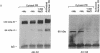Abstract
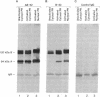
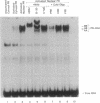
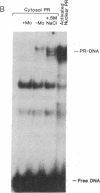
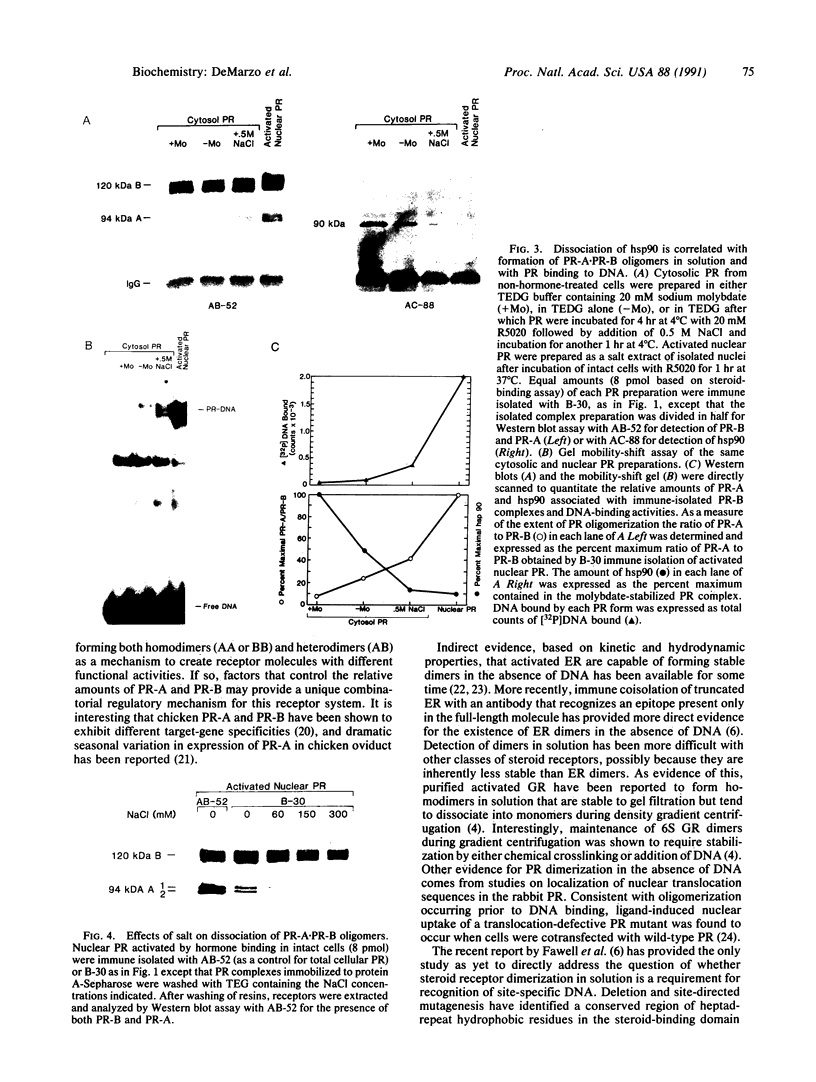
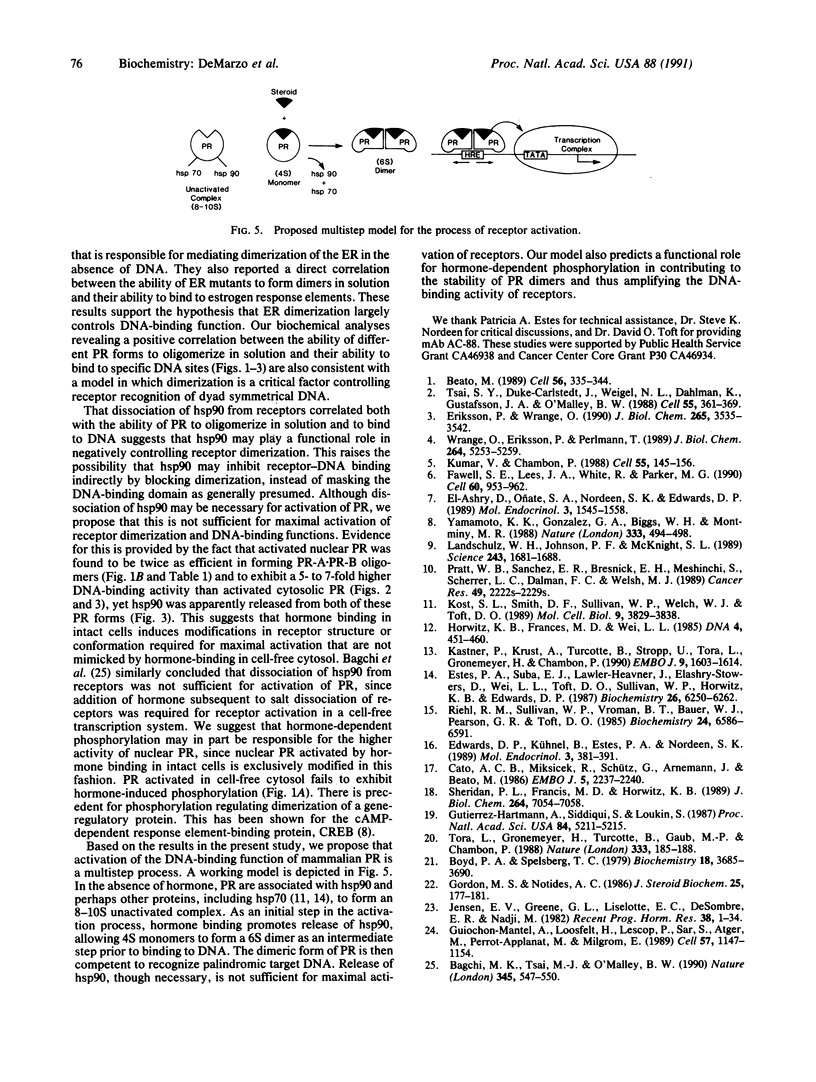
In this study we have demonstrated that dimerization of mammalian progesterone receptors (PR) occurs in the absence of DNA. A specific immune coisolation assay was performed on extracts of T-47D human breast cancer cells with a monoclonal antibody specific for the full-length B form of progesterone receptor (PR-B). This resulted in coisolation of significant amounts of truncated form-A receptors (PR-A), indicating the presence of stable PR-A.PR-B dimers in solution. A positive correlation was observed between the ability of different receptor forms to oligomerize in solution and their ability to bind to specific DNA sequences. The ability to form stable PR-A.PR-B oligomers in the absence of DNA was also found to correlate with release of 90-kDa heat shock protein (hsp90) from the unactivated PR complex. These results support the hypothesis that dimerization in the absence of DNA is an important mechanism controlling receptor DNA-binding function and that hsp90 release may be a key step regulating dimerization. This suggests that hsp90 may function to repress DNA-binding activity indirectly by blocking receptor dimerization.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi M. K., Tsai S. Y., Tsai M. J., O'Malley B. W. Identification of a functional intermediate in receptor activation in progesterone-dependent cell-free transcription. Nature. 1990 Jun 7;345(6275):547–550. doi: 10.1038/345547a0. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Boyd P. A., Spelsberg T. C. Seasonal changes in the molecular species and nuclear binding of the chick oviduct progesterone receptor. Biochemistry. 1979 Aug 21;18(17):3685–3690. doi: 10.1021/bi00584a008. [DOI] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. P., Kühnel B., Estes P. A., Nordeen S. K. Human progesterone receptor binding to mouse mammary tumor virus deoxyribonucleic acid: dependence on hormone and nonreceptor nuclear factor(s). Mol Endocrinol. 1989 Feb;3(2):381–391. doi: 10.1210/mend-3-2-381. [DOI] [PubMed] [Google Scholar]
