Abstract
Bombesin, a pan agonist of the bombesin-like peptide receptor family, elicits potent hypothermia when applied centrally. The signaling mechanisms involved are not known. Here we report that GABAergic preoptic neurons express gastrin-releasing peptide (GRP) receptors and are directly excited by GRP or bombesin. This effect was abolished by a GRP receptor antagonist. A partially overlapping group of preoptic GABAergic neurons express bombesin-like receptor 3 (BRS3), however their activation results in a decrease in firing rate. The excitatory effects of bombesin or GRP were not affected by BRS3 antagonist. GRP activated a Ca2+-dependent inward nonselective cationic current and Ca2+ release from intracellular stores. Our data indicate that GRP receptors mediate the excitatory effects of bombesin in preoptic neurons
Keywords: GRP, Preoptic neuron, BRS3, bombesin
Mammalian bombesin-like peptide receptors, a family of G protein coupled receptors that is comprised of the bombesin-like receptor-3 (BRS3), the gastrin-releasing peptide receptor (GRPR), and the neuromedin B receptor (NMBR) are widely distributed in the central nervous system and modulate metabolism and various behaviors (reviewed in (Ohki-Hamazaki et al., 2005)). Intracerebroventricular injection of bombesin, the pan-agonist for all three receptors, induces a fast (within minutes) and robust (3 - 4 degrees C) hypothermia, however the cellular mechanisms and receptor subtypes involved are not fully understood (Tsushima et al., 2003).
Recent studies have revealed that preoptic GABAergic neurons play an important role in the control of thermoregulatory networks that comprise also neurons of the rostral raphe pallidus and dorsomedial hypothalamus (reviewed in (Morrison et al., 2012)). The firing activity of preoptic GABAergic neurons provides an inhibitory tone on downstream thermoeffector neurons. A decrease in the firing rate of preoptic GABAergic neurons can account for the increased thermogenesis associated with fever (Morrison and Madden, 2014) or other hyperthermic responses (Lundius et al., 2010, Sanchez-Alavez et al., 2010). In this study we have investigated the effects of bombesin on identified preoptic GABAergic neurons and the receptor subtypes involved.
Materials and Methods
Slice Preparation
Coronal tissue slices containing the median preoptic nucleus (MnPO) were prepared from GAD65-GFP mice (28–42 d old) housed in standard conditions as previously described (Lundius et al., 2010) and in accordance to procedures approved by The Scripps Research Institute Animal Welfare Committee. This transgenic mouse line expresses enhanced green fluorescent protein (eGFP) under the control of the regulatory region of mouse glutamic acid decarboxylase (GAD) 65 gene. The mice were a kind gift from Dr. Gabor Szabo (Hungarian Academy of Sciences, Budapest, Hungary). The slice used in our recordings corresponded to the sections located from 0.5 mm to 0.25 mm from Bregma in the mouse brain atlas (Paxinos, 2001).
Whole-cell patch-clamp recording
The aCSF contained (in mM) the following: 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 2 CaCl2, 1 MgSO4, and 10 glucose, osmolarity of 300–305 mOsm, equilibrated with 95% O2 and 5% CO2, pH 7.4. Other salts and agents were added to this medium. A set of experiments was carried out in Ca2+-free aCSF which contained (in mM) 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 0.5 EGTA, 3 MgSO4, and 10 glucose. In a set of experiments the extracellular solution was exchanged with a Na+-free buffer (“NMDG external solution”) containing 155 N-methyl-d-glucamine, 3.5 KCl, 2 CaCl2, 1 MgSO4, 10 glucose, and 10 HEPES (pH 7.4, adjusted with HCl). For cell-attached recordings the patch pipette was filled with aCSF. Whole-cell recordings were carried out using either a K+ or a Cs+ pipette solution. The K+ pipette solution contained (in mM) 130 K-gluconate, 5 KCl, 10 HEPES, 2 MgCl2, 0.5 EGTA, 2 ATP and 1 GTP (pH 7.3) was used in all experiments. The Cs+ pipette solution contained (in mM): 130 Cs-methanesulphonate, 10 CsCl, 10 HEPES, 2 MgCl2, 0.5 EGTA, 2 ATP, 1 GTP (pH 7.4). The electrode resistance after back-filling was 2–5 MΩ. All voltages were corrected for the liquid junction potential (−13 mV and -7 mV, respectively). Data were acquired with a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA) digitized using a Digidata 1320A interface and the Pclamp9.2 software package. The sampling rate for the continuous recordings of spontaneous activity was 50 kHz. The recording chamber was constantly perfused with extracellular solution (2–3 mL·min−1). The agonists were applied locally using a perfusion pencil system (tip diameter 100 μm, Automate Scientific) driven by gravity, while the antagonists were bath-applied. The perfusion was kept on for 90 s in most experiments, except those measuring effects on synaptic activity for which the incubation time was 3-4 min. The temperature of the external solution was controlled with a TC-344B temperature controller and an inline heater (Warner Instruments, Hamden, CT, USA) and was maintained at 36–37°C.
