Abstract
Mycobacterium tuberculosis remains a global health threat largely due to the lengthy duration of curative antibiotic treatment, contributing to medical nonadherence and the emergence of drug resistance. This prolonged therapy is likely due to the presence of M. tuberculosis persisters, which exhibit antibiotic tolerance. Inorganic polyphosphate [poly(P)] is a key regulatory molecule in the M. tuberculosis stringent response mediating antibiotic tolerance. The polyphosphate kinase PPK1 is responsible for poly(P) synthesis in M. tuberculosis, while the exopolyphosphatases PPX1 and PPX2 and the GTP synthase PPK2 are responsible for poly(P) hydrolysis. In the present study, we show by liquid chromatography-tandem mass spectrometry that poly(P)-accumulating M. tuberculosis mutant strains deficient in ppx1 or ppk2 had significantly lower intracellular levels of glycerol-3-phosphate (G3P) and 1-deoxy-xylulose-5-phosphate. Real-time PCR revealed decreased expression of genes in the G3P synthesis pathway in each mutant. The ppx1-deficient mutant also showed a significant accumulation of metabolites in the tricarboxylic acid cycle, as well as altered arginine and NADH metabolism. Each poly(P)-accumulating strain showed defective biofilm formation, while deficiency of ppk2 was associated with increased sensitivity to plumbagin and meropenem and deficiency of ppx1 led to enhanced susceptibility to clofazimine. A DNA vaccine expressing ppx1 and ppk2, together with two other members of the M. tuberculosis stringent response, M. tuberculosis rel and sigE, did not show protective activity against aerosol challenge with M. tuberculosis, but vaccine-induced immunity enhanced the killing activity of isoniazid in a murine model of chronic tuberculosis. In summary, poly(P)-regulating factors of the M. tuberculosis stringent response play an important role in M. tuberculosis metabolism, biofilm formation, and antibiotic sensitivity in vivo.
INTRODUCTION
Mycobacterium tuberculosis remains a major threat to global public health (1). The primary obstacles to eradication of M. tuberculosis infection are the need for combination antibiotic treatment and the prolonged duration of treatment, which is believed to be due to the presence of replication-deficient, antibiotic-tolerant persistent bacteria or persisters (2). An improved understanding of the regulatory pathways underlying persister formation is paramount to the development of novel strategies to more effectively target these persisters, thereby shortening the duration of tuberculosis (TB) treatment.
The stringent response (SR) regulatory molecules inorganic polyphosphate [poly(P)] and hyperphosphorylated guanosine [(p)ppGpp] have been shown to mediate persister formation in bacteria (3–5). Although the mechanism remains unclear, (p)ppGpp induces toxin-antitoxin systems, leading to increased numbers of persisters in Escherichia coli (5, 6). M. tuberculosis expresses two polyphosphate kinases (PPK1, PPK2) and two exopolyphosphatases (PPX1, PPX2) to regulate intracellular poly(P) homeostasis (7–10). PPK1 synthesizes poly(P) through hydrolysis of ATP (4). Although PPK2 enzymes can synthesize poly(P), M. tuberculosis PPK2 has nucleoside diphosphate kinase A-like activity (11), catalyzing poly(P) hydrolysis and ATP synthesis 800-fold faster than poly(P) synthesis (12). The mycobacterial stringent response appears to be a positive-feedback loop, as poly(P) phosphorylates and activates the two-component system MprAB, which induces expression of sigE and M. tuberculosis rel (relMtb) (13), leading to increased synthesis of (p)ppGpp, which inhibits the hydrolysis of poly(P) by PPX2 (10). Poly(P) accumulation is associated with antibiotic tolerance (9–11), and poly(P) deficiency renders M. tuberculosis more sensitive to antibiotics (14). In addition, poly(P) homeostasis is required for M. tuberculosis survival during acute infection in murine lungs (15) and during chronic infection in guinea pig lungs (11, 14). Previously, we found that an M. tuberculosis ppx2 (Rv1026) knockdown strain showed global transcriptional changes, including reduced expression of genes encoding enzymes involved in glycerol-3-phosphate (G3P) synthesis (10). This recombinant strain also displayed altered cell wall thickness and permeability and tolerance to the cell wall-active agent isoniazid. However, it is unknown whether these profound metabolic and phenotypic changes were a specific consequence of ppx2 deficiency or were more generally related to poly(P) accumulation.
In the current study, we studied the metabolome and gene expression of two poly(P)-accumulating strains containing transposon insertions in the ppx1 (MT0516) gene or the ppk2 gene (the ppx1::Tn and ppk2::Tn mutants, respectively) (9, 15). In addition, we determined the susceptibility of each strain to various antibiotics and other stresses, as well as their ability to develop biofilms, a potentially important property of persistent M. tuberculosis infection (16, 17). Finally, in order to investigate the role of the M. tuberculosis stringent response pathway in bacillary persistence during antibiotic treatment of chronic murine M. tuberculosis infection, we evaluated the therapeutic efficacy of an adjuvant DNA vaccine expressing ppx1, ppk2, as well as sigE and relMtb, two other members of the positive-feedback cascade regulating M. tuberculosis intracellular poly(P) homeostasis. Our results suggest that enhanced immunity targeting the M. tuberculosis stringent response pathway promotes host control of mycobacteria during antibiotic treatment in mice.
MATERIALS AND METHODS
Bacteria and growth conditions.
Wild-type M. tuberculosis CDC1551 strains deficient in ppx1 (MT0516) (the ppx1::Tn mutant) and ppk2 (Rv3232c) (the ppk2::Tn mutant) were generated by transposon mutagenesis, and the respective complemented strains, the ppx1::Tn Comp and ppk2::Tn Comp strains, were generated and genetically and phenotypically confirmed as previously described (9, 15). All strains, including wild-type strain M. tuberculosis CDC1551, were grown in Middlebrook 7H9 broth (Difco, Sparks, MD) supplemented with 10% oleic acid-albumin-dextrose-catalase (OADC; Difco), 0.1% glycerol, and 0.05% Tween 80 at 37°C on a roller.
Metabolomics analysis.
Sample preparation and analysis were performed as previously described (10, 18). Briefly, mid-logarithmically growing cultures (optical density [OD] = 0.5) of the ppx1::Tn, ppk2::Tn, and wild-type strains were pelleted, and the samples were extracted in 1 ml of extraction buffer (chloroform-methanol, 2:1) and then concentrated by centrifugal evaporation. The samples were processed and analyzed by ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC/MS/MS) and gas chromatography-mass spectrometry (GC/MS) by Metabolon, Inc. (Durham, NC) (19, 20). Statistical analysis of log-transformed data was performed (10), and Welch's t tests were performed to compare data between experimental groups. Multiple comparisons were accounted for by estimating the false discovery rate (FDR) using q values (21).
Sensitivity to plumbagin, meropenem, and clofazimine by disk diffusion.
Each M. tuberculosis strain was grown in enriched 7H9 broth until mid-log growth phase, and 107 bacteria were plated on Middlebrook 7H10 plates. Plumbagin (10 μl of 20 mM or 100 mM dissolved in ethanol), meropenem (10 μg/ml or 20 μg/ml dissolved in water), or clofazimine (10 μg/ml dissolved in dimethyl sulfoxide) was added to separate sterile disks, which were placed on plates individually. The zone of growth inhibition was measured after 10 days of incubation, and each drug concentration was tested for each strain in triplicate (22).
Biofilm formation assay and crystal violet staining.
Crystal violet staining was performed as described previously (23) with minor modifications. Briefly, mid-log-phase cultures (0.5 ml; density, 106 cells/ml) were grown in 24-well conical-bottom plates in Sauton's medium without detergent and shaking for 5 weeks. The extracellular matrix of the biofilm was measured by crystal violet staining using a FLUOstar Optima reader (BMG Labtech).
RT-PCR.
Real-time PCR (RT-PCR) was performed as previously described (15). The gene-specific primers used are listed in Table S2 in the supplemental material.
Ethidium bromide accumulation/efflux assay and Nile red uptake assays.
The results of the ethidium bromide accumulation and efflux assays were determined by measurement of the florescence intensity (10). For Nile red uptake staining, mid-log-phase cultures were washed with phosphate-buffered saline (PBS) and then stained with 20 μM Nile red (Sigma) (24). In all assays, the cells were incubated in 96-well plates, and analysis was performed at the time points indicated below by the use of excitation at 544 nm and emission at 590 nm with a FLUOstar Optima reader (BMG Labtech). All data were normalized to the reading of each well at time zero.
Antigen preparation.
The open reading frames of the ppx1, ppk2, and sigE genes were amplified from M. tuberculosis CDC1551 and cloned individually into plasmid pET28a using the restriction enzymes NdeI and BamHI (see Table S3 in the supplemental material). A previously generated plasmid expressing relMtb, pET15b[relMtb] (25), was used for expression of the RelMtb protein. The resulting plasmids were used to transform E. coli BL21(DE3) RP competent cells (Stratagene). The transformed bacteria were selected by kanamycin (50 μg/ml) or ampicillin (100 μg/ml), and cloning was confirmed by DNA sequencing. The expression and purification of the protein were performed using standard protocols (Qiagen).
DNA vaccination of mice.
The plasmid pSectag2B was used to express individual genes, ppx1, ppk2, relMtb, and sigE. Briefly, each gene was cloned individually into pSectag2B using the restriction enzymes BamHI and HindIII (see Table S3 in the supplemental material). The insertion was confirmed by sequencing, and the expression of target genes was confirmed by transfection of 293T cells. Each DNA vaccine was delivered as previously described (26, 27), and all procedures were performed according to protocols approved by the Johns Hopkins University Institutional Animal Care and Use Committee. Briefly, each plasmid was delivered by intramuscular injection into the quadriceps femoris muscle of the mice, followed by local electroporation using an ECM830 square wave electroporation system (BTX Harvard Apparatus Company, Holliston, MA, USA). Each of the two-needle array electrodes delivered 15 pulses of 72 V (a 20-ms pulse duration at 200-ms intervals). Ten micrograms of each plasmid was injected into each hind leg once weekly for 3 weeks. One week after the last vaccination, the mice were scarified and the blood and splenocytes were collected (27).
