Abstract
Systemic lupus erythematosus (SLE) is a complicated autoimmune disorder characterized by autoantibodies production, immune complex formation, and immune dysregulation, resulting in damage of multiple organs including the kidney. Lupus nephritis (LN) is the most common severe manifestation of SLE involving the majority of patients. Even though there are a number of reports indicating that interleukin-17 (IL-17) and Th17 cells play important roles in the pathogenesis of LN, the precise molecular mechanisms underline the development of LN have not been totally elucidated. In this review, we briefly summarize general characteristics of T and IL-17 cells in SLE. In addition, we discuss in detail T cell signaling pathways which control IL-17 production in patients with LN and in glomerulonephritis in lupus-prone mice. A better understanding of signaling and gene regulation defects in LN will lead to the identification of novel therapeutic targets and predictive biomarkers for diagnosis and prognosis of this disease.
Keywords: systemic lupus erythematosus, lupus nephritis, interleukin-17, T cells
1. Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disorder of unknown cause that can affect every organ [1]. In particular, lupus nephritis (LN) is one of the most serious manifestations of SLE and the pathogenesis of LN involves a variety of mechanisms [2]. Even though it is considered that dendritic, B, and plasma cells, autoantibodies and complement contribute to the development of LN [3–6], T cell-medicated autoimmunity and glomerular injury are critical for persistent renal damage closely related to impaired quality of life [7, 8]. In addition, recent studies in human SLE and animal models indicate a central role for interleukin-17 (IL-17) in the pathogenesis of LN [9, 10]. Treatment of LN varies with the type of disease based on histological findings classified according to the International Society of Nephrology/Renal Pathology Society (ISN/RPS) criteria [11]. Even though combined immunosuppressive therapy is indicated in patients with diffuse and focal proliferative LN and in many patients with lupus membranous nephropathy [12, 13], there is no optimal treatment for all patients with LN. Accordingly, it is quite important to understand the molecular mechanisms by which T cells and IL-17 induce inflammation that trigger LN development. In addition, better understanding of T cell function and IL-17 production may lead to identification of useful biomarkers for this disease.
In this review, we discuss the evidence that links T cells and IL-17 to LN in both humans and lupus-prone mice and describe recent advances in T cell signaling pathways that lead to IL-17 production in LN. Data indicates that an imbalance between Th17 cells and Tregs along with IL-17-driven inflammation play an important role in the damage of the kidney.
2. T cells in SLE
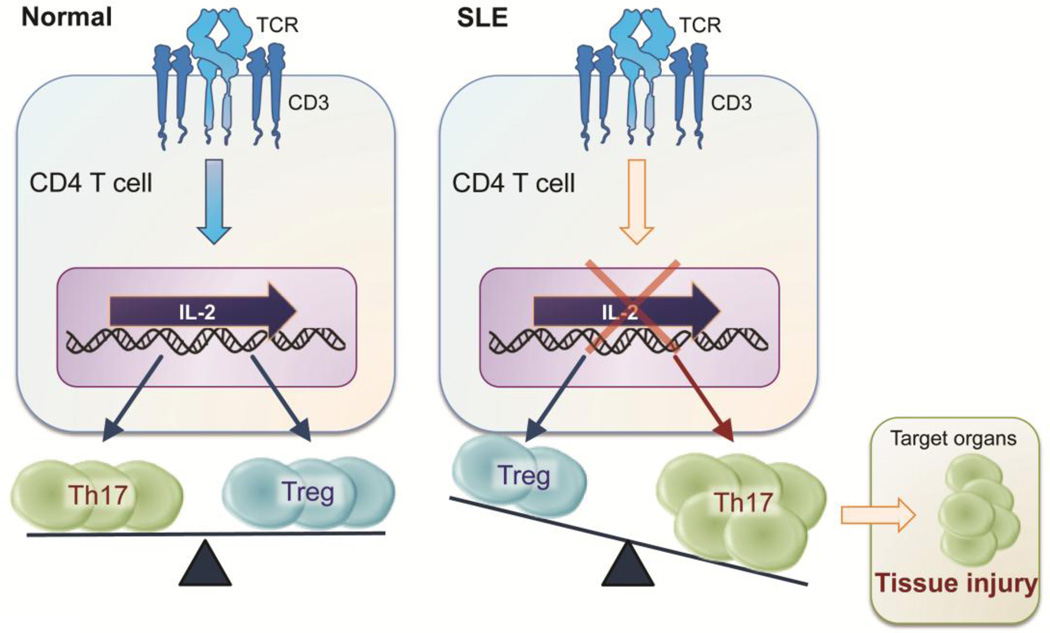
T cells are important players involved in the development and progression of LN in lupus prone mice and SLE patients [14]. Indeed, T cells from patients with SLE present aberrant signaling upon TCR engagement and have an altered gene expression profile [15]. Depletion of T cells or blocking T-cell activation mitigates development of nephritis in lupus prone mice [16, 17]. Of note, the balance between Th17 cells and Tregs is crucially involved in SLE pathogenesis. Limited Treg numbers [18, 19] and impaired function [20] have been observed in patients with SLE, and these defects have been associated with increased lupus disease activity [18]. This imbalance could be caused by deficient IL-2 production since IL-2 is necessary for the maintenance of regulatory T cells and inhibition of Th17 differentiation [21, 22].
