Abstract
Intracranial hemorrhage (ICH) is the most serious bleeding event that occurs in patients with hemophilia; its estimated mortality rate is approximately 20 %, accounting for the largest number of deaths from bleeding. We conducted this single-center, retrospective study to examine the characteristics of and prognostic factors in patients with hemophilia. A comprehensive review of 12 cases of intracranial hemorrhage (ICH) among 10 patients. All 12 cases of ICH in the 10 patients were treated with clotting factor concentrates. Three patients had intracerebral hemorrhage that required neurosurgical intervention. After presenting with ICH, two pediatric patients developed antibodies to clotting factors. Two adult patients with intracerebral hemorrhage died, and the mortality rate was thus 20.0 % (2/10) in our clinical series. Prompt and intensive treatment with clotting factor concentrates may significantly lower the mortality rate among patients with hemophilia presenting with ICH. Our results showed a better prognosis in pediatric patients with intracerebral hemorrhage. Clinicians should pay special attention to the possible development of inhibitors after intensive treatment in pediatric patients. Further studies are needed to examine methods for administering clotting factor concentrates and to determine whether neurosurgical intervention is essential in each case.
Keywords: Hemophilia, Intracranial hemorrhage, Clotting factor concentrates, Neurosurgical interventions
Introduction
Hemophilia is an X-linked congenital bleeding disorder with a frequency of about 1 in 10,000 births. It is caused by a deficiency of coagulation factor VIII (FVIII) or IX (FIX), referred to as hemophilia A or B, respectively, in association with mutations in clotting factor genes [1, 2]. Patients with hemophilia commonly present with joint and muscle bleeding. In these patients, the current standard treatment is to replenish the deficient FVIII or FIX for therapeutic or prophylactic purposes.
Intracranial hemorrhage (ICH) is the most serious bleeding event that occurs in patients with hemophilia; its estimated mortality rate is approximately 20 %, accounting for the largest number of deaths from bleeding [1, 3–5]. ICH may occur spontaneously or due to trauma (spontaneous or traumatic ICH, respectively). Traumatic ICH is more prevalent in children than adults, and head trauma, especially, is one of the most common injuries in childhood [3, 6]. Predisposing risk factors for ICH include the severity of the hemophilia, advanced age, the presence of inhibitors, human immunodeficiency virus (HIV) infection, hypertension, and a history of ICH [1, 6, 7].
Given this background, we conducted the present single-center, retrospective study to examine the characteristics of and prognostic factors in patients with hemophilia through a comprehensive review of 12 cases of ICH among 10 patients. We attempted to identify appropriate treatment methods from this series.
Methods
This single-center, retrospective study involved 12 cases of ICH among 10 patients with hemophilia who were admitted to our hospital from January 2008 to June 2014. Through a retrospective analysis of the patients’ medical records, we evaluated age, sex, weight, the type and severity of hemophilia, the presence of inhibitors, and any history and outcomes of prophylactic treatment for ICH. We also analyzed the characteristics of ICH, such as its location and initial symptoms; the patients’ length of hospital stay; and the methods, outcomes, and sequelae of surgical treatment. Furthermore, we analyzed known predisposing factors for ICH, such as a history of ICH; hepatitis B virus (HBV), hepatitis C virus (HCV), or HIV infection; and hypertension. The current study was approved by the institutional review board of our medical institution (2015-01-029).
Results
Patient Characteristics
In total, 10 patients with hemophilia were diagnosed with 12 cases of ICH. Of these patients, 8 (80 %) and 2 (20 %) had hemophilia A and B, respectively. All patients were classified as having severe hemophilia. Four (33.3 %) cases had high-titer inhibitors at the onset of the ICH. Of the ten patients, five were adults and five were children. The patients developed ICH at a median age of 19 (range 0–49) years (Table 1).
Table 1.
