Abstract
Background
Antiarrhythmic drugs are widely used to treat patients with atrial fibrillation (AF), but the mechanisms conveying their variable effectiveness are not known. Recent data suggested that paired like homeodomain-2 transcription factor (PITX2) might play an important role in regulating gene expression and electrical function of the adult left atrium (LA).
Objectives
After determining LA PITX2 expression in AF patients requiring rhythm control therapy, the authors assessed the effects of Pitx2c on LA electrophysiology and the effect of antiarrhythmic drugs.
Methods
LA PITX2 messenger ribonucleic acid (mRNA) levels were measured in 95 patients undergoing thoracoscopic AF ablation. The effects of flecainide, a sodium (Na+)-channel blocker, and d,l-sotalol, a potassium channel blocker, were studied in littermate mice with normal and reduced Pitx2c mRNA by electrophysiological study, optical mapping, and patch clamp studies. PITX2-dependent mechanisms of antiarrhythmic drug action were studied in human embryonic kidney (HEK) cells expressing human Na channels and by modeling human action potentials.
Results
Flecainide 1 μmol/l was more effective in suppressing atrial arrhythmias in atria with reduced Pitx2c mRNA levels (Pitx2c+/–). Resting membrane potential was more depolarized in Pitx2c+/– atria, and TWIK-related acid-sensitive K+ channel 2 (TASK-2) gene and protein expression were decreased. This resulted in enhanced post-repolarization refractoriness and more effective Na-channel inhibition. Defined holding potentials eliminated differences in flecainide’s effects between wild-type and Pitx2c+/– atrial cardiomyocytes. More positive holding potentials replicated the increased effectiveness of flecainide in blocking human Nav1.5 channels in HEK293 cells. Computer modeling reproduced an enhanced effectiveness of Na-channel block when resting membrane potential was slightly depolarized.
Conclusions
PITX2 mRNA modulates atrial resting membrane potential and thereby alters the effectiveness of Na-channel blockers. PITX2 and ion channels regulating the resting membrane potential may provide novel targets for antiarrhythmic drug development and companion therapeutics in AF.
Key Words: antiarrhythmic drugs, atrial fibrillation, drug targets, electrophysiology, personalized medicine, rhythm control
Abbreviations and Acronyms: AAD, antiarrhythmic drug; APD, action potential duration; ERP, effective refractory period; HEK, human embryonic kidney; LA, left atrium; LAA, left atrial appendage; mRNA, messenger ribonucleic acid; Na, sodium; PITX2, paired like homeodomain-2; PRR, post-repolarization refractoriness; RMP, resting membrane potential; SNP, single nucleotide polymorphism; TASK-2, TWIK-related acid-sensitive K+ channel
Central Illustration
Atrial fibrillation (AF) causes cardiovascular death, frequent hospitalization, and cognitive decline even in patients treated according to guidelines 1, 2, 3. Antiarrhythmic drug (AAD) therapy remains the most commonly used treatment to maintain sinus rhythm in AF patients, but AAD effectiveness remains limited (3). Unfortunately, we lack a basic understanding of why AADs prevent AF over long periods in some patients but not in others 4, 5. Identifying factors that modify the effects of AADs would allow the selection of responsive patients and could help guide development of novel AADs (6).
Paired like homeodomain-2 transcription factor (PITX2) is a transcription factor that regulates the development of the left atrium (LA) and thoracic organs. Its c isoform is expressed in the adult LA and regulates the expression of LA ion channels 7, 8, 9. Low atrial Pitx2 expression renders mice susceptible to AF and shortens the LA action potential 8, 10, 11. In this study, we investigated how atrial PITX2 modifies the effects of AADs.
We detected variable LA PITX2 messenger ribonucleic acid (mRNA) expression in AF patients requiring rhythm control therapy. After finding that low Pitx2c enhanced the effect of flecainide, mediated by a more positive resting membrane potential (RMP), we identified reduced TWIK-related acid-sensitive K+ channel 2 (TASK-2) expression as a possible driver of this effect and replicated these effects in cells expressing human sodium (Na) channels and in a human atrial action potential model.
Methods
All experiments were conducted under the Animals (Scientific Procedures) Act 1986, and approved by the home office (PPL number 30/2967) and the institutional review board at the University of Birmingham. Analyses of human atrial tissue were approved by the institutional review board of Academic Medical Center, Amsterdam, the Netherlands. All patients provided written informed consent.
Left atrial appendages (LAAs) were excised from 95 patients undergoing bilateral thoracoscopic AF ablation either in the AFACT (Atrial Fibrillation Ablation and Autonomic Modulation via Thoracoscopic Surgery) trial (12) or undergoing similar procedures in the same centers using an endoscopic stapling device, snap frozen in liquid nitrogen and stored at –80°C (13). Deoxyribonucleic acid and ribonucleic acid were extracted using DNeasy and RNeasy kits (Qiagen Ltd., Manchester, United Kingdom), respectively. PITX2 mRNA content was quantified by quantitative polymerase chain reaction. Single nucleotide polymorphisms (SNPs) rs2200733, rs6838973, and rs1448818 (14) were identified using TaqMan assays (Thermo Fisher Scientific Inc., Waltham, Massachusetts).
Adult mice (age 12 to 16 weeks) on an MF1 background with normal or reduced (Pitx2c+/−) atrial Pitx2c expression were studied (8).
LA epicardial monophasic action potentials were recorded from Langendorff-perfused murine hearts 8, 15. Programmed stimulation was performed at baseline and with flecainide 1 μmol/l or d,l-sotalol 10 μmol/l. Arrhythmia inducibility and effective refractory period (ERP) were measured by using single right atrial extrastimuli after steady-state pacing in 1-ms decrements 15, 16, 17, 18. Transmembrane action potentials were recorded using borosilicate glass microelectrodes from superfused murine LAs (17), RMP, action potential duration (APD), upstroke velocity, and activation times were analyzed 15, 17, 18.
The human atrial cell model of Courtemanche et al. (19) was used. Pitx2c+/– deficiency was modeled by reducing IK1 conductance by 25% and doubling IKr conductance. Simulations were run in strands of 100 atrial cells (cell length 100 μm). The 5 leftmost cells of the strand were paced (S1) for 2 min at 1,000- and 500-ms basic cycle lengths. Premature stimulation (S2) was applied to determine the ERP and conduction velocity as measured from cells 25 to 75. Values for all other parameters were measured from the 50th cell. For the modeling, post-repolarization refractoriness (PRR) was calculated as the difference between APD at –60 mV repolarization and ERP.
LA cell isolation was performed as previously reported (20). Standard INa and IK1 currents were recorded as previously published 18, 19, 20. Background K+ (TASK-like) currents sensitive to high Ba2+ (10 mM) were measured 21, 22, 23. Human embryonic kidney (HEK) 293 cells stably expressing the human Nav1.5 channel were obtained (SB Ion Channels, Glasgow, UK).
Ribonucleic acid and complementary deoxyribonucleic acid were synthesized from murine LA, (SuperScript VILO, Thermo Fisher Scientific Inc.) to quantify expression of 20 atrial ion channels and genes with suspected PITX2-dependent regulation (9) using custom-designed Taqman low density array plates (Thermo Fisher Scientific Inc.). Western immunoblotting was performed on murine LA tissue lysates with antibodies detecting TASK-2, Kv1.6, Na/K ATPase alpha-1, Na/K ATPase alpha-2, Na/Ca exchanger 1, Serca2a, Nav1.5, or calnexin, using standard methods.
Optical action potentials and calcium ion (Ca2+) transients were recorded in murine LA and analyzed using custom-made MATLAB algorithms (MathWorks, Natick, Massachusetts) as previously described (17).
Statistical analysis
All experiments were performed and analyzed in a blinded fashion. Murine studies were performed and analyzed blinded to genotype in littermate pairs. Categorical data were compared using the Fisher exact test. Numerical data were compared by 2-sided paired parametric Student t tests (e.g., measurements before and after perfusion of flecainide or sotalol) and Wilcoxon signed rank tests. Multiple measurements were assessed by repeated measures of analysis of variance followed by correction for multiple comparison (Bonferroni test) if the overall test was significant. Two-sided p < 0.05 were considered significant. Box plots depict individual measurements (points), mean, and SEM. Statistics and figures were created using Prism 5 (GraphPad Software, San Diego, California).
