Abstract
A novel and highly accurate diagnostic assay platform was established for rapid identification of FKS mutations associated with echinocandin resistance in Candida glabrata. The assay platform uses allele-specific molecular beacon and DNA melt analysis following asymmetric PCR. A dual assay for FKS1 and FKS2 was developed to identify within 3 h the most common and clinically relevant resistance-associated mutations, including 8 FKS1 HS1 (wild type [WT], S629P, F625S, D632Y, D632E [T1896G], D632E [T1896A], I634V, and F625F) and 7 FKS2 HS1 (WT, F659del, F659S, F659V, F659L, S663P, and S663F) genotypes. A blinded panel of 188 C. glabrata clinical isolates was tested by both assays. The molecular diagnostic results from the dual assay were 100% concordant with data obtained from DNA sequencing. This platform has the potential to overcome the deficiencies of existing in vitro susceptibility-based assays to identify echinocandin-resistant C. glabrata and holds promise as a surrogate diagnostic method to better direct echinocandin therapy.
INTRODUCTION
Candida glabrata is the second leading cause of candidemia in the United States, as well as in northern and eastern areas of Europe (1–3). With rapidly expanded usage of echinocandins, the first-line therapy for invasive candidiasis, an alarming trend of rising echinocandin resistance in C. glabrata has posed a serious clinical challenge (4–6). Detection of echinocandin resistance can be assessed phenotypically, using either microdilution or disc diffusion MIC assays performed in accordance with guidance of the Clinical and Laboratory Standards Institute (CLSI) M27-A3 standard (7) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) Definitive Document Edef 7.2 (8). Current echinocandin clinical breakpoints for C. glabrata were established by the CLSI for all echinocandins and by EUCAST for micafungin and anidulafungin. Breakpoints were specifically developed to identify C. glabrata harboring FKS mutations by considering multiple factors, such as β-1,3-d-glucan synthase enzyme kinetics and echinocandin pharmacokinetic and pharmacodynamic data (6, 9). However, despite standardized methodologies, important limitations with the in vitro susceptibility testing cannot be ignored: first, the assay has a time-consuming setup and intrinsically slow turnaround time, requiring 24 to 48 h after isolate recovery; second, interlaboratory variability of caspofungin MICs has limited direct testing of this drug (10); and third, susceptible and resistant populations overlap (11).
Clinical echinocandin resistance in C. glabrata is associated with amino acid substitutions caused by mutations in specific hot-spot (HS) regions of FKS1 and FKS2, which encode the drug target β-1,3-d-glucan synthase. A recent study performed among patients with invasive candidiasis due to C. glabrata has shown that the FKS genotype is a better indicator of echinocandin therapeutic failure than MIC (12).
To date, DNA sequencing is the only means to identify mutations within FKS1 and FKS2 genes. Sequence data are highly informative and accurate. However, targeted gene sequencing is impractical for the regular workflow of clinical microbiology laboratories. It is also costly and time-consuming. There is an emerging need for an alternative molecular tool which allows rapid and accurate discrimination of various FKS mutations in C. glabrata and thereafter has the potential to become part of clinical laboratory routine tests to direct echinocandin therapy.
Molecular beacons (MBs) are small stem-loop-structured DNA oligonucleotides that are ideal for typing single-nucleotide polymorphisms (13). In a classical way, MBs are utilized as amplification reporters in real-time PCR where each probe is specific for a defined genotype and labeled with a differently colored fluorophore. By the same token, a comprehensive FKS genotyping assay will require a large number of probes to cover the full spectrum of nucleic acid variations within the target region. Multiple PCR assays will therefore be needed, and optimization of assay conditions is extremely demanding due to complex oligonucleotide interactions in a multiplex format. In contrast, a more desirable and feasible approach to distinguish between closely related genotypes is to use asymmetric PCR in conjunction with MB probe-based melting curve analysis (14, 15). During asymmetric PCR, a single-stranded amplicon is produced, allowing the probe to anneal and generate fluorescence at low temperature. The fluorescence intensity decreases when the probe slowly dissociates from the target as a result of gradually increased temperature in the subsequent melting analysis. The temperature at which the probe-target hybrids melt apart (Tm) is determined on the curve plotted by the fluorescence intensity change as a function of temperature. The Tm value varies when the target changes from perfectly matched sequence to mismatched sequence in the testing system, thereby providing a solid basis for wild-type (WT)/non-WT discrimination.
