Abstract
Clostridium difficile causes infections of the colon in susceptible patients. Specifically, gut dysbiosis induced by treatment with broad-spectrum antibiotics facilitates germination of ingested C. difficile spores, expansion of vegetative cells, and production of symptom-causing toxins TcdA and TcdB. The current standard of care for C. difficile infections (CDI) consists of administration of antibiotics such as vancomycin that target the bacterium but also perpetuate gut dysbiosis, often leading to disease recurrence. The monoclonal antitoxin antibodies actoxumab (anti-TcdA) and bezlotoxumab (anti-TcdB) are currently in development for the prevention of recurrent CDI. In this study, the effects of vancomycin or actoxumab/bezlotoxumab treatment on progression and resolution of CDI were assessed in mice and hamsters. Rodent models of CDI are characterized by an early severe phase of symptomatic disease, associated with high rates of morbidity and mortality; high intestinal C. difficile burden; and a disrupted intestinal microbiota. This is followed in surviving animals by gradual recovery of the gut microbiota, associated with clearance of C. difficile and resolution of disease symptoms over time. Treatment with vancomycin prevents disease initially by inhibiting outgrowth of C. difficile but also delays microbiota recovery, leading to disease relapse following discontinuation of therapy. In contrast, actoxumab/bezlotoxumab treatment does not impact the C. difficile burden but rather prevents the appearance of toxin-dependent symptoms during the early severe phase of disease, effectively preventing disease until the microbiota (the body's natural defense against C. difficile) has fully recovered. These data provide insight into the mechanism of recurrence following vancomycin administration and into the mechanism of recurrence prevention observed clinically with actoxumab/bezlotoxumab.
INTRODUCTION
Clostridium difficile infections (CDI) are a significant cause of morbidity and mortality in the acute care setting and in the wider health care system (1–3). CDI pathogenesis is associated with use of antimicrobials that disrupt the intestinal microbiota, reducing the host's ability to resist colonization by, and expansion of, C. difficile (4, 5). These conditions allow C. difficile spores to germinate, with resultant production of two toxins, TcdA and TcdB, that cause the symptoms of the disease (6–8). The infection is normally treated with antibiotics such as vancomycin and metronidazole which have potent activity against C. difficile (1, 8). However, the broad antibacterial spectra of these agents perpetuate gut dysbiosis, leading to high rates of disease recurrence following withdrawal of therapy, during which surviving or newly acquired C. difficile spores take advantage of persistent disruption of the gut microbiome to cause another episode of CDI (9–11). The risk of recurrence following a first episode is around 25% but increases significantly with each subsequent episode, often leading to a cycle of recurrence which can be difficult to break (11–13). Therapeutic approaches that minimize deleterious effects to the gut microbiota may therefore reduce rates of recurrent CDI and break the recurrence cycle.
Actoxumab (acto) (previously known as CDA1 or MK-3415A) and bezlotoxumab (bezlo) (previously known as CDB1, MDX-1388, or MK-6072) are human monoclonal antibodies that bind to and neutralize toxins A and B, respectively (14, 15). Bezlotoxumab is in development for prevention of recurrent CDI based on the finding that it lowers rates of CDI recurrence in patients and that coadministration with actoxumab does not provide additional benefit (16, 17). While bezlotoxumab is administered in parallel with standard-of-care antibiotics (vancomycin, metronidazole, or fidaxomicin) during an initial episode of CDI, its long half-life in circulation allows it to function as a prophylactic agent for a potential recurrent episode (15).
The link between a healthy gut microbiota and protection against C. difficile infection has been established both in humans and in animal models (4, 5, 18, 19). The mechanism of protection is thought to be through direct competition for nutrients and environmental niches within the gut, as well as through production of secondary bile acid metabolites that directly inhibit germination of C. difficile spores and growth of vegetative C. difficile (4, 5). Furthermore, several studies over the last few years have demonstrated that the initial effects of antibiotics on the microbiota are reversible and that the period of recovery is anywhere between a few days and several weeks (9, 19, 20). This period of recovery is hypothesized to correspond to the window of susceptibility to recurrence that follows successful cure of a primary episode with antibiotics such as vancomycin.
In this study, we assessed the progression and resolution of CDI over time in hamster and mouse models of antibiotic-induced CDI, in order to better understand the relationships among gut microbiota recovery, C. difficile colonization, and the toxin-dependent intestinal pathology that underlies morbidity and mortality. Furthermore, we examined the impact of vancomycin, an antibiotic widely used as a standard of care for CDI, and of the combination of the antitoxin antibodies actoxumab and bezlotoxumab (acto/bezlo) on these various aspects of disease. Our data suggest that recovery of the intestinal microbiota over time leads to clearance of the infection even in untreated animals and that clinical efficacy of antitoxin antibodies in preventing recurrent CDI may result from prevention of the clinical manifestation of disease during the period of time prior to recovery of the gut microbiota.
MATERIALS AND METHODS
Hamster CDI model.
Pathogen-free Golden Syrian hamsters (Janvier Laboratories, France) were allowed to acclimate to housing for approximately 2 weeks prior to the start of the experiment. Hamsters had free access to sterile food and water and were housed in pairs in sterile individual ventilated cages exposing hamsters at all times to HEPA-filtered sterile air. The CDI model was based on a previously published design (21); details of the timeline are provided in Fig. S1A in the supplemental material. All references to specific times (hours or days) are in relation to the day of C. difficile challenge (day 0). Animals were randomized into three treatment groups that were subdivided into “Survival” arms (n = 10 per treatment group) and “Time point” arms (n = 15 per treatment group). The treatment groups were (i) the acto/bezlo group (subcutaneous administration of actoxumab and bezlotoxumab [50 mg/kg of body weight each] once a day on days −3, −2, −1, and 0), (ii) the vancomycin group (orogastric administration of 10 mg/kg vancomycin [Flynn Pharma Ltd.] twice a day on days 0, 1, 2, 3, and 4), and (iii) the vehicle group (administration of acto/bezlo and vancomycin vehicles using the time points and dosing routes/frequencies described above). The use of a single vehicle group that received both prophylactic subcutaneous injections of acto/bezlo vehicle and therapeutic oral administration of vancomycin vehicle allowed us to minimize the numbers of animals used in the study. It is considered unlikely that this approach had an important impact on the results.
In order to establish susceptibility to CDI, hamsters were administered a single orogastric dose (30 mg/kg) of clindamycin (Villerton, Luxembourg) on day −1. This was followed on day 0 by gastric challenge with 680 CFU C. difficile spores (strain NCTC 13307 [also known as strain 630]; ribotype 012) suspended in 1 ml of phosphate-buffered saline (PBS) (Oxoid, United Kingdom). Hamsters were monitored as frequently as clinically required for signs of CDI with the aim that no hamsters would suffer prolonged severe disease or death. Hamsters that developed severe (<33°C) hypothermia, severe diarrhea, or severe (>20%) weight loss or were found lying prone or were unresponsive were euthanized and considered to have succumbed to disease. Body temperature was assessed through the use of telemetric temperature recording chips (Plexx, the Netherlands) inserted into the skin behind the neck prior to antibiotic preconditioning. The statistical significance of the results of comparisons of mortality data from different treatment groups was assessed by log rank (Mantel-Cox) test.
Mouse CDI model.
Pathogen-free C57BL/6 mice (Harlan, United Kingdom) were obtained at 6 to 8 weeks of age and allowed to acclimate to housing for 7 days prior to the start of the experiment. Mice had free access to sterile food and water and were housed in pairs in sterile individual ventilated cages exposing mice at all times to HEPA-filtered sterile air. The CDI model was based on previously published designs (22–24); details of the timeline are provided in Fig. S1A in the supplemental material. All references to specific times (hours or days) are in relation to the day of C. difficile challenge (day 0). Animals were randomized into three treatment groups that were divided into “Survival” arms (n = 10 per treatment group) and “Time point” arms (n = 15 per treatment group). The treatment groups were (i) the acto/bezlo group (subcutaneous administration of actoxumab and bezlotoxumab [50 mg/kg each] once a day on days −1 and 0), (ii) the vancomycin group (orogastric administration of 10 mg/kg vancomycin twice a day on days 0, 1, 2, 3, and 4), and (iii) the vehicle group (administration of acto/bezlo and vancomycin vehicles using the time points and dosing routes/frequencies described above). The use of a single vehicle group that received both prophylactic subcutaneous injections of acto/bezlo vehicle and therapeutic oral administration of vancomycin vehicle allowed us to minimize the numbers of animals used in the study. It is deemed unlikely that this approach had an important impact on the results.