Results
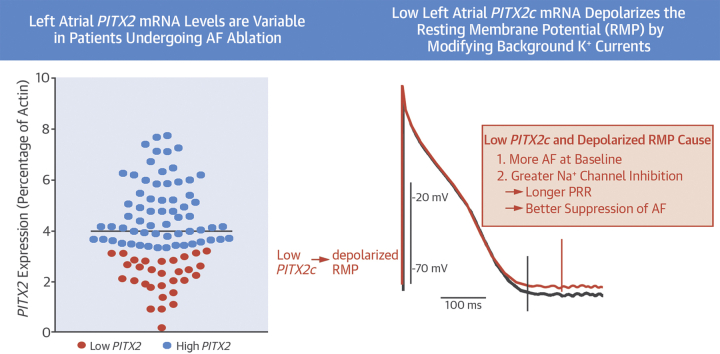
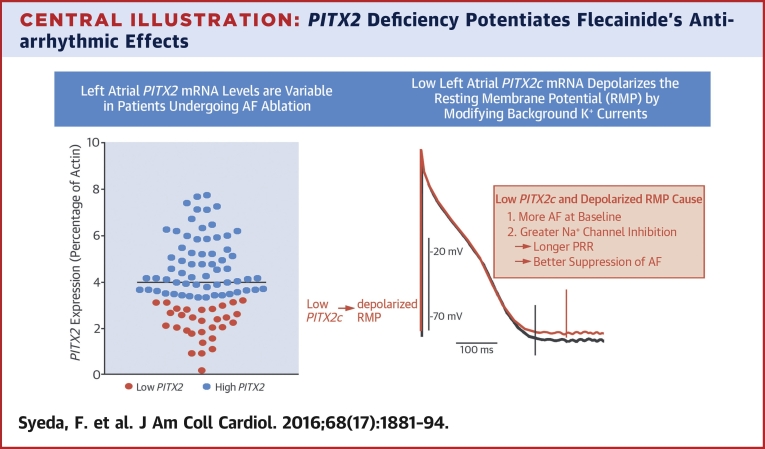
PITX2 mRNA varied markedly in human LAA (Central Illustration) harvested from AF patients (Table 1) (13), suggesting that a 50% lowered PITX2 expression defines a large, potentially clinically relevant group of AF patients. This did not directly correlate with SNP haplotype (Table 2), although we found numerically lower PITX2c levels in patients with 5 risk alleles.
Central Illustration.
PITX2 Deficiency Potentiates Flecainide's Antiarrhythmic Effects
Paired like homeodomain 2 (PITX2) might play an important role in regulating gene expression and electrical function of the left atrium. (Left)Pitx2 messenger ribonucleic acid (mRNA) levels are variable in left atrial appendages of patients undergoing thoracoscopic atrial fibrillation (AF) ablation therapy. The differentiation between “low” (orange) and “high” (blue)PITX2 levels is somewhat arbitrary. (Right) A low left atrial PITX2c mRNA expression slightly depolarizes left atrial resting membrane potential (RMP). A depolarized RMP, in turn, enhances the antiarrhythmic effect of sodium-channel blockers such as flecainide. PRR = post-repolarization refractoriness.
Table 1.
Baseline Characteristics (N= 101)∗
| Age, yrs | 59.7 ± 8.4 (40–76) |
| Male | 79 |
| Congestive heart failure | 6 |
| Hypertension | 34 |
| Age ≥75 yrs | 1 |
| Diabetes | 9 |
| Stroke/transient ischemic attack/embolus | 10 |
| Vascular disease | 10 |
| Female | 22 |
| Age ≥65 yrs | 31 |
| CHA2DS2-VASc score | |
| 0 | 60 |
| 1 | 24 |
| ≥2 | 17 |
| Previous catheter ablation for AF | 20 |
| Type of AF | |
| Paroxysmal | 44 |
| Persistent | 56 |
| Longstanding persistent | 1 |
| AF duration, yrs | 6.0 (1–35) |
| Antiarrhythmic drugs and rate control agents | |
| Quinidine or disopyramide | 4 |
| Flecainide or propafenone | 33 |
| Amiodarone, dronedarone, or sotalol | 41 |
| Beta blockers | 53 |
| Verapamil or diltiazem | 17 |
| Digoxin | 15 |
| Anticoagulant agents (before PVI procedure) | |
| Vitamin K antagonists | 89 |
| Antiplatelets | 6 |
Values are mean ± SD (range), n, or mean (range).
PVI = pulmonary vein isolation.
Left atrial appendages were collected from these patients with atrial fibrillation (AF).
Table 2.
PITX2 mRNA Expression in Left Atrial Appendages From AF Ablation Patients∗
| Risk Alleles | 25% IQR | Median | 75% IQR | Mean | SEM | No. of Patients |
|---|---|---|---|---|---|---|
| 0 | 3.22 | 3.69 | 5.22 | 4.04 | 0.6 | 3 |
| 1 | 2.96 | 4.25 | 6.25 | 4.54 | 0.5 | 13 |
| 2 | 2.65 | 3.78 | 4.75 | 3.94 | 0.3 | 22 |
| 3 | 2.74 | 3.72 | 4.92 | 3.83 | 0.4 | 17 |
| 4 | 3.00 | 4.29 | 5.41 | 4.39 | 0.5 | 10 |
| 5 | 1.96 | 2.66 | 4.66 | 3.10 | 0.7 | 4 |
| 6 | 4.95 | 4.95 | 4.95 | 4.95 | 0.0 | 1 |
IQR = interquartile range; LA = left atrium; other abbreviations as in Table 1.
This dataset was grouped according to the number of risk single nucleotide polymorphism (SNP) alleles for AF on chromosome 4q25 (rs2200733, SNP2 rs6838973, rs1448818 [13]). Although PITX2 mRNA is numerically lower in patients with 5 or 6 risk alleles, we did not find a PITX2 mRNA gradient according to AF risk.
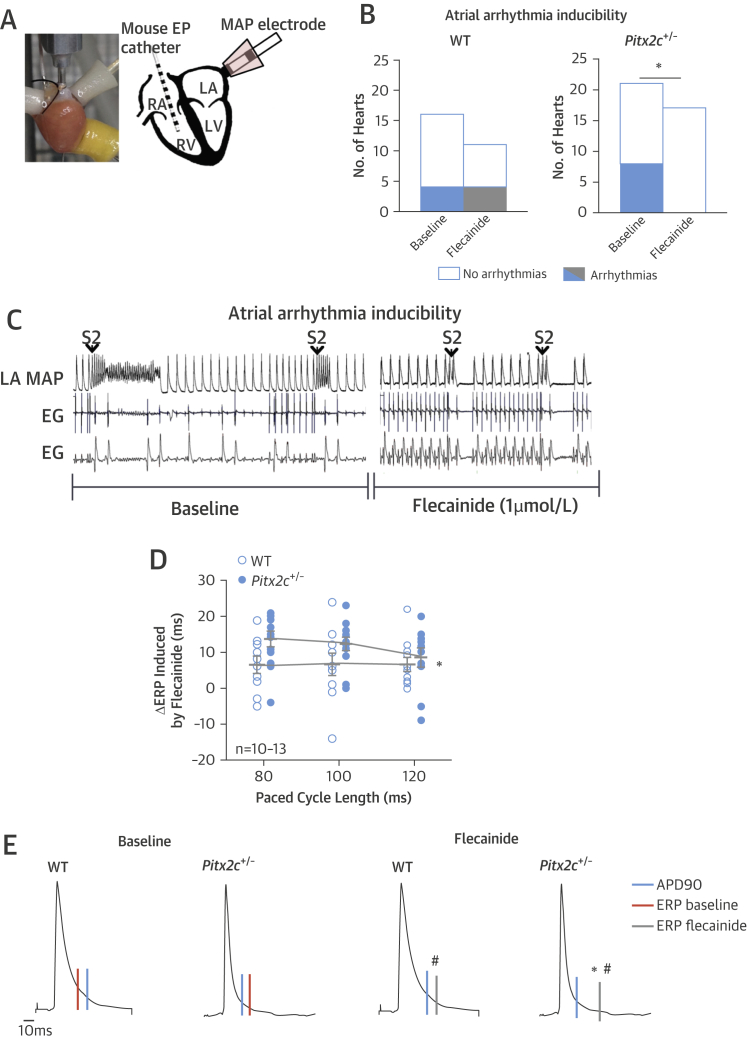
Flecainide suppressed atrial arrhythmias in murine Pitx2c+/– hearts. Flecainide abolished induced atrial arrhythmias in hearts with reduced Pitx2c expression (0 of 17 hearts with atrial arrhythmias) but not in hearts with normal Pitx2c expression (atrial arrhythmias remained in 3 of 12 hearts) (Figures 1A to 1C). Flecainide prolonged ERPs and refractoriness beyond the end of repolarization (PRR) calculated as the difference between ERP and APD90 (ms). Flecainide prolonged PRR more in hearts with reduced Pitx2c expression (Figures 1D and 1E, Table 3). PITX2c+/– hearts had shorter atrial action potentials (8). Flecainide abolished APD differences between Pitx2c+/– and wild-type LA by prolonging early repolarization (APD30, APD50, and APD70) (Table 3). Murine atrial PITX2 expression did not modulate the effects of sotalol on atrial APD or ERP (Table 4).