Importantly, T cells from lupus-prone mice as well as SLE patients exhibit impaired IL-2 production [1, 23–26] and it is partly attributed to the unbalanced activation of cAMP response element binding protein (CREB) and cAMP response element modulator (CREM)α. Increased activation of CREMα, along with a reciprocal decrease in activated CREB, results in impaired IL-2 production by T cells from patients with SLE [27, 28]. In addition, it has been reported that the aberrant functions of NF-κB and activator protein 1 (AP1) in SLE T cells contribute to decreased IL2 transcription [29, 30]. More recently, our lab has reported that administration of low dose IL-2 by using an inducible recombinant adeno-associated virus vector to lupus-prone mice results in increased Treg cell numbers and function, suppressed IL-17 production and tissue damage of kidneys [31]. Taken together, limited IL-2 levels skew the balance between Tregs and Th17 in favor of the latter (Figure 1).
Figure 1.
Lupus patients have an imbalance between Th17 and Treg cells. SLE: Systemic lupus erythematosus, TCR: T cell receptor, Th: T helper, Treg: regulatory T cell.
3. IL-17 in SLE
IL-17 has a potential to induce the production of additional inflammatory cytokines and chemokines and to promote recruitment of inflammatory cells such as monocytes and neutrophils to the inflamed organ [32, 33]. It is a key cytokine involved in the pathogenesis of autoimmune diseases including SLE [10, 33, 34]. Increased number of Th17 cells as well as high serum levels of IL-17 has been demonstrated in SLE patients [35–37]. Although IL-17 is the main cytokine produced by Th17 cells [33], it is also produced by other subsets of T cells including T-cell receptor (TCR)γδ and TCRαβ double negative (DN) T-cells (CD3+CD4−CD8−), macrophages and neutrophils [38, 39]. In addition to IL-17, IL-23 was also found to be crucial for the development of various autoimmune diseases in murine models [40–42] and in humans [43] by promoting Th17 cell–mediated tissue inflammation. There is accumulating evidence indicating the importance of IL-23 in patients with SLE [44–46]. Of note, our group has shown that clinical and pathology findings of LN are mitigated in lupus prone mice with IL-23 receptor deficiency [47] or treated with an anti-IL23 antibody [48]. In clinical settings, trial studies showed the efficacy and safely of treatment with ustekinumab, a human monoclonal antibody that binds to the p40 subunit of IL-23 in subacute cutaneous lupus [49], psoriasis [50], and psoriatic arthritis [51].
Collectively, these findings suggest a critical role of IL-23/IL-17 axis in the pathogenesis of SLE. Further studies are needed to clarify whether IL-23/ IL-17 could be useful in predicting long-term prognosis and its possibility of targets for new treatment strategies in SLE.
4. The role of T cells and IL-17 in lupus nephritis
It has been demonstrated that high levels of IL-17 predict poor histopathological outcome after immunosuppressive therapy in patients with LN [45]. Moreover, it has been reported that the urinary expression of Th17-related genes including IL17 and IL23 is increased in SLE patients and associated with the activity of LN [52].
Our group has proposed that DN T-cells infiltrate the kidneys of patients with LN and reresent the major source for IL-17 [38]. In line with this finding, it has also been shown that DN T-cells as well as Th17 cells produces increased amounts of IL-17 and IFN-γ in kidneys in lupus-prone mice [28]. Even though the precise molecular mechanisms which underline T cell or IL-17 dependent tissue damage of LN are not totally elucidated, there have been some novel findings which demonstrate the contribution of T cell signaling pathways to the expression of LN.
