Abstract
Adolescence marks a time of unique neurocognitive development, in which executive functions reach adult levels of maturation. While many core facets of executive function may reach maturation in childhood, these processes continue to be refined and stabilized during adolescence. We propose that this is mediated, in part, by interactions between the hippocampus and pre-frontal cortex. Specifically, we propose that development of this circuit refines adolescents’ ability to extract relevant information from prior experience to support task-relevant behavior. In support of this model, we review evidence for protracted structural and functional development both within and across the hippocampus and prefrontal cortex. We describe emerging research demonstrating the refinement of adolescents’ ability to integrate prior experiences to support goal-oriented behavior, which parallel hippocampal-prefrontal integration. Finally, we speculate that the development of this circuit is mediated by increases in dopaminergic neuromodulation present in adolescence, which may underlie memory processing, plasticity, and circuit integration. This model provides a novel characterization of how memory and executive systems integrate throughout adolescence to support adaptive behavior.
1. Introduction
Adolescent neurodevelopment has garnered great attention because of the associated propensity towards increased risk taking behavior (e.g., unprotected sex, substance use) and the emergence of psychopathology (e.g., schizophrenia, mood disorders). Prevailing neurodevelopmental models agree that a relative predominance of reward processing over executive functions is core to increased exploration and sensation seeking in adolescence. These processes are thought to garner an adaptive stage of information seeking in service of obtaining experiences that supports independence as adults (Shulman et al., 2016). The process of acquiring information to adapt to environmental demands is present throughout the lifespan. However, during adolescence there are unique elements of information seeking that define it as a unique period compared to earlier development and adulthood. The nature of the rewards that drive information seeking change across development (mother’s face in infancy, to professional satisfaction in adulthood, and social networking in adolescence). Further, the nature of information seeking is qualitatively different. Adolescents partake in sensation seeking behaviors (including exploration and novelty seeking) that incur a risk to survival to acquire new experiences, reflecting a unique quality to information seeking during this time of the lifespan. As proposed in prior neurodevelopmental models (Luna & Wright, 2016; Shulman et al., 2016), adolescents plan actions independent from the guidance of others that prioritize value of immediate rewards while suppressing information regarding negative consequences. By adulthood, the experiences that were accumulated in earlier development are integrated and organized in a manner to support reliable implementation. These prior models are based on a large developmental cognitive neuroscience literature that focuses on the maturation of prefrontal executive systems, dopamine systems associated with reward processing, and the nature of their interaction (Luna & Wright, 2016; Shulman et al., 2016). However, missing from these models are neurodevelopmental mechanisms that detail how adolescents are able to reliably use acquired experiences to support adult levels of cognition.
Here, we extend these prior models by integrating the function of hippocampal memory systems, which could support the ability for prior experiences to influence the specialization of prefrontal systems. Similar to prefrontal cortex and mesolimbic dopamine systems, the hippocampus demonstrates a protracted development through adolescence. Here we propose that developmental trajectories of the hippocampus and hippocampal-prefrontal (HPC-PFC) interactions support the integration and organization of experiences that can influence prefrontal systems. First, we will present our model where we propose that adolescence is a period of specialization in which the maturation of relevant brain systems peaks. This, in turn allows the critical integration of relevant brain processes across the hippocampus and prefrontal cortex that lead to the stabilization of cognition in adulthood. Specifically, we will present brain structural evidence that hippocampal memory and prefrontal executive systems have reached critical levels of maturation and that HPC-PFC interactions strengthen in adolescence. We will then present neurobehavioral evidence that the ability to use prior experiences to facilitate goal-relevant behavior, i.e. the execution of task-relevant demands, is enhanced through adolescence. Finally, we expand upon prior models of interactions between executive and reward systems to provide a mechanistic role for the dopaminergic system in supporting these developmental trajectories. Specifically, we predict that peaks in dopaminergic signaling, which occur in adolescence, not only expand memories of prior experiences, but also promote HPC-PFC interactions to incorporate these prior experiences to support task-relevant behavior. We believe these mechanisms support the ability for adolescents to actively acquire experiences from their environment and prioritize experiences that will continue to support goal-oriented behavior throughout adulthood.
2. A Model for Integration of Prior Experience to Support Adaptive Behavior in Adolescence
In this review, we build upon prior neurodevelopmental models to include the role of information gathering and episodic memory in the maturation and refinement of executive function. Previous models have proposed that executive functions in adolescents are undermined by significant immaturities in prefrontal systems in addition to a hyperactive motivational drive (Shulman et al., 2016; Spear, 2000). We have extended this model to underscore that prefrontal systems have reached a threshold of maturation by adolescence, evidenced by behavioral, functional MRI, and structural MRI studies (Hwang, Hallquist, & Luna, 2013; Luna, Marek, Larsen, Tervo-Clemmens, & Chahal, 2015; Ordaz, Foran, Velanova, & Luna, 2013; Raznahan, Greenstein, Lee, Clasen, & Giedd, 2012), but that access to adult level executive control is limited and variable due to immature connectivity among brain regions underlying different core cognitive functions (Hwang, Ghuman, Manoach, Jones, & Luna, 2016; Hwang, Velanova, & Luna, 2010). We propose that the access to adult levels of executive function in conjunction with enhanced motivational/reward processing in adolescence set a unique drive to use the arsenal of accumulated experiences (i.e., memories). The incorporation of these memories allows for selection and organization of adaptive experiences to support the construction of heuristics needed to transition into adulthood.
Extending upon the above detailed model, we introduce an experience-driven adaptive cognition model of adolescent development (See Figure 1). This model proposes that the refinement of executive function during adolescence results, in part, from the ability to better incorporate prior experiences into task-relevant responses. Although these processes are evident throughout development, the relative peak in maturation of prefrontal and hippocampal systems putatively allows for the strengthening of HPC-PFC integration. We further propose that this integration facilitates the incorporation of relevant prior experiences into executive function. This model builds on prior evidence indicating that the neural systems underlying basic memory processing and executive functions reach critical maturation around adolescence. That is, they are distinctly more mature in adolescence, i.e., similar to adult levels, than in childhood. The ability to predominantly integrate HPC-PFC systems supports the ability to reliably incorporate information acquired during past experiences to support task-relevant behavior. As this integration reaches mature levels, information about prior experiences can be retrieved, either implicitly or explicitly, to inform adaptive behavioral responses. Specifically, during adolescence, changes in structural and functional connectivity promote interactions between neural circuits specialized for episodic memory (i.e., the hippocampus) and executive function (i.e., prefrontal cortex), and these interactions allow for distributed information processing across this circuit. This integration allows individuals to refine their ability to query previous experience of successful goal attainment and generalize that information to engage them readily in concomitant situations with similar goals. Finally, increased dopamine availability may promote the use of acquired experiences in novel contexts and support plasticity for selection and organization of adaptive experiences by enhancing information processing across HPC-PFC circuits and stabilizing this circuitry as individuals approach adulthood.
Figure 1.
Proposed Model of Experience-Driven Maturation. Developmental trajectories within an across prefrontal, hippocampal, and mesolimbic dopamine systems contribute to the refinement of using prior experiences to guide task-related behaviors. Within the prefrontal cortex (blue), there is an early maturation of executive functions including cognitive control, working memory, and task-relevant behaviors prior to adolescence. In parallel within the hippocampus (teal), there is a maturation of memory-related processes that emerge prior to adolescence and are refined throughout adolescence. Critically, increased dopaminergic signaling in the VTA (orange) support both integration and plasticity within and across the hippocampus and prefrontal cortex (red), which results in the ability to distribute information processing across these regions. Together, this developmental trajectory results in the ability to use prior episodes to reliably guide task-relevant behavior. Individual plots summarize the proposed neurodevelopmental trajectories of discrete components of the model (adolescence highlighted in light blue).
This model makes several predictions about individuals’ neurobehavioral profiles across adolescence, many of which have yet to be tested. First, simple behaviors that rely on information within the hippocampus and prefrontal cortex will reach adult levels of maturation before adolescence. Second, the ability to select or integrate across multiple prior experiences in order to facilitate task-relevant goals will be particularly enhanced during adolescence as information processing continues to integrate across the hippocampus and prefrontal cortex. The retrieval of prior experiences in service of task-relevant behavior can manifest as both the accurate selection of context-dependent behavioral responses, i.e. selecting behaviors that are most adaptive given an individual’s environmental cues, or the integration of information across multiple previous episodes to develop heuristics. Although untested, we predict that these developmental trajectories will be concurrent with the peaks in mesolimbic engagement during adolescence, as this is a major determinant of neural plasticity and integration.
Below, we provide empirical research supporting the foundation of the proposed model as well as neurobehavioral data supporting facets of the above predictions. We organize the rest of the review in the following order. First, we review literature examining the role of HPC-PFC integration supporting the retrieval of prior experience in supporting task-relevant behavior (Section 3). Second, we provide structural evidence that hippocampal and prefrontal circuitry reach a threshold of integration during adolescence (Section 4), followed by neurobehavioral evidence in support of our model (Section 5). Finally, we end with predictions of how and why this organization peaks during adolescence, highlighting a predominant role for the dopamine neurotransmitter system (Section 6).
3. Neural Systems Underlying the Integration of Prior Experience and Executive Function
Our model proposes that adolescence represents a time when individuals retrieve acquired experiences to inform task-relevant behavior by extracting information from relevant, previous episodes. This ability becomes relevant when individuals have to inform their current behavioral responses based on information learned in the past. For example, a teen may hesitantly walk into a party at a new place not knowing what to expect. However, when she sees friends she has partied with in the past, she may retrieve those memories, providing a social contextual cue regarding what behaviors and actions may be appropriate to the setting. As the party progresses, those behaviors that were goal concomitant will be reinforced (being jovial and social) and integrated into emerging heuristics while those that proved goal incompatible (being aggressive or vain) will not be incorporated into future behavior. These processes can help individuals generalize over multiple, similar experiences to select the most effective behavioral responses, i.e., the use of heuristics and/or schemas. Accordingly, our model is not limited to a single aspect of cognition, but rather it generalizes across a wide variety of cognitive domains including executive function, learning and memory, and decision-making. Thus, we propose that interactions between the hippocampus and prefrontal cortex can inform the adolescent maturation of behavior drawn from multiple cognitive domains. Before reviewing the developmental literature supporting these processes, we first review the adult literature characterizing how HPC-PFC interactions contribute to integrating prior experiences with task-relevant behavior.
