Abstract
Our objective was to describe the population pharmacokinetics of fluconazole in a cohort of critically ill nonobese, obese, and morbidly obese patients. Critically ill patients prescribed fluconazole were recruited into three body mass index (BMI) cohorts, nonobese (18.5 to 29.9 kg/m2), obese (30.0 to 39.9 kg/m2), and morbidly obese (≥40 kg/m2). Serial fluconazole concentrations were determined using a validated chromatographic method. Population pharmacokinetic analysis and Monte Carlo dosing simulations were undertaken with Pmetrics. Twenty-one critically ill patients (11 male) were enrolled, including obese (n = 6) and morbidly obese (n = 4) patients. The patients mean ± standard deviation (SD) age, weight, and BMI were 54 ± 15 years, 90 ± 24 kg, and 31 ± 9 kg/m2, respectively. A two-compartment linear model described the data adequately. The mean ± SD population pharmacokinetic parameter estimates were clearance (CL) of 0.95 ± 0.48 liter/h, volume of distribution of the central compartment (Vc) of 15.10 ± 11.78 liter, intercompartmental clearance from the central to peripheral compartment of 5.41 ± 2.28 liter/h, and intercompartmental clearance from the peripheral to central compartment of 2.92 ± 4.95 liter/h. A fluconazole dose of 200 mg daily was insufficient to achieve an area under the concentration-time curve for the free, unbound drug fraction/MIC ratio of 100 for pathogens with MICs of ≥2 mg/liter in patients with BMI of >30 kg/m2. A fluconazole loading dose of 12 mg/kg and maintenance dose of 6 mg/kg/day achieved pharmacodynamic targets for higher MICs. A weight-based loading dose of 12 mg/kg followed by a daily maintenance dose of 6 mg/kg, according to renal function, is required in critically ill patients for pathogens with a MIC of 2 mg/liter.
INTRODUCTION
Recent decades have seen an increased prevalence in obesity worldwide (1, 2). More than two-thirds of adults in the U.S. are either overweight or obese, while one-third are obese (3, 4). Mortality and morbidity are higher in obese patients than nonobese patients for different types of infections, such as surgical site infections, community-acquired pneumonia, and peritonitis in peritoneal dialysis patients (5–8). Optimization of dosing is essential to minimize the risk of therapeutic failure in these patients. However, few data are available to guide clinicians for effective antimicrobial dosing in obese patients.
Moreover, dosing in obese critically ill patients is relatively unexplored; thus, it is a highly challenging scenario for clinicians (9, 10). Although only sparse data are available, it is possible that the altered pharmacokinetics (PK) that occur in critically ill patients are heightened by the presence of obesity due to concurrent pathophysiological changes (11, 12). The physiological changes that are likely to occur in obese compared to nonobese patients include reduced regional blood flow, increased cardiac output, and increased fat and lean mass (13). These changes in obese patients are likely to alter the PK/pharmacodynamics (PK/PD) of antimicrobials, thereby requiring dosing adjustments to ensure optimal patient outcomes. As the prevalence of critically ill obese patients increases, clinicians will face an increasing challenge to ensure effective antimicrobial therapy.
Fluconazole is a commonly used triazole antifungal in critically ill patients (14). It is commonly prescribed to treat infections caused by Candida albicans and Candida tropicalis (14). In healthy individuals and non-critically ill patients, fluconazole has a favorable PK and safety profile and exhibits a long half-life (30 h), enabling once-daily dosing. Fluconazole is excreted unchanged in urine, with ≈80% renal clearance. It has low plasma protein binding (11 to 12%) and a moderate volume of distribution (V), about 0.65 to 0.70 liter/kg (15).
In critically ill nonobese patients, fluconazole PK appears to be different from that of healthy subjects (16). This is likely due to organ dysfunction, renal and/or hepatic, as well as fluid shifts and capillary permeability changes that can alter fluconazole clearance and V. There is little or no data about the PK of fluconazole in obese patients, particularly critically ill obese patients. Therefore, it remains unclear whether standard fluconazole dosing regimens will provide sufficient drug exposure.
The aim of this prospective study was to describe the population PK of fluconazole in a cohort of critically ill nonobese, obese, and morbidly obese patients.
MATERIALS AND METHODS
Setting.
This was an observational PK study at a tertiary referral intensive care unit (ICU). Ethical approval was obtained from the local institutional Human Research Ethics Committee (HREC/14/QRBW/88). Written informed consent was obtained from all participants or from their substitute decision-makers.
Study population.
The inclusion criteria for this study were the following: (i) age of ≥18 years, (ii) receiving fluconazole (prophylaxis or treatment), and (iii) body mass index (BMI) of ≥18.5 kg/m2. The exclusion criteria were the following: (i) patients on renal replacement therapy (RRT), (ii) pregnant women, (iii) patients with active bleeding, or (iv) patients with HIV or hepatitis.
Study protocol.
Fluconazole was administered at the direction of the treating intensivist. Participants were categorized into three groups according to their BMI as normal weight (BMI of 18.5 to 29.9 kg/m2), obese (BMI of 30 to 39.9 kg/m2), and morbidly obese (BMI of ≥40 kg/m2). During one dosing interval, blood samples (≈3 ml) were taken from each participant to determine plasma fluconazole concentrations at predose, at 30 min and 60 min, and at 2, 3, 4, 5, 6, 8, 12, and 24 h after dose administration (10 samples for 12-h dosing or 11 samples for 24-h dosing). Other clinical and demographic data were collected on the day of plasma sampling, including age, sex, total body weight (TBW), ideal body weight (IBW), lean body weight (LBW), and BMI (17). Clinical data also were recorded, including sequential organ failure assessment (SOFA) (18) and acute physiology and chronic health evaluation II (APACHE II) (19) scores, as well as serum albumin and creatinine concentrations (SCR) and CLCR estimated by the Cockcroft-Gault equation (separately using TBW, LBW, and IBW) (20). Urine samples were also collected over the dosing interval to determined measured CLCR.
