Abstract
It is not known whether computerized cognitive assessments, like the CogState battery, are sensitive to preclinical cognitive changes or pathology in people at risk for Alzheimer’s disease (AD). In 469 late middle-aged participants from the Wisconsin Registry for Alzheimer’s Prevention (mean age 63.8±7 years at testing; 67% female; 39% APOE4+), we examined relationships between a CogState abbreviated battery (CAB) of seven tests and demographic characteristics, traditional paper-based neuropsychological tests as well as a composite cognitive impairment index, cognitive impairment status (determined by consensus review); and biomarkers for amyloid and tau (CSF phosphorylated-tau/Aβ42 and global PET-PiB burden) and neural injury (CSF neurofilament light protein). CSF and PET-PiB were collected in n=71 and n=91 participants, respectively, approximately four years prior to CAB testing. For comparison, we examined three traditional tests of delayed memory in parallel. Similar to studies in older samples, the CAB was less influenced by demographic factors than traditional tests. CAB tests were generally correlated with most paper-based cognitive tests examined and mapped onto the same cognitive domains. Greater composite cognitive impairment index was associated with worse performance on all CAB tests. Cognitively impaired participants performed significantly worse compared to normal controls on all but one CAB test. Poorer One Card Learning test performance was associated with higher levels of CSF phosphorylated-tau/Aβ42. These results support the use of the CogState battery as measures of early cognitive impairment in studies of people at risk for AD.
Keywords: Amyloid, cognitive impairment, cerebrospinal fluid, biomarkers
Key words not in MeSH database: CogState, preclinical Alzheimer’s disease, computerized cognitive testing, neural injury
1 INTRODUCTION
CogState is a computerized cognitive battery spanning domains of memory, executive function, and speed of processing. It has been shown to have acceptable stability and test-retest reliability with minimal practice effects at short test-retest intervals in groups of healthy controls and patients at various stages of cognitive impairment and dementia [1, 2]. Computerized testing, such as the CogState battery, may hold potential for detecting early cognitive dysfunction associated with preclinical Alzheimer’s disease (AD)[3].
Previous studies have demonstrated differences between healthy controls, Mild Cognitive Impairment (MCI), and AD, with the most pronounced impairments in the latter two groups on CogState tests of learning and memory [4, 5]. The vast majority of studies investigating biomarker correlates of the CogState have focused on neuroimaging biomarkers, with a particular focus on PET amyloid imaging. The majority [3, 6–11] but not all [12] have found an association with amyloid. One study also found an association with hippocampal volume and glucose metabolism [12]. Of note, the majority of published studies that have examined biomarkers and the CogState battery have been performed on two cohorts, the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL) and the Mayo Clinic Study on Aging. The present study adds the largest long-term study of healthy relatives of persons with Alzheimer’s disease, the Wisconsin Registry for Alzheimer’s Prevention (WRAP), to the cohorts simultaneously investigating biomarkers and the CogState battery. Additionally, there is a dearth of information in the current scientific literature on the association between the CogState battery and potentially informative cerebrospinal fluid (CSF) biomarkers for Alzheimer’s pathology and neural injury. This is an important gap to fill as recent work suggests that CSF biomarkers become abnormal in the earliest stages of AD, before changes in amyloid positron emission tomography are apparent [13, 14].
The present study investigated whether CogState is sensitive to pre-dementia cognitive dysfunction and early accumulation of AD pathology during late-midlife. First we explored relationships between CogState tests and demographic characteristics. Next, we examined relationships between CogState tests and three measures of cognitive function: individual scores on traditional paper-based neuropsychological tests, a composite cognitive impairment index, and cognitive status (cognitively impaired vs. cognitively normal). Finally, we investigated sensitivity to underlying AD pathology by examining whether biomarkers for amyloid and tau (CSF phosphorylated-tau/Aβ42 and global PET-PiB burden) and neural injury (CSF neurofilament light protein) predicted CogState performance approximately four years later. We hypothesized that performance on the CogState battery would be relatively robust to demographic variability but would be associated with cognitive functioning as well as biomarkers for AD pathology. Additionally, to evaluate whether the CogState battery may be more robust to demographics and more associated with disease outcomes compared to traditional neuropsychological tests, we also examined relationships of three traditional tests of delayed memory with demographics, cognitive function, and biomarkers and provide effects sizes for comparison between the cognitive measures. By examining relationships between the CogState battery and multiple measures of early cognitive dysfunction as well as biomarkers for amyloid and neural injury in an at-risk cohort, this study investigated whether the CogState battery is sensitive to early cognitive and pathological changes suggestive of incipient AD.
2 MATERIALS AND METHODS
2.1 Participants
WRAP is a longitudinally followed cohort designed to identify biological and lifestyle risk factors associated with development of dementia due to Alzheimer’s disease [15–17]. The WRAP study consists of 1,545 participants (mean age=53.6±6.6 years at first cognitive assessment), of which 72.4% have a parental family history of dementia due to Alzheimer’s disease. In 2014, the CogState was added to the assessment protocol for each visit; data used in this paper represents first CogState for each person, although the overall WRAP visit number varies from 2 to 5 (3.2% of participants were administered the CogState at visit 2, 19.8% at visit 3, 24.5% at visit 4, and 52.5% at visit 5). Participants were selected for the current analyses if they had completed at least one of the seven CogState tests that have been added to the WRAP battery. The University of Wisconsin Institutional Review Board approved all study procedures, informed consent was obtained for all participants, and the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Cognitive status was determined via consensus review conference by a panel of experts in cognitive aging and dementia, including clinical neuropsychologists, physicians, and nurse practitioners for the same WRAP visit that CogState was administered. The consensus panel reviews cognitive performance as well as additional information in the participant’s chart (e.g., medical history, social history, informant reports) to determine final cognitive status. A cut-off of 1.5 standard deviations below a robust normative sample (e.g., low-risk WRAP participants who remained normal throughout the study;[17, 18]) was used to define impairment on cognitive measures. A diagnosis of clinical MCI was based on the NIA-AA criteria [19, 20] including subjective cognitive decline, objective impairment in one or more cognitive domains, and preservation of functional abilities. The construct of early MCI was developed to identify cognitive decline expected to precede a clinical MCI diagnosis, and identifies individuals who exhibit lower than expected performance on neuropsychological measures (e.g., ≤1.5 SD below demographically-corrected robust norms), but do not necessarily report subjective cognitive decline. Of the 469 participants who were administered the abbreviated CogState battery, 10 met criteria for clinical MCI, another 60 exhibited subtle deficits indicative of early MCI, and 6 were classified as having a cognitive impairment primarily due to depression rather than MCI. The clinical MCI and early MCI participants were grouped together into a cognitively impaired group and the remaining 393 unimpaired participants were considered cognitively normal (Table 1). The 6 individuals with potential non-MCI cognitive impairment were included in the total sample but in neither the cognitively impaired nor normal groups. None of the cognitively impaired participants had dementia.
Table 1.
