during waking in adolescent but not adult mice, suggesting a distinctive role for sleep in the adolescent brain 31. Correlational studies in humans have also found associations between sleep behavior and brain development. One such study examined structural MRI scans in 290 children and adolescents between the ages of 5 and 18 years and found that self-reported sleep duration was positively correlated with bilateral hippocampal grey matter volume 32. Another study found an association in adolescents between variability in sleep duration across fourteen days and white mater integrity as measured with diffusion tensor MRI 33. Although this line of research is in its nascent stage, evidence for a role of sleep in brain development is emerging.
Sleep Traits and Associations with Cognition
Several studies have examined the association between sleep neurophysiology and stable metrics of cognitive aptitude. The sleep EEG is an attractive target to identify such associations because the spectrum is stable across consecutive nights of recording, while at the same time unique to an individual 34. Furthermore, we have shown that the morphology of the sleep EEG spectrum is largely preserved in adolescents across several years, despite maturational changes in sleep EEG power and cortical restructuring 29. Thus, the sleep EEG spectrum is trait-like over several years, and recent data indicate high heritability of the sleep EEG spectrum, including in adolescence 35. Several studies have examined the association between the trait-like oscillations in the sleep EEG and stable metrics of cognitive ability, such as intelligence quotient (IQ). These studies describe positive correlations between IQ and sleep spindles magnitude (e.g., power, amplitude, and density) in children 36-38 and adolescents 39. Furthermore, a two-year longitudinal study that measured sleep EEG coherence and waking performance on cognitive tasks of executive function and response inhibition in early adolescents found associations between spindles and cognitive performance 30. In this study, adolescents who showed the greatest increases in intra-hemispheric spindle coherence also manifested the most improvements on these tasks. Because sleep spindles are generated through long-range thalamocortical loops, this activity may carry important information about cortical functioning and circuit integrity that support cognitive function.
Sleep and Learning and Memory
While the above evidence focuses on the association between sleep physiology and intelligence as one marker of how sleep may index the stable and healthy development of cognitive systems across adolescence, a separate yet germane line of studies—mostly in adults—indicates that even short manipulation of sleep can modulate cognition function. Both the restorative benefit of sleep and the detrimental impact of sleep loss have been documented in adults using experimental protocols targeting a number of cognitive domains: attention40, executive function41, reward sensitivity42, emotional regulation 43, and learning and memory44. These studies typically use short perturbations in sleep (e.g., a daytime nap, a night of total sleep deprivation, or sleep restriction over several days); thus, they do not directly measure the long-term consequences of truncated sleep on cognition. They do, however, provide an excellent opportunity for experimental probing of the impact of sleep loss during sensitive developmental windows such as adolescence.
Healthy development of cognitive systems during this phase of life is paramount to intellectual pursuits. Despite the ecological importance of cognitive function during adolescence, a majority of studies investigating sleep-dependent cognition in experimental settings have focused on adults, with a recent emergence of such work in young children. A paucity of such studies exists in adolescents. Thus, whether the adolescent brain is as sensitive to sleep and sleep loss as the adult brain or more sensitive remains poorly understood.
Of the domains of cognition for which sleep is critically involved, perhaps the largest body of research has focused on learning and memory—both for declarative, episodic memories, and non-declarative procedural skills (see 44-46 for reviews). Such studies typically examine either memory consolidation, i.e., retrieval of items learned prior to either sleep or wakefulness, or memory encoding, i.e., the impact of prior sleep on the formation of new memory traces. With respect to memory consolidation, preliminary data from adolescent samples (e.g., 9-16 years) suggests that at least for declarative memories, post-sleep improvement is present as in adults 47-49. While these preliminary results are encouraging, a number of limitations warrant pause. The lack of a direct adult comparison group limits these studies from directly addressing whether the benefit of sleep for memory is systematically different in adolescents and adults. Moreover, the absence of developmental measures (e.g., puberty, hormonal measures, and prospective longitudinal designs) limit these studies from revealing potentially interesting developmental trajectories by which sleep-dependent memory consolidation emerges early in life. Finally, relatively small (e.g., 20 participants) sample sizes combined with the modest effect sizes restrict conclusions about the role of sleep in learning premature. Nevertheless, an exciting new line of research indicates that during adolescent development healthy sleep may aid learning.