Ca2+ imaging
Fura-2 fluorescence signals were acquired with a CCD camera (Hamamatsu ORCA-ER) connected to its frame grabber driven by Slidebook software (Intelligent Imaging Innovations, Denver, CO, USA). An ultra-high-speed wavelength switcher Lambda DG-4 (Sutter Instruments, Novato, CA, USA) provided alternating excitation for ratiometric Fura-2 measurements. The filters were 340HT15 and 380HT15. The illumination source was a standard xenon lamp. The sampling frequency of 0.2 Hz was sufficiently fast to capture responses to bombesin and GRP. At this excitation frequency, photobleaching and phototoxicity were minimal. Fura-2AM loading and data acquisition were carried out as described in our previous studies.
Data analysis and curve fitting
All data represent mean ± SD. Data analysis and curve fitting was carried out using the SigmaPlot software package (Systat Software, Inc., San Jose, CA, USA). One-way Anova with Tukey's post hoc test (P < 0.05) was used for comparison of multiple groups. The concentration–response data of agonist actions were fitted to the Hill equation: E = Emax/{1 + (EC50/[Agonist])n}, where [Agonist] represents the agonist concentration, n is the Hill coefficient and Emax is the maximum effect as a percentage of the maximum histamine response in the same cell. Synaptic activity was quantified and analyzed statistically as described previously (Tabarean et al., 2006, Tabarean, 2012). Briefly, synaptic events were detected and analyzed (amplitude, kinetics, frequency) off-line using a peak detection program (Mini Analysis program, Synaptosoft, Decatur, NJ, USA). Events were detected from randomly selected recording stretches of 2 min before and during bombesin or GRP treatment. Statistical significance of the cumulative distributions of the measured parameters (inter-event interval, amplitude, rise time, time constant of decay) were assessed with the Kolmogorov-Smirnov two-sample test (K-S test, P<0.05) using the Mini Analysis program. The averages for the measured parameters (frequency, amplitude, rise time, time constant of decay) for each experiment were obtained using the Mini Analysis program. Event frequency was calculated by dividing the number of events by the duration (in seconds) of the analyzed recording stretch.
Chemicals
Bag-1 and bantag-1, BRS3 agonist and antagonist, respectively, were a kind gift from Dr Marc Reitman (Merck, Rahway, NJ USA). Bombesin, GRP, BIM 23042 were purchased from Tocris (Ellisville, MO, USA). RC-3095, TTX, CNQX, AP-5 and gabazine, BAPTA-AM were purchased from Sigma (St Louis, MO, USA).
Cell Harvesting, Reverse Transcription and PCR
MnPO neurons in slices were patch-clamped and then harvested into the patch pipette by applying negative pressure. The content of the pipette was expelled in a PCR tube. dNTPs (0.5 mM), 50 ng random primers (Invitrogen) and H2O were added to each cell to a volume of 16 μl. The samples were incubated at 65°C for 5 min and then put on ice for 3 min. First strand buffer (Invitrogen), DTT (5 mM, Invitrogen), RNaseOUT (40 U, Invitrogen) and SuperScriptIII (200 U, Invitrogen) were added to each sample to a volume of 20 μl followed by incubation at room temperature for 5 min, at 50°C for 50 min and then at 75°C for 15 min. After reverse transcription samples were immediately put on ice. 1 μl of RNAse H was added to samples and kept at 37°C for 20 min. PCR assays were carried out using pairs of nested primers (Table 1) as described previously (Lundius et al., 2010, Sethi et al., 2011).