Sample handling, storage, and assay.
Blood samples were placed in an ice bath immediately and centrifuged at 3,000 rpm for 10 min. Plasma samples were stored at −80°C until bioanalysis. Fluconazole was measured in plasma by an ultrahigh-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method with a reverse-phase F5 column on a Shimadzu Nexera2-8030+ system. Unknown samples were assayed in batches alongside calibrators, and quality controls and results were subject to batch acceptance criteria. Plasma (100 μl) was spiked with internal standard (voriconazole), and acetonitrile was added to precipitate proteins. The supernatant was isolated and 1 μl injected onto the UHPLC. For the stationary phase, we used a Phenomenex Kinetex F5 column, 50 by 2.1 mm, 1.7 μm volume, heated to 30°C. For the mobile phase, we used a 75:25 blend of phase A (0.1% formic acid in 10 mM ammonium formate) and phase B (0.1% formic acid in acetonitrile) delivered isocratically at a flow rate of 0.4 ml/min, producing a typical backpressure of 5,500 lb/in2. Fluconazole (retention time of 0.67 min) was well separated from voriconazole (retention time of 3.93 min) in a run time of 5 min. Column eluent was directed to the mass spectrometer from 0.3 to 4.5 min. Ionization was by positive-mode electrospray, with fluconazole and voriconazole being monitored at 306.7g238.2 and 350.0g281.1, respectively. The assay method was validated for linearity, lower limit of quantification, and precision and accuracy using the FDA and European Medicines Agency (EMA) criteria for bioanalysis (21). The method was linear from 0.1 to 20 mg/liter. The precision was within 3.1% and accuracy was within 3.6% at the three levels tested. The stability of fluconazole in plasma and water stored at −80°C was validated for 19 months.
Population pharmacokinetic modeling.
The plasma fluconazole concentrations were fitted to one- and two-compartment models using a nonparametric adaptive grid (NPAG) algorithm within the Pmetrics package for R (Los Angeles, CA, USA) (22, 23).
Demographic and clinical characteristics that were considered biologically plausible for affecting fluconazole PK were tested for inclusion as covariates. Data, including age, sex, TBW, IBW, LBW, BMI, SCR, measured CLCR, CLCR estimated by Cockcroft-Gault equation (separately using TBW, LBW, and IBW), albumin levels, and SOFA and APACHE II scores, were tested. Each of these covariates was plotted against the PK parameter estimates to assess correlation. Covariates were retained in the model if they significantly improved the log likelihood (P < 0.05) and/or improved the goodness-of-fit plots.
Model diagnostics.
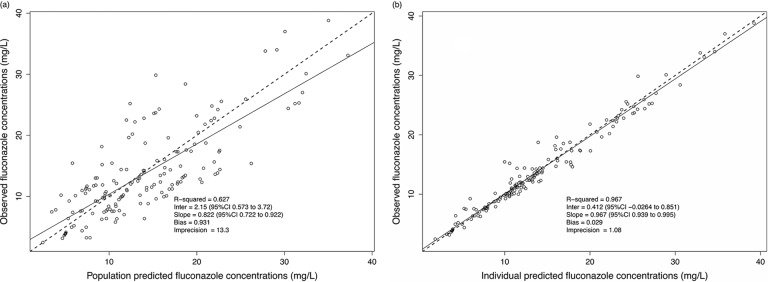
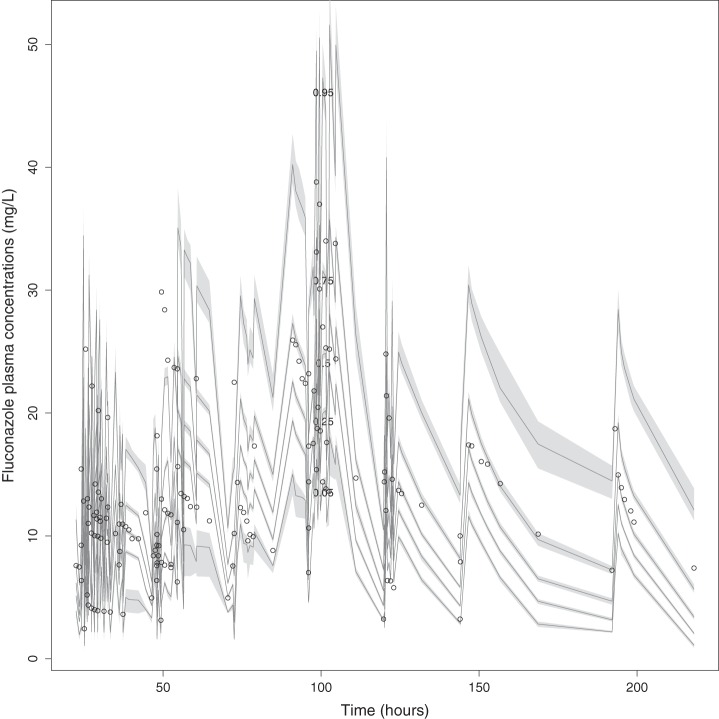
Visual inspection of the observed-predicted (population and individual) concentration scatter plot was undertaken with the coefficient of determination of linear regression of observed-predicted values, and the log-likelihood values from each run were used to evaluate the goodness of fit. Predictive performance evaluation was based on the means of both prediction error (bias) and bias-adjusted squared prediction error (imprecision) of the population and an individual prediction model in the central compartment. A visual predictive check using 1,000 simulations was used to evaluate the suitability of the final covariate model. Additionally, the area under the concentration-time curve (AUC) was calculated using the Bayesian estimates of the final model.
