Abstract
The tumor suppressor p53 has a critical role in maintenance of glucose homeostasis. Phosphorylation of Ser18 in the transaction domain of p53 controls the expression of Zpf385a, a zinc finger protein that regulates adipogenesis and adipose function. Mice with a mutation in p53Ser18 exhibit reduced Zpf385a expression in adipose tissue, adipose tissue-specific insulin resistance, and glucose intolerance. Mice with relative deficits in the transactivation domain of p53 exhibit similar defects in glucose homeostasis, while “Super p53” mice with an increased dosage of p53 exhibit improved glucose tolerance. These data support the role of an ATM—p53 cellular stress axis that helps combat glucose intolerance and insulin resistance and regulates glucose homeostasis.
Keywords: p53, glucose intolerance, mouse genetics
Introduction
p53 is activated by numerous stresses that impact metabolism, including atherosclerosis, trauma, hypoxia, and infections [5, 17, 20, 21, 29, 32, 34]. In addition, p53 is activated directly by glucose levels. In response to hyperglycemia, p53 is induced and phosphorylated at Ser18, Ser376, and Ser390 [6] and mediates hyperglycemia-induced apoptosis in mouse blastocysts [13] and myocytes [6]. p53 transactivates genes involved in the control of glycolysis and oxidative phosphorylation [8] both in vitro and in vivo, such as the glucose transporters Glut1 and Glut4 [24, 31], TIGAR [11], and Sco2 [16]. p53 also regulates the expression of the zinc-finger protein 385a (Zfp385a, also known as hzf), a gene involved in adipocyte function and glucose homeostasis [12].
Animal studies have also implicated p53 in metabolic control. p53 has been shown to play a role in lipid-induced insulin resistance through regulation of senescence [19]. p53 mediates autoimmune disease and the macrophage response in a streptozotocin-induced type 1 diabetes model [35]. Morever, the link of p53 with metabolic control has been established through studies with the p53 regulatory kinase ATM, which is also activated by many of the same cellular stresses as p53. ATM dysfunction is correlated with insulin resistance and defects in glucose homeostasis in both human and mice [2, 18, 22, 23]. ATM is activated by insulin and this can lead to p53Ser18 phosphorylation [33]. In addition, our studies have demonstrated that mice with defects in p53Ser18 phosphorylation, an ATM target, develop insulin resistance [1]. However, important mechanistic questions remain about how p53 contributes to systemic glucose homeostasis and what the specific role of the transcriptional activation domain in this process might be.
We have taken advantage of a unique strain of p53Ser18 mutant mice in which the expression of Zfp385a is reduced specifically in adipose tissue to explore the connection between p53 transcriptional control of metabolic genes and insulin sensitivity. We utilized two additional strains of p53 mutant mice to test the hypothesis that glucose homeostasis depends on the level of p53 activity and the functional capacity of the transactivation domain encoded in the first 40 amino acids of the protein. These studies establish a pivotal role for p53 in metabolic homeostasis.
Materials and Methods
Mouse strains and diet information
The methods employed for the generation and genotyping of p53S18A mice [26], p53−/− mice [4], p44Tg mice [15], and p53super mice [7] have been reported previously. p53S18A mice were backcrossed ten generations on the C57BL/6 genetic background (Jackson Labs). p53super mice were on a C57BL/6 background. p44Tg mice were on an ICR/B6SJL mixed background and mated once to C57BL/6, and wildtype and p44Tg mice were established from intercrosses from the F1 generation. Cohorts of age-matched male mice were established for analysis. The mice were maintained on a standard chow diet. All mice were housed in specific pathogen-free facilities accredited by the American Association for Laboratory Animal Care. The Institutional Animal Care and Use Committees of the University of Massachusetts Medical School approved all studies using animals. Importantly, all animals used in our experiments were determined to be tumor-free.
Glucose and insulin tolerance tests
Glucose homeostasis was examined using a glucose tolerance test (GTT) and an insulin tolerance test (ITT) with methods described previously [1]. Mice were fasted overnight and challenged by intraperitoneal administration of glucose (1g/kg body weight, IMS) or insulin (0.75 units/kg body weight). Blood glucose was measured with an Ascenzia Breeze 2 glucometer (Bayer) and blood insulin was measured by ELISA (Luminex 200, Millipore). Due to their shortened life span, we conducted experiments on p44Tg male mice at 4 months of age rather than the 6–7 month age used for p53S18AB6 mice.
Hyperinsulinemic-euglycemic clamp studies
The clamp studies were performed at the University of Massachusetts Mouse Metabolic Phenotyping Center. Whole body fat and lean mass were non-invasively measured using 1H-MRS (Echo Medical Systems). Following an overnight fast, a 2-hr hyperinsulinemic-euglycemic clamp was conducted in conscious mice with a primed and continuous infusion of human insulin (150 mU/kg body weight priming followed by 2.5 mU/kg/min; Humulin; Eli Lilly), and 20% glucose was infused at variable rates to maintain euglycemia [14]. Whole body glucose turnover was assessed with a continuous infusion of [3-3H]glucose (PerkinElmer) and 2-deoxy-D-[1-14C]glucose (PerkinElmer) was administered as a bolus (10 μCi) at 75 min after the start of clamp to measure insulin-stimulated glucose uptake in individual organs. At the end of the clamp, mice were anesthetized, and tissues were taken for biochemical analysis [14].
