Abstract
While kidney donations stagnate, the number of people in need of kidney transplants continues to grow. Although transplanting culture-grown organs is years away, pursuing the engineering of the kidney de novo is a valid means of closing the gap between the supply and demand of kidneys for transplantation. The structural organization of a mouse kidney is similar to that of humans. Therefore, mice have traditionally served as the primary model system for the study of kidney development. The mouse is an ideal model organism for understanding the complexity of the human kidney. Nonetheless, the elaborate structure of the mammalian kidney makes the discovery of new therapies based on de novo engineered kidneys more challenging. In contrast to mammals, amphibians have a kidney that is anatomically less complex and develops faster. Given that analogous genetic networks regulate the development of mammalian and amphibian nephric organs, using embryonic kidneys of Xenopus laevis (African clawed frog) to analyze inductive cell signaling events and morphogenesis has many advantages. Pioneering work that led to the ability to generate kidney organoids from embryonic cells was carried out in Xenopus. In this review, we discuss how Xenopus can be utilized to compliment the work performed in mammalian systems to understand kidney development.
Keywords: Xenopus, Kidney, Development, Pronephros, Nephron, Induction, Organoid
Xenopus: a unique model system for the study of nephrogenesis
Introduction
Kidneys are essential for the osmoregulation of bodily fluids. An organism achieves osmoregulation by controlling the input and output of water and ions in its body, thus regulating their concentration within body fluids. The nephron is the basic structural and functional unit within the kidney. Adult human kidneys are composed of approximately one million nephrons that regulate osmotic pressure by filtering and removing excess water and waste products from the blood. The ability of kidneys to regulate blood composition is dependent on the process of kidney development. Because mammalian model systems, such as that of the mouse, share many biological characteristics with human kidneys, they have been important resources for understanding human kidney organogenesis and are an invaluable tool for ultimately understanding human kidney development and disease. However, given that kidney development is highly conserved among vertebrates, exploiting some of the notable advantages of amphibian model systems, such as Xenopus laevis (African clawed frog), can facilitate our understanding of kidney organogenesis. The goal of this review is to provide insight into how experimental approaches in the simple, yet functional frog embryonic kidney can complement mammalian models to address open questions underlying the formation and function of the nephron.
Xenopus as a model system
Xenopus offers many advantages that can complement the well-established mammalian models used to study nephrogenesis. The Xenopus female lays a large number of eggs (100–500) that develop externally, allowing for easy manipulation and visualization of all stages of development. In addition to offering experimental simplicity, Xenopus embryos develop functional embryonic kidneys within 2–3 days of fertilization.
The previously characterized Xenopus cell-fate map allows for blastomere-specific microinjection and tissue-targeted disruption of genes [1–4]. Microinjection techniques can be used in combination with fluorescent tracers (chemical dyes and fluorescent constructs) as a method for generating a more detailed fate map and identifying stem and tissue progenitor cells. Furthermore, embryos can also be microinjected with morpholinos or CRISPR/Cas9 for knockdown/knockout experiments and mRNA for overexpression of genes of interest. These techniques make Xenopus a tractable model for pre-screening of DNA or mRNA constructs in order to identify important regulators of nephron formation that can then be further analyzed in mammalian systems. Given the recent availability of sequence data from patients with inherited kidney diseases, Xenopus offers the ability to understand the molecular consequences of these mutations fairly rapidly. Additionally, Xenopus can be used for large-scale chemical screens and identification of potential therapeutic agents. Rapid embryogenesis and the ability to generate large numbers of embryos allow for development of cost-effective high-throughput screens.
Kidney development and function
All vertebrates initially develop simple embryonic kidneys that eventually give rise to more complex adult kidneys. Kidney development begins during embryogenesis when the mesoderm is subdivided into paraxial, intermediate and lateral plate mesoderm. The kidney is derived from the intermediate mesoderm, and its development unfolds in consecutive stages, leading to a gradual increase in organizational complexity and number of nephrons. Both mammals and amphibians initially develop simple embryonic kidneys called pronephroi, with each pronephros consisting of a single nephron. The mammalian pronephros is a nonfunctional structure of early development; however, its formation is required for the establishment of subsequent kidney forms. Amphibians also develop pronephroi, with one pronephros on each side of the tadpole. The amphibian pronephroi serve as fully functional kidneys essential for osmoregulation. Consequently, tadpoles with defects in the formation of the pronephroi develop edema [5–9]. In both mammals and amphibians, the pronephroi are eventually replaced by more complex kidney structures called mesonephroi, which contain numerous nephrons. While the mesonephroi represent the adult kidneys of amphibians, in mammals they serve as transient structures and are the precursors to the development of the adult kidneys, called the metanephroi.
