Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired hemolytic anemia with highly variable clinical symptoms making the diagnosis and prediction of its outcome difficult. It is caused by the expansion of a hematopoietic progenitor cell that has acquired a mutation in the X-linked phosphatidylinositol glycan class A (PIGA) gene that results in deficiency of the glycosylphosphatidylinositol anchor structure responsible for fixing a wide spectrum of proteins particularly CD55 and CD59. The clinical features of this disease arise as a result of complement-mediated hemolysis in unprotected red cells, leukocytes, and platelets as well as the release of free hemoglobin. Patients may present with a variety of clinical manifestations, such as anemia, thrombosis, kidney disease, smooth muscle dystonias, abdominal pain, dyspnea, and extreme fatigue. PNH is an outstanding example of how an increased understanding of pathophysiology may directly improve clinical symptoms and treat disease-associated complications when we inhibit the terminal complement cascade. This topic will discuss PNH overview to assist specialists looking after PNH patients.
Keywords: Complement, Paroxysmal, Phosphatidylinositol
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH), previously Marchiafava–Micheli syndrome, is a rare acquired chronic, debilitating disease, affecting both sexes at any age that is characterized by intravascular hemolysis, thrombosis, impaired bone marrow (BM) function, and a 3–5 % risk of developing leukemia. It is closely related to aplastic anemia (AA) or myelodysplastic syndrome (MDS) [1].Until recently, supportive and symptomatic treatment were the only option for these patients. However, with better understanding of the pathophysiology, the management of these patients has been changed to a specific targeted treatment of the underlying disease process.
Pathphysiology
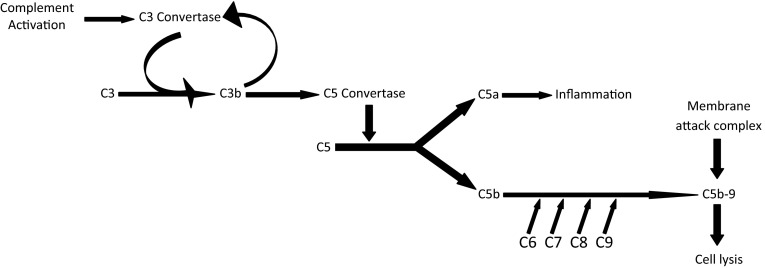
All cells have proteins attached to their membranes, acting as; either a mode of communication with the surrounding extracellular milieu or protection from destruction by complement system. These proteins are attached to the cell membrane by glycolipids such as glycosyl phosphatidylinositol (GPI). The main proteins are; decay-accelerating factor (DAF/CD55), homologous restriction factor (HRF), C8 binding protein, membrane inhibitor of reactive lysis (MIRL), and CD59, which disrupt formation of C3-convertase, and prevents C9 from binding to the cell [2]. PNH occurs as a result of the inability to synthesize GPI anchor that binds these proteins to cell membranes [3] (Figs. 1, 2).
Fig. 1.

Pathphysiology of PNH. †Phosphatidylinositol glycan class A. ‡Glycosyl phosphatidylinositol. Adapted from Boccuni et al. [3]
Fig. 2.
Pathphysiology of hemolysis. Adapted from Reis et al. [8]
The most common defective enzyme in PNH is phosphatidylinositol glycan class A (PIGA), located on the X chromosome, so only one mutation is required in either males or females to prevent the expression of GPI-linked proteins [4].
When this mutation occurs in the early hematopoietic progenitor cells in the BM, all of the produced cells will also have the defect, ranging from a small percentage in some cases to more than 90 % in others. This wide range reflects symptoms variations. As the surface proteins were missing in all blood component membrane; red blood cells (RBCs), white blood cell (WBCs) and platelet, so the previous classification of PNH as purely an acquired hemolytic anemia isn’t valid [5].
Pathogenesis
As a result of defect in protective proteins, uncontrolled amplification of the complement system occur leading to hemolysis in unprotected RBCs, with subsequent release of free hemoglobin in the circulation which is cleared from circulation through binding with haptoglobin.