Analysis of tissue sections
Upon necropsy gross organ analysis was performed. In addition, samples of liver, spleen, thymus, lymph nodes, kidney, heart, pancreas, fat and muscle were removed. Histology was performed using tissue fixed in 10% formalin for 24h, dehydrated and embedded in paraffin. Sections (7μm) were cut, stained using hematoxylin and eosin (American Master Tech Scientific), and examined by a board-certified veterinary pathologist.
Insulin treatment and immunoblot analysis
Mice were fasted overnight and injected with 1.5 U of insulin and tissues were harvested 30 min later. Tissues were homogenized in triton lysis buffer [20 mM Tris (pH 7.4), 1% Triton X-100,10% glycerol, 137 mM NaCl, 2 mM EDTA, 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 10 μg/mL of aprotinin and leupeptin]. Tissue extracts (50 μg) were examined by immunoblot analysis using antibodies to Akt, phospho-Ser473 Akt and phospho-Thr308 Akt (Cell Signaling).
Cytokine analysis
Cytokines in plasma were measured by multiplexed ELISA using a Luminex 200 machine (Millipore) and serum mouse adipokine, adiponectin, and cytokine kits (Millipore).
Blood lipid analysis
Triglycerides were measured using the CardioCheck meter and Triglyceride Strips (Polyment Technoogy Systems, Indianapolis, IN).
RNA preparation and analysis
RNA was prepared from tissues collected in RNA-later (Ambion, Life Technologies, Grand Island, NY) and snap frozen in liquid nitrogen. Total RNA was prepared with RNeasy kits (Qiagen, Valencia, CA) following manufacturer’s instructions. The purified RNA was subjected to an additional DNAse treatment (Ambion, Life Technologies, Grand Island, NY) to ensure removal of contaminating genomic DNA prior to final column purification. The relative expression of mRNA was examined by quantitative PCR analysis. cDNA was prepared using Superscript III (Invitrogen, Life Technologies, Grand Island, NY) with random hexamers and 0.5μg - 1μg of RNA per tissue. Quantitative real-time PCR was performed on a Biorad iCycler using SyBr Green master mix (Biorad, Hercules, CA). The primer sequences for the murine genes were: Gapdh (5’-CTTCACCACCATGGAGAAGGC-3’; 5’-GGCATGGACTGTGGTCAT-3’); p53 (5’-TGAAACGCCGACCTATCCTTA-3’; 5’-GGCACAAACACGAACCTCAAA-3’); Mdm2 (5’-TGACACCAGAGCTTAGTCCTG-3’; 5’-GCGTCTCGTAACGAATAAGGC-3’); Zfp385a (5’-ACATTGAGCACCGCTATGTCT-3’; 5’-CTCTCTTGGATGAGGGTCTGATA-3’); Sesn1 (5’-GTGGACCCAGAACGAGATGACGTGGC -3’; 5’-GACACTGTGGAAGGCAGCTATGTGC -3’); Sesn2 (5’-TCCGAGTGCCATTCCGAGAT-3’; 5’-TCCGGGTGTAGACCCATCAC-3’); Sesn3 (5’-GCGAGGAGAAGAACATTTGCC-3’; 5’-CCAAACATACAGTGAACATAGT-3’). All samples were examined in triplicate and values were normalized for baseline expression and for expression of Gapdh. Calculations of values were made using the ΔΔCt method. Statistical significance was calculated using CT values.
Data analysis
To calculate statistical changes in metabolic parameters, statistically significant differences (P < 0.050) between groups were examined using the two-tailed Student’s T-test. Microsoft Excel was used for statistical calculations.
RESULTS
p53S18A(B6) mice have reduced glucose tolerance, but normal insulin sensitivity
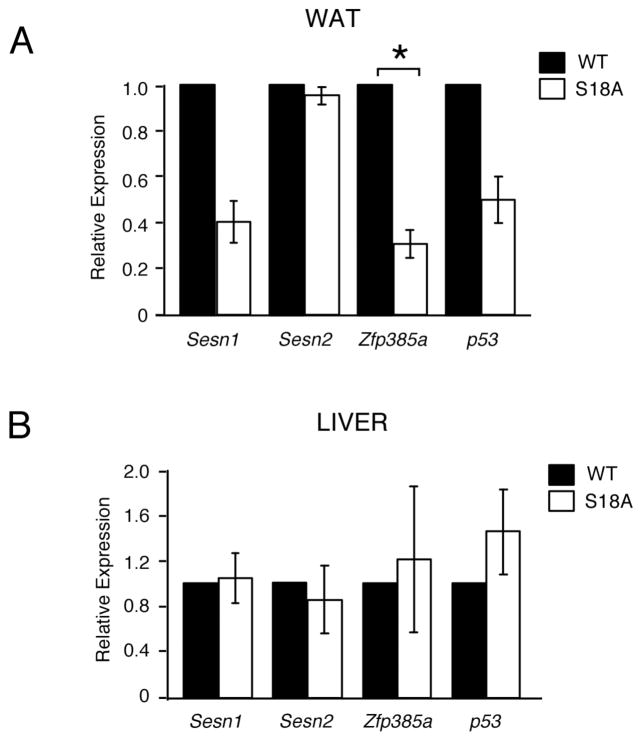
We previously reported that mice defective in phosphorylated p53Ser18 (the equivalent of Ser15 in human p53) develop both glucose intolerance and insulin resistance [1]. In addition, we reported p53Ser18-dependent regulation of the anti-oxidant sestrin genes (sesn1 and sesn2) and adipogenesis regulator Zfp385 (also known as hzf) [1]. As shown in Fig. 1A, we have subsequently discovered that a sub-strain of p53Ser18 mutant mice on a C57Bl/6 genetic background, which we have designated as p53S18A(B6) mice, exhibit decreased expression of Zfp385a, but not sesn1 or sesn2, selectively in adipose tissue. By comparison, expression levels of Zfp385a, sesn1 and sesn2 were the same in other tissues from wild type and p53S18A(B6) mice, as shown for liver in Fig. 1B.