Based on these physiochemical properties of nucleic acids, we have established a molecular diagnostic assay platform for rapid genotyping of FKS1 HS1 and FKS2 HS1 of C. glabrata. Assay performance was evaluated on a blinded panel of clinical C. glabrata isolates representing a full spectrum of FKS1 and FKS2 genotype varieties in the fungal pathogen repository at the University of Pittsburgh Medical Center (UPMC).
MATERIALS AND METHODS
Candida glabrata strains and culture conditions.
A total of 262 C. glabrata strains were included in the present study. Seventy-four echinocandin-susceptible and -resistant strains stocked in the Perlin laboratory collection at the Public Health Research Institute were used for assay development and reference library establishment, and 188 C. glabrata isolates recovered from patients at UPMC were used for the validation study in a blind fashion. Strains were grown on yeast extract peptone dextrose (YPD) agar plates prior to testing.
Echinocandin susceptibility testing.
Echinocandin (caspofungin, micafungin, and anidulafungin) MICs were determined for reference strains in the Perlin collection according to CLSI protocol M27-A3 (Table 1) (7).
TABLE 1.
MIC distributions for reference C. glabrata strains used for assay development
| HS1 genotype (no. of isolates) | MIC range (μg/ml) |
||
|---|---|---|---|
| Anidulafungin | Caspofungin | Micafungin | |
| FKS1 | |||
| WT (15) | 0.06–0.25 | 0.06–0.25 | 0.06–0.12 |
| S629P (4) | 4–8 | 8–32 | 2–8 |
| F625S (3) | 2 | 2 | 0.5 |
| D632E (T1896G) (2) | 2 | 2 | 2 |
| D632E (T1896A) (2) | 1 | 0.5–1 | 0.25 |
| D632Y (1) | 4 | 4 | 1 |
| I634Va (4) | 0.06 | 0.06 | 0.03 |
| F625F (6) | 0.06–0.12 | 0.06–0.25 | 0.03 |
| FKS2 | |||
| WT (15) | 0.06–0.25 | 0.06–0.25 | 0.06–0.12 |
| F659del (3) | 2 | 8 | 4 |
| F659S (3) | 1–4 | 1–32 | 0.12–4 |
| F659V (4) | 1 | 4 | 0.25–1 |
| F659L (1) | 1 | 2 | 0.12 |
| S663P (8) | 1–8 | 4–32 | 0.5–32 |
| S663F (3) | 4 | 4 | 4 |
DNA extraction.
C. glabrata DNA was prepared by incubating a single colony of a strain in 100 μl of extraction buffer (60 mM NaHCO3, 250 mM KCl, 50 mM Tris, pH 9.5) at 95°C for 10 min followed by an addition of 100 μl of 2% bovine serum albumin (16).
Primers and probe design.
Two sets of asymmetric PCR primers were designed to amplify the FKS1 HS1 and FKS2 HS1 regions of C. glabrata. The excess primer for FKS1 HS1 amplification was CgF1H1-X (5′-GGGTTACTGTTTTTGCTGCT-3′), and the limiting primer was CgF1H1-L (5′-GAACCCCACCAGTATTCACCAGTACA-3′). The FKS2 HS1 region was amplified by using excess primer CgF2H1-X (5′-TGGGTTACAGTTTTTGCTGC-3′) and limiting primer CgF2H1-L (5′-AACCCCACCAATACTCACCAGTACATC-3′). Two molecular beacons were designed targeting the WT sequences of FKS1 HS1 and FKS2 HS1. The sequences of the two WT MBs were CgF1H1-RWT (5′-6-carboxyfluorescein [FAM]-CGCGACTGGATCTCTTAGAGATAGAATCAAGAAGGTCGCG-DABCYL-3′) and CgF2H1-RWT (5′-FAM-CGCGACAGGGTCTCTTAGAGACAAAATCAAGAAGTCGCG-DABCYL-3′) (underlining signifies the stem portion of the molecular beacon). It was noticed that some susceptible strains carrying a silent mutation (C1875T) at codon F625 in FKS1 HS1 generated melting profiles very similar to that produced by a rare D632E-resistant mutant carrying a T1896A mutation. To differentiate between susceptible strains carrying the silent mutation and D632E (T1896A) mutants, we designed an F625F silent mutation-specific MB labeled with a different fluorophore, Cg-F1H1-RF625F (5′-HEX-CGCGCAAAAAGTAGTATGATTCACGCG-DABCYL-3′). This probe can be simply added to reactions with “unresolved F625F/D632E (T1896A) classifications” as a post-FKS1 HS1 test to quickly rule out that the T1896A mutation caused D632E.
