Abstract
From an epidemiological point of view, hepatitis E is an old infection in Iran, but only recently has its importance as a public health concern been considered from research and public health standpoints. As such, there is still a long road ahead to clarify the real burden of hepatitis E virus (HEV) infection in Iran. According to the available epidemiological studies, the seroprevalence of HEV infection among pregnant women is between 3.6% and 7.4%, and among Iranian children is between 0.9% to 8.5%, varying by geographic regions within the country and directly dependent upon the sanitary status of each. In addition to evaluating the sanitation level of a society, community-based seroprevalence studies of HEV infection demonstrate the most prevalent risk factors, the major routes of transmission, and the epidemiological patterns of HEV among that country’s population. In this review, the current knowledge about the pathogenesis and epidemiology of HEV infection in pregnant women and children in Iran, as well as the recent advances in diagnosis, prevention and treatment of HEV infection have been summarized.
Keywords: Hepatitis E virus, Pregnant women, Children, Epidemiology, Iran
Introduction
Hepatitis E virus (HEV) infection is generally an enterically transmitted viral hepatitis with asymptomatic or acute self-limited manifestations.1 However, progression to fulminant liver failure has also been reported in high-risk groups, such as pregnant women and patients with underlying liver problems.1–7 Chronicity is rare and is mostly observed in immunocompromised and immunosuppressed patients, such as patients who have received organ transplant, are infected with human immunodeficiency virus (HIV) or suffer from haematological malignancies.5,8–14 Therefore, despite the benign clinical presentation of hepatitis E in the general population, it is considered as an important health concern, especially in those high-risk populations.
According to the epidemiological data, 3.3 million acute cases and 20 million new cases of hepatitis E are diagnosed each year globally.3,15 Despite the mortality rate of 1–2% in the general population,16 10–25% of pregnant women and >75% of patients with underlying liver disease lose their lives due to the HEV infection.17,18 According to a report from the World Health Organization (WHO), ∼56600 deaths occur per year due to HEV-related hepatic failure.19 Overall, one-third of the world’s population has been infected with HEV.20,21
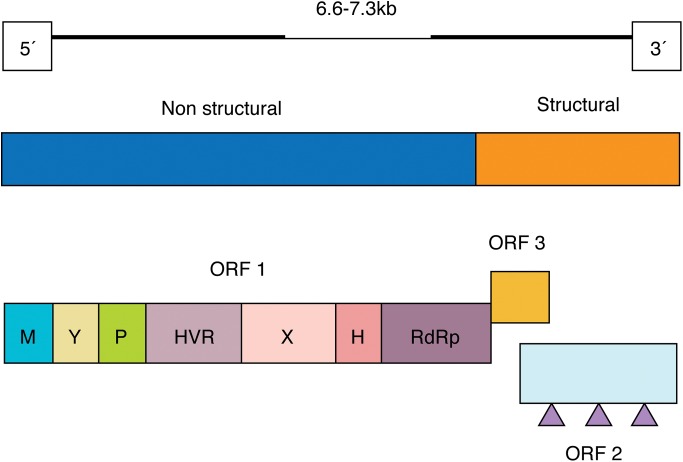
HEV is a small virus in the family Hepeviridae, with a positive single-stranded RNA genome and non-enveloped icosahedral capsid.5,9,22,23 The genome consists of three partially overlapping open reading frames (Fig. 1).5,24,25 HEV is usually transmitted via the faecal-oral route, particularly through contaminated food and water supplies. However, transmission through haemodialysis, organ transplantation, sexual intercourse, placenta, blood and blood product transfusion is also possible, but the importance of each is unknown.1,17,26 Only four genotypes capable of affecting human beings have been identified thus far.3,27 These four genotypes have been classified into the genus Orthohepevirus and the species Orthohepevirus A,1,28 and they differ in their mode of transmission, pathogenicity, severity, mortality rates, geographical and age distribution.1,17,26,29,30
Fig. 1. Molecular structure of hepatitis E virus.1.
(Adapted from Fig. 1 of reference 1, with permission of the publisher).
Abbreviations: M, methyltransferase; Y, Y domain; P, papain-like cysteine protease; HVR, hypervariable region; X, macro-domain; H, helicase; RdRp, RNA-dependent RNA polymerase; Triangle, glycosylation site.
Genotypes 1 and 2 affect human beings and are responsible for large epidemics or acute outbreaks in developing countries. These outbreaks occur due to contamination of drinking water following heavy rainfall or flooding, and are more frequently observed among young adult males.1,3,5,13,26,29,31 Meanwhile, genotypes 3 and 4 use an animal reservoir and are mostly transmitted via consumption of contaminated meat; these two genotypes are the causative agents of locally-acquired sporadic HEV infections in developed countries and mostly affect middle-aged to elderly males.1,3,5,13,29,31–33 These variations in the epidemiological patterns of HEV reflect differences in the level of sanitation, lifestyle, risk factors and status of public health in the different groups and regions across the globe.14,34–36 Therefore, it is important to study the epidemiological patterns of hepatitis E in the different groups and regions to determine the burden of this viral infection.
In Iran, HEV infection is endemic, but the epidemiology of this infection in two of the most vulnerable groups—pregnant women and children—remains unclear. Therefore, this study was conducted to provide a general overview of the pathogenesis and epidemiology of hepatitis E in these two groups in Iran.