In order to establish susceptibility to CDI, mice were given cefoperazone (Sigma, United Kingdom) (0.5 mg/ml in drinking water) for 10 days (days −12 to −2), followed by a single dose (10 mg/kg administered intraperitoneally) of clindamycin on day −1. This was followed on day 0 by gastric challenge with 1.8 × 106 CFU vegetative C. difficile (strain ATCC 43255 [also known as VPI 10463]; ribotype 087) suspended in 0.1 ml of PBS. Mice were monitored as frequently as clinically required for signs of CDI with the aim that no mouse would suffer prolonged severe disease or death. Mice that developed severe (<33°C) hypothermia, severe diarrhea, or severe (>20%) weight loss or were found lying prone or were unresponsive were euthanized and considered to have succumbed to disease. Body temperature was assessed through the use of telemetric temperature recording chips (Plexx, the Netherlands) inserted into the skin behind the neck prior to antibiotic preconditioning. The statistical significance of the results of comparisons of mortality data from different treatment groups was assessed by log rank (Mantel-Cox) test.
Collection of fecal and intestinal content samples and scoring of gross intestinal pathology.
Fecal samples were collected from all surviving animals in the “Survival” arms of the study, on days 1, 2, 5, 10, 15, 21, and 28 postinfection, and resuspended in 0.5 ml saline solution (Baxter Healthcare, United Kingdom) for C. difficile spore and microbiota analyses. Animals in both the “Time point” and “Survival” arms were euthanized at predefined time points (days 1, 2, and 5 for the “Time point” arm and day 28 for the “Survival” arm) or at the time of severe clinical deterioration. Samples (0.5 ml) of luminal content were collected from three distinct intestinal segments (ileum, colon, and cecum) for each animal. Prior to sample collection, gross intestinal pathology was assessed by scoring the ileum, colon, and cecum, according to the scale shown in Table S1 in the supplemental material, and averaging the scores from the three intestinal regions for each animal. The statistical significance of differences in pathology scores across the different treatment groups was determined by two-way analysis of variance (ANOVA) followed by the Tukey posttest.
C. difficile burden in feces and intestinal content.
For spore counts, fecal or intestinal content samples (300 μl) were diluted into 700 μl of 100% industrial methylated spirits (IMS) (VWR, United Kingdom) and subjected to vortex mixing for 10 s. Further dilutions were performed in 70% IMS, and 100 μl of fecal/intestinal content suspensions was plated onto C. difficile agar (Oxoid, United Kingdom), placed into anaerobic jars or cabinets, and cultured at 37°C for 48 h. The burdens in the original samples were determined by back converting based on dilution factors. The limit of detection (LOD) was 17 CFU/ml. For intestinal content data, values obtained for each intestinal segment (small intestine, colon, or cecum) were converted to log10 values and averaged for each animal. The burdens calculated for the individual intestinal segments are shown in Table S2 in the supplemental material.
Microbiota analysis.
Microbiota composition was assessed in mouse fecal and cecum content samples from all three treatment groups described above. In addition to these samples, fecal and cecum samples from naive mice (n = 5) were collected and analyzed for microbiota composition as a comparator group. These animals were randomly selected from age-matched stock that had been held in the vivarium throughout the study period for at least 28 days before the culling. The naive mice were not treated with antibiotics and were housed in the same facilities and handled in the same way as the animals in the treatment groups (identical cages, food, enrichment, light cycles, husbandry staff, etc.) to minimize nonexperimental variables. Microbiota composition was determined in all samples by 16S rRNA gene sequencing. DNA extraction was carried out using a QIAamp Fast DNA stool minikit (Qiagen), and DNA quantification was carried out using a Quant-iT PicoGreen double-stranded DNA (dsDNA) reagent (Invitrogen), both according to the instructions of the manufacturers. PCR amplification was done using high-performance liquid chromatography (HPLC)-purified fusion primers (barcoded) targeting the V3/V5 variable regions of the 16S rRNA gene (target-specific part of the forward primer, 5′-ACTCCTACGGGAGGCAGCAG-3′; target-specific part of the reverse primer, 5′-GGGTTGCGCTCGTTGCGGG-3′). Amplicons were subjected to gel purification using a QIAquick gel extraction kit (Qiagen) and were quantified using an Agilent Bioanalyzer. Sequencing was carried out using Illumina HiSeq v3 in 2-bp-by-300-bp paired-end read mode. Sequences were partitioned into operational taxonomic units (OTU), each representing a distinct cluster with significant sequence divergence from each other cluster (496 OTUs for cecum samples and 420 OTUs for fecal samples) (25). Taxonomic information was assigned to OTUs to the highest degree of specificity allowed by the data (ranging from kingdom to species) (26, 27). Community differences across samples were assessed by first determining weighted UniFrac distances for all pairs of samples and then analyzing the data by principal-coordinate analysis (PCoA) (28). The statistical significance of differences in the relative abundances of organism classes across different samples was determined by two-way ANOVA followed by the Tukey posttest. Relative abundances of individual OTUs of the Clostridia class in cecum samples were clustered using Tibco Spotfire and the unweighted pair group method using average linkages (UPGMA).
Ethical statement.
All animal experiments were performed under UK Home Office License 40/3644, and with local ethical committee clearance (The University of Manchester Standing Committee). All experiments were performed by technicians who had completed at least parts 1 to 3 of the Home Office Personal License course and held current personal licenses. Studies were also approved by the Institutional Animal Care and Use Committee of Merck Research Laboratories, Kenilworth, NJ.
RESULTS
Overview of rodent CDI models.
To assess progression and resolution of CDI, we utilized two common rodent models, the Golden Syrian hamster model (21) and a mouse model first described by Chen et al. (22) and modified by Young and colleagues (23, 24). We assessed mortality, morbidity (clinical signs of disease), intestinal pathology, intestinal and fecal C. difficile burden, and intestinal and fecal microbiota composition at multiple time points following treatment with vehicle, vancomycin, or acto/bezlo (see Fig. S1 in the supplemental material). The rationale for using the acto/bezlo combination rather than bezlotoxumab alone is that the combination is required for maximal protection in rodents (14, 29), despite bezlotoxumab alone being sufficient in piglets (30) and humans (16, 17). At the time of C. difficile challenge, each treatment group was subdivided into two arms, a “survival” arm (n = 10), in which animals were allowed to progress to the end of the study (day 28 postchallenge), and a “time point” arm (n = 15), in which 5 animals were euthanized on each of days 1, 2, and 5 postchallenge, to allow direct comparison of animal results at a given time across all three treatment groups. Mortality and morbidity were assessed in all animals in the “Survival” arm, and fecal samples were collected on days 1, 2, 5, 10, 15, 21, and 28 (for animals that generated them on those days) postchallenge for C. difficile burden and intestinal microbiota analysis. Animals in both the “Time point” and “Survival” arms were euthanized at the prespecified time point (day 1, 2, 5, or 28) or at the time of severe clinical deterioration. Necropsy was carried out at the time of euthanasia to assess gross intestinal pathological scores. Samples of intestinal contents (small intestine, colon, and cecum) were also collected for C. difficile burden and intestinal microbiota analysis.
Disease progression and resolution in hamster CDI model.