In contrast to a beneficial role of sleep in consolidating memories, sleep deprivation— both partial50 and total51,52—impairs the both the initial formation and subsequent consolidation of memories in adults44. Even when multiple nights of partial sleep deprivation are employed, however, adolescent verbal memory appears relatively unaffected53-55, in both younger (e.g., 11-13 years55) and older (e.g., 14-16 years54) cohorts. These studies have proposed the presence of a neural compensation in this young age group. One recent fMRI56 study compared performance on an N-back working memory task in adolescents (mean age = 15 years) who were either sleep restricted (6.5 hours per night) or not (10 hour time-in-bed per night) for one week. Like adults in other similar paradigms57, performance of the sleep-restricted adolescents did not suffer and demonstrated hyperactivity in frontal cortical regions56, which was hypothesized to compensate for the effects of sleep loss, and thus conserve performance on such simple cognitive tasks. Another explanation is that the conserved sleep is sufficient to support cognitive performance. Indeed, in one of the studies noted above, verbal memory performance following sleep restriction was positively correlated with the amount of NREM sleep in the shortened night54. Moreover, preliminary evidence from adults has demonstrated that heightened slow wave activity following sleep deprivation positively indexes a restoration of declarative memory to pre sleep-deprived levels58. It is intriguing, therefore, to consider whether the greater sleep EEG slow wave activity in adolescents as compared to adults may beneficially rescue the ability to learn following small doses of sleep restriction. Ultimately, more studies examining the interplay between sleep loss and cognitive function in adolescents are needed to examine this possibility.
Sleep-dependent Emotion Regulation
Beyond learning and memory, one area receiving much recent attention in the adult sleep literature is sleep-dependent emotional regulation43. Sleep loss is associated both with increased negative mood and heightened emotional reactivity to visual scenes and faces 43, as well as altered emotional memory processing59,60. Thus, sleep loss has been proposed to exert a modulatory influence on the onset of such psychiatric conditions as anxiety and major depression61. Preliminary evidence suggests that sleep loss also negatively affects mood and emotional regulation in adolescents, both following chronic62, and acute63 doses of sleep restriction.
Importantly, the studies outlined above each used experimental manipulation of sleep over a night or two, rather than ecologically valid conditions in childhood and adolescence where sleep loss occurs over a long interval. Studies in adolescent obstructive sleep apnea, for example, a condition that disrupts sleep, reveal impaired learning and memory 64 together with affective and reward processing 65. Moreover, placing the totality of this work in an ecological context, chronic sleep problems early in childhood are associated with heightened risk-taking later in adolescence66. This latter study 66 employed a longitudinal design to identify that heightened risk taking emerges in later adolescence by way of a sleep-mediated deficit in working memory. Thus, while the majority of studies in this review describe children who sleep normally, these examples suggest that such relationships may have real-life consequence for the more pernicious forms of sleep fragmentation and sleep reduction observed chronically in some conditions.
Understanding whether adolescents demonstrate partial resilience of cognitive and affective processes to sleep loss in experimental conditions, therefore, remains an important mechanistic question at both the basic science level and clinical level. At a practical level, the chronic pervasive insufficient sleep seen in teens, and outlined in above sections67,4,7, has a yet unknown impact on the critical cognitive- and emotion-regulating functions outlined here. Moreover, beyond healthy children, it is worth noting the high comorbidity of sleep problems within adolescent psychopathology. For example, PSG recordings in 106 psychiatric inpatients ages of 7 to 16 years, showed that 95% have moderate to severe sleep problems 68. Similar sleep disruption is are seen in a number specific patient populations, including: depression69, ADHD70, impulse control disorders 71, anxiety 72, and bipolar disorder 73, indicating a key role of sleep in adolescent mental health. While beyond the scope of this review, the mechanisms by which psychiatric conditions may moderate (or be moderated by) the developmental trajectories of sleep, brain development, and cognition outlined above, remain poorly understood and topics of active investigation.