Table 1. Primers.
| Primer | External sequence | Amplicon Size | Internal sequence | Amplicon Size |
|---|---|---|---|---|
| GRPR | F: 5′ aaccttcagcgcctaactga 3′ R: 5′ tcagtttgcagccaattctg 3′ |
Bp:437 | F: 5′ acctgaacttggacgtggac 3′ R: 5′ ccaaagccaggctagagatg 3′ |
Bp:219 |
| BRS3 | F:5′ aaagcaccctgaacataccg 3′ R:5′ gtcaccaagaggaggctcag 3′ |
Bp:341 | F: 5′ tgaatcccggaagagaattg 3′ R:5′ aatgctgctggaaggtcttg 3′ |
Bp:233 |
| NMBR | F:5′ tcagaagtagcacgcattgg3′ R:5′ agcaaagggattgacacagg3′ |
Bp:396 | F:5′ acagcatgcataccctaccc 3′ R: 5′ caatcttagccaggcgtttc 3′ |
Bp:242 |
Results
In a first set of experiments we have determined the effects of bombesin as well as selective agonists for NMBR, GRPR and BRS3 on the spontaneous firing activity of preoptic GABAergic neurons. Preoptic GABAergic neurons were identified by using the transgenic mouse line GAD65-GFP (Bali et al., 2005) as previously described (Lundius et al., 2010). To obtain long lasting recordings necessary for sequential testing of several agonists and/or antagonists we have carried out this set of experiments in cell-attached configuration (Vhold=0). Bombesin (1 μM) potently increased the firing rate of 11 out of 25 neurons studied and had no effect on the others. The peptide increased the average firing rate of the neurons from 6.8 ± 3.1 Hz to 19.9 ± 4.7 Hz (n=11). In all 11 neurons excited by bombesin the effect was mimicked by GRP (0.3 μM): an increase in firing rate from 7.1 ± 3.4 Hz to 21.8 ± 5.4 Hz (n=11) (Fig 1A,C). In contrast, the BRS3 agonist bag-1 (10 μM) did not increase the firing rate of any neurons tested (n=25). Instead, the BRS3 agonist decreased the firing rate of 5 of the 11 neurons excited by bombesin and GRP and had no effect on the other neurons. Bag-1 decreased the average firing rate from 7.0 ± 3.5 Hz to 5.2 ± 2.1 Hz (n=5). To further clarify the receptor subtype involved in these responses we have carried out similar experiments in the presence of subtype-selective antagonists (Fig 1B). GRPR antagonist RC-3095 (3 μM) abolished the excitatory effect of bombesin and GRP (Fig 1B,C) in 9 out of 9 neurons tested. In contrast, the BRS3 antagonist bantag-1 (3 μM) (Fig 1B,C) or the NMBR antagonist BIM 23042 (0.3 μM) had no effect on the increase in firing rate elicited by the two peptides in all the neurons tested (n=15 and 12, respectively).
Figure 1. Bombesin and GRP increase the spontaneous firing rate of GABAergic preoptic neurons.

A. Representative recordings of spontaneous firing activity of a preoptic GABAergic neuron recorded before and during local application of GRP (300 nM), bombesin (1 μM) or BRS3 selective agonist bag-1 (10 μM). GRP and bombesin increased the average firing rate from 1.5 Hz to 4.7 Hz and 5.1 Hz, respectively while bag-1 was without effect in this cell.
B. Examples of the effect of bombesin on spontaneous firing activity in control (upper trace) and in the presence of GRPR antagonist RC-3095 (3 μM) (middle trace) or BRS3 antagonist bantag-1 (5 μM) (lower trace). RC-3095 abolished the excitatory effect of bombesin while bantag-1 was without effect.
C. Summary of the effect of GRP (300 nM), bombesin (1 μM), bag-1 (10 μM), in control or during incubation with the antagonists RC-3095 (3 μM) or bantag-1 (5 μM) on the firing activity of GABAergic preoptic neurons. Bars represent means ± S.D. of the normalized firing rate relative to the control. There was a statistically significant difference between groups as determined by one-way ANOVA (F(6,51)=10.15, p=0.0003) followed by Tukey's test relative to control; ** and * indicate statistical significance of P<0.01 and P<0.05, respectively.