After the saturating of haptoglobin, free forms of hemoglobin circulates and binds irreversibly with nitric oxide (NO) resulting in rapid consumption of it with clinical sequelae of NO depletion. As NO plays a critical role in vasomotor tone, so its depletion explain the esophageal spasms, erectile dysfunction, abdominal pain, bloating, back pain, headaches, and pulmonary hypertension which in turn may precipitate heart failure. Unlike erythrocytes, WBCs become activated upon complement-induced damage with production and release of cytokines, resulting in inflammation and fatigue, which is not managed with transfusion because it is not just anemia [6]. The hemostatic balance is maintained by coagulation and fibrinolysis, and is influenced by factors derived from the vessel wall and blood cells. Several mechanisms have been suggested to determine the direction of the balance towards a prothrombotic state in PNH. These include NO depletion, and the release of both free hemoglobin and the procoagulant micro particles into the circulation with platelet activation. Finally, deficiency of GPI-anchored fibrinolytic factors may further disturb the hemostatic balance [7, 8].
Signs and Symptoms
Due to non specific symptoms of PNH, the correct diagnosis may be delayed for months or years. Some of these symptoms are related to hemolysis e.g. red discoloration of urine due to the presence of hemoglobin and hemosidrin, which is more apparent at morning due to concentration of urine overnight. The term “nocturnal” refers to the belief that hemolysis is triggered by acidosis during sleep that activates complement to hemolyze an unprotected cell membrane. However, this observation was later disproved, because hemolysis has been shown to occur throughout the day and is not actually paroxysmal. Others symptoms related to NO depletions e.g. abdominal pain, dysphagia and erectile dysfunction. Attacks of hemoglobinuria may be precipitated by infections, alcohol, exercise, stress or certain medications [9].
Up to 50 % of patients with PNH developed thrombosis almost exclusively in veins, and are the main cause of severe complications and death in PNH. All veins, especially those in the abdomen are susceptible. However, hepatic vein thrombosis (Budd-Chiari syndrome) and sagittal vein thrombosis are the most common sites of thrombosis [10].
Diagnosis
Blood tests show the features of hemolysis; raised bilirubin, reticulocytes, and lactate dehyrogenase (LDH) and low hemoglobin. Gross hemoglobinuria is common during crises, and the urine may contain hemosiderin. The determinations of the absence of specific RBC or WBC membrane proteins (CD59 and CD55) by flow cytometry are the standard for diagnosis.
It is important that at least two GPI-linked antigens are studied to exclude rare congenital deficiencies of single antigens (CD55 and CD59) and polymorphism with individual antigens (CD16), which render them undetectable by some monoclonal antibody clones.
Standard and high-sensitivity flow cytometric procedures for detecting PNH cells are now available. This test can detect 1 % or more PNH cells, (most laboratories report only 10 % or more as a positive result). High-sensitivity analysis (in which as little as 0.01 % PNH cells can be detected) may be helpful in special situations as (AA patients, who may eventually develop PNH, and possibly in those with hypoplastic MDS, to predict responses to immunosuppressive therapy) [11]. Fluorescein proaerolysin (FLAER) is a more accurate test to diagnosis PNH. It binds selectively to the GPI anchor and is more accurate in demonstrating a deficit than simply for CD59 or CD55.
Phenotypic mosaicism is a characteristic feature of PNH. Typically, normal and PNH blood cells coexist in patients with PNH, and the percentage of myeloid cells deficient in GPI-linked proteins is used to indicate the size of the PNH clone. PNH -RBCs, due to their increased sensitivity to complement, have a reduced half-life in circulation. The percentage of PNH RBCs therefore usually does not reflect the size of the PNH clone in the BM. However, the flow cytometric analysis of red blood cells is used to determine the degree of GPI-anchor deficiency, and can identify small PNH clones (<5.0 %). PNH type III cells are defined as cells that completely lack the expression of all GPI-linked proteins, whereas PNH type II cells show some residual expression, and PNH type I cells express the proteins at normal levels [12].
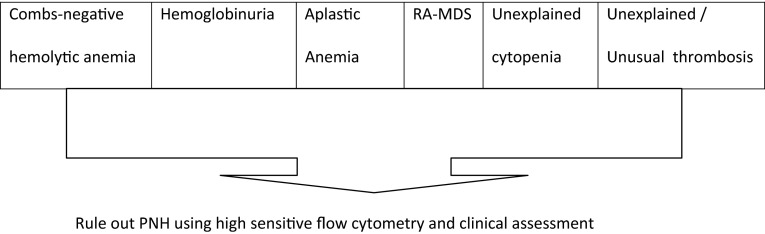
As the presentation is usually more subtle and can be missed or mistaken for some other conditions, especially when PNH when they occur concomitantly with anemia or cytopenias. So, the clinicians must focus on recognizing specific high-risk groups for testing. Guidelines from the International Clinical Cytometry Society (ICCS) and the International PNH Interest Group provide recommendations for identifying high-risk patients [13] (Fig. 3).