Fig. 1. Zfp385a is decreased specifically in WAT of p53S18A(B6) mice.
(A–B) The expression of Sesn 1/2, Zfp385a, p53, and Gapdh mRNA was measured by quantitative real time PCR analysis in 6 month old wildtype mice compared to p53S18A mice in white adipose tissue (WAT) (A) and liver (B). The amount of Gapdh mRNA in each sample was used to calculate relative mRNA expression (mean ± S.E.M.; n = 3, done in triplicate). Statistically significant differences between WT and p53S18A mice are indicated (*, P < 0.05).
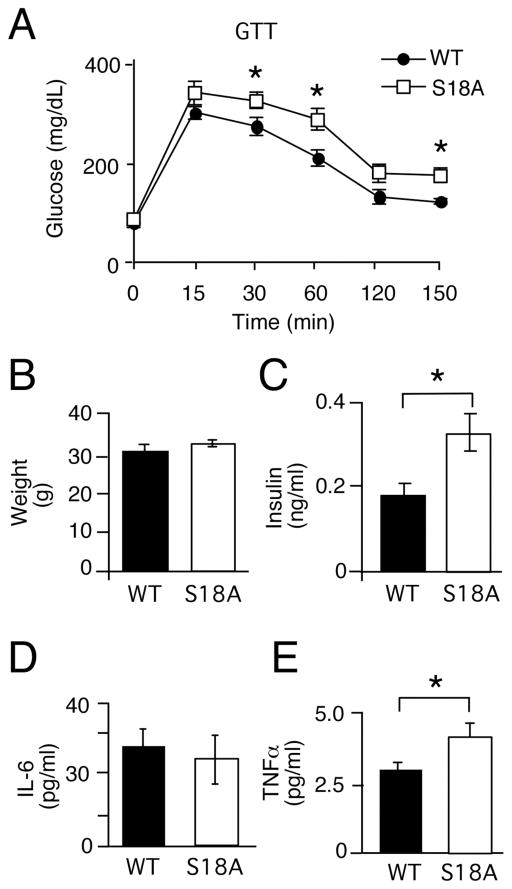
We analyzed glucose metabolism in p53S18A(B6) animals by performing glucose and insulin tolerance tests on tumor-free animals. By 6 months of age, p53S18A(B6) mice on a normal chow diet were glucose intolerant (Fig. 2A). Serum glucose levels were significantly elevated in p53S18A(B6) mice compared to wild type mice at 30 and 60 min following intraperitoneal glucose injection. At 30 min, average peak glucose levels for p53S18A(B6) males were measured to be 337 ± 17 mg/dL compared with 285 ± 19 mg/dL in wild type males. At 60 min, average peak glucose levels for p53S18A(B6) males were 299 ± 22 mg/dL compared with 222 ± 18 mg/dL in wild type mice. And at 150 min, the p53S18A(B6) mice had an elevated mean glucose compared to wild type (188 ± 15 and 135 ± 5 mg/dL, respectively). At the same time, we did not observe overt insulin resistance during an insulin tolerance test (data not shown).
Fig. 2. p53S18A(B6) mice exhibit decreased glucose sensitivity with increased insulin levels.
Wildtype (WT) and p53S18A (S18A) mice were maintained on a standard chow diet. Experiments were performed on 6–7 months old animals. (A) Glucose tolerance test (GTT). Mice fasted overnight were treated with glucose (1g/kg) by intraperitoneal injection. Blood glucose concentration was measured at the indicated times (mean ± S.E.M.; n = 15). (B) Body weight was measured at 6 months (mean ± S.E.M.; n = 15). (C) Insulin measurement in mice fasted overnight (mean ± S.E.M.; n = 9). (D–E) WT and p53S18A mice were fasted overnight and the blood concentration of IL-6 (D) and TNFα(E) was measured (mean ± S.E.M.; n = 12). (A – E) Statistically significant differences between WT and p53S18A mice are indicated (*, P < 0.05).