Asymmetric PCR and molecular beacon-based melting curve analysis.
Asymmetric PCR was carried out on the AriaMx real-time PCR system (Agilent Technologies, CA) in a 20-μl reaction volume using Choice Taq Mastermix (Denville Scientific Inc.). FKS1 HS1 assay contains 30 μM CgF1H1-X, 1 μM CgF1H1-L, 50 nM CgF1H1-RWT MB, and 10 to 25 ng of DNA template. When discriminating F625F and D632E (T1896A), the CgF1H1-RF625F MB was added to the FKS1 HS1 reaction mixture at 25 nM. FKS2 HS1 assay contains 50 μM CgF2H1-X, 1 μM CgF2H1-L, and 50 nM CgF2H1-RWT MB. FKS1 HS1 PCR thermal cycling consisted of 3 min of incubation at 95°C; 45 cycles of 30 s at 95°C, 30 s at 57°C, and 1 min at 72°C; and then incubation at 72°C for 5 min. FKS2 HS1 PCR conditions were 95°C for 3 min; 40 cycles of 30 s at 95°C, 30 s at 60°C, and 1 min at 72°C; and 5 min at 72°C. Immediately after amplification, melting curve analysis was performed at 95°C for 3 min and then 40°C for 30 s, after which it was melted from 40°C to 70°C with a ramp rate of 0.2°C/s.
Colony PCR genotyping.
To further reduce the time to diagnosis, we introduced colony PCR into our diagnostic platform. Instead of using DNA extracts, a sterile toothpick with a touch of testing single colony was dipped into the PCR reaction medium, and then asymmetric PCR and the following melting analysis were performed as described above. At least three representative strains for each FKS genotype (8 FKS1 HS1 genotypes and 7 FKS2 HS1 genotypes) were tested for the efficiency of colony PCR genotyping.
DNA sequencing.
FKS1 HS1 and FKS2 HS1 regions were amplified and sequenced in both directions as previously described (17).
Statistical analysis.
Tm values for each FKS genotype were determined by melting curve analysis using the AriaMx software (version 1.0) (Agilent Technologies). Blind genotyping results by rapid molecular diagnostic assays were compared with DNA sequencing. The Tm distribution of clinical isolates was analyzed by GraphPad Prism 7.01 software. The accuracy of the novel assays discriminating WT from mutated fks genotypes was evaluated by calculating sensitivity and specificity for each assay.
RESULTS
Melting curve analysis-based FKS1/FSK2 HS1 genotyping.
To distinguish echinocandin resistance-associated mutations from the WT in the FKS1/FSK2 HS1 region, we designed two discrimination probes, CgF1H1-RWT and CgF2H1-RWT. Both probes were designed to complement the WT genotype in the target region but possess various energies of binding to non-WT sequences, which reflect mutation and temperature. To enable efficient dissociation of probe-target hybrids for rapid genotyping, asymmetric PCR amplifications were optimized to generate an excess of the sense strand for both targets. Due to the stability difference of the probe-target hybrids, characteristic profiles were produced for different FKS genotypes in the subsequent melting curve analysis. Figure 1A shows melting curves of representative FKS1 HS1 genotypes, including WT, S629P, F625S, D632Y, D632E (T1896G), D632E (T1896A), I634V (just outside the hot-spot region yet not associated with resistance [6, 18, 19]), and F625F (C1875T). Melting curves of mutated genotypes were remarkably different from that of the WT, and the Tm values of mutations were also distinguishable from each other, except for the F625F silent mutation and D632E (T1896A) (Table 2). In the FKS2 HS1 assay, a melting curve with the Tm at 61°C ± 0.2°C was the signature for the WT, and unique melting profiles were also obtained for all mutations tested (F659del, F659S, F659V, F659L, and S663P/F) (Fig. 1B and Table 2).