Participant characteristics
| Sample characteristic | Total Sample (N=469 |
Cognitively Impaired (n=70) |
Cognitively Normal (n=393) |
p-value* |
|---|---|---|---|---|
| Age at CogState (years) | 64.81 (6.6) | 66.26 (6.1) | 63.39 (6.6) | .001 |
| Sex (% female) | 67.0% | 57.1% | 68.4% | .064 |
| APOE4+ | 39.0% | 37.1% | 38.7% | .808 |
| Family History of AD | 74.4% | 67.1% | 75.6% | .137 |
| Education (years) | 16.50 (2.6) | 16.40 (2.9) | 16.53 (2.6) | .703 |
| WRAT reading standard score** | 106.35 (9.2) | 105.17 (11.2) | 106.57 (8.8) | .242 |
| WRAT reading raw score** | 51.17 (4.4) | 50.41 (5.4) | 51.31 (4.2) | .189 |
| Depression (CES-D) | 6.21 (6.6) | 6.30 (5.8) | 5.93 (6.1) | .634 |
| Computer familiarity*** | 4.74 (0.7) | 4.56 (1.0) | 4.77 (0.7) | .097 |
Values are Mean (SD) unless otherwise indicated.
P-value is for chi square or t-test comparing Cognitively Impaired and Cognitively Normal groups.
WRAT reading standard scores in addition to raw scores are reported for easier interpretation, but raw scores were used in all statistical models to main consistency with other variables which were not standardized for age and sex. Computer familiarity was measured on a 1–5 scale where “1” corresponds to using a personal computer “once a year or less” and “5” corresponds to using a personal computer “every day or about every day.” APOE4=apolipoprotein E4 allele. WRAT=Wide Range Achievement Test. CES-D=Center for Epidemiological Studies Depression Scale.
2.2 Measures of cognition
2.2.1 CogState
Select tests from the CogState battery were administered on a laptop to participants after completing the non-computerized assessments. This CogState abbreviated battery (CAB) included a test of delayed visual memory through paired associate learning (Continuous Paired Associate Learning, CPAL), speed of visual processing (Groton Maze timed chase test, GMCT), executive function (Groton Maze learning test, GML), delayed recall (Groton Maze learning test delayed recall, GMR), and working memory (One-card learning, OCL; One-back memory, ONB; and Two-back memory, TWOB). For CPAL, GML, and GMR, total number of errors was assessed; for GMCT moves per second was assessed; and for the three card tasks (OCL, ONB, and TWOB) accuracy was assessed using the arcsine proportion to correct for normality. Data were only included that passed criteria for completion and integrity. To be considered “complete,” at least 75% of all responses needed to be observed for the card tasks (OCL, ONB, TWOB), all 28 steps of the maze path needed to be completed for the Groton Maze tasks (GML, GMR), and all rounds needed to be completed for CPAL; there is no completion check for GMCT. Integrity checks were completed for the three card tasks only and were satisfied if the proportion correct was above chance (at least 50% correct). 99.4–100% of participants passed completion checks for each of the seven tests and 97.5–99.6% passed the integrity checks for each of the three card tasks. Selection of test outcomes, transformations to correct for normality, and tests of completion and integrity were performed per the recommendations from the CogState manual [21]. Additionally, not all participants finished the full CAB, with more missing data for tests administered at the end of the battery. All participants finished at least one test and 98.7% finished all seven tests. Non-completion of the CAB was due to fatigue, frustration, or technical difficulties. Missing data and checks for completion and integrity are summarized in Supplementary Table 1.
2.2.2 Traditional neuropsychological battery
A comprehensive neuropsychological battery was performed at each WRAP visit. For this analysis, non-computer tests were selected that measure memory, language, executive function, or global cognitive function. These included Rey Auditory Verbal Learning Test (RAVLT [22]) total trials 1–5 and delayed recall; Wechsler Memory Scale-Revised (WMS-R [23]) Logical Memory I and II (immediate and delayed recall, respectively); Brief Visuospatial Memory Test-Revised (BVMT-R [24]) immediate and delayed recall; Boston Naming Test–2nd Edition (BNT [25]); Animal Naming [26]; Controlled Oral Word Association Test phonemic fluency (CFL [27]); Stroop Neuropsychological Screening Test color-word interference trial (Stroop [28]); Trail Making Test (TMT [29]) Parts A and B; Wechsler Adult Intelligence Scale-Revised (WAIS-R [30]) Digit Symbol; Wechsler Adult Intelligence Scale-Third Edition (WAIS-III [31]) Letter Number Sequencing and Digit Span subtests; and Mini Mental State Examination (MMSE [32]).
2.2.3 Composite cognitive impairment index
A composite cognitive impairment index (CCII) was calculated using a set of eight cognitive measures: TMT A and B, WAIS-III Digit Span forward and backward, RAVLT total trials 1–5 and delayed recall trial, BNT, and MMSE. Visits were excluded when fewer than four of these measurements were available. We applied the progression score model [33–35] to align individuals along a linear cognitive trajectory based on their longitudinal cognitive measure profiles, adjusting for inter-individual differences in rates of change. The composite cognitive impairment index computed using this method is an individualized summary of the eight cognitive measures, with higher values indicating lower cognitive performance in all measures. Different from previous approaches, we accounted for correlations among cognitive measures. To remove confounding effects of age at entry into WRAP, a composite was estimated at age 65 based on an approximate expression for the time derivative of the CCII.
2.3 Biomarker collection
Some WRAP participants were recruited for biomarker substudies which do not necessarily correspond to a specific WRAP visit. We examined PET and CSF biomarker data, which were collected independently and up to several years prior to the CogState.
2.3.1 PET-PiB
Detailed methods for [C-11] PiB radiochemical synthesis, PiB-PET scanning with a 70 minute dynamic acquisition, and distribution volume ratio map generation using the Logan method and the cerebellum as a reference region have been described previously [36]. PiB-PET images were registered to a T1-weighted anatomical scan collected on a GE 3.0 Tesla MR750 (Waukesha, WI) using an 8 channel head coil [36, 37]. A composite measurement of global amyloid derived from eight bilateral ROIs (angular gyrus, anterior cingulate gyrus, posterior cingulate gyrus, frontal medial orbital gyrus, precuneus, supramarginal gyrus, middle temporal gyrus, and superior temporal gyrus) was calculated as described previously [38, 39]. N=91 participants underwent PiB-PET imaging approximately 4.1 years (SD 0.66, range 2.0–5.3) prior to CAB testing.
2.3.2 Cerebrospinal fluid
CSF was collected as described previously [40, 41]. CSF Aβ42 and phosphorylated-tau (p-tau) were quantified with sandwich ELISAs (INNOTEST β-amyloid1–42 and Phospho-Tau[181P], respectively; Fujirebio Europe, Ghent, Belgium). CSF p-tau/Aβ42 was calculated by dividing CSF p-tau by CSF Aβ42. CSF neurofilament light protein (NFL) was measured with a sandwich ELISA method as described by the manufacturer (NF-light ELISA kit, UmanDiagnostics AB, Umeå, Sweden). N=70 participants underwent baseline lumbar puncture approximately 3.7 years (SD 1.11, range 1.17–5.33) prior to CAB testing.
CSF assays were performed in two batches. We corrected for batch differences using simple linear regression (SLR) on a subset of CSF samples (n=96 from the entire CSF database, not just from individuals who had also undergone CogState testing) that were assayed in both batches. SLR was also used to test whether batch corrections were necessary using null hypothesis tests of a slope of 1 and an intercept of 0. If there was insufficient evidence to suggest that any of these hypotheses should be rejected, raw values from both batches were used; otherwise, predictions were made with SLR on CSF values from batch 2 as if they had been tested in batch 1. All analyses for CSF batch corrections were performed using R version 3.2.3 using the base “lm” function.
2.4 Statistical analyses
Significance was inferred at a Bonferroni-corrected p-value for seven CogState tests (p<.05/7=.007) unless otherwise stated.