HEV in pregnant women in Iran
HEV infection is not only generally a benign self-limited disease, but it can also adversely affect pregnant women and lead to fulminant hepatic failure, especially during the second and third trimesters, subsequently lowering survival of mothers and their fetuses.37–41 Overall, it is reported that 0% to 73% of mortalities in pregnant women are attributed to HEV infection in the endemic regions, representing 0% in Egypt, 3.4% in South India, 42% in Ethiopia and 39.1–73% in North India.39 Besides maternal complications, HEV also causes serious consequences to the foetus following transplacental transmission, which varies in severity from low birth weight, prematurity, mild anicteric neonatal hepatitis or jaundice at birth with full recovery to miscarriage, stillbirth, preterm labour, perinatal death or neonatal death soon after birth.1,27,29,37,38,40,42 No extrahepatic manifestations or chronic carrier state has been reported in children born to mothers with HEV infection.27,38
Such severe complications in pregnancy are not seen with the other known hepatitis viruses.5,22,40 Although the exact mechanism of this excess severity of hepatitis E in pregnancy has not yet been determined, it seems that a combination of host and viral factors contributes to the pathogenesis of hepatitis E during pregnancy.1,37,39
Pregnancy is accompanied by significant changes in maternal hormonal and immunological responses to sustain the foetus.39,40 These immunological changes include inhibition of cell-mediated immunity through secretion of transforming growth factor-beta (TGF-β) and IL-4 and IL-10 cytokines, down-regulation of nuclear factor-kappa B (NF-κB), reduction in T cell activity and cytokine production, along with an alteration in cellular immune regulation towards an increase in CD8 cell counts and a decrease in CD4 cells with predominant T helper 2 (Th2) responses; all of these result in systematic suppression of the maternal immune system and subsequently increase susceptibility to infections.1,37,39,40 Moreover, the steroid hormones including beta-HCG (human chorionic gonadotropin), oestrogen and progesterone increase during pregnancy.5,39,40,42 These hormones have direct inhibitory effects on hepatocytes, cell-mediated immunity, T helper 1 (Th1) cell development and B cell production; moreover, through the decreased expression of NF-κB, they promote lymphocyte apoptosis. In addition, these hormones induce viral replication and the development of Th2 cells.37,39,40,42
Although the above-mentioned hormonal and immunological changes physiologically occur in normal pregnancy, when pregnancy is accompanied by HEV infection these changes increase.39,40,42 As such, HEV-infected pregnant women may suffer from decreased activity or absence of the p65 component of the NF-κB complex, which likely induces acute liver damage.39,40 Moreover, HEV appears to have a direct cytopathogenic effect on hepatic cells or immune-mediated pathogenesis through induction of host inflammatory responses, which may result in destruction of hepatocytes.1,43 Although Th2 responses are predominant in normal pregnancy, a strong cytotoxic immune response may be induced through an alteration in T helper responses towards Th1 following HEV infection aimed at reducing the high viral load, but which in turn results in lower foetal survival rates.42
Foetal infection with HEV will enhance the severity of infection and risk of liver failure in the mother.38–40 Moreover, the inhibitory effects of steroid hormones on hepatocytes can result in hepatic dysfunction when pregnancy is accompanied by HEV infection.40,42 Overall, it seems that HEV by itself does not induce these changes and requires pregnancy as a physiological factor to enhance the risk of hepatic damage. Therefore, pregnant women with hepatitis E are at an increased risk for developing liver failure.39,40
It seems that viral load and genotype can influence severity of HEV infection during pregnancy. As such, high viral load, along with genotypes 1 and 2, known as the more virulent genotypes, contribute to the development of fulminant liver failure (FLF).1,5,37,38,42 In addition, poor maternal nutrition and the use of herbal medicines to relieve symptoms of HEV infection due to side effects are associated with increased severity of hepatitis E.39,40,42,43 Finally, the role of genetic factors, such as variation in human leukocyte antigen (HLA) alleles in different geographical regions, should also be considered in the pathogenesis of HEV infection during pregnancy.39,40
An endotoxin-mediated effect has been proposed as another probable factor in the pathogenesis of FLF in pregnancy. In this process, the Kupffer cells, a type of liver sinusoidal cells, are destroyed by HEV, negating their ability to protect the liver cells against endotoxin derived from gram-negative bacteria of the intestinal tract.40 In addition, release of prostaglandins due to HEV infection can indirectly damage hepatocytes through attraction of neutrophils, which result in inflammation, oedema and cholestasis in liver.40
In endemic regions, the incidence and severity of HEV infection in pregnant women are much higher than that in non-pregnant women and men, with reported maternal mortality rates of 30–100% in the various studies.1,39,40,44 The rate of vertical transmission from mother to foetus varies from 30% to 79%, and sometimes up to 100%, in the different studies as well.27 In addition, mother-to-child transmission via breastfeeding is also possible and mostly occurs during the acute phase of infection.37 In developing countries, HEV infection accounts for approximately 3000 stillbirths annually.5,37 Interestingly, the above-mentioned epidemiological pattern is not observed in all endemic countries.5,44 In Egypt, for example, despite reporting high seroprevalence of HEV among pregnant women, the disease follows a mild or asymptomatic course, with very low mortality.38–40,44 However, the exact reason behind this benign pathogenicity remains unclear, but might be related to the presence of a highly contagious but less virulent strain of the HEV genotype predominantly found in Egypt5,22,38–40,44 or possibly the differences in major histocompatibility complex (MHC) phenotypes in this country as compared to the other endemic areas.5 It can also be explained by the high levels of previous exposure to HEV in early childhood, resulting in long-lasting immunity and probably attenuated infection upon re-exposure to HEV in adulthood.5,38–40 Similar findings have been reported in the United States (US) and Europe, where there is no difference in the severity of HEV infection in pregnant women compared to that in non-pregnant women,38,39,42 while HEV seroprevalence is considerably high but most HEV infections are asymptomatic or remain undiagnosed.44
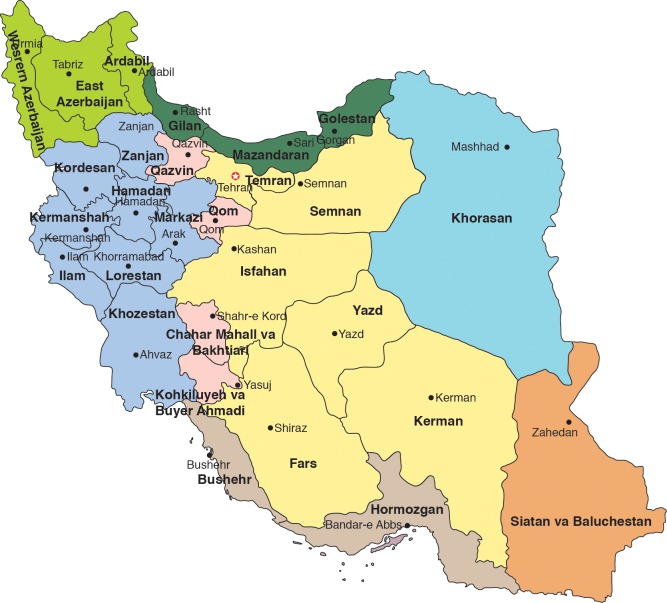
In Iran, however, the epidemiology is even more unclear. There are few reports on the seroprevalence of HEV infection among pregnant women in Iran. In the available studies, however, the seroprevalence of HEV infection varies from 3.6% to 7.6%,45–49 which is more or less similar to HEV seroprevalence in the general population of Iran (Table 1 and Fig. 2). Moreover, age, level of education, parities, stage of gestation and the number of family members have been identified as risk factors for HEV seropositivity among these reported pregnant women.46–48 In particular, increasing gestational age, lower education, third trimester of pregnancy and more parities are associated with high seroprevalence of HEV infection among the pregnant women.46–48 Compared to the other endemic countries,50–52 the seroprevalence of HEV infection among Iranian pregnant women is low.45,47,48 In addition, there is a considerable difference in rate of HEV seroprevalence between Iran and neighbouring countries such as the United Arab Emirates (20.0%)53 and India (33.67%);50 meanwhile, it is similar to that reported for Turkey (7.0%).54
Table 1. Prevalence of hepatitis E virus among pregnant women and children in Iran.