Fig. 3.
Guidelines from the International Clinical Cytometry Society and the International PNH Interest Group provide recommendations for identifying high risk patients. Adapted from Borowitz et al. [13]
The guidelines recommend routine follow-up testing with flow cytometry, repeated every 3 to 6 months initially and then annually thereafter. Repeated testing is recommended to detect clone evolution. Generally, the clone size either remains stable (in approximately 20–50 % of patients over a 5-year period) or increases (in approximately 20 % of patients). In approximately 10–15 % of cases, however, the clone decreases in size or even disappears with time. This routine follow-up testing is recommended regardless of the therapeutic strategy administered, either under observation or under specific treatment [13, 14].
BM examination is not necessary, but if can differentiate between classic and other types of PNH. In addition, it will identify an erythroid and hyperplastic BM during the hemolytic phase or a hypoplastic BM in the aplastic phase. Imaging studies may be indicated in patients with thrombosis [15].
Historically, when PNH was suspected, the sugar-water test was usually the first test done; it relies on enhanced hemolysis of C3-dependent system in isotonic solutions of low ionic strength, although, it is simple, but it is nonspecific; positive results require confirmation by further testing. An alternative is the acid hemolysis test (Ham test); hemolysis usually occurs if blood is acidified with HCL, incubated for 1 h, and centrifuged [16].
Classification
PNH was classified according to presentation, clinical manifestations, and natural history [17]. It can present as any of the following:
Classic PNH, evidence of PNH in the absence of another BM disorders.
PNH in the setting of another specified BM disorders (e.g., PNH/AA or PNH/refractory anemia -MDS).
Subclinical PNH (PNH-sc), abnormalities on flow cytometry without signs of hemolysis.
Due to the possibility of progression from PNH-sc to clinical manifested forms which would require appropriate treatment, we need to ensure monitoring of patients’ PNH clone size.
Clinical Considerations
PNH testing should be done once or repeatedly (at least every 6 mo or annually) in certain situations. During physical examination, there are some points to be considered; fever suggests infection, pallor suggests anemia. For more details, check Table 1 [18].
Table 1.
Diagnostic and physical examination considerations
| PNH tested once | PNH tested repeateda |
|---|---|
| Evidence of hemoglobinuria | All patients with known PNH, to monitor for clone changes |
| Unexplained hemolysis (increased lactate dehydrogenase [LDH] level, absent haptoglobin) | All patients with aplastic anemia (AA) |
| Abdominal or cerebral vein thrombosis | Refractory anemia- myelodysplastic syndrome (RA-MDS) Unexplained cytopnea |
| Clinical finding | Suggestion |
|---|---|
| Hepatomegaly and ascites | Budd-Chiari syndrome |
| Spleenomegally | Splenic vein thrombosis |
| Absent bowel sounds | Bowel necrosis |
| Papilledema | Cerebral vein thrombosis |
| Skin nodules | Dermal vein thrombosis |
Adapted from American Society of Hematology Education Book 2008 [18]
aInitially once every 6 months; then annually
Course and Outcome
PNH is insidious and has a chronic course, with a median survival of about 10.3 years. Morbidity depends on the variable expressions of hemolysis, BM failure, and thrombophilia that define the severity and clinical course of the disease. Death can result from complications such as blood clot formation (thrombosis) or bleeding [19].
Treatment
The appropriate treatment for PNH depends on the severity of symptoms, for asymptomatic patients or those with mild symptoms, active surveillance is appropriate. Over time, the disease may progress and more treatment may be indicated.
According to our understanding of PNH, the treatment is divided into; either supportive care or definite treatment.
Supportive Care
Anemia
In PNH, three types of anemia may be encountered: intravascular hemolysis, inadequate erythropoiesis, and superimposed iron deficiency. Supplemental iron and folic acid are provided, and in severe cases transfusion with leuko-depleted packed RBCs may be needed. Stimulation of erythropoiesis using recombinant erythropoietin therapy has been successful in patients with a moderate decrease in RBC production [20].
Thrombosis
Patients with acute thrombosis are managed with anticoagulation similarly to patients without PNH, then maintenance therapy with the use of an oral anticoagulant.