Baseline characteristics and risk factors of intracranial hemorrhage in patients with hemophilia
| Patient no. | Age/sex | Type of hemophilia | Severity | Possible risk factors | ||||
|---|---|---|---|---|---|---|---|---|
| Inhibitor | Prophylaxis | Viral infection | Previous ICH | Others | ||||
| 1 | 0/M | A | Severe | – | – | – | – | – |
| 2 | 1/M | A | Severe | – | – | – | – | – |
| 3 | 2/M | B | Severe | – | – | – | – | – |
| 4 | 2/M | A | Severe | – | – | – | – | – |
| 5* | 12/M | A | Severe | + | + | – | – | – |
| 6* | 13/M | A | Severe | + | + | – | + | – |
| 7 | 19/M | A | Severe | – | + | – | – | – |
| 8# | 38/M | A | Severe | + | – | HCV | – | – |
| 9 | 38/M | B | Severe | – | – | HCV, HIV | – | Hypertension, Thrombocytopenia |
| 10 | 39/M | A | Severe | – | – | HCV | – | Hypertension |
| 11# | 42/M | A | Severe | + | – | HCV | + | – |
| 12 | 49/M | A | Severe | – | – | HCV | – | – |
*, #, same patient
There were seven (58.3 %) cases of traumatic ICH and five (41.6 %) of spontaneous ICH. One pediatric patient had two ICH episodes. First ICH event was traumatic and the second event was spontaneous. One adult patient had two spontaneous ICH episodes. All patients aged ≤5 years had traumatic ICH. As shown in Table 2, six of the seven (85.7 %) cases of subdural hemorrhage involved traumatic ICH. Of the three cases of intracerebral hemorrhage and intraventricular hemorrhage, two were adult cases of spontaneous ICH and one was a pediatric case of traumatic ICH.
Table 2.
Types of intracranial hemorrhage
| Type of intracranial hemorrhages | n (%) |
|---|---|
| Subdural | 7 (58.3) |
| Subdural and epidural | 1 (8.3) |
| Subarachnoid | 1 (8.3) |
| Intracerebral | 1 (8.3) |
| Intracerebral and intraventricular | 2 (16.7) |
In total, three cases of ICH occurred during prophylaxis in two patients. Of these, one pediatric patient with inhibitors had recurrent ICH episodes. The patients without inhibitors were given FVIII concentrates at a dose of 25 IU/kg three times per week as primary prophylaxis, and activated prothrombin complex concentrates were administered to patients with inhibitors at dose of 50 IU/kg three times per week as secondary prophylaxis. Of all cases, two (20.0 %) had a history of ICH. There were five (41.6 %) HCV-positive cases and one (8.3 %) HIV-positive case. There were no HBV-positive cases (Table 1).
Clinical Courses
All 10 patients with 12 cases of ICH were hospitalized and treated with clotting factor concentrates. The median length of hospital stay was 14 (range 10–37) days. The patients were followed up for a median period of 21 (range 11–55) months.
All patients presented with typical symptoms of ICH. Of these, headache (41.6 %) was the most common. On neurological examination, four patients showed deficits, such as reduced consciousness, hemiparesis, and speech disturbance. Of these patients, three had intracerebral hemorrhage and one had severe subdural hemorrhage. Vomiting and seizures were the presenting symptoms in five patients, and two of these five had seizures.
The hemorrhage and related symptoms improved in 10 cases of ICH following the administration of clotting factor concentrates. In these cases, we replaced the clotting factor concentrates to achieve 100 % of peak FVIII activity. For this purpose, we frequently measured the levels of FVIII or FIX to ensure that they were maintained at a consistent level. The patients with hemophilia A were initially given FVIII concentrates at a dose of 50 IU/kg, which was repeated at 8–12-h intervals.
Two patients were given continuous FVIII concentrates. Of these, one patient with a subdural hemorrhage requiring neurosurgical management achieved improvement in symptoms without surgical management after a 4-day treatment with FVIII concentrates (Fig. 1a). The other with a traumatic intracerebral hemorrhage underwent ICH evacuation followed by an 11-day treatment with FVIII concentrates.
Fig. 1.