5. Aberrant T cell signaling in lupus nephritis
5.1. Protein phosphatase 2A (PP2A)
Protein phosphatase 2A (PP2A) is a multifunctional serine/threonine phosphatase involved in essential cellular processes. Increased expression of PP2A contributes to the dephosphorylation of a number of molecules including Elf-1, SP1, MEK, and CREB and thus plays as a critical role in aberrant T cell function [53–56]. Our group has shown that expression and activity of its catalytic subunit (PP2Ac) is increased in T cells from patients with SLE [55, 56] and that transgenic mice overexpressing PP2Ac in T cells develop glomerulonephritis resulting from increased production of IL-17A and IL-17F [57]. Accordingly, dysregulation of PP2Ac contributes to the pathogenesis of LN by promoting the inflammatory and migration capacity of IL-17 producing T cells.
5.2. Rho-associated protein kinase (ROCK)
Rho-associated protein kinase (ROCK) is a serine-threonine kinase involved mainly in regulating cell migration including T cells [58]. ROCK-mediated phosphorylation of the ezrin/radixin/moesin protein (ERM) leads to the enhancement of the CD44-ERM complex formation [58]. Our group and others have been reported a dysfunction of the ROCK-ERM-CD44 axis in T cells from SLE patients [59–61]. Pharmacological inhibition of ROCK in lupus-prone mice limited T-cells adhesion and migration leading to decreased the kidney pathology [62, 63]. In addition, ROCK2 was reported to facilitate the activity of interferon regulatory factor 4 (IRF4), which is required for the production of IL-17 and IL-21 [63]. Of note, PP2Ac in T cells is also involved in IL-17 production by promoting the ROCK-IRF4 pathway [64]. Collectively, the inhibition of ROCK could represent an important therapeutic regimen for the treatment of LN because it could limit entrance of T cells in to the kidney and the production of IL-17.
5.3. cAMP response element modulator (CREM)
CREM, which has multiple splicing variants encoding different isoforms, is an important component of cAMP-mediated signal transduction during cellular processes including T cell activation. Among isoforms of CREM, CREMα plays an important role in T cell differentiation and IL-17 production in SLE. Previous studies have revealed that T cells from patients with SLE have increased level of CREMα along with aberrant IL-17A expression [65]. Likewise, mice overexpressing CREMα in T cells display increased production of IL-17 and lupus-like disease [66]. Mechanistically, CREMα was found to bind to the IL17 promoter or IL17 locus to enhance its activity at the epigenetic level [65, 67]. In addition, CREMα is essential for the expansion of DN-T cells through epigenetic regulations of CD8a cells in patients with SLE and in lupus prone mice [68, 69]. Taken together, these observations indicate the potential of CREMα to serve as disease biomarker and possible therapeutic target in SLE because lowering its levels may suppress IL-17 production and shrink the pool of pathogenic DN-T cells.
5.4. Calcium/calmodulin-dependent protein kinase IV (CaMK4)
Calcium/calmodulin-dependent protein kinase IV (CaMK4) is a multifunctional serine/threonine kinase that regulates gene expression by activating transcription factors [70]. We have reported previously that CaMK4 is abnormally increased in T cells from patients with SLE [71] and lupus-prone mice [26]. In addition, we have also shown that CaMK4 expression is induced preferentially during Th17 differentiation and necessary for Th17 differentiation [72]. In line with these findings, genetic or pharmacologic inhibition of CaMK4 in MRL/lpr mice resulted in decreased the frequency of IL-17–producing T cells including CD4+ and DN T cells in spleens and in lymph nodes, a significant decrease of autoantibody production, improvement of nephritis and the survival rates [26, 73]. We have presented evidence using a Foxp3 reporter mouse in the MRL/lpr background that CaMK4 inhibition diminishes the accumulation of inflammatory cells followed by a reciprocal increase in Treg cells in the kidneys [74]. We recently demonstrate that CaMK4 facilitates anti-glomerular basement membrane antibody-induced glomerulonephritis in mice through the suppression of the CCR6/CCL20 axis at the inflamed sites [75]. Collectively, CaMK4-mediated aberrant T cell activity facilitates the development of LN via overproduction of IL-17 in lymphoid tissue as well as inflamed kidneys.
5.5 mammalian target of rapamycin complex 1 (mTORC1)
Mammalian target of rapamycin complex 1 (mTORC1) is a serine-threonine kinase that serves as a regulator of cellular metabolism and proliferation [76]. mTORC1 has recently emerged as an important regulator of Th1 and Th17 differentiation [77]. Importantly, CaMK4 [72] and ROCK [78] induce Th17 differentiation through mTORC1 pathway indicating the activation of mTORC1 contributes to Th17/Treg imbalance in autoimmune diseases including SLE. Moreover, recent studies have shown that the blockade by rapamycin reverses the expansion of Th17 cells in SLE patients [79, 80] and that rapamycin blocks glomerulonephritis in lupus prone mice [81]. Taken together, the inhibition of mTORC1 also has robust clinical benefits in patients with lupus nephritis.