At the core of our model is the hypothesis that adolescents encode relevant details of prior experiences and retrieve these memories from the past to inform task-relevant behavior. This aspect of integrating prior experiences taps into a specific aspect of memory processing. Prominent models of memory systems delineate two distinct memory processes: item memory, which retains individual, unitized representations of the environment supported by cortical medial temporal lobe (MTL), including the parahippocampal and perirhinal cortex, and associative memory, which retains flexible representations of relationships amongst discrete items and their contextual environment supported by the hippocampus (Davachi, 2006; Eichenbaum, Yonelinas, & Ranganath, 2007; Ranganath, 2010). We propose that while both are present throughout the lifespan, associative memory plays a predominant role in the ability for adolescents to select and organize experiences to achieve adult levels of cognition. Associative memory is particularly critical for retrieving contextual details from prior experiences to match to new contexts that have compatible task goals. While memory processes are thought to rely on neural circuitry throughout the MTL cortex, associative memory is dependent on intact hippocampal function. Lesions to the hippocampus, which spare the surrounding MTL cortex, result in deficits in associative memory (Diana, Yonelinas, & Ranganath, 2007). Relevant for our model are associative memories encoded by the anterior hippocampus, which supports flexible, relational representations of previous experiences, as opposed to the more rigid representations stored by the posterior hippocampus (Poppenk, Evensmoen, Moscovitch, & Nadel, 2013; Preston & Eichenbaum, 2013). These flexible associative memories may be necessary to generalize relevant information across multiple experiences to apply in novel contexts.
The hippocampus is highly connected to the prefrontal cortex predominantly by the uncinate fasciculus (UF), a white matter tract that provides connectivity among frontal, medial temporal, and limbic structures and has been found to be critical to associative memory (Simons & Spiers, 2003). Human neuroimaging and lesion studies have found that HPC-PFC interactions predict both the encoding and retrieval of associative memory (Blumenfeld & Ranganath, 2007). Further, the integrity of the UF has been found to be associated with associative memory processes (Von Der Heide, Skipper, Klobusicky, & Olson, 2013) but not item memory (Alm, Rolheiser, & Olson, 2016; Thomas, Avram, Pierpaoli, & Baker, 2015). Homologous evidence from animal models supports that lesions to both hippocampus and prefrontal cortex, or connections amongst them, disrupt flexible retrieval of associative memory (Preston & Eichenbaum, 2013). These connections are purported to be organized and specialized so that connections to medial prefrontal cortex support affective processes and those to lateral prefrontal cortex support executive processes (Barbas, 2000). These converging lines of evidence underscore that critical to associative memory is not only the storing of item associations in hippocampus but also its integration with prefrontal systems that support planning and execution of task goals.
In our model, we predict that the retrieval of relevant prior experiences is necessary to perform a variety of complex executive functions, particularly behaviors that show protracted development throughout adolescence such as memory judgments relying on the hippocampus (i.e., associative memory, recollection-based memory) and the consistency of executive function (see Section 5). While research has largely focused on the necessity of prefrontal cortex during executive function, behaviors that require the retrieval of prior contexts for optimal performance additionally rely on the hippocampus. Both lesion and human neuroimaging work have shown a critical role for integrated function of HPC-PFC during executive function. In humans, interactions between the hippocampus and prefrontal cortex have been implicated during successful working memory performance (Sigurdsson & Duvarci, 2015), particularly in tasks that depend on prior information from previous trials. For example, in a spatial working memory paradigm where participants collected rewards in a radial maze, there were greater HPC-PFC interactions when participants had to actively maintain which spatial locations they had previously visited (Bähner et al., 2015). Similarly, the ability to hold items in working memory for extended periods of time relies not only on the prefrontal cortex but also the hippocampus (Dudukovic, Preston, Archie, Glover, & Wagner, 2011; Lewis-Peacock & Postle, 2008; Olsen et al., 2009), a process thought to rely on prospective memory to retrieve stored representations of the items. Convergent evidence from rodent models concur in showing that HPC-PFC interactions support a variety of executive behaviors. For example, lesions to the rodent UF reliably disrupt performance on conditional rule-learning tasks, flexible reversal learning, and delayed match–to-sample tasks (Bussey, Wise, & Murray, 2002; Eacott & Gaffan, 1992; Gaffan, Gaffan, & Harrison, 1988; Gutnikov, Ma, Buckley, & Gaffan, 1997; Parker & Gaffan, 1998). Together, these studies provide evidence for a relevant role for associative memory in executive function and underscore the importance of HPC-PFC connectivity in integrating information about associations with task-relevant behavior.
Recent evidence for value-based decision making has further supported that HPC-PFC interactions are necessary when choice behavior relies on the retrieval of relevant prior experiences (Doll, Simon, & Daw, 2012). While a large literature has shown that decision processes rely on value signals in the striatum, there is further engagement of HPC-PFC circuits when choice necessitates the integration of prior experience to build models of decision contexts. This integration of prior experiences relevant to the concurrent environment with decision-making processes has been referred to as model-based decision-making. These processes are in contrast to model-free decision making, which relies on rigid associations independent of the current environment. Previous human research has shown that adaptive performance during model-based decision-making relies on HPC-PFC function (Doll, Duncan, Simon, Shohamy, & Daw, 2015; Doll, Shohamy, & Daw, 2015; Otto, Raio, Chiang, Phelps, & Daw, 2013; Otto, Skatova, Madlon-Kay, & Daw, 2015). In line with this literature, HPC-PFC interactions have been shown to be critical for the use of heuristics during decision-making, a process which necessitates generalization over multiple prior experiences to make optimal decisions (Gigerenzer & Gaissmaier, 2011). For example, neuroimaging studies have shown that the emergence of heuristics is associated with increased HPC-PFC coupling (Gluth, Sommer, Rieskamp, & Büchel, 2015; Kumaran, Summerfield, Hassabis, & Maguire, 2009). These findings suggest that the ability to perform model-based decision-making would rely on integrated HPC-PFC function, and our model would predict that these behaviors would become predominant during adolescence.
Together, these studies provide empirical support that the ability to extract information from relevant prior experience to support concurrent behavior is associated with HPC-PFC integration. Evidence for this mechanism emerges from multiple fields including associative memory, complex executive function, and model-based decision-making. We next review studies that support that the maturation of HPC-PFC circuits and the developmental refinement of the associated behavioral phenomenon emerge during adolescence. We first review the structural development of hippocampal and prefrontal systems in isolation as well as their integration throughout adolescence (Section 4). Then, we turn to neurobehavioral studies that demonstrate the emergence of memory-executive function interactions and show that it is paralleled by functional HPC-PFC integration (Section 5). Finally, we propose a theoretical framework to understand how and why HPC-PFC interactions are particularly relevant for adolescent development by providing evidence for a modulatory role for dopamine signaling.
4. Structural Development of Integrated Hippocampal-Prefrontal Circuitry
Our model postulates that the ability for retrieval of relevant prior experience to support task-relevant behavior emerges through the development of integrated HPC-PFC circuitry. In support of this model is a substantial and growing body of work demonstrating structural development within and between the hippocampus and prefrontal cortex. This circuit includes some of the most protracted structural changes that have been observed in the developing brain and includes changes not only in the hippocampus and prefrontal cortex, but importantly in the structural pathways mediating their interactions.
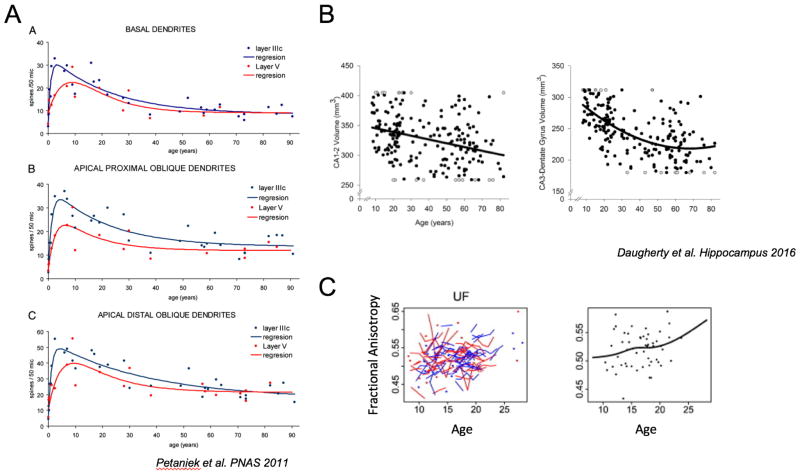
Structural changes in the prefrontal cortex extend through adolescence (Spear, 2000). Recent histological studies in humans indicate that synaptogenesis increases throughout childhood, with pruning proceeding through puberty possibly demarcating a qualitative shift towards prefrontal specialization. Prefrontal cortex pruning persists well into adulthood (See Figure 2a) and may underlie the prominent decreases in prefrontal cortex gray matter volume associated with this time period (Gogtay et al., 2004). Pruning of the prefrontal cortex during adolescence occurs most notably in layer 3 pyramidal cells, as evidenced in post-mortem human studies (Petanjek et al., 2011). Details of this developmental trajectory have been extended using non-human primates as an animal model. Of particular interest for our model, layer 3 prefrontal cortex pyramidal neurons have a relatively high number of dendritic spines, which support the integration of multiple sources of information (Elston, 2000). Further, they are the target of robust, long-distance monosynaptic connections and have a complex network of GABA-ergic and glutamatergic afferents, which are thought to underlie the instantiation of oscillations that can synchronize information processing across discrete neural regions (Elston, 2000; Gonzalez-Burgos & Lewis, 2008). These features of layer 3 prefrontal cortex pyramidal neurons support their role in the refinement of network interactions with discrete regions across the brain. Further, the relatively late maturation of these synaptic connections suggests that neural computations that rely on network integration with the prefrontal cortex (i.e., HPC-PFC interaction) may also rely on the protracted maturation of layer 3 pyramidal neurons. Our model would predict that these developmental changes in the prefrontal cortex support reliable integration of information processing with the hippocampus.