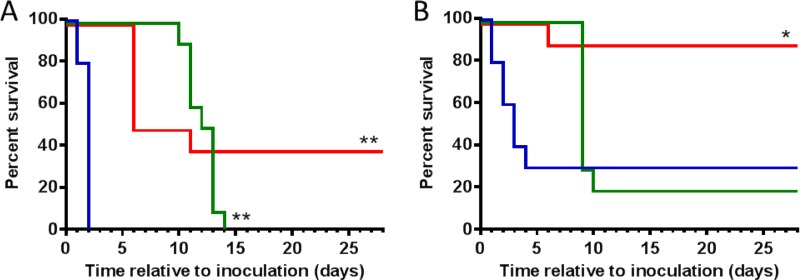
The Golden Syrian hamster spore challenge model has historically been considered the gold standard model of CDI, as the pathology observed in hamster infection reflects many clinical aspects of human infection (31, 32). Similarly to human disease, pathology in the hamster model is primarily localized to the large intestine, including the cecum and colon (31, 33). However, disease is much more severe in the hamster model than in human patients, with hamsters typically succumbing within hours to a few days after infection. In this study, hamsters were preconditioned with clindamycin 24 h prior to challenge with C. difficile spores (strain 630, ribotype 012) (see Fig. S1 in the supplemental material). Vehicle-treated animals experienced signs of morbidity (Table 1), including a drop in body temperature (hypothermia), body weight loss, diarrhea (“wet tail”), and other signs of infection (abdominal distension, proneness, unresponsiveness), and all animals perished within 48 h (Fig. 1A). Animals treated with vancomycin (administered orally for 5 days twice a day, starting 24 h after challenge; see Fig. S1) showed no signs of morbidity until day 10 postinfection, when they started losing weight and showing other symptoms of infection (Table 1), with all animals succumbing by day 14 (Fig. 1A). Animals treated with acto/bezlo showed few of the signs of morbidity observed in the vehicle group, with the exception of body weight loss (Table 1). By day 6, 60% of the animals had reached the weight loss ethical endpoint (≥20% weight loss) (Fig. 1A) and were considered to have succumbed to the infection despite displaying none of the other signs of morbidity, whereas animals that survived beyond the early phase of disease gradually began to regain weight on day 6 and clinical signs eventually disappeared completely. These animals survived until the end of the study on day 28, by which time they were observed to be healthy and thriving.
TABLE 1.
Effects of actoxumab/bezlotoxumab or vancomycin on morbidity in hamster and mouse models of CDI
| Parameter | Resulta |
|||||
|---|---|---|---|---|---|---|
| Hamster |
Mouse |
|||||
| Vehicle | Acto/Bezlo | Vanco | Vehicle | Acto/Bezlo | Vanco | |
| Weight loss | + | +++ | +b | +++ | + | +++b |
| Hypothermia | +++ | + | +++b | +++ | + | ++b |
| Hunched posture/proneness/sluggishness | +++ | + | +++b | ++ | − | ++b |
| Diarrhea | + | + | +b | + | − | +b |
| Primary reason for euthanasia | Multiple clinical signs | Weight loss > 20% | Multiple clinical signs | Multiple clinical signs | Temp < 34°C (1 mouse) | Multiple clinical signs |
Acto/Bezlo, actoxumab/bezlotoxumab; Vanco, vancomycin; −, not observed; +, minimal; ++, moderate; +++, severe.
Clinical signs were delayed compared to vehicle results.
FIG 1.
Effect of acto/bezlo or vancomycin on survival in rodent CDI models. (A) Kaplan-Meier survival curves of infected hamsters in “Survival” arm (n = 10 per group) treated with vehicle (blue line), with acto/bezlo (red line), or with vancomycin (green line). (B) Per panel A but for infected mice (n = 10 per group). **, P < 0.0001 (compared to vehicle group); *, P < 0.01 (compared to vehicle and vancomycin groups).
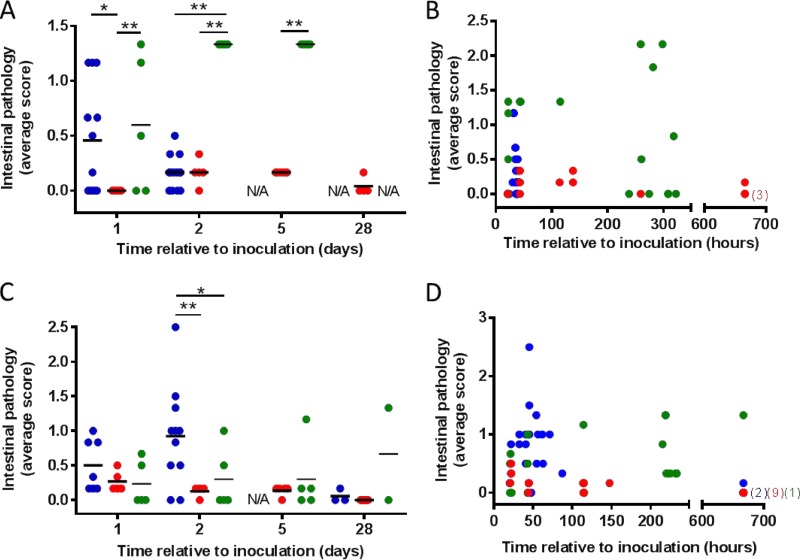
Morbidity and mortality in this model was associated with mild to moderate intestinal pathology characterized by slight inflammation and empty bowels in the small intestine, colon, and cecum in vehicle-treated animals, in particular, on day 1 (Fig. 2A and B; see also Table S1 in the supplemental material). Intestinal pathology was significantly less severe in acto/bezlo-treated hamsters (Fig. 2A); indeed, acto/bezlo-treated animals were largely protected from intestinal damage and maintained low pathology scores throughout the duration of the study (Fig. 2B). Animals treated with vancomycin developed severely inflamed and enlarged intestines with signs of hemorrhage starting on day 1 and continuing throughout the duration of the study, until most of the animals succumbed, between days 10 and 14. The vancomycin-associated pathology was mostly localized to the cecum as has been previously observed (34).
FIG 2.
Effect of acto/bezlo or vancomycin on gross intestinal pathology in rodent CDI models. (A) Intestinal scores for individual infected hamsters treated with vehicle (blue circles), with acto/bezlo (red circles), or with vancomycin (green circles). Samples were assessed at predefined time points (days 1, 2, 5, and 28). Scores are based on severity of pathology as defined in Table S1 in the supplemental material. All scores are averages of individual scores recorded for ileum, colon, and cecum in each animal. *, P < 0.05; **, P < 0.01 (compared to specified groups). N/A, not available (animals in this group did not survive to the planned time point). (B) Per panel A, but scores are shown for all animals across all time points, including animals that succumbed to disease prior to predefined time point. (C) Per panel A but for infected mice. (D) Per panel B but for infected mice. Numbers in brackets indicate number of animals with scores of 0 (overlapping points) on day 28 (672 h) within specified groups (color indicates group as per legend for panel A).