To pursue the issue of insensitivity further, we examined other parameters of metabolic function. Obesity contributes to defects in glucose homeostasis, but in this sub-strain we did not observe a significant difference from wild type in body weight (Fig. 2B) or food consumption (data not shown). We did find a significantly increased level of serum insulin in p53S18A(B6) animals compared to wild type animals (Fig. 2C), which, however, was not associated with insulin resistance, as shown by the insulin tolerance test. Furthermore, although there was no significant increases in circulating IL-6 (Fig. 2D), TNF α levels were elevated (Fig. 2E). Elevated levels of TNFα are associated with insulin resistance and obesity [3, 30]. We also performed a hyperinsulinemic-euglycemic clamp study to examine insulin sensitivity in conscious mice. We examined five 6 month-old animals of each genotype with similar body weights. During the euglycemic clamps, plasma glucose levels were similarly maintained at 140–160 mg/dl in both groups of mice (Fig. S1A). The steady-state glucose infusion rate during the clamp was similar in p53S18A(B6) mice and wild type mice (Fig. S1B). Insulin-stimulated whole body glucose turnover was modestly reduced in wild type compared to p53S18A(B6) mice (Fig. S1C), while whole body glycolysis was slightly reduced in p53S18A(B6) mice compared to wild type (Fig. S1D).
To summarize the results of these metabolic tests, we found that p53S18A(B6) mice have reduced glucose tolerance, but normal whole body insulin sensitivity, in contrast to p53S18A mice, which exhibit both reduced glucose tolerance and reduced insulin sensitivity [1]. One possible explanation might be that glucose intolerance is due to insulin resistance in one, but not all, insulin-sensitive tissues in the p53S18A(B6) sub-line.
p53S18A(B6) mice have impaired function and selective insulin resistance in adipose tissue
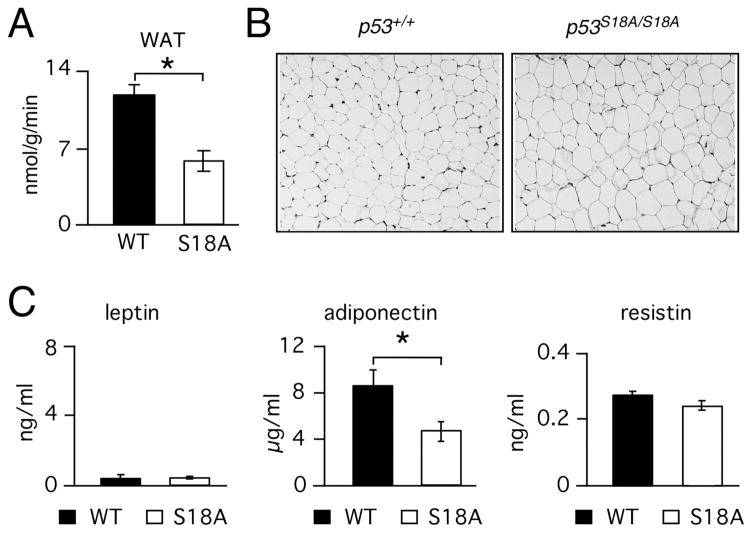
To test this hypothesis, we analyzed insulin sensitivity of peripheral tissues in p53S18A(B6) mice. We found no differences in skeletal muscle insulin sensitivity (Fig. S1E), or in insulin-stimulated whole body lipid and glycogen synthesis, basal hepatic glucose production (HGP), and insulin-stimulated HGP (Fig. S1F, G, and H). Thus, these animals did not exhibit either hepatic or muscle insulin resistance. In contrast, insulin-stimulated glucose uptake by white adipose tissue during the clamp was markedly reduced in p53S18A(B6) mice (Fig. 3A), indicating that insulin resistance was specific to adipose tissue.
Fig. 3. p53S18A(B6) mice mice exhibit adipose tissue insulin resistance and dysfunction.
Wildtype (WT) and p53S18A (S18A) mice were maintained on a standard chow diet. Experiments were performed on 6–7 month old animals. (A) Adipose tissue insulin resistance. Hyperinsulinemia-euglycemic clamp analysis (means ± S.E.M.; n = 5). (B) Representative histological sections from epididymal fat pads from WT and p53S18A mice. (C –E) WT and p53S18A mice were fasted overnight and the blood concentration of leptin (C), adiponectin (D), and resistin (E) were measured (mean ± S.E.M.; n = 6). Statistically significant differences are indicated (*, P < 0.05).
To determine if insulin resistance could be due to adipose tissue dysfunction, we compared white adipose tissue in p53S18A(B6) and wild type mice. Histological analysis revealed that there were no morphological differences, although adipose tissue from p53S18A(B6) animals appeared to be more uniform in size (Fig. 3B). Fasting blood triglyceride levels were similar in p53S18A(B6) mice and wild type mice (data not shown). Circulating plasma leptin levels were also similar (Fig. 3C), but adiponectin levels were lower in p53S18A(B6) animals compared to wild type (Fig. 3D). In contrast, there was no difference in resistin levels (Fig. 3E). Together, these data demonstrate that p53S18A(B6) mice exhibit selective insulin resistance in adipose tissue, which is likely due to defects in adipose tissue function.