6. Conclusion
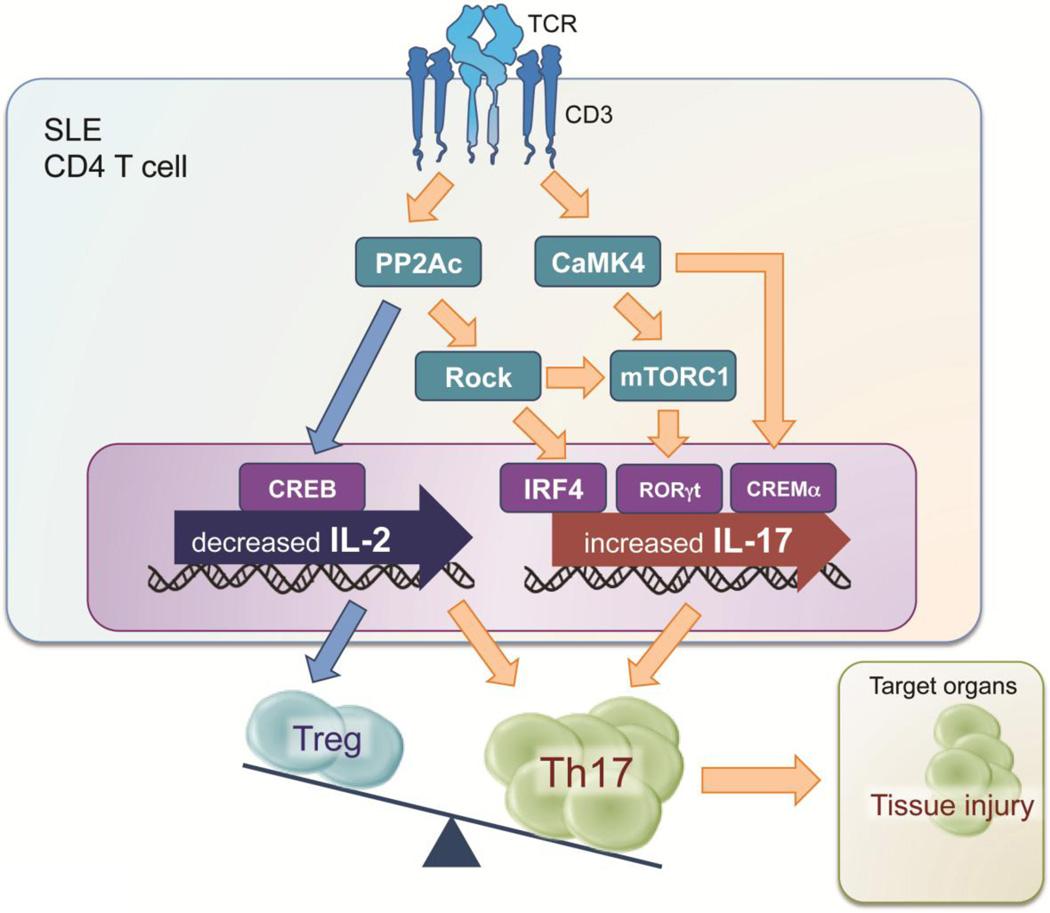
SLE is an autoimmune disease with manifestations in multiple organs. Immune-system aberrations including lack of balance between Th17 and Treg cells, immune complexes, autoantibodies, inflammatory cytokines contribute to the expression of organ damage such as LN. In this article, we reviewed recent advances in molecular immunology of T cells and IL-17 and their clinical implications. We show the aberrant T cell signaling in the pathogenesis of LN in Figure 2. Biologic therapies and small-molecule drugs that target the aberrant T cell signaling and IL-17 production are desired for achievement of a favorable prognosis in patients with LN.
Figure 2.
The aberrant T cell signaling in the pathogenesis of LN. SLE: Systemic lupus erythematosus, TCR: T cell receptor, PP2Ac: Protein phosphatase 2A catalytic subunit, CaMK4: Calcium/calmodulin-dependent protein kinase IV, ROCK: Rho-associated protein kinase, CREB: cAMP response element binding protein, IRF4: interferon regulatory factor 4, CREM: cAMP response element modulator, mTORC1: mammalian target of rapamycin complex 1, Th: T helper, Treg: regulatory T cell.
