Abstract
Background
Myocardial mechanical dyssynchrony induced by the presence of postinfarction scar and/or conduction abnormalities in patients with a left ventricular ejection fraction (LVEF) of < 35 % may be associated with a greater propensity toward inducing serious ventricular arrhythmia [(ventricular tachycardia (VT), ventricular fibrillation (VF)] and sudden cardiac death. The assessment of regional myocardial function using tissue Doppler echocardiography (TDE) allows for noninvasive analysis of regional mechanical dysfunction (LV mechanical dispersion).
Aim
The aim of this study was to evaluate the TDE-based mechanical dispersion as a potential echocardiographic predictor of VT/VF.
Methods
The study group consisted of 47 consecutive ambulatory patients with implanted cardiac resynchronization therapy–defibrillator (CRT-D) devices who were divided into two groups: Group 1 (n = 29) comprised patients with recorded episodes of VT/VF, in whom baseline TDE data were available, and group 2 (n = 18) comprised patients without registered VT/VF in the device memory within 4 years after implantation. LV mechanical dispersion was defined as the standard deviation of the time measured from the beginning of the QRS complex to the peak longitudinal strain in apical four-chamber and two-chamber views. A retrospective quantitative assessment of LV regional deformation was based on the color tissue velocity recordings.
Results
The average time to event after implantation was 345 days. Patients with electrical events demonstrated greater mechanical dispersion: 99.14 ± 33.60 vs. 72.98 ± 19.70, p=0.002.
Conclusion
During the 4-year follow-up, patients with documented VT/VF were characterized by significantly higher LV mechanical dispersion as compared with patients without electrical events. Measurement of LV mechanical dispersion might be helpful in determining the risk of sudden cardiac death.
Keywords: Dilated cardiomyopathy, Sudden cardiac death, Implantable cardioverter–defibrillator, Mechanical dyssynchrony, Ultrasound strain
Zusammenfassung
Hintergrund
Eine myokardiale mechanische Dyssynchronie, die durch eine Narbe nach Infarkt und/oder Erregungsleitungsstörungen bei Patienten mit linksventrikulärer Ejektionsfraktion (LVEF) < 35 % induziert wird, kann mit einem höheren Risiko der Induktion schwerer ventrikulärer Arrhythmien (ventrikuläre Tachykardie, VT; Kammerflimmern) und plötzlichem Herztod einhergehen. Die Untersuchung der regionalen Myokardfunktion mit der Gewebsdopplerechokardiographie (TDE) ermöglicht die nichtinvasive Erkennung einer regionalen mechanischen Dysfunktion (mechanische LV-Dispersion).
Ziel
Ziel der vorliegenden Studie war es, die TDE-basierte mechanische Dispersion als potenziellen echokardiographischen Prädiktor von VT/Kammerflimmern zu untersuchen.
Methoden
Die Studiengruppe bestand aus 47 konsekutiven ambulanten Patienten mit implantiertem CRT-D (kardialer Resynchronisationstherapie-Defibrillator), die in 2 Gruppen aufgeteilt wurden: Gruppe 1 (n = 29) – Patienten mit dokumentierten Episoden von VT/Kammerflimmern, bei denen Ausgangs-TDE-Daten verfügbar waren, und Gruppe 2 (n = 18) – Patienten ohne Registrierung von VT/Kammerflimmern im Gerätespeicher innerhalb von 4 Jahren nach Implantation. Die mechanische LV-Dispersion wurde definiert als Standardabweichung der gemessenen Dauer vom Beginn des QRS-Komplexes bis zur größten longitudinalen Verformung im apikalen 4-Kammer-Blick und 2-Kammer-Blick. Eine retrospektive quantitative Beurteilung der regionalen LV-Verformung basierte auf der Dokumentation der Farb-Gewebedoppler-Geschwindigkeit.
Ergebnisse
Die durchschnittliche Dauer bis zu einem Ereignis nach Implantation betrug 345 Tage. Patienten mit elektrisch registrierten Ereignissen wiesen eine höhere mechanische Dispersion auf (99,14 ± 33,60 vs. 72,98 ± 19,70; p = 0,002).