| Study population | City or province | Location | Year(s) of study | No. of participants | Mean age ± SD (age group) in years | No. of positive cases | HEV seroprevalence | HEV diagnosis | Manufacturer of serology kits used | Authors |
| Pregnant women | Urmia | Northwest | 2011 | 136 | 25.12 ± 4.91 (14 to 39) | 5 | 3.6% | Anti-HEV IgG | DIA.PRO, Italy | Rostamzadeh Khameneh et al.45 |
| Pregnant women | Ahvaz | Southwest | 2010–2011 | 418 | 30 ± 10 | 22 1 |
5.26% 0.23% |
Anti-HEV IgG Anti-HEV IgM |
DIA.PRO, Italy | Rasti et al.46 |
| Pregnant women | Hamadan | 2010–2011 | 1050 | 27.2 ± 5.6 (14 to 49) | 78 | 7.4% | Anti-HEV IgG | DIA.PRO, Italy | Mamani et al.47 | |
| Pregnant women | Gorgan | Northeast | 2010 | 394 | (15 to 47) | 29 | 7.4% | Anti-HEV total antibodies | DIA.PRO, Italy | Tabarraei et al.48 |
| Pregnant women | Gorgan | 2009 | 1200 | 27.41 ± 6.33 (17 to 45) | 76 | 6.3% | Anti-HEV total antibodies | DIA.PRO, Italy | Moradi et al.49 | |
| Children | Sari, Mazandaran | North | 2003 | 255 | (< 10) | 3 | 1.2% | Anti-HEV IgG | DIA.PRO, Italy | Saffar et al.55 |
| Children | Isfahan | Centre | 2005 | 110 | (6 to 9) | 1 | 0.9% | Anti-HEV total antibodies | DIA.PRO, Italy | Ataei et al.58 |
| Children | Kashan | Centre | 2012 | 558 | (1 to 15) | 21 | 3.7% | Anti-HEV IgG | DIA.PRO, Italy | Sharif et al.57 |
| Children | Ahvaz | Southwest | 2006–2007 | 566 | 10 ± 2.7 (6 to 15) | 48 | 8.5% | Anti-HEV IgG | Biokit, Spain | Shamsizadeh et al.56 |
| Children | Shiraz | South | 2011–2012 | 356 | (6 to 15) | 27 2 |
7.6% 0.6% |
Anti-HEV total antibodies Anti-HEV IgM |
DIA.PRO, Italy | Asaei et al.59 |
Abbreviations: No., number; SD, standard deviation; HEV, hepatitis E virus.
Fig. 2. Map of Iran.
Considering the adverse effects of HEV infection on pregnancy outcomes, prevention strategies, appropriate precautions and training programs regarding the possible routes of exposure to HEV as well as routine screening for HEV infection before and during pregnancy are needed to reduce the occurrence and adverse consequences of HEV infection in the pregnant population.46,47 Overall, the importance of HEV infection during pregnancy is underestimated in Iran and there are no current data on the rate of maternal mortality in Iran.
HEV in children in Iran
There are few studies on the seroprevalence of HEV infection among children in Iran. In those studies, seroprevalence rates vary from 0.9% to 8.5% (Table 1 and Fig. 2).55–59 Although the seroprevalence of HEV among children in Iran is not as high as reported for Nepal (16%), Tibet (23.8%) and India (17.75%–24.7%),27 it is still considerably higher than for Taiwan (0.3%), Mongolia (0.6%), Greenland (0.0%), Korea (0%–1%) and Argentina (0.15%).27 Globally, the highest HEV prevalence, ranging from 36.2% to 75.5%, has been reported for children in Egypt.27
The sero-epidemiological patterns of HEV infection among children in Iran are similar to those observed in other endemic countries, such as Bangladesh, Mexico, China, Turkey and Venezuela.27 Those studies have shown that the HEV seroprevalence is low in early childhood but increases with age.27,55,57 Egypt is an exception, with the majority of children being exposed to HEV in early life, as evidenced by 65% of children under 10 years of age being seropositive.27,60 Globally, <10% of children aged under 10 years are HEV seropositive.27
Most cases of HEV infection among children are subclinical.27,39,55 Despite this asymptomatic feature, the mortality rate is considerably high in children with jaundice; one study in Uganda reported a high mortality rate among icteric children, which was even higher than the mortality rates reported among the pregnant women in that country.16 However, there is no report on the mortality rate of HEV infection among children in Iran.
Some studies have evaluated the trends in seroprevalence of HEV infection among children over time and showed a slight but not significant increase in HEV seropositivity. One study from India indicated a significant increase in HEV seroprevalence over time.27 However, due to inadequate studies in Iran, the changes in trends of HEV seroprevalence over time remain unclear.