Highlights.
-
▶
Aberrant T cell signaling causes the development and progression of lupus nephritis.
-
▶
IL-17 and Th17 cells play important roles in the pathogenesis of lupus nephritis.
-
▶
PP2A, ROCK, CREM and CaMK4 facilitate IL-17 production in SLE.
-
▶
T cell-targeted treatments are desired to achieve therapeutic goals in SLE.
Acknowledgments
Financial Support:
This work was supported by a U.S. National Institutes of Health Grant R01AR064350 (to G.C.T.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Rovin BH, Parikh SV. Lupus nephritis: the evolving role of novel therapeutics. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63:677–690. doi: 10.1053/j.ajkd.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rovin BH. The chemokine network in systemic lupus erythematous nephritis. Front Biosci. 2008;13:904–922. doi: 10.2741/2731. [DOI] [PubMed] [Google Scholar]
- 4.Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, Hansen A, Burmester GR, Diamond B, Lipsky PE, Dorner T. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum. 2008;58:1762–1773. doi: 10.1002/art.23498. [DOI] [PubMed] [Google Scholar]
- 5.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 6.Hedrich CM, Crispin JC, Tsokos GC. Epigenetic regulation of cytokine expression in systemic lupus erythematosus with special focus on T cells. Autoimmunity. 2014;47:234–241. doi: 10.3109/08916934.2013.801462. [DOI] [PubMed] [Google Scholar]
- 7.Turner JE, Paust HJ, Steinmetz OM, Panzer U. The Th17 immune response in renal inflammation. Kidney international. 2010;77:1070–1075. doi: 10.1038/ki.2010.102. [DOI] [PubMed] [Google Scholar]
- 8.Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME. Lupus nephritis: a critical review. Autoimmunity reviews. 2012;12:174–194. doi: 10.1016/j.autrev.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apostolidis SA, Crispin JC, Tsokos GC. IL-17-producing T cells in lupus nephritis. Lupus. 2011;20:120–124. doi: 10.1177/0961203310389100. [DOI] [PubMed] [Google Scholar]
- 11.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15:241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 12.Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, Ramsey-Goldman R, Singh K, Khalighi M, Choi SI, Gogia M, Kafaja S, Kamgar M, Lau C, Martin WJ, Parikh S, Peng J, Rastogi A, Chen W, Grossman JM. R. American College of, American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, Boletis J, Cervera R, Dorner T, Doria A, Ferrario F, Floege J, Houssiau FA, Ioannidis JP, Isenberg DA, Kallenberg CG, Lightstone L, Marks SD, Martini A, Moroni G, Neumann I, Praga M, Schneider M, Starra A, Tesar V, Vasconcelos C, van Vollenhoven RF, Zakharova H, Haubitz M, Gordon C, Jayne D, Boumpas DT R. European League Against, D. European Renal Association-European, A. Transplant, Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 2012;71:1771–1782. doi: 10.1136/annrheumdis-2012-201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konya C, Paz Z, Tsokos GC. The role of T cells in systemic lupus erythematosus: an update. Current opinion in rheumatology. 2014;26:493–501. doi: 10.1097/BOR.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 15.Kyttaris VC, Tsokos GC. Targeting lymphocyte signaling pathways as a therapeutic approach to systemic lupus erythematosus. Current opinion in rheumatology. 2011;23:449–453. doi: 10.1097/BOR.0b013e328349a242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wofsy D, Ledbetter JA, Hendler PL, Seaman WE. Treatment of murine lupus with monoclonal anti-T cell antibody. J Immunol. 1985;134:852–857. [PubMed] [Google Scholar]
- 17.Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171:489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 18.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 19.Barath S, Aleksza M, Tarr T, Sipka S, Szegedi G, Kiss E. Measurement of natural (CD4+CD25high) and inducible (CD4+IL-10+) regulatory T cells in patients with systemic lupus erythematosus. Lupus. 2007;16:489–496. doi: 10.1177/0961203307080226. [DOI] [PubMed] [Google Scholar]
- 20.Yan B, Ye S, Chen G, Kuang M, Shen N, Chen S. Dysfunctional CD4+, CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-alpha-producing antigen-presenting cells. Arthritis Rheum. 2008;58:801–812. doi: 10.1002/art.23268. [DOI] [PubMed] [Google Scholar]