FIG 1.
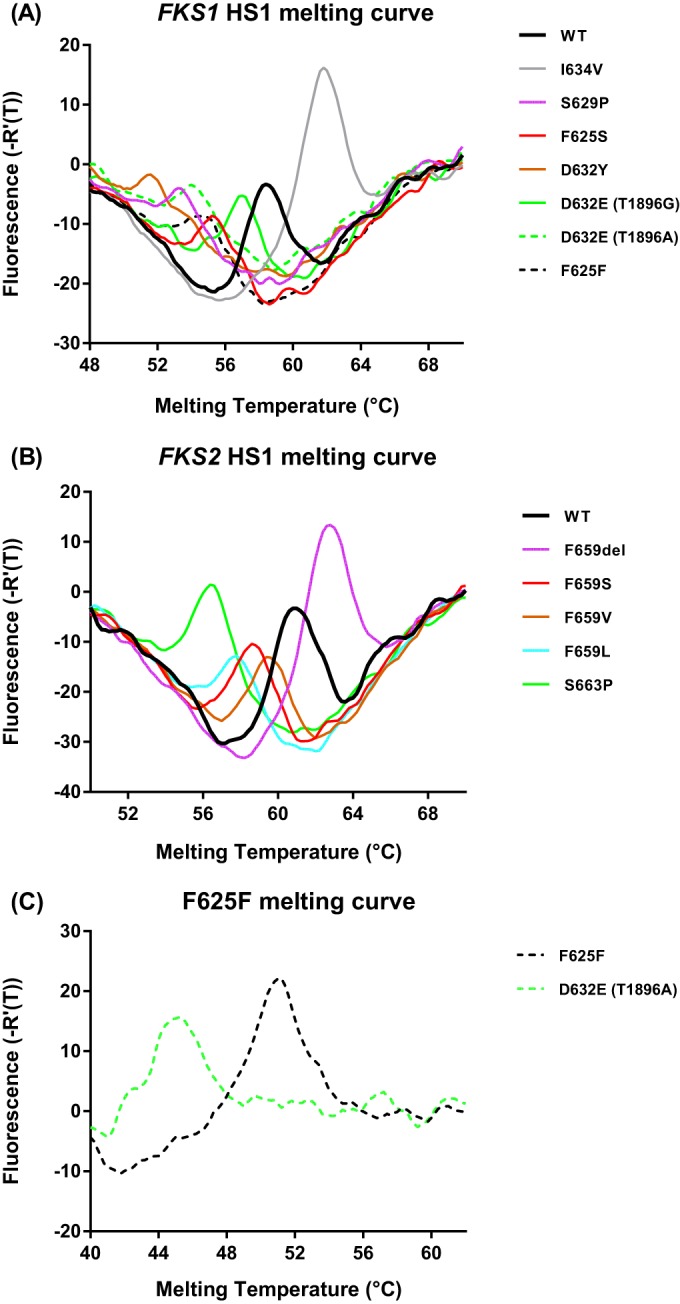
Representative melting profiles for C. glabrata FKS1 HS1 genotypes, including WT, S629P, F625S, D632Y, D632E (T1896G), D632E (T1896A), I634V (not associated with resistance), and F625F (C1875T) (A), and FKS2 HS1 genotypes, including F659del, F659S, F659V, F659L, and S663P (B). (C) Differentiation between F625F and D632E (T1896A) by an F625F-specific MB probe.
TABLE 2.
