Abstract
Purpose
The East Harlem (EH), Central Harlem (CH), and Upper East Side (UES) neighborhoods of New York City are geographically contiguous to tertiary medical care but are characterized by cancer mortality rate disparities. This ecological study aims to disentangle effects of race and neighborhood on cancer deaths.
Methods
Mortality-to-incidence ratios (MIR) were determined using neighborhood-specific data from the New York State Cancer Registry and Vital Records Office (2007–2011). Ecological data on modifiable cancer risk factors from the New York City Community Health Survey (2002–2006) was stratified by sex, age group, race/ethnicity, and neighborhood and modeled against stratified mortality rates to disentangle race/ethnicity and neighborhood using logistic regression.
Results
Significant gaps in mortality rates were observed between the UES and both CH and EH across all cancers, favoring UES. MIRs of both CH and EH were similarly elevated in the range of 0.41–0.44 compared to UES (0.26–0.30). After covariate and multivariable adjustment, black race (OR=1.68; 95% CI: 1.46, 1.93) and EH residence (OR=1.20; 95% CI: 1.07, 1.35) remained significant risk factors in all cancers combined mortality.
Conclusions
Mortality disparities remain among EH, CH, and UES neighborhoods. Both neighborhood and race are significantly associated with cancer mortality, independent of each other. Multivariable adjusted models that include CHS risk factors demonstrate that this mortality gap may be avoidable through community-based public health interventions.
Keywords: neoplasms, urban health, health status disparities, New York City, residence characteristics, healthcare disparities
INTRODUCTION
New York City (NYC) is a densely populated metropolitan area composed of diverse race/ethnicities and a wide range of socioeconomic statuses and is an ideal setting for cancer disparity studies. In particular, NYC Upper Manhattan neighborhoods of Upper East Side (UES), Central Harlem (CH), and East Harlem (EH) are contiguous with similar proximity from tertiary medical hospitals. However, these neighborhoods are characterized by extreme differences in ethnic/racial composition, income, and educational level (Buchholz, Resnick, and Konty 2012), with a larger proportion of non-Hispanic whites, college graduates, and income levels > $100,000 living in the UES. Conversely, high proportions of blacks and Hispanics characterize CH and EH, respectively. Both CH and EH have a lower proportion of college graduates and a higher proportion of individuals below the federal poverty level than the UES (Hashim et al. 2015).
Previous studies focusing on NYC have shown disparities by co-morbidities (Van Wye et al. 2008), screening rates (Richards et al. 2011), and treatment outcomes (Martindale et al. 2014). These NYC studies, including studies of other US locations that focus on cancer mortality (Whitman et al. 2011; Hirschman, Whitman, and Ansell 2007), have focused on disparities according to racial/ethnic differences. Neighborhood has been demonstrated to be a superior indicator of socioeconomic status, encompassing racial/ethnic composition, cultural homogeny and income level (LaVeist et al. 2011). However, studies exploring the effects of neighborhood-based disparities have examined single cancer risk factors, such as smoking habits, or single cancer sites only (Levin et al. 2014; Hanibuchi et al. 2015; Peterson et al. 2015), without taking into account multiple modifiable risk factors such as nutrition, exercise, and screening rates concurrently. More importantly, the role of neighborhood residence as a predictor of overall cancer mortality or mortality from the most common cancers has not been determined for NYC.
This study builds on the previous study on cancer incidence among the same three neighborhoods, in which we found both neighborhood and race played a role in cancer incidence (Hashim et al. 2015). These standardized incidence rates provided insight on the burden of exposure to causes of cancer development. The current study is an attempt to further identify the burden of cancer influenced by incidence, prognosis, and access to treatment through a comparison of standardized mortality rates. Analysis was restricted to the three Upper Manhattan neighborhoods of diverse and distinctive demographic characteristics: Upper East Side (UES), Central Harlem (CH), and East Harlem (EH. Using state and local data sources, this study aimed to: 1) describe mortality rates among the three neighborhoods, including a comparison to incidence rates; and 2) disentangle the effects of race and neighborhood – as a proxy for socioeconomic status- on cancer-caused deaths adjusted for risk factors.
METHODS
The study was conducted in three predefined United Health Fund (UHF) neighborhoods of NYC: UES, CH and EH (Fig. 1). Detailed information about neighborhood zipcodes and demographics of each neighborhood has been reported [2]. In brief, the UES neighborhood is characterized by a larger proportion of college graduates and a higher proportion of non-Hispanic white residents (Table 1).
Fig. 1.
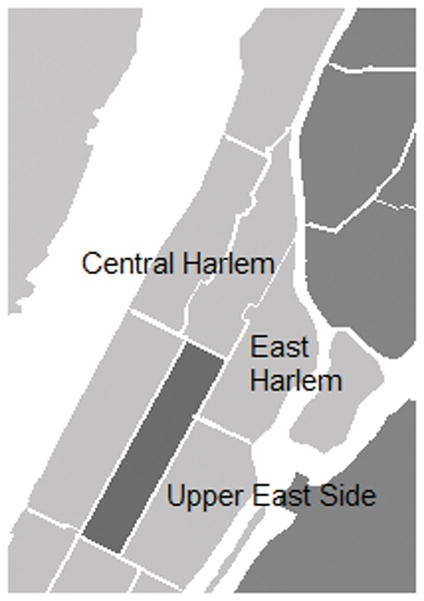
Geographic location three Upper Manhattan neighborhoods. Neighborhoods are clustered by the following zip codes: Upper East Side (10021, 10028, 10044, 10128), Central Harlem (10026, 10027, 10030, 10037, 10039), and East Harlem (10029, 10035).