- Eriksson P., Wrange O. Protein-protein contacts in the glucocorticoid receptor homodimer influence its DNA binding properties. J Biol Chem. 1990 Feb 25;265(6):3535–3542. [PubMed] [Google Scholar]
- Estes P. A., Suba E. J., Lawler-Heavner J., Elashry-Stowers D., Wei L. L., Toft D. O., Sullivan W. P., Horwitz K. B., Edwards D. P. Immunologic analysis of human breast cancer progesterone receptors. 1. Immunoaffinity purification of transformed receptors and production of monoclonal antibodies. Biochemistry. 1987 Sep 22;26(19):6250–6262. doi: 10.1021/bi00393a045. [DOI] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Gordon M. S., Notides A. C. Computer modeling of estradiol interactions with the estrogen receptor. J Steroid Biochem. 1986 Aug;25(2):177–181. doi: 10.1016/0022-4731(86)90414-0. [DOI] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Lescop P., Sar S., Atger M., Perrot-Applanat M., Milgrom E. Mechanisms of nuclear localization of the progesterone receptor: evidence for interaction between monomers. Cell. 1989 Jun 30;57(7):1147–1154. doi: 10.1016/0092-8674(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Hartmann A., Siddiqui S., Loukin S. Selective transcription and DNase I protection of the rat prolactin gene by GH3 pituitary cell-free extracts. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5211–5215. doi: 10.1073/pnas.84.15.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz K. B., Francis M. D., Wei L. L. Hormone-dependent covalent modification and processing of human progesterone receptors in the nucleus. DNA. 1985 Dec;4(6):451–460. doi: 10.1089/dna.1985.4.451. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Greene G. L., Closs L. E., DeSombre E. R., Nadji M. Receptors reconsidered: a 20-year perspective. Recent Prog Horm Res. 1982;38:1–40. doi: 10.1016/b978-0-12-571138-8.50006-8. [DOI] [PubMed] [Google Scholar]