p44Tg mice have reduced glucose tolerance and defects in adipose tissue
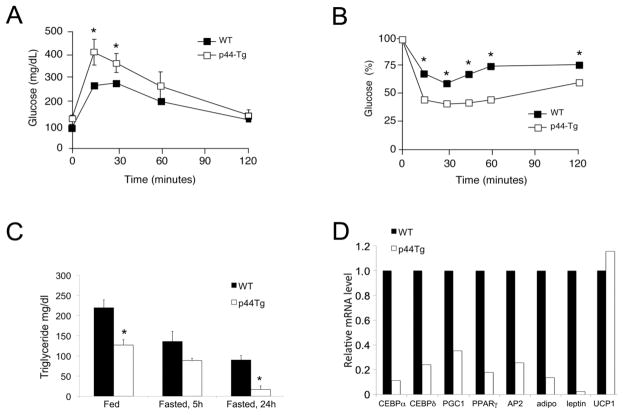
To begin to understand the relationship between the Ser18 mutation, which affects the transactivation domain of p53, and glucose sensitivity and adipose tissue, we analyzed another line of p53 mutant mice. p44Tg mice have an extra (ectopic) allele of p53 that codes for Δ40p53, a p53 isoform lacking the transactivation domain. Expression of both endogenous and ectopic p53 genes results in an imbalance in p53 and Δ40p53 such that there is a higher ratio of Δ40p53 to full-length p53 and a skewed contribution of Δ40p53 to p53 tetramers. The effect can be compared to effect of the Ser18 mutation on the activity of the p53 transcription factor [27]. If the physiological phenotype of p53S18A(B6) mice is linked to the transactivation domain of p53, then p44Tg might exhibit similar changes. To test this hypothesis, we subjected p44Tg mice to a glucose tolerance test and found they exhibited significantly increased glucose levels at 15 and 30 minutes post-injection (Fig. 4A). Thus, like p53S18A(B6) mice, p44Tg mice are glucose intolerant. In contrast to p53S18A(B6) mice, however, p44Tg mice exhibited increased, rather than decreased, sensitivity during an insulin tolerance test (Fig. 4B) and lower than normal triglyceride levels in both the fed and fasted states (Fig. 4C). These data are consistent with published results, demonstrating that, despite a defect in beta cell mass and significantly reduced insulin levels, young p44Tg mice are not hyperglycemic [9]. They do, however, have defects in adipose tissue such that the expression of genes that together regulate adipogenesis (CEBPα, CEBPδ), fatty acid storage (PPARγ, PGC-1, AP2), and systemic glucose metabolism (adiponectin and leptin) are significantly reduced. As shown in Fig. 4D, the levels found in adipose tissue (epididymal fat pad) from p44Tg mice (open bars) were lower than normal adipose tissue (black bars) even at a young age. The level of UCP-1, on the other hand, which is not involved in adipocyte generation or function, was not different. Thus, mutations that impair the ability of p53 to transactivate (or transrepress) target genes, either by interfering with phosphorylation of critical sites (Ser18) or altering the stoichiometry of p53 tetramers with or without the transactivation domain, cause decreased glucose sensitivity that could be attributed at least in part to deficits in adipose tissue.
Fig. 4. p44Tg mice exhibit decreased glucose sensitivity and defects in adipose tissue.
Wildtype (WT) and p44Tg mice were maintained on a standard chow diet. Experiments were performed on 3 – 4 month old animals. (A) Glucose tolerance test (GTT). Mice fasted overnight were treated with glucose (1g/kg) by intraperitoneal injection. Blood glucose concentration was measured at the indicated times (mean ± S.E.M.; n = 5 – 20). (B) Insulin Tolerance Test (ITT). Mice fed ad libitum were treated with insulin (0.75 U/kg) by intraperitoneal injection. Blood glucose was measured at the indicated times (mean ± S.E.M.; n = 5 – 20). Statistically significant differences are indicated (*, P < 0.05). (C) Triglyceride levels under fed or fasted conditions for times indicated. (D) Gene expression in WAT from 3 month old male determined by qPCR.
Increased p53 dosage improves glucose tolerance
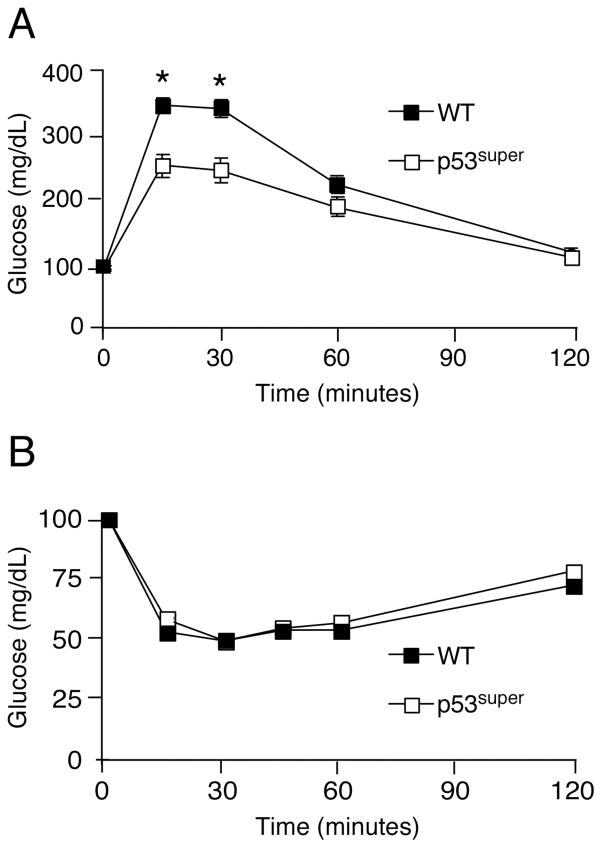
To further confirm that p53Ser18 regulates metabolism, we examined glucose homeostasis in “Super p53” mice [7], which have an additional dose of p53 carried on a BAC transgene, resulting in greater p53Ser18-dependent gene expression. We observed a significant improvement in glucose response in p53super compared to wildtype (WT) mice, as shown by glucose tolerance (Fig. 5A) and insulin tolerance (Fig. 5B). These studies further highlight the importance of p53 pathway in regulation of glucose homeostasis.