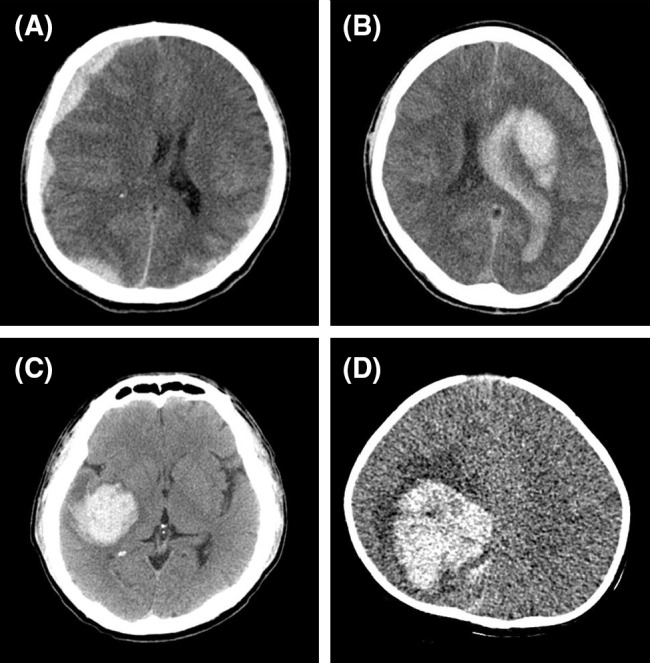
Brain computed tomography (CT) scans in four patients with hemophilia presenting with intracranial hemorrhage. a A 39-year-old man with severe hemophilia A presented with subdural hemorrhage (SDH) and a GCS score of 14. Brain CT showed SDH along both cerebral convexities and the right falx. This was accompanied by the finding that the midline was shifted to the left side. b A 42-year-old man with severe hemophilia A presented with intracranial hemorrhage and a GCS score of 3. Brain CT showed an acute intracerebral hemorrhage of 6.9 × 3.2 × 5.0 cm at the left basal ganglia, an intraventricular hemorrhage with hydrocephalus, severe brain edema, and a subfalcine herniation. c A 38-year-old man with severe hemophilia B presented with ICH and a GCS score of 3. Acute intracerebral hemorrhage was present in the right temporal lobe. d A 7-month-old boy with severe hemophilia A presented with ICH and a GCS score of 9. Brain CT showed an acute intracerebral hemorrhage of 5.0 × 5.0 × 4.0 cm at the right basal ganglia and an intraventricular hemorrhage at the right parietal lobe. Three of these patients (b, c, and d) with intracerebral hemorrhage underwent neurosurgical intervention
The patients with hemophilia B were initially given recombinant FIX concentrates at a dose of 120 IU/kg. In addition, the patients with inhibitors were given activated prothrombin complex concentrates at a dose of 100 IU/kg at 12-h intervals. We adjusted the dose and treatment interval depending on the coagulation factor levels and the clinical course. All patients were recommended to take prophylaxis for the ICH; most of them (8 of 10) received it over a minimum period of 6 months.
Outcomes and Sequelae
Three patients had intracerebral hemorrhage requiring neurosurgical intervention. Because they had decreased mentality and signs of increased intracranial pressure at the time of arrival in the emergency department, they underwent emergency surgery. Two of these three patients who presented with an initial Glasgow coma scale (GCS) score of 3 showed poor outcomes and finally died, although emergency management with clotting factor concentrates and surgical procedures were performed. One patient with a subcortical hemorrhage underwent ICH evacuation following decompressive craniectomy and burr-hole trephination with catheter insertion for aspiration of the hemorrhage. The same procedure was performed for the other patient, who had basal ganglia hemorrhage with intraventricular hemorrhage.
As shown in Fig. 1b, c, computed tomography (CT) showed several risk factors for ICH in both patients. One patient had hypertension, a history of ICH, and high-titer inhibitor, and the other was positive for HCV and HIV and had a low platelet count (36,000 × 106/L), possibly due to HIV infection, at the time of ICH onset. On admission, approximately 7–8 h after the onset of symptoms, both patients received clotting factor concentrates.
One adult patient and one pediatric patient had repeated episodes. The adult patient required emergent neurosurgical intervention and finally expired. The pediatric patient achieved improvement in hemorrhage and related symptoms after treatment with clotting factor concentrates.
One young patient with a traumatic intracerebral hemorrhage underwent ICH evacuation followed by treatment with continuous infusion of FVIII concentrates. This patient had an initial GCS score of 9 and eventually achieved symptom resolution, although there was a residual presence of neurological sequelae on CT scans (Fig. 1d). The mortality rate in our series was 20.0 % (2/10). The clinical courses and prognoses are presented in Table 3.
Table 3.