Figure 2.
Structural development of prefrontal cortex, hippocampus, and the uncinate fasciculus through adolescence. (A) Dendritic spine density from layers III and V of prefrontal cortex from a human postmortem study (Petanjek et al., 2011, Figure 2). Spines peaked in mid-childhood and decreased throughout adolescence, with pruning persisting into the third decade. (B) Volumetric changes in hippocampus throughout the lifespan as assessed by MRI (Daugherty et al., 2016, Figure 3 B,C). These changes were documented across multiple sub-regions of the hippocampus (CA1:top and CA3:bottom) fields continued well into adulthood. (C) Longitudinal DTI analysis of uncinate fasciculus (UF) fiber tract showing age-related changes in fractional anisotropy for raw data (left) and averaged data (right; Simmonds et al., 2014, modified from Supplemental Figure 2).
One reason the development of an integrated circuit across prefrontal cortex and hippocampus may not mature until adolescence is that the hippocampus has also been shown to continue to mature throughout adolescence and well into adulthood. Postnatal development of the hippocampus in humans has been extensively studied in part due to its status as one of few brain regions that undergoes significant adult neurogenesis and protracted myelination throughout much of the lifespan (Benes, 1989; Benes, Turtle, Khan, & Farol, 1994). Of particular interest to our model, accumulating research has shown that there are significant changes to anterior portions of the hippocampus, which supports flexible relational representations of past experiences. Within this region, both human post-mortem (Abrahám et al., 2010) and in-vivo MRI (Lee, Ekstrom, & Ghetti, 2014) studies show increases in volume until early adolescence (13–14 yo), followed by a protracted volumetric decrease (Daugherty, Bender, Raz, & Ofen, 2016; DeMaster, Pathman, Lee, & Ghetti, 2014; Gogtay et al., 2006; Lee et al., 2014; Tamnes et al., 2014) (see Figure 2b). Although these studies contain a number of conflicting results, they generally support volumetric increases during childhood followed by decreases in anterior hippocampus persisting into and throughout adolescence. While the causes of volumetric changes are still speculative, work from a diverse array of human and animal studies has suggested several candidate mechanisms. Namely, human post-mortem studies have suggested that volumetric changes may arise from ongoing myelination (Benes, 1989; Benes et al., 1994). Animal research has complemented this work by showing volumetric changes are also dependent on experience-dependent axonal sprouting identified in rats (Holahan, Rekart, Sandoval, & Routtenberg, 2006; Ramírez-Amaya, Balderas, Sandoval, Escobar, & Bermúdez-Rattoni, 2001), as well as synaptic pruning found in non-human primate and genetic studies (Cocchi, Drago, & Serretti, 2016; Eckenhoff & Rakic, 1991). Critically, it has been proposed that the cumulative effect of these structural changes is the refinement of the anterior hippocampus in service of supporting associative memory (Ghetti & Bunge, 2012). It is of interest to note that human synaptogenesis peaks in prefrontal cortex in late childhood (10 years of age) earlier than anterior hippocampus in adolescence (13 years of age), suggesting that critical maturation of prefrontal systems may facilitate maturation of hippocampal systems, supporting the later predominance of associative memory (Ghetti & Bunge, 2012; Petanjek et al., 2011). However, causal relationships between the developmental trajectories of these two systems have yet to be studied and are targets for future animal research.
We propose that the HPC-PFC integration of information processing is dependent on developmental trajectories of white-matter tracts that facilitate communication across these regions. In particular, there is histological evidence from rat (T. M. Jay & Witter, 1991; Swanson & Cowan, 1977), non-human primate (Barbas & Blatt, 1995; Cavada, Compañy, Tejedor, Cruz-Rizzolo, & Reinoso-Suárez, 2000), and human (Ebeling & von Cramon, 1992) studies that the UF provides the most prominent pathway for HPC-PFC connectivity via relays in MTL cortex (for review, see Amaral & Witter, 1989; Von Der Heide et al., 2013). Most intriguingly to our model, human in-vivo diffusion tractography studies have provided substantial evidence that the UF is one of the latest white matter tracts to develop, showing continued maturation through the third decade of life (Lebel et al., 2012; Olson, Von Der Heide, Alm, & Vyas, 2015; Simmonds, Hallquist, Asato, & Luna, 2014)(See Figure 2c). While UF volume relative to total intracranial volume is constant throughout adolescence (Hasan et al., 2009), a growing number of studies support increased markers of white matter integrity that persist into early adulthood (Barnea-Goraly et al., 2005; Eluvathingal, Hasan, Kramer, Fletcher, & Ewing-Cobbs, 2007; Hasan et al., 2009; Simmonds et al., 2014), which may reflect protracted myelination. Critically, it has been proposed that the developmental trajectories of these white matter tracts support the emergence and refinement of associative memory processes, and thus in line with our model, the use of associative memory to support flexible cognition (Ghetti & Bunge, 2012; Von Der Heide et al., 2013). It is important to note that the UF is not the only white matter tract between MTL and PFC structures that contributes to memory processes. In particular, the cingulum bundle provides a less direct pathway, projecting back from the anterior MTL to occipital regions before tracking around the corpus collosum through parietal and eventually prefrontal regions. Our longitudinal DTI studies indicate that the UF has an even later maturation than the cingulum (Simmonds et al., 2014). While both the UF and cingulum have protracted development, with maturation persisting through the second decade of life (Lebel et al., 2012; Simmonds et al., 2014), we focus on the UF due to its more direct connectivity between the MTL and prefrontal cortex and the extensive lesion studies which have characterized its role in associative memory processes (Von Der Heide et al., 2013).
Generally speaking, adolescence also marks a time in which functional cortical networks, whose organization is already in place, show increased cross-network integration (Marek, Hwang, Foran, Hallquist, & Luna, 2015). Although many properties of core networks of resting state connectivity, including segregation, small-worldness (Fair et al., 2009; Fransson, Aden, Blennow, & Lagercrantz, 2011) and hub organization (Hwang et al., 2013), are largely in place prior to adolescence, interactions among networks, including those centered on MTL regions, continue to mature supporting improvements in executive function (Marek et al., 2015). These data are compatible with our model, in which function within the hippocampus and pre-frontal cortex, i.e., development within core regions, have matured by adolescence, but in which integrated function does not emerge until adolescence due to the maturation of network connectivity. Relating this literature to our proposed model, functional imaging data has shown that the left hippocampus and left orbitofrontal cortex were both connected with the posterior cingulate cortex (PCC) in 13 year olds, but not in 10 year olds (Sherman et al., 2014), suggesting that the refinement of network integration including the HPC and PFC may initiate during early adolescence.
Together, these results support developmental changes in both the intrinsic properties of the anterior hippocampus and the prefrontal cortex that are heightened during adolescence. Our model predicts that during this time period, integrated function of these regions reach a critical threshold that allows for reliable engagement of information processing across these regions. Specifically, we suggest that this evolving circuitry mediates improved performance on tasks that require integrated function of these regions, either by retrieving past episodes that help identify which behaviors are relevant for the current task demands or by allowing integration across multiple episodes to help constrain adaptive behaviors. Importantly, this late timetable of white matter maturation through adolescence supports our proposal that adolescence is a time of unique refinement of HPC-PFC connectivity. We next review neurobehavioral studies that support a refinement of HPC-PFC circuitry throughout adolescence relevant to both of these behavioral profiles.
5. Neurobehavioral Evidence of the Refinement of Integrated Hippocampal-Prefrontal Function throughout Adolescence
In the prior section, we provided evidence for the structural integration across HPC-PFC circuits throughout adolescence. The structural development of these integrated circuits is paralleled by developmental improvements in the ability to reliably extract information from relevant prior experiences in service of task-related behaviors. This is evidenced both by neuroimaging investigations of facilitations in integrated HPC-PFC function as well as behavioral studies indicating a refinement of behaviors relying on this circuitry. Notably, in many of these studies the retrieval of prior experiences is not explicitly probed, but rather theoretical models support that previous experiences are supporting these task relevant behaviors (see Section 3).