Children are representative of a group at risk of HEV infection.27 Recent studies have shown that the seroprevalence of HEV infection among children is related to sanitary status of different parts of the country.55,61 Considering the fact that contaminated drinking water supplies and inappropriate sewage disposal systems are associated with HEV seropositivity, the faecal-oral route is the main route of HEV transmission among Iranian children.55 Therefore, age-specific community-based seroprevalence studies of HEV infection are used to describe the sanitation level and public health status of a society. Overall, these studies have indicated that children in Iran are exposed to HEV and, therefore, preventive strategies are needed to reduce this exposure.55–57
Diagnosis of HEV infection
HEV causes a vast range of clinical presentations, which vary in severity from FLF, liver fibrosis and cirrhosis following chronic hepatitis E, and acute icteric hepatitis to unapparent and asymptomatic infection.1,7,9,15,29,62 Most cases of hepatitis E among children are asymptomatic,27,39 and no cases of chronic hepatitis E have been reported in pregnant women and infants.38 Chronic HEV infection is, however, frequently observed among immunocompromised and immunosuppressed patients.7,9,14,29
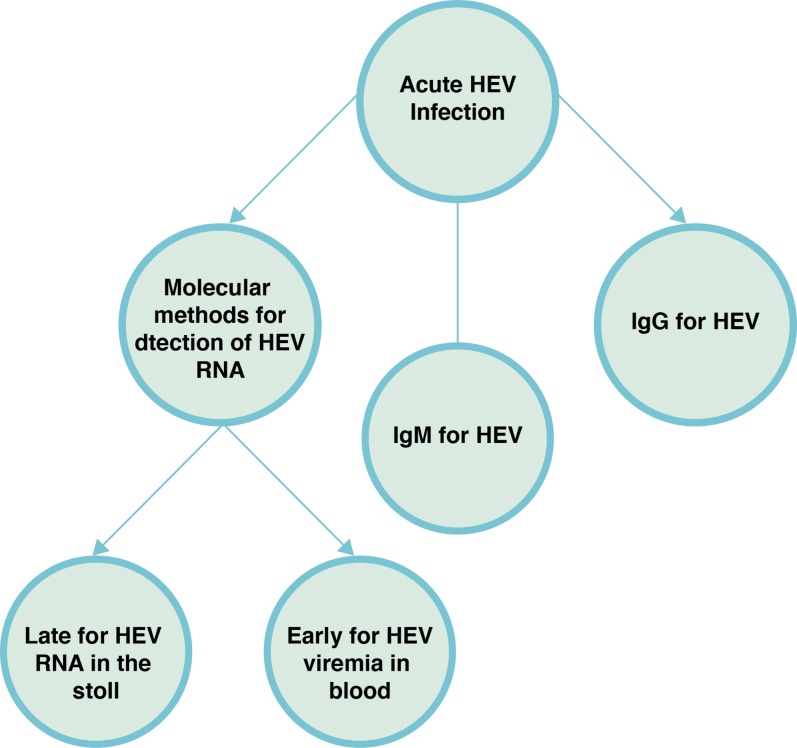
The clinical manifestations of HEV infection are indistinguishable from clinical symptoms of the other viral hepatitis forms.35 In addition, these non-specific symptoms sometimes mask the diagnosis of HEV infection, making laboratory methods the most reliable criteria for diagnosis. The laboratory diagnosis methods are based on detection of HEV RNA in serum or stool samples by nucleic acid amplification techniques (NAT) or of anti-HEV antibodies in serum or plasma samples by serological tests (Fig. 3).1,5,17,63–65
Fig. 3. Laboratory diagnosis of hepatitis E virus infection.38.
(Adapted from Fig. 3 of reference 38, with permission of the publisher).
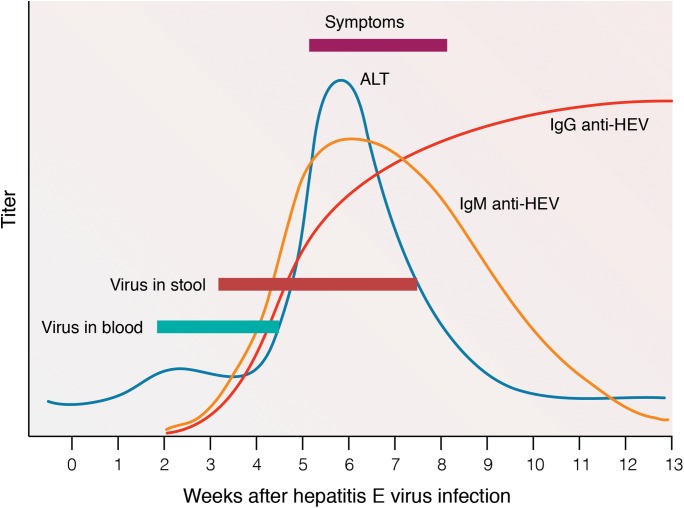
The presence of HEV RNA in blood and stool is short-lived, and becomes undetectable in serum at 3–4 weeks and in stool at 6 weeks after the onset of clinical symptoms (Fig. 4).17,29 In asymptomatic subjects, viremia can last for 4–6 weeks.17 However, a prolonged viremia is also possible, especially among children following acute HEV infection and immunosuppressed patients.4,17 Anti-HEV IgM increases during the acute phase of infection. IgM level remains high for about 8 weeks, but declines rapidly and becomes undetectable in most patients after 3–8 months.5,27 Meanwhile, long-lasting anti-HEV IgG antibodies appear shortly after the increase of IgM and persist for 1–14 years or more after the infection (Fig. 4).5,29,63 Thus, detection of IgM is indicative of acute infection, and the presence of IgG is a sign of previous exposure to HEV.1,29
Fig. 4. Virological markers of hepatitis E virus infection.38.
(Adapted from Fig. 2 of reference 38, with permission of the publisher).
Evaluation of liver parameters is another diagnostic criterion. At onset of clinical symptoms, liver function tests show abnormal findings, such as for the measurements of aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphate, gamma-glutamyl transpeptidase and bilirubin.22,62 However, elevated levels of liver enzymes are short-lived and return to normal about 6 weeks after the onset of clinical symptoms.27
Prevention and treatment of HEV infection
The current preventive strategies primarily aim at reducing exposure to HEV by improving water supply facilities and sewage disposal systems, since the disease is predominantly transmitted via the faecal-oral route.17 Therefore, provision of safe water supplies, sanitary preparation of food, sanitary disposal of human waste and hygienic infrastructure appear to be the most effective preventive measures.1,5,22,30,37,66
Vaccination against HEV is another preventive strategy, although no commercial vaccine has yet become available worldwide.17 Several HEV vaccines have been designed and evaluated in the laboratory setting, including recombinant vaccines consisting of various truncated forms of the capsid protein (HEV 239,67 trpE-C2,68 53 kDa,69 pE2,70 56 kDa,69 rHEV VLP,71 62 kDa72 and T1-ORF273), DNA vaccines (pcHEVORF2 and Lipo-NE-DP74–76) and, more recently, epitope-based vaccines.18,77 Amongst them, only one recombinant vaccine, the HEV 239 vaccine based on a 26 kDa portion of the HEV capsid protein (aa 368–606), has been licensed by China’s State Food and Drug Administration.3,18,78–81 Yet, this vaccine, despite showing promising results in human clinical trials, is not still approved for use in a susceptible population such as pregnant women, children and patients with pre-existing liver problems, and is not available worldwide.16,18,78
Upon disease emergence, the treatment strategy is usually supportive, as the disease is generally asymptomatic or self-limited at this stage.9,17,82 Antiviral treatment is administered only for patients with hepatic complications due to fulminant or chronic HEV infection.9,17 These antiviral therapies include monotherapy with pegylated interferon (PEG-IFN) or ribavirin, or combination therapy with these antiviral agents.9,82,83 Although these antiviral treatments result in clearance of the virus and reduction of the symptomatic period, they harbour their own limitations and side effects.26,83,84 PEG-IFNα has neuropsychiatric side effects and induces influenza-like symptoms, and ribavirin causes impaired renal function and anemia.83–85 Above all, these antiviral agents have teratogenic effects during pregnancy.9,38,84 Therefore, termination of pregnancy or early delivery of the foetus seems to be the most effective option to save mothers.38,39
Conclusions
This review has provided a general overview of the pathogenesis and epidemiology of HEV infection in pregnant women and children in Iran. HEV infection is generally a benign self-limited disease, but it can lead to serious consequences in susceptible populations such as pregnant women. It seems that a combination of host and viral factors contributes to the pathogenesis of hepatitis E in pregnancy; although, the exact reason behind this excess severity during pregnancy is not yet fully understood.