Clinical course and prognosis
| Patient No. | Type of coagulation factor concentrates | Duration of admission (days) | Neurosurgical intervention | Prognosis |
|---|---|---|---|---|
| 1 | Advate®a | 16 | No | No sequelae |
| 2 | Advate® | 14 | No | Antibodies developed |
| 3 | Feiba®b | 10 | No | No sequelae |
| 4 | Feiba® | 12 | No | No sequelae |
| 5 | Advate® | 10 | No | Blurred vision, but recovered |
| 6 | Feiba® | 13 | No | No sequelae |
| 7 | Greenmono®c | 30 | No | Dysarthria, but recovered |
| 8 | Feiba® | 2 | Yes | Expired |
| 9 | Greenmono® | 15 | No | No sequelae |
| 10 | Benefix®d | 12 | No | No sequelae |
| 11 | Benefix® | 2 | Yes | Expired |
| 12 | Greenmono® | 37 | Yes | Seizure, Left side weakness, Antibodies developed |
aRecombinant FVIII concentrates, Baxter Healthcare, Neuchâtel, Switzerland
bActivated prothrombin complex concentrates, Baxter Healthcare, Vienna, Austria
cPlasma-derived FVIII concentrates, Green Cross, Chungbuk, Korea
dRecombinant FIX concentrates, Pfizer, Madrid, Spain
After presenting with ICH, two patients developed inhibitors (antibodies to FVIII). In one of these patients, the low-titer inhibitor (<5 Bethesda Unit (BU)) disappeared spontaneously at postoperative month 5. The other patient had high-titer inhibitor (≥5 BU) and received immune tolerance induction (ITI) for 28 months to remove persistent inhibitors to FVIII. Eradication of inhibitor by ITI was achieved.
Discussion
The most frequently encountered life-threatening event among patients with hemophilia is ICH. Supporting this finding is a report comparing mortality due to ICH between hemophilia populations; this report was based on the United Kingdom Hemophilia Centre Doctor’s Organization database, which has been established over the past two decades [8]. After treatment with FVIII and FIX concentrates became available in the late 1950s and 1960s, there was a dramatic decrease in mortality in patients with hemophilia A or B presenting with ICH from 70 % [9] to 20–30 % [10]. However, according to recent studies, the mortality rate of hemophiliacs with ICH is still about 20 % [3, 11], and there has been no corresponding decrease in the mortality of patients with hemophilia presenting with ICH.
However, there is a paucity of epidemiological data about ICH in patients with hemophilia. The incidence of ICH has been found to range from 2.2 to 7.5 % in patients with hemophilia [5, 6, 12–14]. In one study, the cumulative hazard of ICH for the entire cohort over the comprehensive follow-up period was 26.7 per 1000 patients, and the annual rate of ICH was 2.50 events per 1000 patients (95 % CI = 1.90–3.31) [11]. Patients with hemophilia are 20 to 50 times more likely to develop ICH than the general population, with a reported prevalence of 2.7–12 % and an incidence of 290–748/105 patient-years [3–6].
Trauma is the most common event that precipitates ICH in patients with hemophilia [1]. Our study also showed that the rate of ICH following trauma was 70.0 % (7/10). All cases of ICH occurred after trauma in pediatric patients. Other known predisposing factors for ICH include HIV infection accompanied by immune suppression, the presence of inhibitors, and an age of <5 or >51 years [15]. In the current study, the presence of inhibitors (40.0 %, 4/10) and HCV infection (50.0 %, 5/10) were more prevalent.