- 21.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea J J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O'Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Martin D, Diaz-Zamudio M, Crispin JC, Alcocer-Varela J. Interleukin 2 and systemic lupus erythematosus: beyond the transcriptional regulatory net abnormalities. Autoimmunity reviews. 2009;9:34–39. doi: 10.1016/j.autrev.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 24.Lieberman LA, Tsokos GC. The IL-2 defect in systemic lupus erythematosus disease has an expansive effect on host immunity. J Biomed Biotechnol. 2010;2010:740619. doi: 10.1155/2010/740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humrich JY, Morbach H, Undeutsch R, Enghard P, Rosenberger S, Weigert O, Kloke L, Heimann J, Gaber T, Brandenburg S, Scheffold A, Huehn J, Radbruch A, Burmester GR, Riemekasten G. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci U S A. 2010;107:204–209. doi: 10.1073/pnas.0903158107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koga T, Ichinose K, Mizui M, Crispin JC, Tsokos GC. Calcium/Calmodulin-Dependent Protein Kinase IV Suppresses IL-2 Production and Regulatory T Cell Activity in Lupus. J Immunol. 2012;189:3490–3496. doi: 10.4049/jimmunol.1201785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J Immunol. 2001;166:4216–4222. doi: 10.4049/jimmunol.166.6.4216. [DOI] [PubMed] [Google Scholar]
- 28.Tenbrock K, Juang YT, Tolnay M, Tsokos GC. The cyclic adenosine 5'-monophosphate response element modulator suppresses IL-2 production in stimulated T cells by a chromatin-dependent mechanism. J Immunol. 2003;170:2971–2976. doi: 10.4049/jimmunol.170.6.2971. [DOI] [PubMed] [Google Scholar]
- 29.Wong HK, Kammer GM, Dennis G, Tsokos GC. Abnormal NF-kappa B activity in T lymphocytes from patients with systemic lupus erythematosus is associated with decreased p65-RelA protein expression. J Immunol. 1999;163:1682–1689. [PubMed] [Google Scholar]
- 30.Kyttaris VC, Juang YT, Tenbrock K, Weinstein A, Tsokos GC. Cyclic adenosine 5'-monophosphate response element modulator is responsible for the decreased expression of c-fos and activator protein-1 binding in T cells from patients with systemic lupus erythematosus. J Immunol. 2004;173:3557–3563. doi: 10.4049/jimmunol.173.5.3557. [DOI] [PubMed] [Google Scholar]
- 31.Mizui M, Koga T, Lieberman LA, Beltran J, Yoshida N, Johnson MC, Tisch R, Tsokos GC. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4-CD8- IL-17-producing T cells. J Immunol. 2014;193:2168–2177. doi: 10.4049/jimmunol.1400977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burkett PR, Meyer zu Horste G, Kuchroo VK. Pouring fuel on the fire: Th17 cell, the environment, and autoimmunity. J Clin Invest. 2015;125:2211–2219. doi: 10.1172/JCI78085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moulton VR, Tsokos GC. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest. 2015;125:2220–2227. doi: 10.1172/JCI78087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus. 2000;9:589–593. doi: 10.1191/096120300678828703. [DOI] [PubMed] [Google Scholar]
- 36.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res Ther. 2013;15:R97. doi: 10.1186/ar4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutton CE, Mielke LA, Mills KH. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 40.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 41.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leng RX, Pan HF, Chen GM, Wang C, Qin WZ, Chen LL, Tao JH, Ye DQ. IL-23: a promising therapeutic target for systemic lupus erythematosus. Arch Med Res. 2010;41:221–225. doi: 10.1016/j.arcmed.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 45.Zickert A, Amoudruz P, Sundstrom Y, Ronnelid J, Malmstrom V, Gunnarsson I. IL-17 and IL-23 in lupus nephritis - association to histopathology and response to treatment. BMC Immunol. 2015;16:7. doi: 10.1186/s12865-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rana A, Minz RW, Aggarwal R, Anand S, Pasricha N, Singh S. Gene expression of cytokines (TNF-alpha, IFN-gamma), serum profiles of IL-17 and IL-23 in paediatric systemic lupus erythematosus. Lupus. 2012;21:1105–1112. doi: 10.1177/0961203312451200. [DOI] [PubMed] [Google Scholar]
- 47.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol. 2010;184:4605–4609. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kyttaris VC, Kampagianni O, Tsokos GC. Treatment with anti-interleukin 23 antibody ameliorates disease in lupus-prone mice. BioMed research international. 2013;2013:861028. doi: 10.1155/2013/861028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Souza A, Ali-Shaw T, Strober BE, Franks AG., Jr Successful treatment of subacute lupus erythematosus with ustekinumab. Archives of dermatology. 2011;147:896–898. doi: 10.1001/archdermatol.2011.185. [DOI] [PubMed] [Google Scholar]