HEV infection is an underestimated disease in Iran, most probably due to a lack of HEV consideration in the country’s public health system. Despite being prevalent among children and pregnant women, our current knowledge regarding epidemiology of this viral infection in Iran is scarce. Therefore, further studies are required to more comprehensively determine the incidence and prevalence of HEV infection in these two groups in different regions of Iran. Once these data are obtained, the epidemiological pattern of this neglected infection in Iran will become clearer.
Acknowledgments
We would like to express our sincere gratitude to Professor Saeed Tajbakhsh, Head of the Department of Microbiology and Parasitology at the Bushehr University of Medical Sciences, for all of his support during the writing of this review.
Abbreviations
- HEV
hepatitis E virus
- HIV
human immunodeficiency virus
- WHO
World Health Organization
- TGF-β
transforming growth factor-beta
- NF-κb
nuclear factor-kappa B
- Th2
T helper 2
- HCG
human chorionic gonadotropin
- Th1
T helper 1
- FLF
fulminant liver failure
- HLA
human leukocyte antigen
- MHC
major histocompatibility complex
- NAT
nucleic acid amplification techniques
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- PEG-IFN
pegylated interferon
References
- 1.Pérez-Gracia MT, García M, Suay B, Mateos-Lindemann ML. Current Knowledge on Hepatitis E. J Clin Transl Hepatol. 2015;3:117–126. doi: 10.14218/JCTH.2015.00009. doi:10.14218/JCTH.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taherkhani R, Farshadpour F, Makvandi M, Rajabi Memari H, Samarbafzadeh AR, Sharifi N, et al. Cytokine profiles and cell proliferation responses to truncated ORF2 protein in Iranian patients recovered from hepatitis E infection. J Trop Med. 2015;2015:523560. doi: 10.1155/2015/523560. doi:10.1155/2015/523560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sridhar S, Lau SK, Woo PC. Hepatitis E: A disease of reemerging importance. J Formos Med Assoc. 2015;114:681–690. doi: 10.1016/j.jfma.2015.02.003. doi:10.1016/j.jfma.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajpai M, Gupta E. Transfusion-transmitted hepatitis E: is screening warranted? Indian J Med Microbiol. 2011;29:353–358. doi: 10.4103/0255-0857.90158. doi:10.4103/0255-0857.90158. [DOI] [PubMed] [Google Scholar]
- 5.Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116–138. doi: 10.1128/CMR.00057-13. doi:10.1128/CMR.00057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rockville M. Topic I: Hepatitis E virus (HEV) and blood transfusion safety blood products advisory committee, 104th Meeting; September 20, 2012; FDA Fishers Lane Building 5630 Fishers Lane, Room 1066. 2012. pp. 1–24. [Google Scholar]
- 7.Pischke S, Behrendt P, Bock CT, Jilg W, Manns MP, Wedemeyer H. Hepatitis E in Germany–an under-reported infectious disease. Dtsch Arztebl Int. 2014;111:577–583. doi: 10.3238/arztebl.2014.0577. doi:10.3238/arztebl.2014.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara S, Yokokawa Y, Morino K, Hayasaka K, Kawabata M, Shimizu T. Chronic hepatitis E: a review of the literature. J Viral Hepat. 2014;21:78–89. doi: 10.1111/jvh.12156. doi:10.1111/jvh.12156. [DOI] [PubMed] [Google Scholar]
- 9.Lapa D, Capobianchi MR, Garbuglia AR. Epidemiology of hepatitis E virus in European countries. Int J Mol Sci. 2015;16:25711–25743. doi: 10.3390/ijms161025711. doi:10.3390/ijms161025711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosseini Moghaddam SM. Hepatitis E virus and renal transplantation. Hepat Mon. 2011;11:599–600. doi: 10.5812/kowsar.1735143X.770. doi:10.5812/kowsar.1735143X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamar N. Hepatitis e virus infection in Iranian kidney-transplant patients. Hepat Mon. 2011;11:927–928. doi: 10.5812/kowsar.1735143X.791. doi:10.5812/kowsar.1735143X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fierro NA, Realpe M, Meraz-Medina T, Roman S, Panduro A. Hepatitis E virus: An ancient hidden enemy in Latin America. World J Gastroenterol. 2016;22:2271–2283. doi: 10.3748/wjg.v22.i7.2271. doi:10.3748/wjg.v22.i7.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrestha AC, Faddy HM, Flower RL, Seed CR, Keller AJ. Hepatitis E virus: do locally acquired infections in Australia necessitate laboratory testing in acute hepatitis patients with no overseas travel history? Pathology. 2015;47:97–100. doi: 10.1097/PAT.0000000000000229. doi:10.1097/PAT.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taherkhani R, Farshadpour F. Epidemiology of hepatitis E virus in Iran. World J Gastroenterol. 2016;22:5143–5153. doi: 10.3748/wjg.v22.i22.5143. doi:10.3748/wjg.v22.i22.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bazerbachi F, Haffar S, Garg SK, Lake JR. Extra-hepatic manifestations associated with hepatitis E virus infection: a comprehensive review of the literature. Gastroenterol Rep (Oxf) 2016;4:1–15. doi: 10.1093/gastro/gov042. doi:10.1093/gastro/gov042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH, Nelson KE, Panzner U, Kasture Y, Labrique AB, Wierzba TF. A systematic review of the epidemiology of hepatitis E virus in Africa. BMC Infect Dis. 2014;14:308. doi: 10.1186/1471-2334-14-308. doi:10.1186/1471-2334-14-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marano G, Vaglio S, Pupella S, Facco G, Bianchi M, Calizzani G, et al. Hepatitis E: an old infection with new implications. Blood Transfus. 2015;13:6–17. doi: 10.2450/2014.0063-14. doi:10.2450/2014.0063-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taherkhani R, Farshadpour F. A new strategy for development of hepatitis E vaccine: Epitope-based vaccines. Pathog Infect Dis. 2015;1:e933. [Google Scholar]
- 19.World Health Organization. Hepatitis E, WHO fact sheet; No. 280, updated July 2015. Available at: http://www.who.int/mediacentre/factsheets/fs280/en .