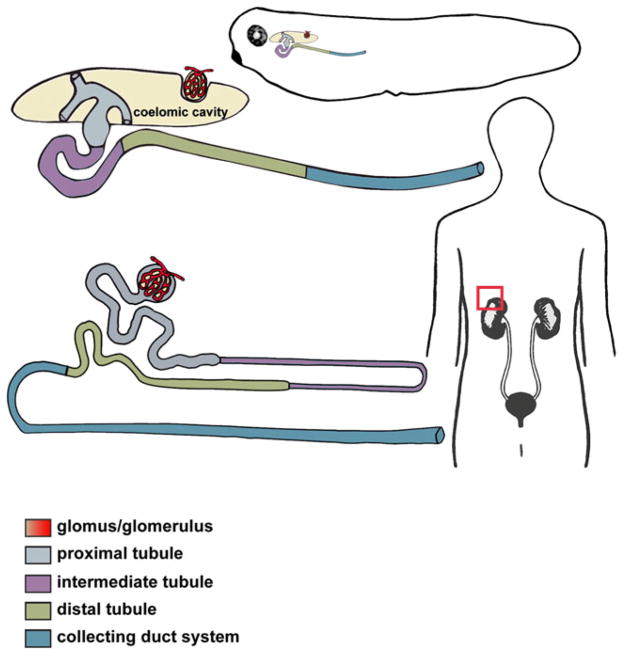
Each Xenopus pronephros consists of a single large nephron that shares structural and functional similarities with the mammalian nephron (Fig. 1). For example, the Xenopus glomus and the glomerulus of the metanephric nephron in mammals are both responsible for blood filtration. Additionally, the Xenopus pronephric and the mammalian metanephric tubules and duct are both responsible for filtering and eliminating waste products, and the common developmental markers of nephric components are also conserved (Table 1) [10–36]. The similarities in segmentation and expression of molecular markers between the Xenopus pronephric and the mammalian metanephric nephron have been covered in detail in multiple reviews [37–42] and therefore will not be discussed here in great detail.
Fig. 1.
Xenopus pronephric and human metanephric nephrons share a conserved segmentation pattern. A schematic representation of an enlarged Xenopus pronephric nephron (top) and mammalian metanephric nephron (bottom), with the distinct tubular compartments labeled. The glomus/glomerulus filters the fluid from the blood plasma through capillary walls into the proximal tubule. The drawing depicts a slight difference in organization of the glomar/glomerular anatomy between the amphibian pronephric and mammalian metanephric nephrons. In mammalian metanephric nephrons, the glomerulus is integrated into the Bowman’s capsule near the proximal tubule, while the Xenopus glomus projects into a body cavity (coelomic cavity). Three branches of pronephric tubule (nephrostomes) use ciliary action to force the glomar filtrate down the pronephric tubule. Together, the tubule segments (proximal, intermediate and distal) and the collecting duct system (connecting tubule and duct) facilitate the process of filtrate reabsorption and transport of wastes products for secretion
Table 1.
| Gene symbol | Gene name | Expression | Role in pronephric development | References |
|---|---|---|---|---|
| lhx1; lim1 | lim homeobox 1 | PR, G, PTD | Required for establishment of the pronephric rudiment Depletion impairs development of glomus and tubules |
[12–14] |
| hnf1b | hnf1 homeobox b | PR, G, PTD | Regulates pronephros development | [15–20] |
| pax8 | paired box 8 | PR, G, PTD | Overexpression promotes ectopic tubulogenesis Morpholino knockdown causes a complete absence of tubule |
[11, 21, 22] |
| pax2 | paired box 2 | PR, G, PTD | Morpholino knockdown leads to impaired tubulogenesis, mainly of the proximal tubules Required following establishment of the pronephric rudiment |
[21–23] |
| wt1 | wilms tumor 1 | PR, G | Overexpression impairs pronephric rudiment specification and formation of proximal tubules Morpholino knockdown inhibits wnt4 expression |
[24–27] |
| gdnf | glial-cell derived neurotropic factor | PTD | Not established | [28, 29] |
| bmp4 | bone morphogenetic protein 4 | PR, PTD | Required for pronephric development | [30] |
| bmp7 | bone morphogenetic protein 7 | PR, PTD | Required for pronephric development | [31–33] |
| wnt4 | wingless-type MMTV integration site family, member 4 | PR, G, PTD | Morpholino knockdown causes absence of pronephric tubules with minimal effect on duct formation Overexpression induces formation of ectopic nephrostomes and glomus at the expense of tubules |
[25, 29, 33] |
| osr1 | odd-skipped related transcription factor 1 | PR, G, PTD | Morpholino knockdown impairs renal development Overexpression promotes ectopic kidney development |
[34] |
| osr2 | odd-skipped related transcription factor 2 | PR, G, PTD | Morpholino knockdown impairs renal development Overexpression promotes ectopic kidney development |
[34] |
PR, Pronephric rudiment; G, glomus; PTD, pronephric tubules and duct
The listed genes are shared between amphibians and mammals and are essential for proper kidney formation in both mouse and frogs
Kidney engineering: the in vitro-induced frog nephron is functional in vivo
Explant and transplantation models
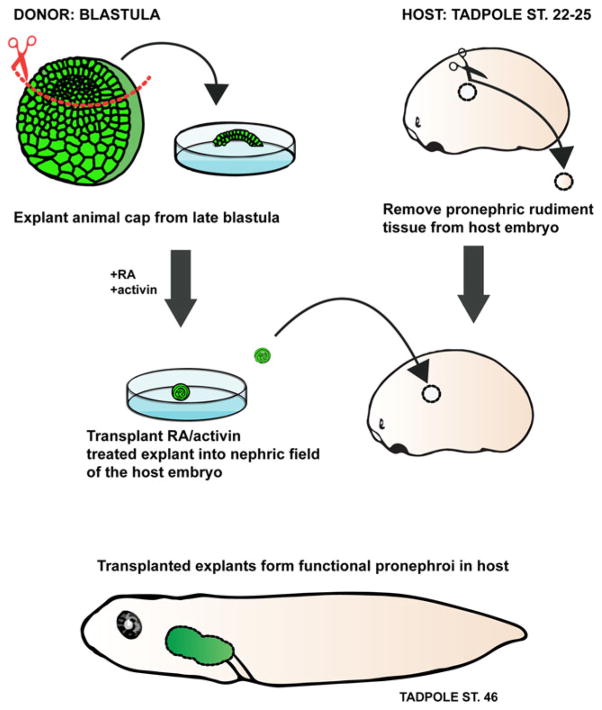