Figure 1.
Atrial Arrhythmia Inducibility in Pitx2c+/– Murine Whole Hearts
(A) Image and schematic representation of the Langendorff-perfused heart. (B) Atrial arrhythmia inducibility in isolated, beating hearts from wild-type (WT) and reduced paired like homeodomain 2 messenger ribonucleic acid (Pitx2c+/–) mice. Flecainide abolished atrial arrhythmia inducibility in Pitx2c+/– hearts only. *p < 0.05 flecainide versus baseline. (C) Representative trace of atrial fibrillation (AF) induced during programmed stimulation at baseline, showing reduced severity of arrhythmias with 1 μmol/l flecainide in Pitx2c+/– atria. (D) Effects of flecainide on atrial effective refractory period (ERP) in wild-type and Pitx2c+/– isolated, beating hearts. Shown is the difference in atrial ERP between baseline and 1 μmol/l flecainide at 80- to 120-ms paced cycle length following a single extrastimulus (S2) in WT and Pitx2c+/– isolated, beating hearts. *p < 0.05 between genotypes across all cycle lengths. (E) Whereas flecainide prolonged ERP in both genotypes, this effect was more pronounced in Pitx2c+/– atria. Flecainide caused post-repolarization refractoriness (PRR), the difference between ERP (orange and grey lines) and APD90(blue lines), in WT and Pitx2c+/– atria. Flecainide-induced PRR in Pitx2c+/– is almost 3 times that of WT atria. *p < 0.05 WT versus Pitx2c+/–. #p < 0.05 baseline versus 1 μmol/l flecainide. APD = action potential duration; EG = intracardiac electrogram; EP = electrophysiology; LA = left atrium; LV = left ventricle; MAP = monophasic action potential; RA = right atrium; RV = right ventricle.
Table 3.
Effect of Flecainide on Refractoriness and Repolarization in Mouse Hearts
| Paced CL, ms | Wild-Type |
Pitx2c+/– |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 120 |
100 |
80 |
120 |
100 |
80 |
|||||||
| Baseline | Flecainide | Baseline | Flecainide | Baseline | Flecainide | Baseline | Flecainide | Baseline | Flecainide | Baseline | Flecainide | |
| LA ERP, ms | ||||||||||||
| 23.5 ± 2.3 (11) | 29.8 ± 3.0 (11) | 22.2 ± 2.1 (11) | 29.6 ± 3.3 (11) | 21.9 ± 2.4 (10) | 28.7 ± 3.5∗ (10) | 30.5 ± 2.4 (11) | 38.5 ± 3.3∗ (11) | 28.0 ± 2.3 (13) | 40.2 ± 2.8∗ (13) | 27.5 ± 2.5 (13) | 41.2 ± 3.0∗† (13) | |
| LA monophasic APD, ms | ||||||||||||
| APD50 | 10.2 ± 1.3 (8) | 14.5 ± 1.7 (8) | 10.8 ± 1 (8) | 11.9 ± 1.6 (8) | 10.4 ± 0.7 (7) | 12.0 ± 1.1 (7) | 12.4 ± 1.1 (15) | 14.4 ± 1.3 (15) | 11.5 ± 1.0 (15) | 12.4 ± 1.1 (15) | 10.6 ± 0.9 (11) | 10.3 ± 1.0 (11) |
| APD70 | 17.8 ± 2.2 (9) | 23 ± 2.1 (9) | 18.4 ± 1.6 (9) | 18.7 ± 2.2 (9) | 18.1 ± 1.2 (8) | 18.1 ± 1.9 (8) | 18.0 ± 1.6 (15) | 19.2 ± 1.8 (15) | 16.0 ± 1.4 (13) | 16.2 ± 1.4 (13) | 14.9 ± 1.0† (10) | 13.1 ± 0.7 (10) |
| APD90 | 31.3 ± 3.0 (8) | 37.4 ± 2.8 (8) | 31.5 ± 2.5 (9) | 29.9 ± 2.9 (9) | 31.0 ± 1.4 (8) | 28.4 ± 2.7 (8) | 28.3 ± 2.2 (13) | 29.9 ± 2.2 (13) | 27.4 ± 2.2 (13) | 26.6 ± 1.5 (13) | 26.8 ± 1.7 (10) | 23.1 ± 1.4 (10) |
| LA transmembrane APD, ms | ||||||||||||
| APD30 | 4.5 ± 0.1 (30) | 5.5 ± 0.3 (22) | 4.5 ± 0.1 (30) | 5.4 ± 0.3 (22) | 4.4 ± 0.1 (30) | 5.2 ± 0.3 (22) | 4.0 ± 0.1 (31) | 4.7 ± 0.2 (24) | 3.9 ± 0.1 (31) | 4.6 ± 0.2 (24) | 3.8 ± 0.1† (31) | 4.4 ± 0.2 (24) |
| APD50 | 6.7 ± 0.2 (30) | 8.2 ± 0.5 (22) | 6.6 ± 0.2 (30) | 8.0 ± 0.4 (22) | 6.4 ± 0.2 (30) | 7.8 ± 0.5 (22) | 5.9 ± 0.2 (31) | 7.1 ± 0.4 (24) | 5.7 ± 0.2 (31) | 7.0 ± 0.4 (24) | 5.6 ± 0.2† (31) | 6.7 ± 0.3 (24) |
| APD70 | 10.5 ± 0.4 (30) | 12.7 ± 0.8 (22) | 10.1 ± 0.4 (30) | 12.1 ± 0.7 (22) | 9.6 ± 0.4 (30) | 11.8 ± 0.7 (22) | 8.9 ± 0.4 (31) | 10.7 ± 0.6 (24) | 8.6 ± 0.4 (31) | 10.3 ± 0.6 (24) | 8.3 ± 0.3† (31) | 9.8 ± 0.5 (24) |
| APD90 | 20.9 ± 1.0 (30) | 23.4 ± 1.5 (22) | 19.9 ± 0.9 (30) | 22.2 ± 1.4 (22) | 18.4 ± 0.8 (30) | 21.6 ± 1.3 (22) | 17.6 ± 0.9 (31) | 20.3 ± 1.1 (24) | 16.5 ± 0.8 (31) | 19.2 ± 1.0 (24) | 15.7 ± 0.8† (31) | 17.9 ± 0.9 (24) |
| LA optical APD, ms | ||||||||||||
| APD30 | 6.1 ± 0.3 (10) | 7.3 ± 0.6 (6) | 6.4 ± 0.8 (10) | 5.9 ± 1.0 (6) | 6.1 ± 0.4 (10) | 6.9 ± 1.3 (6) | 4.9 ± 0.4 (10) | 7.7 ± 0.9 (8) | 4.6 ± 0.3 (10) | 5.4 ± 0.7 (8) | 4.3 ± 0.4† (10) | 5.7 ± 0.7 (8) |
| APD50 | 8.5 ± 0.6 (10) | 10.7 ± 1.2 (6) | 8.9 ± 1.1 (10) | 8.5 ± 1.2 (6) | 8.3 ± 0.7 (10) | 10.3 ± 1.8 (6) | 6.9 ± 0.4 (10) | 10.0 ± 1.0 (8) | 6.6 ± 0.4 (10) | 8.1 ± 0.9 (8) | 6.1 ± 0.4† (10) | 8.0 ± 0.9 (8) |
| APD70 | 11.7 ± 1.2 (10) | 15.0 ± 2.1 (6) | 12.5 ± 1.5 (10) | 12.8 ± 1.7 (6) | 11.5 ± 1.1 (10) | 14.4 ± 2.5 (6) | 9.4 ± 0.0 (10) | 13.3 ± 1.2 (8) | 9.4 ± 0.6 (10) | 11.6 ± 1.5 (8) | 9.1 ± 0.5† (10) | 11.2 ± 1.2 (8) |
Table 4.