Tm values for C. glabrata FKS1 HS1 and FKS2 HS1
| HS1 variant | Tm (°C) | SD (°C) |
|---|---|---|
| FKS1 | ||
| WT | 58.7 | 0.2 |
| I634V (A1900G) | 62 | 0.2 |
| F625S (T1874C) | 55.2 | 0.2 |
| D632E (T1896A); F625F (C1875T)a | 54.2 | 0.4 |
| S629P (T1885C) | 52.8 | 0.2 |
| D632Y (G1894T); D632H (G1894C) | 51.3 | 0.5 |
| FKS2 | ||
| WT | 61 | 0.2 |
| F659del (del1957-1977) | 62.7 | 0.1 |
| F659V (T1975G) | 59.4 | 0.1 |
| F659S (T1976C) | 58.4 | 0.1 |
| F659L (C1977A) | 57.8 | 0 |
| S663P (T1987C); S663F (C1988T) | 56.4 | 0.2 |
Silent mutation can be differentiated from the “true” resistance-associated mutation D632E (T1896A) with the second-tier assay using the F625F-specific MB probe.
To resolve indistinguishable melting signatures between F625F and D632E (T1896A) in the FKS1 HS1 assay, an F625F-specific MB probe was added to the reaction mixture following FKS1 HS1 assay analysis. A second round of melting analysis was then performed on the HEX channel, during which D632E (T1896A) (Tm = 45.4°C ± 0.1°C) was widely separated from the silent mutation (Tm = 51.2°C ± 0.1°C) by an approximately 6°C Tm difference (Fig. 1C).
Direct colony FKS genotyping.
By replacing DNA extracts with direct single-colony picking in the established assay system, we successfully detected all representative strains (Table 1) and generated robust melting profiles for all testing genotypes consistent with those produced by DNA samples. Most importantly, direct genotyping from a colony was completed within 3 h, shortening the turnaround time by 30 min.
Validation experiments.
As a proof of concept, we evaluated the diagnostic performance of this novel FKS genotyping platform using a panel of 188 C. glabrata clinical isolates collected at UPMC. Information on FKS sequences and echinocandin susceptibility was unknown at the time of molecular diagnosis. All isolates were tested in duplicate for the dual assay, and reference strains representing all genotypes used for platform establishment were tested in parallel in every single run. Inclusion of reference strains in each test run was important, as they served as systematic quality controls to ensure the assay's accuracy and reproducibility. It was noted that two isolates repeatedly failed in both assays and were identified subsequently by internal transcribed spacer sequencing as non-C. glabrata (1 Saccharomyces cerevisiae isolate and 1 Candida guilliermondii isolate). Robust and reproducible melting profiles were observed for all other isolates. Compared with Tm values generated by reference strains of various FKS HS1 genotypes, the FKS1 HS1 assay identified 138 WT sequences (Tm = 58.7°C ± 0.2°C), 45 F625F silent mutations (54.3°C ± 0.3°C), and 3 D632 alterations (51.3°C ± 0.5°C) (Fig. 2A). DNA sequencing results confirmed all predicted genotypes and further identified the 3 D632 alterations as 2 D632Y and 1 D632H alteration. The FKS2 HS1 assay detected 176 WT sequences (Tm = 61°C ± 0.2°C) as well as 2 F659del (Tm = 62.7°C ± 0°C), 2 F659S (Tm = 58.4°C ± 0.1°C), 2 F659L (Tm = 57.8°C ± 0°C), and 4 S663P/F (Tm = 56.4°C ± 0.2°C) alterations (Fig. 2B). Molecular typing results were 100% accurate on the F659 codon, and the 4 predicted S663 alterations were identified as 3 S663P and 1 S663F alteration by sequencing. In summary, both FKS1 HS1 and FKS2 HS1 assays have demonstrated very promising diagnostic value for WT/non-WT discrimination with 100% specificity and 100% sensitivity.
FIG 2.