Insufficient Sleep and Behavioral Problems in Teens
When so many adolescents obtain so little sleep 67,4,7, the impact of insufficient sleep on behavior must be catalogued. Many studies show that insufficient sleep is associated with poor emotional functioning in teens without diagnosed psychiatric disorders. For example, in non-clinical samples, less sleep is associated with more depressive symptoms 74, feelings of hopelessness 74 and greater anxiety 75. The risks of short/insufficient sleep in adolescents have also become evident in a number of large epidemiologic studies: Meldrum and Restivo 76 identified increased relative risk for the following behaviors in high school teens (n=15,364) reporting 7, 6, 5, or less than 5 hours a night on school nights: drunk driving, weapon carrying, fighting, contemplated suicide, attempted suicide, smoking, alcohol use, binge drinking, marijuana use, sexual risk taking, and texting while driving. They also reported increased relative risk for obesity with shorter sleep. Wheaton and colleagues 67 summarizing data from over 50,000 US teenagers found that reports of five injury-related risk behaviors were associated with reported school-night sleep length of 7 hours of less. These behaviors were infrequent bicycle helmet use; infrequent seatbelt use; riding with a drinking driver; drinking and driving; and texting while driving.
Other studies have noted that early morning school schedules carry a significant role in the low sleep times of adolescents. When school start times are delayed, sleep is increased, enrollment rates and attendance improve, students sleep less in class, and symptoms of depressed mood are reduced 77, and automobile crash rates in teen drivers are lower 78,79,80,81.
Recent studies are attempting to identify neural mechanisms that underlie findings from experimental study in which adolescents show increased depression, anxiety, vigor and fatigue following sleep deprivation82. That is, how does short or poor sleep affect such emotional processes in adolescents? One longitudinal study, for example, examined insomnia symptoms in early adolescent girls at ages 9 to 13 years and then measured neural reward processing with fMRI several years later 83. This study found that self-reported non-restorative sleep (i.e., reporting feeling unrested upon awaking) at ages 9-13 years was positively associated with the dorsal medial prefrontal cortex (dmPFC) response to reward anticipation and to depressive symptoms several years later. Because of the important role of the dmPFC in affective control, these findings suggest that poor sleep may contribute to depressive affect by disrupting functioning of the dmPFC. Future studies are needed to examine the mechanisms by which adequate sleep may support emotional functioning in adolescents and whether unique processes are at work at this time of life.
Summary: Sleep Matters
To summarize, research overwhelmingly supports an important role for sleep in many areas of adolescent brain function and behavior. What remains largely unknown, is whether sleep supports unique functions in adolescence as compared to children and adults. This gap in our knowledge is due to the limited number of concurrent studies examining given phenomena in children, adolescents and adults. Furthermore, adolescence presents a unique neurodevelopmental milieu and though brain activity during sleep reflects this, it is unknown the degree to which the neuronal benefits of sleep overlap in adolescents and adults. Finally, teens have unique social, cognitive and behavioral demands; does a greater need for sleep during adolescence as compared to adulthood reflect this? For example, a major theory about sleep’s function, the synaptic homeostasis hypothesis, posits that one function of slow wave activity during sleep is the rescaling of synapses after the waking day 84. Therefore, might the greater sleep need and more slow wave activity observed in youth result from greater number of synapses in the teen brain21? Many such questions remain unanswered.
Nonetheless, ensuring well-timed, adequate, and restorative sleep is important for optimal maturation. Fortunately, sleep is a modifiable behavior for many teens and effective interventions exist. At a basic level, practice of good ‘sleep hygiene’ protects sleep 85. Carskadon 86 has suggested the tips listed in Table 1 to help healthy adolescents improve their sleep. In addition, parental limit setting can help: teens whose parents set a bedtime of 10:00 pm or earlier, for example, had fewer depressive symptoms and less suicidal ideation as compared to teens whose parents set a bedtime of midnight or later 87. For youngsters who have more significant sleep problems, recent studies show that sleep-based interventions, such as cognitive behavioral therapy for insomnia (CBT-I), are effective 88,89. In summary, sleep is a potentially important therapeutic target in adolescence with emotional, cognitive, or behavioral problems.
Table 1.