Sometimes, heparin can exacerbate the thrombotic problem, possibly by activating complement, which can be prevented using inhibitors of the cyclooxygenase system, such as aspirin and ibuprofen. Unfortunately, some patients will continue to develop blood clots despite aggressive anti-coagulation agents. Primary prophylaxis with warfarin may be used, but it remains controversial, based on a lack of high-quality evidence, with the increased risk of bleeding in patients with concomitant thrombocytopenia. Medications that increase the risk for thrombosis, such as oral contraceptive pills, should be avoided [21].
Corticosteroids
Steroids may slow the breakdown of RBCs by suppressing the immune system. Because of corticosteroids will not cure the disease, numerous side-effects and in a minority of patients it can be useful; so should be discontinued if they do not show significant benefit in 4–6 weeks [22].
Anti-complement Therapy
Most of the complications in PNH are attributable to the absence of CD55 and CD59, results in complement-mediated hemolysis. Anti-complement therapy is the most effective way to treat the symptoms.
In 2007, eculizumab, an anti-complement antibody targeting the CD5 complement, was approved by the Food and Drug Administration (FDA) [23]. Treatment with this drug appears to change the natural history of the disease, reduces intravascular hemolysis, decreases or eliminates the need for blood transfusions, markedly reduces the risk for thrombosis, and improves quality of life.
Importantly, eculizumab does not alter the underlying defect of the disease. Thus, it is not curative; so treatment should be continued life-long or until spontaneous remission, which occurred only in a minority of patients. The standard schedule in adults is 600 mg intravenously once per week for the first 4 weeks, followed by 900 mg intravenously 1 week later, followed by 900 mg intravenously once every 2 weeks thereafter indefinitely. The maintenance dose is fixed and is not dependent on weight or body surface area. Breakthrough hemolysis in some is evidenced near end of treatment cycle due to difference in rate of drug metabolism and also can occur in small minority (10 %) of patients due to an inadequate dosing schedule. The eculizumab level must remain above 35 μg/mL. The recommended dose adjustment for those patients is 1200 mg every 2 weeks or be shortened to 12–13 days [24].
Withdrawal hemolysis can occur by stopping therapy for any reason, as accumulation of PNH RBC increases over time by protecting type II and III PNH cells from destruction due to therapy, which can potentially trigger a massive hemolysis.
The Efficacy of Eculizumab
Hillmen et al. evaluated the long-term safety and efficacy of eculizumab in 195 patients with PNH over 66 months, the Phase III TRIUMPH (Transfusion Reduction Efficacy and Safety Clinical Investigation, a Randomized, Multicenter, Double-Blind, Placebo-Controlled, Using Eculizumab in PNH) study or the Phase III SHEPHERD (Safety in Hemolytic PNH Patients Treated With Eculizumab: A Multi-Center Open-Label Research Design) study were eligible to participate.
Efficacy assessments were performed at least every 2 weeks from the time of initiation of eculizumab therapy. Efficacy endpoints included patient survival, degree of hemolysis, thrombotic events (TEs), mean change from baseline in hemoglobin and the number of units of transfused packed RBCs administered.
All patients showed a reduction in LDH levels, which was sustained over the course of treatment (median reduction of 86.9 % at 36 months). Incidence of TEs decreased by 81.8 %, with 96.4 % of patients remaining free of TEs with improvement in renal function; 93.1 % of patients exhibited improvement or stabilization in chronic kidney disease score at 36 months. Transfusion independence increased by 90.0 % from baseline, with the number of RBCs units transfused decreasing by 54.7 %. Eculizumab was well tolerated, with no evidence of cumulative toxicity.
Researchers concluded that long-term treatment with eculizumab resulted in sustained improvement in patient’s outcome by rapidly reducing hemolysis and significantly reducing the frequency of severe and life-threatening morbidities, improving patient’s survival [25], which is corresponding to the study done by Kelly et al. [19].
Infection Prophylaxis
Eculizumab increases the risk of life-threatening Neisserial infections, including Neisseria meningitidis. The risk in patients with terminal complement deficiencies is approximately 0.5 % per year, so all patients should be vaccinated with the tetravalent vaccine (meningococcal A/C/Y/W-135 vaccine) at least 2 weeks prior to receiving the first dose. For patients who must receive eculizumab emergently, antibiotics may be given to bridge the time needed for vaccination to become effective [26].