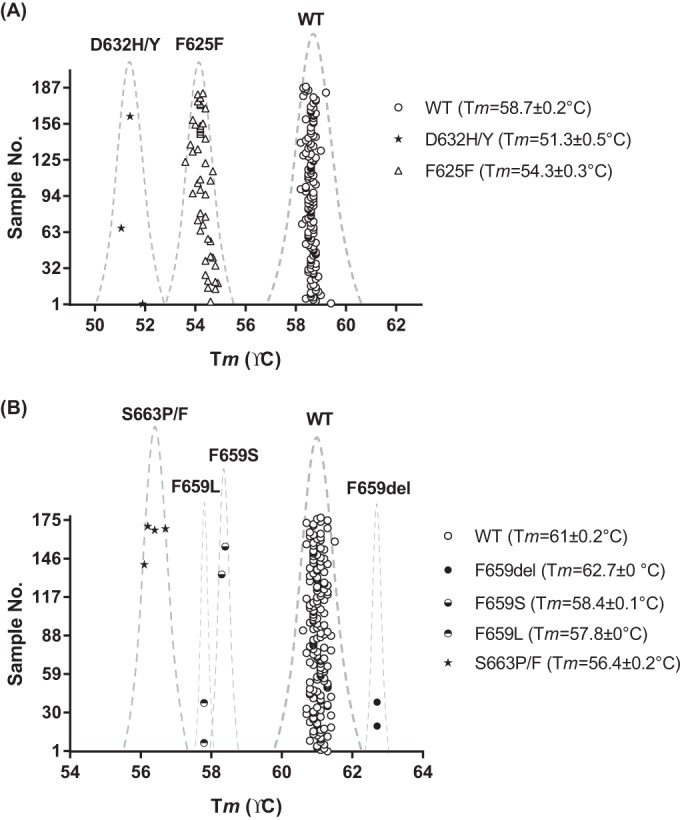
FKS1 HS1 (A) and FKS2 HS1 (B) Tm distributions of 186 blind-tested C. glabrata clinical isolates.
DISCUSSION
FKS HS mutation is the major mechanism of echinocandin resistance in C. glabrata, providing an underlying rationale for developing rapid molecular detection of FKS genotypes. The goal of current echinocandin breakpoints is to distinguish C. glabrata isolates with a WT FKS from those containing FKS mutations that are unlikely to respond to echinocandin therapy (9). While mutations at almost every amino acid position within the FKS hot spot 1 and 2 regions confer some degree of resistance (20), recent studies have shown that the majority of mutations that affect C. glabrata susceptibility to the echinocandins are located within FKS1 and FKS2 HS1 regions. Accordingly, these regions are the primary targets for molecular assays to detect drug resistance (6, 21).
Given this goal, we have developed a novel diagnostic platform for rapid FKS genotyping of C. glabrata isolates. This platform, taking advantage of the strong single-nucleotide polymorphism recognition capability of molecular beacons and incorporating asymmetric PCR, accurately discriminated WT from various mutated FKS genes within 3 h. Using reference strains in our in-house collection, signature melting profiles and corresponding Tm values were generated for both WT and non-WT populations. Specific mutations are identified either individually or in a small cluster with distinguishable and consistent Tm values, demonstrating the robust discrimination power of this novel platform. Furthermore, with the adaptation of colony PCR in the testing system, the turnaround time was reduced by 30 min without losing the discrimination power. In the subsequent proof-of-concept clinical performance evaluation study, all blinded C. glabrata clinical isolates were successfully identified as either WT or mutated FKS genotypes, with 100% accuracy compared to DNA sequencing results.
Promising data were achieved from both assay development and clinical validation, but we did notice a minor issue with the FKS1 HS1 assay. Tm values were indistinguishable between the silent mutation F625F and a weakly resistant genotype, D632E (T1896A). In theory, such unresolved susceptible/resistant classification is a concern for directing antifungal therapy. In reality, however, it may be only trivial to the diagnostic value of this FKS genotyping method, because the frequency of T1896A mutation-caused D632E is very low (estimated as <0.5% of all FKS HS mutations) based on several large-scale epidemiological studies on FKS mutations (6, 12, 18, 19, 22, 23). Nevertheless, an F625F silent mutation-specific MB probe was designed to address this susceptible/resistant uncertainty. This silent mutation probe was added to the reaction mixture following FKS1 HS1 analysis and used as a second-tier assay. Using this method, all reactions with the F625F/D632E (T1896A) Tm values in the clinical isolate blind testing were subjected to a second melting analysis, and a unanimous F625F genotype which has no influence on susceptibility was identified for all suspected isolates, concordant with DNA sequencing results. It should be pointed out that the second-tier assay does require opening reaction tubes for the addition of the second beacon, which may carry the risk of contamination. However, this risk is relatively low, since the second-tier assay only involves a melting curve analysis without an amplification procedure. Technically, a duplex assay combining both FKS1 HS1 WT probe with the F625F probe is the best option for high-throughput genotyping, but the melting curve resolution of the original WT probe was noticeably decreased with the addition of the F625F probe (data not shown). Presumably, the majority of unresolved strains in the primary FKS1 HS1 test are susceptible strains harboring F625F silent mutation as a result of the very low frequency of D632E (T1896A); therefore, the need for a definite discrimination between these two genotypes is low. The two-tier assay strategy is currently recommended for comprehensive FKS1 HS1 genotyping.