- 20.World Health Organization. Viral hepatitis report by the Secretariat; A62/22. Available at: apps.who.int/gb/ebwha/pdf_files/A62/A62_22-en.pdf .
- 21.Khuroo MS, Khuroo MS. Hepatitis E: an emerging global disease - from discovery towards control and cure. J Viral Hepat. 2016;23:68–79. doi: 10.1111/jvh.12445. doi:10.1111/jvh.12445. [DOI] [PubMed] [Google Scholar]
- 22.Teshale EH, Hu DJ. Hepatitis E: Epidemiology and prevention. World J Hepatol. 2011;3:285–291. doi: 10.4254/wjh.v3.i12.285. doi:10.4254/wjh.v3.i12.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian DY, Chen Y, Xia NS. Significance of serum IgA in patients with acute hepatitis E virus infection. World J Gastroenterol. 2006;12:3919–3923. doi: 10.3748/wjg.v12.i24.3919. doi:10.3748/wjg.v12.i24.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farshadpour F, Taherkhani R, Makvandi M, Rajabi Memari H, Samarbafzadeh AR. Codon-optimized expression and purification of truncated ORF2 protein of hepatitis E virus in escherichia coli. Jundishapur J Microbiol. 2014;7:e11261. doi: 10.5812/jjm.11261. doi:10.5812/jjm.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Zhuang H. Hepatitis E: an overview and recent advances in vaccine research. World J Gastroenterol. 2004;10:2157–2162. doi: 10.3748/wjg.v10.i15.2157. doi: 10.3748/WJG.v10.i15.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee GY, Poovorawan K, Intharasongkroh D, Sa-Nguanmoo P, Vongpunsawad S, Chirathaworn C, et al. Hepatitis E virus infection: Epidemiology and treatment implications. World J Virol. 2015;4:343–355. doi: 10.5501/wjv.v4.i4.343. 10.5501/wjv.v4.i4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verghese VP, Robinson JL. A systematic review of hepatitis E virus infection in children. Clin Infect Dis. 2014;59:689–697. doi: 10.1093/cid/ciu371. doi:10.1093/cid/ciu371. [DOI] [PubMed] [Google Scholar]
- 28.Farshadpour F, Makvandi M, Taherkhani R. Design, construction and cloning of truncated ORF2 and tPAsp-PADRE-truncated ORF2 gene cassette from hepatitis E virus in the pVAX1 expression vector. Jundishapur J Microbiol. 2015;8:e26035. doi: 10.5812/jjm.26035. doi:10.5812/jjm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed A, Ali IA, Ghazal H, Fazili J, Nusrat S. Mystery of hepatitis E virus: recent advances in its diagnosis and management. Int J Hepatol. 2015;2015:872431. doi: 10.1155/2015/872431. doi:10.1155/2015/872431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mushahwar IK. Hepatitis E virus: molecular virology, clinical features, diagnosis, transmission, epidemiology, and prevention. J Med Virol. 2008;80:646–658. doi: 10.1002/jmv.21116. doi:10.1002/jmv.21116. [DOI] [PubMed] [Google Scholar]
- 31.Yazbek S, Kreidieh K, Ramia S. Hepatitis E virus in the countries of the Middle East and North Africa region: an awareness of an infectious threat to blood safety. Infection. 2016;44:11–22. doi: 10.1007/s15010-015-0807-5. doi:10.1007/s15010-015-0807-5. [DOI] [PubMed] [Google Scholar]
- 32.Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. doi: 10.1016/S1473-3099(08)70255-X. doi:10.1016/S1473-3099(08)70255-X. [DOI] [PubMed] [Google Scholar]
- 33.Sayed IM, Vercauteren K, Abdelwahab SF, Meuleman P. The emergence of hepatitis E virus in Europe. Future Virology. 2015;10:763–778. doi:10.2217/fvl.15.29. [Google Scholar]
- 34.Jamali R. Epidemiologic studies on viral hepatitis: a short review. Thrita. 2014;3:e15376. doi:10.5812/thrita.15376. [Google Scholar]
- 35.Farshadpour F, Taherkhani R, Makvandi M. Prevalence of hepatitis E virus among adults in south-west of Iran. Hepat Res Treat. 2015;2015:759589. doi: 10.1155/2015/759589. doi:10.1155/2015/759589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadi Ghezeldasht S, Miri R, Hedayatimoghadam M, Shamsian A, Bidkhori H, Fathimoghadam F, et al. Population movement and virus spreading: HEV spreading in a Pilgrimage city, Mashhad in northeast Iran; an example. Hepat Mon. 2013;13:e10255. doi: 10.5812/hepatmon.10255. doi:10.5812/hepatmon.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhry SA, Verma N, Koren G. Hepatitis E infection during pregnancy. Can Fam Physician. 2015;61:607–608. [PMC free article] [PubMed] [Google Scholar]
- 38.El Sayed Zaki M, El Razek MM, El Razek HM. Maternal-fetal hepatitis E transmission: is it underestimated? J Clin Transl Hepatol. 2014;2:117–123. doi: 10.14218/JCTH.2014.00006. doi:10.14218/JCTH.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28:1190–1199. doi: 10.1111/j.1478-3231.2008.01840.x. doi:10.1111/j.1478-3231.2008.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar A, Begum N. Hepatitis E in pregnancy: an insight into etiopathogenesis. JIMSA. 2010;23:281–283. [Google Scholar]
- 41.Taherkhani R, Makvandi M, Farshadpour F. Development of enzyme-linked immunosorbent assays using 2 truncated ORF2 proteins for detection of IgG antibodies against hepatitis E virus. Ann Lab Med. 2014;34:118–126. doi: 10.3343/alm.2014.34.2.118. doi:10.3343/alm.2014.34.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kar P. Hepatitis E virus infection during pregnancy: why is the disease stormy? Medicine Update. 2012;22:459–462. [Google Scholar]