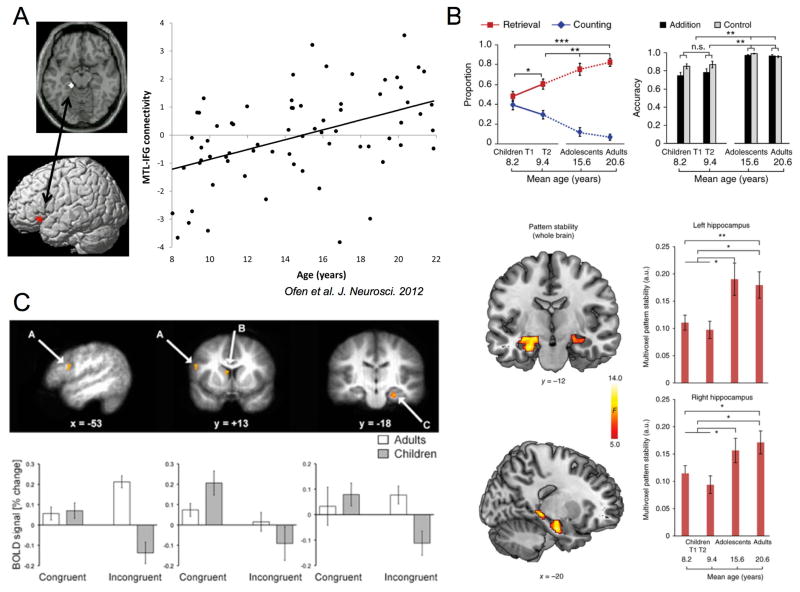
While more simple forms of memory (i.e., item memory, familiarity) mature relatively early (i.e., pre-adolescence), associative memory continues to develop towards adult levels through adolescence. Prior to adolescence, the basic building blocks of memory processing have reached maturation. Children are able to perform at adult levels in simple stimulus-response learning tasks, implicit priming tasks, and declarative memory tasks that do not require the retrieval details of prior experiences (Billingsley, Lou Smith, & Pat McAndrews, 2002; Brainerd, Holliday, & Reyna, 2004; Finn et al., 2016; Ghetti & Angelini, 2008; Meulemans, Van der Linden, & Perruchet, 1998; Piolino et al., 2007). However, more complex forms of associative memory that support the retrieval of details of prior experiences continue to develop through adolescence (Billingsley et al., 2002; Brainerd et al., 2004; Cycowicz, Friedman, Snodgrass, & Duff, 2001). A study of memory processing across 6 to 18 year olds showed a prolonged maturation of recollection-based judgments, which reflect associative memory. Conversely, simple forms of familiarity-based memory did not change after childhood (i.e., 8 years old) (Ghetti & Angelini, 2008). Neuroimaging evidence has further shown that the maturation of associative memory processes is related to neurodevelopment of integrated HPC-PFC circuitry. FMRI studies have shown greater reliance on the prefrontal cortex during associative memory throughout adolescence into early adulthood (Ofen et al., 2007) as well as greater functional coupling between the hippocampus and prefrontal cortex (Menon, Boyett-Anderson, & Reiss, 2005; Ofen, Chai, Schuil, Whitfield-Gabrieli, & Gabrieli, 2012)(See Figure 3b). Importantly, anterior portions of the hippocampus may be particularly relevant for adolescent neurodevelopment of associative memory. In adolescents and adults, but not children, the volume of the anterior hippocampus as well as functional activation of the anterior hippocampus and prefrontal cortex predict better associative memory performance (DeMaster et al., 2014). Critically, research investigating pre-adolescent development have detailed increases in associative memory processes as well as changes in connectivity between the hippocampus and prefrontal cortex (Riggins, Geng, Blankenship, & Redcay, 2016). However, the extant evidence above shows that these processes approach adult levels in adolescence. Together, these findings suggest that the initiation of prefrontal-hippocampal integration may begin in childhood, but these processes do not reach a critical point of maturation until adolescence. In turn, our model predicts that the reliability in retrieving appropriate memories to support behavior doesn’t emerge until adolescence, although some markers of these processes appear earlier.
Figure 3.
Functional neurodevelopment of experience-dependent behaviors. (A) Functional connectivity between the hippocampus and prefrontal cortex during performance of a declarative memory task increased with age in conjunction with age-related improvements in the hit rate of recognizing previously viewed scenes (Ofen et al., 2012, Figure 4). (B) Increased reliance on memory-based, rather than counting-based, math strategies from childhood through adolescence (top) was accompanied by improved stability of hippocampal pattern representations (bottom) (Qin et al., 2014, Figures 1,5). (C) Lateral prefrontal cortex (left), dorsal striatum (middle), and hippocampus (right) showed significant age-related changes in their condition-specific responses to a context-dependent reward task (Voss et al., 2015, Figure 3). Prefrontal cortex and hippocampus in particularly showed greater activation in young adults compared to early adolescents in their response to context shifts, supporting improvements in context-guided, reward-related responses during adolescence.
The same protracted developmental trajectories described above for associative memory are paralleled by development of executive functions that are theorized to rely on the accurate retrieval of previous experiences and integrated HPC-PFC function. The basic tenants of executive function and their underlying neural substrates have been shown to be established by early childhood, with evidence that many of these properties emerge as early as infancy (Garon, Bryson, & Smith, 2008; Jones, Rothbart, & Posner, 2003). However, the fidelity and stability of these processes continue to mature throughout adolescence. For example, while the ability to generate an executive response at the single trial level is available early in development, developmental improvements are seen in the rate of correct responses, their precision, and their consistency (Luna et al., 2015; McIntosh et al., 2014; Tamnes, Fjell, Westlye, Østby, & Walhovd, 2012). Within our theoretical framework, the ability to reliably select the appropriate behaviors could be stabilized by prefrontal interactions with hippocampus. Specifically, this refinement in executive function emerges from being able to generalize over multiple previous experiences to develop heuristics of behavioral repertoires that reduce variability. In line with this interpretation, behavioral evidence has indicated an increased reliance on heuristics during problem-solving in adolescents compared to children, supporting that development to adult levels of cognition is marked by refinement of heuristics (Borst, Aïte, & Houdé, 2015).
Although relatively few studies have investigated functional HPC-PFC interactions during the maturation of executive systems, there is some extant data to support our theoretical model. In a neurodevelopmental working memory study, researchers characterized HPC-PFC interactions during a delayed match-to-sample task using a longitudinal design tracking individuals from early to late adolescence (Finn, Sheridan, Kam, Hinshaw, & D’Esposito, 2010). Working memory performance improved across adolescence, which paralleled differences in functional connectivity between the prefrontal cortex and hippocampus. During early adolescence, there was significant HPC-PFC coupling during both high and low load trials; however, by late adolescence, there was only significant interactions for high load trials. Further, HPC-PFC interactions predicted behavioral performance at both time points. Importantly, theoretical work suggests that performance on delayed match-to-sample tasks that utilize a longer delay, such as those in the described study, can rely in part on retrieval of the identity of the target stimulus from episodic memory. Given this theoretical framework, the above findings suggest that in early adolescence, individuals begin to show the ability to integrate information processing across the prefrontal cortex and hippocampus, and maturation throughout adolescence leads to further refinement of this circuitry. A similar refinement of HPC-PFC interactions was recently demonstrated in a neurodevelopmental study investigating problem solving in the math domain (Qin et al., 2014)(see Figure 3a). In this study, individuals completed arithmetic problems that could either be solved using procedure-based strategies (i.e, counting) or memory-based strategies (i.e., retrieval of prior-encoded information). The investigators found that the use of associative memory strategies increased throughout adolescence into adulthood. Further, retrieval-based strategies were associated with increased HPC-PFC coupling when age was matched. Finally, adolescent development was associated with greater stability in hippocampal representations of retrieval-based strategies, a mechanism that would theoretically reduce variability in problem-solving behavior. These findings are in line with our theoretical model in that adolescents refine their ability to generalize over multiple prior experiences and use this information for task-relevant behaviors. Together, these behavioral and neuroimaging studies suggest that the integrated function of HPC-PFC is refined throughout adolescent neurodevelopment, and this integrated circuitry supports the implementation of relevant experience-based heuristics to support flexibility in executive function.
Finally, evidence from value-based decision-making has further supported that the retrieval of relevant information from prior experiences to support choice behavior does not reach adult levels of maturation until adolescence. Extant research has shown that adolescents have the ability to use simple stimulus-response relationships to make reward decisions incorporating prediction error signaling to guide future choice (Hartley & Somerville, 2015). However, recent studies investigating model-based decision-making, which relies on building models from prior representations of the environment, show that the capacity to retrieve memories to support choice reaches adult levels in adolescence. A recent study showed that the execution of cognitive models based on experiential priors, however, does not emerge until early adolescence and does not plateau until adulthood (Decker, Otto, Daw, & Hartley, 2016). In line with these findings, recent studies have shown that adults, unlike children and adolescents, are more likely to use learned heuristics or instructions to execute value-based decisions (Decker, Lourenco, Doll, & Hartley, 2015; Kwak, Payne, Cohen, & Huettel, 2015). Although these studies were behavioral, the processing involved is putatively thought to rely on HPC-PFC interactions (Doll et al., 2012). Similarly, a recent neuroimaging study explicitly investigated how maturation of HPC-PFC interactions supports related decision-making behaviors (Voss, O’Neil, Kharitonova, Briggs-Gowan, & Wakschlag, 2015)(See Figure 3c). In this study, participants first performed a simple stimulus-response learning task with feedback learning. Halfway through learning, a reversal manipulation was introduced in which adaptive behavior necessitated the retrieval of prior contextual information. Simply stated, participants could not accurately perform the task unless they were able to retrieve and discriminate between memories of contextual demands presented earlier in the task. Results indicated that neural and behavioral markers of simple stimulus-response learning were similar in early versus late adolescents. However, late adolescents showed a relative advantage of retrieving prior contexts to update choice behavior compared to young adolescents, and behavioral advantages were associated with greater engagement of both prefrontal cortex and hippocampus. While we focused our review to the human literature, a large body of animal research has shown parallel developmental trajectories in learning and decision-making. For example, research has shown that many simple learning processes, like fear conditioning, are intact in the adolescent rodent, however, how these learned associations are used to guide choice behavior continues to develop into adulthood (see Hunt et al., 2016 in the same issue).
Functional neuroimaging and behavioral studies alike support the proposal that adolescence marks a time period in which there is a refinement in HPC-PFC integration. Support for this neurodevelopmental model spans multiple cognitive domains including learning and memory, executive function, and value-based decision making. The extant evidence indicates that this neurodevelopmental integration supports refinements in the ability to integrate prior experiences, mediated by the hippocampus, to dynamically enhance task-relevant behavior, mediated by the prefrontal cortex. These interactions may be particularly relevant for stabilizing context-specific heuristics to implement behaviorally-appropriate responses in varied environments as adults.