2.4.1 Correlations between CAB, demographics, and traditional neuropsychological tests
For dichotomous characteristics (sex, parental family history of AD, and APOE4 carriage), t-tests were performed on the seven CogState variables. For continuous variables (total years of education; literacy as measured by baseline Wide Range Achievement Test reading raw score; age at testing; depression as measured by the Center for Epidemiologic Studies Depression Scale; and traditional paper-based neuropsychological tests) and ordinal variables (e.g., computer familiarity as measured on a Cognitive Activities questionnaire), we performed Spearman rank-order correlations. Cohen’s d were calculated for t-tests and effect sizes of .2, .5., and .8 are interpreted as small, medium, and large, respectively. Correlation coefficients of .1, .3., and .5 are interpreted as small, medium, and large effect sizes, respectively [42].
To determine whether CAB is more robust to education and other demographic characteristics compared to traditional paper-based tests, we also examined correlations between demographics and select traditional neuropsychological tests. Numerous studies have identified delayed episodic memory as one of the earliest cognitive domains to become impaired in AD [43–45], likely during the preclinical timeframe; therefore, to reduce the number of multiple comparisons, we selected three tests of delayed memory from our neuropsychological battery: RAVLT delayed recall, Logical Memory delayed recall, and BVMT-R delayed recall. For the analyses described here and as follows, these three delayed recall tests served to provide context for interpreting the CAB findings compared to more traditional neuropsychological testing formats.
2.4.2 ANCOVA comparing cognitive groups on CAB performance
Scores on the CAB of cognitively impaired participants were compared to cognitively normal controls by ANCOVA controlling for age, literacy, sex, APOE4 positivity, family history of AD, and computer familiarity. Effect sizes by partial eta squared are reported. Small, medium, and large effect sizes for eta squared are .01, .06, and .14, respectively [46]. We did not compare cognitive groups on the select traditional neuropsychological tests identified in section 2.4.1 because these tests were evaluated during diagnostic consensus conference.
2.4.3 Associations between the individual cognitive tests and composite cognitive impairment index
In addition to examining individual neuropsychological tests, we investigated whether CCII, which takes advantage of longitudinally measured cognition up to (and including) the visit at which the CAB was administered, is associated with performance on the CAB. We ran regression analysis for each of the seven CogState tests, with the CAB test as the dependent variable and CCII as the independent variable of interest, controlling for age at CAB testing, literacy, sex, APOE4, family history of AD, and computer familiarity. Variance inflation factors (VIF) and tolerance were assessed and deemed normal if tolerance was greater than .1 and VIF was less than 10. Cohen’s f2 for hierarchical regression, R2, and R2-change (the change in R2 after adding CCII to the model) are reported. Cohen’s f2 of 0.02, 0.15, and 0.35 are considered small, medium, and large, respectively [46]. For comparison, parallel models were run for the three traditional delayed recall tests except RAVLT delayed which was used to calculate CCII.
2.4.4 Cognition and biomarker associations
2.4.4.1. Biomarker normalization and dichotomization
Although PiB burden was skewed to the right, traditional transformations were ineffective at improving normality. Instead, we chose to examine PiB burden untransformed (with and without an outlier) as a continuous variable and as a dichotomous variable (i.e., PiB positive vs. PiB negative) with the goal of capturing the hypothesized underlying binomial distribution [47]. A cut-off value for PiB positivity was determined using receiver operating characteristic (ROC) analysis in pROC R Statistical Package [48] bootstrapping 2000 times with replacement and stratification of sample. We used expert visual ratings of PiB positive or negative that have been described previously as the diagnostic groups [36, 37]. Supplementary Figure 1 depicts the ROC plot with an area under the curve of .974. A threshold was determined using Youden’s Index which identifies the PiB burden value that maximizes both sensitivity and specificity [49]. A threshold of 1.19 was identified which corresponded to sensitivity of .938 and specificity of .917.
2.4.4.2. Cognition and biomarkers associations
We performed Spearman correlations between CAB variables and three biomarkers of interest: CSF p-tau/Aβ42, CSF NFL, and global PiB burden (with and without an outlier). T-tests were performed to compare CogState performance between PiB+ and PiB− groups. Furthermore, we investigated promising correlations (significant or trending) through multiple regressions with CAB scores as the dependent variable and biomarker as the independent variable of interest. In addition to the covariates used in the CCII regression models, we additionally controlled for the interval from biomarker collection to CAB testing (CSF to CAB 44.3±13.5 months; PET-PiB to CAB 49.6±7.9 months) because the biomarker assessments were conducted at various time points prior to administration of the CAB. Comparable models with ANCOVA were performed for PiB positivity. VIF and tolerance were again inspected. Because we expected smaller effect sizes, we optimized statistical power by not adjusting for multiple comparisons in these cognition/biomarker analyses (i.e., p<.05 was considered significant). For comparison, we also analyzed the relationships between the three traditional neuropsychological tests (see 2.4.1) and biomarkers.
3 RESULTS
Participant characteristics are summarized in Table 1.
3.1 Correlations between the CAB, demographics, and traditional neuropsychological tests
None of the seven CAB scores or traditional delayed memory scores differed significantly by APOE4 status or family history. Females performed better on CPAL (fewer errors; p<.001, Cohen’s d= 0.39) and on GMCT (more moves per second; p=.004, Cohen’s d=−0.27), similar to traditional verbal memory tests [RAVLT delayed (p<.001, Cohen’s d=−0.81) and Logical Memory delayed (p=.005, Cohen’s d=−0.26)]. Spearman rank-order correlation coefficients are reported in Table 2 for age, education, literacy, depression, and computer familiarity. Effect sizes for all associations with demographic and CAB variables were small except between age and GMCT and age and TWOB, which were both moderate. A large effect size was observed for sex on RAVLT delayed and a moderate effect size was observed for literacy on Logical Memory delayed. All other effect sizes were small.
Table 2.
Spearman rank-order correlations between demographic characteristics and CogState and traditional delayed recall tests
| Cognitive Test | Age at CogState (years) | Education (years) | WRATreading raw score | Depression (CES-D) | Computer familiarity |
|---|---|---|---|---|---|
| CPAL errors | .234** | −.023 | −.122* | .034 | −.004 |
| GMCT moves/sec | −.452** | .126** | .140** | −.073 | .224** |
| GML errors | .286** | −.048 | −.113* | .059 | −.026 |
| GMR errors | .174** | −.035 | −.103* | .110* | −.036 |
| OCL accuracy | −.233** | −.013 | .090 | −.026 | −.002 |
| ONB accuracy | −.085 | −.001 | −.023 | −.110* | −.017 |
| TWOB accuracy | −.315** | .090 | .156** | −.017 | .050 |
| RAVLT delayed | −.244** | .152** | .244** | −.052 | .139** |
| Logical Memory delayed | −.141** | .232** | .343** | −.107* | .150** |
| BVMT-R delayed | −.243** | .160** | .197** | −.065 | −.058 |
Spearman correlation coefficients are reported.
p<.05;
p<.007. Moderate correlations (>.3) are bolded. CPAL= Continuous Paired Associate Learning; GMCT=Groton Maze timed chase test; GML= Groton Maze learning test; GMR=Groton Maze learning test delayed recall; OCL=One-card learning; ONB=One-back memory; TWOB=Two-back memory. RAVLT= Rey Auditory Verbal Learning Test; BVMT= Brief Visuospatial Memory Test. Accuracy was transformed using the arcsine proportion to correct for normality.