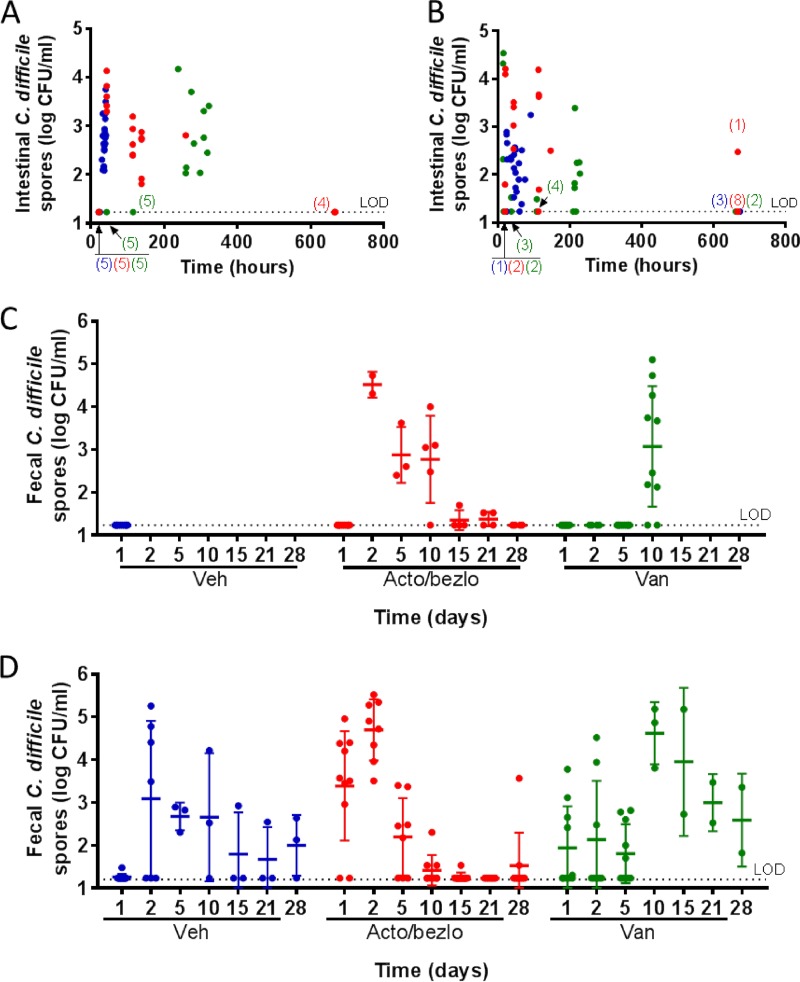
C. difficile spore levels were assessed in intestinal content (Fig. 3A) and feces (Fig. 3C) and found to be below the limit of detection (LOD) in the vehicle group on day 1 (24 h post-spore challenge), with intestinal content levels rising dramatically by 48 h (∼100 to 10,000 CFU/ml). Burdens on day 2 in feces or at later time points in intestinal content could not be assessed for the animals, since all vehicle-treated animals had succumbed to disease within 48 h. In vancomycin-treated animals, C. difficile spore counts remained below the LOD throughout the vancomycin treatment period (days 1 to 5 [<120 h] postchallenge) but rose by days 10 to 14 (>240 h, when all animals succumbed to disease) to levels comparable to those observed in vehicle-treated animals on day 2 (up to ∼10,000 CFU/ml). As with vehicle-treated animals, the rise in C. difficile burden immediately preceded death in all vancomycin-treated animals. In acto/bezlo-treated animals, a rise in spore counts in intestinal content and feces similar to that observed in vehicle-treated animals was observed on day 2 (48 h), and levels began to decrease on day 5 (120 h). Spore counts decreased to <100 CFU/ml by days 15 and 21 in feces and were below the LOD in both feces and intestinal content by the end of the study, on day 28 (672 h).
FIG 3.
Effect of acto/bezlo or vancomycin on C. difficile burdens in intestinal content and feces of infected animals in rodent CDI models. (A) Levels of C. difficile spores (in CFU/ml, expressed on log10 scale) in intestinal content of individual infected hamsters treated with vehicle (blue circles), with acto/bezlo (red circles), or with vancomycin (green circles). Samples were obtained at predefined time points or at the time of death for animals that did not survive to predefined time points. Since not all animals were sacrificed at exactly the same time on each specified day, the data are plotted at the time (expressed in hours) when the animals were sacrificed. All values are averages of individual values recorded for ileum, colon, and cecum in each animal (see Table S2 in the supplemental material). Numbers in parentheses indicate numbers of animals with burdens below the limit of detection (LOD) (overlapping points) at indicated time points within specified groups (color indicates group per legend above). (B) Per panel A legend but for infected mice. (C) Levels of C. difficile spores (in CFU per milliliter, expressed on log10 scale) in feces of infected hamsters treated with vehicle (Veh; blue circles), with acto/bezlo (red circles), or with vancomycin (Van; green circles). Samples were obtained from the “Survival” arm (see Fig. S1) at predefined time points (days 1, 2, 5, 10, 15, 21, and 28). Averages ± standard deviations are indicated for each time point and treatment. (D) Per panel C but for infected mice. The limit of detection (LOD) for this assay was 17 CFU/ml as indicated by the dotted line.
Disease progression and resolution in mouse CDI model.
Mouse models of CDI are emerging as an alternative to hamster models and offer several advantages, including the possibility of the use of more widely available mouse-specific reagents and of assessment of the roles of host genes in disease through genetic manipulation (22, 31). In this study, mice were preconditioned with antibiotics and challenged with C. difficile (strain VPI 10463, ribotype 087) (see Fig. S1 in the supplemental material). Similarly to the hamsters, vehicle-treated mice experienced signs of morbidity (Table 1) that included changes in body temperature (hypothermia and/or pyrexia), body weight loss, diarrhea (loose stools), hunched posture, dehydration, and sluggishness; 70% of animals succumbed to disease within 4 days postchallenge (Fig. 1B), with the remaining animals gradually recovering from disease and surviving until the end of the study (day 28). Animals treated with vancomycin showed few signs of morbidity until day 9 postinfection, when animals started exhibiting symptoms of infection similar to those seen with the vehicle-treated animals at earlier time points. By day 10, 80% of vancomycin-treated animals had perished, with the remaining 2 animals recovering and surviving until the end of the study (Fig. 1B). Animals treated with acto/bezlo were largely devoid of symptoms, with a single mouse succumbing to disease on day 6 due to severe hypothermia (Fig. 1B and Table 1). The remaining animals (90%) were healthy and thriving throughout the duration of the study.
The pathological effects of C. difficile infection on the intestines were assessed by using a scoring system similar to that used in hamsters (see Table S1 in the supplemental material). Vehicle-treated animals developed mild to severe pathology in the small intestine, colon, and cecum, ranging from a normal appearance with loose content to a highly inflamed appearance with signs of hemorrhaging (Fig. 2C and D). Pathology was most severe on day 2, showed signs of diminishing on days 3 to 4, and was fully resolved in animals surviving to day 28. Treatment with vancomycin largely prevented intestinal pathology throughout the duration of the study, although high pathology scores were recorded sporadically in individual animals at various time points. Animals treated with acto/bezlo showed few to no signs of intestinal pathology at any time point.
C. difficile spore burdens in intestinal contents and feces followed patterns similar to those observed in hamsters, although the data were generally more variable in the mouse. In vehicle- and acto/bezlo-treated mice, spore levels were high at early time points (up to 100,000 CFU/ml or more on days 1 to 2 [h 24 to 48]) in both intestinal content (Fig. 3B) and feces content (Fig. 3D), started to decrease by day 5 to day 10 (>120 h), and were low (<100 CFU/ml) or below the LOD in mice surviving to 28 days (672 h). Spore counts in mice treated with vancomycin were low to moderate at early time points while vancomycin was still being administered (<1,000 CFU/ml at day 1 to day 5 [up to 120 h], with individual mice having higher levels even at those early time points), increased sharply starting on day 10 (240 h) (10,000 to 100,000), and decreased to below the LOD (in intestinal content) or to moderate levels (100 to 1,000 CFU/ml in feces) on day 28 (672 h).
Changes in intestinal and fecal microbiota in mice.
Previous studies have shown that treatment with preconditioning antibiotics disrupts the gut microbiota in mice but that dysbiosis resolves at least partially over time following discontinuation of antibiotic treatment (19, 20, 35). We assessed the impact of cefoperazone/clindamycin treatment and C. difficile challenge on the gut microbiota in mice and characterized the effects of vancomycin or antitoxin treatment on recovery of the microbiota over 28 days. We focused on mouse samples from both cecum content and feces to enable us to compare changes over time across different treatment paradigms (including vehicle), since hamsters treated with vehicle succumb to disease by day 2, precluding a direct comparison across all time points and all treatment groups in this species.