Table 1.
Descriptive characteristics of Community Health Survey respondents by United Health Fund (UHF) New York City neighborhoods: East Harlem, Central Harlem, and Upper East Side, 2002–2006
| Category | Missing n (% of total surveyed ) | Characteristic | Category total | Upper East Side | East Harlem | Central Harlem | P-value |
|---|---|---|---|---|---|---|---|
| Neighborhood | 0 | Number of participants (%) | 2958 | 1181 (40) | 761 (26) | 1016 (34) | |
| Age | 154 (5.2) | Age, years (mean, sd) | 2804 | 1109/1181 58.7±15 |
736/761 54.7±14 |
959/1016 55.3±15 |
0.06 |
| 154 (5.2) | Age groups | <0.001 | |||||
| 35–44 years (%) | 750(27) | 244 (22) | 215(29) | 291(30) | |||
| 45–54 years (%) | 659(24) | 222 (23) | 196 (27) | 241 (23) | |||
| 55–64 years (%) | 543(19) | 230 (21) | 132 (18) | 181(19) | |||
| 65–74 years (%) | 426(15) | 177 (16) | 111 (15) | 138 (14) | |||
| 75+ years (%) | 426(15) | 217 (20) | 82 (11) | 127 (13) | |||
| Gender | 0.01 | ||||||
| 0 | Females (%) | 1829 (62) | 691 (59) | 488 (64) | 650 (64) | ||
| Males (%) | 1129 (38) | 490 (41) | 273 (36) | 366 (36) | |||
| Race/ethnicity | 0 | Black, Non, Hispanic (%) | 993 (34) | 34 (3) | 232 (31) | 727 (72) | <0.001 |
| 0 | Hispanic, all races (%) | 682 (23) | 71 (6) | 453 (60) | 158 (16) | ||
| 0 | White, Non, Hispanic | 1283 (4) | 1076 (91) | 76 (10) | 131 (13) | ||
| Household income | 656 (22) | Poverty level ≥30% based on zipcode (%) | 839 (36) | 32 (4) | 408 (64) | 399 (50) | <0.001 |
| Education | 32 (0.1) | College degree (%) | 1340 (46) | 870 (74) | 145 (19) | 325 (32) | <0.001 |
| Self, assessed health | 22 (0.7) | General health rated as “fair/poor” (%) | 733 (25) | 151 (13) | 277 (37) | 305 (30) | <0.001 |
| 7 (0.2) | US born (%) | 2397 (81) | 961 (82) | 596 (78) | 840 (83) | 0.05 | |
| Sexual identity | 190 (8.0) | Homosexual and bisexual (%) | 118 (5) | 63 (7) | 15 (3) | 40 (5) | 0.02 |
| Neighborhood safety | 14 (2.4) | Feel “extremely safe” in neighborhood (%) | 95 (17) | 68 (33) | 12 (7) | 15 (8) | <0.001 |
| Body Mass Index | 208 (7.0) | Body mass index, (kg/m2) mean, sd | 2750 | 1105/1181 24.6±4.4 |
702/761 28.5±7.0 |
943/1016 27.8±5.7 |
<0.001 |
| Co-morbities | 9 (0.4) | Diabetes mellitus (%) | 273 (12) | 51 (6) | 102 (17) | 120 (16) | <0.001 |
| 17 (2.9) | Hypercholesterolemia (%) | 191 (34) | 83 (40) | 49 (30) | 59 (31) | 0.08 | |
| 522 (29) | Hypertension (%) | 705 (38) | 219 (29) | 181 (41) | 305 (45) | <0.001 | |
| 14 (0.6) | Current asthma (%) | 136 (6) | 35 (4) | 50 (8) | 51 (7) | <0.001 | |
| Health Screening | 971 (41) | Ever colonoscopy (%) | 893 (64) | 448 (71) | 182 (55) | 263 (59) | <0.001 |
| 250 (17) | Mammogram in past 2 years (%) | 1002 (80) | 393 (79) | 258 (82) | 351 (79) | 0.43 | |
| 70 (4.6) | PAP smear in past 3 years (%) | 1233 (86) | 477 (86) | 313 (85) | 443 (86) | 0.93 | |
| 105 (5.8) | HIV test in past 12 months (%) | 373 (22) | 77 (11) | 114 (27) | 182 (30) | <0.001 | |
| 11 (0.4) | Has a primary care physician (%) | 2471 (84) | 1044 (89) | 584 (77) | 843 (83) | 0.001 | |
| Access to Health care | 43 (1.5) | Uninsured (%) | 46 (8) | 61 (5) | 91 (12) | 94 (9) | <0.001 |
| Lifestyle risk factors | |||||||
| Tobacco smoking | 29 (1.0) | Current smokers (%) | 613 (21) | 172 (15) | 191 (25) | 250 (25) | <0.001 |
| Past smokers (%) | 892 (30) | 467 (40) | 165 (19) | 260 (29) | <0.001 | ||
| Never smokers (%) | 1424 (49) | 532 (45) | 396 (53) | 496 (49) | <0.001 | ||
| 6 (2.6) | Exercise in past 30 days (%) | 1676 (73) | 739 (85) | 405 (64) | 532 (67) | <0.001 | |
| 50 (2.2) | Binge drinking (%) | 245 (11) | 92 (11) | 75 (3.3) | 78 (10) | 0.53 | |
| 46 (4.0) | Fruit and vegetable servings/day ≥5 (%) | 117 (11) | 75 (18) | 18 (6) | 24 (6) | <0.001 | |
| 582 (20) | ≥3 sex partners in lifetime (%) | 148 (6) | 70 (8) | 31 (5) | 47 (6) | 0.18 |
Incidence and Mortality data
Age, neighborhood residence at diagnosis, race/ethnicity, and cancer incidence based on tumor classification by the International Classification of Oncology, third edition (ICD-O-3) were provided by the New York State Cancer Registry (NYSCR) for the years 2007–2011. Cancer mortality data using the same tumor ICD-O-3 classification was provided by the New York Department of Health and Mental Hygiene’s (DOHMH) Vital Records Office (2007–2011). Cancer data were aggregated by age, sex, race/ethnicity, neighborhood. Age was categorized by 10-year age groups for those 35 years to 75+ years. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, and Hispanic of all races. Age-specific cancer mortality rates were calculated for each age-sex-neighborhood-race/ethnicity stratum by dividing the number of deaths by the NYC Census stratum-specific population for 2007–2011.