ELISA.
Antigen-specific antibody responses were measured by enzyme-linked immunoabsorbent assay (ELISA) as described previously (28), with minor modifications in the coating and serum incubation steps. The 96-well microplate was coated with 1 μg/ml of purified RelMtb or SigE protein overnight. For PPK2 or PPX1, the coating concentration was 5 μg/ml. After blocking, sera from vaccinated mice were diluted 1:100 with PBS, and the diluted sera were added to wells and incubated at room temperature for 2 h.
Intracellular cytokine staining and flow cytometry analysis.
To detect antigen-specific CD4+ T-cell responses by intracellular staining for gamma interferon (IFN-γ), splenocytes were stimulated individually with one of the purified recombinant proteins, PPK2, PPX1, RelMtb, or SigE (10 μg/ml), for 24 h at 37°C before addition of GolgiPlug cocktail (BD Pharmingen, San Diego, CA) overnight. After incubation, the splenocytes were washed once with FACScan buffer and then stained with phycoerythrin-conjugated monoclonal rat anti-mouse CD4. The cells were permeabilized using a Cytofix/Cytoperm kit (BD Pharmingen, San Diego, CA). Intracellular IFN-γ was stained with fluorescein isothiocyanate-conjugated rat anti-mouse IFN-γ and allophycocyanin-conjugated rat anti-mouse tumor necrosis factor alpha (TNF-α; BD Pharmingen, San Diego, CA). Flow cytometry was performed with a FACSCalibur flow cytometer, and the results were analyzed with FlowJo software.
Aerosol infection of mice with M. tuberculosis and DNA vaccine study endpoints.
The stringent response (SR) vaccine, containing 5 μg of each plasmid expressing ppx1, ppk2, relMtb, or sigE was delivered as described above once weekly for 4 weeks. The empty vector, pSectag2B (20 μg), was given as sham vaccine. At 3 weeks after the last vaccine dose, mice were aerosol infected with ∼100 bacilli of wild-type M. tuberculosis CDC1551. Mice were sacrificed on days 14 and 56 after aerosol challenge, and the lungs were homogenized and plated for determination of the number of CFU (15) to evaluate the protective efficacy of the SR vaccine. Separate groups of naive, vaccinated, and sham-vaccinated mice were treated with isoniazid (10 mg/kg of body weight) by esophageal gavage once daily (5 days/week) for 4 weeks beginning on day 14 after aerosol challenge. The lungs were homogenized and plated after 56 days of isoniazid treatment to determine the number of CFU. A partial sample of each infected lung was fixed with 10% buffered formaldehyde, processed, and paraffin embedded for histological staining with hematoxylin and eosin. Morphometric analysis of histology sections was performed as previously described (29).
Statistical analysis.
Data from at least three biological replicates were used to calculate means and standard deviations (SDs) for graphing purposes. Statistical analysis employed the unpaired Student's t test, and a P value of <0.05 was considered statistically significant.
RESULTS
Polyphosphate accumulation alters the metabolic profile of M. tuberculosis.
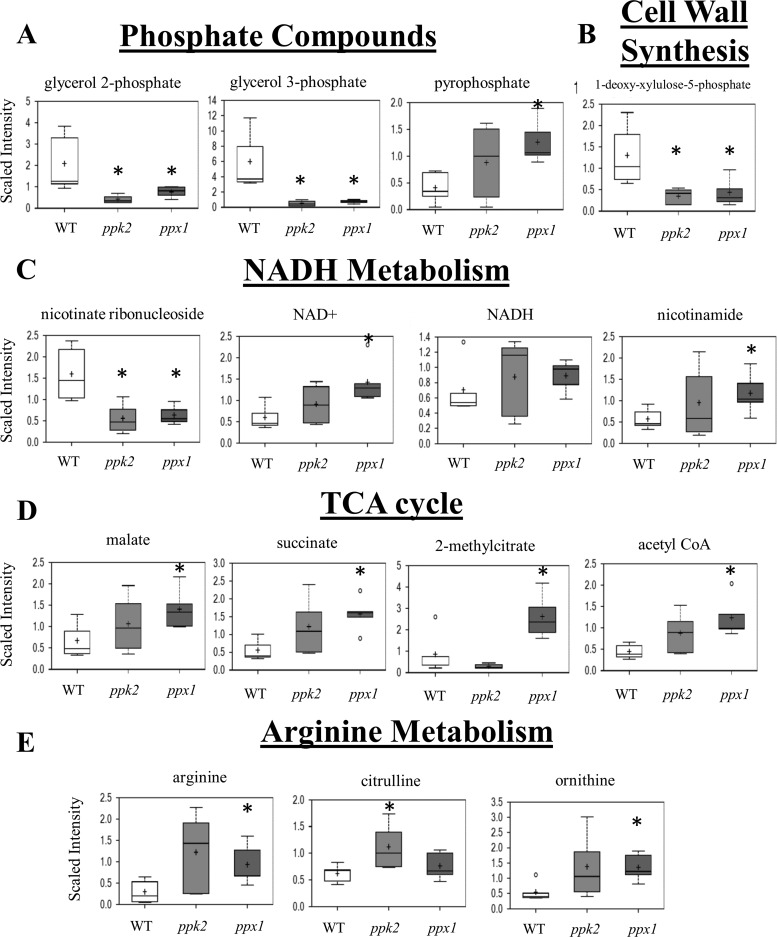
Previously, we have shown that M. tuberculosis mutant strains deficient in ppk2 or ppx1 accumulate poly(P) relative to the level of poly(P) accumulated by the isogenic wild-type strain (9, 15). The ppx1 mutant showed higher levels of expression of the relMtb, sigE, and mprB genes by RT-PCR during mid-log-phase growth (9), while the ppk2-deficient mutant showed a higher level of expression of sigE (change in the threshold cycle value [ΔCT] = 1.26; P = 0.035) but a lower level of expression of mprB (−ΔCT = −0.75; P = 0.017) and unchanged expression of relMtb (−ΔCT = −0.25; P = 0.4) compared to the level of expression by the isogenic wild-type strain during late-log phase growth after normalization to the level of sigA expression. We used metabolomics to further characterize the physiological changes in these two poly(P)-accumulating mutants (see Table S1 in the supplemental material). In addition to the accumulation of poly(P) and pyrophosphate, both strains had significantly lower levels of glycerol-3-phosphate (G3P) and glycerol-2-phosphate (G2P) (Fig. 1A). The peptidoglycan biosynthetic metabolite 1-deoxy-xylulose-5-phosphate (DXP), which is required for the synthesis of isoprenoid (30), was also less abundant in the mutant strains (Fig. 1B). These changes suggest that peptidoglycan synthesis may be affected by poly(P) accumulation. NAD is a cofactor utilized in several redox reactions that are vital to metabolism. The levels of the oxidized form of the metabolite NAD+ were increased in the ppx1::Tn mutant relative to those in wild-type bacilli (Fig. 1C). The level of nicotinate ribonucleoside, an intermediate in the salvage pathway of NAD, was significantly reduced in both the ppk2::Tn and ppx1::Tn mutants. The level of nicotinic acid mononucleotide (NaMN), an intermediate in the de novo synthesis pathway of NAD, was relatively lower in both strains. These findings suggest that the intracellular NAD pool may be regenerated by salvage pathways in these mutant bacteria.
FIG 1.
Polyphosphate accumulation alters M. tuberculosis metabolism. Metabolites altered in the M. tuberculosis ppk2-deficient and ppx1-deficient mutants relative to the wild-type strain include those belonging to the following categories: phosphate compounds (A), cell wall synthesis pathways (B), NADH metabolism (C), the citric acid (TCA) cycle (D), and arginine metabolism (E). WT, wild-type strain; ppk2, ppk2::Tn mutant; ppx1, ppx1::Tn mutant. The data represent 5 independent samples. *, P < 0.05 compared to the wild type.
Lipid oxidation and citrate cycle activity increase during polyphosphate accumulation in the ppk2 and ppx1 mutant strains.
The ppx1::Tn strain showed an increased abundance of several metabolites indicative of increased lipid oxidation, including acetyl coenzyme A (CoA), propionyl-CoA, and 2-methylcitrate (see Table S1 in the supplemental material). Acetyl-CoA can be formed from the oxidation of even-numbered-chain fatty acids, and propionyl-CoA is often generated from the oxidation of odd-numbered-chain fatty acids. Both acetyl-CoA and propionyl-CoA can enter the citric acid cycle to produce precursors for amino acids, lipids, and energy. When propionyl-CoA enters the citric acid cycle, the metabolite 2-methylcitrate is generated. In addition to acetyl-CoA and 2-methylcitrate, the levels of several other citric acid cycle intermediates were elevated in the ppx1::Tn strain, including malate and succinate (Fig. 1D). An increase in the amounts of citric acid cycle intermediates was also observed in the ppk2::Tn mutant, possibly reflecting a need to generate larger amounts of metabolites involved in energy production, such as NADH.
Several amino acid metabolites were more abundant in the poly(P)-accumulating strains, especially in the ppx1::Tn mutant (see Table S1 in the supplemental material). The arginine deiminase pathway provides a mechanism by which ATP can be generated from the metabolism of arginine. In this pathway, arginine is converted to citrulline, which is then converted to ornithine and carbamoyl phosphate. ATP can be generated when carbamoyl phosphate is dephosphorylated by carbamate kinase (31, 32). This pathway is known to be particularly active in response to environmental stress (33, 34) and is of special interest since poly(P) accumulation is associated with bacterial stress resistance. The levels of arginine, citrulline, and ornithine were higher in the ppk2::Tn and ppx1::Tn mutants than in the wild type (Fig. 1E). The increase in the amounts of the intermediates of this pathway may reflect an increased demand for energy and could serve as a potential mechanism by which these strains resist different stresses.