6. A Role for Dopaminergic Neuromodulation in Guiding the Maturation of Integrated Hippocampal-Prefrontal Function throughout Adolescence
Thus far, we have provided evidence for the development of distributed information processing throughout the prefrontal cortex and hippocampus throughout adolescence, which is associated with the ability to effectively retrieve relevant prior experiences in service of task-relevant behavior. Open questions remain, however, as to why the neurodevelopment of this circuit is particularly sensitive to adolescence. Although not directly tested in a neurodevelopmental framework, we theorize that maturational changes in dopamine signaling and its associated neuromodulation may be particularly relevant for these processes. Dopaminergic neuro-modulation reaches its peak during adolescence and begins to decline in adulthood (detailed below). Elevated dopamine may contribute to propel the type of exploration that relies on associative memory processes as novel contexts are engaged independent from supervision or guidance. Importantly, given the critical role for dopamine in plasticity, network integration, and information processing (Durstewitz & Seamans, 2002; Jay, 2003; Lisman & Grace, 2005), heightened levels of dopamine during adolescence could provide the neurochemical foundation to support HPC-PFC integration.
The dopamine system undergoes prolonged maturation that continues throughout late adolescence into early adulthood (Dahl, 2004; Padmanabhan & Luna, 2014; Spear, 2000; Wahlstrom, White, & Luciana, 2010). Behaviorally, adolescents show developmental changes in behaviors tightly coupled with dopaminergic function, including reward sensitivity, novelty seeking, and exploration, which are expressed less leading into adulthood (Dahl, 2004; Spear, 2000). Sensation seeking is characterized by pursuing reward despite evident risks, which may support the active exploration of new contexts and experiences. Memories of these novel experiences can than be used in the service of specialization during executive function. This marked increase in dopamine-related behaviors during adolescence parallels significant changes in dopamine signaling. Animal models have indicated that peak levels of dopamine signaling occur during puberty, particularly in regions relevant for motivation and reward learning (i.e., ventral striatum) (Andersen, Dumont, & Teicher, 1997; McCutcheon et al., 2012; Padmanabhan & Luna, 2014; Tarazi, Tomasini, & Baldessarini, 1999; Tarazi et al., 1999; Teicher, Andersen, & Hostetter Jr., 1995; Wahlstrom et al., 2010). Similarly, human post-mortem studies have shown that the most dynamic changes in dopamine receptors occur during adolescence (Haycock et al., 2003; Meng, Ozawa, Itoh, & Takashima, 1999; Weickert et al., 2007). Further, the structural and functional integrity of dopaminergic targets in humans, like the ventral striatum, continue to develop throughout late adolescence (Raznahan et al., 2014; Wahlstrom et al., 2010). FMRI studies indicate a peak in striatal reactivity in adolescence during reward processing, believed to mirror the aforementioned evidence in animal models (Padmanabhan & Luna, 2014).
Multiple properties of dopamine suggest that its relatively high signaling during adolescence would support the theoretical foundations of our model. First, dopamine in the prefrontal cortex has been shown to mediate a variety of executive processes, including working memory, cognitive control, and action selection (Arnsten & Li, 2005; Goldman-Rakic, 1996; Robbins & Arnsten, 2009). The ability of dopamine to facilitate prefrontal cortex signal-to-noise processing is thought to underlie the ability to actively stabilize task-compatible representations, and inhibit task-incompatible representations, to support behavior (Cohen, Braver, & Brown, 2002; Durstewitz & Seamans, 2002; Durstewitz, Seamans, & Sejnowski, 2000; Winterer et al., 2004). Similar processes have also been shown to mediate the prefrontal cortex’s role in memory retrieval (Floresco & Phillips, 2001; Phillips, Ahn, & Floresco, 2004).
Dopaminergic mechanisms in the hippocampus may also foster the use of prior relevant experience in service of adaptive behavior (Shohamy & Adcock, 2010). Dopamine, particularly the enhanced dopamine signaling in adolescence, results in increased novelty-seeking and exploratory behaviors (Ikemoto & Panksepp, 1999), specifically behaviors that reflect hippocampal-mediated, active acquisition of information from novel environments, rather than simple orientation to novel stimuli. Beyond information seeking, animal models have shown that dopamine enhances episodic memory encoding and stabilizes active representations into long-term memory (Lisman & Grace, 2005; Wang & Morris, 2010). More recently, human research shows that interactions between mesolimbic dopamine systems and hippocampus enhances associative memory encoding (Adcock, Thangavel, Whitfield-Gabrieli, Knutson, & Gabrieli, 2006; Murty & Adcock, 2014; Shohamy & Wagner, 2008; Wolosin, Zeithamova, & Preston, 2012), resulting in rich representations of prior experiences. These findings suggest that the increases in dopamine signaling during adolescence independently increase the number of experiences stored in long-term memory as well as the fidelity of retrieved memory representations during goal-relevant behavior.
More relevant to our proposed model, dopaminergic neuromodulation has further been shown to directly support the integration of information across HPC-PFC. Dopamine signaling from the ventral tegmental area (VTA), the source of mesolimbic dopamine neurons, has been proposed to set rhythmic patterns of excitatory firing to synchronize neural signaling across discrete regions (Tibor Koós & Tepper, 2002; T. Koós & Tepper, 1999). Rodent research has shown synchronized activity across the VTA, PFC, and hippocampus as well as dopaminergic neuromodulation of HPC-PFC interactions during spatial working memory (Seamans, Floresco, & Phillips, 1998). Of particular relevance, dopamine may specifically influence prefrontal-dependent retrieval of prior experiences from the hippocampus. Research has demonstrated that dopamine-dependent modulation over HPC-PFC connectivity was necessary to perform a working memory task that required retrieval of responses on previous trials, i.e., a spatial delayed-alternation task (Goto & Grace, 2008). Its peak in availability may serve as a trigger for associative memory processing to become predominant. These findings support that enhanced dopaminergic signaling in adolescents may directly facilitate information processing across the prefrontal cortex and hippocampus.
Beyond supporting information processing across the prefrontal cortex and hippocampus, dopamine is known to play a key role in plasticity by stabilizing the integration of discrete networks into adulthood (Jay, 2003). A long history of research has implicated dopamine signaling in both learning and plasticity. Rodent studies have demonstrated that hippocampal long-term potentiation (LTP), a cellular marker of experience-dependent plasticity, is mediated by dopamine (Huang & Kandel, 1995; Li, Cullen, Anwyl, & Rowan, 2003). For example, application of dopamine within the hippocampus mimics neurophysiological profiles induced by standard experimental protocols of experience-dependent plasticity, and dopaminergic antagonists will block plasticity during these protocols (Huang & Kandel, 1995). Similarly, dopamine has been shown to enhance neuroplasticity and reorganization of cortical circuits (Bao, Chan, and Merzenich 2001). Most relevant to our model, animal research has shown that plasticity across hippocampal-prefrontal circuits is driven by dopaminergic neuromodulation (Gurden, Takita, & Jay, 2000; Gurden, Tassin, & Jay, 1999; Jay, 2003). For example, the magnitude of dopamine levels in the prefrontal cortex has been shown to predict the magnitude of plasticity between the hippocampus and prefrontal cortex, as measured by LTP (Gurden et al., 1999). Within our framework, during childhood dopamine neuromodulation may not be strong enough to establish reliable network integration, but during adolescence elevated dopamine signaling could support long-lasting communication across the hippocampus and prefrontal cortex.
Critically, the role of dopamine in guiding plasticity across these circuits may be specifically driven by mechanisms of reward learning, which is amplified during adolescent development. Computational models have suggested that behaviors that lead to reward result in the release of dopamine and that this dopamine release stabilizes the associated neural circuitry (Frank, Loughry, & O’Reilly, 2001; Montague, Hyman, & Cohen, 2004). If the integration of function across HPC-PFC circuits yields higher reward rates, dopamine signaling could reinforce integrated processing across this circuit. During adolescence, there is increased sensitivity to rewarding outcomes, which would result in greater amounts of dopamine release and greater opportunity to refine HPC-PFC circuits. Further, during adolescence, there are increases in exploratory and novelty-seeking behaviors, which give individuals the opportunity to refine this circuit in a variety of novel situations. This type of reward-guided plasticity would in turn allow adolescents to establish connectivity across the hippocampus and prefrontal cortex to flexibly support information processing across diverse contextual domains. As individuals move into adulthood, decreasing of dopamine signaling would dampen the hypersensitivity from rewards, stabilizing the degree of plasticity to adaptive levels necessary for reliable consistent adult level functioning. Together, these different lines of research provide compelling support for adolescence being a unique period of plasticity and refinement of HPC-PFC circuits for the establishment of contextually-relevant responses to guide and optimize goal-oriented behaviors.
Together, these literatures provide a viable mechanism as to how prefrontal-hippocampal integration may reach a critical threshold during adolescence. Namely, dopamine increases the stability of prior experiences in long-term memory while simultaneously increasing signal-to-noise processing within the prefrontal cortex. In addition, greater dopamine may serve to motivate the search for novel experiences regardless of risk that can support independence. Further, dopamine has properties that would allow information processing to be distributed between nodes of this circuit, as opposed to being processed independently in the hippocampus or prefrontal cortex. Finally, dopamine has properties that allow these changes in network connectivity to be refined based off of behavioral success and to stabilize these networks not only during adolescence, but throughout adulthood. However, the role of development in integrating these processes has yet to be tested. Further research is warranted in relating changes in dopamine signaling with both the integration of appropriate prior experiences in task-relevant behavior and HPC-PFC integration.