Xenopus tissue transplants and explants have been successfully used to study pronephric development. Frog embryos are relatively large and have significant healing capacity, making them amenable to numerous microsurgical assays. The pronephric cells are specified by late gastrula stages, and isolated mesodermal explants of the presumptive pronephric region can form glomus tissue and nephric tubules in culture [43–45]. Additionally, the development of the pronephros can be initiated in vitro from a mass of undifferentiated cells called the animal cap [46]. The animal cap consists of pluripotent presumptive ectoderm cells derived from the late blastula, and it serves as a useful model for elucidating the mechanisms of kidney induction and development ex vivo. Activin A, a member of the transforming growth factor beta (TGF-β) superfamily, and retinoic acid (RA), a metabolite of vitamin A, are well-known regulators of differentiation and proliferation of mesodermal tissues. At specific doses, activin A and RA can induce the formation of nephric tubules in isolated animal cap explants. In vitro-generated pronephric organoids express known molecular markers involved in nephrogenesis (lhx1, grem1, cret, osr1, osr2, hnf1b, pax8) and closely recapitulate the process of in vivo renal tubulogenesis [47–49]. Transplantation experiments performed in frogs showed that in vitro-induced pronephroi are capable of replacing and restoring the function of the native pronephros in a host embryo in which both kidney rudiments had been removed (Fig. 2) [8]. Renal function in this study was assayed by suppression of edema. Although the success of these transplantations was not high (21 %), it is remarkable that pronephroi derived from reprogrammed immature ectoderm have the capacity to adapt and function in the bilaterally pronephrectomized host embryo.
Fig. 2.
Xenopus in vitro-induced kidney organoids. Isolated animal cap (presumptive ectodermal tissue) explants (green) treated with activin and retinoic acid (RA) differentiate to form pronephric organoids in culture. The animal cap tissue treated with activin and RA can be transplanted into a host embryo which lacks kidney primordia. The in vitro-induced pronephric tissue is capable of functioning in vivo and compensates for the loss of the host kidneys by suppressing edema formation
The ultimate goal of regenerative medicine is to engineer renal tissue that will replace kidney function in patients following transplantation. Mouse and rat renal organoids derived in vitro from embryonic kidney rudiments are capable of in vivo implantation, and although short-lived, they do show some functional capability [50, 51]. Since in vitro-induced Xenopus pronephroi are capable of in vivo function, they are amenable for studies with the aim to improve survival and function of the cultured renal tissue. The frog kidney organoids can be used in combination with techniques to downregulate or overexpress genes, providing insights into the regulatory steps of in vitro kidney formation. Since the Xenopus renal organoids develop in serum-free media, the process of nephron assembly can be studied in response to controlled stimuli. The required use of animal serum containing growth factors for in vitro mammalian kidney cultures may make the results more difficult to interpret, and the variable composition of animal serum from different batches could trigger different experimental outcomes [51–53]. Moreover, Xenopus embryos and tissue explants can be used for high-throughput in vivo phenotypic drug screens and are suitable models for drug development [54–56].
All animal system models have experimental advantages and limitations. For example, mammalian kidney culture systems allow direct observation of adult kidney development, but the simultaneous branching of multiple nephrons in metanephric kidneys can make it difficult to untangle the underlying molecular mechanisms of induction, patterning and tubulogenesis. The embryonic kidney of Xenopus is structurally simple, but does enable study of the induction signals and analysis of the cellular mechanisms at the single nephron level. Since the mammalian kidney is a complicated organ to grow de novo in culture, using the simple Xenopus pronephros to study the inductive and cellular events of kidney organogenesis can promote our understanding of the beginnings of its formation. For example, the animal cap induction experiments described above served as the basis for development of the protocol for induction of mouse and human stem cells into renal epithelia [57, 58].