Anti-complement Therapies in Development
Nafamostat is a synthetic serine protease inhibitor of C1s and other proteases currently is only approved in a limited market (Japan and Korea).Other small molecule inhibitors of C1s have also been characterized; however, to our knowledge, the role of these inhibitors in transfusion-related indications has not been reported [27].
C1 esterase inhibitor—C1 esterase inhibitor (C1INH) is a concentrate derived from human plasma that is used to treat C1 esterase deficiency, responsible for hereditary or acquired angioedema. It inhibits the early stages of complement activation and associated inflammatory proteases [28].
Compstatin, a cyclical peptide that binds C3 preventing C3 activation of all three complement pathways. Analogs of compstatin are in various stages of pre-clinical and clinical development for indications such as age-related macular degeneration (AMD), hemolytic uraemic syndrome (HUS), dense deposit disease (DDD), transplantation, and hemodialysis. Moreover, compstatin analogs have been demonstrated to inhibit hemolysis of PNH RBCs in an in vitro system [29].
Definite Treatment
Hematopoietic Stem Cell Transplantation (HSCT)
Allogeneic bone marrow transplantation (ABMT) has been the mainstay of curative therapy for PNH. With the development of eculizumab for PNH, the indications for ABMT in this setting have changed. It should be offered to patients with severe disease (i.e., patients with life threatening thrombosis or dangerously low blood counts; AA/PNH–MDS/PNH) because high transplant-related mortality, especially when using unrelated or mismatched donors (15–20 % chance of death).Moreover, ABMT is a reasonable option after eculizumab therapy failure and in life-threatening cytopenias [30].
Finally, we must know that eculizumab is not available in all countries, in this condition BMT is the only option.
Special Circumstances
PNH in Pregnancy
During pregnancy, the risk of complications for both mother and fetus are increased. Worsening cytopenias may complicate the management of pregnancy but the main concern is the occurrence of thrombosis, which is responsible for the high mortality rates in pregnant PNH patients. A pregnant patient with PNH should be managed by a hematologist and an obstetrician specializing in high-risk pregnancy. The following considerations must be addressed: iron and folate supplementation, transfusions support and thromboembolic risk reduction, usually by low molecular weight heparin. The prophylactic anticoagulation is often initiated during the third trimester and continues it for 6–12 weeks postpartum. There is accumulating evidence of improved maternal outcomes in women with PNH who received eculizumab during pregnancy, without additional evidence of increased fetal risks. Based on these data, Usuki et al. proposed a protocol for the management of a pregnancy with PNH, as shown in Fig. 4 [31, 32].
Fig. 4.

Proposed protocol for the management of a pregnancy with PNH. Adapted from Usuki et al. [32]
Pediatric PNH
PNH is a rare disease in children with a high rate of BM failure along with a low rate of hemoglobinuria at presentation, and a high rate of thrombosis. Delay in diagnosis is common, so we recommend appropriate PNH testing in all patients with AA, MDS, unexplained Coombs-negative hemolysis, or thrombosis. Based on the lack of spontaneous remissions and poor long-term survival (80 % at 5 years, 60 % at 10 years, and only 28 % at 20 years), HSCT is the recommended treatment of childhood PNH. However, the high prevalence of hemolysis and thrombosis should warrant the consideration of early treatment with anti-complement therapy [33, 34].
Dysphagia, Male Impotence, Abdominal Pain
Especially during hemolytic exacerbations,and due to NO depletion with consequently esophageal spasm, many patients with PNH are troubled by dysphagia, odynophagia, and impotance. Sildenafil citrate and pharmacologically related compounds have shown efficacy in the treatment of hypercontractile motility disorders of the esophagus, and erectile dysfunction. Agents such as oral or dermal nitroglycerine that supply NO pharmacologically have also shown efficacy [35].
Some patients with PNH are debilitated by recurrent episodes of colicky abdominal pain. The etiology of the abdominal pain is largely speculative, but thrombosis or vascular spasm of mesenteric vessels appears to play a role in some cases. Vigorous hydration and pain control are the mainstays of management, but mesenteric vein thrombosis can result in intestinal infarction necessitating surgical intervention. Still to be determined are the roles of anticoagulation, complement inhibition, and NO supplementation in the management of abdominal pain of PNH [36].