Two patients with several predisposing factors died, both of whom had intracerebral hemorrhage. We assume that their death occurred partly as a result of delayed treatment with coagulation factor concentrates. This suggests that mortality in patients with hemophilia presenting with ICH is subject to the site of hemorrhage, particularly intracerebral hemorrhage, as well as delayed treatment with coagulation factor concentrates. The mortality rate was 20.0 % (2/10) in our clinical series of patients. This is consistent with previous reports showing variability in mortality ranging from 0 to 25 % in patients with hemophilia presenting with ICH [5, 6, 11, 16]. Two of our three patients who underwent neurosurgical intervention died, but this was likely the result of the ICH characteristics and patient conditions, not the neurosurgical intervention. In a large trial of intracerebral hemorrhage in patients without hemophilia, patients with a GCS score of ≥9 had a trend toward more favorable outcomes with early surgery [17]. When deciding on neurosurgical intervention versus medical treatment, various factors should be considered, including the patient’s condition. In the present series, 3 (37.5 %, 3/8) developed neurological complications, including transient neurological abnormalities (blurred vision and dysarthria) and two patients achieved complete recovery from symptoms. Previous studies have shown variability in the incidence of long-term sequelae, ranging from 9.4 to 22.7 % [5, 6, 11, 16]. This is also consistent with our results. Inhibitors to FVIII developed in young patients who were diagnosed with hemophilia following the ICH event. The patients’ first clotting factor exposure at a high dose was thought to have contributed to inhibitor development. The only proven strategy for eradicating inhibitors and achieving antigen-specific tolerance to clotting factors is ITI [18, 19]. ITI involves the repeated administration of FVIII concentrates over a period of weeks to years, with the goal of inducing antigen-specific tolerance [20].
Patients with severe hemophilia are recommended to take prophylaxis through the routine administration of factor concentrates. This treatment is reportedly effective in preventing joint hemarthrosis [21]. One cohort study examined whether prophylaxis is effective in lowering the risk of ICH and found that it was effective in significantly lowering the risk of ICH in HIV-negative patients with severe hemophilia or those with inhibitors [7]. In that study, the mortality was 19.6 % and its burden was shifted to adults. Thus, the increased use of prophylaxis in the pediatric age group might be associated with decreased mortality due to ICH in children. Three of our patients survived after prophylaxis.
A long-term prophylaxis regimen has been advocated in children and adults with severe ICH [1]. This is based on the findings that neovascularization in the brain area, where a hemorrhagic stroke may frequently occur, may lead to fragility of the vessels that are vulnerable to hemorrhagic events with even minimal insult. Patients presenting with ICH are therefore recommended to take secondary prophylaxis for a minimum period of 6 months [22].
This study has some limitations that should be considered. The sample size was small, so it is difficult to compare between different age groups or whether the patients had risk factors or not.
In summary, central nervous system hemorrhage is one of the most common causes of death in patients with hemophilia. Prompt and intensive treatment with coagulation factor concentrates may significantly lower the mortality in patients with hemophilia presenting with ICH. It is also important to decide on neurosurgical intervention and perform intensive treatments, such as continuous infusion of coagulation factor concentrates, through a multidisciplinary approach. Adult patients with hemophilia and known predisposing factors for ICH are recommended to take prophylaxis, which is essential for preventing recurrence and lowering mortality. Clinicians should pay special attention to the possible development of inhibitors after intensive treatment in pediatric patients. Further studies are needed to examine methods for administering coagulation factor concentrates, lower the rate of the recurrence of ICH, and determine whether it is necessary to perform neurosurgical interventions.
Compliance with Ethical Standards
Disclosures
None declared.
References
- 1.Lee CA, Berntorp EE, Hoots WK, editors. Textbook of hemophilia. 2. Blackwell: Blackwell Publishing; 2010. pp. 394–396. [Google Scholar]