DNA sequencing has been the only technique available for definite identification of FKS mutations. Thus far, only two studies have reported development of rapid FKS genotyping assays (21, 24). Despite good diagnostic performance, both assays suffer from complicated assay design and setup. Compared to these assays, advantages of the present platform include (i) simple design modality, where a single MB probe and one set of primers allow the detection of a wide spectrum of mutations in the target region; (ii) ease of data interpretation, as the FKS genotype of the testing strain can be easily identified by comparing the Tm value with those generated by reference strains representing various mutations in the target region; (iii) multiplex potential, because the simple design modality allows probe build-up and the high specificity of each assay (no cross-reactivity) allows for probe combination; and (iv) expandable readiness for novel/unreported mutation detection, because the assay will be able to pick up a novel mutation by simply incorporating the corresponding genotype into the library without the need for assay redesign.
We acknowledge a minor limitation of our assay. Since the reference strains used for assay development do not cover the full FKS mutation spectrum, there is a chance that the Tm values of untested mutation genotypes are not distinguishable from other mutations already included in our reference library. However, this will only have minimal impact on our platform, because the uncovered mutations account for only <10% of the mutants and would be <1% of the entire C. glabrata population. Furthermore, no cross-reaction between untested mutations and the WT is expected, because probes were designed to have complete sequence match only with the WT.
In summary, we have developed a rapid and accurate diagnostic platform that has the potential to overcome the deficiencies of existing MIC-based assays to identify echinocandin-resistant C. glabrata, and it holds the promise to be a surrogate diagnostic method to better direct echinocandin therapy.
ACKNOWLEDGMENTS
We thank Salvatore Marras for his technical assistance with assay development.
This study was supported by a Merck Investigator grant (53563) to D.S.P. and Y.Z. and NIH grants R01AI109025 to D.S.P. and K08AI114883 to R.K.S.
REFERENCES
- 1.Lockhart SR, Iqbal N, Cleveland AA, Farley MM, Harrison LH, Bolden CB, Baughman W, Stein B, Hollick R, Park BJ, Chiller T. 2012. Species identification and antifungal susceptibility testing of Candida bloodstream isolates from population-based surveillance studies in two U.S. cities from 2008 to 2011. J Clin Microbiol 50:3435–3442. doi: 10.1128/JCM.01283-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guinea J. 2014. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20(Suppl 6):S5–S10. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Horn D. 2012. Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance) registry, 2004-2008. Diagn Microbiol Infect Dis 74:323–331. doi: 10.1016/j.diagmicrobio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfaller MA, Castanheira M, Lockhart SR, Ahlquist AM, Messer SA, Jones RN. 2012. Frequency of decreased susceptibility and resistance to echinocandins among fluconazole-resistant bloodstream isolates of Candida glabrata. J Clin Microbiol 50:1199–1203. doi: 10.1128/JCM.06112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, Schaffner W, Beldavs ZG, Chiller TM, Park BJ, Cleveland AA, Lockhart SR. 2014. Role of FKS mutations in Candida glabrata: MIC values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother 58:4690–4696. doi: 10.1128/AAC.03255-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Document M27-A3. CLSI, Wayne, PA. [Google Scholar]