Tips for Teens to Improve Sleep (from 86)
|
Conclusion
Recent and emerging data indicate a key role for sleep in supporting cognitive function and mental well-being in adolescence. Furthermore, sleep and brain development are bidirectionally related – brain maturation is reflected in the sleep EEG and sleep may play a role in shaping the brain. Hence, the chronic insufficient and poorly timed sleep that is endemic amongst adolescents is of concern. Public health interventions targeting sleep can promote sleep during this important developmental period. For example, delaying school start times results in longer weeknight sleep and produces cascading positive effects, such as improved school attendance, less tardiness and better grades (reviewed in 67). Indeed, the American Academy of Pediatrics has endorsed a policy that middle schools and high schools not begin the day until 8:30 am or later 90.
Figure 1. Determinants and Consequences of Adolescent Sleep.
This diagram outlines the central theme of the review: a complex milieu of determinants and consequences of sleep interact during the sensitive window of adolescence. Center: Developmental shifts in sleep behavior (e.g., sleep need and timing) and physiology (e.g., slow waves and sleep spindles) index maturational changes in sleep and circadian regulatory systems emerging along with developmental trajectories of grey and white matter within the brain. Top: Several moderating and mediating extrinsic pressures can alter sleep behavior and physiology beyond intrinsic developmental forces, including school schedules, extra-curricular activities, and technology use. Bottom: Healthy development of sleep during adolescence underlies a variety of functional outcomes. Together this framework highlights sleep during adolescence as a “perfect storm” (Carskadon, 2011): a sensitive time-period where developmental changes, together with (mal)-adaptive environmental forces, may yield powerful consequences for behavior and cognition.
Highlights.
During adolescence, sleep behavior and physiology undergo significant maturation
Changes to the biological systems regulating sleep result in later bedtimes, while rise times, driven by school start times, remain unchanged or move earlier. This results in a pattern of chronic insufficient sleep for many teens.
Sleep in adolescence supports learning, memory, attention, cognition, and emotion processing
Maturational changes to brain structure and function are reflected in the sleep EEG
Sleep may offer a unique opportunity to probe the developing brain
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (AA13252 to MAC) and the National Institute of l Health (T32MH019927 to JMS; PI: Spirito)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tesler N, Gerstenberg M, Huber R. Developmental changes in sleep and their relationships to psychiatric illnesses. Current opinion in psychiatry. 2013;26:572–579. doi: 10.1097/YCO.0b013e328365a335. doi:10.1097/YCO.0b013e328365a335. [DOI] [PubMed] [Google Scholar]
- 2.Kotagal S. Sleep in Neurodevelopmental and Neurodegenerative Disorders. Seminars in pediatric neurology. 2015;22:126–129. doi: 10.1016/j.spen.2015.03.003. doi:10.1016/j.spen.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28:1446–1454. doi: 10.1093/sleep/28.11.1446. [DOI] [PubMed] [Google Scholar]
- 4.Foundation NS. 2006 Sleep In America Poll Summary Findings. 2006 [Google Scholar]
- 5.Crowley SJ, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PloS one. 2014;9:e112199. doi: 10.1371/journal.pone.0112199. doi:10.1371/journal.pone.0112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheaton AG, Olsen EO, Miller GF, Croft JB. Sleep Duration and Injury-Related Risk Behaviors Among High School Students - United States, 2007-2013. MMWR. Morbidity and mortality weekly report. 2016;65:337–341. doi: 10.15585/mmwr.mm6513a1. doi:10.15585/mmwr.mm6513a1. [DOI] [PubMed] [Google Scholar]
- 7.Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115:250–256. doi: 10.1542/peds.2004-0815G. [DOI] [PubMed] [Google Scholar]
- 8.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 9.Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–881. doi: 10.1093/sleep/21.8.871. [DOI] [PubMed] [Google Scholar]
- 10.Association AP. Diagnostic and Statistical Manual of Mental Disorders. (5th) 2013 [Google Scholar]