Electrophysiological Effects of Sotalol
| Paced CL, ms | Wild-Type |
Pitx2c+/– |
||||||
|---|---|---|---|---|---|---|---|---|
|
120 |
100 |
120 |
100 |
|||||
| Baseline | Sotalol | Baseline | Sotalol | Baseline | Sotalol | Baseline | Sotalol | |
| LA ERP, ms | ||||||||
| 38.7 ± 7.8 (7) | 33.9 ± 6.3 (7) | 32.2 ± 6.1 (6) | 29.2 ± 5.3 (6) | 39.3 ± 4.0 (4) | 26.8 ± 3.5 (4) | 37.0 ± 5.7 (4) | 24.0 ± 3.7 (4) | |
| LA APD, ms | ||||||||
| APD50 | 11.5 ± 1.2 (9) | 13.4 ± 1.2 (9) | 10.9 ± 2.0 (7) | 12.2 ± 1.3 (7) | 10.8 ± 1.1 (7) | 11.2 ± 1.0 (7) | 8.3 ± 0.9 (4) | 11.1 ± 1.7 (4) |
| APD70 | 17.6 ± 2.2 (9) | 20.0 ± 1.9 (9) | 16.0 ± 1.3 (7) | 18.2 ± 2.3 (7) | 16.5 ± 1.6 (7) | 17.3 ± 1.2 (7) | 13.0 ± 1.5 (4) | 17.0 ± 1.9 (4) |
| APD90 | 30.7 ± 3.2 (9) | 33.5 ± 2.7 (9) | 29.0 ± 1.9 (7) | 30.9 ± 3.2 (7) | 29.7 ± 2.7 (7) | 31.2 ± 2.0 (7) | 23.8 ± 2.8 (4) | 29.6 ± 2.9 (4) |
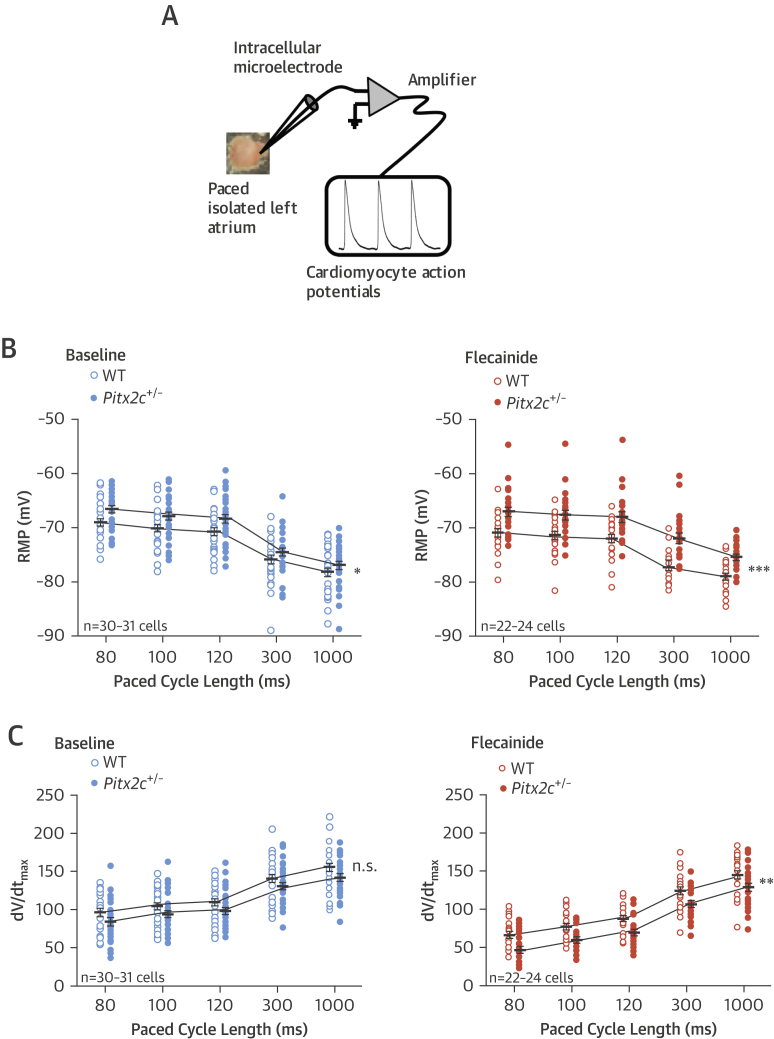
RMP was slightly depolarized in LA murine cells with reduced Pitx2c expression (range of mean depolarization 1.2 to 2.4 mV over 5 cycle lengths; all p < 0.05) (Figures 2A and 2B). Atrial Pitx2c levels did not significantly affect dV/dtmax (100-ms paced cycle length: wild-type: 104.4 ± 4.3 V/s; Pitx2c+/–: 93.7 ± 4.5 V/s) (Figure 2C). Flecainide did not modify atrial RMP (Figure 2B) but reduced action potential amplitude consistent with its Na-channel blocking effect, specifically at 100-ms cycle length: wild-type baseline: 77.5 ± 1.2 mV (n = 30); wild-type flecainide: 71.3 ± 1.2 mV (n = 31); Pitx2c+/– baseline: 73.4 ± 1.3 mV (n = 22); and Pitx2c+/– flecainide: 65.1 ± 1.45 mV (n = 24).
Figure 2.
Resting Membrane Potential and Maximum Upstroke Velocity
(A) Schematic representation of sharp microelectrode electrode recordings from the superfused whole LA. (B) Resting membrane potential (RMP) in WT and Pitx2c+/– LA at baseline and with 1 μmol/l flecainide. Pitx2c+/– LA have depolarized RMP. The difference between WT and Pitx2c+/– is exaggerated with flecainide. *p < 0.05; ***p < 0.001 across all cycle lengths. (C) Flecainide unmasked a lower maximum upstroke velocity (dV/dtmax) in Pitx2c+/– LA compared to WT. **p < 0.01 versus WT. Abbreviations as in Figure 1.