M. tuberculosis polyphosphate accumulation is associated with altered expression of G3P-related genes.
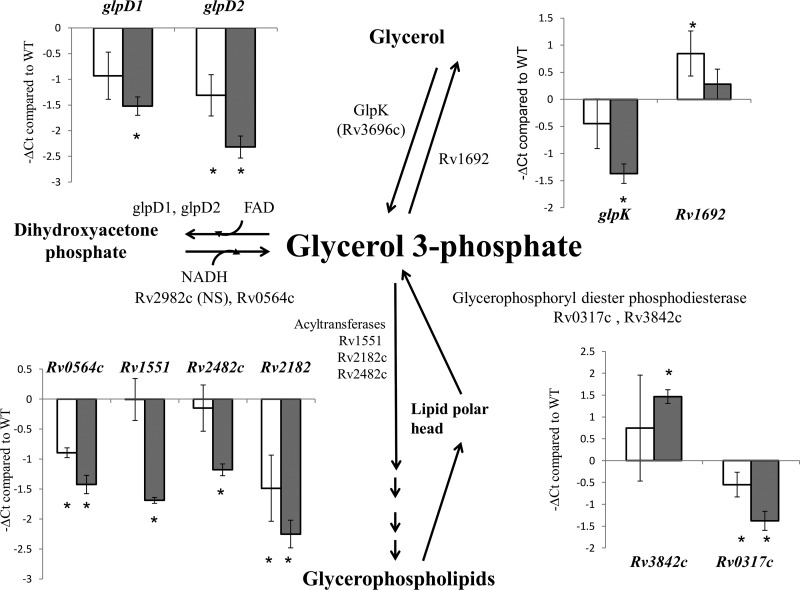
On the basis of the findings presented in our previous report (10) and those from the current study, M. tuberculosis poly(P) accumulation is associated with significantly decreased levels of G3P. This may be due to either decreased de novo synthesis of G3P or increased turnover. Several M. tuberculosis genes encode enzymes that regulate G3P homeostasis (35). Using RT-PCR, we analyzed the expression levels of these genes in the ppk2 and ppx1 mutant strains (Fig. 2). The genes encoding proteins responsible for G3P synthesis from dihydroxyacetone phosphate (Rv0564c) or hydrolysis (glpD1, glpD2) were significantly downregulated in the ppx1::Tn mutant compared to their level of regulation in the wild type. Among the genes involved in recycling of the glycerophospholipid polar head, Rv2182c was significantly downregulated in both mutant strains. The gene encoding the enzyme responsible for the synthesis of G3P from glycerol (glpK) was downregulated in the ppx1::Tn mutant, while the gene encoding the enzyme responsible for G3P hydrolysis was upregulated in the ppk2::Tn mutant. Thus, poly(P) accumulation associated with a deficiency of ppk2 or ppx1 led to altered expression of genes involved in G3P homeostasis, contributing to a reduced intracellular G3P content.
FIG 2.
RT-PCR of M. tuberculosis genes related to glycerol-3-phosphate (G3P) metabolism during poly(P) accumulation. The CT of each gene was normalized to that of the housekeeping gene sigA in each strain, and this value was subtracted from the similarly normalized CT of each gene in the wild-type strain to yield the change in CT (ΔCT). Open bars, ppk2::Tn; gray bars, ppx1::Tn; error bars, SDs from at least three replicates; NS, no significant difference.
Poly(P) homeostasis is required for M. tuberculosis biofilm formation.
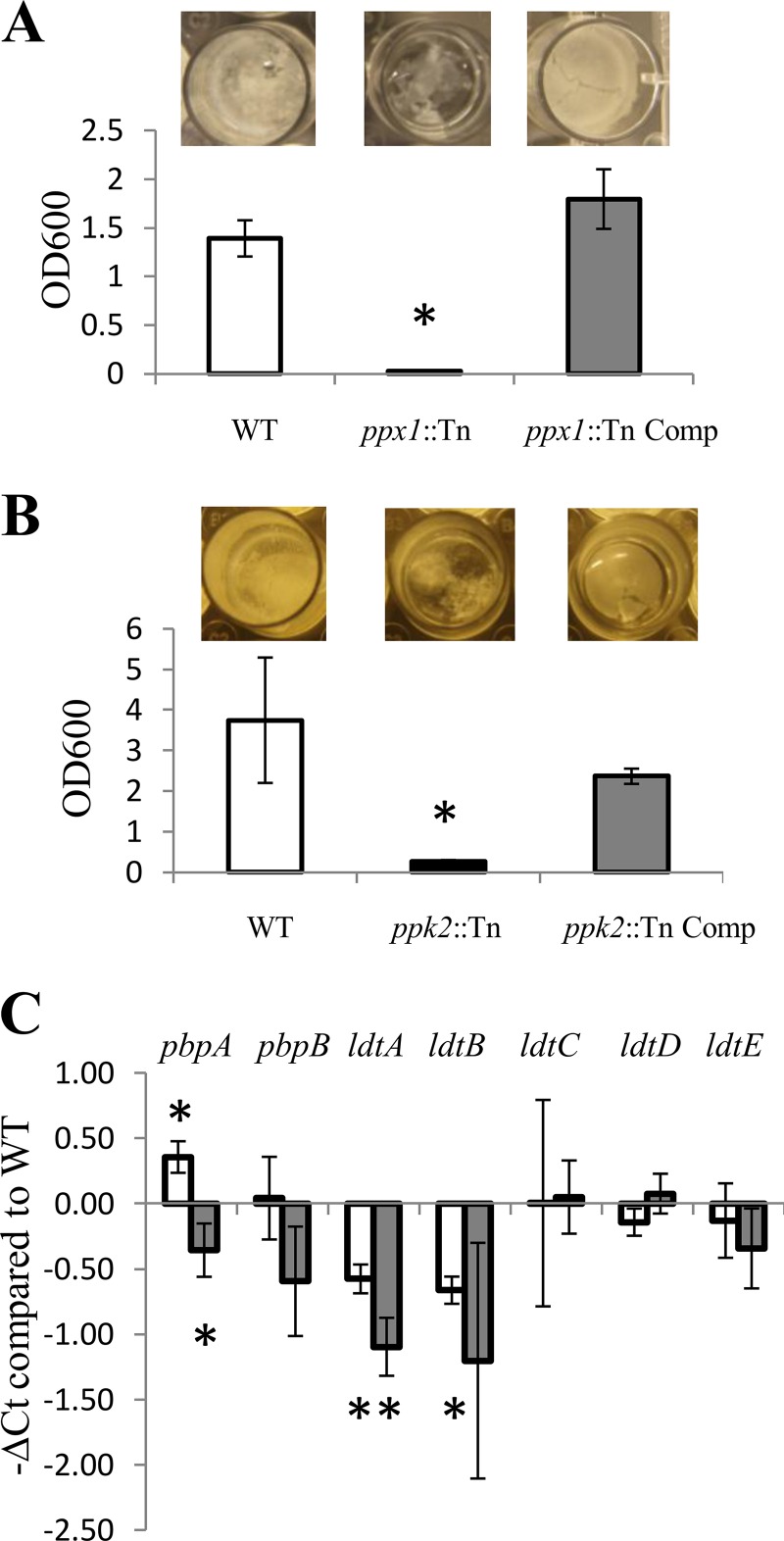
We found that biofilm formation was impaired in a poly(P)-accumulating strain deficient in the exopolyphosphatase ppx2 gene (10). However, it is unclear if poly(P) homeostasis is generally required for biofilm formation or if this phenomenon was related specifically to PPX2 deficiency. The ppx1::Tn and ppk2::Tn mutants and their respective complemented strains were grown in Sauton's medium for 5 to 6 weeks. As shown in Fig. 3, neither of the two mutant strains formed pellicle biofilms at the air-fluid interface. The pellicle formed by the ppk2:Tn complemented strain was less dense than that formed by the wild type, and the density of the pellicle formed by the ppx1::Tn complemented strain was similar to that of the pellicle formed by the wild type. Crystal violet staining corroborated the finding that M. tuberculosis poly(P) accumulation is associated with defective biofilm formation. It is unlikely that these results can be explained by defective growth in the mutants, since these differences persisted even after prolonged observation of cultures until the 8th week (data not shown).
FIG 3.
Polyphosphate accumulation alters expression of peptidoglycan biosynthesis genes and reduces biofilm formation. (A, B) The ppx1::Tn mutant (A) and the ppk2::Tn mutant (B) were incubated in Sauton's medium lacking detergent for 5 weeks, and biofilms were assessed by crystal violet staining (*, P < 0.05 relative to the wild type; n = 3). OD600, OD at 600 nm. (C) RT-PCR of M. tuberculosis genes related to peptidoglycan biosynthesis during mid-log phase of growth. The CT of each gene was normalized to that of the housekeeping gene sigA in each strain, and this value was subtracted from the similarly normalized CT of each gene in the wild-type strain to yield the change in CT (ΔCT). Open bars, ppk2::Tn mutant; gray bars, ppx1::Tn mutant; error bars, SDs. *, P < 0.05 relative to the wild type; n = 3.
Deficiency of ppk2 leads to increased sensitivity to plumbagin and meropenem, while ppx1 deficiency increases sensitivity to clofazimine.
Previous studies have shown that meropenem inhibits both the d,d-carboxypeptidase and l,d-transpeptidase of M. tuberculosis (36). l,d-Transpeptidase plays an important role in cell wall remodeling during mycobacterial entry into stationary phase (37). We studied the sensitivity of the ppk2::Tn and ppx1::Tn mutants to meropenem by the disk diffusion method (Table 1). The ppk2::Tn mutant showed increased sensitivity to meropenem at 10 μg/ml and 20 μg/ml, and the wild-type sensitivity pattern was partially restored in the ppk2::Tn complemented strain. Similarly, the ppx1::Tn mutant showed trends toward increased susceptibility to meropenem similar to those for the ppk2::Tn mutant, although this did not reach statistical significance. Next, we tested whether poly(P) accumulation altered mycobacterial resistance to oxidative stress (38). The ppk2::Tn mutant was more sensitive to the naphthoquinone plumbagin, and wild-type resistance was restored in the complemented strain. Similarly, the ppx1::Tn mutant showed mildly increased sensitivity to plumbagin compared to the wild type (P = 0.1). We then tested the effect of poly(P) accumulation on sensitivity to clofazimine, which destabilizes the bacterial membrane and alters redox cycling (39). Although ppk2 deficiency did not alter clofazimine susceptibility, the ppx1::Tn mutant was more sensitive to clofazimine (P = 0.006), and wild-type levels of susceptibility were restored in the complemented strain.