- 11.Dewald JF, Short MA, Gradisar M, Oort FJ, Meijer AM. The Chronic Sleep Reduction Questionnaire (CSRQ): a cross-cultural comparison and validation in Dutch and Australian adolescents. Journal of sleep research. 2012;21:584–594. doi: 10.1111/j.1365-2869.2012.00999.x. doi:10.1111/j.1365-2869.2012.00999.x. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep & breathing = Schlaf & Atmung. 2012;16:913–917. doi: 10.1007/s11325-011-0585-y. [DOI] [PubMed] [Google Scholar]
- 13.de Zambotti M, Baker FC, Colrain IM. Validation of Sleep-Tracking Technology Compared with Polysomnography in Adolescents. Sleep. 2015;38:1461–1468. doi: 10.5665/sleep.4990. doi:10.5665/sleep.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David F, et al. Essential thalamic contribution to slow waves of natural sleep. J Neurosci. 2013;33:19599–19610. doi: 10.1523/JNEUROSCI.3169-13.2013. doi:10.1523/JNEUROSCI.3169-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achermann P, Borbely AA. In: Principles and Practices of Sleep Medicine. 5th Kryger MH, Roth T, Dement WC, editors. Elsevier; 2011. [Google Scholar]
- 16.Borbély AA. A two process model of sleep regulation. Human neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- 17.Steriade M, Deschenes M, Domich L, Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. Journal of neurophysiology. 1985;54:1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- 18.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neuroscience and biobehavioral reviews. 2012;35:1154–1165. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Campbell IG, Grimm KJ, de Bie E, Feinberg I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5740–5743. doi: 10.1073/pnas.1120860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarokh L, Van Reen E, LeBourgeois M, Seifer R, Carskadon MA. Sleep EEG provides evidence that cortical changes persist into late adolescence. Sleep. 2011;34:1385–1393. doi: 10.5665/SLEEP.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarokh L, Carskadon MA. Developmental changes in the human sleep EEG during early adolescence. Sleep. 2010 doi: 10.1093/sleep/33.6.801. ePUB ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell IG, Feinberg I. Longitudinal trajectories of non-rapid eye movement delta and theta EEG as indicators of adolescent brain maturation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5177–5180. doi: 10.1073/pnas.0812947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchmann A, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21:607–615. doi: 10.1093/cercor/bhq129. [DOI] [PubMed] [Google Scholar]
- 24.Kurth S, et al. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J Neurosci. 2010;30:13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarokh L, Carskadon MA, Achermann P. Developmental changes in brain connectivity assessed using the sleep EEG. Neuroscience. 2010;171:622–634. doi: 10.1016/j.neuroscience.2010.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shinomiya S, Nagata K, Takahashi K, Masumura T. Development of sleep spindles in young children and adolescents. Clinical EEG. 1999;30:39–43. doi: 10.1177/155005949903000203. [DOI] [PubMed] [Google Scholar]
- 28.Campbell IG, Feinberg I. Maturational Patterns of Sigma Frequency Power Across Childhood and Adolescence: A Longitudinal Study. Sleep. 2016;39:193–201. doi: 10.5665/sleep.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarokh L, Carskadon MA, Achermann P. Trait-like characteristics of the sleep EEG across adolescent development. J Neurosci. 2011;31:6371–6378. doi: 10.1523/JNEUROSCI.5533-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarokh L, Carskadon MA, Achermann P. Early adolescent cognitive gains are marked by increased sleep EEG coherence. PloS one. 2014;9:e106847. doi: 10.1371/journal.pone.0106847. doi:10.1371/journal.pone.0106847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maret S, Faraguna U, Nelson AB, Cirelli C, Tononi G. Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nature neuroscience. 2012;14:1418–1420. doi: 10.1038/nn.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taki Y, et al. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. NeuroImage. 2012;60:471–475. doi: 10.1016/j.neuroimage.2011.11.072. doi:10.1016/j.neuroimage.2011.11.072. [DOI] [PubMed] [Google Scholar]