A,B. Recordings were carried out in cell-attached configuration (Vhold = 0 mV)
To understand the cellular mechanism involved in the robust excitation of preoptic GABAergic neurons by GRP we have then carried out whole-cell voltage-clamp recordings. We found that GRP (0.3 μM) activated an apparent inward current that averaged 21.8 ± 3.9 pA (n=11), effect that was mimicked by bombesin (1 μM)(n=11)(Fig 2A,B). This effect was not associated with any changes in the properties of spontaneous (s) EPSCs and IPSCs. Since at the -60 mV holding potential few sIPSCs are detected (because of the small driving force for Cl-) we have carried out a set of experiments with Cs+ pipette solution at a holding potential of -20 mV. The parameters describing the characteristics of sEPSCs (at -60 mV) and sIPSCs (at 0 mV) recorded from preoptic GABAergic neurons in control as well as during incubation with GRP (0.3 μM) or bombesin (1 μM) are summarized in Table 2.
Figure 2. GRP and bombesin activate an inward current in GABAergic preoptic neurons.

A. GRP (0.3 μM) activates an inward current of 19 pA (upper trace), response that is abolished by GRPR antagonist RC-3095 (3 μM) (second trace). After wash-out of the antagonist bombesin (1 μM) activated a similar inward current (20 pA, third trace), while bag-1 (10 μM) was without effect (lower trace). The recoding was carried out in whole-cell configuration, Vhold=-60 mV.
B. Summary of the amplitudes of the inward current activated by GRP (0.3 μM), bombesin (1 μM), bag-1 (10 μM), in control or during incubation with the antagonists RC-3095 (3 μM) or bantag-1 (5 μM) in GABAergic preoptic neurons. Bars represent means ± S.D. There was a statistically significant difference between groups as determined by one-way ANOVA (F(5,54)=118.66, p=0.0011) followed by Tukey's test relative to the respective control; ** and * indicate statistical significance of P<0.01 and P<0.05, respectively.
C. GRPR and BRS3 but not NMBR transcripts are present in MnPO GABAergic neurons. Representative gels from a batch of 7 GABAergic preoptic neurons in which bombesin activated an inward current. The expected sizes of the PCR products are (in base pairs) 219, 233, and 242, respectively. Negative (−) control was amplified from a harvested cell without reverse-transcription, and positive control (+) was amplified using 1 ng of hypothalamic mRNA.
Table 2. Characteristics of synaptic activity in control and during GRP or bombesin incubation.
| Parameter | control | GRP | Bombesin |
|---|---|---|---|
| sEPSC frequency (Hz) | 7.2 ± 2.5 (11) | 7.4 ± 2.6 (6) | 7.5 ± 2.9 (5) |
| sEPSC amplitude (pA) | 26.4 ± 6.1 (11) | 25.1 ± 6.3 (6) | 27.2 ± 6.8 (5) |
| sEPSC tau decay (ms) | 4.1±1.4 (11) | 4.0±1.5 (6) | 4.1 ± 1.7 (5) |
| sEPSC time to peak (ms) | 1.5±0.3 (11) | 1.5±0.4 (6) | 1.6±0.3 (5) |
| sIPSC frequency (Hz) | 5.9 ± 2.2 (9) | 5.6 ± 2.4 (5) | 5.8 ± 2.1 (4) |
| sIPSC amplitude (pA) | 32.5 ± 4.9 (9) | 30.2 ± 4.6 (5) | 31.7 ± 5.3 (4) |
| sIPSC tau decay (ms) | 9.6 ± 3.0 (9) | 10.1 ± 3.8 (5) | 9.8 ± 3.3 (4) |
| sIPSC time to peak (ms) | 3.2 ± 0.8 (9) | 2.9 ± 0.9 (5) | 3.1 ± 1.0 (4) |
The activation of an inward current was blocked by incubation with GRPR antagonist RC-3095 (3 μM) (Fig 2A, B) in all neurons tested (n=9) but was not affected by incubations with either BRS3 antagonist bantag-1 (3 μM) or NMBR antagonist BIM 23042 (0.3 μM). Furthermore the BRS3 agonist bag-1 (10 μM) did not activate inward currents in any of the neurons tested (n=12).
Single cell reverse transcription-PCR (scRT/PCR) analysis was performed for 20 MnPO GABAergic neurons in which we tested the effect of bombesin. GRPR transcripts were detected in 11 out of 12 neurons in which bombesin (1 μM) activated an inward current and in none of the remaining 8 neurons. BRS3 transcripts were found in 4 out of 12 neurons in which bombesin activated an inward current and in 1 out of the remaining 8 neurons. NMBR transcripts were not detected in any of the 20 neurons analyzed. Fig 2C illustrates such an analysis of a group of 7 neurons responsive to bombesin.