Summary and Recommendations
Ultimately, the goal of treatment for these patients is to manage the chronic complement activity and, as a consequence, ameliorate symptoms, improve quality of life, and prevent the severe disease-related complications. Optimal disease control requires early intervention and treatment addressing the underlying hemolysis.
A major barrier to the use of eculizumab is the cost, which has been estimated to be in the range of 400,000 US dollars (approximately 300,000 Euros) per year. Since this agent has no effect on the underlying cellular abnormality in PNH, continuous treatment is required to suppress intravascular hemolysis and/or thrombosis; this further increases the cost.
All patients with PNH should have baseline testing that includes a CBC with differential, reticulocyte count, LDH, biochemical profile, flow cytometry, iron studies.
The approach to therapy depends on presence or absence of symptoms; asymptomatic patients who have a small clone and do not have clinically significant hemolysis are generally monitored every 6–12 months. For patients with symptomatic PNH, the approach to therapy depends on the severity of symptoms and the degree of hemolysis.
Figure 5 shows suggested algorithm for PNH treatment.
Fig. 5.
Algorithm for the therapy of patients with paroxysmal nocturnal hemoglobinuria. †Supportive treatment is found through the entire course of the disease, when it is needed (RBC) transfusions for severe anemia; supplemental iron for iron deficiency, supplemental folic acid, appropriate analgesia for pain paroxysms and anticoagulation for acute and chronic venous thrombosis. ‡Patients with life threatening thrombosis or dangerously low blood counts, severe aplastic anemia and some high-risk myelodysplastic syndromes. §All patients should be vaccinated against N. meningitidis at least 2 weeks prior to the first dose of eculizumab
Continued investigation of new approaches to therapy aimed at obviating the extravascular hemolysis that limits eculizumab efficacy in some patients is warranted.
New drugs that prove effective in PNH will be useful for treating other complement-mediated disorders too, so efforts to develop these new therapies will have benefits beyond PNH.
Compliance with Ethical Standards
Conflict of interest
The authors certify that is no potential or actual conflict of interest related to this research.
References
- 1.Kelly RJ, Hill A, Arnold LM, Khursigara G, Kanagasundaram NS, Hillmen P. Eculizumab for patients with paroxysmal nocturnal hemoglobinuria is effective during the maintenance of hemodialysis for end stage renal failure. Leuk Res. 2011;35:560–562. doi: 10.1016/j.leukres.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190:3831–3838. doi: 10.4049/jimmunol.1203487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boccuni P, Vecchio DL, Di Noto R, Rotoli B. Glycosyl phosphatidylinositol (GPI)-anchored molecules and the pathogenesis of paroxysmal nocturnal hemoglobinuria. Crit Rev Oncol/Hematol. 2000;33:25–43. doi: 10.1016/S1040-8428(99)00052-9. [DOI] [PubMed] [Google Scholar]
- 4.Krawitz PM, Höchsmann B, Murakami Y, Teubner B, Krüger U, Klopocki E, Neitzel H, Hoellein A, Schneider C, Parkhomchuk D, Hecht J, Robinson PN, Mundlos S, Kinoshita T, Schrezenmeier H. A case of paroxysmal nocturnal hemoglobinuria caused by a germline mutation and a somatic mutation in PIGT. Blood. 2013;122:1312–1315. doi: 10.1182/blood-2013-01-481499. [DOI] [PubMed] [Google Scholar]
- 5.Luzzatto Lucio. PNH from mutations of another PIG gene. Blood. 2013;122:1099–1100. doi: 10.1182/blood-2013-06-508556. [DOI] [PubMed] [Google Scholar]
- 6.Parker Charles. Paroxysmal nocturnal hemoglobinuria. Curr Opin Hematol. 2012;19:141–148. doi: 10.1097/MOH.0b013e328351c348. [DOI] [PubMed] [Google Scholar]