Kidney nephrogenesis: in vivo imaging of the frog pronephros
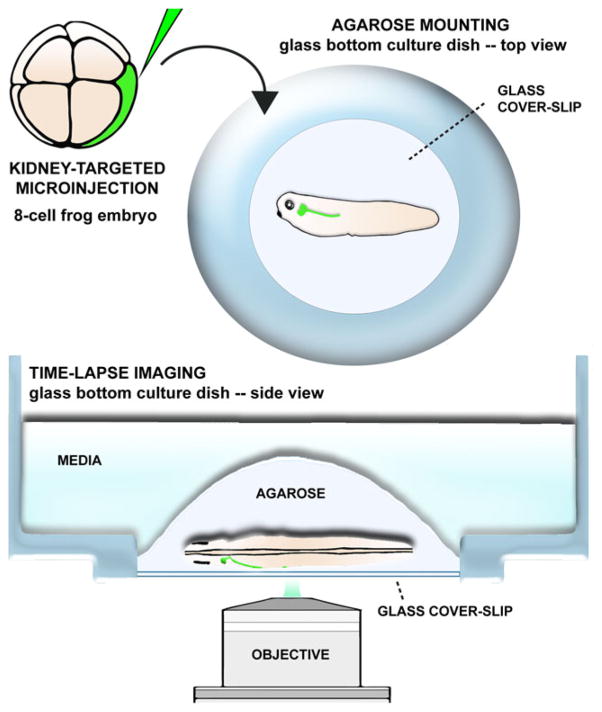
Xenopus is a particularly useful model for in vivo studies of nephrogenesis. Given that Xenopus embryos develop outside of the mother and the developing kidney can be visualized through the surface ectoderm, the formation of the pronephric tubules can be visualized in whole embryos in vivo (Fig. 3). Whole-animal imaging allows individual renal progenitor cells to be tracked in their physiological environment as they differentiate, migrate and interact to assemble the pronephros.
Fig. 3.
In vivo imaging of the frog pronephros. Xenopus fate-map of early development permits microinjections of blastomeres fate-mapped to kidney cell progenitors and it can be utilized for targeted delivery of fluorophores to developing pronephros. Microinjected embryos can be immobilized in low melting-point agarose and subjected to time-lapse imaging. This sample preparation allows for in vivo analysis of cell behaviors and molecular dynamics underlying pronephros formation
In vivo pronephros morphogenesis
Kidney studies in mammalian models are carried out in either fixed tissue or organ culture, which has been the primary means of performing live imaging in mammalian systems. Previous studies using fixed tissues have suggested that mouse kidney tubules elongate through the process of convergent extension, a process that was first described in Xenopus [59]. Intercalation of neighboring cells with one another serves to extend the length of a tissue [59, 60] and, in the case of the kidney, forms a narrower and longer tubule. A study from mice in fixed tissue suggests that Wnt9b produced in the ureteric bud and collecting ducts regulates the diameter of the developing kidney tubules through the regulation of convergent extension cell movements [61]. This study from the laboratory of T.J. Carroll was the first to suggest that vertebrate kidney tubule diameter is regulated by convergent extension. These experiments were carried out in fixed tissue, so this process had yet to be visualized through live imaging. Subsequently, studies in Xenopus implicated a role for convergence and extension in kidney tubulogenesis, and more recently, a study by Lienkamp et al. utilized live imaging in Xenopus kidneys to directly test the hypothesis that convergent extension movements are responsible for kidney tubulogenesis [62–64]. Kidney tissue was labeled using an injected mRNA encoding membrane-bound green fluorescent protein (GFP), and the movements of individual cells within the developing kidney were tracked in real time within their native environment in the embryo (Fig. 3). This experiment supported the conclusions drawn from the previous work by the Carroll research group [61] that was done using fixed mouse kidney tissue, showing that convergent extension movements are involved in kidney tubulogenesis.
Work in Xenopus also suggests how cell movements result in the convergence and extension of the kidney tubules. Live imaging of the forming pronephric kidney in Xenopus demonstrates that cells within the developing proximal and intermediate tubules undergo convergent extension by forming rosettes consisting of four to seven neighboring cells [64]. Rosettes, which were first described in the germ band epithelia of Drosophila, are pinwheel-shaped structures that form when groups of cells line up and then rapidly reorganize to elongate along a particular axis of a tissue [65]. Cells forming rosette-shaped structures have been described in mouse kidney tubules. Although it is postulated that the cell rosettes described in kidney tubules contribute to lumen development, the cellular movements that occur during rosette formation and resolution have not been determined in mice. The rosette structures visualized in real time in the developing Xenopus kidney are similar to the structures seen in the fixed tissue of the collecting duct of mice. In Xenopus, time-lapse imaging supports the findings that rosette structures in the kidney tubules are involved in convergent extension movements. Cells within a rosette constrict to form wedges with points that meet in the center of the rosette. Once the rosette is formed, the cells intercalate with one another, elongating on an axis roughly perpendicular to the axis in which the cells were originally oriented. These convergent extension movements serve to elongate the tubules of the developing Xenopus kidney. The unique ability to target developing kidney tissue in live Xenopus embryos has allowed researchers to clearly visualize how convergent extension movements and rosette formation and resolution form the tubules of the kidney.
Modulating pronephros morphogenesis
In addition to using Xenopus embryonic kidneys to elucidate the cellular movements that underlie nephrogenesis, Xenopus embryos can be used to study the effect of a gene on kidney formation. For example, Lienkamp et al. used time lapse confocal imaging to assess the role of inversin in the developing Xenopus pronephros [66]. Mutations in inversin are known to cause type II nephronophthisis in humans, a juvenile genetic disorder known to cause defects in kidney development and renal failure. Inversin is also believed to facilitate a switch between the canonical Wnt signaling and planar cell polarity pathways [67]. Although inversin mutations in humans and mice cause kidney defects, the exact cause of these defects are unknown. The authors co-injected a translation-blocking inversin morpholino to knockdown inversin expression with mRNA encoding membrane-associated GFP and histone 2B-red fluorescent protein to label cell membranes and nuclei, respectively. Confocal time-lapse microscopy was used to track individual cell movements in the developing pronephros. Two types of cell movements were detected, with one type causing the proximal and early intermediate tubules to extend in a dorsal to ventral direction, and the other movement type consisting of cells moving from the distal portion of the intermediate tubules towards the head of the embryo. Combined, these two types of cell movements resulted in the proximal tubule elongating and extending in a ventral direction, with the distal-to-proximal cell movement leading to looping and extension of the intermediate tubule. Knockdown of inversin was found to decrease only the proximal and intermediate tubule extension in the dorsal to ventral direction. Although the authors tracked movements of individual cells in this study, they did not mention whether convergent extension movements are involved in the extension of the proximal or intermediate tubules. However, these cellular movements occur simultaneously with the convergent extension movements described in the paragraph above.