Fig. 5. Super p53 mice exhibit increased glucose sensitivity.
Wildtype (WT) and p53super/+ mice were maintained on a standard chow diet. Experiments were performed on 4 months old animals. (A) Glucose tolerance test (GTT). Mice fasted overnight were treated with glucose (1g/kg) by intraperitoneal injection. Blood glucose concentration was measured at the indicated times (mean ± S.E.M.; n = 10). (B) Insulin Tolerance Test (ITT). Mice fed ad libitum were treated with insulin (0.75 U/kg) by intraperitoneal injection. Blood glucose was measured at the indicated times (mean ± S.E.M.; n = 10). The mice were fasted overnight and treated without or with insulin (1.5 U/kg body mass) by intraperitoneal injection (30 mins). Statistically significant differences are indicated (*, P < 0.05).
Discussion
p53 is a tumor suppressor and regulator of cellular stress. Various cellular stresses connected with defective metabolism, including atherosclerosis, trauma, hypoxia, and infections, are associated with p53 activation and phosphorylation of p53 at Ser18 [5, 17, 20, 21, 29, 32, 34]. ATM, the kinase that phosphorylates p53Ser18, is also activated by these same stresses. In addition, patients with mutations in the ATM gene, which cause ataxia telangiectasia, exhibit an increased risk of developing insulin resistance and type 2 diabetes [2], and loss of Atm in mice has been implicated in insulin resistance and metabolic disease [25]. Furthermore, we have demonstrated a defect in glucose homeostasis in animals expressing a mutation at p53Ser18, an ATM target site [1].
p53Ser18 mutant animals (p53S18A mice) on a mixed genetic background exhibited increased oxidative stress, likely due to loss of sestrin gene expression in the liver, which contributed to defects in glucose homeostasis [1]. However, the animals also exhibited deficits in Zfp385a expression and in levels of circulating adipokines. We subsequently identified a sub-strain of p53S18A mice that exhibited adipose-specific loss of p53Ser18-dependent Zfp385a expression. We report in this study that p53S18A animals on a C57BL/6 background [p53S18A(B6) mice] had a significant reduction in Zfp385a expression in adipose tissue, but no changes in sesn expression levels in the liver (Fig. 1A, B). This enabled us to study the effects of loss of p53Ser18-dependent expression of Zfp385a in adipose tissue.
p53S18A(B6) mice exhibited glucose intolerance at 6 months of age (Fig. 2), confirming our previous findings that the ATM phosphorylation site on murine p53Ser18 exerts a protective role in maintaining glucose homeostasis [1]. The animals exhibited increased circulating plasma insulin levels, which could have indicated insulin resistance, but none was observed in a serum insulin tolerance test (data not shown). However, clamp studies did identify insulin resistance specifically in adipose tissue (Fig. 3A), with none observed in liver or muscle, suggesting a defect in adipose tissue function. Consistent with this, we found lower levels of circulating adiponectin. Although the mechanism is unclear, we propose that it is the loss of Zfp385a that is interfering with adipose tissue function, perhaps by altering the translation of CEBPα, as suggested in a previous report [12]. The insulin resistance in adipose tissue, decreased Zfp385a expression in white adipose tissue, decreased circulating levels of adiponectin levels, as well as increased TNFα levels suggest that the defect in glucose tolerance in p53S18A(B6) mice is due to defects in adipose tissue function. This is an important finding and confirms the role of adipose tissue in overall glucose homeostasis.
To test our hypothesis that p53 positively regulates glucose homeostasis, we analyzed glucose handling in mice with an extra dose of p53, which we predicated would lead to improved glucose homeostasis. "Super p53 mice" (p53super mice) have an ectopic p53 locus in which p53 expression is under normal genomic control. These mice exhibited significantly improved glucose homeostasis at 6 months of age (Fig. 5). Although the mechanism for this improved glucose tolerance is not clear, it has been reported that Super p53 mice exhibit increased sestrin gene expression, suggesting that they might have decreased oxidative stress. Alternatively, they might have improved adipose tissue function, which could contribute to improved glucose homeostasis.
We also examined glucose handling in a line of mutant p53 mice in which an ectopic allele of p53 encoding a specific p53 isoform (Δ40p53) alters the balance between this isoform and full-length p53. Δ40p53 is identical to p53 except that it is missing the first 40 amino acids, including Ser18. Thus, mice with an extra dose of Δ40p53 (p44Tg mice) compared to wild type mice have a reduced amount of Ser18, whereas Super p53 mice have an increased amount of Ser18. Consistent with these Ser18 dosage differences, we confirmed previously published results [8] that p44Tg mice exhibit decreased glucose tolerance (Fig. 4). However, we observed improved insulin sensitivity in these same mice, which would suggest that the tissues are not insulin resistant but that some aspect of insulin production or secretion is defective. Along these lines, a recent study demonstrated that defects in glucose tolerance were coupled to decreased beta cell mass and lower insulin levels in p44Tg mice [9].