The majority of neuropsychological test scores and CAB scores were significantly correlated. A correlation matrix is provided as Table 3 with moderate correlations in bold. Within CogState correlations are reported in Supplementary Table 2.
Table 3.
Spearman rank-order correlations between traditional neuropsychological tests and CogState
| Neuropsychological Test | CPAL | GMCT | GML | GMR | OCL | ONB | TWOB |
|---|---|---|---|---|---|---|---|
| Memory | |||||||
| RAVLT total trials 1–5 | −.478** | .301** | −.270** | −.266** | .279** | .095* | .197** |
| RAVLT delayed | −.477** | .278** | −.249** | −.247** | .319** | .112* | .205** |
| Logical Memory immediate | −.298** | .238** | −.255** | −.236** | .186** | .010 | .188** |
| Logical Memory delayed | −.332** | .245** | −.291** | −.257** | .204** | .000 | .181** |
| BVMT-R immediate | −.486** | .314** | −.347** | −.317** | .279** | .075 | .296** |
| BVMT-R delayed | −.453** | .266** | −.378** | −.340** | .246** | .116* | .292** |
| Language | |||||||
| Boston Naming | −.160** | .110* | −.158** | −.198** | .084 | −.006 | .107* |
| CFL fluency | −.119* | .236** | −.068 | −.057 | .093* | .082 | .136** |
| Animal Naming | −.285** | .386** | −.166** | −.192** | .141** | .053 | .215** |
| Executive function | |||||||
| Stroop color-word interference | −.183** | .427** | −.161** | −.140** | .193** | .134** | .313** |
| TMT Part A | .241** | −.475** | .194** | .177** | −.207** | −.102* | −.324** |
| TMT Part B | .298** | −.448** | .252** | .206** | −.316** | −.127* | −.406** |
| WAIS-R Digit Symbol | −.239** | .499** | −.177** | −.176** | .172** | .159** | .336** |
| WAIS-III Letter Number | −.233** | .251** | −.203** | −.166** | .182** | .091 | .273** |
| WAIS-III Digit Span Forward | −.192** | .168** | −.187** | −.121* | .168** | .081 | .245** |
| WAIS-III Digit Span Backward | −.219** | .177** | −.227** | −.256** | .147** | .064 | .231** |
| Global cognitive function | |||||||
| MMSE | −.115* | .184** | −.145** | −.125* | .201** | .077 | .199** |
Spearman correlation coefficients are reported.
p<.05;
p<.007. Moderate correlations (>.3) are bolded. RAVLT= Rey Auditory Verbal Learning Test; BVMT= Brief Visuospatial Memory Test; TMT=Trail Making Test; WAIS=Wechsler Adult Intelligence Scale (-R=Revised); MMSE= Mini Mental State Exam; CPAL= Continuous Paired Associate Learning; GMCT=Groton Maze timed chase test; GML= Groton Maze learning test; GMR=Groton Maze learning test delayed recall; OCL=One-card learning; ONB=One-back memory; TWOB=Two-back memory.
3.2 CAB performance by cognitive status
After controlling for risk factors and demographics, CAB performance differed between individuals who were cognitively impaired and cognitively normal controls for all CAB tests (p<.007, Table 4, Figure 1) except ONB. All effect sizes were small except GMR, which was moderate. There were several other significant (p<.05) predictors in the CogState models. Age and literacy were significant predictors of CogState performance in every model except ONB. Sex was a significant predictor for CPAL, GML, and GMR. APOE4 was a significant predictor of CPAL and GML. Computer familiarity was significantly associated with GMCT and family history was significantly associated with OCL.
Table 4.
CogState performance by cognitive status
| Cognitive Test | Cognitively Impaired (n=70) | Cognitively Normal (n=393) | F | p-value | Partial eta-squared |
|---|---|---|---|---|---|
| CPAL errors | 116.29 (53) | 77.55 (50.3) | 22.15 | <.001 | .048 |
| GMCT moves/sec | 0.96 (.26) | 1.16 (.22) | 26.47 | <.001 | .055 |
| GML errors | 62.72 (17) | 51.75 (16.3) | 18.19 | <.001 | .039 |
| GMR errors | 11.68 (5.1) | 8.17 (4.4) | 30.38 | <.001 | .065 |
| OCL accuracy | 0.97 (.09) | 1.02 (.09) | 9.58 | .002 | .021 |
| ONB accuracy | 1.37 (.14) | 1.39 (.13) | 0.31 | .576 | .001 |
| TWOB accuracy | 1.18 (.15) | 1.27 (.13) | 16.59 | <.001 | .036 |
CPAL= Continuous Paired Associate Learning; GMCT=Groton Maze timed chase test; GML= Groton Maze learning test; GMR=Groton Maze learning test delayed recall; OCL=One-card learning; ONB=One-back memory; TWOB=Two-back memory. Accuracy was transformed using the arcsine proportion to correct for normality.
Figure 1.
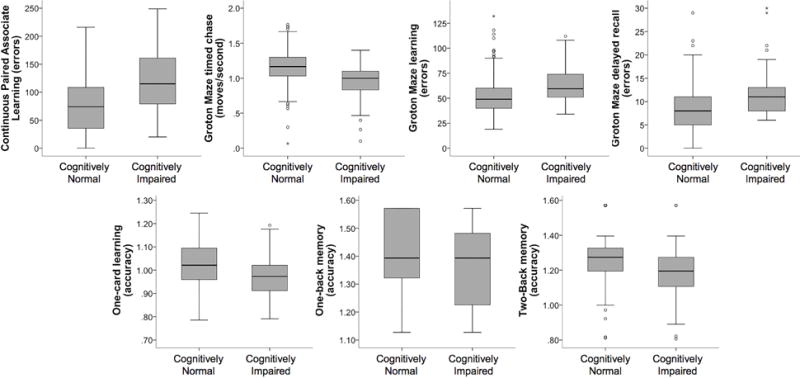
Boxplots depicting comparison of Cognitively Normal and Cognitively Impaired groups on mean performance on CogState tests. Accuracy was transformed using the arcsine proportion to correct for normality.
3.3 Associations between individual cognitive tests and composite cognitive impairment index
VIF and tolerance were in the normal range for all models. CCII significantly predicted performance on all CAB tests (p<.007, Table 5, Figure 2). Age at CAB testing (CPAL, GMCT, GML, OCL, TWOB) and sex (GML, GMR, TWOB) were common additional predictors of CAB performance (p<.05). Computer familiarity also significantly predicted GMCT only (p<.05). Logical memory delayed and BVMT-R delayed also significantly predicted CCII (p<.007, Table 5). Effect sizes were moderate for CPAL, GMR, Logical Memory delayed, and BVMT-R delayed; all others were small.
Table 5.