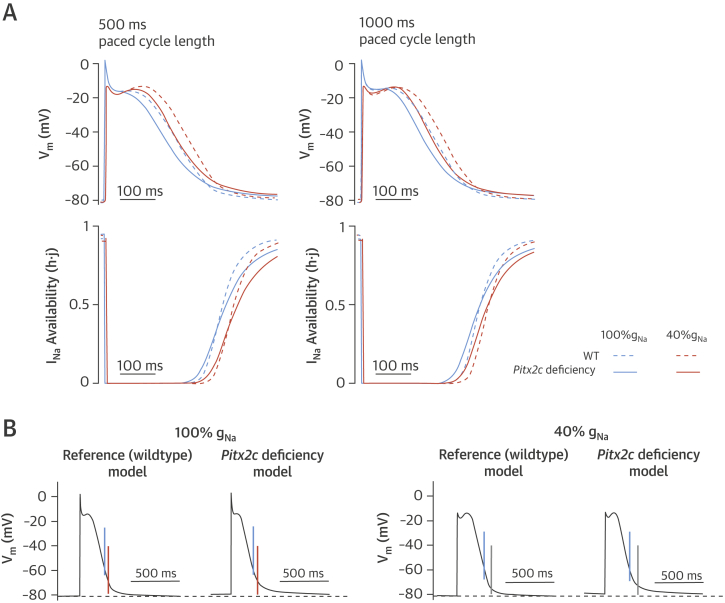
Because the Courtemanche–Ramirez–Nattel model does not incorporate background K+ currents (19), we simulated a depolarized RMP in this model by a 25% reduction in IK1. This reduced the RMP at 500-ms paced cycle length by 2 mV from 79.9 mV (“normal PITX2”) to –77.9 mV (“low PITX2”). Na channels recovered from inactivation more slowly upon partial INa block (50% or 60%) (Figure 3A). Furthermore, PRR was enhanced in the PITX2 deficiency model (Figure 3B and Table 5). Inhibition of INa reduced upstroke velocity (dV/dtmax) and conduction velocity in both models, and reproduced the prolongation of PRR (Figure 3B).
Figure 3.
Modeling of the Electrophysiological Consequences of Pitx2c Deficiency
(A) Propagated action potentials (top) simulated with the Courtemanche–Ramirez–Nattel model modified to reflect the more positive RMP of murine Pitx2c+/– atria and the effect of 60% sodium current (INa) block at pacing cycle lengths of 500 and 1,000 ms, with 2-min pre-pacing, and corresponding time courses of the product of the 2 inactivation gates h and j of INa(bottom), reflecting INa availability. (B) Reduced sodium conductance (gNa) increased post-repolarization refractoriness (PRR) in the reference model and the Pitx2c deficiency model. After reducing gNa, PRR was greater in the Pitx2c deficiency model than in the reference model (grey lines). Lines denote APD60(blue) and ERP (orange: with 100% gNa; grey: with 40% gNa). Abbreviations as in Figures 1 and 2.
Table 5.
Electrophysiological Effects of Reduced Sodium Conductance in a Human Atrial Model
| Paced CL, ms | Wild-Type Model |
Pitx2c Deficiency Model |
||
|---|---|---|---|---|
| 500 | 1,000 | 500 | 1,000 | |
| RMP, mV | ||||
| gNa, % | ||||
| 100 | -79.92 | -81.28 | -77.90 | -79.61 |
| 50 | -79.60 | -81.12 | -77.33 | -79.37 |
| 40 | -79.43 | -81.01 | -76.99 | -79.23 |
| APD at repolarization to -60 mV, ms | ||||
| gNa, % | ||||
| 100 | 217 | 253 | 206 | 226 |
| 50 | 239 | 266 | 233 | 239 |
| 40 | 248 | 273 | 245 | 245 |
| ERP, ms | ||||
| gNa, % | ||||
| 100 | 266 | 301 | 261 | 280 |
| 50 | 308 | 335 | 316 | 320 |
| 40 | 327 | 352 | 342 | 339 |
| PRR, ms | ||||
| gNa, % | ||||
| 100 | 49 | 48 | 55 | 54 |
| 50 | 69 | 69 | 83 | 81 |
| 40 | 79 | 79 | 97 | 94 |
| Conduction velocity, cm/s | ||||
| gNa, % | ||||
| 100 | 49.5 | 50.0 | 50.3 | 50.5 |
| 50 | 36.9 | 37.0 | 37.5 | 37.5 |
| 40 | 32.9 | 32.7 | 33.0 | 33.1 |
gNa = reduced sodium conductance; PRR = post-repolarization refractoriness; RMP = resting membrane potential; other abbreviations as in Table 3.
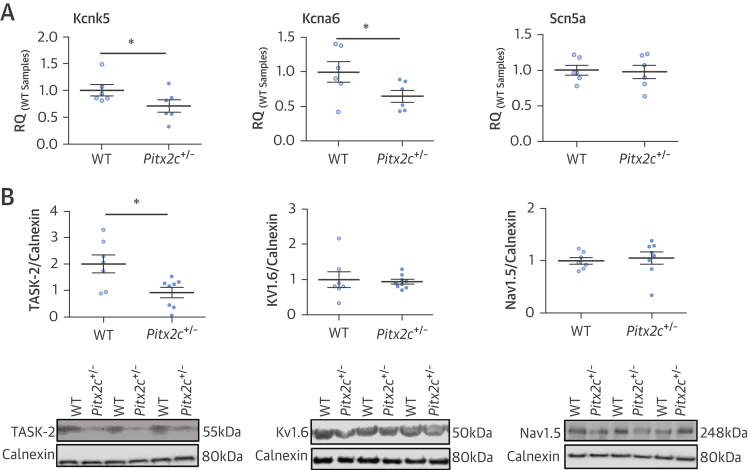
Kcna6 and Kcnk5 mRNA expression were reduced in Pitx2c+/– murine LA (Figure 4A, Online Table 1), whereas mRNA concentrations of 20 other ion channels or related genes were not altered. Kv1.6 protein concentration was unaltered, whereas TASK-2 protein concentration was reduced in murine atria with reduced Pitx2c expression (Figure 4B). Nav1.5 mRNA and protein expression were not changed (Figures 4A and 4B).
Figure 4.
mRNA and Protein Expression in Murine LA
(A) Relative quantity (RQ) of messenger ribonucleic acid (mRNA) of selected ion channels in LA from Pitx2c+/– and WT mice. Expression levels were measured relative to WT sample 1. Kcnk5 encodes TWIK-related acid-sensitive K+ channel 2 (TASK-2), Kcna6 encodes Kv1.6, and Scn5a encodes Nav1.5. *p < 0.05. (B) Concentration of TASK-2, KV1.6, and Nav1.5 proteins relative to calnexin (arbitrary units). Representative immunoblots are displayed below the corresponding dot plot. *p < 0.05. Abbreviations as in Figures 1 and 2.
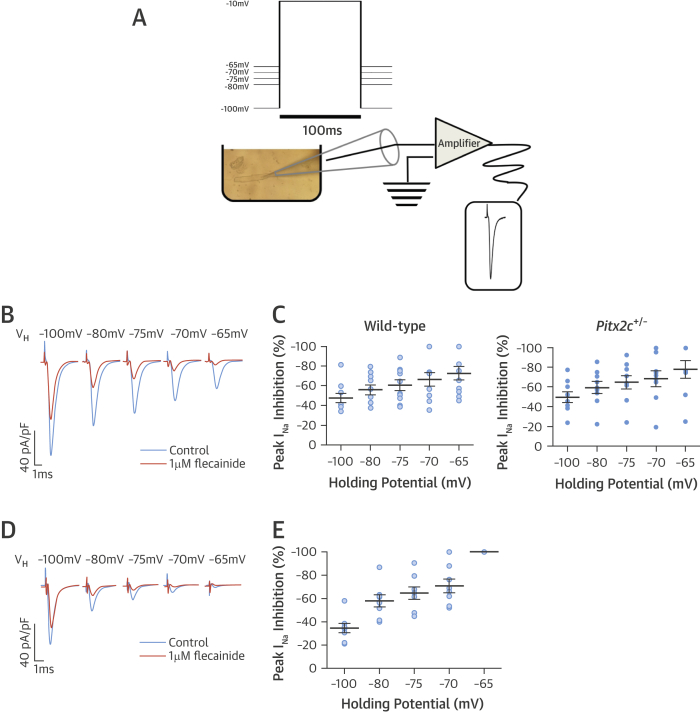
Atrial Pitx2c expression did not modify peak Na+ currents (INa) recorded from isolated murine cardiomyocytes at holding potentials ranging from –100 to –65 mV (Figures 5A to 5C). Peak INa was reduced at more depolarized holding potentials (Figure 5). Flecainide inhibited INa better at more positive holding potentials (inhibition at –70 mV: 68 ± 5%; inhibition at –65 mV: 75 ± 5%; n = 86 cells from n = 17 atria) in cells from murine atria with normal or reduced Pitx2c expression, suggesting that the greater efficiency of flecainide in atria with reduced Pitx2c expression is secondary to RMP depolarization (Figure 5C). Consistent with this, flecainide inhibited human Nav1.5 channels expressed in HEK cells more potently at more depolarized test potentials (–65 to –75 mV) (Figures 5D and 5E).