Because Δ40p53 interferes with the function of the p53 transcription factor [8, 12, and [28]], the extra dose of Δ40p53 in p44Tg mice could easily lead to changes in the expression of p53 target genes, such as Zfp385a, and perturbation of glucose homeostasis. This could explain why the expression of an array of genes involved in adipogenesis and adipocyte-specific utilization of glucose was significantly lower in white adipose tissue from p44Tg mice compared to that from normal mice. In all p53 models, changes in glucose tolerance (decreased in p53S18A(B6) and p44Tg mice and increased in Super p53 mice) did not occur until the mice were middle-aged. Thus, it is unlikely that p53 exerts a direct affect on insulin signaling per se. However, altered transcription of other p53 target genes that regulate metabolism, such as Glut-1 or -4 [24, 31], TIGAR [11], or Sco2 [16], could contribute substantially to the metabolic phenotype of these various mouse strains. The results presented in this paper link functional deficits in the transactivation domain of the p53 protein, which stem from completely different p53 mutations in isolated strains of p53 mutant mice, to adipose tissue-specific defects that contribute to impaired glucose handling. How these defects in relatively young animals lead to serious consequences, such as diabetes, when they are older will be an important question to pursue.
Conclusions
We have utilized a mouse model wherein p53Ser18 is no longer phosphorylated to study the contribution of p53 activation to glucose homeostasis. We have identified animals that exhibit adipose, but not liver, specific defects of p53Ser18-dependent transcription, such as Zfp385a. These animals demonstrate glucose intolerance. In addition, we examined glucose homeostasis in two additional models of p53 function, one in which p53 transcriptional activity is perturbed and another with increased p53 activity. Mice with an increased dosage of p53 exhibited better than normal glucose homeostasis. The results of this study support a model in which normal p53 function acts as a barrier to glucose intolerance and insulin resistance.
Acknowledgments
We thank Roger Davis for critical reading of the manuscript, Judy O’Reilly for invaluable technical assistance, David Garlick for histology analysis, and Ceren Acer and Charissa Cottonham for assistance with real-time PCR analysis. We thank Dr. Serrano for the p53super mice. C.D. was supported by a Fuller Fellowship from the American Cancer Society. These studies were supported by a Pilot & Feasibility grant from the UMASS Diabetes Endocrinology Research Center (NIH P30 DK32520) (to H.K.S.), a Worcester Foundation Annual Research Award from the Worcester Foundation for Biomedical Research (to H.K.S.) and by grants from the NIH (DK80756 to J.K.K. and AG26094 to H.S.), the American Diabetes Association (7-07-RA-80 to J.K.K.), and the Ellison Medical Research Foundation (to H.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Core resources, including the UMASS Mouse Metabolic Phenotyping Center, supported by the Diabetes Endocrinology Research Center grant DK32520 were also used. Hayla K. Sluss and Jason K. Kim are members of the UMass DERC (DK32520).
Footnotes
Conflict of interest statement. The authors declare they have no conflict of interest.
References
- 1.Armata HL, Golebiowski D, Jung DY, Ko HJ, Kim JK, Sluss HK. Requirement of the ATM/p53 tumor suppressor pathway for glucose homeostasis. Mol Cell Biol. 2010;30:5787–5794. doi: 10.1128/MCB.00347-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bar RS, Levis WR, Rechler MM, Harrison LC, Siebert C, Podskalny J, Roth J, Muggeo M. Extreme insulin resistance in ataxia telangiectasia: defect in affinity of insulin receptors. N Engl J Med. 1978;298:1164–1171. doi: 10.1056/NEJM197805252982103. [DOI] [PubMed] [Google Scholar]
- 3.Borst SE. The role of TNF-alpha in insulin resistance. Endocrine. 2004;23:177–182. doi: 10.1385/ENDO:23:2-3:177. [DOI] [PubMed] [Google Scholar]
- 4.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 5.Eizenberg O, Faber-Elman A, Gottlieb E, Oren M, Rotter V, Schwartz M. Direct involvement of p53 in programmed cell death of oligodendrocytes. Embo J. 1995;14:1136–1144. doi: 10.1002/j.1460-2075.1995.tb07097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Cao I, Garcia-Cao M, Martin-Caballero J, Criado LM, Klatt P, Flores JM, Weill JC, Blasco MA, Serrano M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. Embo J. 2002;21:6225–6235. doi: 10.1093/emboj/cdf595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb E, Vousden KH. p53 regulation of metabolic pathways. Cold Spring Harb Perspect Biol. 2010:a001040. doi: 10.1101/cshperspect.a001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hinault C, Kawamori D, Liew CW, Maier B, Hu J, Keller SR, Mirmira RG, Scrable H, Kulkarni RN. Delta40 Isoform of p53 controls beta-cell proliferation and glucose homeostasis in mice. Diabetes. 2011;60:1210–1222. doi: 10.2337/db09-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinault C, Kawamori D, Liew CW, Maier B, Hu J, Keller SR, Mirmira RG, Scrable H, Kulkarni RN. Δ 40 Isoform of p53 Controls β-Cell Proliferation and Glucose Homeostasis in Mice. Diabetes. 