CCII as a predictor of CogState and traditional neuropsychological test performance
| Cognitive Test | β-coefficient | T | p-value | f2 | R2 | R2 change |
|---|---|---|---|---|---|---|
| CPAL errors | 22.201 | 10.864 | <.001 | 0.2644 | .304 | .184 |
| GMCT moves/sec | −.041 | −4.546 | <.001 | 0.0459 | .324 | .031 |
| GML errors | 4.823 | 6.835 | <.001 | 0.0985 | .198 | .082 |
| GMR errors | 1.765 | 9.045 | <.001 | 0.1846 | .209 | .145 |
| OCL accuracy | −.023 | −5.515 | <.001 | 0.0682 | .135 | .059 |
| ONB accuracy | −.017 | −2.730 | .007 | 0.0690 | .030 | .017 |
| TWOB accuracy | −.039 | −6.697 | <.001 | 0.0996 | .197 | .080 |
| Logical Memory delayed | −2.37 | −8.798 | <.001 | 0.1693 | .291 | .120 |
| BVMT-R delayed | −.652 | −8.769 | <.001 | 0.1680 | .256 | .125 |
CPAL= Continuous Paired Associate Learning; GMCT=Groton Maze timed chase test; GML= Groton Maze learning test; GMR=Groton Maze learning test delayed recall; OCL=One-card learning; ONB=One-back memory; TWOB=Two-back memory. RAVLT= Rey Auditory Verbal Learning Test; BVMT= Brief Visuospatial Memory Test. Accuracy was transformed using the arcsine proportion to correct for normality.
Figure 2.
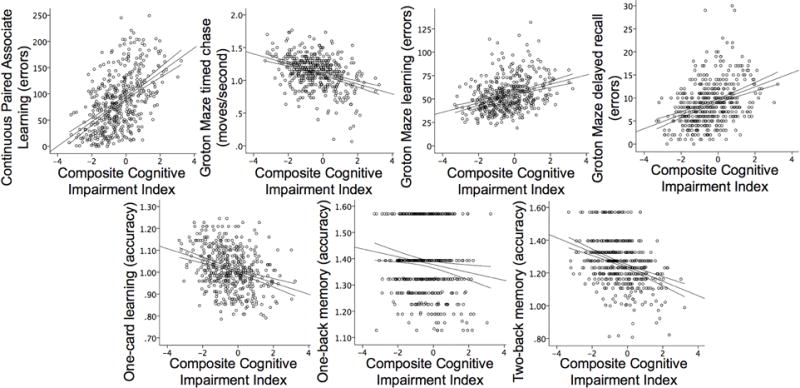
Relationships between CogState tests and a composite cognitive impairment index estimated at age 65. 95% confidence intervals for the regression line are displayed. Accuracy was transformed using the arcsine proportion to correct for normality.
3.4 Associations between cognition and biomarkers
Based on null hypothesis testing, p-tau/Aβ42 but not NFL required batch correction. Overall, biomarkers were not strongly associated with the CAB or delayed recall scores in the subset with CSF (n=70) or PiB (n=91), with significant and trending associations only present for the CSF biomarkers. Spearman correlations are reported in Table 6. When significant or trending correlations were investigated further in regression and ANCOVA models, only CSF p-tau/Aβ42 (Figure 3) was a significant predictor of OCL performance (β=−1.13, t=−3.09, f2=.162, R2=.203, R2-change=.129, p=.003).
Table 6.
Biomarker correlations with CogState and traditional neuropsychological tests
| Cognitive Test | Biomarker | |||
|---|---|---|---|---|
| PiB burden | PiB burden (outlier removed) |
CSF p-tau/Aβ42 |
CSF NFL | |
| CPAL errors | −.002 | −.023 | .203 | .305 |
| GMCT moves/sec | −.083 | −.089 | −.102 | −.296 |
| GML errors | .030 | .006 | .118 | .219 |
| GMR errors | .057 | .035 | .165 | .217 |
| OCL accuracy | −.141 | −.115 | −.347 | −.204 |
| ONB accuracy | .072 | .111 | −.018 | −.148 |
| TWOB accuracy | −.120 | −.121 | −.007 | −.069 |
| RAVLT delayed | .120 | .105 | −.235 | −.378 |
| Logical Memory delayed | −.053 | −.026 | −.199 | −.300 |
| BVMT-R delayed | −.121 | −.100 | −.192 | −.257 |
Spearman correlation coefficients are reported. Significant (p<.05) results are bolded. Trends (p<.1) are italicized. Accuracy was transformed using the arcsine proportion to correct for normality.
Figure 3.
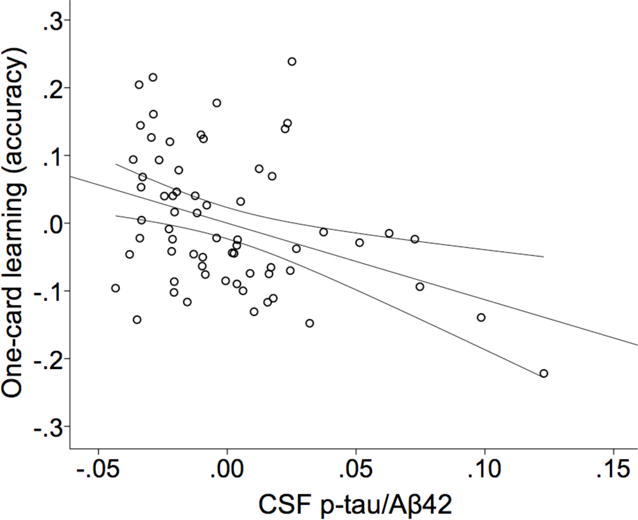
Partial regression plot of CSF p-tau/Aβ42 and One-card learning performance as measured by arcsine-corrected accuracy. 95% confidence interval for the regression line is displayed. R2=.139.
4 DISCUSSION
Computer-based psychological batteries offer several advantages over traditional psychological (often paper-and-pencil-based) tests including reduced testing time and administrative training, standardization of test administration, accurate measures of response latencies, and reduced risk of human error [50, 51]. Consequently, there has been a shift in interest to computer-administrated psychological batteries. The CogState battery is one such computerized battery that has been shown to have good accuracy, efficiency, and stability for repeated assessment, as well as demonstrated sensitivity to cognitive impairment and cognitive change [52, 53]. Here we evaluated performance on an abbreviated CogState battery at a single time point in relation to demographics characteristics, traditional neuropsychological tests, cognitive status, a composite cognitive score, and biomarkers in a late-middle-aged sample from the WRAP cohort. We also sought to provide a context for assessing the sensitivity of the select CogState tests by examining traditional gold-standard tests of delayed memory in parallel. Our findings that select CogState tests were associated with several measures of early cognitive impairment and a CSF biomarker for AD pathology support the use of the CogState battery as a neuropsychological testing tool during the preclinical timeframe.
Our results are consistent with previous studies of CogState showing generally weak relationships with demographic variables and weak to moderate associations with traditional neuropsychological tests [5, 11, 54]. Associations with demographic characteristics were generally small, with the most consistent relationships observed with age and sex. GMCT was most affected by demographic characteristics like computer familiarity, education, and literacy. Traditional delayed memory tests were more strongly and consistently associated with demographic characteristics (e.g., all three tests were significantly correlated with age, education, and literacy) than CogState measures, supporting the theory that the CogState battery is more robust to education level compared to traditional paper-based neuropsychological tests.
The majority of the CAB and traditional tests were significantly correlated, and moderate correlation coefficients were generally observed between tests of comparable cognitive domains. CPAL was moderately correlated with almost all traditional tests of memory examined. The Groton Maze tests combine skills of executive function, learning, and memory and correspondingly were moderately correlated with traditional neuropsychological tests of memory (RAVLT, BVMT) and executive function (Stroop, TMT, WAIS-R Digit Symbol), as well as Animal Naming. Interestingly, GMCT, which is generally considered a task to introduce subjects to the Groton Maze learning and delayed recall tasks, had the most frequent associations with other neuropsychological tests of the three maze paradigms; GML and GMR were both only moderately correlated with BVMT-R immediate and delayed recall. Of the card tests, OCL, a visual memory test, was moderately correlated with RAVLT delayed recall and TMT Part B; and TWOB, a test of working memory, was moderately correlated with three executive functioning tasks: Stroop, TMT Parts A and B, and WAIS-R Digit Symbol. Moderate correlations between CogState and traditional neuropsychological tests, therefore, were generally consistent with the domains they are expected to probe.