- 43.Bernuau J, Nicand E, Durand F. Hepatitis E-associated acute liver failure in pregnancy: an Indian puzzle. Hepatology. 2008;48:1380–1382. doi: 10.1002/hep.22619. doi:10.1002/hep.22619. [DOI] [PubMed] [Google Scholar]
- 44.Navaneethan U. Seroprevalence of hepatitis E infection in pregnancy - more questions than answers. Indian J Med Res. 2009;130:677–679. [PubMed] [Google Scholar]
- 45.Rostamzadeh Khameneh Z, Sepehrvand N, Khalkhali HR. Seroprevalence of hepatitis E among pregnant women in Urmia, Iran. Hepat Mon. 2013;13:e10931. doi: 10.5812/hepatmon.10931. doi:10.5812/hepatmon.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rasti M, Samarbafzadeh A, Neisi N, Makvandi M, Najafifard S, Sharifat M, et al. Study on the seroprevalence of hepatitis E virus infection in pregnant women referring to Imam Khomeini general hospital in Ahvaz. Jentashapir J Health Res. 2014;5:101–105. [Google Scholar]
- 47.Mamani M, Zamani M, Hashemi SH, Keramat F. Seroprevalence of antibodies to hepatitis E virus among pregnant women. Avicenna J Clin Microbiol Infect. 2015;2:e25339. doi:10.17795/ajcmi-25339. [Google Scholar]
- 48.Tabarraei A, Moradi A, Rodgari D, Javid N, Bakhshandeh Nosrat S. Anti-hepatitis E virus seroprevalence in pregnant women, in Gorgan, Iran, North East of Caspian Sea. International Conference on Life Science and Technology; Singapore. 2011. [Google Scholar]
- 49.Moradi A, Besharat S, Minaiifar MM, Roshandel G, Tabaraii A. Seroepidemiologic assessment of hepatitis E virus in women of reproductive age, Gorgan. Zahedan J Res Med Sci. 2010;12:44–47. [Google Scholar]
- 50.Begum N, Devi SG, Husain SA, Ashok K, Kar P. Seroprevalence of subclinical HEV infection in pregnant women from north India: a hospital based study. Indian J Med Res. 2009;130:709–713. [PubMed] [Google Scholar]
- 51.Stoszek SK, Abdel-Hamid M, Saleh DA, El Kafrawy S, Narooz S, Hawash Y, et al. High prevalence of hepatitis E antibodies in pregnant Egyptian women. Trans R Soc Trop Med Hyg. 2006;100:95–101. doi: 10.1016/j.trstmh.2004.12.005. doi:10.1016/j.trstmh.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Adjei AA, Tettey Y, Aviyase JT, Adu-Gyamfi C, Obed S, Mingle JA, et al. Hepatitis E virus infection is highly prevalent among pregnant women in Accra, Ghana. Virol J. 2009;6:108. doi: 10.1186/1743-422X-6-108. doi:10.1186/1743-422X-6-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar RM, Uduman S, Rana S, Kochiyil JK, Usmani A, Thomas L. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur J Obstet Gynecol Reprod Biol. 2001;100:9–15. doi: 10.1016/s0301-2115(01)00448-1. doi:10.1016/S0301-2115(01)00448-1. [DOI] [PubMed] [Google Scholar]
- 54.Oncu S, Oncu S, Okyay P, Ertug S, Sakarya S. Prevalence and risk factors for HEV infection in pregnant women. Med Sci Monit. 2006;12:CR36–39. [PubMed] [Google Scholar]
- 55.Saffar MJ, Farhadi R, Ajami A, Khalilian AR, Babamahmodi F, Saffar H. Seroepidemiology of hepatitis E virus infection in 2–25-year-olds in Sari district, Islamic Republic of Iran. East Mediterr Health J. 2009;15:136–142. [PubMed] [Google Scholar]
- 56.Shamsizadeh A, Nikfar R, Makvandi M, Shamsizadeh N. Seroprevalence of hepatitis E virus infection in children in the Southwest of Iran. Hepat Mon. 2009;9:261–264. [Google Scholar]
- 57.Reza Sharif A, Reza Sharif M, Taghavi Ardekani A, Madani M, Kheirkhah D, Afzali H. Seroepidemiology of hepatitis E in children of Kashan, 2012. Iranian J Infect Dis Trop Med. 2014;18:31–36. [Google Scholar]
- 58.Ataei B, Nokhodian Z, Javadi AA, Kassaian N, Shoaei P, Farajzadegan Z, et al. Hepatitis E virus in Isfahan Province: a population-based study. Int J Infect Dis. 2009;13:67–71. doi: 10.1016/j.ijid.2008.03.030. doi:10.1016/j.ijid.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 59.Asaei S, Ziyaeyan M, Moeini M, Jamalidoust M, Behzadi MA. Seroprevalence of hepatitis A and E virus infections among healthy population in Shiraz, Southern Iran. Jundishapur J Microbiol. 2015;8:e19311. doi: 10.5812/jjm.19311v2. doi:10.5812/jjm.19311v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fix AD, Abdel-Hamid M, Purcell RH, Shehata MH, Abdel-Aziz F, Mikhail N, et al. Prevalence of antibodies to hepatitis E in two rural Egyptian communities. Am J Trop Med Hyg. 2000;62:519–523. doi: 10.4269/ajtmh.2000.62.519. [DOI] [PubMed] [Google Scholar]
- 61.Taremi M, Mohammad Alizadeh AH, Ardalan A, Ansari S, Zali MR. Seroprevalence of hepatitis E in Nahavand, Islamic Republic of Iran: a population-based study. East Mediterr Health J. 2008;14:157–162. [PubMed] [Google Scholar]
- 62.Teshale EH, Hu DJ, Holmberg SD. The two faces of hepatitis E virus. Clin Infect Dis. 2010;51:328–334. doi: 10.1086/653943. doi:10.1086/653943. [DOI] [PubMed] [Google Scholar]
- 63.Vasickova P, Psikal I, Kralik P, Widen F, Hubalek Z, Pavlik I. Hepatitis E virus: a review. Veterinarni Medicina. 2007;52:365–384. [Google Scholar]
- 64.Aggarwal R. Diagnosis of hepatitis E. Nat Rev Gastroenterol Hepatol. 2013;10:24–33. doi: 10.1038/nrgastro.2012.187. doi:10.1038/nrgastro.2012.187. [DOI] [PubMed] [Google Scholar]