2011;60:1210–1222. doi: 10.2337/db09-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang P, Du W, Wang X, Mancuso A, Gao X, Wu M, Yang X. p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat Cell Biol. 13:310–316. doi: 10.1038/ncb2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawagishi H, Wakoh T, Uno H, Maruyama M, Moriya A, Morikawa S, Okano H, Sherr CJ, Takagi M, Sugimoto M. Hzf regulates adipogenesis through translational control of C/EBPalpha. Embo J. 2008;27:1481–1490. doi: 10.1038/emboj.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keim AL, Chi MM, Moley KH. Hyperglycemia-induced apoptotic cell death in the mouse blastocyst is dependent on expression of p53. Mol Reprod Dev. 2001;60:214–224. doi: 10.1002/mrd.1080. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Higashimori T, Park SY, Choi H, Dong J, Kim YJ, Noh HL, Cho YR, Cline G, Kim YB, Kim JK. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 15.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650– 1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 17.Mercer J, Figg N, Stoneman V, Braganza D, Bennett MR. Endogenous p53 protects vascular smooth muscle cells from apoptosis and reduces atherosclerosis in ApoE knockout mice. Circ Res. 2005;96:667–674. doi: 10.1161/01.RES.0000161069.15577.ca. [DOI] [PubMed] [Google Scholar]
- 18.Miles PD, Treuner K, Latronica M, Olefsky JM, Barlow C. Impaired insulin secretion in a mouse model of ataxia telangiectasia. Am J Physiol Endocrinol Metab. 2007;293:E70–74. doi: 10.1152/ajpendo.00259.2006. [DOI] [PubMed] [Google Scholar]
- 19.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 20.Moon C, Kim S, Wie M, Kim H, Cheong J, Park J, Jee Y, Tanuma N, Matsumoto Y, Shin T. Increased expression of p53 and Bax in the spinal cords of rats with experimental autoimmune encephalomyelitis. Neurosci Lett. 2000;289:41–44. doi: 10.1016/s0304-3940(00)01253-2. [DOI] [PubMed] [Google Scholar]
- 21.Sakhi S, Bruce A, Sun N, Tocco G, Baudry M, Schreiber SS. p53 induction is associated with neuronal damage in the central nervous system. Proc Natl Acad Sci U S A. 1994;91:7525–7529. doi: 10.1073/pnas.91.16.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schalch DS, McFarlin DE, Barlow MH. An unusual form of diabetes mellitus in ataxia telangiectasia. N Engl J Med. 1970;282:1396–1402. doi: 10.1056/NEJM197006182822503. [DOI] [PubMed] [Google Scholar]
- 23.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab. 2006;4:377–389. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Schwartzenberg-Bar-Yoseph F, Armoni M, Karnieli E. The tumor suppressor p53 down-regulates glucose transporters GLUT1 and GLUT4 gene expression. Cancer Res. 2004;64:2627–2633. doi: 10.1158/0008-5472.can-03-0846. [DOI] [PubMed] [Google Scholar]
- 25.Shoelson SE. Banking on ATM as a new target in metabolic syndrome. Cell Metab. 2006;4:337–338. doi: 10.1016/j.cmet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Sluss HK, Armata H, Gallant J, Jones SN. Phosphorylation of serine 18 regulates distinct p53 functions in mice. Mol Cell Biol. 2004;24:976–984. doi: 10.1128/MCB.24.3.976-984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turenne GA, Paul P, Laflair L, Price BD. Activation of p53 transcriptional activity requires ATM's kinase domain and multiple N-terminal serine residues of p53. Oncogene. 2001;20:5100–5110. doi: 10.1038/sj.onc.1204665. [DOI] [PubMed] [Google Scholar]
- 28.Ungewitter E, Scrable H. Delta40p53 controls the switch from pluripotency to differentiation by regulating IGF signaling in ESCs. Genes Dev. 2010;24:2408–2419. doi: 10.1101/gad.1987810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Vlijmen BJ, Gerritsen G, Franken AL, Boesten LS, Kockx MM, Gijbels MJ, Vierboom MP, van Eck M, van De Water B, van Berkel TJ, Havekes LM. Macrophage p53 deficiency leads to enhanced atherosclerosis in APOE*3-Leiden transgenic mice. Circ Res. 2001;88:780–786. doi: 10.1161/hh0801.089261. [DOI] [PubMed] [Google Scholar]
- 30.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner H, Karnieli E, Rauscher FJ, LeRoith D. Wild-type and mutant p53 differentially regulate transcription of the insulin-like growth factor I receptor gene. Proc Natl Acad Sci U S A. 1996;93:8318–8323. doi: 10.1073/pnas.93.16.8318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamanishi Y, Boyle DL, Pinkoski MJ, Mahboubi A, Lin T, Han Z, Zvaifler NJ, Green DR, Firestein GS. Regulation of joint destruction and inflammation by p53 in collagen-induced arthritis. Am J Pathol. 2002;160:123–130. doi: 10.1016/S0002-9440(10)64356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–898. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 34.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis - the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng SJ, Lamhamedi-Cherradi SE, Wang P, Xu L, Chen YH. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes. 2005;54:1423–1428. doi: 10.2337/diabetes.54.5.1423. [DOI] [PubMed] [Google Scholar]