PTA.
Monte Carlo simulations (n = 1,000) were performed using Pmetrics software to determine the probability of target attainment (PTA) for PK/PD targets of 25 and 100 for ratios of the AUC for the free, unbound drug fraction to the MIC (fAUC/MIC) for various measured CLCR values and patient BMI (24–26). A free fraction of 12% was used for all simulations.
Fixed fluconazole loading doses (LD) of 200 mg, 400 mg, or 800 mg delivered intravenously for 12 or 24 h (infusion rate of 200 mg/60 min) were simulated from 0 to 24 h at three different levels of renal function (measured CLCR of 30, 50, and 150 ml/min) and three BMI groups of 20, 30, and 40 kg. The PTA for achieving fAUC/MIC of 25 and 100 were calculated.
Weight-based fluconazole loading doses of 3 mg/kg, 6 mg/kg, 9 mg/kg, 12 mg/kg, and 15 mg/kg from 0 to 24 h for BMI of 30 kg/m2 with measured CLCR of 50 ml/min were simulated. We also simulated fluconazole maintenance doses of 3 mg/kg/day, 6 mg/kg/day, 9 mg/kg/day, 12 mg/kg/day, and 15 mg/kg/day from 96 to 120 h for BMI of 30 kg/m2 (after a loading dose of 12 mg/kg on day 1) with three measured CLCR levels of 30, 50, and 150 ml/min.
FTA calculation.
The fAUC/MIC data for the susceptible pathogens C. albicans and C. tropicalis were used according to MIC distributions from the European Committee for Antimicrobial Susceptibility and Testing (EUCAST) database (25) to determine the fractional target attainment (FTA). The FTA describes the pharmacodynamic exposure (PTA) of fluconazole against a MIC distribution. This FTA threshold was attained when the value was ≥90%. Susceptible MIC distributions of both pathogens (MIC of ≤2 mg/liter) were used to determine the FTA for directed therapy.
Statistical analysis.
Continuous variables are presented as means (with standard deviations [SD]) or medians (with interquartile ranges) as appropriate. Categorical variables are expressed as absolute numbers and relative frequencies. The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to test for normality. A one-way analysis of variance (ANOVA) was used to test for differences in demographic and clinical data between the BMI categories. Linear regression was used to describe correlations between patient weight metrics in the three BMI categories with the fluconazole volume of distribution in the central compartment (Vc) and clearance. All statistical analyses were performed using the statistical software package IBM-SPSS statistics 22.0 (IBM, New York, USA). A P value of <0.05 was considered statistically significant.
RESULTS
Demographic and clinical data.
Twenty-one critically ill patients (11 male) were enrolled in the study; 11 nonobese, 6 obese, and 4 morbidly obese patients. In total, 215 blood samples were obtained from the participants. The demographics and clinical characteristics of the respective BMI categorizations are shown in Table 1. Only patients' TBW, LBW, and BMI were significantly different between the three BMI categorizations (P < 0.05).
TABLE 1.
Demographic and clinical data
| Variablea | Mean (SD) value by wt group |
P value | |||
|---|---|---|---|---|---|
| All (n = 21) | Normal wt (n = 11) | Obese (n = 6) | Morbidly obese (n = 4) | ||
| Age (yr) | 54 (15) | 55 (18) | 53 (15) | 54 (7) | 0.97 |
| wt (kg) | 90 (24) | 74 (10) | 98.1 (17) | 122 (23) | <0.001 |
| IBW (kg) | 64 (10) | 67 (7) | 66 (12) | 55 (11) | 0.09 |
| LBW (kg) | 56 (10) | 56 (6) | 62.5 (11) | 47 (10) | 0.03 |
| Height (cm) | 171 (11) | 173 (8) | 172 (13) | 163 (13) | 0.29 |
| Sex (no. [%] male) | 11 (52) | 7 (64) | 3 (50) | 1 (25) | |
| BMI (kg/m2) | 31 (9) | 25 (3) | 33 (3) | 46 (6) | <0.001 |
| SCR (μmol/liters) | 74 (35) | 62 (21) | 83 (40) | 94 (52) | 0.22 |
| Measured CLCR (liters/h) | 106 (54) | 108 (45) | 89 (27) | 125 (103) | 0.60 |
| CG-TBW (liters/h) | 142 (85) | 127 (57) | 135 (66) | 192 (159) | 0.43 |
| CG-IBW (liters/h) | 102 (52) | 113 (47) | 89 (40) | 90 (84) | 0.60 |
| CG-LBW (liters/h) | 89 (45) | 95 (41) | 84 (38) | 78 (72) | 0.81 |
| SOFA score | 5 (2) | 5 (2) | 6 (3) | 3 (1) | 0.29 |
| APACHE II score | 20 (5) | 19 (2) | 21 (7) | 22 (8) | 0.48 |
CG-TBW, estimated CLCR using Cockcroft-Gault equation based on total body weight; CG-IBW, estimated CLCR using Cockcroft-Gault equation based on ideal body weight; CG-LBW, estimated CLCR using Cockcroft-Gault equation based on lean body weight.
Pharmacokinetic model building.
Fluconazole PK was best described using a two-compartment linear model. The model goodness of fit was improved by inclusion of the covariates measured CLCR (normalized to the population mean of 105 ml/min) for fluconazole clearance and BMI (normalized to 30 kg/m2) for fluconazole Vc. Furthermore, addition of both resulted in a statistically significant improvement in the log likelihood from the previous model (P < 0.05).