TABLE 1.
Sensitivity of each strain to plumbagin, meropenem, and clofazimine by the disk diffusion method
| Drug (concn) | Inhibition zone diam (mm) for wild-type strain |
ppk2::Tn mutant |
ppk2::Tn Comp strain |
ppx1::Tn mutant |
ppx1::Tn Comp strain |
||||
|---|---|---|---|---|---|---|---|---|---|
| Inhibition zone diam (mm) | P value | Inhibition zone diam (mm) | P value | Inhibition zone diam (mm) | P value | Inhibition zone diam (mm) | P value | ||
| Plumbagin | |||||||||
| 20 mM | 25 ± 1.7 | 32.7 ± 4.1 | 0.042 | 24.3 ± 0.6 | 0.561 | 25.7 ± 2.1 | 0.692 | 23.3 ± 0.6 | 0.189 |
| 100 mM | 44 ± 1 | 49.3 ± 1.2 | 0.004 | 45.3 ± 4.2 | 0.618 | 47.3 ± 2.5 | 0.100 | 40.7 ± 2.3 | 0.083 |
| Meropenem | |||||||||
| 10 μg/ml | 17.4 ± 1.2 | 24 ± 2 | 0.007 | 20.6 ± 1.2 | 0.024 | 19.4 ± 2.4 | 0.251 | 16 ± 0 | 0.116 |
| 20 μg/ml | 26.6 ± 1.154 | 29.3 ± 1.2 | 0.047 | 31.3 ± 3.1 | 0.069 | 31.3 ± 3.2 | 0.077 | 32.6 ± 4.9 | 0.110 |
| Clofazimine (10 μg/ml) | 18.3 ± 0.57 | 18.6 ± 2.3 | 0.820 | 17.33 ± 1.2 | 0.251 | 21 ± 1 | 0.006 | 20.3 ± 1.2 | 0.055 |
Deficiency of ppx1 or ppk2 alters expression of peptidoglycan biosynthesis genes but does not alter cell wall permeability.
The ppk2::Tn and ppx1::Tn mutants showed increased susceptibility to meropenem, suggesting potential changes in the mycobacterial cell wall structure associated with poly(P) accumulation. Our metabolomics analysis revealed a decreased abundance of 1-deoxy-d-xylulose-5-phosphate in both mutants. To investigate the possibility that poly(P) accumulation leads to altered peptidoglycan biosynthesis, we used RT-PCR to evaluate the expression of relevant genes (40). The genes encoding l,d-transpeptidase, ldtA and ldtB, were downregulated in both mutant strains (41). The ppk2 mutant showed higher levels of expression of pbpA, while the ppx1 mutant showed lower levels of expression than the wild type (Fig. 3C).
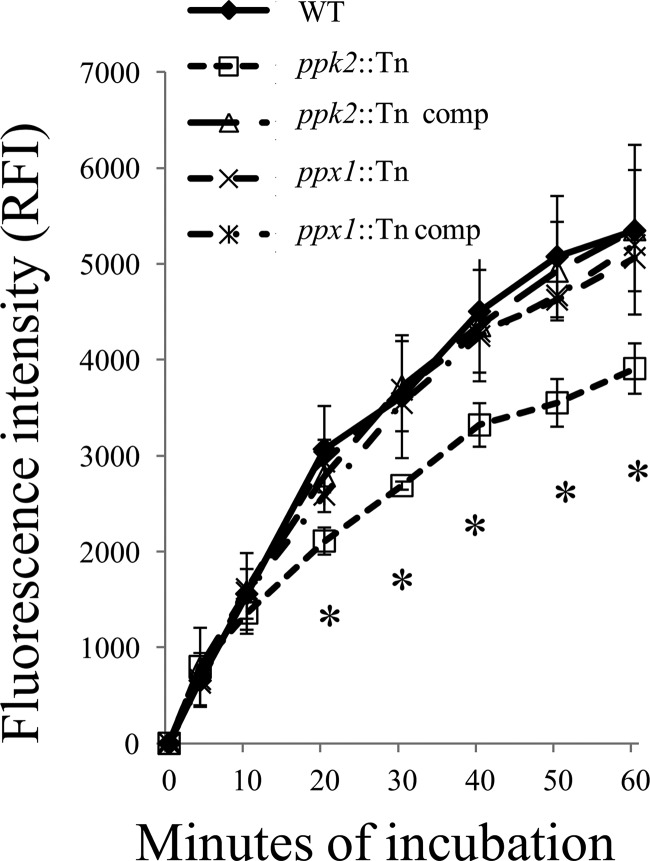
During macrophage infection, M. tuberculosis can acquire phenotypic tolerance to antibiotics through induction of efflux pumps (42). Previously, we found that a poly(P)-accumulating strain (ppx2 knockdown) had altered cell wall permeability based on the results of an ethidium bromide uptake assay (10). In the current study, we did not observe any significant difference in ethidium bromide accumulation or efflux pump activity between each mutant and the respective complemented and wild-type strains (data not shown). For further evaluation of cell wall permeability, we used Nile red staining to determine whether deficiency of ppx1 or ppk2 was associated with altered rates of diffusion of lipophilic molecules across the M. tuberculosis cell wall (24). Nile red staining was equivalent between the ppx1::Tn mutant and the wild type, but the ppk2::Tn mutant showed reduced Nile red accumulation relative to the wild type (Fig. 4). The ppk2 complemented strain showed uptake of Nile red similar to that of the wild type.
FIG 4.
Decreased Nile red staining in the ppk2-deficient mutant. Each strain was grown to mid-log phase and stained with 20 μM Nile red. The fluorescence intensity was normalized to the initial signal. Data represent mean values ± SDs for three biological replicates. *, P < 0.05 when comparing ppk2::Tn mutants to the wild type or the pp2::Tn Comp strain. RFI, relative fluorescence intensity.
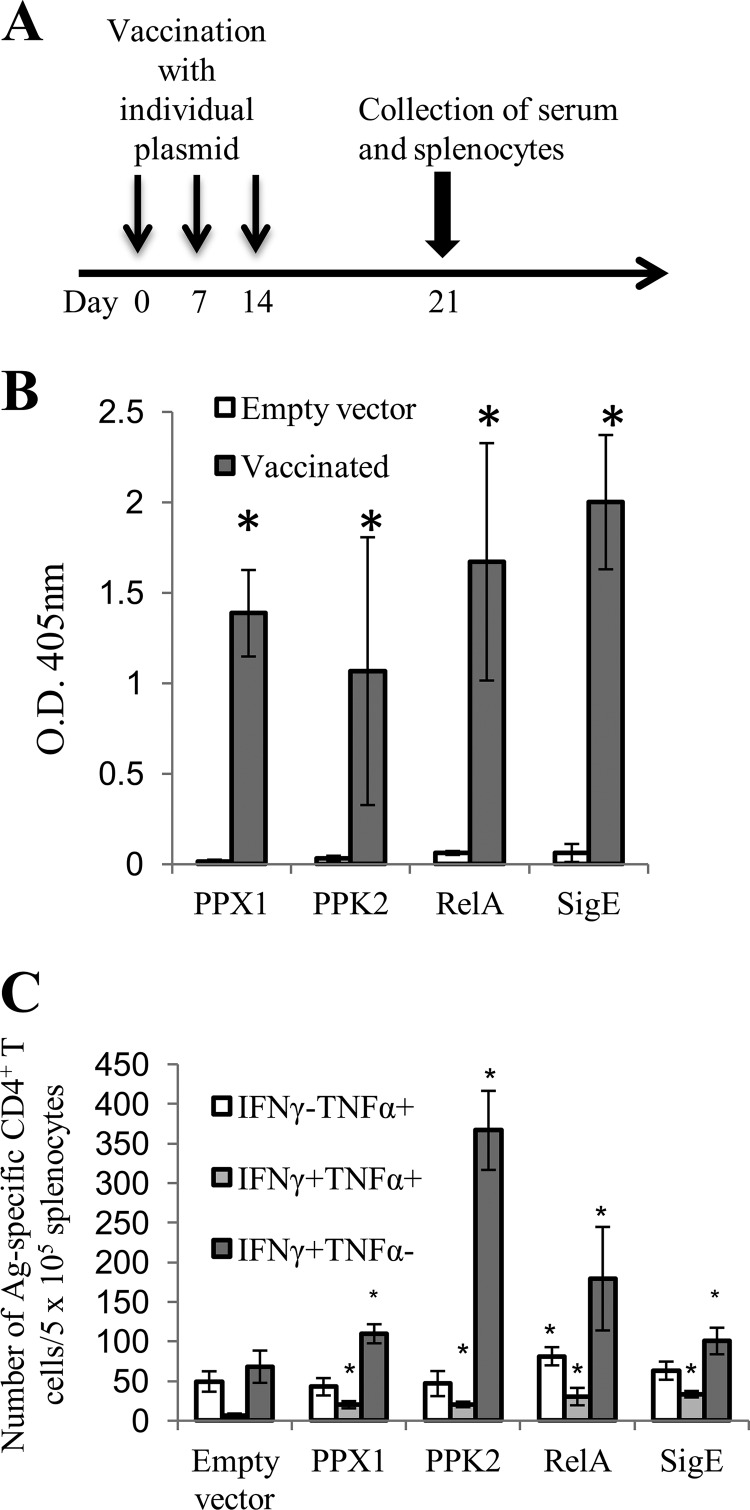
DNA vaccination with poly(P)-regulatory genes of the M. tuberculosis stringent response pathway generates antigen-specific CD4+ T-cell responses and immunoglobulin.