- 2.Srivastava A, Brewer AK, Mauser-Bunschoten EP, Key NS, Kitchen S, Llinas A, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):1–47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 3.Stieltjes N, Calvez T, Demiguel V, Torchet MF, Briquel ME, Fressinaud E, et al. Intracranial haemorrhages in French haemophilia patients (1991–2001): clinical presentation, management and prognosis factors for death. Haemophilia. 2005;11(5):452–458. doi: 10.1111/j.1365-2516.2005.01090.x. [DOI] [PubMed] [Google Scholar]
- 4.Ljung RC. Intracranial haemorrhage in haemophilia A and B. Br J Haematol. 2008;140(4):378–384. doi: 10.1111/j.1365-2141.2007.06949.x. [DOI] [PubMed] [Google Scholar]
- 5.Antunes SV, Vicari P, Cavalheiro S, Bordin JO. Intracranial haemorrhage among a population of haemophilic patients in Brazil. Haemophilia. 2003;9(5):573–577. doi: 10.1046/j.1365-2516.2003.00789.x. [DOI] [PubMed] [Google Scholar]
- 6.Gruskin KD, Schutzman SA. Head trauma in children younger than 2 years: are there predictors for complications? Arch Pediatr Adolesc Med. 1999;153(1):15–20. doi: 10.1001/archpedi.153.1.15. [DOI] [PubMed] [Google Scholar]
- 7.Witmer C, Presley R, Kulkarni R, Soucie JM, Manno CS, Raffini L. Associations between intracranial haemorrhage and prescribed prophylaxis in a large cohort of haemophilia patients in the United States. Br J Haematol. 2011;152(2):211–216. doi: 10.1111/j.1365-2141.2010.08469.x. [DOI] [PubMed] [Google Scholar]
- 8.Darby SC, Kan SW, Spooner RJ, Giangrande PL, Hill FG, Hay CR, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110(3):815–825. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein A. Intracranial bleeding in hemophilia. Arch Neurol. 1960;3(2):141–157. doi: 10.1001/archneur.1960.00450020021005. [DOI] [PubMed] [Google Scholar]
- 10.de Tezanos Pinto M, Fernandez J, Perez Bianco PR. Update of 156 episodes of central nervous system bleeding in hemophiliacs. Haemostasis. 1992;22(5):259–267. doi: 10.1159/000216333. [DOI] [PubMed] [Google Scholar]
- 11.Zanon E, Iorio A, Rocino A, Artoni A, Santro R, Tagliaferri A, et al. Intracranial haemorrhage in the Italian population of haemophilia patients with and without inhibitors. Haemophilia. 2012;18(1):39–45. doi: 10.1111/j.1365-2516.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 12.Klinge J, Auberger K, Auerswald G, Brackmann HH, Mauz-Körholz C, Kreuz W, et al. Prevalence and outcome of intracranial haemorrhage in haemophiliacs—a survey of the paediatric group of the German society of thrombosis and haemostasis (GTH) Eur J Pediatr. 1999;158(3):S162–S165. doi: 10.1007/PL00014346. [DOI] [PubMed] [Google Scholar]
- 13.Tarantino MD, Gupta SL, Brusky RM. The incidence and outcome of intracranial haemorrhage in newborns with haemophilia: analysis of the nationwide inpatient sample database. Haemophilia. 2007;13(4):380–382. doi: 10.1111/j.1365-2516.2007.01492.x. [DOI] [PubMed] [Google Scholar]
- 14.Nelson MD, Jr, Maeder MA, Usner D, Mitchell WG, Fenstermacher MJ, Wilson DA, et al. Prevalence and incidence of intracranial haemorrhage in a population of children with haemophilia. Haemophilia. 1999;5(5):306–312. doi: 10.1046/j.1365-2516.1999.00338.x. [DOI] [PubMed] [Google Scholar]
- 15.Nagel K, Pai MK, Paes BA, Chan AK. Diagnosis and treatment of intracranial hemorrhage in children with hemophilia. Blood Coagul Fibrinolysis. 2013;24(1):23–27. doi: 10.1097/MBC.0b013e32835975d6. [DOI] [PubMed] [Google Scholar]
- 16.Patiroglu T, Ozdemir MA, Unal E, Torun YA, Coskun A, Menku A, et al. Intracranial hemorrhage in children with congenital factor deficiencies. Childs Nerv Syst. 2011;27(11):1963–1966. doi: 10.1007/s00381-011-1519-5. [DOI] [PubMed] [Google Scholar]
- 17.Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patient with spontaneous supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387–397. doi: 10.1016/S0140-6736(05)70233-6. [DOI] [PubMed] [Google Scholar]
- 18.Dimichele DM, Hoots WK, Pipe SW, Rivard GE, Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Hemophilia. 2007;13(s1):1–22. doi: 10.1111/j.1365-2516.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- 19.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12(1):991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 20.Hay CR, Baglin TP, Collins PW, Hill FG, Keeling DM. The diagnosis and management of factor VIII and IX inhibitors: a guideline from the UK Haemophilia Centre Doctors’ Organization (UKHCDO) Br J Haematol. 2000;111(1):78–90. doi: 10.1046/j.1365-2141.2000.02327.x. [DOI] [PubMed] [Google Scholar]
- 21.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 22.Bianco RP, Neme D, Candela M, de Tezanos pinto M. Secondary prophylaxis with rFVIIa in hemophilia and inhibitors:recommendations from an experts committee from argentina. Medicina. 2010;70(3):209–214. [PubMed] [Google Scholar]