Cancer risk factor data
Data on demographics, health, and lifestyle risk factors were obtained from the New York City Community Health Survey (CHS) for 2002–2006 [2]. The CHS is an annual, cross-sectional telephone survey using a stratified random-digit dial sampling design to ensure adequate sample per neighborhood conducted by the Department of Health and Mental Hygiene in all NYC neighborhoods. The 2002–2006 period was selected to allow for a 5-year latency to determine associations with 2007–2011 cancer incidence and mortality rates. A total of 2974 individuals with survey responses from 2002–2006 comprised the independent variable analyzed dataset of, of whom 170 were excluded due to missing data on age and most other variables. For each age-sex-race/ethnicity-neighborhood stratum, CHS responses were converted to prevalence for binary and categorical responses, and arithmetic means for continuous responses.
Statistical analysis
CHS risk/protective factor prevalence and means were calculated by each age-sex-race/ethnicity-neighborhood stratum. Significant differences in CHS means and proportions between neighborhoods were measured using ANOVA and chi-square test, respectively. The methodology for incidence rate analysis has been previously reported (Hashim et al. 2015).
To determine cancer mortality rates for each neighborhood, mortality rates for those ≥35 years old were standardized by method of direct standardization to the 2000 US population. Age-standardized cancer mortality rates were compared by computing standardized rate estimates and standardized rate ratios (SRR) to account for age distributions differences among neighborhoods (Breslow, Day, and Cancer 1986). Age-standardized mortality-to-incidence ratios (MIR) were calculated to serve as an indicator of survival or the level of diagnostic activity (Asadzadeh Vostakolaei et al. 2011).
To disentangle the effects of race and neighborhood on mortality, rates of overall cancer and specific cancer mortality (2007–2011) were stratified by neighborhood, age category (35–44, 45–54, 55–64, 65–74, and ≥75 years), sex, and race/ethnicity (White, Black, and Hispanic). Two models were tested. Basic logistic regression models were weighted by population for each stratum, and included cancer death as the outcome variable to determine effect estimates for sex, age group, race, and neighborhood covariates for all cancers and specific cancer mortality. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated, applying age 35–44 years, males, whites, and UES residents as referents. The second set of logistic regression models included all variables from the basic models, plus CHS-response prevalence and means as independent variables. Stepwise logistic regression techniques were used to determine which CHS-response variables best predicted cancer mortality using a threshold p-value of 0.10. Final model selection was based on a balance between goodness-of-fit and parsimony. To determine whether the second set of logistic regression models that included the CHS-response variables on modifiable risk factors differed significantly from the basic logistic regression models, log-likelihood ratio differences between the two models were calculated per cancer site. All statistical analyses were conducted using SAS® software, Version 9.4.
RESULTS
Overall cancer mortality
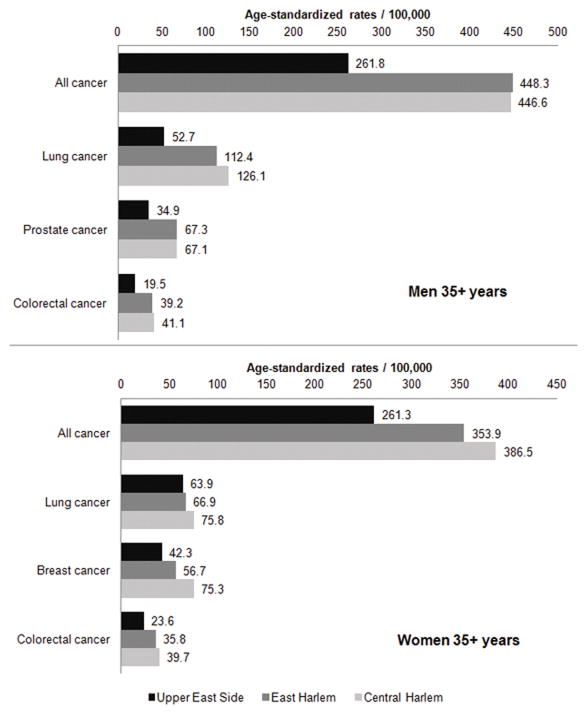
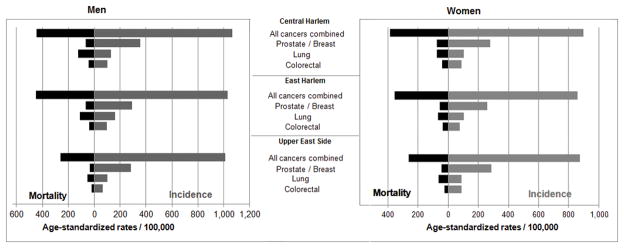
A total of 12,251 cancer cases and 4268 cancer deaths were included in this analysis. Cancers of the lung, breast, colon and rectum, and prostate represent nearly half all cancer deaths. Age-standardized mortality rates for overall cancer mortality were significantly higher in both men and women aged 35+ years in East and Central compared to UES (SRR=1.52 and 1.59; p<0.001 for both, respectively) (Fig. 2). Mortality rates between East and Central Harlem did not differ significantly from each another (SRR=1.04; p=0.33). For men, the overall cancer mortality rate were 1.7-times higher (p<0.001) in East and Central Harlem compared to UES; for women, 1.4-times higher (p<0.001) in East and Central Harlem. MIR of overall cancer mortality in Harlem neighborhoods were > 0.40 for both sexes while in UES, MIR were lower: 0.30 for women and 0.26 for men (Fig. 3).