Although previous reports have highlighted the importance of poly(P)-regulatory genes in antibiotic tolerance and persistence in animal lungs (9, 15), it remains to be determined whether the stringent response pathway can be therapeutically targeted during chronic M. tuberculosis infection. To test this possibility, we first sought to determine whether specific immunity could be engendered to specific poly(P)-regulatory factors by DNA vaccination. We cloned four poly(P)-regulatory genes, relMtb, sigE, ppk2, and ppx, individually into the eukaryotic expression plasmid pSectag2B. Separate groups of C57BL/6J mice were vaccinated intramuscularly with each of the plasmids carrying the individual genes or the empty vector once weekly for 3 weeks, and electroporation was performed immediately following each injection (Fig. 5A). Significant IgG responses to each antigen were detected by ELISA in the serum of vaccinated mice 1 week after the last DNA vaccine dose (Fig. 5B). Next, splenocytes from vaccinated mice were collected and stimulated with each recombinant protein (10 μg/ml) for 24 h. DNA vaccination with each poly(P)-regulatory gene generated significant antigen-specific IFN-γ-producing CD4+ T cells compared with vaccination with the vector control (Fig. 5C). Only DNA vaccination with relMtb generated significant antigen-specific TNF-α-producing CD4+ T cells compared with vaccination with the vector control.
FIG 5.
Immunogenicity of DNA vaccines targeting poly(P)-regulatory genes. (A) Six- to 8-week-old female C57BL/6J mice (n = 3 or 4) were vaccinated with DNA plasmid (pSectag2B) encoding relMtb, sigE, ppx1, or ppk2 or the empty vector, as illustrated in the scheme. Vaccination and electroporation were performed once weekly for 3 weeks. At 1 week after the last vaccination, sera and splenocytes were collected from each group. (B) ELISA detection of antigen-specific IgG responses from the different mouse vaccination groups. (C) Summary of antigen (Ag)-specific CD4+ T-cell responses (positive intracellular staining for IFN-γ [IFN-γ+] and TNF-α [TNF-α+]) following DNA vaccination. Splenocytes from the individual vaccinated groups were stimulated with 10 μg/ml of each recombinant protein for 24 h at 37°C, and then GolgiPlug cocktail (1 μl/ml) was added overnight. The cells were then stained with anti-mouse CD4, followed by positive intracellular staining for IFN-γ and TNF-α. The data were acquired with a FACSCalibur flow cytometer and analyzed with FlowJo software. *, P < 0.05 compared to the empty vector control. IFN-γ− and TNF-α−, negative intracellular staining for IFN-γ and TNF-α, respectively.
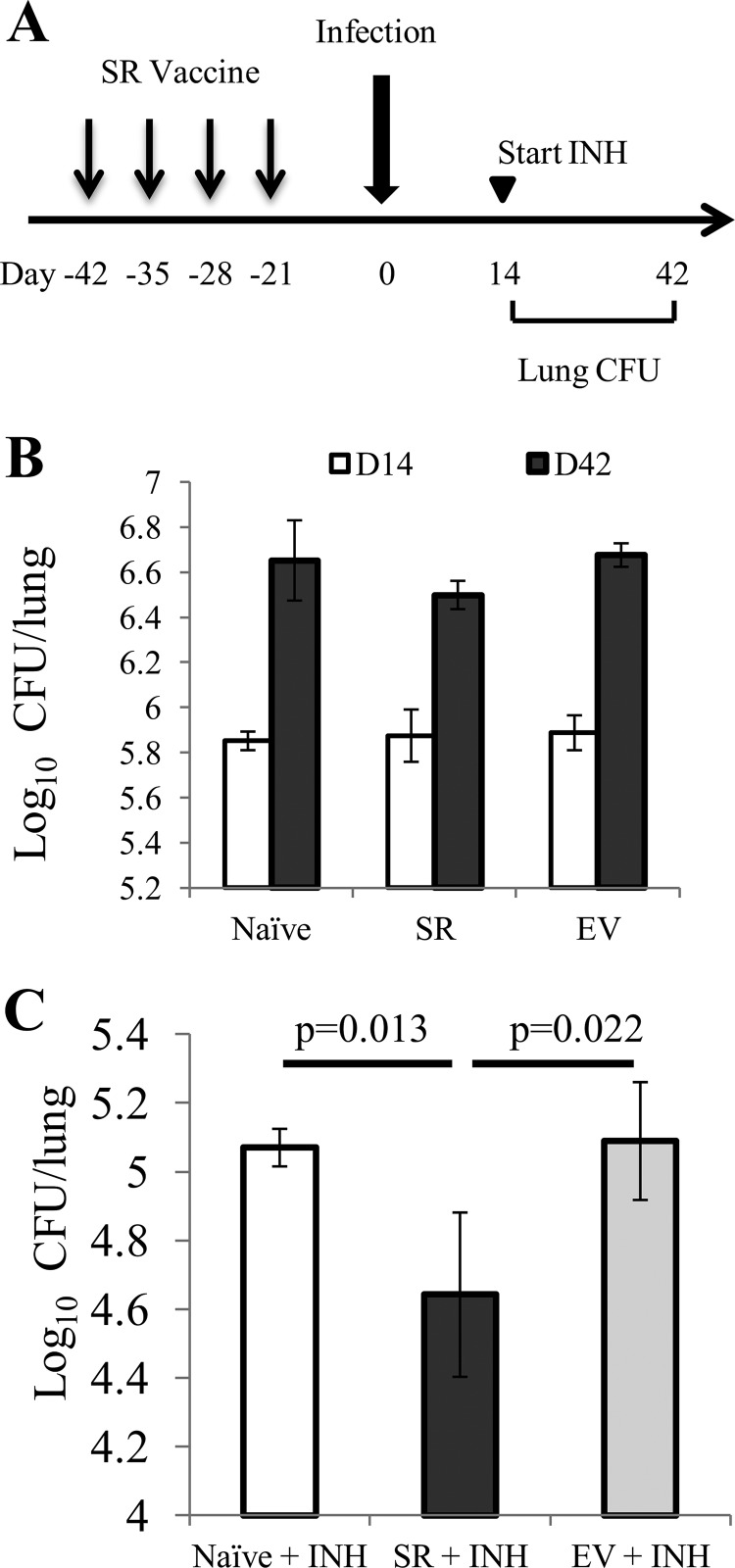
Immunity engendered by DNA vaccination with poly(P)-regulatory genes enhances the bactericidal activity of isoniazid in mice.
To address the potential preventive and therapeutic efficacy of immunity induced by relMtb, sigE, ppk2, and ppx1, we combined the four individual DNA plasmids expressing each of these poly(P)-regulatory genes, yielding the stringent response (SR) DNA vaccine. C57BL/6J mice were vaccinated either with the SR vaccine (5 μg of each plasmid) or with the empty vector control (20 μg) once weekly for 4 weeks (Fig. 6A). These mice were aerosol challenged with virulent M. tuberculosis strain CDC1551 3 weeks after the last vaccine dose. The lung bacillary burden was not significantly different between vaccinated mice and naive mice at 14 days and 42 days after aerosol challenge (Fig. 6B). A separate group of mice received human-equivalent doses of isoniazid once daily (5 days/week) by esophageal gavage beginning on day 14 after aerosol challenge (43). Following 28 days of treatment with isoniazid, we observed a significant (P = 0.013) reduction in the number of CFU in the lungs of the SR-vaccinated group relative to the number in the lungs of the group receiving the sham vaccine (Fig. 6C). Analysis of lung histology revealed pulmonary inflammation which was commensurate with the lung bacillary burden (see Fig. S2 in the supplemental material). As far as we are aware, this is the first study to show that immunity to poly(P)-regulatory factors of the stringent response pathway enhances the bactericidal activity of the first-line drug isoniazid.
FIG 6.
Immunity to key poly(P)-regulatory factors of the M. tuberculosis stringent response augments the bactericidal activity of isoniazid in mice. (A) Experimental scheme. C57BL/6J mice were vaccinated intramuscularly once weekly with the SR vaccine comprising four different DNA plasmids, each of which carried the relMtb, sigE, ppx1, or ppk2 gene, or with a sham vaccine (empty vector [EV]). A separate group of mice received no vaccination (naive). Three weeks later, all mice were aerosol infected with wild-type M. tuberculosis. Beginning on day 14 after aerosol infection, subgroups of mice in each group were treated orally with human-equivalent doses of isoniazid for 4 weeks. INH, isoniazid at 10 mg/kg by esophageal gavage once daily (5 days/week). (B) Lung bacillary burden (log10 number of CFU) on day 14 (D14) and day 42 (D42) following M. tuberculosis aerosol challenge of naive mice and those receiving SR vaccine or sham vaccine (empty vector). (C) Lung bacillary burden (log10 number of CFU) at day 42 of isoniazid treatment (n = 4 animals per data point).
DISCUSSION
The bacterial stringent response appears to play a key role in antibiotic tolerance (2, 44). Poly(P) may serve as a phosphate donor, activating downstream stringent response genes, including mprAB, sigE, and relMtb, thereby leading to antibiotic tolerance in M. tuberculosis (7, 9, 10, 15, 45). Previously, we found that the ppk2-deficient mutant showed attenuated growth during the acute phase of infection in the murine model but did not show reduced persistence beyond day 56 after infection (15). The ppx1-deficient mutant showed a persistence defect in the guinea pig model (9). Similarly, when the ppk2 gene was disrupted by a mycobacteriophage, the resulting ppk2-deficient M. tuberculosis strain showed reduced persistence in guinea pigs (11). Our metabolomics analysis revealed that poly(P) accumulation alters the intracellular G3P content in M. tuberculosis (Fig. 1). Transcriptional analysis of both mutant strains showed reduced expression of genes involved in G3P synthesis and recycling (Fig. 2). Importantly, G3P can be used as a scaffold for phospholipid biosynthesis (46). Overexpression of glycerol-3-phosphate dehydrogenase in E. coli leads to reduced intracellular G3P levels and increased formation of persister cells following exposure to antibiotics (47). G3P and G2P levels are reduced in M. tuberculosis when cholesterol is the sole carbon source (48). During chronic M. tuberculosis infection of mice, the use of cholesterol is important for bacillary survival (49). Cholesterol accumulation alters M. tuberculosis cell wall permeability to rifampin and masks cell wall antigen from binding antibody (50). We have shown previously that a poly(P)-accumulating strain (ppx2 knockdown) exhibited significant downregulation of the G3P dehydrogenase gene glpD2 (10), which is also downregulated in M. tuberculosis persisters (10, 51). Taken together, these findings suggest that poly(P) serves an important role in downregulating G3P synthesis, which may promote persister formation and antibiotic tolerance (52).