- 33.Telzer EH, Goldenberg D, Fuligni AJ, Lieberman MD, Galvan A. Sleep variability in adolescence is associated with altered brain development. Developmental cognitive neuroscience. 2015;14:16–22. doi: 10.1016/j.dcn.2015.05.007. doi:10.1016/j.dcn.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckelmüller J, Landolt HP, Stassen HH, Achermann P. Trait-like individual differences in the human sleep electroencephalogram. Neuroscience. 2006;138:351–356. doi: 10.1016/j.neuroscience.2005.11.005. doi:10.1016/j.neuroscience.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Hamann C, Rupp D, Leuenberger S, Schmidt S, Tarokh L. Associated Professional Sleep Societies. :A21. [Google Scholar]
- 36.Hoedlmoser K, et al. Slow sleep spindle activity, declarative memory, and general cognitive abilities in children. Sleep. 2014;37:1501–1512. doi: 10.5665/sleep.4000. doi:10.5665/sleep.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geiger A, et al. Sleep electroencephalography topography and children's intellectual ability. Neuroreport. 2012;23:93–97. doi: 10.1097/WNR.0b013e32834e7e8f. doi:10.1097/WNR.0b013e32834e7e8f. [DOI] [PubMed] [Google Scholar]
- 38.Geiger A, et al. The sleep EEG as a marker of intellectual ability in school age children. Sleep. 2011;34:181–189. doi: 10.1093/sleep/34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bodizs R, Gombos F, Ujma PP, Kovacs I. Sleep spindling and fluid intelligence across adolescent development: sex matters. Frontiers in human neuroscience. 2014;8:952. doi: 10.3389/fnhum.2014.00952. doi:10.3389/fnhum.2014.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma N, Dinges DF, Basner M, Rao H. How acute total sleep loss affects the attending brain: a meta-analysis of neuroimaging studies. Sleep. 2015;38:233–240. doi: 10.5665/sleep.4404. doi:10.5665/sleep.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Killgore WD. Effects of sleep deprivation on cognition. Progress in brain research. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 42.Greer SM, Goldstein AN, Knutson B, Walker MP. A Genetic Polymorphism of the Human Dopamine Transporter Determines the Impact of Sleep Deprivation on Brain Responses to Rewards and Punishments. J Cogn Neurosci. 2016:1–8. doi: 10.1162/jocn_a_00939. doi:10.1162/jocn_a_00939. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annual review of clinical psychology. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. doi:10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol. 2013;23:R774–788. doi: 10.1016/j.cub.2013.07.025. doi:10.1016/j.cub.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diekelmann S, Born J. The memory function of sleep. Nature reviews. 2010;11:114–126. doi: 10.1038/nrn2762. doi:10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 46.Walker MP, Stickgold R. Overnight alchemy: sleep-dependent memory evolution. Nature reviews. 2010;11:218. doi: 10.1038/nrn2762-c1. author reply 218, doi:10.1038/nrn2762-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Potkin KT, Bunney WE., Jr. Sleep improves memory: the effect of sleep on long term memory in early adolescence. PloS one. 2012;7:e42191. doi: 10.1371/journal.pone.0042191. doi:10.1371/journal.pone.0042191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groch S, et al. Memory cueing during sleep modifies the interpretation of ambiguous scenes in adolescents and adults. Developmental cognitive neuroscience. 2016;17:10–18. doi: 10.1016/j.dcn.2015.10.006. doi:10.1016/j.dcn.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prehn-Kristensen A, et al. Reduced sleep-associated consolidation of declarative memory in attention-deficit/hyperactivity disorder. Sleep medicine. 2011;12:672–679. doi: 10.1016/j.sleep.2010.10.010. doi:10.1016/j.sleep.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 50.Van Der Werf YD, et al. Sleep benefits subsequent hippocampal functioning. Nature neuroscience. 2009;12:122–123. doi: 10.1038/nn.2253. doi:10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- 51.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nature neuroscience. 2007;10:385–392. doi: 10.1038/nn1851. doi:10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 52.Drummond SP, et al. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–657. doi: 10.1038/35001068. doi:10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 53.Voderholzer U, et al. Sleep restriction over several days does not affect long-term recall of declarative and procedural memories in adolescents. Sleep medicine. 2011;12:170–178. doi: 10.1016/j.sleep.2010.07.017. doi:10.1016/j.sleep.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 54.Kopasz M, et al. No persisting effect of partial sleep curtailment on cognitive performance and declarative memory recall in adolescents. Journal of sleep research. 2010;19:71–79. doi: 10.1111/j.1365-2869.2009.00742.x. doi:10.1111/j.1365-2869.2009.00742.x. [DOI] [PubMed] [Google Scholar]