Lienkamp et al. also tested the role of inversin in elongation of the distal tubule [66]. The authors co-injected mRNA encoding the photo-convertible fluorophore Kaede with a translation-blocking inversin morpholino in Xenopus embryos to knockdown inversin expression, resulting in embryos displaying green fluorescence in the trunk and kidney region. Photo conversion of Kaede from green to red resulted in a red stripe on the side of the developing embryo. As the embryo developed, red cells in the distal tubule migrated beyond the original photo-converted stripe in a posterior to anterior direction at a similar rate as cells in an embryo injected with a control morpholino. These data suggest that inversin does not play a crucial role in posterior to anterior cell movement in the distal tubule, but instead it is necessary for proximal and intermediate tubule development. This information on cell movement during early nephrogenesis would have been difficult to obtain from fixed samples or from more complex model organisms.
To date, mouse kidney tubulogenesis has been primarily studied in fixed tissue specimens taken from embryos at various stages of development, and live imaging of Xenopus embryonic kidneys has supported work done in mice. Surgical removal and ex vivo culture of the complex mouse kidney has provided valuable information on early kidney development and supplemented work that was previously performed using fixed tissue [68–71]. Future work on kidney development and function could be complemented with Xenopus, which has the advantage of having a simple, fully functional model of a nephron: the pronephros. The use of a less complex model organism would allow the researcher to address concerns related to ex vivo organ culture, such as possible alterations of tissue development due to lack of normal environmental factors, as well as limited tissue life-span in culture. Therefore, in vivo imaging of Xenopus pronephric development can be used to verify and supplement the data collected in fixed tissue and ex vivo mouse studies.
Perspectives
The cellular movements responsible for kidney tubulogenesis are conserved between mammals and frogs. The ease of manipulating gene expression in Xenopus through knockdown or overexpression coupled with the ability to perform time-lapse imaging makes Xenopus a promising model system to study the effects of perturbations in gene expression on cell movements during nephron assembly. CRISPR/Cas9 genome editing has also been successfully used to create knockout Xenopus frogs, allowing researchers to selectively knockout genes suspected to play a role in kidney tubulogenesis [71–74]. In addition, Xenopus proximal tubules have been reported to regenerate after surgical removal, making Xenopus a promising model of vertebrate kidney regeneration after acute kidney injury [75]. Similarly, animal cap explant assays take advantage of the ability to explant tissue from a Xenopus embryo and induce kidney development ex vivo. These assays, coupled with the new transgenic lines expressing fluorescent proteins in the kidney and live imaging technologies that are currently available, make kidney engineering and regeneration experiments in Xenopus feasible [42]. Additionally, Xenopus is a good candidate for multi-model studies involving human genome-wide association studies, mouse organoid cultures and cell culture work. In this context, the ease of tissue manipulation and live imaging techniques available in Xenopus can complement the mutant lines available in mice and cell cultures. Taken together, Xenopus is a unique vertebrate model system for the study of kidney development.
Acknowledgments
We would like to thank Lance Davidson, Mark Corkins and Soeren Lienkamp for critical reading of the manuscript and thoughtful suggestions. This work was funded by a National Institutes of Health NIDDK grant (K01DK092320) and startup funding from the Department of Pediatrics at the University of Texas McGovern Medical School.