Measured CLCR, Cockcroft-Gault (based on total body weight), and modified diet in renal disease equations were each tested as potential covariates for fluconazole clearance. None of these CLCR measures resulted in a statistically significant decrease in the log likelihood. However, of these, the lowest log likelihood was associated with inclusion of measured CLCR in the model. The addition of this covariate improved the agreement between the observed and population-predicted concentrations as well as distribution of observed data within the visual predictive check, so it was retained in the final model.
Addition of BMI as a descriptor of Vc to the final covariate model was performed even though there was no positive correlation evident between Vc and BMI. This decision was based on biological plausibility and because the addition resulted in a clear improvement of the observed and population-predicted concentration goodness-of-fit plot as well as the visual predictive check plot.
The final covariate model was TVCL = CL × CLCR/105 and TVVC = Vc × BMI/30, where TVCL is the typical value of fluconazole clearance and TVVc is the typical value of fluconazole distribution in the central compartment.
The mean ± SD population PK parameter estimates from the final covariate model are shown in Table 2. The diagnostic plots and visual predictive check confirmed the appropriateness of the model as shown in Fig. 1 and 2, respectively. The final covariate model was for Monte Carlo dosing simulations.
TABLE 2.
Parameter estimates for fluconazole from the final covariate two-compartment population PK model
| Parametera | Mean value (SD) | Coefficient of variation (%) | Median |
|---|---|---|---|
| CL (liters/h) | 0.95 (0.48) | 50.47 | 0.86 |
| Vc (liters) | 15.10 (11.78) | 78.07 | 8.55 |
| KCP (h−1) | 5.41 (2.28) | 42.21 | 5.42 |
| KPC (h−1) | 2.92 (4.95) | 170.00 | 1.04 |
CL, population clearance of fluconazole; Vc, population volume of distribution of central compartment; KCP, rate constant for fluconazole distribution from the central to peripheral compartment; KPC, rate constant for fluconazole distribution from the peripheral to central compartment.
FIG 1.
Diagnostic plot for the final population pharmacokinetic covariate model. (a) Observed fluconazole concentrations versus population-predicted fluconazole concentration (R2 = 0.627). (b) Observed fluconazole concentrations versus individual-predicted fluconazole concentration (R2 = 0.967). CI, confidence interval.
FIG 2.
Visual predictive check of fluconazole plasma data.
Dosing simulations.
Monte Carlo simulations and PTA for achieving fAUC/MIC of 25 and 100 for fixed 200-mg, 400-mg, and 800-mg daily doses are presented in Table 3. For an fAUC/MIC of 25, the results showed that all morbidly obese patients failed to achieve the PK/PD target of a MIC of 2 mg/liter at lower fluconazole doses (200 mg daily). For higher doses (800 mg daily), there was failure to achieve an fAUC/MIC of 25 for a MIC of 8 mg/liter. For an fAUC/MIC of 100, all simulated morbidly obese patients failed to achieve the PK/PD target for a MIC of 0.5 mg/liter at a fixed 200-mg daily dose and for a MIC of 2 mg/liter at a fixed 800-mg daily dose.
TABLE 3.
Fluconazole PTA at different BMIs, CLCR, and loading dosage regimens
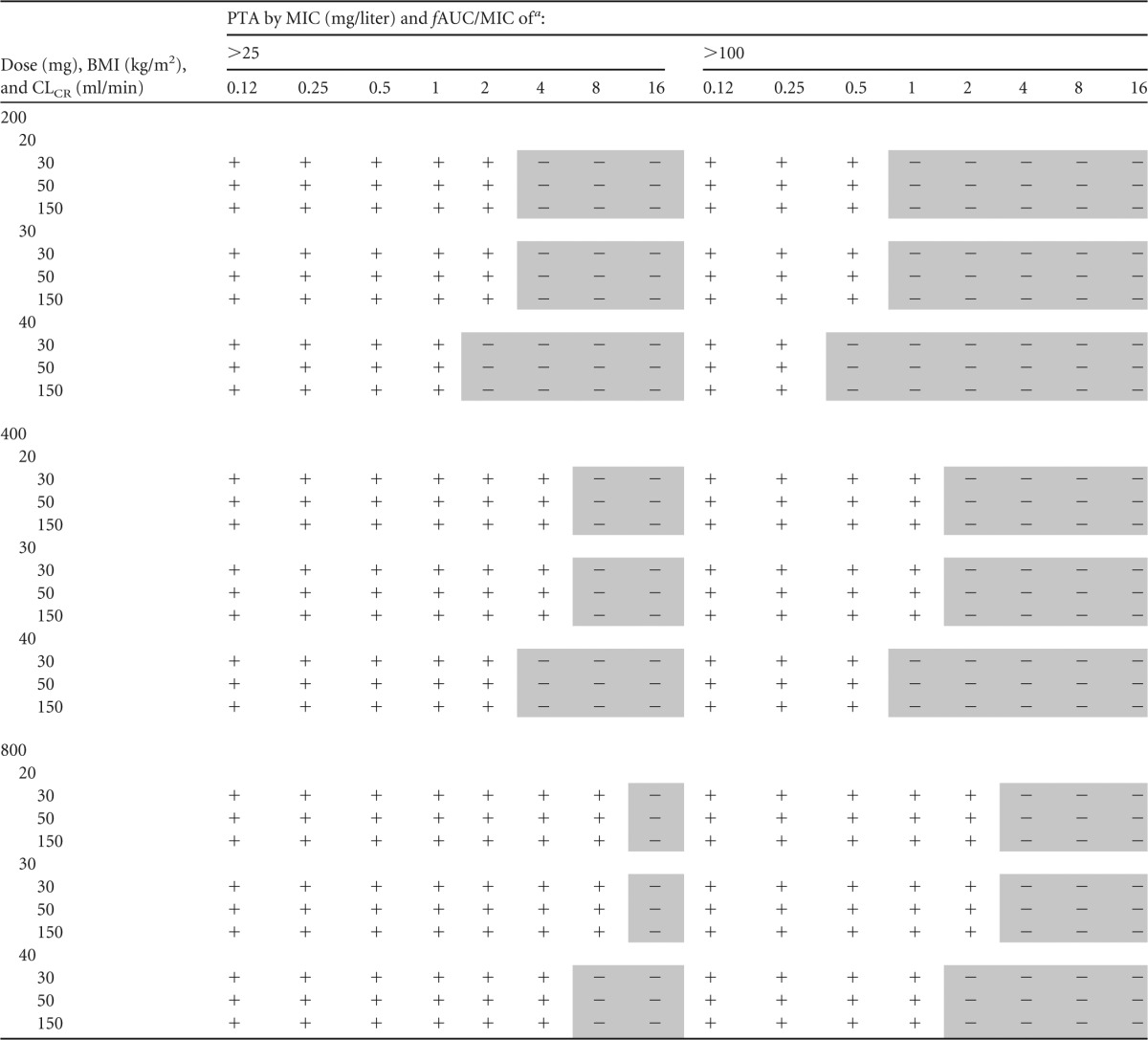
Fluconazole target MIC was defined according to EUCAST susceptibility breakpoints (2 mg/liter). A plus sign indicates that at least 90% of fluconazole PTA is achieved; a minus sign (shaded) indicates fluconazole PTA attainment failed to achieve 90%.