Schlussfolgerung
Während der 4-jährigen Nachbeobachtungsphase wiesen die Patienten mit Dokumentation von VT/Kammerflimmern eine signifikant höhere mechanische LV-Dispersion auf als die Patienten ohne elektrisch registrierte Ereignisse. Die Messung der mechanischen LV-Dispersion könnte zur Ermittlung des Risikos für einen plötzlichen Herztod von Nutzen sein.
Schlüsselwörter: Dilatative Kardiomyopathie, Plötzlicher Herztod, ICD, Mechanische Dyssynchronie, Sonographische Verformung
Epidemiological studies indicate that sudden cardiac death is the cause of 20–159 deaths per 100,000 residents per year in Europe and 84–200 deaths/100,000 residents/year in the United States. According to the current recommendations of cardiological societies, patients with a history of myocardial infarction and reduced ejection fraction (EF < 35 %) should be protected by cardioverter–defibrillator implantation. Impaired EF has been shown to be a marker of increased cardiovascular mortality and sudden cardiac death [1, 2], but it has relatively low sensitivity for detecting arrhythmia and sudden cardiac death risk [3].
The diagnosis of mechanical dyssynchrony induced by the presence of infarction scar and/or conduction abnormalities in patients with an EF of < 35 % may be associated with a greater propensity for inducing serious ventricular arrhythmia [ventricular tachycardia (VT), ventricular fibrillation (VF)] and sudden cardiac death. The assessment of regional myocardial function using tissue Doppler echocardiography (TDE) allows for noninvasive analysis of the regional mechanical dysfunction (LV mechanical dispersion). Therefore, the aim of this study was to evaluate mechanical dispersion as an echocardiographic predictor of VT/VF.
Patients and methods
The study group consisted of 47 consecutive patients with cardiac resynchronization therapy defibrillator (CRT-D) devices implanted in the Department of Cardiology, Congenital Heart Diseases and Electrotherapy, of the Silesian Center for Heart Diseases in Zabrze between 2008 and 2009. All patients who were included in the study met the criteria for CRT-D implantation, had an EF of < 35 %, and were followed up for more than 3 years. The study population was divided into two groups: Group 1 (n = 29) comprised patients who had recorded episodes of arrhythmic events; group 2 (n = 18) comprised patients who did not have any registered arrhythmic events within 4 years after implantation.
Arrhythmic events were defined as ventricular arrhythmias that required appropriate antitachycardia pacing or shock released by an implantable cardioverter–defibrillator (ICD) and included both VF and sustained VT (sVT). All data on arrhythmic events were reviewed retrospectively by a physician experienced in clinical pacing.
Echocardiography
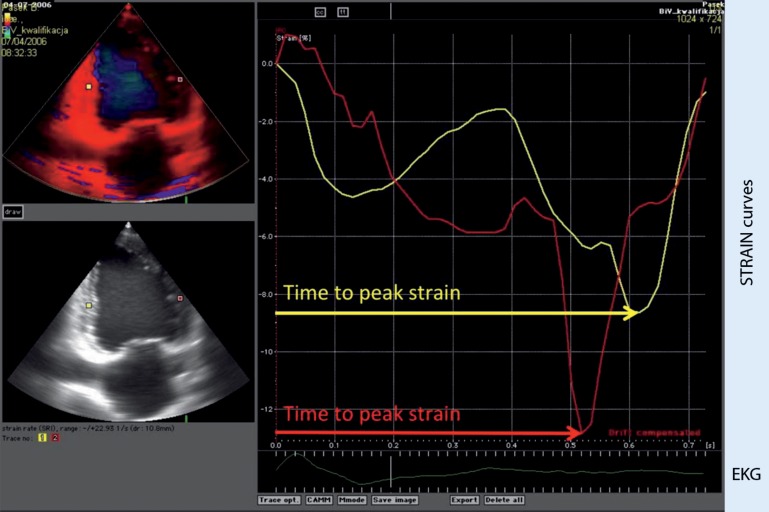
An echocardiographic study including standard measurement and TDE was completed for each patient before device implantation using the Vivid 5 System (GE-Vingmed, GE Healthcare). Digital echocardiographic recordings were analyzed retrospectively using ECHOPAC 9.0 software (GE Healthcare). A quantitative assessment of left ventricular (LV) regional deformation was based on the color tissue velocity recordings. In our study, global LV function parameters were calculated: LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), interventricular septum (IVS) thickness, LV posterior wall (LVPW) thickness, end-systolic volume (ESV), end-diastolic volume (EDV), as well as regional LV function parameters—strain parameters (time to peak strain and postsystolic strain) and velocity parameters (time to onset velocity, time to peak velocity, and time to end of systole) (Fig. 1). LVEF was assessed according to Simpson’s biplane method. Myocardial strain was calculated based on the color tissue velocity data recorded in two-chamber and four-chamber apical view. Subsequently, longitudinal strain was quantified for each myocardial segment [4]. LV mechanical dispersion was defined as the standard deviation of the time measured from the beginning of the QRS complex to peak longitudinal segmental strain.