- 55.Carskadon MA, Harvey K, Dement WC. Acute Restriction of Nocturnal Sleep in Children. Perceptual and motor skills. 1981;53:103–112. [Google Scholar]
- 56.Beebe DW, Difrancesco MW, Tlustos SJ, McNally KA, Holland SK. Preliminary fMRI findings in experimentally sleep-restricted adolescents engaged in a working memory task. Behav Brain Funct. 2009;5:9. doi: 10.1186/1744-9081-5-9. doi:10.1186/1744-9081-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drummond SP, Gillin JC, Brown GG. Increased cerebral response during a divided attention task following sleep deprivation. Journal of sleep research. 2001;10:85–92. doi: 10.1046/j.1365-2869.2001.00245.x. [DOI] [PubMed] [Google Scholar]
- 58.Saletin JM, et al. Human Hippocampal Structure: A Novel Biomarker Predicting Mnemonic Vulnerability to, and Recovery from, Sleep Deprivation. J Neurosci. 2016;36:2355–2363. doi: 10.1523/JNEUROSCI.3466-15.2016. doi:10.1523/JNEUROSCI.3466-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Payne JD, Kensinger EA. Sleep Leads to Changes in the Emotional Memory Trace: Evidence from fMRI. Journal of cognitive neuroscience. 2011;23:1285–1297. doi: 10.1162/jocn.2010.21526. [DOI] [PubMed] [Google Scholar]
- 60.Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychol Sci. 2008;19:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. doi:PSCI2157 [pii] 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker MP, Harvey AG. Obligate symbiosis: sleep and affect. Sleep medicine reviews. 2010;14:215–217. doi: 10.1016/j.smrv.2010.02.003. doi:10.1016/j.smrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 62.Baum KT, et al. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 2014;55:180–190. doi: 10.1111/jcpp.12125. doi:10.1111/jcpp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep deprivation in adolescents and adults: changes in affect. Emotion. 2010;10:831–841. doi: 10.1037/a0020138. doi:10.1037/a0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rhodes SK, et al. Neurocognitive deficits in morbidly obese children with obstructive sleep apnea. J Pediatr. 1995;127:741–744. doi: 10.1016/s0022-3476(95)70164-8. [DOI] [PubMed] [Google Scholar]
- 65.McNally KA, Shear PK, Tlustos S, Amin RS, Beebe DW. Iowa gambling task performance in overweight children and adolescents at risk for obstructive sleep apnea. J Int Neuropsychol Soc. 2012;18:481–489. doi: 10.1017/S1355617711001937. doi:10.1017/S1355617711001937. [DOI] [PubMed] [Google Scholar]
- 66.Thomas AG, Monahan KC, Lukowski AF, Cauffman E. Sleep problems across development: a pathway to adolescent risk taking through working memory. J Youth Adolesc. 2015;44:447–464. doi: 10.1007/s10964-014-0179-7. doi:10.1007/s10964-014-0179-7. [DOI] [PubMed] [Google Scholar]
- 67.Wheaton AG, Chapman DP, Croft JB. School Start Times, Sleep, Behavioral, Health, and Academic Outcomes: A Review of the Literature. The Journal of school health. 2016;86:363–381. doi: 10.1111/josh.12388. doi:10.1111/josh.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shahid A, Khairandish A, Gladanac B, Shapiro C. Peeking into the minds of troubled adolescents: the utility of polysomnography sleep studies in an inpatient psychiatric unit. Journal of affective disorders. 2012;139:66–74. doi: 10.1016/j.jad.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 69.Lovato N, Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep medicine reviews. 2014;18:521–529. doi: 10.1016/j.smrv.2014.03.006. doi:10.1016/j.smrv.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Owens JA. Sleep disorders and attention-deficit/hyperactivity disorder. Current psychiatry reports. 2008;10:439–444. doi: 10.1007/s11920-008-0070-x. [DOI] [PubMed] [Google Scholar]