Fig. 2.
All combined cancer mortality and major cancer mortalities for men and women in three contiguous New York City Upper Manhattan neighborhoods.
Fig. 3.
Mortality-to-Incidence ratios for all cancers combined and the four commonest cancers for men and women 35+ years, 2007–2011.
In basic multivariate models, adjusting for sex, age, race/ethnicity, and neighborhood, black race and EH residence were associated with significantly higher risks of overall cancer mortality (Table 2). In the second set of models in which CHS responses on modifiable risk factors were tested, black race and EH residence risk estimates were attenuated, but remained significant. Never smoking tobacco had the largest magnitude of association in the negative direction with cancer mortality (OR=0.50; 95% CI: 0.40, 0.65).
Table 2.
Associations of neighborhood and race/ethnicity with all cancers combined and the four most common sites mortality rate for 35+ years, 2007–2011
| Cancer | Model | Black vs. White racea | Hispanic vs. White racea | East Harlem vs. Upper East Sidea | Central Harlem vs. Upper East Sidea | Significant risk factor variables and direction of association |
|---|---|---|---|---|---|---|
| All cancers combined | 1 | 1.72 (1.54, 1.92) | 0.99 (0.88, 1.11) | 1.24 (1.11, 1.39) | 1.06 (0.95, 1.19) | -- |
| 2 | 1.68 (1.46, 1.93) | 0.86 (0.73, 1.02) | 1.20 (1.07, 1.35) | 1.06 (0.95, 1.96) | Diabetes + Never smoking − Told had high blood pressure − Asthma episode in past 10 months + Private insurance - |
|
| Lung | 1 | 2.00 (1.58, 2.53) | 0.91 (0.71, 1.18) | 1.18 (0.93, 1.51) | 1.01 (0.79, 1.28) | |
| 2 | 1.13 (0.73, 1.74) | 0.46 (0.28, 0.76) | 1.02 (0.79, 1.33) | 0.89 (0.69, 1.15) | Current smoking + College graduation - |
|
| Prostate | 1 | 3.05 (1.20, 4.67) | 1.33 (0.84, 2.12) | 1.15 (0.84, 2.12) | 0.82 (0.53, 1.28) | |
| 2 | 2.25 (1.19, 4.23) | 1.34 (0.79, 2.27) | 0.50 (0.23, 1.08) | 0.49 (0.25, 0.97) | Married − Self-rated best general health - |
|
| Breast (female) | 1 | 1.66 (1.14, 2.41) | 0.69 (0.45, 1.05) | 1.20 (0.81, 1.78) | 1.21 (0.83, 1.77) | |
| 2 | 4.16 (2.12, 8.15) | 1.19 (0.69, 2.04) | 1.09 (0.67, 1.78) | 1.04 (0.62, 1.73) | Mammogram − Told had high blood pressure - |
|
| Colorectal | 1 | 1.63 (1.12, 2.36) | 1.17 (0.80, 1.71) | 1.34 (0.92, 1.95) | 1.27 (0.87, 1.85) | |
| 2 | 1.48 (1.01, 2.18) | 1.01 (0.67, 1.52) | 1.41 (0.97, 2.06) | 1.34 (0.92, 1.98) | Asthma episode in past 10 months + |
Associations are represented as odds ratios (OR) and 95% confidence intervals (95% CI).
Model 1 includes age group in 10-year increments, sex, race/ethnicity, and neighborhood.
Model 2 includes all terms in Model 1 in addition to significant Community Health Survey risk factor variables.
Site-specific cancer mortality
Neighborhood comparisons of male age-standardized mortality rates from colorectal cancers (SRR=2.08; p<0.001 for both East and Central Harlem), lung cancer (SRR=2.35; p<0.001 for CH and SRR=2.08; p<0.001 for EH), were at least 2-times significantly higher in East and Central Harlem neighborhoods than in UES for men. East and Central Harlem residents had nearly double the prostate cancer mortality rates (SRR=1.93; p<0.001 for both) than UES residents. Age-standardized mortality rates in the commonest female cancers were significantly larger in CH (SRR=1.75; p<0.001) and EH (SRR=1.58; p<0.001 for colorectal cancer and in CH (SRR=1.80; p<0.001) and EH (SRR=1.37; p=0.02) for breast cancer. Lung cancer mortality rates among women were SRR=1.24 higher (p=0.04) in CH compared to UES. Other comparisons among the three neighborhoods were not significant.
MIRs for lung cancer were lowest in UES men (0.51) and highest in CH men (0.97). The UES was the only neighborhood in which women had higher MIR (0.71) than men. In basic multivariate models, blacks had twice the risk of lung cancer compared to whites, adjusting for sex, age, and neighborhood. In the second set of multivariate models that included CHS responses, this association was not significant after adjusting for current smoking status and college graduation. Within the same model, Hispanic ethnicity was associated with a lower risk of lung cancer mortality (OR=0.46; 95% CI: 0.28, 0.76).