FTA.
The FTA for the different scenarios are shown in Tables 4 and 5. For an fAUC/MIC of 25, all fluconazole dosing regimens successfully achieved the 90% target for directed therapy (MIC distribution of ≤2 mg/liter). For an fAUC/MIC of 100, the lower fluconazole dose (i.e., 200 mg daily) achieved 90% coverage of C. albicans for directed therapy only in patients in the normal weight group at the lower measured CLCR (30 and 50 ml/min).
TABLE 4.
FTA at fAUC/MIC of >25 for fluconazole LD (0 to 24 h) at different BMIs and CLCR for directed therapy (MIC of ≥2 mg/liter)
| LD (mg), BMI (kg/m2), and CLCR (ml/min) | FTA (%) |
|
|---|---|---|
| C. albicans | C. tropicalis | |
| 200 | ||
| 20 | ||
| 30 | 99.1 | 98.7 |
| 50 | 99.1 | 98. 7 |
| 150 | 98.1 | 98.4 |
| 30 | ||
| 30 | 99.0 | 98.0 |
| 50 | 99.2 | 98.0 |
| 150 | 99.5 | 98.4 |
| 40 | ||
| 30 | 98.5 | 96.1 |
| 50 | 98.5 | 96.0 |
| 150 | 98.0 | 93.0 |
| 400 | ||
| 20 | ||
| 30 | 99.9 | 99.5 |
| 50 | 99.9 | 99.5 |
| 150 | 99.9 | 99.5 |
| 30 | ||
| 30 | 99.7 | 99.3 |
| 50 | 99.7 | 99.2 |
| 150 | 99.9 | 99.5 |
| 40 | ||
| 30 | 99.5 | 98.7 |
| 50 | 99.5 | 98.7 |
| 150 | 99.5 | 98.6 |
| 800 | ||
| 20 | ||
| 30 | 99.5 | 99.9 |
| 50 | 99.5 | 99.9 |
| 150 | 99.5 | 99.9 |
| 30 | ||
| 30 | 99.9 | 99.7 |
| 50 | 99.9 | 99.7 |
| 150 | 99.9 | 99.9 |
| 40 | ||
| 30 | 99.9 | 99.5 |
| 50 | 99.9 | 99.5 |
| 150 | 99.9 | 99.5 |
TABLE 5.
FTA at fAUC/MIC of >100 for fluconazole LD (0 to 24 h) at different BMIs and CLCR for directed therapy (MIC of ≥2 mg/liter)
| LD (mg), BMI (kg/m2), and CLCR (ml/min) | FTA (%)a |
|
|---|---|---|
| C. albicans | C. tropicalis | |
| 200 mg | ||
| 20 | ||
| 30 | 94.4 | 81.9 |
| 50 | 93.6 | 79.3 |
| 150 | 89.5 | 65.7 |
| 30 | ||
| 30 | 89.9 | 68.8 |
| 50 | 89.3 | 66.8 |
| 150 | 89.5 | 65.7 |
| 40 | ||
| 30 | 86.1 | 59.6 |
| 50 | 85.6 | 58.2 |
| 150 | 80.7 | 47.3 |
| 400 mg | ||
| 20 | ||
| 30 | 98.4 | 95.7 |
| 50 | 98.2 | 94.7 |
| 150 | 96.6 | 87.7 |
| 30 | ||
| 30 | 96.7 | 88.9 |
| 50 | 96.4 | 87.9 |
| 150 | 96.6 | 87.7 |
| 40 | ||
| 30 | 94.8 | 83.3 |
| 50 | 94.5 | 82.4 |
| 150 | 92.4 | 75.2 |
| 800 mg | ||
| 20 | ||
| 30 | 99.5 | 98.7 |
| 50 | 99.5 | 98.7 |
| 150 | 99.5 | 98.4 |
| 30 | ||
| 30 | 99.2 | 97.8 |
| 50 | 99.2 | 97.7 |
| 150 | 99.5 | 98.4 |
| 40 | ||
| 30 | 98.5 | 96.1 |
| 50 | 98.5 | 95.8 |
| 150 | 97.7 | 92.7 |
Dose, BMI, and CLCR values that did not achieve the target of fractional target attainment against at least 90% of isolates are indicated by boldface.