The role of the arginine deiminase pathway during chronic bacterial infections remains controversial. Previous studies have reported the importance of this pathway during microaerobic survival of Pseudomonas aeruginosa in the airways of patients with cystic fibrosis (53). l-Arginine deiminase degradation may serve as an alternative carbohydrate utilization pathway during chronic infection in P. aeruginosa (54). Infection with Mycobacterium bovis BCG leads to enhanced arginine uptake by IFN-γ-stimulated macrophages (55). However, an M. tuberculosis strain deficient in the arginine deiminase gene (arcA) did not show defective survival during chronic murine infection (31). Our data raise the possibility that poly(P) accumulation leads to upregulation of the arginine deiminase pathway, perhaps reflecting a switch to alternative energy sources. Remodeling of the citric acid cycle, including increased production of succinate and decreased production of α-ketoglutarate, has been observed during M. tuberculosis adaptation to hypoxia and following bacillary exposure to antibiotics (56, 57). In E. coli, citrate and succinate were found to accumulate during exposure to bactericidal antibiotics (58). In the current study, we found that deficiency of ppx1 was associated with a significantly increased abundance of succinate and malate. These trends were also observed during ppk2 deficiency, although they did not achieve statistical significance. Our data suggest that poly(P) accumulation leads to remodeling of the citric acid cycle, although the mechanisms by which these metabolic changes are regulated and how they contribute to antibiotic tolerance remain to be elucidated.
Prior work has focused on the role of poly(P) as a molecular chaperone for stabilizing proteins (59). In the current study, we found accumulation of several amino acid metabolites in the two poly(P)-accumulating strains, particularly in the ppx1::Tn mutant. These findings differ from those of a previously reported poly(P)-accumulating strain deficient in ppx2 (10). This discrepancy may potentially be explained by differences in the function of the two M. tuberculosis exopolyphosphatases, PPX1 and PPX2. Specifically, PPX1 hydrolyzes short-chain poly(P) (60), while PPX2 predominantly hydrolyzes long-chain poly(P) (10). The length of individual molecules may dictate the function of poly(P) (59). In addition, these mutant strains were generated using distinct genetic techniques. The previously reported ppx2-deficient mutant was generated using a tetracycline-inducible system to overexpress the antisense version of the ppx2 gene, thereby knocking down its expression, whereas the ppx1-deficient mutant used in the current study contains a transposon insertion at bp 732 (total gene length, 1,035 bp), likely disrupting the ppx1 function. Further studies are needed to elucidate the role of short-chain and long-chain poly(P) in the regulation of individual M. tuberculosis metabolic processes.
Poly(P) homeostasis appears to be required for the formation of biofilms. Thus, poly(P) accumulation in Campylobacter jejuni (61) and P. aeruginosa (62) was associated with defective biofilm formation. Furthermore, deficiency of a polyphosphate kinase responsible for poly(P) synthesis in P. aeruginosa also resulted in defective biofilms (63). Null mutants of PPK and PPX in Bacillus cereus showed similar defects in biofilm formation (64). Consistent with these findings in other bacteria, we have shown previously (10) and in the current study that maintenance of the intracellular poly(P) balance is required for biofilm formation in M. tuberculosis. Interestingly, a recent study found that ppk2 deficiency did not alter biofilm formation in M. tuberculosis (11). Notably, the parental strain used to generate the mutant in our study was CDC1551. This strain has previously been shown to form less biofilm than strain H37Rv (23), which was used by Singh et al. (11). Furthermore, Singh et al. used homologous recombination with temperature-sensitive mycobacteriophages to delete the ppk2 open reading frame (11), while our study used transposon mutagenesis to disrupt ppk2. Relatively little is known about the mechanism by which poly(P) levels modulate biofilm formation. In E. coli, poly(P) hydrolysis during stationary phase appears to trigger the formation of biofilms via the LuxS quorum-sensing system (65). Future studies will investigate the role of decreased G3P, remodeling of the citric acid cycle, and altered NADH metabolism on M. tuberculosis biofilm formation.
Intracellular accumulation of poly(P) has been linked to M. tuberculosis growth restriction and tolerance to the bactericidal drug isoniazid (9, 15). Our metabolomics analysis revealed alterations in cell wall lipid composition and DXP levels during poly(P) accumulation. By RT-PCR, both mutants showed reduced expression of the l,d-transpeptidase genes, ldtA and ldtB, which play an important role in peptidoglycan formation of nonreplicating mycobacteria (41). Unlike the ppx1-deficient mutant, the ppk2-deficient mutant showed increased sensitivity to the l,d-transpeptidase inhibitor meropenem (36), as well as increased expression of pbpA. Further studies are required to understand the role of poly(P) accumulation in peptidoglycan synthesis.
The respiration and electron transport pathways are required for the transition of M. tuberculosis into persistence and antibiotic tolerance (66, 67). We used the oxidative agent plumbagin to determine if poly(P) accumulation alters sensitivity to inhibitors of respiratory electron transport (38, 68). The ppk2::Tn mutant was more sensitive than the wild type and the ppx1-deficient mutant to plumbagin, suggesting that ppk2 deficiency shifts bacteria to a greater dependence on the citric acid cycle and the electron transport chain (69). Furthermore, the ppx1-deficient mutant showed increased susceptibility to clofazimine, which destabilizes the bacterial membrane and targets the redox cycling pathway by enzymatic reduction of the drug by NDH-2, the primary respiratory chain NADH:quinone oxidoreductase of mycobacteria, and nonenzymatic oxidation of reduced clofazimine by O2, yielding reactive oxygen species (39, 70). Future studies will address whether the discrepancies in antibiotic sensitivity between these two poly(P)-accumulating strains result from differences in the enzymatic function of PPX1 and PPK2.
Recently, there has been significant interest in host-directed therapy to treat TB (71). Although BCG vaccination does not enhance the bactericidal activity of chemotherapy in the murine model (72), a DNA vaccine expressing heat shock protein 65 has been shown to synergize with conventional antitubercular drugs, further reducing the bacterial burden in the lungs of M. tuberculosis-infected mice or nonhuman primates (72, 73). A fragment whole-cell lysate therapeutic vaccine, RUTI, has shown efficacy in generating protective immunity in preclinical studies (74, 75). However, the primary factors responsible for TB immunity remain unknown. In the current study, we have shown that a DNA vaccine targeting key poly(P)-regulatory factors of the M. tuberculosis stringent response generates IgG and antigen-specific CD4+ T cells, but these immune responses did not offer protection against M. tuberculosis challenge in the murine model. However, we observed a significant reduction in lung bacillary burden when immunity to these M. tuberculosis stringent response factors was combined with isoniazid. One potential explanation for this phenomenon is that the population of persistent bacilli in untreated, chronically infected mice is relatively small (76), but exposure to isoniazid further induces the formation of persisters (77, 78). In favor of this hypothesis, isoniazid exposure induces M. tuberculosis expression of relMtb, which is required for persister formation (2, 44, 79). Further studies are required to determine the utility of the SR vaccine as a therapeutic vaccine in shortening the duration of treatment in combination with the standard antitubercular regimen or for treating latent M. tuberculosis infection.
In summary, poly(P) accumulation appears to induce changes in M. tuberculosis metabolism, resulting in altered susceptibility to oxidative stresses and antibiotic exposure and defective biofilm formation. Poly(P) chain length may play an important role in these processes, since we observed several differences between the ppk2-deficient and ppx1-deficient mutants. Immunity targeting key poly(P)-regulatory factors of the M. tuberculosis stringent response augments the tuberculocidal activity of isoniazid in mouse lungs. Future studies will focus on the utility of the stringent response as a potential new target in host-directed therapy for TB.
Supplementary Material
ACKNOWLEDGMENTS
We have no conflicts of interest to declare.
This work was supported by NIH grants R01HL106786 and UH2AI122309 to P.C.K.
The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01139-16.