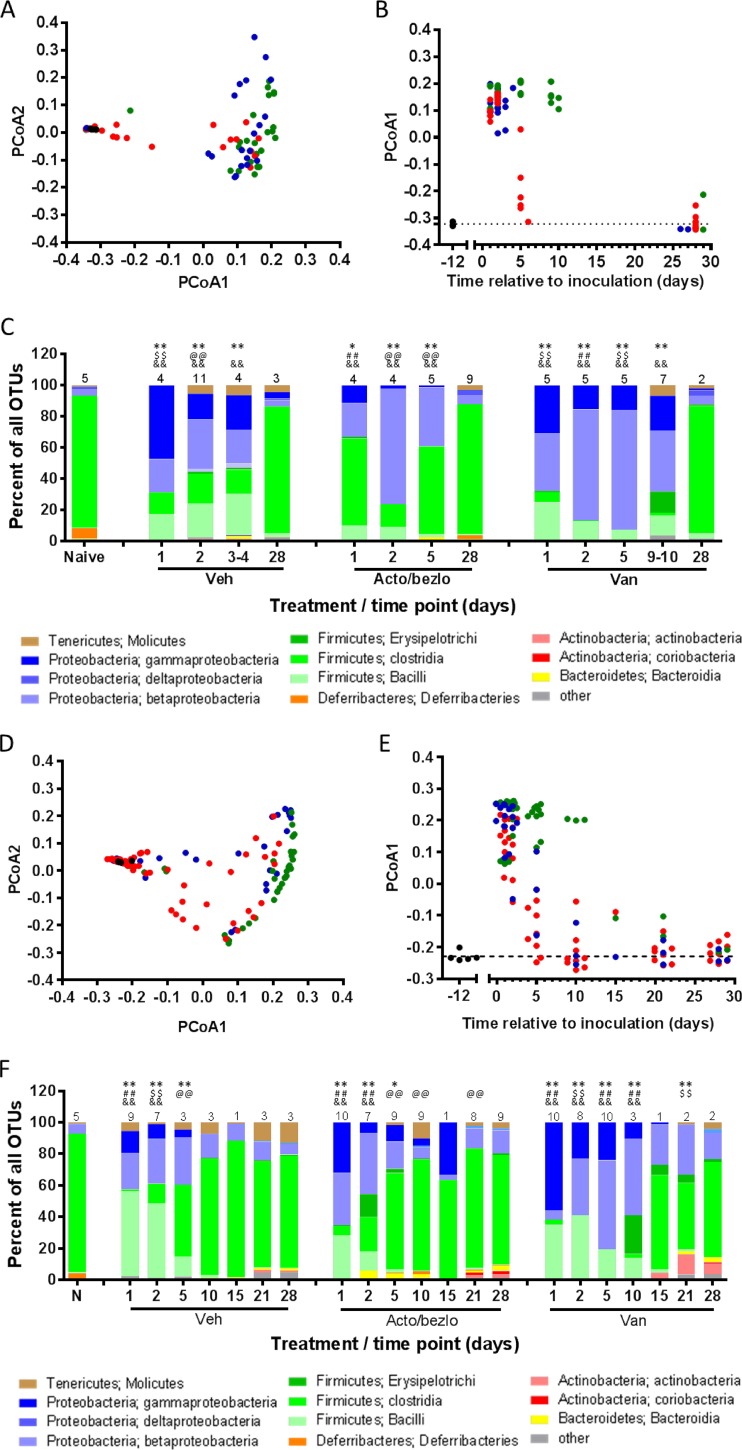
The microbiota composition of all samples was determined through 16S rRNA gene sequencing from amplified DNA isolated from fecal or cecum content samples. The sequences obtained were partitioned into operational taxonomic units (OTUs), with taxonomic information assigned to the highest degree of specificity allowed by the data. Beta diversity (diversity across samples) was assessed by principal-coordinate analysis (PCoA) of weighted UniFrac distances across all samples. The two axes accounting for most of the diversity, PCoA1 (51.8% and 51.6% of the diversity in cecum and fecal samples, respectively) and PCoA2 (12.0% and 16.6% of the diversity in cecum and fecal samples, respectively), are plotted in Fig. 4A and D for cecum and fecal samples, respectively. To gain insight into how microbiota composition changed over time, PCoA1 data were plotted against time relative to C. difficile challenge (Fig. 4B and E). The data show that, regardless of treatment, the composition of all samples was vastly different from that of samples from naive mice in early samples (day 1 to 2) but began to normalize (i.e., become more like naive samples) starting on day 5 for vehicle- and acto/bezlo-treated samples. PCoA1 values for these two groups were largely back to the baseline values (similar to those determined for the naive samples) by day 10. The situation was vastly different for the vancomycin-treated samples, with the PCoA1 values remaining high (similar to those determined for day 1 to day 2) through day 10 and returning to baseline only on days 15 to 21. The patterns revealed by analysis of cecum (Fig. 4B) and fecal (Fig. 4E) samples were similar, although more fecal samples were available for analysis, providing additional information, during days 5 to 28.
FIG 4.
Effect of acto/bezlo or vancomycin on microbiota composition of cecum content and feces in infected mice. (A) Beta diversity (principal-coordinate analysis of weighted UniFrac distances) of cecum content of samples from uninfected naive mice (black circles) or from individual infected mice treated with vehicle (blue circles), with acto/bezlo (red circles), or with vancomycin (green circles). Samples were obtained at predefined time points (days 1, 2, 5, and 28) or at the time of death for animals that did not survive to predefined time points. PCoA1 (accounting for 51.8% of the diversity) is plotted versus PCoA2 (accounting for 12.0% of the diversity). (B) Changes in PCoA1 values (taken from panel A and analyzed per time relative to C. difficile challenge, in various treatment groups [see panel A legend]). The dotted line represents the average PCoA1 value for naive animals. Day 28 points for vehicle and vancomycin-treated animals are offset for clarity. (C) Relative abundances of organisms (identified by phylum and class as indicated in the key below the panel) in the cecum content of samples described in panel A. Values represent averages of the results determined for all animals within the same treatment group at the same time point (numbers of animals per group are indicated above the bars); relative microbiota compositions for individual animals are shown in Fig. S2 in the supplemental material. *, P < 0.01; **, P < 0.0001 (compared to naive animals); ##, P < 0.0001 (compared to mice receiving other treatments at same time point); @@, P < 0.0001 (compared to vancomycin-treated mice at same time point); $$, P < 0.0001 (compared to acto/bezlo-treated mice at same time point). (D) Per panel A but for fecal samples. Samples were obtained from the “Survival” arm (see Fig. S1 in the supplemental material) at predefined time points (days 1, 2, 5, 10, 15, 21, and 28). PCoA1 accounts for 51.6% of the diversity, and PCoA2 accounts for 16.6% of the diversity. (E) Per panel B but for fecal samples. Values are staggered at each time point for clarity. (F) Per panel C but for fecal samples obtained from the “Survival” arm (see Fig. S1) at predefined time points (days 1, 2, 5, 10, 15, 21, and 28).
The average relative abundances of organisms at the class level identified at each time point and for each treatment group are shown in Fig. 4C (cecum) and F (feces), with data from individual animals shown in Fig. S2 in the supplemental material. The microbiota of the naive mice was composed largely of members of the Firmicutes (mostly of the class Clostridia), with minimal levels of proteobacterial species present. Cefoperazone and clindamycin treatment caused a shift to a composition consisting of a majority of proteobacteria (a mix of beta- and gammaproteobacteria), with decreases in the relative abundance of Clostridia species and increases in the relative abundance of Bacilli species, which are members of another class within the Firmicutes phylum. There was also a numerical decrease in the relative abundance of Deferribacteres spp. in both cecum and fecal samples, although the difference was not statistically significant. As shown in Fig. 4C and F, the relative abundance of Clostridia in vehicle- and acto/bezlo-treated samples began to increase at the expense of proteobacteria and bacilli, starting on or around day 5, and the microbiota composition was statistically indistinguishable from that of naive animals by day 10 (fecal samples only, as no cecum samples were collected between day 5 and day 28). In vancomycin-treated animals, the profiles remained significantly different from those determined for naive mice even on day 21 for fecal samples and up to day 9 to day 10 for cecum samples (no additional cecum samples were obtained between days 10 and 28). Regardless of the treatment or sample type, microbiota compositions had largely returned to baseline (similar to those seen with naive mice) by day 28. Although the levels of actinobacteria (in feces) and tenericutes (in feces and cecum) were numerically higher in all day 28 samples and deferribacteres had not returned to the same relative levels as those present in naive mice, these differences were small and not statistically significant. The statistical significance of comparisons of results observed across time points and treatments is shown in Fig. 4C and F for microbiota composition overall and in Table S2 and S3 for specific classes of organisms.