- 7.Luzzatto L, Gianfaldoni G, Notaro R. Management of paroxysmal nocturnal haemoglobinuria: a personal view. Br J Haematol. 2011;153:709–720. doi: 10.1111/j.1365-2141.2011.08690.x. [DOI] [PubMed] [Google Scholar]
- 8.Reis E, De Angelis RA, Chena H, Resuellob RG, Ricklina D, John D. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology. 2015;220:476–482. doi: 10.1016/j.imbio.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devalet B, Mullier F, Chatelain B, Dogné JM, Chatelain C. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015 doi: 10.1111/ejh.12543. [DOI] [PubMed] [Google Scholar]
- 10.Weitz I, Meyers G, Lamy T, Cahn JY, Uranga MT, García Vela JA, Sanz MA, Severino B, Kelly RJ, Hillmen P, Hill A. Cross-sectional validation study of patient-reported outcomes in patients with paroxysmal nocturnal haemoglobinuria. Intern Med J. 2013;43:298–307. doi: 10.1111/j.1445-5994.2012.02924.x. [DOI] [PubMed] [Google Scholar]
- 11.Wanachiwanawin W, Siripanyaphinyo U, Piyawattanasakul N, Kinoshita T. A cohort study of the nature of paroxysmal nocturnal hemoglobinuria clones and PIG-A mutations in patients with aplastic anemia. Eur J Haematol. 2006;76:502–509. doi: 10.1111/j.0902-4441.2005.t01-1-EJH2467.x. [DOI] [PubMed] [Google Scholar]
- 12.Parker CJ. Management of paroxysmal nocturnal hemoglobinuria in the era of complement inhibitory therapy. Hematol Am Soc Hematol Educ Progr. 2011;2011:21–29. doi: 10.1182/asheducation-2011.1.21. [DOI] [PubMed] [Google Scholar]
- 13.Borowitz MJ, Craig FE, Digiuseppe JA, Illingworth AJ, Rosse W, Sutherland DR, Wittwer CT, Richards SJ. On behalf of the clinical cytometry society. Guidelines for the diagnosis and monitoring of paroxysmal nocturnal hemoglobinuria and related disorders by flow cytometry. Cytom B Clin Cytom. 2010;78:211–230. doi: 10.1002/cyto.b.20525. [DOI] [PubMed] [Google Scholar]
- 14.Sugimori C, Mochizuki K, Qi Z, Sugimori N, Ishiyama K, Kondo Y, Yamazaki H, Takami A, Okumura H, Nakao S. Origin and fate of blood cells deficient in glycosylphosphatidylinositol-anchored protein among patients with bone marrow failure. Br J Haematol. 2009;147:102–112. doi: 10.1111/j.1365-2141.2009.07822.x. [DOI] [PubMed] [Google Scholar]
- 15.Schrezenmeier H, Muus P, Socié G, Szer J, Urbano-Ispizua A, Maciejewski JP, Brodsky RA, Bessler M, Kanakura Y, Rosse W, Khursigara G, Bedrosian C, Hillmen P. Baseline characteristics and disease burden in patients in the international paroxysmal nocturnal hemoglobinuria registry. Haematologica. 2014;99:922–929. doi: 10.3324/haematol.2013.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodsky RA. How I treat paroxysmal nocturnal hemoglobinuria. Blood. 2009;113:6522–6527. doi: 10.1182/blood-2009-03-195966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, Rosse W, Socié G. International PNH interest group diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699–3709. doi: 10.1182/blood-2005-04-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monica B, Jeffrey H. The pathophysiology of disease in patients with paroxysmal nocturnal hemoglobinuria. Am Soc Hematol Educ Book. 2008;1:104–110. doi: 10.1182/asheducation-2008.1.104. [DOI] [PubMed] [Google Scholar]
- 19.Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117:6786–6792. doi: 10.1182/blood-2011-02-333997. [DOI] [PubMed] [Google Scholar]
- 20.Kahng J, Kim Y, Kim JO, Koh K, Lee WJ, Han K. A novel marker for screening paroxysmal nocturnal hemoglobinuria using routine complete blood count and cell population data. Ann Lab Med. 2015;35:35–40. doi: 10.3343/alm.2015.35.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121:4985–4996. doi: 10.1182/blood-2012-09-311381. [DOI] [PubMed] [Google Scholar]
- 22.Hill A, Hillmen P, Richards SJ, Elebute D, Marsh JC, Chan J, Mojcik CF, Rother RP. Sustained response and long-term safety of eculizumab in paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:2559–2565. doi: 10.1182/blood-2005-02-0564. [DOI] [PubMed] [Google Scholar]