- 50.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, Li S, Dooley LT, Gordon KB P. s. investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 51.McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM, Doyle MK, Group PS. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet. 2013;382:780–789. doi: 10.1016/S0140-6736(13)60594-2. [DOI] [PubMed] [Google Scholar]
- 52.Kwan BC, Tam LS, Lai KB, Lai FM, Li EK, Wang G, Chow KM, Li PK, Szeto CC. The gene expression of type 17 T-helper cell-related cytokines in the urinary sediment of patients with systemic lupus erythematosus. Rheumatology (Oxford) 2009;48:1491–1497. doi: 10.1093/rheumatology/kep255. [DOI] [PubMed] [Google Scholar]
- 53.Juang YT, Wang Y, Jiang G, Peng HB, Ergin S, Finnell M, Magilavy A, Kyttaris VC, Tsokos GC. PP2A dephosphorylates Elf-1 and determines the expression of CD3zeta and FcRgamma in human systemic lupus erythematosus T cells. J Immunol. 2008;181:3658–3664. doi: 10.4049/jimmunol.181.5.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juang YT, Rauen T, Wang Y, Ichinose K, Benedyk K, Tenbrock K, Tsokos GC. Transcriptional activation of the cAMP-responsive modulator promoter in human T cells is regulated by protein phosphatase 2A-mediated dephosphorylation of SP-1 and reflects disease activity in patients with systemic lupus erythematosus. J Biol Chem. 2011;286:1795–1801. doi: 10.1074/jbc.M110.166785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katsiari CG, Kyttaris VC, Juang YT, Tsokos GC. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. J Clin Invest. 2005;115:3193–3204. doi: 10.1172/JCI24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sunahori K, Juang YT, Tsokos GC. Methylation status of CpG islands flanking a cAMP response element motif on the protein phosphatase 2Ac alpha promoter determines CREB binding and activity. J Immunol. 2009;182:1500–1508. doi: 10.4049/jimmunol.182.3.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crispin JC, Apostolidis SA, Rosetti F, Keszei M, Wang N, Terhorst C, Mayadas TN, Tsokos GC. Cutting edge: protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17-dependent mechanism. J Immunol. 2012;188:3567–3571. doi: 10.4049/jimmunol.1200143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JH, Katakai T, Hara T, Gonda H, Sugai M, Shimizu A. Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. The Journal of cell biology. 2004;167:327–337. doi: 10.1083/jcb.200403091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, Tung K, Tsokos GC. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. J Immunol. 2007;178:1938–1947. doi: 10.4049/jimmunol.178.3.1938. [DOI] [PubMed] [Google Scholar]
- 60.Crispin JC, Keenan BT, Finnell MD, Bermas BL, Schur P, Massarotti E, Karlson EW, Fitzgerald LM, Ergin S, Kyttaris VC, Tsokos GC, Costenbader KH. Expression of CD44 variant isoforms CD44v3 and CD44v6 is increased on T cells from patients with systemic lupus erythematosus and is correlated with disease activity. Arthritis Rheum. 2010;62:1431–1437. doi: 10.1002/art.27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Isgro J, Gupta S, Jacek E, Pavri T, Duculan R, Kim M, Kirou KA, Salmon JE, Pernis AB. Enhanced rho-associated protein kinase activation in patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65:1592–1602. doi: 10.1002/art.37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stirzaker RA, Biswas PS, Gupta S, Song L, Bhagat G, Pernis AB. Administration of fasudil, a ROCK inhibitor, attenuates disease in lupus-prone NZB/W F1 female mice. Lupus. 2012;21:656–661. doi: 10.1177/0961203312436862. [DOI] [PubMed] [Google Scholar]
- 63.Biswas PS, Gupta S, Chang E, Song L, Stirzaker RA, Liao JK, Bhagat G, Pernis AB. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120:3280–3295. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Apostolidis SA, Rauen T, Hedrich CM, Tsokos GC, Crispin JC. Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling. J Biol Chem. 2013;288:26775–26784. doi: 10.1074/jbc.M113.483743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rauen T, Hedrich CM, Juang YT, Tenbrock K, Tsokos GC. cAMP-responsive element modulator (CREM)alpha protein induces interleukin 17A expression and mediates epigenetic alterations at the interleukin-17A gene locus in patients with systemic lupus erythematosus. J Biol Chem. 2011;286:43437–43446. doi: 10.1074/jbc.M111.299313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lippe R, Ohl K, Varga G, Rauen T, Crispin JC, Juang YT, Kuerten S, Tacke F, Wolf M, Roebrock K, Vogl T, Verjans E, Honke N, Ehrchen J, Foell D, Skryabin B, Wagner N, Tsokos GC, Roth J, Tenbrock K. CREMalpha overexpression decreases IL-2 production, induces a T(H)17 phenotype and accelerates autoimmunity. J Mol Cell Biol. 2012;4:121–123. doi: 10.1093/jmcb/mjs004. [DOI] [PubMed] [Google Scholar]