- 71.Scott N, et al. Sleep patterns in children with ADHD: a population-based cohort study from birth to 11 years. Journal of sleep research. 2013;22:121–128. doi: 10.1111/j.1365-2869.2012.01054.x. doi:10.1111/j.1365-2869.2012.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gregory AM, et al. Prospective longitudinal associations between persistent sleep problems in childhood and anxiety and depression disorders in adulthood. Journal of abnormal child psychology. 2005;33:157–163. doi: 10.1007/s10802-005-1824-0. [DOI] [PubMed] [Google Scholar]
- 73.Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. Journal of affective disorders. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- 74.Fredriksen K, Rhodes J, Reddy R, Way N. Sleepless in Chicago: tracking the effects of adolescent sleep loss during the middle school years. Child development. 2004;75:84–95. doi: 10.1111/j.1467-8624.2004.00655.x. [DOI] [PubMed] [Google Scholar]
- 75.Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep medicine reviews. 2010;14:227–238. doi: 10.1016/j.smrv.2009.10.007. doi:10.1016/j.smrv.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 76.Meldrum RC, Restivo E. The behavioral and health consequences of sleep deprivation among U.S. high school students: relative deprivation matters. Preventive medicine. 2014;63:24–28. doi: 10.1016/j.ypmed.2014.03.006. doi:10.1016/j.ypmed.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Wahlstrom K. Changing Times: Findings From the First Longitudinal Study of Later High School Start Times. 2002;86:3–21. [Google Scholar]
- 78.Danner F, Phillips B. Adolescent sleep, school start times, and teen motor vehicle crashes. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2008;4:533–535. [PMC free article] [PubMed] [Google Scholar]
- 79.Wahlstrom K, et al. Center for Applied Research and Educational Improvement. University of Minnesota; St Paul, MN: 2014. Examining the Impact of Later School Start Times on the Health and Academic Performance of High School Students: A MultiSite Study. [Google Scholar]
- 80.Vorona RD, et al. Adolescent crash rates and school start times in two central Virginia counties, 2009-2011: a follow-up study to a southeastern Virginia study, 2007-2008. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2014;10:1169–1177. doi: 10.5664/jcsm.4192. doi:10.5664/jcsm.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vorona RD, et al. Dissimilar teen crash rates in two neighboring southeastern Virginia cities with different high school start times. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine. 2011;7:145–151. [PMC free article] [PubMed] [Google Scholar]
- 82.Short MA, Louca M. Sleep deprivation leads to mood deficits in healthy adolescents. Sleep medicine. 2015;16:987–993. doi: 10.1016/j.sleep.2015.03.007. doi:10.1016/j.sleep.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 83.Casement MD, Keenan KE, Hipwell AE, Guyer AE, Forbes EE. Neural Reward Processing Mediates the Relationship between Insomnia Symptoms and Depression in Adolescence. Sleep. 2016;39:439–447. doi: 10.5665/sleep.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep medicine reviews. 2006;10:49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 85.Buxton OM, et al. Sleep in the modern family: protective family routines for child and adolescent sleep. Sleep health. 2015;1:15–27. doi: 10.1016/j.sleh.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carskadon MA. In: The Encyclopedia of Sleep. Kushida C, editor. Academic Press; 2013. pp. 86–87. [Google Scholar]
- 87.Gangwisch JE, et al. Earlier parental set bedtimes as a protective factor against depression and suicidal ideation. Sleep. 2010;33:97–106. doi: 10.1093/sleep/33.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Bruin EJ, Oort FJ, Bogels SM, Meijer AM. Efficacy of internet and group-administered cognitive behavioral therapy for insomnia in adolescents: a pilot study. Behavioral sleep medicine. 2014;12:235–254. doi: 10.1080/15402002.2013.784703. doi:10.1080/15402002.2013.784703. [DOI] [PubMed] [Google Scholar]
- 89.Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clinical psychology review. 2005;25:629–644. doi: 10.1016/j.cpr.2005.04.007. doi:10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 90.Adolescent Sleep Working, G. Committee on, A. Council on School, H. School start times for adolescents. Pediatrics. 2014;134:642–649. doi: 10.1542/peds.2014-1697. doi:10.1542/peds.2014-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]