Figure 5.
Effect of Membrane Potential on Sodium-Channel Inhibition
(A) Schematic representation of patch clamp experiments carried out in isolated atrial cardiomyocytes. Human embryonic kidney (HEK) cells were patch clamped using the same setup. (B) Representative traces showing INa measured by patch clamping in isolated atrial cardiomyocytes, at baseline and with flecainide, at increasing holding potentials (–100 to –65 mV). (C) Reduction in peak INa was enhanced with increased holding potentials, irrespective of cardiomyocyte origin. (D) Representative traces showing INa in Nav1.5-transfected HEK cells at baseline and with flecainide at increasing holding potentials (–100 to –65 mV). (E) Reduction in peak INa with flecainide in Nav1.5-transfected HEK cells was enhanced with increased holding potentials. The greatest difference in percent reduction was between –70 and –65 mV. Abbreviations as in Figures 1, 2, and 3.
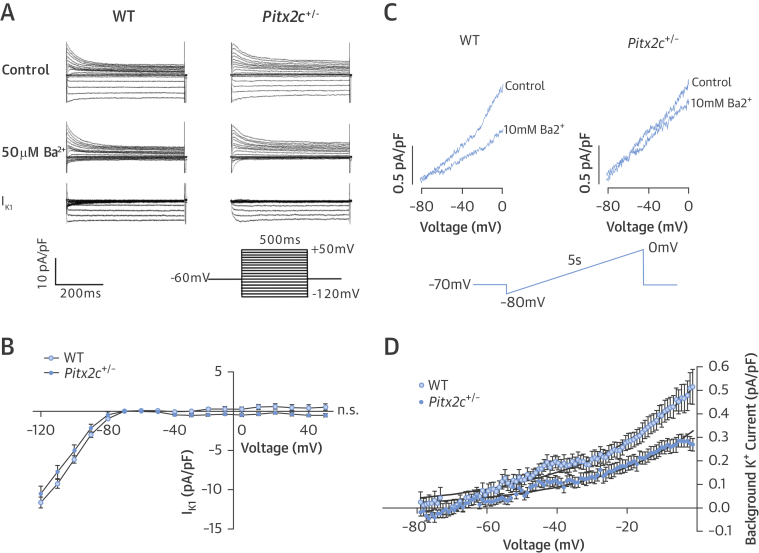
Background K+ currents, which include TASK currents, were reduced in Pitx2c+/– murine atria, whereas IK1 did not differ between genotypes (Figure 6).
Figure 6.
Currents Responsible for RMP
(A) Representative raw traces showing IK1 currents (50 μmol/l Ba2+ sensitive) in isolated WT and Pitx2c+/– LA cardiomyocytes at test potentials ranging from –120 to 50mV. (B) No difference is seen in the Ik1 current/voltage relationship for LA cardiomyocytes between WT and Pitx2c+/– LA at test potentials ranging from –120 to 50 mV. (C) Representative raw traces show background current response to 10 mmol/l Ba2+ in isolated WT and Pitx2c+/– LA cardiomyocytes. (D)Pitx2c+/– cardiomyocytes had significantly reduced background K+ (10 mmol/l Ba2+ sensitive) currents than WT cardiomyocytes. *p < 0.05. Abbreviations as in Figures 1, 2, and 3.
Reduced Pitx2c expression did not alter atrial conduction velocities or activation patterns (Online Figures 1A to 1C, Table 6), consistent with published data (8). We found that 1 μmol/l flecainide decreased atrial conduction velocities without differences between wild-type and Pitx2c+/– mice (Online Figures 1B and 1C). Calcium transient relaxation times at 50% relaxation were not different between wild-type and Pitx2c+/– (Online Figures 1D and 1E). Flecainide 1 μmol/l shortened 50% Ca2+ relaxation times by approximately 10% and decreased Ca2+ transient amplitude by approximately 50% in murine atria with normal and reduced Pitx2c expression (Online Figures 1E and 1F). Additionally, expression of the Na/Ca exchanger Serca2a and Na/K ATPase alpha-1 and alpha-2 subunit protein did not differ between wild-type and Pitx2c+/– atria (Online Figure 2).
Table 6.
Electrical Activation Time and Conduction Velocity in Isolated Atria in the Presence of Flecainide (1 μmol/l)
| Wild-Type | Pitx2c+/– | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Paced CL, ms | 1,000 | 300 | 120 | 100 | 80 | 1,000 | 300 | 120 | 100 | 80 |
| Activation time (isolated left atrium), ms | 6 ± 0.3 (22) | 6 ± 0.3 (22) | 9 ± 0.5 (22) | 12 ± 1.0 (22) | 16 ± 1.4 (22) | 6 ± 0.2 (24) | 7 ± 0.3 (24) | 12 ± 0.9 (24) | 13 ± 0.9 (24) | 18 ± 1.2 (24) |
| Conduction velocity (optical mapping), cm/s | — | 30 ± 1.8 (8) | 25 ± 2.4 (8) | 25 ± 1.9 (8) | 23 ± 1.9 (8) | — | 29 ± 1.5 (8) | 26 ± 1.6 (8) | 25 ± 1.6 (8) | 23 ± 1.6 (8) |
Values are mean ± SEM (number of atria).
— = not applicable; other abbreviations as in Table 3.
Discussion
This study demonstrated that LA PITX2 mRNA concentrations vary in patients with AF requiring rhythm control therapy (Central Illustration). Furthermore, flecainide increases PRR and suppresses arrhythmias more effectively in atria with halved Pitx2c expression, mediated by a more depolarized RMP (Central Illustration). Drug-induced PRR is thought to prevent arrhythmias, as reactivation can then occur only after full recovery of excitability, avoiding slow propagation during the vulnerable period 16, 24, 25. We found similar effects in cells expressing human Na channels and in the Courtemanche–Ramirez–Nattel model of human atrial action potentials.
Thus, this study highlighted modulation of the atrial RMP by PITX2, possibly mediated by background currents such as TASK-2, as a target for AAD therapy, including atrial-selective therapy. Furthermore, the results suggested that markers for atrial PITX2 expression may identify AF patients who benefit from Na-channel blocker therapy (Central Illustration).
Low atrial PITX2 expression was identified as an important determinant of the antiarrhythmic effects of Na channel blockers. Low LA Pitx2c mRNA depolarized atrial RMP (Figure 2), consistent with a previous report (11). A depolarized RMP increased flecainide-induced PRR (Figure 1) 26, 27, 28, 29, 30. The conduction-slowing effect of flecainide was not modulated by reduced atrial Pitx2c (Online Figure 1), an important surrogate for drug safety. Both the modeling experiments (Figure 3) and the experiments in HEK cells expressing human Na channels (Figure 5) confirmed that small changes in RMP can markedly modulate Na-channel inhibition.
Resting membrane potential
Open-state Na-channel blockers such as flecainide and propafenone bind preferentially to Na channels integrated in membranes with slightly depolarized resting potentials, where more channels are in the open or inactivated state 31, 32. Our data can be interpreted as suggesting that AAD combinations that include a Na-channel blocker with a membrane potential modifying substance, such as amiodarone 16, 33 or the combination of dronedarone and ranolazine 29, 34, 35, may have synergistic antiarrhythmic effects because they modulate atrial RMP and thereby enhance the effect of Na-channel blockade. Further studies of such drug combinations and the relationship between their effectiveness and the patient’s atrial PITX2 mRNA levels are warranted. Our data also suggested that such combined effects may be of special relevance in patients who have a depolarized RMP, such as secondary to low LA PITX2. Because PITX2 expression is confined to the LA in the heart, AAD therapy that leverages modifications in RMP may achieve “atrial-specific” AAD therapy.