MIR for prostate cancers were 0.19, 0.22, and 0.12 for CH, EH, and UES, respectively. For both prostate and colorectal cancers, basic multivariate models showed that black race had a greater risk for prostate cancer; mortality risks decreased by 80% when CHS-responses were included in the second set of models. Within the second set of models, CH residence was associated with a 51% lower mortality risk versus UES.
MIR for breast cancer among CH women was the highest (0.27) compared to EH (0.22) and UES (0.15). In basic multivariate models, breast cancer mortality was significantly higher for blacks and mortality associations with neighborhood were not significant. In the second model, breast cancer risk in blacks increased 250% after CHS-response adjustment for mammography in the past 2 years and hypertension; both mammography and hypertension were negatively associated with breast cancer mortality in this model.
MIR for colorectal cancers among UES men (0.31), were lower than East and Central Harlem (both 0.40). For women, colorectal cancer MIRs were 0.47 and 0.46 in East and Central Harlem, respectively, and 0.26 in UES. In the basic multivariate model, black race was again significantly higher than for whites; when mortality was modeled for CHS-responses in the second analysis, mortality risks decreased by 15%.
DISCUSSION
This ecological study confirms disparities in all cancers combined mortality as well as lung, breast, prostate, and colorectal cancer mortality among three contiguous Upper Manhattan NYC neighborhoods, despite similar proximity to health care facilities. Using two multivariate logistic regression models, adjusting for known confounders and CHS population survey responses, ethnicity/race and neighborhood each showed an association with the cancer mortality, independent of each other. When mortality is adjusted for modifiable risk factors in addition to neighborhood, sex, and age, disparities persist races among race/ethnicity differences for the most common cancers, particularly between blacks versus whites.
This conclusion differs slightly from the previous study comparing cancer incidence among the three neighborhoods which demonstrated wide variations in incidence in which neighborhood was significantly associated with all cancers combined after CHS adjustment. Thus, this underscores the notion that the burden of exposure to cancer risk factors is higher in Central and East Harlem than in the Upper East Side. Mortality disparities are, too, influenced by neighborhood risk factors, but also by race/ethnicity. Moreover, across the commonest cancer sites, black race was associated with mortality ; this association had a higher magnitude than neighborhood of residence. These overall findings are consistent with a robust literature that has demonstrated the presence of race/ethnicity and socioeconomic status in relation to health disparities in the United States (Aizer et al. 2014; Baquet and Commiskey 2000; Du, Fang, and Meyer 2008; Newman 2014).
Cancer mortality is influenced by a continuum that spans prevention of modifiable risk factors, cancer screening, medical diagnosis, treatment, and other determinants of survival (Myers C, Hakenewerth A, Olson C Kerker B, Krauskopf M, Tavares A, Perlman S, Greene C 2011). Breast, prostate, and colorectal cancer incidence and mortality are particularly sensitive to screening. Although colorectal and prostate screening data could not be adequately captured by CHS due to missing responses, previous studies examining race/ethnicity alone have shown similar screening patterns (Richards et al. 2011; Thorpe et al. 2013). Additionally, the effectiveness of prostate cancer on mortality reduction remains controversial (Cuzick et al. 2014) For other cancers, causes of disparities that have been studied include treatment and follow-up (Myers C, Olson EC, Ramaswamy C 2008), tumor grade and stage at diagnosis (Islami et al. 2013), and referral patterns (Gage-Bouchard et al. 2014).
A greater lung cancer risks among blacks was attenuated once smoking and education were adjusted for, suggesting that effective interventions for modifiable risk factors would be effective in closing the mortality gap. The larger mortality rate of lung cancer cases among men than women likely reflects a combination of increased smoking and occupational exposures in men in East and Central Harlem neighborhoods. Current smoking prevalence is 28% higher in Harlem neighborhoods than Upper East Side for men and 22% higher for women, by the same neighborhood contrast. Moreover, smoking rates prior to the CHS survey time period, may have been less different among neighborhoods in women than in men. Although UES women have the highest incidence of breast cancer out of the three neighborhoods, women in CH have a 56% higher death rate. Higher CH mortality is consistent with racial/ethnic disparities since blacks constitute the majority of CH residents and have experienced higher breast cancer mortality rate as a racial subgroup despite a city-wide decrease in breast cancer since 1994 (Myers C, Hakenewerth A, Olson C Kerker B, Krauskopf M, Tavares A, Perlman S, Greene C 2011). That this disparity remains despite screening adjustments suggests that race/ethnicity and neighborhood of residence are not the only factors influencing the cancer care continuum and contributing to a wide mortality disparity.
Despite high levels of poverty and lower education, Hispanics’ cancer mortality did not differ significantly from whites; in the case of lung cancer, Hispanic ethnicity was protective compared to whites. These findings are consistent with the “Hispanic paradox”—the epidemiologic phenomenon in which Hispanics tend to live longer than whites despite socioeconomic disadvantage (Ruiz, Steffen, and Smith 2013). Cancer mortality rates among Hispanics are declining and have been observed in SEER data from 2002 to 2009; the incidence rates among men for all cancers combined decreased annually by 2.3% among Hispanics and 1.4% among whites; for women, the decrease was and 1.4% per year for Hispanics and 1.3% for whites (American Cancer Society 2012). This decreased risk may partly be explained by the adaptation of US cultural risk factors associated with cancer incidence and mortality. Cultural protective factors for cancer screening and follow-up among Hispanics have been documented in previous studies (Chan et al. 2014) and include familial support or interconnectedness (Moore de Peralta, Holaday, and McDonell 2014), lower levels of smoking compared to non-Hispanic whites (Haiman et al. 2006; Hyland et al. 2005; Pinsky 2006), and protective reproductive patterns (lower age at primigravida and lower parity) (Sweeney et al. 2008; Martin et al. 2010; Chlebowski et al. 2005). Out of the 11 nations that constitute the largest NYC immigrant population sources, Latin American countries make up greater than half (New York City Department of City Planning 2005) and 40% of foreign-born Manhattan residents are from Latin America (Burden and Shama 2013). The contribution of SES to cancer mortality among Hispanics may be either modified by additional variables of US cultural and lifestyle adaptation or reflective of a “healthy migrant” effect (Palloni and Arias 2004) compared to US-born Manhattan residents.