Curiously, although ONB is included in CogState’s recommended Alzheimer’s Battery [4], it was the most weakly correlated with any traditional neuropsychological tests, often not reaching even liberal thresholds for statistical significance (i.e., p<.05). Performance on ONB was also the only CAB test that did not differ between cognitively normal and cognitively impaired groups. Given the relative health and younger age of our sample, we suspect this test was too easy for our participants and resulted in a marked ceiling effect. Indeed, participants only made on average two errors on ONB with one-fourth of the sample making zero errors and 93% of participants making five or fewer errors. This contrasts with the other two card tasks: an average of five errors were made on TWOB with only 7% making zero errors, and an average of 26 errors were made on OCL and no participants made fewer than 10 errors. Others have found differences between diagnostic groups on ONB test using reaction time instead of accuracy, which could be less prone to ceiling effects and may be more applicable in cohorts without clinical dementia [11]. Its major function in the battery we selected was to serve as a warm up test for the more difficult TWOB task. Our results suggest that ONB accuracy is less useful in late middle-age.
One of the earliest studies of the CogState battery showed that 15 patients with MCI declined within a one-year period on a CogState memory task (Continuous Learning Test) compared to age, education, IQ, and gender matched controls; while decline was not detectable using routine memory tests in either group [55]. While we were not able to address decline in CogState performance across groups with only one time point of CAB administration, we did incorporate the extensive longitudinal data that has been collected in WRAP using traditional neuropsychological tests to create a composite of cognitive impairment. Unlike simple z-score composites, the CCII adjusts for inter-individual differences in rates of change, removes confounding effects of age at study entry, and accounts for correlations among cognitive measures. This type of cognitive impairment index could be a useful tool against which to measure novel tests of cognitive/clinical status and progression, like the CogState battery. Both the CAB and traditional delayed memory tests were associated with CCII. Effect sizes were moderate for CPAL, GMR, Logical Memory delayed, and BVMT-R delayed with the largest effect size observed for CPAL. Since GMR, CPAL, Logical Memory delayed recall, and BVMT-R delayed recall all measure delayed memory, it would seem that this cognitive domain is either driving the CCII calculation or that delayed recall tests—either computerized or not—are the most sensitive to early cognitive decline, as measured by this unique composite cognitive impairment index.
With the exception of ONB, cognitively impaired individuals performed significantly worse on the CAB tests compared to cognitively normal controls. The difference was generally small, with the most marked difference observed for GMR, a tests of delayed memory, suggesting that GMR is most sensitive to early cognitive dysfunction among the CAB variables.
After correcting for covariates, only CSF p-tau/Aβ42 was associated with worse performance on OCL test, which uses a pattern separation paradigm to measure visual memory. Most previous studies that have found an association between biomarkers and CogState tests have evaluated intra-individual cognitive decline based on longitudinally acquired CogState testing rather than a single time point [3, 6–8, 56]. In contrast, a study with a single CogState battery evaluation did not find an association between CogState test performance and amyloid PET [12]. The latter study did, however, find relatively weak associations between CogState test performance and FDG-PET hypometabolism and smaller hippocampal volumes, suggesting that a single time point could still be informative of underlying pathology. While we were able to detect a relationship between a CSF measure of co-occurring amyloid and tau pathology and one CogState test but none of the three traditional delayed memory tests, it remains unclear whether CogState tests at one time point would substantially improve inference about underling pathology beyond what is possible with traditional paper-based neuropsychological tests.
4.1 Limitations
The primary limitations of this study are that biomarkers were collected several years before CAB administration and that we do not yet have serial CAB testing, both of which constrain our ability to make stronger inferences about the CogState battery and underlying pathology. The correlational analyses between the CAB and traditional tests may have also been affected by the number of times of previous administration on the pencil-and-paper tests which are known to have practice effects [57, 58]. Due to testing duration considerations, we only selected two of the four CogState card tasks; while the selected tasks utilize the cognitive domains of primary interest to this study (learning and memory), this may limit the comparability to other studies which used all four card tasks. It is also worth noting again the smaller samples sizes in the biomarkers analyses; it is possible that we lacked sufficient power to detect important associations between these cognitive tests and underlying pathology; indeed, although most correlations between p-tau/Aβ42 and the CAB tests were considered not significant, all were in the expected direction. Additionally, our study cohort was largely Caucasian and well educated, and so generalizability is restricted. This homogeneity may have also reduced our ability to detect demographic correlates of the CAB. It will be important to perform similar studies with CogState in more diverse populations. Longitudinal clinical outcomes will be important for evaluating prognostic utility of the CAB.
4.2 Conclusions
Overall this study provided support for the use of the CAB in evaluating cognitive function during late-middle-age. The present study is unique in that the WRAP participants are younger and cognitively healthier than the typical clinical MCI groups that have been investigated in prior studies; the population is also enriched for higher risk of developing MCI and dementia due to parental history of AD. Although prior studies provide evidence that the CogState battery can differentiate between healthy controls and clinical MCI in older age, this study suggests that it is also sensitive to decline in early MCI, before clinical symptoms and multiple objective cognitive impairments are apparent. It further provides evidence for an association between one CogState test in particular (OCL) and an important pathological marker for preclinical AD, CSF p-tau/Aβ42. However, it also suggests that CogState at a single time point may not substantially improve preclinical AD detection over traditional neuropsychological tests. Still, its administration offers several advantages over paper-based tests, which make it desirable for large, longitudinal studies with demographic variability. Future directions will focus on longitudinally collected CogState data in the WRAP cohort and examination of a greater array of biomarkers.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (SCJ, AG021155, AG027161), (SA, AG000213, P50 AG033514), and (BBB, AG037639); by P30 HD003352; by a Clinical and Translational Science Award (UL1RR025011) to the University of Wisconsin, Madison; by the Neuroscience & Public Policy Program (RK, SES-0849122); by the Neuroscience Training Program (MH, T32GM007507); by the Medical Scientist Training Program (T32GM008692); by the Wisconsin Alzheimer’s Institute Lou Holland Fund; and by the Swedish Research Council, the Swedish Brain Foundation, the Knut and Alice Wallenberg Foundation, and Torsten Söderberg’s Foundation to the University of Gothenburg. Portions of this research were supported by the Veterans Administration including facilities and resources at the Geriatric Research Education and Clinical Center of the William S. Middleton Memorial Veterans Hospital, Madison, WI; by the Intramural Research Program of the National Institute on Aging, National Institutes of Health; and by the Michael J. Fox Foundation for Parkinson’s Research, MJFF Research Grant ID: 9310.
The authors gratefully acknowledge Allen Wenzel, Nia Norris, Shawn Bolin, Diane Wilkinson, Lisa Bluder, Susan Schroeder, Emily Groth, Nancy Davenport-Sis, Amy Hawley, Sandra Harding, Jennifer Oh, Chuck Illingworth, and the support of researchers and staff at the the Wisconsin Alzheimer’s Institute, Wisconsin Alzheimer’s Disease Research Center, and the University of Wisconsin-Madison for their assistance in recruitment, data collection, and data analysis. Above all, we wish to thank our dedicated volunteers for their participation in this research.