Fig. 1.
Tissue Doppler echocardiography-based longitudinal strain curves derived from mid-inferior and mid-anterior segments in two-chamber view
Statistical analysis
Continuous parameters are expressed as means with standard deviations, categorical variables are presented as numbers and percentages. Comparative analysis between groups was performed using Student’s t test for continuous variables and the chi-square test, as appropriate, for dichotomous parameters. All p values less than 0.05 were considered significant. The dispersion value with optimal sensitivity and specificity was identified with the use of receiver-operating characteristics.
Results
Post-CRT-D implantation arrhythmic events were recorded in 29 patients (group 1), whereas 18 patients did not experience arrhythmia (group 2). Four patients of group 1 and two of group 2 received a CRT-D for secondary prevention (13.79 vs. 11.11 %, p = 0.789). The clinical characteristics of the patients are presented in Table 1. There were no significant differences between groups according to age, sex, and body mass index. No significant differences between groups were observed for ischemic diseases, chronic heart failure, arterial hypertension, hyperlipidemia, and atrial fibrillation. Patients suffering from cardiomyopathy predominated in group 1 (79.31 % in group 1 vs. 44.44 % in group 2, p = 0.014). There was no significant difference in QRS duration, mean blood pressure, hematocrit, INR, and potassium level between the two groups. No significant differences between groups were observed regarding the intake of beta-adrenergic blocking agents and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers.
Table 1.
Clinical characteristics of study population
| Group 1 patients with VT/VF during follow-up (n = 29) | Group 2 patients without VT/VF during follow-up (n = 18) | p | |
|---|---|---|---|
| Age (years) | 66.38 ± 8.81 | 68.50 ± 12.23 | NS |
| Sex (M = 1) n (%) | 24 (82 %) | 12 (67 %) | NS |
| BMI | 28.02 ± 5.54 | 27.54 ± 4.26 | NS |
| Ischemic etiology, n (%) | 15 (52 %) | 14 (78 %) | NS |
| Arterial hypertension, n (%) | 11 (38 %) | 12 (67 %) | NS |
| Cardiomyopathy, n (%) | 23 (79 %) | 8 (44 %) | 0.014 |
| DM total, n (%) | 11 (38 %) | 5 (28 %) | NS |
| Hyperlipidemia, n (%) | 7 (24 %) | 5 (28 %) | NS |
| Atrial fibrillation, n (%) | 9 (31 %) | 5 (28 %) | NS |
| QRS duration (ms) | 161.59 ± 32.34 | 179.94 ± 35.42 | NS |
| Mean blood pressure (mmHg) | 96.94 ± 10.10 | 100.97 ± 12.26 | NS |
| K (mmol/l) | 4.51 ± 0.49 | 4.44 ± 0.40 | NS |
| Pre-implantation PCI, n (%) | 10 (34 %) | 6 (33 %) | NS |
| Beta-blocker, n (%) | 25 (86 %) | 16 (89 %) | NS |
| ACEI/ARB, n (%) | 24 (83 %) | 15 (83 %) | NS |
ACEI/ARB angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, BMI body mass index, DM diabetes mellitus, K potassium, NS not significant, PCI percutaneous coronary intervention, VF ventricular fibrillation, VT ventricular tachycardia
The average time to event after implantation was 345 ± 317 days. Echocardiographic findings are presented in Table 2. There were significant differences between groups 1 and 2 with respect to the timing of mechanical dispersion: (99.14 ± 33.60 vs. 72.98 ± 19.70 ms, p = 0.002). However, there were no significant differences between groups in terms of other echocardiographic parameters.