To understand the nature of the inward current activated by GRP we performed recordings at several holding potentials and have substituted some of the cations in our solutions. The interpolated reversal potential for the GRP-activated inward current was -4.3 mV or -3.9 mV and when K+ or Cs+ pipette solution were used, respectively (Fig 3 A,B). Finally, we have also carried out recordings in NMDG external solution (Fig 3C). In this solution GRP did not activate an inward current in all neurons tested (n=6). This observation indicates that Na+ is the main ion responsible for the inward current and that Ca2+ has little permeability through the respective channels.
Figure 3. GRP activates a cationic current in preoptic GABAergic neurons.

A. Responses to GRP (0.3 μM) recorded at three holding potentials. TTX (1 μM) and blockers of synaptic inputs (20 μM CNQX, 50 μM AP-5 and 5 μM gabazine) were present in the external solution.
B. I-V relationship for the peak inward current activated by GRP (0.3 μM) recorded with K+ pipette solution (filled circles) and Cs+ pipette solution (open circles). Each point represents the average from n=5 neurons.
C. Responses to GRP (0.3 μM) recorded in control aCSF (left trace) and in NMDG external solution (right trace) at -60 mV holding potential. TTX (1 μM) was present in all external solutions.
GRPR signaling involves the Gq-PLC-[Ca2+]i pathway in some preparations (Karlsson and Ahren, 1996, Hellmich et al., 1997, Sancho et al., 2010) therefore we have investigated the effect of bombesin (1 μM), GRP (0.3 μM) and BRS3 agonist bag-1 (10 μM) on [Ca2+]i in acutely dissociated neurons loaded with fura-2AM. Bombesin and GRPR evoked very similar responses in 13 of 35 neurons studied while bag-1 had no effect (n=35) (Fig 3A). The [Ca2+]i response to GRP and bombesin were fully blocked by GRPR antagonist RC-3095 (3 μM) (data not shown). Fig 3B depicts the concentration-response relationship for [Ca2+]i changes induced by bombesin and GRP. Data were fit by Hill functions with EC50 of 0.14 μM and 0.05 μM, respectively and slope factors of 0.9 and 1.2, respectively. To test whether Ca2+ entry from the extracellular medium contributed to the [Ca2+]i response to GRP we have carried out experiments in Ca2+-free extracellular solution. The responses were little affected (Fig 4 C) strongly indicating that the cation is released from intracellular stores.
Figure 4. GRP and bombesin induce Ca2+ release from intracellular stores in preoptic neurons.
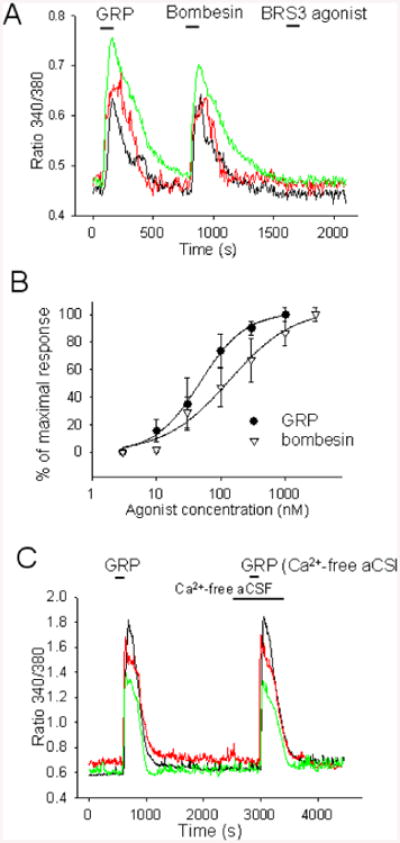
A. [Ca2+]i responses to GRP (0.3 μM) and bombesin (1 μM) from 3 preoptic neurons. TTX (1 μM), CNQX (20 μM), AP-5 (50 μM), and bicuculline (20 μM) were present in all external solutions.
B. Concentration-response curve for the activation of [Ca2+]i responses to bombesin and GRP Each point represents the average of data collected from 13 different preoptic neurons. The data were fitted with a Hill function. The fit yielded an EC50 of 0.05 μM and 0.14 μM for GRP and bombesin respectively.
C. [Ca2+]i responses to GRP (0.3 μM) from 3 preoptic neurons in control aCSF and in Ca2+-free aCSF. TTX (1 μM), CNQX (20 μM), AP-5 (50 μM), and bicuculline (20 μM) were present in all external solutions.