References
- 1.Hammers D, Spurgeon E, Ryan K, Persad C, Heidebrink J, Barbas N, Albin R, Frey K, Darby D, Giordani B. Reliability of repeated cognitive assessment of dementia using a brief computerized battery. Am J Alzheimers Dis Other Demen. 2011;26:326–333. doi: 10.1177/1533317511411907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim YY, Jaeger J, Harrington K, Ashwood T, Ellis KA, Stoffler A, Szoeke C, Lachovitzki R, Martins RN, Villemagne VL, Bush A, Masters CL, Rowe CC, Ames D, Darby D, Maruff P. Three-month stability of the CogState brief battery in healthy older adults, mild cognitive impairment, and Alzheimer’s disease: results from the Australian Imaging, Biomarkers, and Lifestyle-rate of change substudy (AIBL-ROCS) Arch Clin Neuropsychol. 2013;28:320–330. doi: 10.1093/arclin/act021. [DOI] [PubMed] [Google Scholar]
- 3.Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, Harrington K, Martins RN, Masters CL, Szoeke C, Savage G, Villemagne VL, Rowe CC, Group AR Abeta and cognitive change: examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimers Dement. 2014;10:743–751 e741. doi: 10.1016/j.jalz.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Maruff P, Lim YY, Darby D, Ellis KA, Pietrzak RH, Snyder PJ, Bush AI, Szoeke C, Schembri A, Ames D, Masters CL, Group AR Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer’s disease. BMC Psychol. 2013;1:30. doi: 10.1186/2050-7283-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim YY, Ellis KA, Harrington K, Ames D, Martins RN, Masters CL, Rowe C, Savage G, Szoeke C, Darby D, Maruff P, The Aibl Research G Use of the CogState Brief Battery in the assessment of Alzheimer’s disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol. 2012;34:345–358. doi: 10.1080/13803395.2011.643227. [DOI] [PubMed] [Google Scholar]
- 6.Darby DG, Brodtmann A, Pietrzak RH, Fredrickson J, Woodward M, Villemagne VL, Fredrickson A, Maruff P, Rowe C. Episodic memory decline predicts cortical amyloid status in community-dwelling older adults. J Alzheimers Dis. 2011;27:627–637. doi: 10.3233/JAD-2011-110818. [DOI] [PubMed] [Google Scholar]
- 7.Lim YY, Pietrzak RH, Bourgeat P, Ames D, Ellis KA, Rembach A, Harrington K, Salvado O, Martins RN, Snyder PJ, Masters CL, Rowe CC, Villemagne VL, Maruff P. Relationships between performance on the Cogstate Brief Battery, neurodegeneration, and Abeta accumulation in cognitively normal older adults and adults with MCI. Arch Clin Neuropsychol. 2015;30:49–58. doi: 10.1093/arclin/acu068. [DOI] [PubMed] [Google Scholar]
- 8.Lim YY, Ellis KA, Harrington K, Pietrzak RH, Gale J, Ames D, Bush AI, Darby D, Martins RN, Masters CL, Rowe CC, Savage G, Szoeke C, Villemagne VL, Maruff P. Cognitive decline in adults with amnestic mild cognitive impairment and high amyloid-beta: prodromal Alzheimer’s disease? J Alzheimers Dis. 2013;33:1167–1176. doi: 10.3233/JAD-121771. [DOI] [PubMed] [Google Scholar]
- 9.Lim YY, Villemagne VL, Pietrzak RH, Ames D, Ellis KA, Harrington K, Snyder PJ, Martins RN, Masters CL, Rowe CC, Maruff P, Australian Imaging B, Lifestyle Research G APOE epsilon4 moderates amyloid-related memory decline in preclinical Alzheimer’s disease. Neurobiol Aging. 2015;36:1239–1244. doi: 10.1016/j.neurobiolaging.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Thai C, Lim YY, Villemagne VL, Laws SM, Ames D, Ellis KA, Rainey-Smith SR, Martins RN, Masters CL, Rowe CC, Maruff P, Australian Imaging B, Lifestyle Research G Amyloid-Related Memory Decline in Preclinical Alzheimer’s Disease Is Dependent on APOE epsilon4 and Is Detectable over 18-Months. PLoS One. 2015;10:e0139082. doi: 10.1371/journal.pone.0139082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mielke MM, Machulda MM, Hagen CE, Edwards KK, Roberts RO, Pankratz VS, Knopman DS, Jack CR, Jr, Petersen RC. Performance of the CogState computerized battery in the Mayo Clinic Study on Aging. Alzheimers Dement. 2015;11:1367–1376. doi: 10.1016/j.jalz.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mielke MM, Weigand SD, Wiste HJ, Vemuri P, Machulda MM, Knopman DS, Lowe V, Roberts RO, Kantarci K, Rocca WA, Jack CR, Jr, Petersen RC. Independent comparison of CogState computerized testing and a standard cognitive battery with neuroimaging. Alzheimers Dement. 2014;10:779–789. doi: 10.1016/j.jalz.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattsson N, Insel PS, Donohue M, Landau S, Jagust WJ, Shaw LM, Trojanowski JQ, Zetterberg H, Blennow K, Weiner MW, Alzheimer’s Disease Neuroimaging I Independent information from cerebrospinal fluid amyloid-beta and florbetapir imaging in Alzheimer’s disease. Brain. 2015;138:772–783. doi: 10.1093/brain/awu367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmqvist S, Mattsson N, Hansson O, Alzheimer’s Disease Neuroimaging I Cerebrospinal fluid analysis detects cerebral amyloid-beta accumulation earlier than positron emission tomography. Brain. 2016;139:1226–1236. doi: 10.1093/brain/aww015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 16.Jonaitis E, La Rue A, Mueller KD, Koscik RL, Hermann B, Sager MA. Cognitive activities and cognitive performance in middle-aged adults at risk for Alzheimer’s disease. Psychology and aging. 2013;28:1004–1014. doi: 10.1037/a0034838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koscik RL, La Rue A, Jonaitis EM, Okonkwo OC, Johnson SC, Bendlin BB, Hermann BP, Sager MA. Emergence of mild cognitive impairment in late middle-aged adults in the wisconsin registry for Alzheimer’s prevention. Dementia and geriatric cognitive disorders. 2014;38:16–30. doi: 10.1159/000355682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark LR, Koscik RL, Nicholas CR, Okonkwo OC, Engelman CD, Bratzke LC, Hogan KJ, Mueller KD, Bendlin BB, Carlsson CM, Asthana S, Sager MA, Hermann BP, Johnson SC. Mild Cognitive Impairment in Late Middle Age in the Wisconsin Registry for Alzheimer’s Prevention Study: Prevalence and Characteristics Using Robust and Standard Neuropsychological Normative Data. Arch Clin Neuropsychol. 2016 doi: 10.1093/arclin/acw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cogstate. Cogstate: Melbourne, Australia. 2011. [Google Scholar]
- 22.Schmidt M. Rey Auditory Verbal Learning Test: A Handbook. Western Psychological Services; Los Angeles, California: 1996. [Google Scholar]
- 23.Wechsler D. Wechsler Memory Scale-Revised. The Psychological Corporation, Harcourt Brace Jovanovich, inc for Psychological Corp; New York: 1987. [Google Scholar]
- 24.Benedict RHB, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial Memory Test: Studies of normal performance, reliability, and validity. Psychological Assessment. 1996;8:145–153. [Google Scholar]
- 25.Kaplan E, Goodglass H, Weintraub S (2001), ed. PRO-ED I, Austin, TX.