7. Conclusions
Adolescence is a time characterized by heightened sensation-seeking and a newly acquired access to prefrontal executive function that motivates exploration and experience gathering. We introduce the Experience-Driven Adaptive Cognitive Model of adolescence that integrates the retrieval of relevant prior experiences with refinement in prefrontal executive function. Together, this integration may underlie the ability to establish adaptive heuristics critical for adult level functioning. We identified two interrelated brain mechanisms, HPC-PFC integration and dopaminergic neuromodulation over this circuitry, that support this distinct adolescent mode of operation. The model proposes that elevated dopamine signaling in adolescence strengthens HPC-PFC interactions, allowing increased flexibility in the use of prior experience to guide task-relevant behavior. In the current review, we highlight the role of this circuit in the domains of episodic memory, executive function, and decision-making. Critically, prior research has implicated a role of integrating prior experiences with task-relevant demands in other areas of cognition, including attention (Hutchinson & Turk-Browne, 2012), social cognition (Murty, FeldmanHall, Hunter, Phelps, & Davachi, 2016; Rubin, Watson, Duff, & Cohen, 2014), and emotion processing (LaBar & Cabeza, 2006; Murty, Ritchey, Adcock, & LaBar, 2010). Further, emerging evidence suggests that behaviors associated with these cognitive domains may rely on interactions between the hippocampus and prefrontal cortex (Shohamy & Turk-Browne, 2013). Future areas of empirical and theoretical research will need to test how our proposed model generalizes to these other domains. While the hippocampus and prefrontal cortex are each capable of a wide range of functions prior to adolescence, this increased coupling compels experience gathering. While this increased motivation to gather novel experiences can lead to a variety of risk taking behavior which could be maladaptive, these processes may be critical to the adaptive transition to adult levels of executive function.
This period of active coordination of major brain systems may reflect a period of unique plasticity to afford selection of optimal approaches for decision-making. As such, this is an adaptive period where obtaining a broad range of experiences driven by reward expectation may support greater specialization. This stage of active specialization, however, may also be particularly vulnerable to the emergence of psychopathology including schizophrenia, mood disorders, and substance use disorders, all of which have been associated with abnormal function in pre-frontal, hippocampal, and dopamine systems. The nature of vulnerability during this period of development highlights the critical role of experience in affecting neurodevelopmental trajectories throughout the lifespan. For example, previous research in animal models and humans have demonstrated that exposure to drugs of abuse during adolescence results in alterations in HPC volume and neurophysiology that has downstream consequences on drug abuse and affective disorders (see Spear, 2016 and Silveri et al, 2016 in the same issue), which may have downstream consequences on HPC-PFC interactions. Characterizing this period of adolescent development, and its sensitivity to drugs of abuse and other environmental insults, is critical to a greater understanding of normative increases in sensation seeking and psychopathology.
In summary, the ability to incorporate experience to task relevant behavior is a core process throughout development. Earlier in development, this mechanism becomes critical in the discovery of the environment and accumulation of relevant experiences. However, executive systems are immature, undermining the ability to plan goal directed behavior and limiting the extent of self-generated exploration. By adolescence, an arsenal of relevant experiences has become available. These experiences in conjunction with adult-level prefrontal executive function allows exploration with an unprecedented ability to plan goal directed behavior independent from adult assistance. During adolescence, the relative maturation of prefrontal executive systems, hippocampal memory systems, and increased dopamine processing may support a qualitative shift in the dynamic integration amongst these systems. This integration could support a qualitative change from experience accumulation to the selection and organization of prior experiences in order to support future behavior. A peak in sensation seeking, driven by heightened dopamine signaling, and the relative maturation of HPC-PFC interactions may support the prioritization of integrating prior experiences in a flexible manner to individually specialize behaviors to environmental demands. As a consequence, the ability to readily and dynamically integrate hippocampal and prefrontal systems affords cognition that is enhanced by the integration of experience.
Highlights.
Retrieval of relevant prior experience can facilitate executive function.
Adolescents refine the ability to integrate prior experience to support task behavior.
Hippocampal-prefrontal integration continues to mature throughout adolescence.
Dopamine signaling during adolescence may support hippocampal-prefrontal integration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Abrahám H, Vincze A, Jewgenow I, Veszprémi B, Kravják A, Gömöri E, Seress L. Myelination in the human hippocampal formation from midgestation to adulthood. International Journal of Developmental Neuroscience: The Official Journal of the International Society for Developmental Neuroscience. 2010;28(5):401–410. doi: 10.1016/j.ijdevneu.2010.03.004. http://doi.org/10.1016/j.ijdevneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50(3):507–517. doi: 10.1016/j.neuron.2006.03.036. http://doi.org/10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Alm KH, Rolheiser T, Olson IR. Inter-individual variation in fronto-temporal connectivity predicts the ability to learn different types of associations. NeuroImage. 2016;132:213–224. doi: 10.1016/j.neuroimage.2016.02.038. http://doi.org/10.1016/j.neuroimage.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. http://doi.org/10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/−)-7-OH-DPAT. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1997;356(2):173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biological Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. http://doi.org/10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bähner F, Demanuele C, Schweiger J, Gerchen MF, Zamoscik V, Ueltzhöffer K, … Meyer-Lindenberg A. Hippocampal-dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: a human translational imaging study. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2015;40(7):1674–1681. doi: 10.1038/npp.2015.13. http://doi.org/10.1038/npp.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin. 2000;52(5):319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5(6):511–533. doi: 10.1002/hipo.450050604. http://doi.org/10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, … Reiss AL. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral Cortex (New York, NY: 1991) 2005;15(12):1848–1854. doi: 10.1093/cercor/bhi062. http://doi.org/10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophrenia Bulletin. 1989;15(4):585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51(6):477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, Lou Smith M, Pat McAndrews M. Developmental patterns in priming and familiarity in explicit recollection. Journal of Experimental Child Psychology. 2002;82(3):251–277. doi: 10.1016/s0022-0965(02)00007-3. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2007;13(3):280–291. doi: 10.1177/1073858407299290. http://doi.org/10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Borst G, Aïte A, Houdé O. Inhibition of misleading heuristics as a core mechanism for typical cognitive development: evidence from behavioural and brain-imaging studies. Developmental Medicine and Child Neurology. 2015;57(Suppl 2):21–25. doi: 10.1111/dmcn.12688. http://doi.org/10.1111/dmcn.12688. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Holliday RE, Reyna VF. Behavioral measurement of remembering phenomenologies: so simple a child can do it. Child Development. 2004;75(2):505–522. doi: 10.1111/j.1467-8624.2004.00689.x. http://doi.org/10.1111/j.1467-8624.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Wise SP, Murray EA. Interaction of ventral and orbital prefrontal cortex with inferotemporal cortex in conditional visuomotor learning. Behavioral Neuroscience. 2002;116(4):703–715. [PubMed] [Google Scholar]
- Cavada C, Compañy T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suárez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex (New York, NY: 1991) 2000;10(3):220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cocchi E, Drago A, Serretti A. Hippocampal Pruning as a New Theory of Schizophrenia Etiopathogenesis. Molecular Neurobiology. 2016;53(3):2065–2081. doi: 10.1007/s12035-015-9174-6. http://doi.org/10.1007/s12035-015-9174-6. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Braver TS, Brown JW. Computational perspectives on dopamine function in prefrontal cortex. Current Opinion in Neurobiology. 2002;12(2):223–229. doi: 10.1016/s0959-4388(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Snodgrass JG, Duff M. Recognition and source memory for pictures in children and adults. Neuropsychologia. 2001;39(3):255–267. doi: 10.1016/s0028-3932(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. http://doi.org/10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Daugherty AM, Bender AR, Raz N, Ofen N. Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus. 2016;26(2):220–228. doi: 10.1002/hipo.22517. http://doi.org/10.1002/hipo.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16(6):693–700. doi: 10.1016/j.conb.2006.10.012. http://doi.org/10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Decker JH, Lourenco FS, Doll BB, Hartley CA. Experiential reward learning outweighs instruction prior to adulthood. Cognitive, Affective & Behavioral Neuroscience. 2015;15(2):310–320. doi: 10.3758/s13415-014-0332-5. http://doi.org/10.3758/s13415-014-0332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker JH, Otto AR, Daw ND, Hartley CA. From Creatures of Habit to Goal-Directed Learners: Tracking the Developmental Emergence of Model-Based Reinforcement Learning. Psychological Science. 2016;27(6):848–858. doi: 10.1177/0956797616639301. http://doi.org/10.1177/0956797616639301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Lee JK, Ghetti S. Structural development of the hippocampus and episodic memory: developmental differences along the anterior/posterior axis. Cerebral Cortex (New York, NY: 1991) 2014;24(11):3036–3045. doi: 10.1093/cercor/bht160. http://doi.org/10.1093/cercor/bht160. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. http://doi.org/10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Doll BB, Duncan KD, Simon DA, Shohamy D, Daw ND. Model-based choices involve prospective neural activity. Nature Neuroscience. 2015;18(5):767–772. doi: 10.1038/nn.3981. http://doi.org/10.1038/nn.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Shohamy D, Daw ND. Multiple memory systems as substrates for multiple decision systems. Neurobiology of Learning and Memory. 2015;117:4–13. doi: 10.1016/j.nlm.2014.04.014. http://doi.org/10.1016/j.nlm.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll BB, Simon DA, Daw ND. The ubiquity of model-based reinforcement learning. Current Opinion in Neurobiology. 2012;22(6):1075–1081. doi: 10.1016/j.conb.2012.08.003. http://doi.org/10.1016/j.conb.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudukovic NM, Preston AR, Archie JJ, Glover GH, Wagner AD. High-resolution fMRI reveals match enhancement and attentional modulation in the human medial temporal lobe. Journal of Cognitive Neuroscience. 2011;23(3):670–682. doi: 10.1162/jocn.2010.21509. http://doi.org/10.1162/jocn.2010.21509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The computational role of dopamine D1 receptors in working memory. Neural Networks: The Official Journal of the International Neural Network Society. 2002;15(4–6):561–572. doi: 10.1016/s0893-6080(02)00049-7. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nature Neuroscience. 2000;3(Suppl):1184–1191. doi: 10.1038/81460. http://doi.org/10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D. Inferotemporal-frontal Disconnection: The Uncinate Fascicle and Visual Associative Learning in Monkeys. The European Journal of Neuroscience. 1992;4(12):1320–1332. doi: 10.1111/j.1460-9568.1992.tb00157.x. [DOI] [PubMed] [Google Scholar]
- Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochirurgica. 1992;115(3–4):143–148. doi: 10.1007/BF01406373. [DOI] [PubMed] [Google Scholar]
- Eckenhoff MF, Rakic P. A quantitative analysis of synaptogenesis in the molecular layer of the dentate gyrus in the rhesus monkey. Brain Research. Developmental Brain Research. 1991;64(1–2):129–135. doi: 10.1016/0165-3806(91)90216-6. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. http://doi.org/10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston GN. Pyramidal cells of the frontal lobe: all the more spinous to think with. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(18):RC95. doi: 10.1523/JNEUROSCI.20-18-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cerebral Cortex (New York, NY: 1991) 2007;17(12):2760–2768. doi: 10.1093/cercor/bhm003. http://doi.org/10.1093/cercor/bhm003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, … Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Computational Biology. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. http://doi.org/10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AS, Kalra PB, Goetz C, Leonard JA, Sheridan MA, Gabrieli JDE. Developmental dissociation between the maturation of procedural memory and declarative memory. Journal of Experimental Child Psychology. 2016;142:212–220. doi: 10.1016/j.jecp.2015.09.027. http://doi.org/10.1016/j.jecp.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CLH, Hinshaw S, D’Esposito M. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(33):11062–11067. doi: 10.1523/JNEUROSCI.6266-09.2010. http://doi.org/10.1523/JNEUROSCI.6266-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behavioral Neuroscience. 2001;115(4):934–939. [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cognitive, Affective & Behavioral Neuroscience. 2001;1(2):137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cerebral Cortex (New York, NY: 1991) 2011;21(1):145–154. doi: 10.1093/cercor/bhq071. http://doi.org/10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- Gaffan EA, Gaffan D, Harrison S. Disconnection of the amygdala from visual association cortex impairs visual reward-association learning in monkeys. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1988;8(9):3144–3150. doi: 10.1523/JNEUROSCI.08-09-03144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Smith IM. Executive function in preschoolers: A review using an integrative framework. Psychological Bulletin. 2008;134(1):31–60. doi: 10.1037/0033-2909.134.1.31. http://doi.org/10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from the dual-process signal detection model. Child Development. 2008;79(2):339–358. doi: 10.1111/j.1467-8624.2007.01129.x. http://doi.org/10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Bunge SA. Neural changes underlying the development of episodic memory during middle childhood. Developmental Cognitive Neuroscience. 2012;2(4):381–395. doi: 10.1016/j.dcn.2012.05.002. http://doi.org/10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigerenzer G, Gaissmaier W. Heuristic decision making. Annual Review of Psychology. 2011;62:451–482. doi: 10.1146/annurev-psych-120709-145346. http://doi.org/10.1146/annurev-psych-120709-145346. [DOI] [PubMed] [Google Scholar]
- Gluth S, Sommer T, Rieskamp J, Büchel C. Effective Connectivity between Hippocampus and Ventromedial Prefrontal Cortex Controls Preferential Choices from Memory. Neuron. 2015;86(4):1078–1090. doi: 10.1016/j.neuron.2015.04.023. http://doi.org/10.1016/j.neuron.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, … Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. http://doi.org/10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM, … Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16(8):664–672. doi: 10.1002/hipo.20193. http://doi.org/10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1996;351(1346):1445–1453. doi: 10.1098/rstb.1996.0129. http://doi.org/10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophrenia Bulletin. 2008;34(5):944–961. doi: 10.1093/schbul/sbn070. http://doi.org/10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine modulation of hippocampal-prefrontal cortical interaction drives memory-guided behavior. Cerebral Cortex (New York, NY: 1991) 2008;18(6):1407–1414. doi: 10.1093/cercor/bhm172. http://doi.org/10.1093/cercor/bhm172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(22):RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurden H, Tassin JP, Jay TM. Integrity of the mesocortical dopaminergic system is necessary for complete expression of in vivo hippocampal-prefrontal cortex long-term potentiation. Neuroscience. 1999;94(4):1019–1027. doi: 10.1016/s0306-4522(99)00395-4. [DOI] [PubMed] [Google Scholar]
- Gutnikov SA, Ma YY, Buckley MJ, Gaffan D. Monkeys can associate visual stimuli with reward delayed by 1 s even after perirhinal cortex ablation, uncinate fascicle section or amygdalectomy. Behavioural Brain Research. 1997;87(1):85–96. doi: 10.1016/s0166-4328(96)02259-0. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Somerville LH. The neuroscience of adolescent decision-making. Current Opinion in Behavioral Sciences. 2015;5:108–115. doi: 10.1016/j.cobeha.2015.09.004. http://doi.org/10.1016/j.cobeha.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, … Ewing-Cobbs L. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Research. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. http://doi.org/10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. Journal of Neurochemistry. 2003;87(3):574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Rekart JL, Sandoval J, Routtenberg A. Spatial learning induces presynaptic structural remodeling in the hippocampal mossy fiber system of two rat strains. Hippocampus. 2006;16(6):560–570. doi: 10.1002/hipo.20185. http://doi.org/10.1002/hipo.20185. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(7):2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS, Burk JA, Barnet RC. Adolescent transiions in reflexive and non-reflexive behavior: Review of fear conditoninig and impulse control in rodent models. Neuroscience and Biobehavioral Reviews. 2016 doi: 10.1016/j.neubiorev.2016.06.026. In-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Turk-Browne NB. Memory-guided attention: control from multiple memory systems. Trends in Cognitive Sciences. 2012;16(12):576–579. doi: 10.1016/j.tics.2012.10.003. http://doi.org/10.1016/j.tics.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Ghuman AS, Manoach DS, Jones SR, Luna B. Frontal preparatory neural oscillations associated with cognitive control: A developmental study comparing young adults and adolescents. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.05.017. http://doi.org/10.1016/j.neuroimage.2016.05.017. [DOI] [PMC free article] [PubMed]
- Hwang K, Hallquist MN, Luna B. The development of hub architecture in the human functional brain network. Cerebral Cortex (New York, NY: 1991) 2013;23(10):2380–2393. doi: 10.1093/cercor/bhs227. http://doi.org/10.1093/cercor/bhs227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. http://doi.org/10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Research. Brain Research Reviews. 1999;31(1):6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jay TM. Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Progress in Neurobiology. 2003;69(6):375–390. doi: 10.1016/s0301-0082(03)00085-6. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. The Journal of Comparative Neurology. 1991;313(4):574–586. doi: 10.1002/cne.903130404. http://doi.org/10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- Jones LB, Rothbart MK, Posner MI. Development of executive attention in pre-school children. Developmental Science. 2003;6(5):498–504. http://doi.org/10.1111/1467-7687.00307. [Google Scholar]
- Koós T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABA-ergic interneurons. Nature Neuroscience. 1999;2(5):467–472. doi: 10.1038/8138. http://doi.org/10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koós T, Tepper JM. Dual cholinergic control of fast-spiking interneurons in the neostriatum. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22(2):529–535. doi: 10.1523/JNEUROSCI.22-02-00529.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Summerfield JJ, Hassabis D, Maguire EA. Tracking the emergence of conceptual knowledge during human decision making. Neuron. 2009;63(6):889–901. doi: 10.1016/j.neuron.2009.07.030. http://doi.org/10.1016/j.neuron.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Payne JW, Cohen AL, Huettel SA. The Rational Adolescent: Strategic Information Processing during Decision Making Revealed by Eye Tracking. Cognitive Development. 2015;36:20–30. doi: 10.1016/j.cogdev.2015.08.001. http://doi.org/10.1016/j.cogdev.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews. Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. http://doi.org/10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage. 2012;60(1):340–352. doi: 10.1016/j.neuroimage.2011.11.094. http://doi.org/10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- Lee JK, Ekstrom AD, Ghetti S. Volume of hippocampal subfields and episodic memory in childhood and adolescence. NeuroImage. 2014;94:162–171. doi: 10.1016/j.neuroimage.2014.03.019. http://doi.org/10.1016/j.neuroimage.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Lewis-Peacock JA, Postle BR. Temporary activation of long-term memory supports working memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(35):8765–8771. doi: 10.1523/JNEUROSCI.1953-08.2008. http://doi.org/10.1523/JNEUROSCI.1953-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nature Neuroscience. 2003;6(5):526–531. doi: 10.1038/nn1049. http://doi.org/10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–713. doi: 10.1016/j.neuron.2005.05.002. http://doi.org/10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annual Review of Neuroscience. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. http://doi.org/10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Wright C. Adolescent brain development: implciations to the juvenile criminal justice system. American Psychological Association; 2016. [Google Scholar]