To determine whether [Ca2+]i plays a role in the activation of the inward current activated by GRP we have carried out a set of experiments in slices pre-incubated with BAPTA-AM (1 mM) for 60 min. The inward currents activated by GRP (300 nM) in neurons from these slices had significantly reduced amplitudes averaging 7.7±3.2 pA (n = 11, P<0.01 unpaired t-test).
Discussion
This data establishes for the first time that GABAergic preoptic neurons express GRPR and are potently excited by bombesin and GRP. A subset of neurons also expresses BRS3, however their activation did not contribute to the bombesin induced excitation, on the contrary it resulted in a small but significant reduction in firing rate. NMBR transcripts were not detected in any neurons studied and NMBR antagonist did not affect the responses to bombesin and GRP thus excluding a contribution of this receptor subtype.
Intracerebroventricular injection of bombesin results in robust hypothermia and experiments with selective antagonists have indicated that GRPR rather than NMBR are responsible for this response (Tsushima et al., 2003). However, the observation that NMBR-/- mice develop a reduced hypothermia to bombesin (but not to GRP) (Ohki-Hamazaki et al., 1999), leaves open a possible role of NMBR activation in hypothermia. Our data suggest that the GABAergic preoptic neurons are the likely target of bombesin and GRP in hypothermia and that a possible contribution of NMBR may take place at a different target.
GABAergic preoptic neurons exert an inhibitory tone on thermoeffector neurons and their inhibition results in hyperthermia (Morrison et al., 2008). Thus the observed reduction in firing rate of GABAergic preoptic neurons in response to BRS3-selective agonist may represent the mechanism by which central application of the same agonist induces a small hyperthermia in mice (Metzger et al., 2010). While the endogenous ligand for BRS3 is not known its co-expression with GRPR suggests that it may play a role to balance the activation of GRPR. More importantly, the data reported here suggests that excitation of GABAergic preoptic neurons may represent a mechanism for inducing hypothermia.
Several studies have reported excitatory effects of bombesin on central neurons and the mechanisms involved appear to be cell type-specific including modulation of K+ conductances, activation of a nonselective conductance, and the activation of a Na+/Ca2+ exchanger (Pinnock and Woodruff, 1991, Reynolds and Pinnock, 1997, Lee et al., 1999, van den Pol et al., 2009, Gamble et al., 2011, Hermes et al., 2013). Our data indicate that in GABAergic preoptic neurons GRP activates Ca2+ release from intracellular stores and a Ca2+ -dependent cationic conductance, however the specific ion channel subunits and second messengers involved remain to be established.
Highlights.
GABAergic preoptic neurons express BRS3 and/or GRP receptors.
GRP, in contrast to BRS3 agonist, mimics the excitatory effect of bombesin
GRP and bombesin activate a Ca2+-dependent inward nonselective cationic current
GRP and bombesin activate Ca2+ release from intracellular stores
Acknowledgments
This work was supported by the National Institutes of Health Grants NS082255 and NS094800 (IVT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bali B, Erdelyi F, Szabo G, Kovacs KJ. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neuroscience letters. 2005;380:60–65. doi: 10.1016/j.neulet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Kudo T, Colwell CS, McMahon DG. Gastrin-releasing peptide modulates fast delayed rectifier potassium current in Per1-expressing SCN neurons. Journal of biological rhythms. 2011;26:99–106. doi: 10.1177/0748730410396678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmich MR, Battey JF, Northup JK. Selective reconstitution of gastrin-releasing peptide receptor with G alpha q. Proc Natl Acad Sci U S A. 1997;94:751–756. doi: 10.1073/pnas.94.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes ML, Kolaj M, Coderre EM, Renaud LP. Gastrin-releasing peptide acts via postsynaptic BB2 receptors to modulate inward rectifier K+ and TRPV1-like conductances in rat paraventricular thalamic neurons. J Physiol. 2013;591:1823–1839. doi: 10.1113/jphysiol.2012.249227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S, Ahren B. Gastrin-releasing peptide mobilizes calcium from intracellular stores in HIT-T15 cells. Peptides. 1996;17:909–916. doi: 10.1016/0196-9781(96)00146-5. [DOI] [PubMed] [Google Scholar]