The higher fluconazole dosing regimen (800 mg daily) achieved 90% coverage of susceptible C. albicans and C. tropicalis in all BMI groups at different levels of measured CLCR (Fig. 2).
FTA at different fluconazole weight-based loading and maintenance doses.
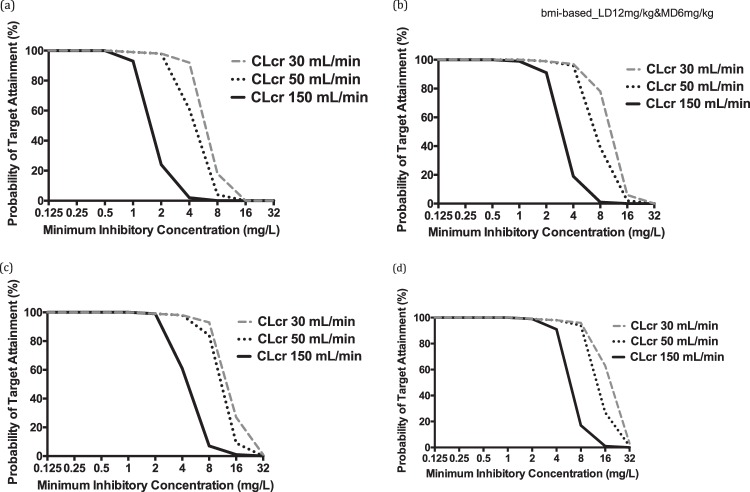
The FTA for various weight-based dosing regimens from 0 to 24 h and separately from 96 to 120 h are shown in Fig. 3 and 4. These data show that a loading dose of at least 12 mg/kg was required for the fAUC/MIC target of 100. Furthermore, a maintenance dose of at least 6 mg/kg/day (TBW) was required to achieve an fAUC/MIC of 100 (assessed between 96 and 120 h of therapy).
FIG 3.
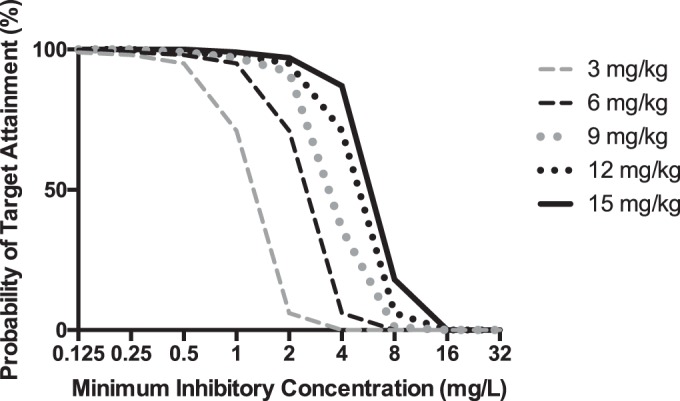
Probability of target attainment (fAUC/MIC of 100) from 0 to 24 h for different fluconazole loading doses (milligrams per kilogram) for patients with a BMI of 30 kg/m2 and CLCR of 50 ml/min. The PK/PD target is achieved when the value is ≥90% coverage.
FIG 4.
Probability of target attainment (fAUC/MIC of 100) from 96 to 120 h for a patient with BMI of 30 kg/m2 at simulated CLCR values of 30, 50, and 150 ml/min and administered different maintenance doses of fluconazole: 3 mg/kg/day (a), 6 mg/kg/day (b), 9 mg/kg/day (c), and 12 mg/kg/day (d). The PK/PD target is achieved when the value is ≥90% coverage.
DISCUSSION
Key findings.
This is the first population PK study of fluconazole in critically ill nonobese, obese, and morbidly obese patients. Fluconazole PK/PD targets of an fAUC/MIC of >25 (for fungistatic effect [24]) and of >100 (for a high probability of cure for Candida spp. with a MIC of up to 2 mg/liter [24]) were both tested due to the uncertainty of which PK/PD ratios should be targeted to ensure successful treatment. We found that a fluconazole dosing regimen of 200 mg/day was sufficient to achieve the PK/PD target of an fAUC/MIC of >25. However, a higher fluconazole dosing regimen, 400 mg/day or 800 mg/day, was required to achieve the higher target of an fAUC/MIC of >100. We believe a more accurate approach to dosing may be achieved through the use of a weight-based approach which includes a loading dose of 12 mg/kg on day 1 and a maintenance dose guided by measured CLCR.
Relationship with previous papers.
Despite the common use of fluconazole in the ICU, there is a significant shortage of robust PK studies of this drug for obese critically ill patients. Lopez and Phillips, in a recent case report of a morbidly obese patient (BMI of 84.0 kg/m2 [272 kg]) on RRT, reported the use of a 12-mg/kg fluconazole loading dose (based on LBW) followed by a maintenance dose of 6 mg/kg (27–29). The resulting fAUC was 163.8 mg · h/liter (assuming 11% protein binding), which would be sufficient to achieve the PK/PD target of an fAUC/MIC of >25 but not quite the higher PK/PD target of an fAUC/MIC of >100. In another case report of a morbidly obese patient (BMI of 48.3 kg/m2; measured CLCR of 125 ml/min), Cohen et al. reported the use of a 1,200-mg daily dose, administered as a 6-h infusion, with the resulting measured fAUC of 494.4 mg &3x00B7; h/liter (assuming 11% protein binding). This dose was sufficient to achieve both the lower and higher PK/PD targets (30). Both case reports on critically ill patients support the use of higher-than-standard doses in obese patients.