RMP is maintained by an intricate balance of different transmembrane currents and is closely related to the potassium equilibrium potential. We identified that PITX2 modifies expression of the genes encoding Kv1.6 and TASK-2 (Figure 4). Complete deletion of PITX2 regulates other potassium and Na channels such as Kcnj2 8, 36, which alter the RMP, but these were not responsible for the depolarized RMP observed in our study. Two-pore domain potassium channels, such as TASK-2, contribute to RMP in various cells, including skeletal and cardiac muscle 37, 38. To date, an altered function of the TASK-1 channel and of IK1 has been implicated in atrial remodeling and AF 39, 40. This study demonstrated that TASK-2 is expressed in atrial myocardium (Figure 4B), suggesting that a reduced function of TASK-2 could depolarize RMP (Figures 1 and 5) 8, 11, analogous to the effect of TASK-2 in neuronal and cartilage tissue 41, 42.
Developing clinical markers for patients with depolarized RMP
It will be challenging to directly assess LA RMP in AF patients, but our data suggested that differences in atrial RMP could explain the effectiveness of Na-channel blockers in carriers of common gene variants on chromosome 4q25 (43), although LA PITX2 levels are modulated by factors other than SNP status (Table 2) (44). It seems desirable to develop and validate drivers that modify RMP and clinical markers for patients prone to a depolarized atrial RMP to select appropriate AADs for individual patients in the future, thus enabling personalized AAD selection 6, 45.
Study limitations
This study provided robust evidence that LA PITX2 expression varies in AF patients and that reduced PITX2c expression enhances the antiarrhythmic effects of Na-channel blockers by modulating atrial RMP. The study was partly motivated by the assumption that gene variants on chromosome 4q25 modify PITX2 expression, an assumption that has not been definitively proven 9, 11, 44, 46. Our analysis (Table 2) and that of others indicate that SNP status does not always correlate with PITX2 levels 47, 48. Our findings are relevant to AAD therapy even if the presumed link between PITX2 expression and genetic variants on chromosome 4q25 proves elusive. The mechanisms by which reduced PITX2 mRNA concentrations shorten the LA action potential at high heart rates remain to be fully elucidated 8, 20. Validating our findings in patients is desirable but will be challenging because access to fresh LA cardiomyocytes and LA tissue is limited.
Due to the novelty of our findings, we could not perform a priori power calculations for our mechanistic experiments, and we analyzed several functional parameters to identify potential mechanisms conveying the antiarrhythmic effects of flecainide in atria with low Pitx2c concentrations. Our findings thus require independent validation.
Conclusions
This study shows that low LA PITX2 mRNA levels increase atrial RMP and thereby increase the effectiveness of flecainide (Central Illustration). This finding calls for appropriately designed clinical studies to assess whether AF patients with low atrial PITX2 levels respond favorably to Na-channel blockade. Further studies exploring the relevance of TASK channels to atrial RMP also are warranted.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: PITX2, a transcription factor linked to left–right asymmetry in the chest during development, modulates the expression of LA ion channels maintaining the RMP and modulates the antiarrhythmic effects of Na-channel blockers.
TRANSLATIONAL OUTLOOK: Clinical studies are needed to assess whether reduced PITX2 expression identifies patients with AF who respond favorably to Na-channel blocking drugs.
Acknowledgments
The authors thank Sian Marie O’Brien, Sarah Hopkins, Syeeda Nashitha Kabir, Pushpa Patel, and Charles Carey for technical support; Marta Coric for help with HEK cells; and Ilaria Piccini for advice on TLDA.
Footnotes
This work was supported by the European Union (EUTRAF 25105 to Drs. Kirchhof and Rohr; and Grant Agreement No. 633196 [CATCH ME] to Drs. Kirchhof and Fabritz); British Heart Foundation (FS/13/43/30324 to Drs. Kirchhof and Fabritz); Leducq Foundation to Dr. Kirchhof; Physical Science of Imaging in Biomedical Sciences (PSIBS) University of Birmingham for TY to Dr. Fabritz (EP/F50053X/1); DFG (FA 413 3/1) to Dr. Fabritz; Swiss National Science Foundation (138297) to Dr. Rohr; and Boehringer Ingelheim Foundation to Mr. Kuhlmann. Dr. de Groot is supported by NWO/ZonMW VIDI Grant 016.146.310. Dr. Riley is currently employed by Bio-Techne (R&D Products). Dr. Fabritz has received further institutional research grant support from DFG, MRC, and Gilead Inc. Dr. Kirchhof has received further research support from the German Centre for Heart Research and from several drug and device companies active in atrial fibrillation; and has received honoraria from several such companies. Drs. Syeda, Fabritz, and Kirchhof are listed as inventors on a patent (WO2015/140571) held by the University of Birmingham on genotype-specific antiarrhythmic drug therapy of atrial fibrillation. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Drs. Syeda and Holmes contributed equally to this work.
For an expanded Methods section as well as supplemental figures and a table, please see the online version of this article.
Appendix
References
- 1.Chugh S.S., Havmoeller R., Narayanan K. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P., Benussi S., Kotecha D. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC Endorsed by the European Stroke Organisation (ESO) Eur Heart J. 2016 Aug 27 [E-pub ahead of print] [Google Scholar]
- 4.Kirchhof P., Andresen D., Bosch R. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet. 2012;380:238–246. doi: 10.1016/S0140-6736(12)60570-4. [DOI] [PubMed] [Google Scholar]
- 5.Schotten U., Verheule S., Kirchhof P., Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 6.Fabritz L., Guasch E., Antoniades C. Expert consensus document: defining the major health modifiers causing atrial fibrillation: a roadmap to underpin personalized prevention and treatment. Nat Rev Cardiol. 2015;13:230–237. doi: 10.1038/nrcardio.2015.194. [DOI] [PubMed] [Google Scholar]
- 7.Li N., Dobrev D., Wehrens X.H. PITX2: a master regulator of cardiac channelopathy in atrial fibrillation? Cardiovasc Res. 2016;109:345–347. doi: 10.1093/cvr/cvw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchhof P., Kahr P.C., Kaese S. PITX2c is expressed in the adult left atrium, and reducing Pitx2c expression promotes atrial fibrillation inducibility and complex changes in gene expression. Circ Cardiovasc Genet. 2011;4:123–133. doi: 10.1161/CIRCGENETICS.110.958058. [DOI] [PubMed] [Google Scholar]
- 9.Kahr P.C., Piccini I., Fabritz L. Systematic analysis of gene expression differences between left and right atria in different mouse strains and in human atrial tissue. PLoS One. 2011;6:e26389. doi: 10.1371/journal.pone.0026389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Klysik E., Sood S. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci U S A. 2010;107:9753–9758. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinchilla A., Daimi H., Lozano-Velasco E. PITX2 insufficiency leads to atrial electrical and structural remodeling linked to arrhythmogenesis. Circ Cardiovasc Genet. 2011;4:269–279. doi: 10.1161/CIRCGENETICS.110.958116. [DOI] [PubMed] [Google Scholar]
- 12.Driessen A.H., Berger W.R., Krul S.P. Ganglion plexus ablation in advanced atrial fibrillation. The (AFACT) study. J Am Coll Cardiol. 2016;68:1155–1165. doi: 10.1016/j.jacc.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Krul S.P., Driessen A.H., van Boven W.J. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions: first results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:262–270. doi: 10.1161/CIRCEP.111.961862. [DOI] [PubMed] [Google Scholar]