Table 2.
Echocardiographic measurements in all patients
| Group 1 patients with VT/VF during follow-up (n = 29) | GR 2 patients without VT/VF during follow-up (n = 18) | p | |
|---|---|---|---|
| Septal flash, n (%) | 14 (48 %) | 10 (56 %) | NS |
| EF (%) | 27.95 ± 10.68 | 28.87 ± 8.69 | NS |
| ESV (ml) | 191.71 ± 90.05 | 172.73 ± 73.52 | NS |
| EDV (ml) | 249.89 ± 108.69 | 215.46 ± 79.99 | NS |
| LVES dimension (mm) | 67.70 ± 12.43 | 67.67 ± 7.74 | NS |
| LVED dimension (mm) | 55.53 ± 16.38 | 54.67 ± 10.13 | NS |
| IVSD (mm) | 11.44 ± 5.12 | 10.33 ± 2.73 | NS |
| IVSS (mm) | 14.29 ± 5.70 | 14.17 ± 3.82 | NS |
| LVPWD (mm) | 10.50 ± 2.39 | 10.20 ± 1.48 | NS |
| LVPWS (mm) | 13.86 ± 4.04 | 13.40 ± 2.88 | NS |
| Mechanical dispersion (ms) | 99.14 ± 33.60 | 72.98 ± 19.70 | 0.002 |
EDV end-diastolic volume, EF ejection fraction, ESV end-systolic volume, IVSD interventricular septum diastolic diameter, IVSS interventricular septum systolic diameter, LVES left ventricular end systolic, LVED left ventricular end diastolic, LVPWD left ventricular posterior wall diastolicdiameter, LVPWS left ventricular posterior wall systolic diameter, NS not significant
Patients with ischemic heart failure
Among the analyzed patients, those with ischemic etiology (patients after myocardial infarction and patients suffering from coronary artery disease or ischemic cardiomyopathy) were selected and divided into two groups: group ISCH 1 comprising patients with VT/VF (n = 15) during follow-up and group ISCH 2 comprising patients without VT/VF events during follow-up (n = 14). The results and echocardiographic characteristics for patients with heart failure of ischemic etiology are presented in Table 3. The average time to event post-device implantation in this group was 289 ± 267 days. Mechanical dispersion was greater in patients with documented arrhythmic events (group ISCH 1; 97.80 ± 30.06 vs. 74.15 ± 15.72 ms; p = 0.014).
Table 3.