In a study of critically ill nonobese patients with normal renal function, a standard fluconazole dose of 400 mg daily (<6 mg/kg) resulted in suboptimal exposures, leading to failure in achieving PK/PD targets (31). The authors suggested that higher fluconazole doses are required in some patients with higher body weights. Therefore, individualized weight-based fluconazole dosing is recommended to ensure PK/PD target achievement. The results of the aforementioned study support the current study, which demonstrated that fluconazole at lower dosing failed to achieve the desired PK/PD in various scenarios. In a population PK study of fluconazole in critically ill patients receiving continuous veno-venous hemodiafiltration (CVVHDF), fluconazole clearance was higher than previously observed (32). Thus, this study recommended that higher fluconazole dosing is required in patients undergoing CVVHDF. The authors recommended a fluconazole loading dose of ≥12 mg/kg followed by a 6-mg/kg maintenance dose. This recommendation matches the findings of the current study, which showed that patients with measured CLCR of ≥150 ml/min (i.e., higher drug clearances) require higher loading (12 mg/kg) and maintenance (at least 6 mg/kg) doses.
Implications of study findings.
Fluconazole remains an important antifungal drug to prevent and treat Candida spp. infection. Given the increased prevalence and resistance of non-albicans Candida infections, critically ill patients, including the obese and morbidly obese, should be given antifungal therapy to optimize clinical outcomes as well as to minimize resistance. EUCAST recommends that a fluconazole PK/PD target of an fAUC/MIC of >100 be used for high probability of cure. Accordingly, we found that a standard fluconazole dose (200 mg daily) was insufficient for treatment of susceptible C. albicans and C. tropicalis in nonobese, obese, and morbidly obese critically ill patients. Thus, if a fixed dosing approach is to be used, higher fluconazole doses (400 mg daily or higher) should be considered for directed therapy. However, therapy for C. albicans and C. tropicalis might require even higher fluconazole doses (i.e., ≥800 mg daily).
When testing a weight-based dosing approach, we found that a fluconazole loading dose of 12 mg/kg/day followed by a maintenance dose of 6 mg/kg/day achieved PK/PD targets regardless of patient weight and BMI. This finding results from our observation that the fluconazole Vc was related to BMI, of which weight is a central determinant. We also found that fluconazole clearance is correlated with measured CLCR. Consequently, the fluconazole maintenance dose should be calculated according to renal function assessed by measured CLCR.
Study limitations.
Although this study is the first population PK examination of fluconazole in critically ill nonobese, obese, and morbidly obese patients, it has some limitations we would like to declare. First, the sample size was relatively small, particularly for the number of obese and morbidly obese patients, which was not sufficient for quantifying the effect of fluconazole exposure on patients' outcomes. Furthermore, describing all possible correlations between PK parameters and covariates may not have been possible due to the sample size. Although a larger sample size may have enabled additional covariate relationships to be demonstrated, we believe that BMI and measured CLCR are likely to be the two most significant determinants of dosing. Second, we did not measure fluconazole plasma concentrations at the site of infection, which may provide better mechanistic data regarding the effectiveness of dosing. Third, blood samples were collected only from one dosing interval, and levels may change within patients over time.
Conclusions.
In this study, which included critically ill obese and morbidly obese patients, we demonstrated that fluconazole clearance was correlated with measured CLCR, while Vc was best correlated with BMI. The results of this study suggest that a higher fixed fluconazole dose (i.e., ≥400 mg daily) is required in these patients when they are infected by less susceptible Candida spp. (MIC of ≥2 mg/liter). Our results show that a loading dose of 12 mg/kg followed by a maintenance dose of 6 or 12 mg/kg/day is required to achieve either the low or high PK/PD target. Finally, loading doses should be weight based, whereas maintenance doses should be prescribed according to renal function. Further clinical studies of fluconazole are warranted to determine the effect of optimized dosing on clinical outcome.
ACKNOWLEDGMENTS
We recognize funding from the Australian National Health and Medical Research Council for Centre of Research Excellence (APP1099452), funding from the Intensive Care Foundation, and for A.S.A. from The Saudi Arabian Culture Mission (SACM), affiliate to the Royal Embassy of Saudi Arabia. J.A.R. received a Career Development Fellowship (APP1048652) and M.S.R a Research Fellowship (APP1107356) from the National Health and Medical Research Council of Australia.
We have no conflicts of interest to declare.