Footnotes
Conflict of interest statement: The authors declare that they have no conflicts of interest
References
- 1.Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol. 1987;119:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- 2.Moody SA. Fates of the blastomeres of the 32-cell stage Xenopus embryo. Dev Biol. 1987;122:300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- 3.Bauer DV, Huang S, Moody SA. The cleavage stage origin of Spemann’s organizer: analysis of the movements of blastomere clones before and during gastrulation in Xenopus. Development. 1994;120:1179–1189. doi: 10.1242/dev.120.5.1179. [DOI] [PubMed] [Google Scholar]
- 4.DeLay BD, Krneta-Stankic V, Miller RK. Technique to target microinjection to the developing Xenopus kidney. J Vis Exp. 2016:e53799. doi: 10.3791/53799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxén L. Organogenesis of the kidney. Cambridge University Press; Cambridge: 1987. [Google Scholar]
- 6.Vize PD, Woolf AS, Bard JBL. The kidney: from normal development to congenital disease. Academic Press; New York: 2003. [Google Scholar]
- 7.Romagnani P, Lasagni L, Remuzzi G. Renal progenitors: an evolutionary conserved strategy for kidney regeneration. Nat Rev Nephrol. 2013;9:137–146. doi: 10.1038/nrneph.2012.290. [DOI] [PubMed] [Google Scholar]
- 8.Chan TC, Ariizumi T, Asashima M. Model system for organ engineering: transplantation of in vitro induced embryonic kidney. Naturwissenschaften. 1999;86:224–227. doi: 10.1007/s001140050602. [DOI] [PubMed] [Google Scholar]
- 9.Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) : a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. North-Holland Publ Co; Amsterdam: 1967. [Google Scholar]
- 10.Rak-Raszewska A, Hauser PV, Vainio S. Organ in vitro culture: what have we learned about early kidney development? Stem Cells Int. 2015;2015:959807. doi: 10.1155/2015/959807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francipane MG, Lagasse E. Pluripotent stem cells to rebuild a kidney: the lymph node as a possible developmental niche. Cell Transplant. 2015 doi: 10.3727/096368915X688632. [DOI] [PubMed] [Google Scholar]
- 12.Taira M, Otani H, Jamrich M, Dawid IB. Expression of the LIM class homeobox gene Xlim-1 in pronephros and CNS cell lineages of Xenopus embryos is affected by retinoic acid and exogastrulation. Development. 1994;120:1525–1536. doi: 10.1242/dev.120.6.1525. [DOI] [PubMed] [Google Scholar]
- 13.Carroll TJ, Vize PD. Synergism between Pax-8 and lim-1 in embryonic kidney development. Dev Biol. 1999;214:46–59. doi: 10.1006/dbio.1999.9414. [DOI] [PubMed] [Google Scholar]
- 14.Haldin CE, Masse KL, Bhamra S, Simrick S, Kyuno J, Jones EA. The lmx1b gene is pivotal in glomus development in Xenopus laevis. Dev Biol. 2008;322:74–85. doi: 10.1016/j.ydbio.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Dudziak K, Mottalebi N, Senkel S, Edghill EL, Rosengarten S, Roose M, Bingham C, Ellard S, Ryffel GU. Transcription factor HNF1beta and novel partners affect nephrogenesis. Kidney Int. 2008;74:210–217. doi: 10.1038/ki.2008.149. [DOI] [PubMed] [Google Scholar]
- 16.Wild W, Pogge von Strandmann E, Nastos A, Senkel S, Lingott-Frieg A, Bulman M, Bingham C, Ellard S, Hattersley AT, Ryffel GU. The mutated human gene encoding hepatocyte nuclear factor 1beta inhibits kidney formation in developing Xenopus embryos. Proc Natl Acad Sci USA. 2000;97:4695–4700. doi: 10.1073/pnas.080010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohn S, Thomas H, Turan G, Ellard S, Bingham C, Hattersley AT, Ryffel GU. Distinct molecular and morphogenetic properties of mutations in the human HNF1beta gene that lead to defective kidney development. J Am Soc Nephrol. 2003;14:2033–2041. doi: 10.1097/01.asn.0000078808.70309.c4. [DOI] [PubMed] [Google Scholar]
- 18.Weber H, Strandmann EP, Holewa B, Bartkowski S, Zapp D, Zoidl C, Ryffel GU. Regulation and function of the tissue-specific transcription factor HNF1 (LFB1) during Xenopus development. Int J Dev Biol. 1996;40:297–304. [PubMed] [Google Scholar]
- 19.Demartis A, Maffei M, Vignali R, Barsacchi G, De Simone V. Cloning and developmental expression of LFB3/HNF1 beta transcription factor in Xenopus laevis. Mech Dev. 1994;47:19–28. doi: 10.1016/0925-4773(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 20.Bartkowski S, Zapp D, Weber H, Eberie G, Zoidi C, Senkel S, Klein-Hitpass L, Ryfell GU. Developmental regulation and tissue distribution of the liver transcription factor LFB1 (HNF1) in Xenopus laevis. Mol Cell Biol. 1993;13:421–431. doi: 10.1128/mcb.13.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buisson I, Le Bouffant R, Futel M, Riou JF, Umbhauer M. Pax8 and Pax2 are specifically required at different steps of Xenopus pronephros development. Dev Biol. 2015;397:175–190. doi: 10.1016/j.ydbio.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Heller N, Brändli AW. Xenopus Pax-2/5/8 orthologues: novel insights into pax gene evolution and identification of pax-8 as the earliest marker for otic and pronephric cell lineages. Dev Genet. 1999;24:208–219. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<208::AID-DVG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Heller N, Brändli AW. Xenopus pax-2 displays multiple splice forms during embryogenesis and pronephric kidney development. Mech Dev. 1997;69:83–104. doi: 10.1016/s0925-4773(97)00158-5. [DOI] [PubMed] [Google Scholar]