Echocardiographic measurements in patients with heart failure of ischemic etiology
| ISCH 1 patients with VT/VF during follow-up (n = 15) | ISCH 2 patients without VT/VF during follow-up (n = 14) | p | |
|---|---|---|---|
| Septal flash, n (%) | 8 (53 %) | 9 (64 %) | NS |
| EF (%) | 29.33 ± 10.25 | 29.85 ± 8.91 | NS |
| ESV (ml) | 180.00 ± 79.33 | 173.00 ± 77.50 | NS |
| EDV (ml) | 252.67 ± 89.58 | 224.27 ± 81.74 | NS |
| LVES dimension (mm) | 66.50 ± 11.97 | 68.10 ± 7.20 | NS |
| LVED dimension (mm) | 56.22 ± 16.20 | 54.50 ± 9.20 | NS |
| IVSD (mm) | 11.50 ± 4.87 | 10.50 ± 1.29 | NS |
| IVSS (mm) | 13.00 ± 6.32 | 15.00 ± 1.15 | NS |
| LVPWS (mm) | 10.75 ± 3.01 | 10.67 ± 1.15 | NS |
| LVPWD (mm) | 13.67 ± 5.28 | 14.67 ± 2.08 | NS |
| Mechanical dispersion (ms) | 97.80 ± 30.06 | 74.15 ± 15.72 | 0.014 |
EDV end-diastolic volume, EF ejection fraction, ESV end-systolic volume, IVSD interventricular septum diastolic diameter, IVSS interventricular septum systolic diameter, LVES left ventricular end systolic, LVED left ventricular end diastolic, LVPWD left ventricular posterior wall diastolic diameter, LVPWS left ventricular posterior wall systolic diameter, NS not significant
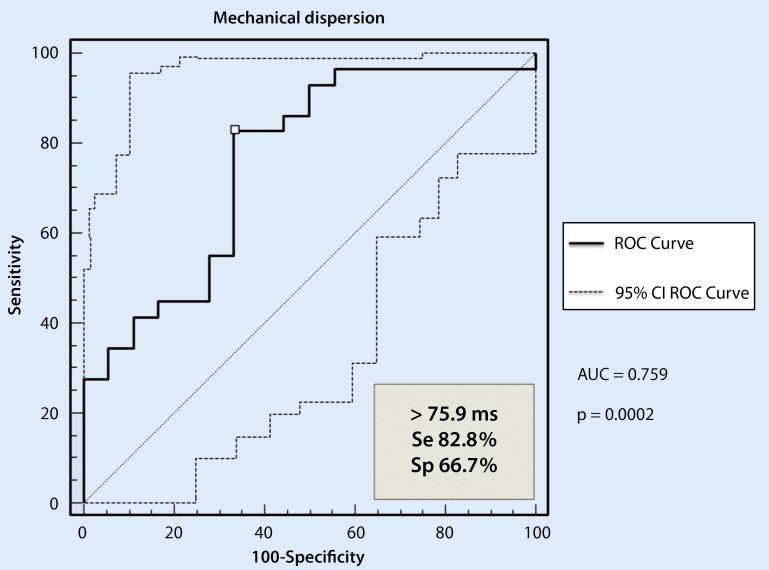
ROC curve analysis
As presented in Fig. 2, the cutoff value for mechanical dispersion of 76 ms provided 67 % specificity and 83 % sensitivity. Measurements of mechanical dispersion and global strain in ischemic patients add important information about the risk of sudden cardiac death apart from information provided by the EF. In patients with a preserved or slightly reduced EF, mechanical dispersion above 76 ms identified ischemic patients with an increased risk of sudden cardiac death.
Fig. 2.
ROC curve for the ability of mechanical dispersion to identify arrhythmic events during a 4-year follow-up in ischemic patients
Discussion
The etiology of sudden cardiac death is multifactorial. Several risk factors have been identified, such as decreased LVEF, history of coronary artery disease, cigarette smoking, hypertension, obesity, male sex, heart failure, ventricular arrhythmias, increased ventricular ectopy, diminished heart rate variability, baroreflex sensitivity, and heart rate profile during exercise [5]. Randomized clinical trials indicate that 84 % of sudden deaths are due to ventricular tachyarrhythmias, while bradyarrhythmias were responsible for 16 % of sudden cardiac deaths. VF was the most common ventricular tachyarrhythmia, usually secondary to VT, whereas the rest of the arrhythmias were caused by torsade de pointes [6]. The best method to prevent sudden cardiac death in high-risk patients is ICD. ICD reduces mortality in patients selected for primary prevention of SCD on the basis of reduced LVEF. ICD can prevent sudden cardiac death caused by bradyarrhythmias, torsade de pointes associated with congenital long-QT syndrome (LQTS), and pause-dependent VT [7]. An impaired EF (< 35 %) is the main indication for cardioverter–defibrillator implantation in patients after myocardial infarction. Hypertrophic obstructive cardiomyopathy, long QT and Brugada syndromes, and idiopathic VF are additional indications. Although LVEF is still regarded as a good predictor of ventricular arrhythmias [8, 9], it has several limitations in terms of predicting sudden cardiac death. In the Oregon Sudden Unexpected Death Study, only 30 % of sudden cardiac death cases met the criteria; 65 % of patients, who had LV function measured before sudden cardiac death, did not have severe LV dysfunction [10]. Other clinical studies indicated that 52 % patients with sudden cardiac death had some decrease in LV systolic function, while 30 % had severely decreased LV systolic function. Therefore, based on current LVEF guidelines for sudden cardiac death prevention, only 30 % would have qualified as candidates for a prophylactic ICD. Patients who had sudden cardiac death and normal LVEF were more often female, younger, more likely to have a seizure disorder, and more likely to be taking antiepileptics compared with patients with decreased LVEF [11–16].