- 8.Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope W. 2012. EUCAST technical note on the EUCAST definitive document EDef 7.2: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin Microbiol Infect 18:E246–E247. doi: 10.1111/j.1469-0691.2012.03880.x. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 14:164–176. doi: 10.1016/j.drup.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Florl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arendrup MC, Garcia-Effron G, Lass-Florl C, Lopez AG, Rodriguez-Tudela JL, Cuenca-Estrella M, Perlin DS. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and Iso-Sensitest media. Antimicrob Agents Chemother 54:426–439. doi: 10.1128/AAC.01256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shields RK, Nguyen MH, Press EG, Kwa AL, Cheng S, Du C, Clancy CJ. 2012. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive candidiasis due to Candida glabrata. Antimicrob Agents Chemother 56:4862–4869. doi: 10.1128/AAC.00027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marras SA, Kramer FR, Tyagi S. 2003. Genotyping SNPs with molecular beacons. Methods Mol Biol 212:111–128. [DOI] [PubMed] [Google Scholar]
- 14.El-Hajj HH, Marras SA, Tyagi S, Shashkina E, Kamboj M, Kiehn TE, Glickman MS, Kramer FR, Alland D. 2009. Use of sloppy molecular beacon probes for identification of mycobacterial species. J Clin Microbiol 47:1190–1198. doi: 10.1128/JCM.02043-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakravorty S, Kothari H, Aladegbami B, Cho EJ, Lee JS, Roh SS, Kim H, Kwak H, Lee EG, Hwang SH, Banada PP, Safi H, Via LE, Cho SN, Barry CE III, Alland D. 2012. Rapid, high-throughput detection of rifampin resistance and heteroresistance in Mycobacterium tuberculosis by use of sloppy molecular beacon melting temperature coding. J Clin Microbiol 50:2194–2202. doi: 10.1128/JCM.00143-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brillowska-Dabrowska A, Saunte DM, Arendrup MC. 2007. Five-hour diagnosis of dermatophyte nail infections with specific detection of Trichophyton rubrum. J Clin Microbiol 45:1200–1204. doi: 10.1128/JCM.02072-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Healey KR, Zhao Y, Perez WB, Lockhart SR, Sobel JD, Farmakiotis D, Kontoyiannis DP, Sanglard D, Taj-Aldeen SJ, Alexander BD, Jimenez-Ortigosa C, Shor E, Perlin DS. 2016. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat Commun 7:11128. doi: 10.1038/ncomms11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyda ND, John J, Kilic A, Alam MJ, Lasco TM, Garey KW. 2014. FKS mutant Candida glabrata: risk factors and outcomes in patients with candidemia. Clin Infect Dis 59:819–825. doi: 10.1093/cid/ciu407. [DOI] [PubMed] [Google Scholar]
- 19.Pfaller MA, Diekema DJ, Jones RN, Castanheira M. 2014. Use of anidulafungin as a surrogate marker to predict susceptibility and resistance to caspofungin among 4,290 clinical isolates of Candida by using CLSI methods and interpretive criteria. J Clin Microbiol 52:3223–3229. doi: 10.1128/JCM.00782-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arendrup MC, Perlin DS. 2014. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis 27:484–492. doi: 10.1097/QCO.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham CD, Bolden CB, Kuykendall RJ, Lockhart SR. 2014. Development of a Luminex-based multiplex assay for detection of mutations conferring resistance to echinocandins in Candida glabrata. J Clin Microbiol 52:790–795. doi: 10.1128/JCM.03378-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimbeck AJ, Iqbal N, Ahlquist AM, Farley MM, Harrison LH, Chiller T, Lockhart SR. 2010. FKS mutations and elevated echinocandin MIC values among Candida glabrata isolates from U.S. population-based surveillance. Antimicrob Agents Chemother 54:5042–5047. doi: 10.1128/AAC.00836-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castanheira M, Woosley LN, Messer SA, Diekema DJ, Jones RN, Pfaller MA. 2014. Frequency of fks mutations among Candida glabrata isolates from a 10-year global collection of bloodstream infection isolates. Antimicrob Agents Chemother 58:577–580. doi: 10.1128/AAC.01674-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudiuk C, Gamarra S, Leonardeli F, Jimenez-Ortigosa C, Vitale RG, Afeltra J, Perlin DS, Garcia-Effron G. 2014. Set of classical PCRs for detection of mutations in Candida glabrata FKS genes linked with echinocandin resistance. J Clin Microbiol 52:2609–2614. doi: 10.1128/JCM.01038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