REFERENCES
- 1.Lawn SD, Zumla AI. 2011. Tuberculosis. Lancet 378:57–72. doi: 10.1016/S0140-6736(10)62173-3. [DOI] [PubMed] [Google Scholar]
- 2.Cohen NR, Lobritz MA, Collins JJ. 2013. Microbial persistence and the road to drug resistance. Cell Host Microbe 13:632–642. doi: 10.1016/j.chom.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulaev I, Kulakovskaya T. 2000. Polyphosphate and phosphate pump. Annu Rev Microbiol 54:709–734. doi: 10.1146/annurev.micro.54.1.709. [DOI] [PubMed] [Google Scholar]
- 4.Rao NN, Gomez-Garcia MR, Kornberg A. 2009. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem 78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 5.Maisonneuve E, Castro-Camargo M, Gerdes K. 2013. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell 154:1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 6.Germain E, Roghanian M, Gerdes K, Maisonneuve E. 2015. Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases. Proc Natl Acad Sci U S A 112:5171–5176. doi: 10.1073/pnas.1423536112. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Sureka K, Dey S, Datta P, Singh AK, Dasgupta A, Rodrigue S, Basu J, Kundu M. 2007. Polyphosphate kinase is involved in stress-induced mprAB-sigE-rel signalling in mycobacteria. Mol Microbiol 65:261–276. doi: 10.1111/j.1365-2958.2007.05814.x. [DOI] [PubMed] [Google Scholar]
- 8.Sureka K, Sanyal S, Basu J, Kundu M. 2009. Polyphosphate kinase 2: a modulator of nucleoside diphosphate kinase activity in mycobacteria. Mol Microbiol 74:1187–1197. doi: 10.1111/j.1365-2958.2009.06925.x. [DOI] [PubMed] [Google Scholar]
- 9.Thayil SM, Morrison N, Schechter N, Rubin H, Karakousis PC. 2011. The role of the novel exopolyphosphatase MT0516 in Mycobacterium tuberculosis drug tolerance and persistence. PLoS One 6:e28076. doi: 10.1371/journal.pone.0028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang YM, Bandyopadhyay N, Rifat D, Rubin H, Bader JS, Karakousis PC. 2015. Deficiency of the novel exopolyphosphatase Rv1026/PPX2 leads to metabolic downshift and altered cell wall permeability in Mycobacterium tuberculosis. mBio 6:e02428. doi: 10.1128/mBio.02428-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh M, Tiwari P, Arora G, Agarwal S, Kidwai S, Singh R. 2016. Establishing virulence associated polyphosphate kinase 2 as a drug target for Mycobacterium tuberculosis. Sci Rep 6:26900. doi: 10.1038/srep26900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shum KT, Lui EL, Wong SC, Yeung P, Sam L, Wang Y, Watt RM, Tanner JA. 2011. Aptamer-mediated inhibition of Mycobacterium tuberculosis polyphosphate kinase 2. Biochemistry 50:3261–3271. doi: 10.1021/bi2001455. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Lee SS, Xu W. 2003. Crystallization and characterization of polyphosphate kinase from Escherichia coli. Biochem Biophys Res Commun 305:997–1001. doi: 10.1016/S0006-291X(03)00886-6. [DOI] [PubMed] [Google Scholar]
- 14.Singh R, Singh M, Arora G, Kumar S, Tiwari P, Kidwai S. 2013. Polyphosphate deficiency in Mycobacterium tuberculosis is associated with enhanced drug susceptibility and impaired growth in guinea pigs. J Bacteriol 195:2839–2851. doi: 10.1128/JB.00038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang YM, Belchis DA, Karakousis PC. 2013. The polyphosphate kinase gene ppk2 is required for Mycobacterium tuberculosis inorganic polyphosphate regulation and virulence. mBio 4:e00039-13. doi: 10.1128/mBio.00039-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand A, Verma P, Singh AK, Kaushik S, Pandey R, Shi C, Kaur H, Chawla M, Elechalawar CK, Kumar D, Yang Y, Bhavesh NS, Banerjee R, Dash D, Singh A, Natarajan VT, Ojha AK, Aldrich CC, Gokhale RS. 2015. Polyketide quinones are alternate intermediate electron carriers during mycobacterial respiration in oxygen-deficient niches. Mol Cell 60:637–650. doi: 10.1016/j.molcel.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackart DF, Hascall-Dove L, Caceres SM, Kirk NM, Podell BK, Melander C, Orme IM, Leid JG, Nick JA, Basaraba RJ. 2014. Expression of antimicrobial drug tolerance by attached communities of Mycobacterium tuberculosis. Pathog Dis 70:359–369. doi: 10.1111/2049-632X.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galagan JE, Minch K, Peterson M, Lyubetskaya A, Azizi E, Sweet L, Gomes A, Rustad T, Dolganov G, Glotova I, Abeel T, Mahwinney C, Kennedy AD, Allard R, Brabant W, Krueger A, Jaini S, Honda B, Yu WH, Hickey MJ, Zucker J, Garay C, Weiner B, Sisk P, Stolte C, Winkler JK, Van de Peer Y, Iazzetti P, Camacho D, Dreyfuss J, Liu Y, Dorhoi A, Mollenkopf HJ, Drogaris P, Lamontagne J, Zhou Y, Piquenot J, Park ST, Raman S, Kaufmann SH, Mohney RP, Chelsky D, Moody DB, Sherman DR, Schoolnik GK. 2013. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499:178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boudonck KJ, Mitchell MW, Nemet L, Keresztes L, Nyska A, Shinar D, Rosenstock M. 2009. Discovery of metabolomics biomarkers for early detection of nephrotoxicity. Toxicol Pathol 37:280–292. doi: 10.1177/0192623309332992. [DOI] [PubMed] [Google Scholar]
- 20.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. 2009. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 21.Storey JD, Tibshirani R. 2003. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zacharia VM, Manzanillo PS, Nair VR, Marciano DK, Kinch LN, Grishin NV, Cox JS, Shiloh MU. 2013. cor, a novel carbon monoxide resistance gene, is essential for Mycobacterium tuberculosis pathogenesis. mBio 4:e00721-13. doi: 10.1128/mBio.00721-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang JM, Layre E, Sweet L, Sherrid A, Moody DB, Ojha A, Sherman DR. 2012. The polyketide Pks1 contributes to biofilm formation in Mycobacterium tuberculosis. J Bacteriol 194:715–721. doi: 10.1128/JB.06304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu WX, Zhang L, Mai JT, Peng RC, Yang EZ, Peng C, Wang HH. 2014. The Wag31 protein interacts with AccA3 and coordinates cell wall lipid permeability and lipophilic drug resistance in Mycobacterium smegmatis. Biochem Biophys Res Commun 448:255–260. doi: 10.1016/j.bbrc.2014.04.116. [DOI] [PubMed] [Google Scholar]
- 25.Avarbock D, Salem J, Li LS, Wang ZM, Rubin H. 1999. Cloning and characterization of a bifunctional RelA/SpoT homologue from Mycobacterium tuberculosis. Gene 233:261–269. doi: 10.1016/S0378-1119(99)00114-6. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Peng S, Qiu J, Miao J, Yang B, Jeang J, Hung CF, Wu TC. 2015. Intravaginal HPV DNA vaccination with electroporation induces local CD8+ T-cell immune responses and antitumor effects against cervicovaginal tumors. Gene Ther 22:528–535. doi: 10.1038/gt.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng S, Song L, Knoff J, Wang JW, Chang YN, Hannaman D, Wu TC, Alvarez RD, Roden RB, Hung CF. 2014. Control of HPV-associated tumors by innovative therapeutic HPV DNA vaccine in the absence of CD4+ T cells. Cell Biosci 4:11. doi: 10.1186/2045-3701-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng WF, Hung CF, Hsu KF, Chai CY, He L, Ling M, Slater LA, Roden RB, Wu TC. 2001. Enhancement of Sindbis virus self-replicating RNA vaccine potency by targeting antigen to endosomal/lysosomal compartments. Hum Gene Ther 12:235–252. doi: 10.1089/10430340150218387. [DOI] [PubMed] [Google Scholar]
- 29.Dutta NK, Illei PB, Jain SK, Karakousis PC. 2014. Characterization of a novel necrotic granuloma model of latent tuberculosis infection and reactivation in mice. Am J Pathol 184:2045–2055. doi: 10.1016/j.ajpath.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heuston S, Begley M, Gahan CG, Hill C. 2012. Isoprenoid biosynthesis in bacterial pathogens. Microbiology 158:1389–1401. doi: 10.1099/mic.0.051599-0. [DOI] [PubMed] [Google Scholar]
- 31.Surken M, Keller C, Rohker C, Ehlers S, Bange FC. 2008. Anaerobic arginine metabolism of Mycobacterium tuberculosis is mediated by arginine deiminase (arcA), but is not essential for chronic persistence in an aerogenic mouse model of infection. Int J Med Microbiol 298:657–661. doi: 10.1016/j.ijmm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE III, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 33.Deng X, Weerapana E, Ulanovskaya O, Sun F, Liang H, Ji Q, Ye Y, Fu Y, Zhou L, Li J, Zhang H, Wang C, Alvarez S, Hicks LM, Lan L, Wu M, Cravatt BF, He C. 2013. Proteome-wide quantification and characterization of oxidation-sensitive cysteines in pathogenic bacteria. Cell Host Microbe 13:358–370. doi: 10.1016/j.chom.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladjouzi R, Bizzini A, van Schaik W, Zhang X, Rince A, Benachour A, Hartke A. 2015. Loss of antibiotic tolerance in Sod-deficient mutants is dependent on the energy source and arginine catabolism in enterococci. J Bacteriol 197:3283–3293. doi: 10.1128/JB.00389-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larrouy-Maumus G, Biswas T, Hunt DM, Kelly G, Tsodikov OV, de Carvalho LP. 2013. Discovery of a glycerol 3-phosphate phosphatase reveals glycerophospholipid polar head recycling in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 110:11320–11325. doi: 10.1073/pnas.1221597110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar P, Arora K, Lloyd JR, Lee IY, Nair V, Fischer E, Boshoff HI, Barry CE III. 2012. Meropenem inhibits d,d-carboxypeptidase activity in Mycobacterium tuberculosis. Mol Microbiol 86:367–381. doi: 10.1111/j.1365-2958.2012.08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavollay M, Arthur M, Fourgeaud M, Dubost L, Marie A, Veziris N, Blanot D, Gutmann L, Mainardi JL. 2008. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by l,d-transpeptidation. J Bacteriol 190:4360–4366. doi: 10.1128/JB.00239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasan MR, Rahman M, Jaques S, Purwantini E, Daniels L. 2010. Glucose 6-phosphate accumulation in mycobacteria: implications for a novel F420-dependent anti-oxidant defense system. J Biol Chem 285:19135–19144. doi: 10.1074/jbc.M109.074310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R. 2012. Clofazimine: current status and future prospects. J Antimicrob Chemother 67:290–298. doi: 10.1093/jac/dkr444. [DOI] [PubMed] [Google Scholar]