- Marek S, Hwang K, Foran W, Hallquist MN, Luna B. The Contribution of Network Organization and Integration to the Development of Cognitive Control. PLoS Biology. 2015;13(12):e1002328. doi: 10.1371/journal.pbio.1002328. http://doi.org/10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. Journal of Neurophysiology. 2012;108(6):1620–1630. doi: 10.1152/jn.00077.2012. http://doi.org/10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Vakorin V, Kovacevic N, Wang H, Diaconescu A, Protzner AB. Spatiotemporal dependency of age-related changes in brain signal variability. Cerebral Cortex (New York, NY: 1991) 2014;24(7):1806–1817. doi: 10.1093/cercor/bht030. http://doi.org/10.1093/cercor/bht030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng SZ, Ozawa Y, Itoh M, Takashima S. Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Research. 1999;843(1–2):136–144. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Brain Research. Cognitive Brain Research. 2005;25(1):379–385. doi: 10.1016/j.cogbrainres.2005.07.007. http://doi.org/10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Meulemans T, Van der Linden M, Perruchet P. Implicit sequence learning in children. Journal of Experimental Child Psychology. 1998;69(3):199–221. doi: 10.1006/jecp.1998.2442. http://doi.org/10.1006/jecp.1998.2442. [DOI] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431(7010):760–767. doi: 10.1038/nature03015. http://doi.org/10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Murty VP, Adcock RA. Enriched encoding: reward motivation organizes cortical networks for hippocampal detection of unexpected events. Cerebral Cortex (New York, NY: 1991) 2014;24(8):2160–2168. doi: 10.1093/cercor/bht063. http://doi.org/10.1093/cercor/bht063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, FeldmanHall O, Hunter LE, Phelps EA, Davachi L. Episodic memories predict adaptive value-based decision-making. Journal of Experimental Psychology. General. 2016;145(5):548–558. doi: 10.1037/xge0000158. http://doi.org/10.1037/xge0000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Ritchey M, Adcock RA, LaBar KS. fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia. 2010;48(12):3459–3469. doi: 10.1016/j.neuropsychologia.2010.07.030. http://doi.org/10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Chai XJ, Schuil KDI, Whitfield-Gabrieli S, Gabrieli JDE. The development of brain systems associated with successful memory retrieval of scenes. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(29):10012–10020. doi: 10.1523/JNEUROSCI.1082-11.2012. http://doi.org/10.1523/JNEUROSCI.1082-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JDE. Development of the declarative memory system in the human brain. Nature Neuroscience. 2007;10(9):1198–1205. doi: 10.1038/nn1950. http://doi.org/10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Nichols EA, Chen J, Hunt JF, Glover GH, Gabrieli JDE, Wagner AD. Performance-related sustained and anticipatory activity in human medial temporal lobe during delayed match-to-sample. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(38):11880–11890. doi: 10.1523/JNEUROSCI.2245-09.2009. http://doi.org/10.1523/JNEUROSCI.2245-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Von Der Heide RJ, Alm KH, Vyas G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Developmental Cognitive Neuroscience. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. http://doi.org/10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2013;33(46):18109–18124. doi: 10.1523/JNEUROSCI.1741-13.2013. http://doi.org/10.1523/JNEUROSCI.1741-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto AR, Raio CM, Chiang A, Phelps EA, Daw ND. Working-memory capacity protects model-based learning from stress. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(52):20941–20946. doi: 10.1073/pnas.1312011110. http://doi.org/10.1073/pnas.1312011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto AR, Skatova A, Madlon-Kay S, Daw ND. Cognitive control predicts use of model-based reinforcement learning. Journal of Cognitive Neuroscience. 2015;27(2):319–333. doi: 10.1162/jocn_a_00709. http://doi.org/10.1162/jocn_a_00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Luna B. Developmental imaging genetics: linking dopamine function to adolescent behavior. Brain and Cognition. 2014;89:27–38. doi: 10.1016/j.bandc.2013.09.011. http://doi.org/10.1016/j.bandc.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A, Gaffan D. Memory after frontal/temporal disconnection in monkeys: conditional and non-conditional tasks, unilateral and bilateral frontal lesions. Neuropsychologia. 1998;36(3):259–271. doi: 10.1016/s0028-3932(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(32):13281–13286. doi: 10.1073/pnas.1105108108. http://doi.org/10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial pre-frontal cortex predicts accuracy of memory on a delayed response task. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2004;24(2):547–553. doi: 10.1523/JNEUROSCI.4653-03.2004. http://doi.org/10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piolino P, Hisland M, Ruffeveille I, Matuszewski V, Jambaqué I, Eustache F. Do school-age children remember or know the personal past? Consciousness and Cognition. 2007;16(1):84–101. doi: 10.1016/j.concog.2005.09.010. http://doi.org/10.1016/j.concog.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends in Cognitive Sciences. 2013;17(5):230–240. doi: 10.1016/j.tics.2013.03.005. http://doi.org/10.1016/j.tics.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Current Biology: CB. 2013;23(17):R764–773. doi: 10.1016/j.cub.2013.05.041. http://doi.org/10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Cho S, Chen T, Rosenberg-Lee M, Geary DC, Menon V. Hippocampal-neocortical functional reorganization underlies children’s cognitive development. Nature Neuroscience. 2014;17(9):1263–1269. doi: 10.1038/nn.3788. http://doi.org/10.1038/nn.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Amaya V, Balderas I, Sandoval J, Escobar ML, Bermúdez-Rattoni F. Spatial long-term memory is related to mossy fiber synaptogenesis. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21(18):7340–7348. doi: 10.1523/JNEUROSCI.21-18-07340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20(11):1263–1290. doi: 10.1002/hipo.20852. http://doi.org/10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Greenstein D, Lee NR, Clasen LS, Giedd JN. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11366–11371. doi: 10.1073/pnas.1203350109. http://doi.org/10.1073/pnas.1203350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw PW, Lerch JP, Clasen LS, Greenstein D, Berman R, … Giedd JN. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):1592–1597. doi: 10.1073/pnas.1316911111. http://doi.org/10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Geng F, Blankenship SL, Redcay E. Hippocampal functional connectivity and episodic memory in early childhood. Developmental Cognitive Neuroscience. 2016;19:58–69. doi: 10.1016/j.dcn.2016.02.002. http://doi.org/10.1016/j.dcn.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AFT. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annual Review of Neuroscience. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. http://doi.org/10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RD, Watson PD, Duff MC, Cohen NJ. The role of the hippocampus in flexible cognition and social behavior. Frontiers in Human Neuroscience. 2014;8:742. doi: 10.3389/fnhum.2014.00742. http://doi.org/10.3389/fnhum.2014.00742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1998;18(4):1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Developmental Cognitive Neuroscience. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. http://doi.org/10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends in Cognitive Sciences. 2010;14(10):464–472. doi: 10.1016/j.tics.2010.08.002. http://doi.org/10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Turk-Browne NB. Mechanisms for widespread hippocampal involvement in cognition. Journal of Experimental Psychology. General. 2013;142(4):1159–1170. doi: 10.1037/a0034461. http://doi.org/10.1037/a0034461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating memories in the human brain: hippocampal-midbrain encoding of overlapping events. Neuron. 2008;60(2):378–389. doi: 10.1016/j.neuron.2008.09.023. http://doi.org/10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L. The dual systems model: Review, reappraisal, and reaffirmation. Developmental Cognitive Neuroscience. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. http://doi.org/10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Duvarci S. Hippocampal-Prefrontal Interactions in Cognition, Behavior and Psychiatric Disease. Frontiers in Systems Neuroscience. 2015;9:190. doi: 10.3389/fnsys.2015.00190. http://doi.org/10.3389/fnsys.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Dager AD, Cohen-Gilbert JE, Sneider JT. Neurobiological signatures associated with alocohol and drug use in the human adolescent brain. Neuroscience and Biobehavioral Reviews. 2016 doi: 10.1016/j.neubiorev.2016.06.042. In-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Hallquist MN, Asato M, Luna B. Developmental stages and sex differences of white matter and behavioral development through adolescence: a longitudinal diffusion tensor imaging (DTI) study. NeuroImage. 2014;92:356–368. doi: 10.1016/j.neuroimage.2013.12.044. http://doi.org/10.1016/j.neuroimage.2013.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews. Neuroscience. 2003;4(8):637–648. doi: 10.1038/nrn1178. http://doi.org/10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neuroscience and Biobehavioral Reviews. 2016 doi: 10.1016/j.neubiorev.2016.07.026. In-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. The Journal of Comparative Neurology. 1977;172(1):49–84. doi: 10.1002/cne.901720104. http://doi.org/10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Fjell AM, Westlye LT, Østby Y, Walhovd KB. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(3):972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. http://doi.org/10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Engvig A, Grydeland H, Krogsrud SK, Østby Y, … Fjell AM. Regional hippocampal volumes and development predict learning and memory. Developmental Neuroscience. 2014;36(3–4):161–174. doi: 10.1159/000362445. http://doi.org/10.1159/000362445. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Developmental Neuroscience. 1999;21(1):43–49. doi: 10.1159/000017365. http://doi.org/17365. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89(2):167–172. doi: 10.1016/0165-3806(95)00109-q. http://doi.org/10.1016/0165-3806(95)00109-Q. [DOI] [PubMed] [Google Scholar]
- Thomas C, Avram A, Pierpaoli C, Baker C. Diffusion MRI properties of the human uncinate fasciculus correlate with the ability to learn visual associations. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2015;72:65–78. doi: 10.1016/j.cortex.2015.01.023. http://doi.org/10.1016/j.cortex.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain: A Journal of Neurology. 2013;136(Pt 6):1692–1707. doi: 10.1093/brain/awt094. http://doi.org/10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, O’Neil JT, Kharitonova M, Briggs-Gowan MJ, Wakschlag LS. Adolescent development of context-dependent stimulus-reward association memory and its neural correlates. Frontiers in Human Neuroscience. 2015;9:581. doi: 10.3389/fnhum.2015.00581. http://doi.org/10.3389/fnhum.2015.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience and Biobehavioral Reviews. 2010;34(5):631–648. doi: 10.1016/j.neubiorev.2009.12.007. http://doi.org/10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Morris RGM. Hippocampal-Neocortical Interactions in Memory Formation, Consolidation, and Reconsolidation. Annual Review of Psychology. 2010;61(1):49–79. doi: 10.1146/annurev.psych.093008.100523. http://doi.org/10.1146/annurev.psych.093008.100523. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, … Akil M. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2007;144(3):1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. http://doi.org/10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR. Prefrontal broadband noise, working memory, and genetic risk for schizophrenia. The American Journal of Psychiatry. 2004;161(3):490–500. doi: 10.1176/appi.ajp.161.3.490. http://doi.org/10.1176/appi.ajp.161.3.490. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Reward modulation of hippocampal subfield activation during successful associative encoding and retrieval. Journal of Cognitive Neuroscience. 2012;24(7):1532–1547. doi: 10.1162/jocn_a_00237. http://doi.org/10.1162/jocn_a_00237. [DOI] [PMC free article] [PubMed] [Google Scholar]