- 26.Rosen WG. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980;2:135–146. [Google Scholar]
- 27.Benton AL, Hamsher KS, Sivan AB. Multilingual aphasia examination. Psychological Assessment Resources; Lutz, FL: 1994. [Google Scholar]
- 28.Trenerry MR, Crosson B, J. D, Leber WR (1989), ed. Resources PA, Odessa, FL.
- 29.Reitan R. Validity of the Trail Making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 30.Wechsler D (1987), ed. Corporation TP, San Antonio.
- 31.Wechsler D. San Antonio, TX. 1997. [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.Bilgel M, Jedynak B, Wong DF, Resnick SM, Prince JL. Temporal Trajectory and Progression Score Estimation from Voxelwise Longitudinal Imaging Measures: Application to Amyloid Imaging. Inf Process Med Imaging. 2015;24:424–436. doi: 10.1007/978-3-319-19992-4_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jedynak BM, Lang A, Liu B, Katz E, Zhang Y, Wyman BT, Raunig D, Jedynak CP, Caffo B, Prince JL, Alzheimer’s Disease Neuroimaging I A computational neurodegenerative disease progression score: method and results with the Alzheimer’s disease Neuroimaging Initiative cohort. Neuroimage. 2012;63:1478–1486. doi: 10.1016/j.neuroimage.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bilgel M, Prince JL, Wong DF, Resnick SM, Jedynak BM. A multivariate nonlinear mixed effects model for longitudinal image analysis: Application to amyloid imaging. Neuroimage. 2016 doi: 10.1016/j.neuroimage.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, Hillmer AT, Wooten DW, Murali D, Barnhart TE, Hall LT, Racine AM, Klunk WE, Mathis CA, Bendlin BB, Gallagher CL, Carlsson CM, Rowley HA, Hermann BP, Dowling NM, Asthana S, Sager MA. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiology of aging. 2013 doi: 10.1016/j.neurobiolaging.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Racine AM, Adluru N, Alexander AL, Christian BT, Okonkwo OC, Oh J, Cleary CA, Birdsill A, Hillmer AT, Murali D, Barnhart TE, Gallagher CL, Carlsson CM, Rowley HA, Dowling NM, Asthana S, Sager MA, Bendlin BB, Johnson SC. Associations between white matter microstructure and amyloid burden in preclinical Alzheimer’s disease: A multimodal imaging investigation. Neuroimage Clin. 2014;4:604–614. doi: 10.1016/j.nicl.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, Sager MA, Asthana S, Johnson SC, Benca RM. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36:2568–2576. doi: 10.1016/j.neurobiolaging.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Racine AM, Koscik RL, Nicholas CR, Clark LR, Okonkwo OC, Oh JM, Hillmer AT, Murali D, Barnhart TE, Betthauser TJ, Gallagher CL, Rowley HA, Dowling NM, Asthana S, Bendlin BB, Blennow K, Zetterberg H, Carlsson CM, Christian BT, Johnson SC. Cerebrospinal fluid ratios with Abeta42 predict preclinical brain beta-amyloid accumulation. Alzheimers Dement (Amst) 2016;2:27–38. doi: 10.1016/j.dadm.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida RP, Schultz SA, Austin BP, Boots EA, Dowling NM, Gleason CE, Bendlin BB, Sager MA, Hermann BP, Zetterberg H, Carlsson CM, Johnson SC, Asthana S, Okonkwo OC. Effect of Cognitive Reserve on Age-Related Changes in Cerebrospinal Fluid Biomarkers of Alzheimer Disease. JAMA Neurol. 2015;72:699–706. doi: 10.1001/jamaneurol.2015.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starks EJ, Patrick O’Grady J, Hoscheidt SM, Racine AM, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, Puglielli L, Asthana S, Dowling NM, Gleason CE, Anderson RM, Davenport-Sis NJ, DeRungs LM, Sager MA, Johnson SC, Bendlin BB. Insulin Resistance is Associated with Higher Cerebrospinal Fluid Tau Levels in Asymptomatic APOEvarepsilon4 Carriers. J Alzheimers Dis. 2015;46:525–533. doi: 10.3233/JAD-150072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 43.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 44.Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annu Rev Psychol. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen J. Erlbaum. 1988. [Google Scholar]
- 47.Villain N, Chetelat G, Grassiot B, Bourgeat P, Jones G, Ellis KA, Ames D, Martins RN, Eustache F, Salvado O, Masters CL, Rowe CC, Villemagne VL, Group AR Regional dynamics of amyloid-beta deposition in healthy elderly, mild cognitive impairment and Alzheimer’s disease: a voxelwise PiB-PET longitudinal study. Brain. 2012;135:2126–2139. doi: 10.1093/brain/aws125. [DOI] [PubMed] [Google Scholar]
- 48.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Muller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 50.Snyder PJ, Jackson CE, Petersen RC, Khachaturian AS, Kaye J, Albert MS, Weintraub S. Assessment of cognition in mild cognitive impairment: a comparative study. Alzheimers Dement. 2011;7:338–355. doi: 10.1016/j.jalz.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wild K, Howieson D, Webbe F, Seelye A, Kaye J. Status of computerized cognitive testing in aging: a systematic review. Alzheimers Dement. 2008;4:428–437. doi: 10.1016/j.jalz.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fredrickson J, Maruff P, Woodward M, Moore L, Fredrickson A, Sach J, Darby D. Evaluation of the usability of a brief computerized cognitive screening test in older people for epidemiological studies. Neuroepidemiology. 2010;34:65–75. doi: 10.1159/000264823. [DOI] [PubMed] [Google Scholar]
- 53.Zygouris S, Tsolaki M. Computerized cognitive testing for older adults: a review. Am J Alzheimers Dis Other Demen. 2015;30:13–28. doi: 10.1177/1533317514522852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammers D, Spurgeon E, Ryan K, Persad C, Barbas N, Heidebrink J, Darby D, Giordani B. Validity of a brief computerized cognitive screening test in dementia. J Geriatr Psychiatry Neurol. 2012;25:89–99. doi: 10.1177/0891988712447894. [DOI] [PubMed] [Google Scholar]
- 55.Maruff P, Collie A, Darby D, Weaver-Cargin J, Masters C, Currie J. Subtle memory decline over 12 months in mild cognitive impairment. Dement Geriatr Cogn Disord. 2004;18:342–348. doi: 10.1159/000080229. [DOI] [PubMed] [Google Scholar]
- 56.Mielke MM, Machulda MM, Hagen CE, Christianson TJ, Roberts RO, Knopman DS, Vemuri P, Lowe VJ, Kremers WK, Jack CR, Jr, Petersen RC. Influence of amyloid and APOE on cognitive performance in a late middle-aged cohort. Alzheimers Dement. 2015 doi: 10.1016/j.jalz.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldberg TE, Harvey PD, Wesnes KA, Snyder PJ, Schneider LS. Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimers Dement (Amst) 2015;1:103–111. doi: 10.1016/j.dadm.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beglinger LJ, Gaydos B, Tangphao-Daniels O, Duff K, Kareken DA, Crawford J, Fastenau PS, Siemers ER. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol. 2005;20:517–529. doi: 10.1016/j.acn.2004.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