- 24.Taelman V, Van Campenhout C, Sölter M, Pieler T, Bellefroid EJ. The Notch-effector HRT1 gene plays a role in glomerular development and patterning of the Xenopus pronephros anlagen. Development. 2006;133:2961–2971. doi: 10.1242/dev.02458. [DOI] [PubMed] [Google Scholar]
- 25.Carroll TJ, Wallingford JB, Vize PD. Dynamic patterns of gene expression in the developing pronephros of Xenopus laevis. Dev Genet. 1999;24:199–207. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<199::AID-DVG3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 26.Carroll TJ, Vize PD. Wilms tumor suppressor gene is involved in the development of disparate kidney forms: evidence from expression in the Xenopus pronephros. Dev Dyn. 1996;206:131–138. doi: 10.1002/(SICI)1097-0177(199606)206:2<131::AID-AJA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 27.Murugan S, Shan J, Kühl SJ, Tata A, Pietilä I, Kühl M, Vainio SJ. WT1 and Sox11 regulate synergistically the promoter of the Wnt4 gene that encodes a critical signal for nephrogenesis. Exp Cell Res. 2012;318:1134–1145. doi: 10.1016/j.yexcr.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Kyuno J, Jones EA. GDNF expression during Xenopus development. Gene Expr Patterns. 2007;7:313–317. doi: 10.1016/j.modgep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, Sariola H, Pachnis V. GDNF signalling through the Ret receptor tyrosine kinase. Nature. 1996;381:789–793. doi: 10.1038/381789a0. [DOI] [PubMed] [Google Scholar]
- 30.Hawley SH, Wünnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- 31.Alarcón P, Rodríguez-Seguel E, Fernández-González A, Rubio R, Gómez-Skarmeta JL. A dual requirement for Iroquois genes during Xenopus kidney development. Development. 2008;135:3197–3207. doi: 10.1242/dev.023697. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, Krinks M, Kleinwaks L, Moos M. A novel Xenopus homologue of bone morphogenetic protein-7 (BMP-7) Genes Funct. 1997;1:259–271. doi: 10.1046/j.1365-4624.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- 33.Saulnier DME, Ghanbari H, Brändli AW. Essential function of Wnt-4 for Tubulogenesis in the Xenopus pronephric kidney. Dev Biol. 2002;248:13–28. doi: 10.1006/dbio.2002.0712. [DOI] [PubMed] [Google Scholar]
- 34.Tena JJ, Neto A, de la Calle-Mustienes E, Bras-Pereira C, Casares F, Gómez-Skarmeta JL. Odd-skipped genes encode repressors that control kidney development. Dev Biol. 2007;301:518–531. doi: 10.1016/j.ydbio.2006.08.063. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Vize PD. Proximo-distal specialization of epithelial transport processes within the Xenopus pronephric kidney tubules. Dev Biol. 2004;271:322–338. doi: 10.1016/j.ydbio.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 36.Raciti D, Reggiani L, Geffers L, Jiang Q, Bacchion F, Subrizi AE, Clements D, Tindal C, Davidson DR, Kaissling B, Brandli AW. Organization of the pronephric kidney revealed by large-scale gene expression mapping. Genome Biol. 2008;9:R84. doi: 10.1186/gb-2008-9-5-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brändli AW. Towards a molecular anatomy of the Xenopus pronephric kidney. Int J Dev Biol. 1999;43:381–395. [PubMed] [Google Scholar]
- 38.Jones EA. Xenopus: a prince among models for pronephric kidney development. J Am Soc Nephrol. 2005;16:313–321. doi: 10.1681/ASN.2004070617. [DOI] [PubMed] [Google Scholar]
- 39.Wessely O, Tran U. Xenopus pronephros development—past, present, and future. Pediatr Nephrol. 2011;26:1545–1551. doi: 10.1007/s00467-011-1881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingert RA, Davidson AJ. The zebrafish pronephros: a model to study nephron segmentation. Kidney Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- 41.Vize PD, Seufert DW, Carroll TJ, Wallingford JB. Model systems for the study of kidney development: use of the pronephros in the analysis of organ induction and patterning. Dev Biol. 1997;188:189–204. doi: 10.1006/dbio.1997.8629. [DOI] [PubMed] [Google Scholar]
- 42.Miller RK, Lee M, McCrea PD. Chapter 12: The Xenopus Pronephros: A Kidney Model Making Leaps toward Understanding Tubule Development. In: Kloc M, Kubiak JZ, editors. Xenopus Development. Oxford: John Wiley & Sons; 2014. pp. 215–238. [DOI] [Google Scholar]
- 43.Vize PD, Jones EA, Pfister R. Development of the Xenopus pronephric system. Dev Biol. 1995;171:531–540. doi: 10.1006/dbio.1995.1302. [DOI] [PubMed] [Google Scholar]
- 44.Brennan HC, Nijjar S, Jones EA. Glomus specification and induction in Xenopus. Development. 1999;126:5847–5856. doi: 10.1242/dev.126.24.5847. [DOI] [PubMed] [Google Scholar]
- 45.Brennan HC, Nijjar S, Jones EA. The specification of the pronephric tubules and duct in Xenopus laevis. Mech Dev. 1998;75:127–137. doi: 10.1016/s0925-4773(98)00094-x. [DOI] [PubMed] [Google Scholar]
- 46.Moriya N, Uchiyama H, Asashima M. Induction of pronephric tubules by activin and retinoic acid in presumptive ectoderm of Xenopus laevis. Dev Growth Differ. 1993;35:123–128. doi: 10.1111/j.1440-169X.1993.00123.x. [DOI] [PubMed] [Google Scholar]
- 47.Drews C, Senkel S, Ryffel GU. The nephrogenic potential of the transcription factors osr1, osr2, hnf1b, lhx1 and pax8 assessed in Xenopus animal caps. BMC Dev Biol. 2011;11:5. doi: 10.1186/1471-213X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Osafune K, Nishinakamura R, Komazaki S, Asashima M. In vitro induction of the pronephric duct in Xenopus explants. Dev Growth Differ. 2002;44:161–167. doi: 10.1046/j.1440-169x.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 49.Uochi T, Asashima M. Sequential gene expression during pronephric tubule formation in vitro in Xenopus ectoderm. Dev Growth Differ. 1996;38:625–634. doi: 10.1046/j.1440-169X.1996.t01-5-00006.x. [DOI] [PubMed] [Google Scholar]