- 40.Pavelka MS Jr, Mahapatra S, Crick DC. 2014. Genetics of peptidoglycan biosynthesis. Microbiol Spectr 2:MGM2-0034-2013. doi: 10.1128/microbiolspec.MGM2-0034-2013. [DOI] [PubMed] [Google Scholar]
- 41.Dubee V, Triboulet S, Mainardi JL, Etheve-Quelquejeu M, Gutmann L, Marie A, Dubost L, Hugonnet JE, Arthur M. 2012. Inactivation of Mycobacterium tuberculosis l,d-transpeptidase LdtMt(1) by carbapenems and cephalosporins. Antimicrob Agents Chemother 56:4189–4195. doi: 10.1128/AAC.00665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams KN, Szumowski JD, Ramakrishnan L. 2014. Verapamil, and its metabolite norverapamil, inhibit macrophage-induced, bacterial efflux pump-mediated tolerance to multiple anti-tubercular drugs. J Infect Dis 210:456–466. doi: 10.1093/infdis/jiu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutta NK, Pinn ML, Karakousis PC. 2014. Reduced emergence of isoniazid resistance with concurrent use of thioridazine against acute murine tuberculosis. Antimicrob Agents Chemother 58:4048–4053. doi: 10.1128/AAC.02981-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boutte CC, Crosson S. 2013. Bacterial lifestyle shapes stringent response activation. Trends Microbiol 21:174–180. doi: 10.1016/j.tim.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanyal S, Banerjee SK, Banerjee R, Mukhopadhyay J, Kundu M. 2013. Polyphosphate kinase 1, a central node in the stress response network of Mycobacterium tuberculosis, connects the two-component systems MprAB and SenX3-RegX3 and the extracytoplasmic function sigma factor, sigma E. Microbiology 159:2074–2086. doi: 10.1099/mic.0.068452-0. [DOI] [PubMed] [Google Scholar]
- 46.Kanehisa M, Goto S, Hattori M, Aoki-Kinoshita KF, Itoh M, Kawashima S, Katayama T, Araki M, Hirakawa M. 2006. From genomics to chemical genomics: new developments in KEGG. Nucleic Acids Res 34:D354–D357. doi: 10.1093/nar/gkj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spoering AL, Vulic M, Lewis K. 2006. GlpD and PlsB participate in persister cell formation in Escherichia coli. J Bacteriol 188:5136–5144. doi: 10.1128/JB.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin JE, Pandey AK, Gilmore SA, Mizrahi V, McKinney JD, Bertozzi CR, Sassetti CM. 2012. Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem Biol 19:218–227. doi: 10.1016/j.chembiol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandey AK, Sassetti CM. 2008. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A 105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brzostek A, Pawelczyk J, Rumijowska-Galewicz A, Dziadek B, Dziadek J. 2009. Mycobacterium tuberculosis is able to accumulate and utilize cholesterol. J Bacteriol 191:6584–6591. doi: 10.1128/JB.00488-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keren I, Minami S, Rubin E, Lewis K. 2011. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. mBio 2:. doi: 10.1128/mBio.00100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 53.Hogardt M, Heesemann J. 2010. Adaptation of Pseudomonas aeruginosa during persistence in the cystic fibrosis lung. Int J Med Microbiol 300:557–562. doi: 10.1016/j.ijmm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 54.Palmer KL, Aye LM, Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peteroy-Kelly M, Venketaraman V, Connell ND. 2001. Effects of Mycobacterium bovis BCG infection on regulation of l-arginine uptake and synthesis of reactive nitrogen intermediates in J774.1 murine macrophages. Infect Immun 69:5823–5831. doi: 10.1128/IAI.69.9.5823-5831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eoh H, Rhee KY. 2013. Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 110:6554–6559. doi: 10.1073/pnas.1219375110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nandakumar M, Nathan C, Rhee KY. 2014. Isocitrate lyase mediates broad antibiotic tolerance in Mycobacterium tuberculosis. Nat Commun 5:4306. doi: 10.1038/ncomms5306. [DOI] [PubMed] [Google Scholar]
- 58.Belenky P, Ye JD, Porter CB, Cohen NR, Lobritz MA, Ferrante T, Jain S, Korry BJ, Schwarz EG, Walker GC, Collins JJ. 2015. Bactericidal antibiotics induce toxic metabolic perturbations that lead to cellular damage. Cell Rep 13:968–980. doi: 10.1016/j.celrep.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gray MJ, Wholey WY, Wagner NO, Cremers CM, Mueller-Schickert A, Hock NT, Krieger AG, Smith EM, Bender RA, Bardwell JC, Jakob U. 2014. Polyphosphate is a primordial chaperone. Mol Cell 53:689–699. doi: 10.1016/j.molcel.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi MY, Wang Y, Wong LL, Lu BT, Chen WY, Huang JD, Tanner JA, Watt RM. 2012. The two PPX-GppA homologues from Mycobacterium tuberculosis have distinct biochemical activities. PLoS One 7:e42561. doi: 10.1371/journal.pone.0042561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malde A, Gangaiah D, Chandrashekhar K, Pina-Mimbela R, Torrelles JB, Rajashekara G. 2014. Functional characterization of exopolyphosphatase/guanosine pentaphosphate phosphohydrolase (PPX/GPPA) of Campylobacter jejuni. Virulence 5:521–533. doi: 10.4161/viru.28311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallarato LA, Sanchez DG, Olvera L, Primo ED, Garrido MN, Beassoni PR, Morett E, Lisa AT. 2014. Exopolyphosphatase of Pseudomonas aeruginosa is essential for the production of virulence factors, and its expression is controlled by NtrC and PhoB acting at two interspaced promoters. Microbiology 160:406–417. doi: 10.1099/mic.0.074773-0. [DOI] [PubMed] [Google Scholar]
- 63.Fraley CD, Rashid MH, Lee SS, Gottschalk R, Harrison J, Wood PJ, Brown MR, Kornberg A. 2007. A polyphosphate kinase 1 (ppk1) mutant of Pseudomonas aeruginosa exhibits multiple ultrastructural and functional defects. Proc Natl Acad Sci U S A 104:3526–3531. doi: 10.1073/pnas.0609733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi X, Rao NN, Kornberg A. 2004. Inorganic polyphosphate in Bacillus cereus: motility, biofilm formation, and sporulation. Proc Natl Acad Sci U S A 101:17061–17065. doi: 10.1073/pnas.0407787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grillo-Puertas M, Villegas JM, Rintoul MR, Rapisarda VA. 2012. Polyphosphate degradation in stationary phase triggers biofilm formation via LuxS quorum sensing system in Escherichia coli. PLoS One 7:e50368. doi: 10.1371/journal.pone.0050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baek SH, Li AH, Sassetti CM. 2011. Metabolic regulation of mycobacterial growth and antibiotic sensitivity. PLoS Biol 9:e1001065. doi: 10.1371/journal.pbio.1001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Black PA, Warren RM, Louw GE, van Helden PD, Victor TC, Kana BD. 2014. Energy metabolism and drug efflux in Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:2491–2503. doi: 10.1128/AAC.02293-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mathew R, Kruthiventi AK, Prasad JV, Kumar SP, Srinu G, Chatterji D. 2010. Inhibition of mycobacterial growth by plumbagin derivatives. Chem Biol Drug Des 76:34–42. doi: 10.1111/j.1747-0285.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 69.Reddy PJ, Ray S, Sathe GJ, Prasad TS, Rapole S, Panda D, Srivastava S. 2015. Proteomics analyses of Bacillus subtilis after treatment with plumbagin, a plant-derived naphthoquinone. OMICS 19:12–23. doi: 10.1089/omi.2014.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yano T, Kassovska-Bratinova S, Teh JS, Winkler J, Sullivan K, Isaacs A, Schechter NM, Rubin H. 2011. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem 286:10276–10287. doi: 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallis RS, Hafner R. 2015. Advancing host-directed therapy for tuberculosis. Nat Rev Immunol 15:255–263. doi: 10.1038/nri3813. [DOI] [PubMed] [Google Scholar]
- 72.Silva CL, Bonato VL, Coelho-Castelo AA, De Souza AO, Santos SA, Lima KM, Faccioli LH, Rodrigues JM. 2005. Immunotherapy with plasmid DNA encoding mycobacterial hsp65 in association with chemotherapy is a more rapid and efficient form of treatment for tuberculosis in mice. Gene Ther 12:281–287. doi: 10.1038/sj.gt.3302418. [DOI] [PubMed] [Google Scholar]
- 73.Kita Y, Hashimoto S, Nakajima T, Nakatani H, Nishimatsu S, Nishida Y, Kanamaru N, Kaneda Y, Takamori Y, McMurray D, Tan EV, Cang ML, Saunderson P, Dela Cruz EC, Okada M. 2013. Novel therapeutic vaccines [(HSP65 + IL-12)DNA-, granulysin- and Ksp37-vaccine] against tuberculosis and synergistic effects in the combination with chemotherapy. Hum Vaccin Immunother 9:526–533. doi: 10.4161/hv.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nell AS, D'Lom E, Bouic P, Sabate M, Bosser R, Picas J, Amat M, Churchyard G, Cardona PJ. 2014. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: randomized, placebo-controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS One 9:e89612. doi: 10.1371/journal.pone.0089612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cardona PJ. 2006. RUTI: a new chance to shorten the treatment of latent tuberculosis infection. Tuberculosis (Edinb) 86:273–289. doi: 10.1016/j.tube.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 76.Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. 2009. A replication clock for Mycobacterium tuberculosis. Nat Med 15:211–214. doi: 10.1038/nm.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmad Z, Klinkenberg LG, Pinn ML, Fraig MM, Peloquin CA, Bishai WR, Nuermberger EL, Grosset JH, Karakousis PC. 2009. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J Infect Dis 200:1136–1143. doi: 10.1086/605605. [DOI] [PubMed] [Google Scholar]
- 78.Karakousis PC, Williams EP, Bishai WR. 2008. Altered expression of isoniazid-regulated genes in drug-treated dormant Mycobacterium tuberculosis. J Antimicrob Chemother 61:323–331. [DOI] [PubMed] [Google Scholar]
- 79.Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE III. 2004. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem 279:40174–40184. doi: 10.1074/jbc.M406796200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.