REFERENCES
- 1.Dixon JB. 2015. The global burden of obesity and diabetes, p 1–6. In Schauer PR, Schirmer BD, Brethauer S (ed), Minimally invasive bariatric surgery. Springer, New York, NY. [Google Scholar]
- 2.Keating C, Backholer K, Gearon E, Stevenson C, Swinburn B, Moodie M, Carter R, Peeters A. 2015. Prevalence of class-I, class-II and class-III obesity in Australian adults between 1995 and 2011-12. Obesity Res Clinical Pract 9:553–562. doi: 10.1016/j.orcp.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Overweight and obesity statistics. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: https://www.niddk.nih.gov/health-information/health-statistics/Pages/overweight-obesity-statistics.aspx. [Google Scholar]
- 4.Fryar CD, Carroll MD, Ogden CL. 2015. Prevalence of overweight, obesity, and extreme obesity among adults: United States, 1960-1962 through 2011-2012. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 5.Löfgren M, Poromaa IS, Stjerndahl JH, Renström B. 2004. Postoperative infections and antibiotic prophylaxis for hysterectomy in Sweden: a study by the Swedish National Register for Gynecologic Surgery. Acta Obstet Gynecol Scand 83:1202–1207. doi: 10.1111/j.0001-6349.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 6.Olsen MA, Mayfield J, Lauryssen C, Polish LB, Jones M, Vest J, Fraser VJ. 2003. Risk factors for surgical site infection in spinal surgery. J Neurosurg Spine 98:149–155. doi: 10.3171/spi.2003.98.2.0149. [DOI] [PubMed] [Google Scholar]
- 7.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. 2000. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in us men and women. Arch Intern Med 160:3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 8.McDonald SP, Collins JF, Rumpsfeld M, Johnson DW. 2004. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 24:340–346. [PubMed] [Google Scholar]
- 9.Lisboa T, Rello J, Richart C, Anzueto A, El Solh AA. 2009. Obesity and critical care. Clin Pulm Med 16:202–211. doi: 10.1097/CPM.0b013e3181ad2171. [DOI] [Google Scholar]
- 10.Alobaid AS, Hites M, Lipman J, Taccone FS, Roberts JA. 2016. Effect of obesity on the pharmacokinetics of antimicrobials in critically ill patients: a structured review. Int J Antimicrob Agents 47:259–268. doi: 10.1016/j.ijantimicag.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Pai MP, Bearden DT. 2007. Antimicrobial dosing considerations in obese adult patients. Pharmacotherapy 27:1081–1091. doi: 10.1592/phco.27.8.1081. [DOI] [PubMed] [Google Scholar]
- 12.Blot SI, Pea F, Lipman J. 2014. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11. doi: 10.1016/j.addr.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Jain R, Chung S, Jain L, Khurana M, Lau S, Lee J, Vaidyanathan J, Zadezensky I, Choe S, Sahajwalla C. 2011. Implications of obesity for drug therapy: limitations and challenges. Clin Pharmacol Ther 90:77–89. doi: 10.1038/clpt.2011.104. [DOI] [PubMed] [Google Scholar]
- 14.Lewis RE. 2011. Current concepts in antifungal pharmacology. Mayo Clin Proc 86:805–817. doi: 10.4065/mcp.2011.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashley C, Currie A. 2004. The renal drug handbook, vol 2. Radcliffe Medical Press, San Francisco, CA. [Google Scholar]
- 16.Sinnollareddy M, Peake SL, Roberts MS, Lipman J, Roberts JA. 2012. Using pharmacokinetics and pharmacodynamics to optimise dosing of antifungal agents in critically ill patients: a systematic review. Int J Antimicrob Agents 39:1–10. doi: 10.1016/j.ijantimicag.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Green B, Duffull SB. 2004. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 58:119–133. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710. [DOI] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 21.Center for Drug Evaluation and Research. 2001. Guidance for industry: bioanalytical method validation. Food and Drug Administration, U.S. Department of Health and Human Services, Washington, DC. [Google Scholar]
- 22.Tatarinova T, Neely M, Bartroff J, van Guilder M, Yamada W, Bayard D, Jelliffe R, Leary R, Chubatiuk A, Schumitzky A. 2013. Two general methods for population pharmacokinetic modeling: non-parametric adaptive grid and non-parametric Bayesian. J Pharmacokinet Pharmacodyn 40:189–199. doi: 10.1007/s10928-013-9302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neely M, van Guilder M, Yamada W, Schumitzky A, Jelliffe R. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a non-parametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mouton JW. 2007. Pharmacokinetics and pharmacodynamics of azoles. Infect Dis Ther Ser 44:327. [Google Scholar]
- 25.European Committee on Antimicrobial Susceptibility Testing. 2015. EUCAST fluconazole rationale document version 0.0.8. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/Antifungal_breakpoints_v_8.0_November_2015.pdf.
- 26.Andes D, Van Ogtrop M. 1999. Characterization and quantitation of the pharmacodynamics of fluconazole in a neutropenic murine disseminated candidiasis infection model. Antimicrob Agents Chemother 43:2116–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts JA, Choi GY, Joynt GM, Paul SK, Deans R, Peake S, Cole L, Stephens D, Bellomo R, Turnidge J, Wallis SC, Roberts MS, Roberts DM, Lassig-Smith M, Starr T, Lipman J. 2016. Sampling antibiotics in renal replacement therapy (SMARRT): an observational pharmacokinetic study in critically ill patients. BMC Infect Dis 16:103. doi: 10.1186/s12879-016-1421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts DM, Roberts JA, Roberts MS, Liu X, Nair P, Cole L, Lipman J, Bellomo R. 2012. Variability of antibiotic concentrations in critically ill patients receiving continuous renal replacement therapy: a multicentre pharmacokinetic study. Crit Care Med 40:1523–1528. doi: 10.1097/CCM.0b013e318241e553. [DOI] [PubMed] [Google Scholar]
- 29.Lopez ND, Phillips KM. 2014. Fluconazole pharmacokinetics in a morbidly obese, critically ill patient receiving continuous venovenous hemofiltration. Pharmacotherapy 34:e162–e168. doi: 10.1002/phar.1470. [DOI] [PubMed] [Google Scholar]
- 30.Cohen LG, DiBiasio A, Lisco SJ, Hurford WE. 1997. Fluconazole serum concentrations and pharmacokinetics in an obese patient. Pharmacotherapy 17:1023–1026. [PubMed] [Google Scholar]
- 31.Sinnollareddy MG, Roberts JA, Lipman J, Akova M, Bassetti M, De Waele JJ, Kaukonen K-M, Koulenti D, Martin C, Montravers P. 2015. Pharmacokinetic variability and exposures of fluconazole, anidulafungin, and caspofungin in intensive care unit patients: data from multinational Defining Antibiotic Levels in Intensive Care Unit (DALI) Patients study. Crit Care 19:33. doi: 10.1186/s13054-015-0758-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel K, Roberts JA, Lipman J, Tett SE, Deldot ME, Kirkpatrick CM. 2011. Population pharmacokinetics of fluconazole in critically ill patients receiving continuous venovenous hemodiafiltration: using Monte Carlo simulations to predict doses for specified pharmacodynamic targets. Antimicrob Agents Chemother 55:5868–5873. doi: 10.1128/AAC.00424-11. [DOI] [PMC free article] [PubMed] [Google Scholar]