- 50.Moon KH, Ko IK, Yoo JJ, Atala A. Kidney diseases and tissue engineering. Methods. 2015 doi: 10.1016/j.ymeth.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Rak-Raszewska A, Hauser VP, Vainio S. Organ in vitro culture: what have we learned about early kidney development? Stem Cells. 2015;2015:959807. doi: 10.1155/2015/959807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ekblom P, Miettinen A, Virtanen I, Wahlström T, Dawnay A, Saxén L. In vitro segregation of the metanephric nephron. Dev Biol. 1981;84:88–95. doi: 10.1016/0012-1606(81)90373-0. [DOI] [PubMed] [Google Scholar]
- 53.Thesleff I, Ekblom P. Role of transferrin in branching morphogenesis, growth and differentiation of the embryonic kidney. J Embryol Exp Morph. 1984;82:147–161. [PubMed] [Google Scholar]
- 54.Kyuno J, Massé K, Jones EA. A functional screen for genes involved in Xenopus pronephros development. Mech Dev. 2008;125:571–586. doi: 10.1016/j.mod.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Tomlinson ML, Hendry AE, Wheeler GN. Methods in molecular biology. Humana Press; Clifton: 2012. Xenopus protocols. [DOI] [PubMed] [Google Scholar]
- 56.Schmitt SM, Gull M, Brändli AW. Engineering Xenopus embryos for phenotypic drug discovery screening. Adv Drug Deliv Rev. 2014;69–70:225–246. doi: 10.1016/j.addr.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 57.Kim D, Dressler GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16:3527–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 58.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526:564–568. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 59.Keller R, Danilchik M, Gimlich R, Shih J. The function and mechanism of convergent extension during gastrulation of Xenopus laevis. J Embryol Exp Morpholog. 1985;89(Suppl):185–209. [PubMed] [Google Scholar]
- 60.Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 61.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCoy KE, Zhou X, Vize PD. Non-canonical wnt signals antagonize and canonical wnt signals promote cell proliferation in early kidney development. Dev Dyn. 2011;240(6):1558–66. doi: 10.1002/dvdy.22626.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller RK, Canny SG, Jang CW, Cho K, Ji H, Wagner DS, Jones EA, Habas R, McCrea PD. Pronephric tubulogenesis requires Daam1-mediated planar cell polarity signaling. J Am Soc Nephrol. 2011;22(9):1654–64. doi: 10.1681/ASN.2010101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, Walz G. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat Genet. 2012;44:1382–1387. doi: 10.1038/ng.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blankenship JT, Backovic ST, Sanny JSP, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 66.Lienkamp S, Ganner A, Boehlke C, Schmidt T, Arnold SJ, Schäfer T, Romaker D, Schuler J, Hoff S, Powelske C, Eifler A, Krönig C, Bullerkotte A, Nitschke R, Kuehn EW, Kim E, Burkhardt H, Brox T, Ronneberger O, Gloy J, Walz G. Inversin relays Frizzled-8 signals to promote proximal pronephros development. Proc Natl Acad Sci USA. 2010;47:20388–20393. doi: 10.1073/pnas.1013070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabella OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37(5):537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srinivas S, Goldberg MR, Watanabe T, D’Agati V, Al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet. 1999;24:241–251. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<241::AID-DVG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 69.Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht J, Asai N, Takahashi M, Ohgami N, Kato M, Mendelsohn C, Costantini F. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17(2):199–209. doi: 10.1016/j.devcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Packard A, Georgas K, Michos O, Riccio P, Cebrian C, Combes A, Ju A, Ferrer-Vaquer A, Hadjantonakis AK, Zong H, Little MH, Costantini F. Luminal mitosis drives epithelial cell dispersal within the branching ureteric bud. Dev Cell. 2013;27(3):319–330. doi: 10.1016/j.devcel.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan X, Schnell U, Karner CM, Small EV, Carroll TJ. A Cre-inducible fluorescent reporter for observing apical membrane dynamics. Genesis. 2015;53:285–293. doi: 10.1002/dvg.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blitz IL, Biesinger J, Xie X, Cho KWY. Biallelic genome modification in F0 Xenopus tropicalis embryos using the CRISPR/Cas system. Genesis. 2013;51:827–834. doi: 10.1002/dvg.22719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakayama T, Fish MB, Fisher M, Oomen-Hajagos J, Thomsen GH, Grainger RM. Simple and efficient CRISPR/Cas9-mediated targeted mutagenesis in Xenopus tropicalis. Genesis. 2013;51:835–843. doi: 10.1002/dvg.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang F, Shi Z, Cui Y, Guo X, Shi Y-B, Chen Y. Targeted gene disruption in Xenopus laevis using CRISP/Cas9. Cell Biosci. 2015;5:15. doi: 10.1186/s13578-015-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caine ST, Mclaughlin KA. Regeneration of functional pronephric proximal tubules after partial nephrectomy in Xenopus laevis. Dev Dyn. 2013;242:219–229. doi: 10.1002/dvdy.23916. [DOI] [PubMed] [Google Scholar]