The data described above suggest that the microbiota of mice can completely revert to baseline (naive) composition within ∼10 days following an initial disruption with antibiotics (with C. difficile challenge perhaps also contributing to the dysbiosis). To verify this assertion, we looked at how specific OTUs vary over time and across treatment groups. We focused our analysis on the classes Clostridia, Bacilli, Betaproteobacteria, and Gammaproteobacteria, as these were the only classes of organisms with results that varied significantly across different samples (see Tables S3 and S4 in the supplemental material). For the classes Bacilli, Betaproteobacteria, and Gammaproteobacteria, overall changes in the relative abundances of specific OTUs mirrored observations made at the class level (data not shown). Most of the organisms identified as belonging to the class Bacilli were of the Lactobacillus and Enterococcus genera, both of which were largely absent in naive samples, increased in abundance at early time points (day 1 to day 2), and decreased back to naive levels by day 5, except in vancomycin-treated samples, where levels of Lactobacillus (but not Enterococcus) persisted up to day 10. The vast majority of betaproteobacteria were of the genus Sutterella, were absent in naive animals, appeared by day 1, and decreased back to naive levels by day 5 in vehicle- and acto/bezlo-treated animals and by day 21 in vancomycin-treated animals. The gammaproteobacteria were a mix of Enterobacteriaceae (Escherichia, Klebsiella, Morganella, and Trabulsiella spp.) and Moraxellaceae (mostly Acinetobacter rhizosphaerae), appeared early, and decreased in abundance after day 5 in vehicle- and acto/bezlo-treated mice but persisted up to day 10 in vancomycin-treated mice.
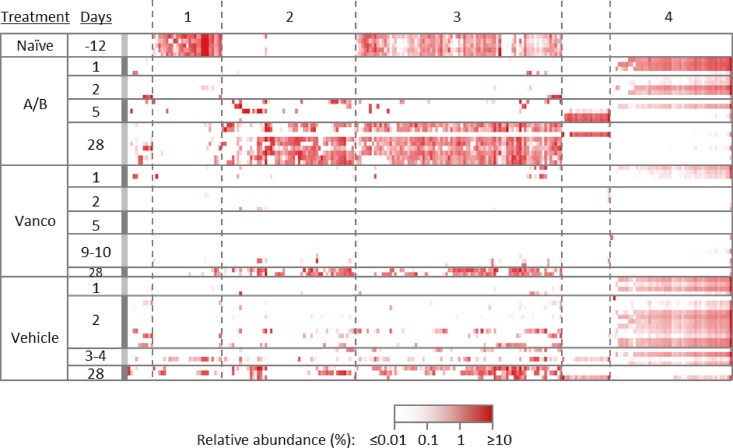
In contrast to changes in the relative abundances of specific OTUs in these three classes of organisms, changes in OTUs of the members of the class Clostridia showed distinct patterns in terms of changes in relative abundance over time following treatment with cefoperazone/clindamycin (Fig. 5). Generally, OTUs clustered in four distinct patterns that included (1) organisms that were present at a high level in naive animals, decreased in abundance with cefoperazone/clindamycin treatment, and did not return by day 28; (2) organisms that were absent in naive animals, began to colonize the gut on day 5, and persisted through day 28; (3) organisms that were present at a high level in naive animals, decreased in abundance with cefoperazone/clindamycin treatment in vehicle- and acto/bezlo-treated mice, and began to reappear around day 5; and (4) organisms that were absent in naive animals, increased to a high level upon cefoperazone/clindamycin treatment in vehicle- and acto/bezlo-treated mice, started to disappear on day 5, and were largely absent by day 28. Organisms in the class Clostridia were mostly of the Lachnospiraceae and Ruminococcaceae families, distributed more or less uniformly across the four patterns described above. Notably, vancomycin-treated mice had low levels of all organisms in the class Clostridia through day 10.
FIG 5.
Changes in the relative abundances of individual OTUs in the Clostridia class in cecum samples of infected mice. The heat map shows relative abundances of individual OTUs of organisms from the Clostridia class in cecum samples from individual uninfected naive mice or from individual infected mice treated with vehicle, with acto/bezlo (A/B), or with vancomycin (Vanco). Samples were obtained from the “Time point” arm (see Fig. S1 in the supplemental material) at predefined time points (days 1, 2, 5, and 28) or at the time of death for animals that did not survive to predefined time points. Results were observed to cluster into 4 distinct patterns of abundance across time points and within treatment groups (clusters 1, 2, 3, and 4 as indicated at the top of the figure).
DISCUSSION
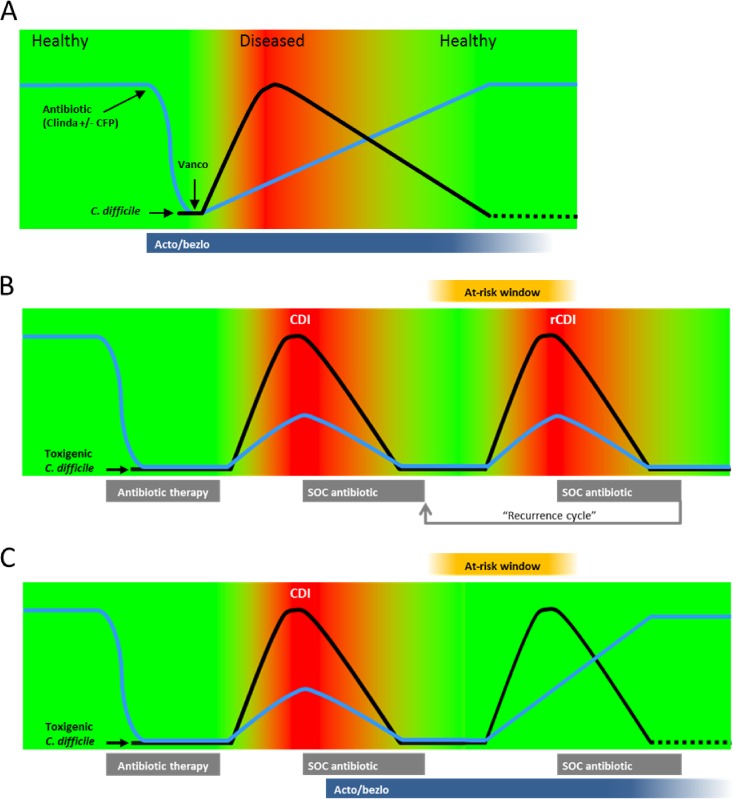
In this study, we characterized the progression and resolution of disease in two rodent models of CDI. As summarized in the model shown in Fig. 6A, CDI in both hamsters and mice is characterized by an early severe phase of disease, associated with (i) a rapid expansion of C. difficile burden enabled by antibiotic-induced gut dysbiosis, (ii) intestinal damage presumably mediated by the C. difficile toxins TcdA and TcdB, and (iii) various clinical signs of infection, including body weight loss, changes in body temperature, diarrhea, and other signs of morbidity. Disease is fatal in all untreated hamsters and in a majority of untreated mice. Animals that survive the early phase of infection start recovering from disease symptoms, as a gradual reestablishment of a healthy gut microbiota leads to clearance of the infection (see Fig. S3A in the supplemental material, showing the inverse relationship between fecal microbiota recovery and C. difficile levels in vehicle- and acto/bezlo-treated mice). By the end of the study on day 28, surviving animals had fully recovered from disease, displayed no symptoms or intestinal pathology, had low C. difficile intestinal burdens, and had a gut microbiota composition rich in members of the Firmicutes phylum (Clostridia) and poor in the Proteobacteria phylum, similar to naive animals. Treatment with vancomycin delayed disease by blocking the initial expansion of C. difficile levels for the duration of treatment but ultimately did not prevent high morbidity and mortality. This was likely due to the negative impact of vancomycin on recovery of the gut microbiota (Fig. 4); while the antibacterial activity of vancomycin prevented growth of C. difficile, it also delayed recolonization of the gut by protective organisms (see Fig. S3B) such that, once vancomycin pressure was removed, the remaining C. difficile spores germinated and grew, colonizing the gut and causing full-fledged disease. Treatment with acto/bezlo, in contrast, had a limited impact (if any) on intestinal C. difficile levels and on recovery of the microbiota following antibiotic-induced gut dysbiosis (see Fig. S3C) but prevented mortality/morbidity during the early phase of disease by neutralizing the pathogenic determinants of CDI, the toxins TcdA and TcdB, which cause the damage and inflammation of the intestine that underlie the symptoms of disease. The protection against morbidity and mortality afforded by acto/bezlo allowed animals to survive the early phase of disease and to benefit from recovery of the gut microbiota that eventually led to clearance of the infection (Fig. 6A).