- 14.Lubitz S.A., Lunetta K.L., Lin H. Novel genetic markers associate with atrial fibrillation risk in Europeans and Japanese. J Am Coll Cardiol. 2014;63:1200–1210. doi: 10.1016/j.jacc.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blana A., Kaese S., Fortmuller L. Knock-in gain-of-function sodium channel mutation prolongs atrial action potentials and alters atrial vulnerability. Heart Rhythm. 2010;7:1862–1869. doi: 10.1016/j.hrthm.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Kirchhof P., Degen H., Franz M.R. Amiodarone-induced postrepolarization refractoriness suppresses induction of ventricular fibrillation. J Pharmacol Exp Ther. 2003;305:257–263. doi: 10.1124/jpet.102.046755. [DOI] [PubMed] [Google Scholar]
- 17.Yu T.Y., Syeda F., Holmes A.P. An automated system using spatial oversampling for optical mapping in murine atria. Development and validation with monophasic and transmembrane action potentials. Prog Biophys Mol Biol. 2014;115:340–348. doi: 10.1016/j.pbiomolbio.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemoine M.D., Duverger J.E., Naud P. Arrhythmogenic left atrial cellular electrophysiology in a murine genetic long QT syndrome model. Cardiovasc Res. 2011;92:67–74. doi: 10.1093/cvr/cvr166. [DOI] [PubMed] [Google Scholar]
- 19.Courtemanche M., Ramirez R.J., Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998;275:H301–H321. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- 20.Holmes A.P., Yu T.Y., Tull S. A regional reduction in Ito and IKACh in the murine posterior left atrial myocardium is associated with action potential prolongation and increased ectopic activity. PLoS One. 2016;11:e0154077. doi: 10.1371/journal.pone.0154077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbuti A., Ishii S., Shimizu T., Robinson R.B., Feinmark S.J. Block of the background K(+) channel TASK-1 contributes to arrhythmogenic effects of platelet-activating factor. Am J Physiol Heart Circ Physiol. 2002;282:H2024–H2030. doi: 10.1152/ajpheart.00956.2001. [DOI] [PubMed] [Google Scholar]
- 22.Buckler K.J. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498(Pt 3):649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limberg S.H., Netter M.F., Rolfes C. TASK-1 channels may modulate action potential duration of human atrial cardiomyocytes. Cell Physiol Biochem. 2011;28:613–624. doi: 10.1159/000335757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchhof P., Fabritz L., Franz M.R. Post-repolarization refractoriness versus conduction slowing caused by class I antiarrhythmic drugs: antiarrhythmic and proarrhythmic effects. Circulation. 1998;97:2567–2574. doi: 10.1161/01.cir.97.25.2567. [DOI] [PubMed] [Google Scholar]
- 25.Milberg P., Frommeyer G., Uphaus T. Electrophysiologic profile of dronedarone on the ventricular level: beneficial effect on postrepolarization refractoriness in the presence of rapid phase 3 repolarization. J Cardiovasc Pharmacol. 2012;59:92–100. doi: 10.1097/FJC.0b013e3182377a11. [DOI] [PubMed] [Google Scholar]
- 26.Frommeyer G., Schmidt M., Clauss C. Further insights into the underlying electrophysiological mechanisms for reduction of atrial fibrillation by ranolazine in an experimental model of chronic heart failure. Eur J Heart Fail. 2012;14:1322–1331. doi: 10.1093/eurjhf/hfs163. [DOI] [PubMed] [Google Scholar]
- 27.Milberg P., Frommeyer G., Ghezelbash S. Sodium channel block by ranolazine in an experimental model of stretch-related atrial fibrillation: prolongation of interatrial conduction time and increase in post-repolarization refractoriness. Europace. 2013;15:761–769. doi: 10.1093/europace/eus399. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda K., Watanabe J., Yagi T. A sodium channel blocker, pilsicainide, produces atrial post-repolarization refractoriness through the reduction of sodium channel availability. Tohoku J Exp Med. 2011;225:35–42. doi: 10.1620/tjem.225.35. [DOI] [PubMed] [Google Scholar]
- 29.Burashnikov A., Sicouri S., Di Diego J.M., Belardinelli L., Antzelevitch C. Synergistic effect of the combination of ranolazine and dronedarone to suppress atrial fibrillation. J Am Coll Cardiol. 2010;56:1216–1224. doi: 10.1016/j.jacc.2010.08.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirchhof P., Engelen M., Franz M.R. Electrophysiological effects of flecainide and sotalol in the human atrium during persistent atrial fibrillation. Basic Res Cardiol. 2005;100:112–121. doi: 10.1007/s00395-005-0513-4. [DOI] [PubMed] [Google Scholar]
- 31.Anno T., Hondeghem L.M. Interactions of flecainide with guinea pig cardiac sodium channels. Importance of activation unblocking to the voltage dependence of recovery. Circ Res. 1990;66:789–803. doi: 10.1161/01.res.66.3.789. [DOI] [PubMed] [Google Scholar]
- 32.Nitta J., Sunami A., Marumo F., Hiraoka M. States and sites of actions of flecainide on guinea-pig cardiac sodium channels. Eur J Pharmacol. 1992;214:191–197. doi: 10.1016/0014-2999(92)90118-n. [DOI] [PubMed] [Google Scholar]
- 33.Singh B.N., Singh S.N., Reda D.J. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352:1861–1872. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 34.Burashnikov A., Di Diego J.M., Zygmunt A.C. Atrial-selective sodium channel block as a strategy for suppression of atrial fibrillation. Ann N Y Acad Sci. 2008;1123:105–112. doi: 10.1196/annals.1420.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiffel J.A., Camm A.J., Belardinelli L. The HARMONY trial: combined ranolazine and dronedarone in the management of paroxysmal atrial fibrillation: mechanistic and therapeutic synergism. Circ Arrhythm Electrophysiol. 2015;8:1048–1056. doi: 10.1161/CIRCEP.115.002856. [DOI] [PubMed] [Google Scholar]
- 36.Tao Y., Zhang M., Li L. Pitx2, an atrial fibrillation predisposition gene directly regulates ion transport and intercalated disc genes. Circ Cardiovasc Genet. 2014;7:23–32. doi: 10.1161/CIRCGENETICS.113.000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reyes R., Duprat F., Lesage F. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- 38.Enyedi P., Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 39.Harleton E., Besana A., Chandra P. TASK-1 current is inhibited by phosphorylation during human and canine chronic atrial fibrillation. Am J Physiol Heart Circ Physiol. 2015;308:H126–H134. doi: 10.1152/ajpheart.00614.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang B., Soka M., Christensen A.H. Genetic variation in the two-pore domain potassium channel, TASK-1, may contribute to an atrial substrate for arrhythmogenesis. J Mol Cell Cardiol. 2014;67:69–76. doi: 10.1016/j.yjmcc.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Clark R.B., Kondo C., Belke D.D., Giles W.R. Two-pore domain K(+) channels regulate membrane potential of isolated human articular chondrocytes. J Physiol. 2011;589:5071–5089. doi: 10.1113/jphysiol.2011.210757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kindler C.H., Paul M., Zou H. Amide local anesthetics potently inhibit the human tandem pore domain background K+ channel TASK-2 (KCNK5) J Pharmacol Exp Ther. 2003;306:84–92. doi: 10.1124/jpet.103.049809. [DOI] [PubMed] [Google Scholar]
- 43.Ellinor P.T., Lunetta K.L., Albert C.M. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–675. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gore-Panter S.R., Hsu J., Barnard J. PANCR, the PITX2 Adjacent noncoding RNA, is expressed in human left atria and regulates PITX2c expression. Circ Arrhythm Electrophysiol. 2016;9:e003197. doi: 10.1161/CIRCEP.115.003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirchhof P., Sipido K.R., Cowie M.R. The continuum of personalized cardiovascular medicine: a position paper of the European Society of Cardiology. Eur Heart J. 2014;35:3250–3257. doi: 10.1093/eurheartj/ehu312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holmes A.P., Kirchhof P. Pitx2 adjacent noncoding RNA: a new, long, noncoding kid on the 4q25 Block. Circ Arrhythm Electrophysiol. 2016;9:e003808. doi: 10.1161/CIRCEP.115.003808. [DOI] [PubMed] [Google Scholar]
- 47.Martin R.I., Babaei M.S., Choy M.K. Genetic variants associated with risk of atrial fibrillation regulate expression of PITX2, CAV1, MYOZ1, C9orf3 and FANCC. J Mol Cell Cardiol. 2015;85:207–214. doi: 10.1016/j.yjmcc.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Gore-Panter S.R., Hsu J., Hanna P. Atrial fibrillation associated chromosome 4q25 variants are not associated with PITX2c expression in human adult left atrial appendages. PLoS One. 2014;9:e86245. doi: 10.1371/journal.pone.0086245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.