Dear Editor,
Paraneoplastic pemphigus (PNP) is a rare autoimmune muco-cutaneous blistering disorder seen most commonly in the setting of an underlying lymphoreticular malignancy [1]. Given its resemblance to host of dermatological conditions, PNP is often misdiagnosed and culminate in death of patients. A 60-year old man presented to our hematology clinic in January 2013 with generalized lymphadenopathy and significant weight loss. Personal history was significant for smoking (20 pack years) and alcohol intake (90 ml/day of whisky for 15 years). FNAC of inguinal lymph node suggested a low grade non-Hodgkin’s lymphoma (NHL) and biopsy with immunohistochemistry characterized it as Follicular Lymphoma (FL) grade 1 (CD-20+). PET–CT identified FDG avid lymph nodes on both sides of diaphragm and bone marrow examination showed infiltration by FL (Ann Arbor IVB, FLIPI score: 3). Investigations suggested: Hb-16.5 gm/dl, white cell count—15.4 × 109/l, and platelets—134 × 103/l. LDH and β2 microglobulin were 293 U/L (180–248 U/L) and 4173 ng/ml (1010–1730 ng/ml) respectively. Viral markers (HBsAg, anti-HCV, HIV) were negative and 2D-Echocardiography was normal. Patient was administered 6 cycles Bendamustine-Rituximab (BR, Bendamustine—90 mg/m2 on D1 and D2; Rituximab—375 mg/m2 on D1) immuno-chemotherapy on a 4 weekly basis. Patient achieved a complete remission at the end of 6 cycles as assessed by PET–CT and bone marrow evaluation. Patient thereafter received maintenance therapy with Rituximab (375 mg/m2) at 3 monthly intervals. After 6 cycles of maintenance Rituximab (Progression free survival of 18 months), he developed a new lymph node in left inguinal area which was confirmed as relapsed FL grade 1 on a repeat biopsy (re-stage Ann Arbor IIIA). After a loss to follow-up of 4 months, patient presented to us in January 2016 with complaints of painful peri-anal lesions of 1 month duration which were followed after 10 days by oral ulcers associated with pain while swallowing food. Patient was febrile (axillary temperature-101 °F) and had tachycardia (PR-112/min) at presentation. General examination revealed marked oral ulcerations covered with thick muco-purulent secretions, hemorrhagic crusting of lips and nasal cavity and peri-anal excoriations (Fig. 1). Systemic examination revealed coarse crepitations in bilateral lung fields. Chest X-ray suggested bilateral bronchopneumonia which was treated with 7 days of parenteral antibiotics (azithromycin plus amoxicillin/clavulanic acid). Oral mucosal biopsy revealed acantholysis and tomb stone appearance of the basal layer suggestive of pemphigus Vulgaris (Fig. 2). Direct immunofluorescence (DIF) was negative for IgG, IgA, IgM and C3 deposition. Enzyme Linked Immunosorbent Assay (ELISA) identified anti-desmoglein (Dsg) 3 antibody in the patient’s serum (anti-Dsg 1 negative). Based on clinical and immune-pathological findings, patient was diagnosed as Paraneoplastic pemphigus and was administered oral prednisolone (1 mg/kg/day). He failed a 2 weeks trial of oral corticosteroids and developed altered behavior and visual hallucinations. MRI brain and Cerebrospinal fluid analysis were normal. Patient was diagnosed as steroid induced psychosis which improved with oral haloperidol (5 mg/day). Oral corticosteroids were gradually tapered and rituximab was instituted on a weekly regimen (375 mg/m2). Oral and peri-anal lesions showed dramatic improvement following 4 doses of weekly rituximab. Patient started accepting orally and had a significant improvement in his performance status. He was