A variety of mechanisms in heart failure can lead to sudden cardiac death such as remodeling of the myocardium, altered neurohumoral signaling, slowed conduction, impaired repolarization, poor coupling of myocardium, and delayed-paced ventricular activation. These factors make the myocardium susceptible to arrhythmia triggers [17]. The presence of scar tissue in the myocardium after myocardial infarction causes electrical heterogeneity, changes in expression of ion channels, delayed electrical conduction, a dispersed recovery of excitability, and dispersed electrical repolarization. Electrophysiological testing could be an objective screening tool; however, it is invasive, expensive, and impractical. Therefore, there is a need for a sensitive tool for evaluating the risk of sudden cardiac death.
Electrical dispersion results in altered myocardial function. Regional and global myocardial function and timing can be evaluated by tissue Doppler imaging of strain [18]. Mechanical dispersion, which can be defined as heterogeneous contraction assessed by myocardial strain, is useful for the noninvasive measurement of LV function and the timing of myocardial contraction, and it may be a good predictor of arrhythmic episodes. Clinical trials indicated mechanical dispersion to be a good tool for predicting VT/VF in patients with long QT syndrome [19]. Moreover, mechanical dispersion by myocardial strain is related to episodes of ventricular arrhythmia in patients with ARVC.
The concept of using ultrasound-based mechanical dyssynchrony as a marker of sudden cardiac death was introduced for the first time by Norwegian researchers [20]. The results of our study confirmed to some extent their findings that greater mechanical dispersion occurs in patients with documented severe ventricular arrhythmias. Mechanical dispersion may become an important parameter for evaluating the risk of sudden cardiac death and the necessity for cardioverter–defibrillator implantation. Segmental contractility disorders are the result of electrical abnormalities that appear in the place of postinfarction scars. The asynchronous work of all segments causes hemodynamic cardiac inefficiency, and therefor there is a need for ICD therapy. Measurement of mechanical dispersion might help to better select patients who will be at risk of severe ventricular arrhythmia and sudden cardiac death. This parameter may be useful in clinical work for selecting patients who are in need of better care so as to avoid sudden cardiac death.
Study limitations
It was a retrospective, pilot study covering a relatively small group of patients. Moreover, consecutive patients formed the study group, derived from a hospital database with complete digital echo and clinical data.
Conclusion
This study demonstrates that during a 4-year follow-up, patients with documented VT/VF were characterized by significantly higher LV mechanical dispersion compared with patients without electrical events. The measurement of LV mechanical dispersion might be helpful in determining the risk of sudden cardiac death. The determination of LV mechanical dispersion in each individual patient could be helpful in selecting patients for ICD therapy. However, this new ultrasound-based parameter of mechanical dispersion requires further validation in a bigger cohort of patients.
Compliance with ethical standards
Conflict of interest
G. Banasik, O. Segiet, M. Elwart, M. Szulik, R. Lenarczyk, Z. Kalarus, and T. Kukulski state that there are no conflicts of interest.