FIG 6.
Models of progression and resolution of disease in CDI. (A) Schematic representation of disease progression and resolution in rodent models of CDI based on data from this study. Green areas represent lack of symptomatology; red areas represent the presence of symptomatology. The blue line represents the state of the gut microbiota; the black line represents C. difficile burden. Times of clindamycin/cefoperazone [Antibiotic (Clinda ± CFP)], vancomycin (Vanco), and acto/bezlo (Acto/bezlo) administration, and of C. difficile challenge, are indicated. (B) Model of primary and recurrent CDI progression and resolution in humans. Colors and lines are per panel A. Relative times of administration of CDI-causing broad-spectrum antibiotics (“Antibiotic therapy”) and of standard-of-care CDI therapy (“SOC antibiotic,” representing vancomycin, metronidazole, or fidaxomicin) are indicated. “At-risk window” box indicates the periods of susceptibility to recurrent CDI due to sustained dysbiosis following administration of SOC antibiotics. “Recurrence cycle” arrow indicates potential repeat episodes of recurrent CDI (rCDI). (C) Per panel B legend but in the context of acto/bezlo (“Acto/bezlo”) treatment during a primary episode of CDI, leading to protection against recurrent CDI throughout the at-risk window.
These results provide insight into the mechanism of prevention of recurrent disease observed in clinical trials performed with acto/bezlo (15), as summarized in Fig. 6B and C. Since colonization of the gut by C. difficile is facilitated by antibiotic-mediated disruption of the normal gut biota, there is a period of time, following successful cure of an initial episode of CDI with standard-of-care antibiotics such as vancomycin (the “at-risk window” in Fig. 6B and C), during which the gut microbiome has not yet recovered and patients are at risk for recurrence (9, 10, 36). Paradoxically, the standard of care for recurrent episodes of CDI consists of a new course of antibiotics, including vancomycin, perpetuating a recurrence cycle that can be difficult to break (Fig. 6B). Thus, if our data in rodent models of CDI are indeed predictive of disease recurrence in humans, a single infusion of antibodies (which have a long half-life in circulation [15]) administered during the initial episode may be able to prevent recurrence of CDI by neutralizing toxins produced following outgrowth of persistent or newly acquired C. difficile spores within this at-risk window, until the microbiota has recovered sufficiently to provide natural protection against CDI (Fig. 6C). Treatment with acto/bezlo may break the recurrence cycle by preventing the symptoms of a recurrent infection, obviating the need for additional antibiotics, and allowing the gut microbiota to fully recover, thus preventing future episodes of CDI (Fig. 6C).
The microbiota data from this study are largely comparable to those from previous studies, specifically with regard to intestinal composition in naive mice, impact of CDI-enabling antibiotics, and subsequent recovery (19, 20, 35, 37, 38). Published data have shown that the intestinal/fecal microbiota of naive mice is characterized by a predominance of Firmicutes (Clostridia and Bacilli), with a low to moderate relative abundance of Bacteroidetes (a minor component of the gut microbiome in our study) and Proteobacteria. Mice treated with clindamycin (20) or with cefoperazone (19, 35)—the two CDI-enabling antibiotics administered in this study—show a decrease in the relative abundance of Clostridia and an increase in the abundance of Bacilli and Proteobacteria, although the relative levels of the latter in antibiotic-treated mice were higher in our study than in previously published studies. Treatment with vancomycin has also previously been shown to induce profound and long-lasting shifts in microbiota composition (38), consistent with our own observations that vancomycin-treated mice show a delayed recovery of the relative abundances of Bacilli and Proteobacteria compared to vehicle- and acto/bezlo-treated mice. The largely minor differences between the current study and other published studies with respect to microbiota composition can likely be ascribed to different sources of mice, to types of feed or bedding, and/or to other environmental factors. Alternatively, differences with other studies may be due to an effect of C. difficile itself since we did not assess the microbiota of antibiotic-treated animals that were not challenged with C. difficile. Notably, the composition of the gut microbiota in mice in this study and others is comparable to that of humans, at least at the level of organism phyla, with a high proportion of Firmicutes and a low proportion of Proteobacteria prior to antibiotic administration and a reversal of these proportions following vancomycin treatment (9, 39).
Despite the comparable microbiota compositions observed at the class level in comparisons of naive mice to fully recovered (day 28) C. difficile-challenged mice (Fig. 4), the two groups differ with respect to the specific makeup of organisms, in particular, those in the Clostridia class (Fig. 5). Indeed, while the patterns were similar for the majority of Clostridia OTUs (categories 3 and 4 in Fig. 5), a significant number of organisms were either abundant in naive animals and absent in day 28 animals (category 1) or absent in naive animals and abundant in day 28 animals (category 2). This may have been due to environmental factors that changed from the time that the mice were first acquired for the study to the end of the study on day 28. Alternatively, changes to the gut environment that occur during the disease state, including the presence of C. difficile itself, may have a long-lasting impact on the recovery of specific organisms in the class Clostridia. These results, combined with previously published data, highlight the complexities involved in the dynamic effects of antibiotics on the microbial profile of the gut.
While the data presented here suggest that neutralization of TcdA and TcdB has a limited impact on C. difficile burden and on the gut microbiota, they cannot entirely rule out direct or indirect effects of toxins on C. difficile colonization, sporulation, germination, or growth in the gut or on reestablishment of the normal gut microbiota. Indeed, the toxins have been shown to increase C. difficile adherence to polarized epithelial cells in vitro (40), suggesting a role of toxins in C. difficile colonization. Additionally, neutralization of TcdA and TcdB may indirectly facilitate recolonization of the gut by commensal organisms by keeping important ecological niches within the gut wall intact (i.e., by limiting damage to the intestinal wall). Nevertheless, it is clear that the high-level shifts from a Clostridia-rich composition in naive mice to a Proteobacteria/Bacilli-rich composition following antibiotic treatment and back to a Clostridia-rich composition upon recovery follow similar courses in vehicle- and acto/bezlo-treated animals.
Overall, our data expand our understanding of CDI disease progression, relapse, and resolution in rodents. Specifically, we have shown that CDI resolves on its own in animals that survive the initial severe phase of disease, due to the gradual recovery of the gut microbiota leading to clearance and eventual elimination of C. difficile from the intestine. Our evaluation of C. difficile burden and microbiota composition over time also strengthens our current understanding of the mechanistic basis for the recurrence/relapse observed with vancomycin. Specifically, treatment with vancomycin delays recovery of the gut microbiota, allowing residual C. difficile spores to germinate and cause disease once vancomycin therapy is discontinued. Finally, our report enhances our understanding of how the antitoxin antibodies actoxumab and bezlotoxumab protect against disease, presumably by neutralizing the damage-causing toxins of C. difficile, leading to prevention of the clinical manifestation of disease, while having a limited impact on C. difficile burden and on recovery of the gut microbiota following a course of antibiotics. Treatment with antitoxin antibodies therefore represents a microbiota-sparing approach to the prevention of CDI, underscoring its successful use in the prevention of recurrent disease in human patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Elmar Schilling, Christiana Cicicopol-Boicu, and Lukas Windhager at Eurofins Genomics (Germany) for carrying out the microbiota experiments and James Mortimer for help with the Tibco Spotfire analyses.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00974-16.