discharged in a stable condition and is planned for R-CHOP therapy for underlying FL. Described initially by Anhalt et al. [2], PNP has now been recognized as a multi-system disorder and renamed as paraneoplastic autoimmune Multiorgan syndrome [3]. Although virtually any mucosa may be involved, painful oral erosions are the earliest and a hallmark feature and its absence argues against a diagnosis of PNP [4]. Polymorphous cutaneous lesions follows mucosal involvement (although isolated mucosal disease has been described) and may resemble PV, Pemphigus foliaceus (PF), Bullous Pemphigoid (BP), lichen planus (LP), Steven-Johnson’s syndrome (SJS), Graft-versus-host disease (GVHD) and herpes stomatitis [1, 5]. Bronchiolitis obliterans (20 %) is the most dreaded complication of PNP due to sloughing of respiratory epithelium from immune complex deposition in the basement membrane and imparts a poor prognosis [6]. PNP may precede (1/3rd) or develop following the diagnosis (2/3rd) of the underlying neoplasm [1]. Haematological neoplasms account for the majority of cases (84 %) including NHL (38 %), Chronic lymphocytic leukemia (CLL, 18 %), castleman disease (18 %), thymoma (5.5 %), waldenstrom’s macroglobulinemia (1.2 %) and Hodgkin’s lymphoma (0.6 %). Non-haematological neoplasms (16 %, carcinoma, sarcoma, melanoma) accounts for the remaining cases [7]. Eighty-nine percent of the NHL associated with PNP are of B cell type. NHL subtypes includes CLL (43 %), FL (27 %), T-cell lymphoma (11 %), indolent B-cell lymphoma (7 %) and B-cell lymphoma unspecified (7 %) and Diffuse large B-cell lymphoma (5 %) indicating that indolent NHL is most commonly associated with PNP [8]. DIF shows IgG and C3 deposition in inter-cellular areas and may also show linear/granular deposition along basement membrane [6]. Immune-precipitation identifies specific antibodies (envoplakin/periplakin/plectin) in the sera and is considered gold standard [1]. Although DIF and immune-precipitation tests are supportive, they may be negative in about 50 and 25 % cases respectively and their absence doesn’t rule out the diagnosis of PNP [1]. Choi et al. [1] identified both Dsg-1 and 3(30 %), only Dsg-1 (40 %), only Dsg-3 (10 %) and none (20 %) in PNP cases. PNP runs an aggressive course in the setting of a malignant disorder (90 % mortality rate in 2 years, mean survival of 3 months from diagnosis) and treatment aimed at the underlying malignancy is usually unsuccessful [7]. Corticosteroids are initial agents followed by azathioprine, methotrexate, Cyclophosphamide and mycophenolate in steroid refractory cases. Rituximab offers a reasonable therapeutic option in PNP associated with B-cell NHL considering its efficacy and acceptable side-effect profile. Hoque et al. [9] summarized 6 cases of FL with PNP and half of them responded to rituximab (50 %) and remaining (50 %) didn’t respond. Our patient developed peri-anal lesions as the first manifestation of PNP. Although peri-anal lesions are reported in cases of PNP, it is rarely an initial presentation. Since, DIF and serological tests may be negative in a reasonable proportion of cases; a high index of suspicion must be maintained to facilitate early diagnosis and treatment. Considering the poor performance status and co-morbidities of these patients, we advocate early use of rituximab to avoid steroid toxicity and mortality.
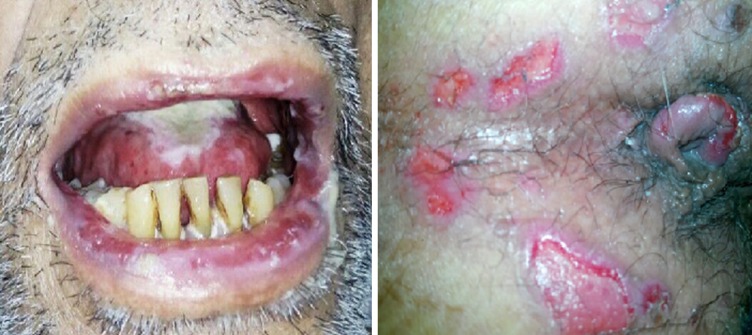
Fig. 1.
clinical pictures of the patient showing oral mucosal lesions associated with hemorrhagic crust (left) and peri-anal erosions (right)
Fig. 2.