Causes of disparities remains complex and influenced by multiple layers of factors at the individual, family, community, and societal levels (LaVeist et al. 2011). Targeting any particular cancer risk factor on the cancer care continuum, whether exposure, screening, or treatment accessibility, requires an approach that address these multiple levels (LaVeist et al. 2011). In order to be effective, these policies should initially be neighborhood-based and focused on building organizations that address opportunity inequalities within communities. Policymakers must engage in seeking to not only improve health care resources within the disadvantaged communities but also to foster the affordability of nutritious food, the safety of recreational parks, and the strengthening of community-based networks (Small, Jacobs, and Massengill 2008), which would facilitate long-term healthy decisions that will reduce the cancer mortality gap. Furthermore, affordable healthcare for those earning lower incomes and for those whose employers may not provide private health coverage that includes lower costs for physician specialist visits, may enable better access to cancer treatment to those living in neighborhoods with higher poverty rates.
Our findings must be understood in light of its limitations. As a fallacy of an ecological study design, risk factor exposures and case-by-case mortality variations within neighborhoods and race/ethnicities could not be adjusted for on an individual level. Confounding variables of co-morbid illnesses (including cardiovascular disease and chronic obstructive pulmonary disease), family history, past medical history, occupational exposures, and levels of air pollution were not captured by the CHSs for the 2002–2006 timeframe and could not be adjusted for, although disparities for many of these confounders also exist among race/ethnicity and neighborhoods (Kheirbek et al. 2013; Kass et al. 2015). Additionally, collective sociocultural behaviors affecting these and other areas on the cancer-care continuum would have not been possible to adjust for at the individual level using the provided data resources.
In conclusion, wide mortality disparities persist among EH, CH, and UES neighborhoods. Both neighborhood and race are significantly associated with cancer mortality, independent of each other. There is a larger effect of race/ethnicity than neighborhood that persists despite adjustment for modifiable cancer risk factors. However, many of these effects attenuated after adjustment, which implores a need for effective public health interventions and legislation. Although many captured risk factors were consistent with previous studies (Myers C, Olson EC, Ramaswamy C 2008), non-economically driven influences, such as variables of acculturation, variations in neighborhood air-quality (Kheirbek et al. 2013; Kass et al. 2015), and adherence to recommended screening and treatment, are worth investigating in NYC as well as other metropolitan populations with wide demographic and cultural differences.
Acknowledgments
We would like to thank Berton Freedman at the New York City Vital Records Office for providing the aggregated cancer mortality data and Maria Schymura at the New York State Cancer Registry for providing cancer incidence data. The project was partially supported by the Tisch Cancer Institute and by NIEHS P30 grant # ES023515 (The Mount Sinai Transdisciplinary Center on Early Environmental Exposures).
Footnotes
Conflict of Interest Statement: All authors declare no conflicts of interest.
References
- Aizer Ayal A, Wilhite Tyler J, Chen Ming-Hui, Graham Powell L, Choueiri Toni K, Hoffman Karen E, Martin Neil E, Trinh Quoc-Dien, Hu Jim C, Nguyen Paul L. Lack of Reduction in Racial Disparities in Cancer-Specific Mortality over a 20-Year Period. Cancer. 2014;120(10):1532–39. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts & Figures for Hispancis/Latinos 2012–2014. Atlanta: American Cancer Society; 2012. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-034778.pdf. [Google Scholar]
- Asadzadeh Vostakolaei Fatemeh, Karim-Kos Henrike E, Janssen-Heijnen Maryska LG, Visser Otto, Verbeek André LM, Kiemeney Lambertus ALM. The Validity of the Mortality to Incidence Ratio as a Proxy for Site-Specific Cancer Survival. European Journal of Public Health. 2011;21(5):573–77. doi: 10.1093/eurpub/ckq120. [DOI] [PubMed] [Google Scholar]
- Baquet CR, Commiskey P. Socioeconomic Factors and Breast Carcinoma in Multicultural Women. Cancer. 2000;88(5 Suppl):1256–64. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1256::aid-cncr13>3.0.co;2-3. http://www.ncbi.nlm.nih.gov/pubmed/10705364. [DOI] [PubMed] [Google Scholar]
- Breslow Norman E, Day Nicholas E International Agency for Research on Cancer. Statistical Methods in Cancer Research Volume II-The Design and Analysis of Cohort Studies (IARC Scientific Publications) Statistical Methods in Cancer Research. 1986;1 [PubMed] [Google Scholar]
- Buchholz N, Resnick S, Konty K. The New York City Community Health Survey Atlas. The New York City Department of Health and Mental Hygiene. 2012 http://www.nyc.gov/html/doh/downloads/pdf/epi/nyc_comhealth_atlas10.pdf.
- Burden Amanda M, Shama Fatima. The Newest New Yorkers. Population Division-New York City Department of City Planning. 2013 http://www.nyc.gov/html/dcp/pdf/census/nny2013/nny_2013.pdf.