REFERENCES
- 1.Bassetti M, Villa G, Pecori D, Arzese A, Wilcox M. 2012. Epidemiology, diagnosis and treatment of Clostridium difficile infection. Expert Rev Anti Infect Ther 10:1405–1423. doi: 10.1586/eri.12.135. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CP, LaMont JT. 2008. Clostridium difficile—more difficult than ever. N Engl J Med 359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 3.Rupnik M, Wilcox MH, Gerding DN. 2009. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 4.Buffie CG, Pamer EG. 2013. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theriot CM, Young VB. 2015. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol 69:445–461. doi: 10.1146/annurev-micro-091014-104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jank T, Aktories K. 2008. Structure and mode of action of clostridial glucosylating toxins: the ABCD model. Trends Microbiol 16:222–229. doi: 10.1016/j.tim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Pruitt RN, Lacy DB. 2012. Toward a structural understanding of Clostridium difficile toxins A and B. Front Cell Infect Microbiol 2:28. doi: 10.3389/fcimb.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rineh A, Kelso MJ, Vatansever F, Tegos GP, Hamblin MR. 2014. Clostridium difficile infection: molecular pathogenesis and novel therapeutics. Expert Rev Anti Infect Ther 12:131–150. doi: 10.1586/14787210.2014.866515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abujamel T, Cadnum JL, Jury LA, Sunkesula VC, Kundrapu S, Jump RL, Stintzi AC, Donskey CJ. 2013. Defining the vulnerable period for re-establishment of Clostridium difficile colonization after treatment of C. difficile infection with oral vancomycin or metronidazole. PLoS One 8:e76269. doi: 10.1371/journal.pone.0076269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson S. 2009. Recurrent Clostridium difficile infection: causality and therapeutic approaches. Int J Antimicrob Agents 33(Suppl 1):S33–S36. doi: 10.1016/S0924-8579(09)70014-7. [DOI] [PubMed] [Google Scholar]
- 11.Zanella Terrier MC, Simonet ML, Bichard P, Frossard JL. 2014. Recurrent Clostridium difficile infections: the importance of the intestinal microbiota. World J Gastroenterol 20:7416–7423. doi: 10.3748/wjg.v20.i23.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFarland LV, Elmer GW, Surawicz CM. 2002. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol 97:1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 13.Shields K, Araujo-Castillo RV, Theethira TG, Alonso CD, Kelly CP. 2015. Recurrent Clostridium difficile infection: from colonization to cure. Anaerobe 34:59–73. doi: 10.1016/j.anaerobe.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babcock GJ, Broering TJ, Hernandez HJ, Mandell RB, Donahue K, Boatright N, Stack AM, Lowy I, Graziano R, Molrine D, Ambrosino DM, Thomas WD Jr. 2006. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect Immun 74:6339–6347. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, Nichol G, Thomas WD Jr, Leney M, Sloan S, Hay CA, Ambrosino DM. 2010. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 16.Gerding D, Wilcox M, Poxton I, Kelly C, Birch T, Bouza E, Lee C, Kim YS, Yoshida J, Gabryelski L, Pedley A, Taylor A, Guris D, Kartsonis N, Dorr MB. 2015. Phase 3 double-blind study of bezlotoxumab (BEZ) alone & with actoxumab (ACT) for prevention of recurrent C. difficile infection (rCDI) in patients on standard of care (SoC) antibiotics (MODIFY II). Abstr 55th Intersci Conf Antimicrob Agents Chemother, abstr 2028. [Google Scholar]
- 17.Wilcox M, Gerding D, Poxton I, Kelly C, Nathan R, Rahav G, Cornely OA, Jenkin G, Jensen W, Eves K, Pedley A, Tipping R, Guris D, Kartsonis N, Dorr MB. 2015. Phase 3 double-blind study of actoxumab (ACT) & bezlotoxumab (BEZ) for prevention of recurrent C. difficile infection (rCDI) in patients on standard of care (SoC) antibiotics (MODIFY I). Abstr 55th Intersci Conf Antimicrob Agents Chemother, abstr 1805. [Google Scholar]
- 18.Miezeiewski M, Schnaufer T, Muravsky M, Wang S, Caro-Aguilar I, Secore S, Thiriot DS, Hsu C, Rogers I, DeSantis T, Kuczynski J, Probst AJ, Chehoud C, Steger R, Warrington J, Bodmer JL, Heinrichs JH. 2015. An in vitro culture model to study the dynamics of colonic microbiota in Syrian golden hamsters and their susceptibility to infection with Clostridium difficile. ISME J 9:321–332. doi: 10.1038/ismej.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theriot CM, Koenigsknecht MJ, Carlson PE Jr, Hatton GE, Nelson AM, Li B, Huffnagle GB, Li Z, Young VB. 2014. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun 5:3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. 2015. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517:205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razaq N, Sambol S, Nagaro K, Zukowski W, Cheknis A, Johnson S, Gerding DN. 2007. Infection of hamsters with historical and epidemic BI types of Clostridium difficile. J Infect Dis 196:1813–1819. doi: 10.1086/523106. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Katchar K, Goldsmith JD, Nanthakumar N, Cheknis A, Gerding DN, Kelly CP. 2008. A mouse model of Clostridium difficile-associated disease. Gastroenterology 135:1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 23.Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB. 2011. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes 2:145–158. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theriot CM, Koumpouras CC, Carlson PE, Bergin II, Aronoff DM, Young VB. 2011. Cefoperazone-treated mice as an experimental platform to assess differential virulence of Clostridium difficile strains. Gut Microbes 2:326–334. doi: 10.4161/gmic.19142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eren AM, Morrison HG, Lescault PJ, Reveillaud J, Vineis JH, Sogin ML. 2015. Minimum entropy decomposition: unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J 9:968–979. doi: 10.1038/ismej.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 27.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 6:610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lozupone C, Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Chen X, Hernandez LD, Lipari P, Flattery A, Chen SC, Kramer S, Polishook JD, Racine F, Cape H, Kelly CP, Therien AG. 2015. Toxin-mediated paracellular transport of antitoxin antibodies facilitates protection against Clostridium difficile infection. Infect Immun 83:405–416. doi: 10.1128/IAI.02550-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steele J, Mukherjee J, Parry N, Tzipori S. 2013. Antibody against TcdB, but not TcdA, prevents development of gastrointestinal and systemic Clostridium difficile disease. J Infect Dis 207:323–330. doi: 10.1093/infdis/jis669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutton ML, Mackin KE, Chakravorty A, Lyras D. 2014. Small animal models for the study of Clostridium difficile disease pathogenesis. FEMS Microbiol Lett 352:140–149. doi: 10.1111/1574-6968.12367. [DOI] [PubMed] [Google Scholar]
- 32.Price AB, Larson HE, Crow J. 1979. Morphology of experimental antibiotic-associated enterocolitis in the hamster: a model for human pseudomembranous colitis and antibiotic-associated diarrhoea. Gut 20:467–475. doi: 10.1136/gut.20.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keel MK, Songer JG. 2006. The comparative pathology of Clostridium difficile-associated disease. Vet Pathol 43:225–240. doi: 10.1354/vp.43-3-225. [DOI] [PubMed] [Google Scholar]
- 34.Boss SM, Gries CL, Kirchner BK, Smith GD, Francis PC. 1994. Use of vancomycin hydrochloride for treatment of Clostridium difficile enteritis in Syrian hamsters. Lab Anim Sci 44:31–37. [PubMed] [Google Scholar]
- 35.Antonopoulos DA, Huse SM, Morrison HG, Schmidt TM, Sogin ML, Young VB. 2009. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun 77:2367–2375. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly CP. 2012. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect 18(Suppl 6):21–27. doi: 10.1111/1469-0691.12046. [DOI] [PubMed] [Google Scholar]
- 37.Koenigsknecht MJ, Theriot CM, Bergin IL, Schumacher CA, Schloss PD, Young VB. 2015. Dynamics and establishment of Clostridium difficile infection in the murine gastrointestinal tract. Infect Immun 83:934–941. doi: 10.1128/IAI.02768-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis BB, Buffie CG, Carter RA, Leiner I, Toussaint NC, Miller LC, Gobourne A, Ling L, Pamer EG. 2015. Loss of microbiota-mediated colonization resistance to Clostridium difficile Infection with oral vancomycin compared with metronidazole. J Infect Dis 212:1656–1665. doi: 10.1093/infdis/jiv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seekatz AM, Aas J, Gessert CE, Rubin TA, Saman DM, Bakken JS, Young VB. 2014. Recovery of the gut microbiome following fecal microbiota transplantation. mBio 5:e00893-14. doi: 10.1128/mBio.00893-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasendra M, Barrile R, Leuzzi R, Soriani M. 2014. Clostridium difficile toxins facilitate bacterial colonization by modulating the fence and gate function of colonic epithelium. J Infect Dis 209:1095–1104. doi: 10.1093/infdis/jit617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.