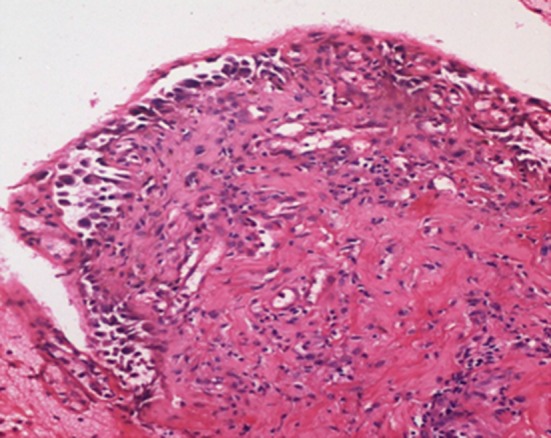
Photomicrograph showing suprabasal bulla with tombstone appearance of basal cells and presence of acantholytic cells in the bulla cavity. Roof of the bulla is eroded (Hematoxylin and Eosin, ×200)
Compliance with Ethical Standards
Conflict of interest
The authors disclose no conflict of interest.
Consent for Publication
The authors declare that prior written and informed consent was obtained from the patient prior to publication of any material relating to the patient and his identity.
Ethical Statement
The authors declare that the article abides by the ethical considerations as laid under Helsinki’s Declaration.
Contributor Information
Ankur Jain, Email: drankur589@yahoo.in.
Gaurav Prakash, Email: drgp04@gmail.com.
Ram V. Nampoothiri, Email: ramvnampoothiri@gmail.com
Dipankar De, Email: de.dipankar@pgimer.edu.in.
Amanjit Bal, Email: docaman5@hotmail.com.
Alka Khadwal, Email: alkakhadwal@hotmail.com.
Deepesh Lad, Email: deepesh.kem@gmail.com.
Pankaj Malhotra, Email: malhotrapankaj@hotmail.com.
Subhash Varma, Email: suvarma@hotmail.com.
References
- 1.Choi Y, Nam KH, Lee JB, Lee JY, Ihm CW, Lee SE, et al. Retrospective analysis of 12 Korean patients with paraneoplastic pemphigus. J Dermatol. 2012;39:973–981. doi: 10.1111/j.1346-8138.2012.01655.x. [DOI] [PubMed] [Google Scholar]
- 2.Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, et al. Paraneoplastic pemphigus: an autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323:1729–1735. doi: 10.1056/NEJM199012203232503. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen V, Ndoye A, Bassler K, Schultz L, Shields M, Ruben B, et al. Classification, clinical manifestation and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome. Arch Dermatol. 2001;137:193–206. [PubMed] [Google Scholar]
- 4.Hidalgo I, Martinez F, Grau C, Gil I, Azón A. Follicular lymphoma with paraneoplastic autoimmune multiorgan syndrome. Actas Dermosifiliogr. 2012;103:244–246. doi: 10.1016/j.ad.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Bialy-Golan A, Brenner S, Anhalt GJ. Paraneoplastic pemphigus: oral involvement as the sole manifestation. Acta Derm Venereol. 1996;76:253–254. doi: 10.2340/0001555576253254. [DOI] [PubMed] [Google Scholar]
- 6.Allen CM, Camisa C. Paraneoplastic pemphigus: a review of the literature. Oral Dis. 2000;6:208–214. doi: 10.1111/j.1601-0825.2000.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 7.Tilakaratne W, Dissanayake M. Paraneoplastic pemphigus: a case report and review of literature. Oral Dis. 2005;11:326–329. doi: 10.1111/j.1601-0825.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- 8.Morikawa K, Tsuji T, Yamasaki H, Egashira S, Kaguchi A, Kido M, et al. Paraneoplastic pemphigus occurs most commonly in indolent B cell lymphoma. Acta Haematol. 2014;132:73–74. doi: 10.1159/000357109. [DOI] [PubMed] [Google Scholar]
- 9.Hoque SR, Black MM, Cliff S. Paraneoplastic pemphigus associated with CD20-positive follicular non-Hodgkin’s lymphoma treated with rituximab: a third case resistant to Rituximab therapy. Clin Exp Dermatol. 2007;32:172–175. doi: 10.1111/j.1365-2230.2006.02331.x. [DOI] [PubMed] [Google Scholar]