References
- 1.Emond M, Mock MB, Davis KB, et al. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation. 1994;90:2645–2657. doi: 10.1161/01.CIR.90.6.2645. [DOI] [PubMed] [Google Scholar]
- 2.Quinones MA, Greenberg BH, Kopelen HA, et al. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: significance of left ventricular hypertrophy. Studies of left ventricular dysfunction. J Am Coll Cardiol. 2000;35:1237–1244. doi: 10.1016/S0735-1097(00)00511-8. [DOI] [PubMed] [Google Scholar]
- 3.Buxton AE, Lee KL, Hafley GE, et al. Limitations of ejection fraction for prediction of sudden death risk in patients with coronary artery disease: lessons from the MUSTT study. J Am Coll Cardiol. 2007;50:1150–1157. doi: 10.1016/j.jacc.2007.04.095. [DOI] [PubMed] [Google Scholar]
- 4.Pirat B, Khoury DS, Hartley CJ, et al. A novel feature-tracking echocardiographic method for the quantitation of regional myocardial function: validation in an animal model of ischemia- reperfusion. J Am Coll Cardiol. 2008;51:651–659. doi: 10.1016/j.jacc.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cupples LA, Gagnon DR, Kannel WB. Long- and short-term risk of sudden cardiac death: population at risk. Circulation. 1992;85(1):I11–18. [PubMed] [Google Scholar]
- 6.Zipes DP. Sudden cardiac death: future approaches. Circulation. 1992;85(1):I160–166. [PubMed] [Google Scholar]
- 7.Passman R, Kadish A. Sudden death prevention with implantable devices. Circulation. 2007;116:561–571. doi: 10.1161/CIRCULATIONAHA.106.655704. [DOI] [PubMed] [Google Scholar]
- 8.Bigger JT, Jr, Fleiss JL, Kleiger R, et al. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984;69:250–258. doi: 10.1161/01.CIR.69.2.250. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg H, McMaster P, Dwyer EM., Jr Left ventricular dysfunction after acute myocardial infarction: results of a prospective multicenter study. J Am Coll Cardiol. 1984;4:867–874. doi: 10.1016/S0735-1097(84)80045-5. [DOI] [PubMed] [Google Scholar]
- 10.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–1166. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Buxton AE, Lee KL, Fisher JD, Josephson ME, et al. Multicenter Unsustained Tachycardia Trial Investigators. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med. 1999;341:1882–1890. doi: 10.1056/NEJM199912163412503. [DOI] [PubMed] [Google Scholar]
- 12.Moss AJ, Hall WJ, Cannom DS, et al. Multicenter Automatic Defibrillator Implantation Trial Investigators. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med. 1996;335:1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 13.Moss AJ, Zareba W, Jackson Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 14.Myerburg RJ, Mitrani R, Interian A, Jr, Castellanos A. Interpretation of outcomes of antiarrhythmic clinical trials: design features and population impact. Circulation. 1998;97:1514–1521. doi: 10.1161/01.CIR.97.15.1514. [DOI] [PubMed] [Google Scholar]
- 15.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, et al. Out-of-hospital cardiac arrest in the 1990s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–1505. doi: 10.1016/S0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 16.Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest—the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204–1209. doi: 10.1016/S0195-668X(03)00191-X. [DOI] [PubMed] [Google Scholar]
- 17.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 18.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 19.Haugaa KH, Edvardsen T, Leren TP, Gran JM, Smiseth OA, Amlie JP. Left ventricular mechanical dispersion by tissue Doppler imaging: a novel approach for identifying high-risk individuals with long QT syndrome. Eur Heart J. 2009;30:330–337. doi: 10.1093/eurheartj/ehn466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haugaa KH, Smedsrud MK, Steen T, et al. Mechanical dispersion assessed by myocardial strain in patients after myocardial infarction for risk prediction of ventricular arrhytmia. JACC Cardiovasc Imaging. 2010;3(3):247–256. doi: 10.1016/j.jcmg.2009.11.012. [DOI] [PubMed] [Google Scholar]