- 23.Dmytrijuk A, Robie-Suh K, Cohen MH, Rieves D, Weiss K, Pazdur R. FDA report eculizumab for the treatment of patients with Paroxysmal nocturnal hemoglobinuria. Oncologist. 2008;13:993–1000. doi: 10.1634/theoncologist.2008-0086. [DOI] [PubMed] [Google Scholar]
- 24.Röth A, Dührsen U, Schrezenmeier H, Schubert J. Paroxysmal nocturnal hemoglobinuria. Dtsch Med Wochenschr. 2009;134:404–409. doi: 10.1055/s-0028-1124013. [DOI] [PubMed] [Google Scholar]
- 25.Hillmen P, Muus P, Röth A, Elebute MO, Risitano AM, Schrezenmeier H, Szer J, Browne P, Maciejewski JP, Schubert J, Urbano-Ispizua A, de Castro C, Socié G, Brodsky RA. Long-term safety and efficacy of sustained eculizumab treatment in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 2013;162:62–73. doi: 10.1111/bjh.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Struijk GH, Bouts AH, Rijkers GT, Kuin EA, ten Berge IJ, Am Bemelman FJ, Transplant J. Meningococcal sepsis complicating eculizumab treatment despite prior vaccination. Am J Transplant. 2013;13:819–820. doi: 10.1111/ajt.12032. [DOI] [PubMed] [Google Scholar]
- 27.Lee YK, Lee HW, Choi KH, Kim BS. Ability of Nafamostat Mesilate to prolong filter patency during continuous renal replacement therapy in patients at high risk of bleeding: a randomized controlled study. PLoS ONE. 2014;9:e108737. doi: 10.1371/journal.pone.0108737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busse P, Bygum A, Edelman J, Lumry W, Machnig T, Martinez-Saguer I, Rojavin M. Safety of c1-esterase inhibitor in acute and prophylactic therapy of hereditary angioedema: findings from the ongoing international berinert patient registry. Allergy Clin Immunol Pract. 2015;3(2):213–219. doi: 10.1016/j.jaip.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013;190:3838–3847. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brodsky RA. Stem cell transplantation for paroxysmal nocturnal hemoglobinuria. Haematologica. 2010;95:855–856. doi: 10.3324/haematol.2010.023176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly JR, Höchsmann B, Szer J, Kulasekararaj A, de Guibert S, Röth A, Weitz IC, Armstrong E, Risitano MA, Patriquin CJ, Terriou L, Muus P, Hill A, Turner MP, Schrezenmeier H, de Latour RP. Eculizumab in pregnant patients with paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2015;373:1032–1039. doi: 10.1056/NEJMoa1502950. [DOI] [PubMed] [Google Scholar]
- 32.Usuki K, Urabe A, Kawaguchi T, Miyasaka N, Miura O, Morishita E, Arima N, Morita Y, Nishiwaki K, Ninomiya H, Gotoh A, Imashuku S, Shichishima T, Nishimura J, Kanakura Y. Management of pregnancy in paroxysmal nocturnal hemoglobinuria (PNH): a report of 10 cases from the working group on pregnancy of The Japan PNH Study Group. Blood. 2013;15:122. [Google Scholar]
- 33.Curran KJ, Kernan NA, Prockop SE, Scaradavou A, Small TN, Kobos R, Castro-Malaspina H, Araten D, DiMichele D, O’Reilly RJ, Boulad F. Paroxysmal nocturnal hemoglobinuria in pediatric patients. Pediatr Blood Cancer. 2012;59:525–529. doi: 10.1002/pbc.23410. [DOI] [PubMed] [Google Scholar]
- 34.Woodard P, Wang W, Pitts N, Benaim E, Horwitz E, Cunningham J, Bowman L. Successful unrelated donor bone marrow transplantation for paroxysmal nocturnal hemoglobinuria. Bone Marrow Transplant. 2001;27:589–592. doi: 10.1038/sj.bmt.1702827. [DOI] [PubMed] [Google Scholar]
- 35.Bortolotti M, Pandolfo N, Giovannini M, Mari C, Miglioli M. Effect of sildenafil on hypertensive lower oesophageal sphincter. Eur J Clin Invest. 2002;32:682–685. doi: 10.1046/j.1365-2362.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- 36.van den Heuvel-Eibrink MM, Bredius RG, te Winkel ML, Tamminga R, de Kraker J, Schouten-van Meeteren AY, Bruin M, Korthof ET. Childhood paroxysmal nocturnal haemoglobinuria (PNH), a report of 11 cases in the Netherlands. Br J Haematol. 2005;128:571–577. doi: 10.1111/j.1365-2141.2004.05337.x. [DOI] [PubMed] [Google Scholar]