- Chan Kitty S, Roberts Eric, McCleary Rachael, Buttorff Christine, Gaskin Darrell J. Community Characteristics and Mortality: The Relative Strength of Association of Different Community Characteristics. American Journal of Public Health. 2014;104(9):1751–58. doi: 10.2105/AJPH.2014.301944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski Rowan T, Chen Zhao, Anderson Garnet L, Rohan Thomas, Aragaki Aaron, Lane Dorothy, Dolan Nancy C, et al. Ethnicity and Breast Cancer: Factors Influencing Differences in Incidence and Outcome. Journal of the National Cancer Institute. 2005;97(6):439–48. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- Cuzick Jack, Thorat Mangesh A, Andriole Gerald, Brawley Otis W, Brown Powel H, Culig Zoran, Eeles Rosalind A, et al. Prevention and Early Detection of Prostate Cancer. The Lancet. Oncology. 2014;15(11):e484–92. doi: 10.1016/S1470-2045(14)70211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Xianglin L, Fang Shenying, Meyer Tamra E. Impact of Treatment and Socioeconomic Status on Racial Disparities in Survival among Older Women with Breast Cancer. American Journal of Clinical Oncology. 2008;31(2):125–32. doi: 10.1097/COC.0b013e3181587890. [DOI] [PubMed] [Google Scholar]
- Gage-Bouchard Elizabeth A, Rodriguez Elisa M, Saad-Harfouche Frances G, Miller Austin, Erwin Deborah O. Factors Influencing Patient Pathways for Receipt of Cancer Care at an NCI-Designated Comprehensive Cancer Center. PloS One. 2014;9(10):e110649. doi: 10.1371/journal.pone.0110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman Christopher A, Stram Daniel O, Wilkens Lynne R, Pike Malcolm C, Kolonel Laurence N, Henderson Brian E, Le Marchand Loïc. Ethnic and Racial Differences in the Smoking-Related Risk of Lung Cancer. New England Journal of Medicine. 2006;354(4):333–42. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- Hanibuchi Tomoya, Nakaya Tomoki, Honjo Kaori, Ikeda Ai, Iso Hiroyasu, Inoue Manami, Sawada Norie, Tsugane Shoichiro. Neighborhood Contextual Factors for Smoking among Middle-Aged Japanese: A Multilevel Analysis. Health & Place. 2015 Jan;31:17–23. doi: 10.1016/j.healthplace.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Hashim Dana, Farhat Zeinab, Wallenstein Sylvan, Manczuk Marta, Holcombe Randall F, Thorpe Lorna, Schymura Maria J, Lucchini Roberto G, Boffetta Paolo. Standardized Cancer Incidence Disparities in Upper Manhattan New York City Neighborhoods: The Role of Race/ethnicity, Socioeconomic Status, and Known Risk Factors. European Journal of Cancer Prevention_: The Official Journal of the European Cancer Prevention Organisation (ECP) 2015 Jul; doi: 10.1097/CEJ.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman Jocelyn, Whitman Steven, Ansell David. The Black:white Disparity in Breast Cancer Mortality: The Example of Chicago. Cancer Causes & Control. 2007;18(3):323–33. doi: 10.1007/s10552-006-0102-y. [DOI] [PubMed] [Google Scholar]
- Hyland Andrew, Rezaishiraz Hamed, Bauer Joseph, Giovino Gary A, Michael Cummings K. Characteristics of Low-Level Smokers. Nicotine & Tobacco Research_: Official Journal of the Society for Research on Nicotine and Tobacco. 2005;7(3):461–68. doi: 10.1080/14622200500125369. [DOI] [PubMed] [Google Scholar]
- Islami Farhad, Kahn Amy R, Bickell Nina A, Schymura Maria J, Boffetta Paolo. Disentangling the Effects of Race/ethnicity and Socioeconomic Status of Neighborhood in Cancer Stage Distribution in New York City. Cancer Causes & Control_: CCC. 2013;24(6):1069–78. doi: 10.1007/s10552-013-0184-2. [DOI] [PubMed] [Google Scholar]
- Kass Daniel, Kheirbek Iyad, Wheeler K, Walters S, Pezeshki G. Air Pollution and the Health of New Yorkers: The Impact of Fine Particles and Ozone. [Accessed March 18];New York: New York City Department of Health and Mental Hygiene. 2015 http://www.nyc.gov/html/doh/downloads/pdf/eode/eode-air-quality-impact.pdf.