- Lee K, Dixon AK, Gonzalez I, Stevens EB, McNulty S, Oles R, Richardson PJ, Pinnock RD, Singh L. Bombesin-like peptides depolarize rat hippocampal interneurones through interaction with subtype 2 bombesin receptors. J Physiol. 1999;518(Pt 3):791–802. doi: 10.1111/j.1469-7793.1999.0791p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundius EG, Sanchez-Alavez M, Ghochani Y, Klaus J, Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci. 2010;30:4369–4381. doi: 10.1523/JNEUROSCI.0378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JM, Gagen K, Raustad KA, Yang L, White A, Wang SP, Craw S, Liu P, Lanza T, Lin LS, Nargund RP, Guan XM, Strack AM, Reitman ML. Body Temperature as a Mouse Pharmacodynamic Response to Bombesin Receptor Subtype-3 (BRS-3) Agonists and Other Potential Obesity Treatments. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00404.2010. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ. Central nervous system regulation of brown adipose tissue. Comprehensive Physiology. 2014;4:1677–1713. doi: 10.1002/cphy.c140013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Frontiers in endocrinology. 2012;3 doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Iwabuchi M, Maekawa F. Development and function of bombesin-like peptides and their receptors. The International journal of developmental biology. 2005;49:293–300. doi: 10.1387/ijdb.041954ho. [DOI] [PubMed] [Google Scholar]
- Ohki-Hamazaki H, Sakai Y, Kamata K, Ogura H, Okuyama S, Watase K, Yamada K, Wada K. Functional properties of two bombesin-like peptide receptors revealed by the analysis of mice lacking neuromedin B receptor. J Neurosci. 1999;19:948–954. doi: 10.1523/JNEUROSCI.19-03-00948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterlin ZA, Kozloski J, Mao BQ, Tsiola A, Yuste R. Optical probing of neuronal circuits with calcium indicators. Proc Natl Acad Sci U S A. 2000;97:3619–3624. doi: 10.1073/pnas.97.7.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock RD, Woodruff GN. Bombesin excites a subpopulation of 5-hydroxytryptamine-sensitive neurones in the rat dorsal raphe nucleus in vitro. J Physiol. 1991;440:55–65. doi: 10.1113/jphysiol.1991.sp018695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds T, Pinnock RD. Neuromedin C decreases potassium conductance and increases a non-specific conductance in rat suprachiasmatic neurones in brain slices in vitro. Brain research. 1997;750:67–80. doi: 10.1016/s0006-8993(96)01332-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Osborn O, Mitsukawa K, Schaefer J, Dubins J, Holmberg KH, Klein I, Klaus J, Gomez LF, Kolb H, Secrest J, Jochems J, Myashiro K, Buckley P, Hadcock JR, Eberwine J, Conti B, Bartfai T. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 2010;59:43–50. doi: 10.2337/db09-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho V, Moody TW, Mantey SA, Di Florio A, Uehara H, Coy DH, Jensen RT. Pharmacology of putative selective hBRS-3 receptor agonists for human bombesin receptors (BnR): affinities, potencies and selectivity in multiple native and BnR transfected cells. Peptides. 2010;31:1569–1578. doi: 10.1016/j.peptides.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi J, Sanchez-Alavez M, Tabarean IV. Kv4.2 mediates histamine modulation of preoptic neuron activity and body temperature. PloS one. 2011;6:e29134. doi: 10.1371/journal.pone.0029134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV. Persistent histamine excitation of glutamatergic preoptic neurons. PloS one. 2012;7:e47700. doi: 10.1371/journal.pone.0047700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV, Korn H, Bartfai T. Interleukin-1beta induces hyperpolarization and modulates synaptic inhibition in preoptic and anterior hypothalamic neurons. Neuroscience. 2006;141:1685–1695. doi: 10.1016/j.neuroscience.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Tsushima H, Mori M, Fujiwara N, Moriyama A. Pharmacological characteristics of bombesin receptor mediating hypothermia in the central nervous system of rats. Brain research. 2003;969:88–94. doi: 10.1016/s0006-8993(03)02281-9. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Yao Y, Fu LY, Foo K, Huang H, Coppari R, Lowell BB, Broberger C. Neuromedin B and gastrin-releasing peptide excite arcuate nucleus neuropeptide Y neurons in a novel transgenic mouse expressing strong Renilla green fluorescent protein in NPY neurons. J Neurosci. 2009;29:4622–4639. doi: 10.1523/JNEUROSCI.3249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