- 67.Hedrich CM, Crispin JC, Rauen T, Ioannidis C, Apostolidis SA, Lo MS, Kyttaris VC, Tsokos GC. cAMP response element modulator alpha controls IL2 and IL17A expression during CD4 lineage commitment and subset distribution in lupus. Proc Natl Acad Sci U S A. 2012;109:16606–16611. doi: 10.1073/pnas.1210129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hedrich CM, Rauen T, Crispin JC, Koga T, Ioannidis C, Zajdel M, Kyttaris VC, Tsokos GC. cAMP-responsive element modulator alpha (CREMalpha) trans-represses the transmembrane glycoprotein CD8 and contributes to the generation of CD3+CD4-CD8- T cells in health and disease. J Biol Chem. 2013;288:31880–31887. doi: 10.1074/jbc.M113.508655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hedrich CM, Crispin JC, Rauen T, Ioannidis C, Koga T, Rodriguez Rodriguez N, Apostolidis SA, Kyttaris VC, Tsokos GC. cAMP responsive element modulator (CREM) alpha mediates chromatin remodeling of CD8 during the generation of CD3+ CD4- CD8- T cells. J Biol Chem. 2014;289:2361–2370. doi: 10.1074/jbc.M113.523605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Racioppi L, Means AR. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: novel routes for an ancient traveller. Trends Immunol. 2008;29:600–607. doi: 10.1016/j.it.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, Kyttaris VC, Tsokos GC. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koga T, Hedrich CM, Mizui M, Yoshida N, Otomo K, Lieberman LA, Rauen T, Crispin JC, Tsokos GC. CaMK4-dependent activation of AKT/mTOR and CREM-alpha underlies autoimmunity-associated Th17 imbalance. J Clin Invest. 2014;124:2234–2245. doi: 10.1172/JCI73411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ichinose K, Rauen T, Juang YT, Kis-Toth K, Mizui M, Koga T, Tsokos GC. Cutting edge: Calcium/Calmodulin-dependent protein kinase type IV is essential for mesangial cell proliferation and lupus nephritis. J Immunol. 2011;187:5500–5504. doi: 10.4049/jimmunol.1102357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koga T, Mizui M, Yoshida N, Otomo K, Lieberman LA, Crispin JC, Tsokos GC. KN-93, an inhibitor of calcium/calmodulin-dependent protein kinase IV, promotes generation and function of Foxp3(+) regulatory T cells in MRL/lpr mice. Autoimmunity. 2014;47:445–450. doi: 10.3109/08916934.2014.915954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koga T, Otomo K, Mizui M, Yoshida N, Umeda M, Ichinose K, Kawakami A, Tsokos GC. CaMK4 facilitates the recruitment of IL-17-producing cells to target organs through the CCR6/CCL20 axis in Th17-driven inflammatory diseases. Arthritis & rheumatology. 2016 doi: 10.1002/art.39665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 77.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gentry EG, Henderson BW, Arrant AE, Gearing M, Feng Y, Riddle NC, Herskowitz JH. Rho Kinase Inhibition as a Therapeutic for Progressive Supranuclear Palsy and Corticobasal Degeneration. J Neurosci. 2016;36:1316–1323. doi: 10.1523/JNEUROSCI.2336-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lai ZW, Borsuk R, Shadakshari A, Yu J, Dawood M, Garcia R, Francis L, Tily H, Bartos A, Faraone SV, Phillips P, Perl A. Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol. 2013;191:2236–2246. doi: 10.4049/jimmunol.1301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kato H, Perl A. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+ CD4-CD8- double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J Immunol. 2014;192:4134–4144. doi: 10.4049/jimmunol.1301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Caza TN, Fernandez DR, Talaber G, Oaks Z, Haas M, Madaio MP, Lai ZW, Miklossy G, Singh RR, Chudakov DM, Malorni W, Middleton F, Banki K, Perl A. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis. 2014;73:1888–1897. doi: 10.1136/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed] [Google Scholar]