- Kastner P., Krust A., Turcotte B., Stropp U., Tora L., Gronemeyer H., Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990 May;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost S. L., Smith D. F., Sullivan W. P., Welch W. J., Toft D. O. Binding of heat shock proteins to the avian progesterone receptor. Mol Cell Biol. 1989 Sep;9(9):3829–3838. doi: 10.1128/mcb.9.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989 Mar 31;243(4899):1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Sanchez E. R., Bresnick E. H., Meshinchi S., Scherrer L. C., Dalman F. C., Welsh M. J. Interaction of the glucocorticoid receptor with the Mr 90,000 heat shock protein: an evolving model of ligand-mediated receptor transformation and translocation. Cancer Res. 1989 Apr 15;49(8 Suppl):2222s–2229s. [PubMed] [Google Scholar]
- Riehl R. M., Sullivan W. P., Vroman B. T., Bauer V. J., Pearson G. R., Toft D. O. Immunological evidence that the nonhormone binding component of avian steroid receptors exists in a wide range of tissues and species. Biochemistry. 1985 Nov 5;24(23):6586–6591. doi: 10.1021/bi00344a042. [DOI] [PubMed] [Google Scholar]
- Sheridan P. L., Francis M. D., Horwitz K. B. Synthesis of human progesterone receptors in T47D cells. Nascent A- and B-receptors are active without a phosphorylation-dependent post-translational maturation step. J Biol Chem. 1989 Apr 25;264(12):7054–7058. [PubMed] [Google Scholar]
- Tora L., Gronemeyer H., Turcotte B., Gaub M. P., Chambon P. The N-terminal region of the chicken progesterone receptor specifies target gene activation. Nature. 1988 May 12;333(6169):185–188. doi: 10.1038/333185a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Wrange O., Eriksson P., Perlmann T. The purified activated glucocorticoid receptor is a homodimer. J Biol Chem. 1989 Mar 25;264(9):5253–5259. [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- el-Ashry D., Oñate S. A., Nordeen S. K., Edwards D. P. Human progesterone receptor complexed with the antagonist RU 486 binds to hormone response elements in a structurally altered form. Mol Endocrinol. 1989 Oct;3(10):1545–1558. doi: 10.1210/mend-3-10-1545. [DOI] [PubMed] [Google Scholar]