- Kheirbek Iyad, Johnson Sarah, Kazuhiko Ito, Matte Thomas, Kass Daniel. New York City Trends in Air Pollution and Its Health Consequences. New York: New York City Department of Health and Mental Hygiene; 2013. http://www.nyc.gov/html/doh/downloads/pdf/environmental/air-quality-report-2013.pdf. [Google Scholar]
- LaVeist Thomas, Pollack Keshia, Thorpe Roland, Fesahazion Ruth, Gaskin Darrell. Place, Not Race: Disparities Dissipate in Southwest Baltimore When Blacks and Whites Live under Similar Conditions. Health Affairs (Project Hope) 2011;30(10):1880–87. doi: 10.1377/hlthaff.2011.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin KA, Dundas R, Miller M, McCartney G. Socioeconomic and Geographic Inequalities in Adolescent Smoking: A Multilevel Cross-Sectional Study of 15 Year Olds in Scotland. Social Science & Medicine (1982) 2014 Apr;107:162–70. doi: 10.1016/j.socscimed.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Joyce A, Hamilton Brady E, Sutton Paul D, Ventura Stephanie J, Mathews TJ, Osterman Michelle JK. Births: Final Data for 2008. National Vital Statistics Reports_: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2010;59(1):1, 3–71. http://www.ncbi.nlm.nih.gov/pubmed/22145497. [PubMed] [Google Scholar]
- Martindale Stacey, Singh Awinder, Wang Hua, Steinberg Ashley, Homsi Amer, Zhang Haidi, Go Alan, Pappas Peter. Racial Disparities in Survival and Age-Related Outcome in Postsurgery Breast Cancer Patients in a New York City Community Hospital. ISRN Oncology. 2014 Jan;2014:1–9. doi: 10.1155/2014/694591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore de Peralta Arelis, Holaday Bonnie, McDonell James R. Factors Affecting Hispanic Women’s Participation in Screening for Cervical Cancer. Journal of Immigrant and Minority Health. 2014 Feb; doi: 10.1007/s10903-014-9997-7. [DOI] [PubMed] [Google Scholar]
- Myers C, Hakenewerth A, Olson C, Kerker B, Krauskopf M, Tavares A, Perlman S, Greene C, Farley T. Health Disparities in New York City: Disparities in Breast, Colorectal and Cervical Cancers in New York City. New York: New York City Department of Health and Mental Hygiene; 2011. http://www.nyc.gov/html/doh/downloads/pdf/episrv/disparitiestwosum_colorectal.pdf. [Google Scholar]
- Myers C, Olson EC, Ramaswamy C, Kerker B. Breast Cancer Screening among New York City Women. NYC Vital Signs. 2008 http://www.nyc.gov/html/doh/downloads/pdf/survey/survey-2008mammogram.pdf.
- New York City Department of City Planning. Ten Largest Sources of the Foreign-Born by County New York Metropolitan Region2000. New York City Department of City Planning. 2005 http://home2.nyc.gov/html/dcp/pdf/census/nny_table_5_4.pdf.
- Newman Lisa A. Breast Cancer Disparities: High-Risk Breast Cancer and African Ancestry. Surgical Oncology Clinics of North America. 2014;23(3):579–92. doi: 10.1016/j.soc.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Palloni Alberto, Arias Elizabeth. Paradox Lost: Explaining the Hispanic Adult Mortality Advantage. Demography. 2004;41(3):385–415. doi: 10.1353/dem.2004.0024. http://www.ncbi.nlm.nih.gov/pubmed/15461007. [DOI] [PubMed] [Google Scholar]
- Peterson Caryn E, Rauscher Garth H, Johnson Timothy P, Kirschner Carolyn V, Freels Sally, Barrett Richard E, Kim Seijeoung, Fitzgibbon Marian L, Joslin Charlotte E, Davis Faith G. The Effect of Neighborhood Disadvantage on the Racial Disparity in Ovarian Cancer-Specific Survival in a Large Hospital-Based Study in Cook County, Illinois. Frontiers in Public Health. 2015 Jan;3:8. doi: 10.3389/fpubh.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky Paul F. Racial and Ethnic Differences in Lung Cancer Incidence: How Much Is Explained by Differences in Smoking Patterns? (United States) Cancer Causes & Control_: CCC. 2006;17(8):1017–24. doi: 10.1007/s10552-006-0038-2. [DOI] [PubMed] [Google Scholar]
- Richards Catherine A, Kerker Bonnie D, Thorpe Lorna, Olson Carolyn, Krauskopf Marian S, Silver Lynn S, Weber Thomas K, Winawer Sidney J. Increased Screening Colonoscopy Rates and Reduced Racial Disparities in the New York Citywide Campaign: An Urban Model. The American Journal of Gastroenterology. 2011;106(11):1880–86. doi: 10.1038/ajg.2011.191. [DOI] [PubMed] [Google Scholar]
- Ruiz John M, Steffen Patrick, Smith Timothy B. Hispanic Mortality Paradox: A Systematic Review and Meta-Analysis of the Longitudinal Literature. American Journal of Public Health. 2013;103(3):e52–60. doi: 10.2105/AJPH.2012.301103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small ML, Jacobs EM, Massengill RP. Why Organizational Ties Matter for Neighborhood Effects: Resource Access through Childcare Centers. Social Forces. 2008;87(1):387–414. doi: 10.1353/sof.0.0079. [DOI] [Google Scholar]
- Sweeney Carol, Baumgartner Kathy B, Byers Tim, Giuliano Anna R, Herrick Jennifer S, Murtaugh Maureen A, Slattery Martha L. Reproductive History in Relation to Breast Cancer Risk among Hispanic and Non-Hispanic White Women. Cancer Causes & Control_: CCC. 2008;19(4):391–401. doi: 10.1007/s10552-007-9098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe Roland J, Bowie Janice V, Wilson-Frederick Shondelle M, Coa Kisha I, Laveist Thomas A. Association between Race, Place, and Preventive Health Screenings among Men: Findings from the Exploring Health Disparities in Integrated Communities Study. American Journal of Men’s Health. 2013;7(3):220–27. doi: 10.1177/1557988312466910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wye Gretchen, Kerker Bonnie D, Matte Thomas, Chamany Shadi, Eisenhower Donna, Frieden Thomas R, Thorpe Lorna. Obesity and Diabetes in New York City, 2002 and 2004. Preventing Chronic Disease. 2008;5(2):A48. http://www.ncbi.nlm.nih.gov/pubmed/18341783. [PMC free article] [PubMed] [Google Scholar]
- Whitman Steven, Ansell David, Orsi Jennifer, Francois Teena. The Racial Disparity in Breast Cancer Mortality. Journal of Community Health. 2011;36(4):588–96. doi: 10.1007/s10900-010-9346-2. [DOI] [PubMed] [Google Scholar]