- 65.Dreier J, Juhl D. Autochthonous hepatitis e virus infections: a new transfusion-associated risk? Transfus Med Hemother. 2014;41:29–39. doi: 10.1159/000357098. doi:10.1159/000357098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shahzad F, Atiq M, Ejaz S, Hameed S. Hepatitis E: review of a disease endemic in Pakistan. J Pak Med Assoc. 2001;51:166–169. [PubMed] [Google Scholar]
- 67.Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, et al. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine. 2005;23:2893–2901. doi: 10.1016/j.vaccine.2004.11.064. doi:10.1016/j.vaccine.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 68.Purdy MA, McCaustland KA, Krawczynski K, Tam A, Beach MJ, Tassopoulos NC, et al. Expression of a hepatitis E virus (HEV)-trpE fusion protein containing epitopes recognized by antibodies in sera from human cases and experimentally infected primates. Arch Virol. 1992;123:335–349. doi: 10.1007/BF01317268. doi:10.1007/BF01317268. [DOI] [PubMed] [Google Scholar]
- 69.Robinson RA, Burgess WH, Emerson SU, Leibowitz RS, Sosnovtseva SA, Tsarev S, et al. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr Purif. 1998;12:75–84. doi: 10.1006/prep.1997.0817. doi:10.1006/prep.1997.0817. [DOI] [PubMed] [Google Scholar]
- 70.Zhang JZ, Ng MH, Xia NS, Lau SH, Che XY, Chau TN, et al. Conformational antigenic determinants generated by interactions between a bacterially expressed recombinant peptide of the hepatitis E virus structural protein. J Med Virol. 2001;64:125–132. doi: 10.1002/jmv.1027. doi:10.1002/jmv.1027. [DOI] [PubMed] [Google Scholar]
- 71.Li TC, Takeda N, Miyamura T, Matsuura Y, Wang JC, Engvall H, et al. Essential elements of the capsid protein for self-assembly into empty virus-like particles of hepatitis E virus. J Virol. 2005;79:12999–13006. doi: 10.1128/JVI.79.20.12999-13006.2005. doi:10.1128/JVI.79.20.12999-13006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McAtee CP, Zhang Y, Yarbough PO, Fuerst TR, Stone KL, Samander S, et al. Purification and characterization of a recombinant hepatitis E protein vaccine candidate by liquid chromatography-mass spectrometry. J Chromatogr B Biomed Appl. 1996;685:91–104. doi: 10.1016/0378-4347(96)00143-0. doi:10.1016/0378-4347(96)00143-0. [DOI] [PubMed] [Google Scholar]
- 73.Huang WJ, Zhang HY, Harrison TJ, Lan HY, Huang GY, Wang YC. Immunogenicity and protective efficacy in rhesus monkeys of a recombinant ORF2 protein from hepatitis E virus genotype 4. Arch Virol. 2009;154:481–488. doi: 10.1007/s00705-009-0335-7. doi:10.1007/s00705-009-0335-7. [DOI] [PubMed] [Google Scholar]
- 74.Kamili S, Spelbring J, Carson D, Krawczynski K. Protective efficacy of hepatitis E virus DNA vaccine administered by gene gun in the cynomolgus macaque model of infection. J Infect Dis. 2004;189:258–264. doi: 10.1086/380801. doi:10.1086/380801. [DOI] [PubMed] [Google Scholar]
- 75.Kamili S, Spelbring J, Krawczynski K. DNA vaccination against hepatitis E virus infection in cynomolgus macaques. J Gastroenterol Hepatol. 2002;17(Suppl 3):S365–S369. doi: 10.1046/j.1440-1746.17.s3.29.x. doi:10.1046/j.1440-1746.17.s3.29.x. [DOI] [PubMed] [Google Scholar]
- 76.Arankalle VA, Lole KS, Deshmukh TM, Srivastava S, Shaligram US. Challenge studies in Rhesus monkeys immunized with candidate hepatitis E vaccines: DNA, DNA-prime-protein-boost and DNA-protein encapsulated in liposomes. Vaccine. 2009;27:1032–1039. doi: 10.1016/j.vaccine.2008.11.097. doi:10.1016/j.vaccine.2008.11.097. [DOI] [PubMed] [Google Scholar]
- 77.Taherkhani R, Farshadpour F, Makvandi M. Design and production of a multiepitope construct derived from hepatitis E virus capsid protein. J Med Virol. 2015;87:1225–1234. doi: 10.1002/jmv.24171. doi:10.1002/jmv.24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu T, Li SW, Zhang J, Ng MH, Xia NS, Zhao Q. Hepatitis E vaccine development: a 14 year odyssey. Hum Vaccin Immunother. 2012;8:823–827. doi: 10.4161/hv.20042. doi:10.4161/hv.20042. [DOI] [PubMed] [Google Scholar]
- 79.Zhang J, Liu CB, Li RC, Li YM, Zheng YJ, Li YP, et al. Randomized-controlled phase II clinical trial of a bacterially expressed recombinant hepatitis E vaccine. Vaccine. 2009;27:1869–1874. doi: 10.1016/j.vaccine.2008.12.061. doi:10.1016/j.vaccine.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J, Shih JW, Wu T, Li SW, Xia NS. Development of the hepatitis E vaccine: from bench to field. Semin Liver Dis. 2013;33:79–88. doi: 10.1055/s-0033-1338116. doi:10.1055/s-0033-1338116. [DOI] [PubMed] [Google Scholar]
- 81.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. doi:10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 82.Abbas Z, Afzal R. Hepatitis E: when to treat and how to treat. Antivir Ther. 2014;19:125–131. doi: 10.3851/IMP2705. doi:10.3851/IMP2705. [DOI] [PubMed] [Google Scholar]
- 83.Nijskens CM, Pas SD, van der Eijk AA, de Man RA. Hepatitis E in Europe: diagnosis and treatment. EMJ Gastroenterol. 2015;4:121–127. [Google Scholar]
- 84.Debing Y, Neyts J. Antiviral strategies for hepatitis E virus. Antiviral Res. 2014;102:106–118. doi: 10.1016/j.antiviral.2013.12.005. doi:10.1016/j.antiviral.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat. 2015;22:965–973. doi: 10.1111/jvh.12403. doi:10.1111/jvh.12403. [DOI] [PubMed] [Google Scholar]